Abstract
The correct regulation of organ size is a fundamental developmental process, the failure of which can compromise organ function and organismal integrity. Consequently, the mechanisms that regulate organ size have been subject to intense research. This research has highlighted four classes of mechanism that are involved in organ size regulation: physiology, plasticity, patterning and physical force. Nevertheless, how these mechanisms are integrated and converge on the cellular process that regulate organ growth is unknown. One group of animals where this integration is beginning to be achieved is in the insects. Here, I review the different mechanisms that regulate organ size in insects, and describe our current understanding of how these mechanisms interact. The genes and hormones involved are remarkably conserved in all animals, so these studies in insects provide a precedent for future research on organ size regulation in mammals.
Key words: size regulation, insulin, insect, hormone, imaginal disc, hypoplasia, morphogens, adhesion
Introduction
The regulation of final organ size is a fundamental and fundamentally important developmental process. To a great extent organ size reflects organ function, and this function may be compromised if organ size is not regulated correctly. Myriad medical conditions result from the inappropriate over or undergrowth of different organs and so the problem of size regulation is of considerable biomedical interest. The problem is a complex one. Over the last couple of decades, a number of different size regulatory mechanisms have been uncovered. Broadly speaking, these mechanisms fall into four categories: (1) physiological mechanisms that regulate the duration of organ growth; (2) plasticity mechanisms that regulate organ size in response to changes in environmental factors; (3) patterning mechanisms that regulate organ size by controlling the growth and differentiation of tissues within the organ; and (4) physical mechanisms that regulate organ size in response to mechanical force. Research into each of these four areas has been largely independent. However, all four mechanisms must at some level be integrated and converge on the cellular processes that regulate cell growth and division. A major challenge to understanding the mechanisms that regulate organ size is elucidating how this integration is achieved.
One group of animals where an integrative understanding of organ size regulation is beginning to be achieved is the insects, in particular the fruit fly, Drosophila melanogaster. Here, I review our current knowledge of organ size regulation in Drosophila. I will explore the mechanisms by which physiology, plasticity, pattern and physical force affect organ size, and, where possible, describe our understanding of how these different mechanisms interact. As will become clear, the genes and signaling pathways involved are remarkably similar between Drosophila and mammals. Thus a comprehensive understanding of how organ size is regulated in insects is informative of how organ size is regulated in animals in general.
Physiology
Hormones in Drosophila development.
Organ growth is not an autonomous process. It occurs within the context of a developing body. In particular, growth must be coordinated across the whole organism so that each organ reaches its appropriate size at the appropriate time. This coordination of growth, and the cessation of growth, is achieved through hormonal mechanisms. Among all animals, these mechanisms have perhaps been best elucidated in holometabolous insects, such as Drosophila.
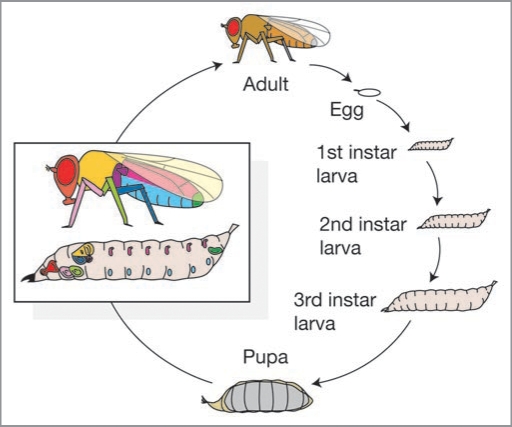
Drosophila are typical holometabolous insects: they begin life as worm-like larvae, molting through three larval instars before undergoing complete metamorphosis as a pupa and eventually eclosing into their adult form (Fig. 1).1,2 Adult flies, like all arthropods, have a stiff exoskeleton and so they can not grow. Consequently, adult body size is entirely regulated by growth during the premetamorphic larval stages. Further, in holometabolous insects the adult organs are not visible until after metamorphosis. Instead, they grow as imaginal discs within the developing larvae, each disc corresponding to an adult structure (Fig. 1). During metamorphosis these imaginal discs differentiate and evaginate to form the adult organs. Just as adult body size is determined by premetamorphic growth, so too is adult organ size determined by growth of the imaginal discs during the larval stages.
Figure 1.
The life cycle of Drosophila melanogaster. Larvae molt through three larval instars before metamorphosing into their adult form. Drosophila is a holometabolous insect and its adult organs develop as imaginal discs within the larva. Inset shows approximate positions of imaginal discs. Colors relate disc to corresponding adult organ.
The duration of each larval instar, and hence the duration of larval growth, is regulated by the release of hormones that control molting: ecdysteroids, prothoracicotropic hormone (PTTH), eclosion hormone (EH) and juvenile hormone (JH). The action of these hormones has been best elucidated in large lepidopterans, like the tobacco hornworm (Manduca sexta)1,3 and the silk moth (Bombyx mori),4,5 although the details are thought to be similar in all holometabolous insects, including Drosophila. Molts are initiated by the production of PTTH from neurosecretory glands in the brain.6 PTTH in turn stimulates the synthesis and release of ecdysone from the prothoracic gland (PG).7 It is the rise and subsequent fall in ecdysteroids (the metabolic derivatives of ecdysone) that initiates and coordinates the synthesis of new cuticle with growth and development of internal organs, in preparation for molting to the new instar.8 Molting itself is caused by EH, which is produced by neurosecretory cells in the brain when ecdysteroids titres fall to a low level.9
JH levels are maintained throughout larval development, and its presence during the release of ecdysone ensures that a larva molts to the next larval instar rather than undergoes metamorphosis. During the final larval instar, however, JH levels decline. In Manduca sexta, when the titre falls to essentially undetectable levels, this de-represses the release of PTTH,10 which again stimulates the release of a small pulse of ecdysone causing the larva to stop feeding and find a site for pupation. This is followed by a more substantial release of ecdysone, initiating pupation itself, followed by an even greater release of ecdysone that causes the imaginal discs to stop growing, differentiate and evaginate (Fig. 2).
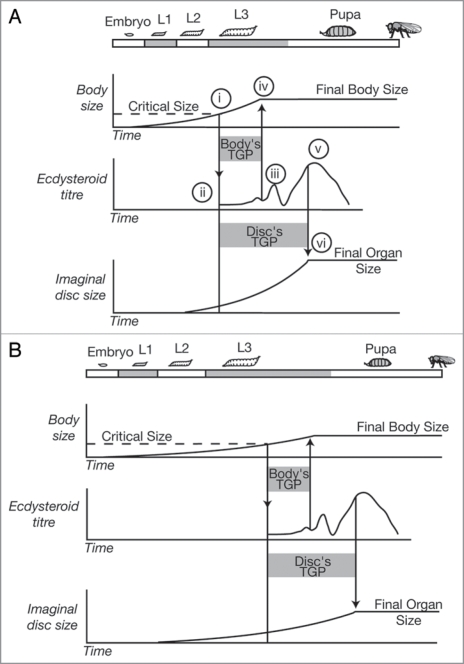
Figure 2.
The physiological regulation of body and organ size in Drosophila. (A) Larvae grow until they reach a critical size at the beginning of the third larval instar (i), which initiates a hormonal cascade that ultimately causes the release of ecdysteroids (ii). When the ecdysteroids rise above a certain level (iii) it causes the larvae to stop feeding and begin wandering (iv) stopping body growth. Subsequent peaks in the ecdysteroid titre (v) cause pupation and the cessation of organ growth (vi). (B) A reduction in nutrition slows growth and delays attainment of critical size, extending total developmental time. Attainment of critical size initiates the same hormonal cascade that brings about the cessation of body and imaginal disc growth. The temporal dynamics of this cascade are unaffected by nutrition. Slow growth of the body and imaginal discs now reduces the amount of growth they can achieve during their TGPs, reducing final body and organ size. Hormones other than ecdsyteroids may be involved in the cessation of disc growth. L1–L3, first to third larval instar; TGP, terminal growth period. Adapted from ref. 11.
The release of ecdysone, JH, PTTH and EH regulates and coordinates key developmental stages in holometabolous insects. Importantly, final body and organ size is controlled by growth within the periods delimitated by these hormonal cascades. Thus the mechanisms that regulate the timing of hormone release also regulate final body and organ size. Very little is known of how the timing of larval-larval molts is regulated. Much more is known, however, of the mechanisms that regulate the timing of metamorphosis, which defines the cessation of larva growth and hence fixes maximum adult body and organ size.
Critical size and the timing of metamorphosis.
In Drosophila, the timing of metamorphosis is regulated by a larva reaching a size checkpoint called critical size (or critical weight) in the final larval instar. Attainment of critical size is associated with initiation of the hormonal cascade that ends in metamorphosis (Fig. 2A(i)). There is, however, temporal separation between the attainment of critical size and the elevation in the ecdysteroid titer that causes the larva to stop feeding and ends body growth. This delay provides a final period of growth for the larvae, called the terminal growth period (TGP), during which Drosophila larvae can more than triple their mass.11,12 Body size in Drosophila is therefore regulated by the critical size, plus the amount of growth achieved during the TGP.11,13–15 Organ size is also regulated by the size of the organs at critical size plus the amount of growth they achieve during their TGPs. However, because the organs stop growing after the beginning of pupation, the TGPs of the organs are longer than the TGP of the body13 (Fig. 2A(v and vi)).
The central role that critical size plays in regulating body and organ size has lead to it being intensely studied by a number of researchers. Critical size was first described in Drosophila by Beadle et al. in the 1930s,16 but the link between attainment of a critical size and the hormonal cascade that ends in metamorphosis was elucidated in Manduca sexta.3,10,17 In this species, attainment of a particular body size is associated with a decline in the JH titre, de-repressing the release of PTTH and ecdysone, and initiating metamorphosis. Initially, critical size was seen as being a readout of body size.17 More recently, researchers have begun to use Drosophila to explore the details of how critical size is assessed.
In Drosophila, the attainment of critical size can be delayed by limiting larval growth through malnutrition or starvation. Insulin/insulin-like growth factor signaling (IIS) is the major signaling pathway regulating growth with respect to nutrition, not just in Drosophila but in all animals (see below). We have shown that, like starvation, a reduction in insulin/insulin-like growth factor signaling (IIS) early in development is sufficient to delay the attainment of critical size.18 Conversely, increasing IIS by upregulating the production of Drosophila insulin-like peptides (dILPs) from insulin-producing cells (IPCs) in the brain causes precocious metamorphosis.19 Critical size may therefore be monitored by an organ or organs, the growth of which is in turn regulated by IIS. One prediction of this hypothesis is that stimulating IIS in this organ alone should accelerate the organ’s growth and cause it to initiate metamorphosis prematurely. If the rest of the body were growing at a normal rate, this would result in an apparent decrease in critical size, reducing final adult size.
Mirth et al.20 and Caldwell et al.21 independently found that stimulating the IIS-pathway in the prothoracic gland accelerates its growth, reduces critical size and causes precocious metamorphosis, resulting in smaller adults. On the other hand, suppressing the IIS in the PG has the opposite effect, extending the duration of growth and resulting in body and organ overgorwth. These effects appear to be a consequence of altering the synthesis of ecysteroids in the PG. Larvae with IIS upregulated in the PG show an increase in transcription of ecdysone synthesizing genes and in ecdysone-regulated genes.21,22 Further, feeding 20-hydroxyecdysone to larvae in which IIS in the PG is suppressed rescues the overgrowth phenotype.22 This is consistent with the hypothesis that it is reduced ecdysteroid synthesis that retards metamorphosis in these larvae. Collectively, these data suggest that critical size is controlled by IIS-regulated growth of the PG, which synthesizes ecdysone only when it reaches a particular size.
IIS is not, however, the only activator of ecdysteroidgenesis in the PG. PTTH also stimulates the synthesis of ecdysteroids, and ablation of the PTTH-producing neurosecretory cells in the Drosophila brain decreases ecdysteroid titers, delays metamorphosis and increases final body size.23 In Manduca sexta, PTTH appears to act through phospho-ERK to activate ecdysone synthesis.24 Phospho-ERK is also a target of the Ras-Raf-MAPK pathway, which suggests that PTTH may activate ecdysteroidgenesis via activation of this pathway.19 Indeed, Caldwell et al. found that increasing Ras-Raf-MAPK signaling in the PG, like increasing IIS, also results in precocious metamorphosis and a reduction in final body size.21 However, in this case the effects are seen without changing growth of the PG. Interestingly, PTTH transcription in wild-type flies shows a cyclic profile during the third larval instar,23 which suggests that PTTH imposes circadian rhythm on the release of ecdysone and the timing of the resulting developmental events. In Drosophila this periodicity in PTTH expression may account for separate peaks in ecdysteroids that occur at the attainment of critical size, the cessation of feeding and the initiation of pupariation.23
Critical size therefore appears to be regulated by at least two mechanisms in Drosophila: (1) IIS-regulated growth of the PG and (2) stimulation of the PG by PTTH.19,21,25 Both of these mechanisms communicate different types of information to the PG: the IIS communicates size information while the PTTH communicates temporal information.19 Under this model, reduced nutrition slows IIS-regulated growth of the PG. Metamorphosis is delayed because the periodic peaks of PTTH are insufficient to initiate ecdysteroidgenesis without the action of IIS. Only when the PG has grown to a particular size, or ‘accumulated’ a particular level of IIS, is ecdysone synthesis initiated and critical size attained. Similarly, ablation of PTTH-producing cells may delay critical size until IIS in the PG is sufficient to initiate the production of ecdysone alone, without the action of PTTH.
Critical size and organ growth.
While IIS-regulated growth of the PG and periodic signaling via PTTH are both involved in the attainment of critical size, we might also expect the imaginal discs to be involved. After all, attainment of critical size is not just fundamental to the cessation of growth of the body as a whole, but also the organs within it. Indeed, earlier studies in Drosophila have shown that damage to individual developing imaginal discs causes a delay in metamorphosis.26–29 This is apparently to allow repair of the damaged discs, with disc cells adjusting their proliferation rates to regenerate to their normal size and shape.30,31 The extent of developmental retardation is affected not only by the extent of tissue damage, but also when the damage occurs.27 Damage before the beginning of the third-larval instar retards metamorphosis, while the effect is reduced or eliminated for older larvae.31 These data hint that critical size may also be regulated by the developing imaginal discs.
If imaginal discs were used in size assessment, then slowing their growth alone should cause larvae to miscalculate their body size resulting in an increase in critical size. To test this we measured critical size in flies in which the growth of the imaginal discs was retarded while the growth of the body as a whole was ostensibly unaffected.12 We used two methods to slow organ growth without affecting body growth.
First, we slowed the growth of the imaginal discs using X-rays. X-irradiation induces DNA damage, causing cell death and reducing the subsequent rate of cell division. In holometabolous insects, only imaginal tissue grows through cell division. Larval tissue, such as the PG, grow through endoreplication. Consequently, X-rays only affect growth in the imaginal discs.
Second, we slowed growth of the wing imaginal discs alone using targeted RNAi against ribosomal protein S3 (RpS3). RpS3 is a member of the Minute class of genes, which reduce the rate of cell division and cause developmental delay but do not affect final body and organ size. Due to the apparently cell-autonomous effects of Minute genes on cell proliferation, they have been commonly used to explore growth regulation in the imaginal discs.32–34 For RpS3 at least, the severity of the Minute phenotype depends on the extent to which a mutant allele decreases RpS3 mRNA abundance.35 Reducing RpS3 mRNA using RNAi in the wings therefore produces larvae with slow-growing Minute wings in an otherwise wild-type body.
In both experiments, damage to, or slow growth of, the imaginal discs caused an increase in critical size and a delay in the timing of metamorphosis. Thus we concluded that critical size is regulated by a signal from the growing imaginal discs. Unlike the signal from the PG, the imaginal signal is not contingent on IIS-regulated growth, since altering IIS in the wing imaginaldiscs alone does not affect critical size. Further, unlike the signal from the PTTH-producing cells, complete removal of the imaginal signal (by removing all the imaginal tissue with high doses of X-rays) neither increases critical size nor final body size.
Attainment of critical size appears, therefore, to be contingent on two types of signals, one from the larval tissue and one from the imaginal tissue (Fig. 3). The larval signal is a positive one and not contingent on cell division. This signal likely involves IIS-regulated growth of the PG and the periodic release of PTTH from neurosecretory cells in the brain. In contrast the imaginal signal is contingent on cell division, and may be initiated when the imaginal discs reach a particular size or pattern. This signal cannot be a positive one, since critical size is unaffected by complete removal of the discs. Rather, the discs may either stop releasing an inhibitory signal, or stop attenuating a positive signal when they reach a particular target size.
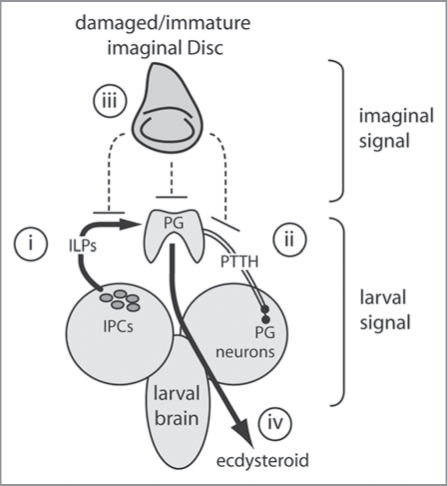
Figure 3.
A model of critical size regulation in Drosophila. Critical size is regulated by larval and imaginal signals. The larvae signals comprise a (i) nutritional/size signal from the IPCs, and (ii) a temporal signal from the PTTH-producing neurons. The imaginal signal (iii) is inhibitory and may affect the action of dILPs or PTTH on the PG, or inhibit ecdysteroidgenesis directly. It is the balance of these signals that ultimately regulates the release of ecdysteroids (iv).
Where the imaginal signal impinges on the larval signal is unclear. It is likely that the imaginal signal acts upstream of the PG, since driving IIS in the PG initiates premature pupariation in larvae with presumably underdeveloped organs. The imaginal discs produce their own insulin-like peptides (dILPs) albeit at a low level.36 Thus slow growing imaginal discs may inhibit IIS-growth of the PG by affecting circulatory dILP levels.37 However, ablation of the discs will also eliminate their dILP production, inconsistent with the observation that disc-less larvae have the same critical size as wild-type larvae, and reach it at the same time. Alternatively, the imaginal discs may influence the release of PTTH from the brain.
Critical size and target organ size.
The finding that organ growth affects developmental timing suggests that the imaginal discs “know” what size they are and communicate this to the body as a whole. There are a couple of possible mechanisms by which this could be achieved.
One possibility is that the imaginal discs have a ‘target state’ which they must achieve before they de-inhibit the critical-size signal from the larval tissue (Fig. 3). This target state may represent a particular organ size or tissue pattern.38 There is considerable evidence that imaginal discs do have a target size, at least as far as final disc size is concerned. When imaginal discs are excised and cultured in a growth-permissive environment they autonomously stop cell division at approximately the same size as that of non-excised discs at metamorphosis.39 A series of elegant experiments by Martin and Morata34 demonstrated that when growth is retarded in the anterior part of the wing imaginal disc relative to the posterior part, the fast-growing posterior compartment slows then stops cell proliferation autonomously and allows the slow-growing compartment to catch up. By pupariation, both compartments are the correct size, and the wing disc is its ’normal’ shape. Thus wing imaginal discs appear to sense how big they need to be at pupariation and do not grow past this size. Interestingly, mammalian organs also appear to have a target size. Infant rat hearts and kidneys transplanted into adult rats continue to grow at the same rate and to the same size as hearts and kidneys in situ in infant rats.40,41
While there is good evidence that imaginal discs have a target final size, there is no comparable evidence that have a target state for critical size, which occurs well before the imaginal discs have stopped growing. Further, damage to the discs after attainment of critical size does not delay metamorphosis,27 indicating that attainment of target final size does not regulate the physiological mechanisms that control pupation.
An alternative hypothesis is that slowing the growth of imaginal discs, either using X-rays, or through the expression of RpS3. RNAi, causes damage to the disc, and that while a disc “perceives” itself as damaged it inhibits the attainment of critical size. Under this hypothesis, it is the maintenance of an abnormal state that retards development, rather than a delay in achievement of a target state. An abnormal state may be sensed through elevated levels of cell death or through disruption in the sequence of morphogen expression that patterns the developing imaginal disc.
Whatever method the discs use to communicate their developmental delay to the mechanisms that regulate critical size, they must also communicate their retarded state to the other discs in the body. This is because, despite the extended developmental period available to these other discs, they do not overgrow. These other organs may autonomously stop development at their target final size to prevent overgrowth. Alternatively, the growth of these other discs may be slowed, so that growth among discs is coordinated throughout development.
Coordinating the cessation of growth among organs.
Collectively, current data paint an increasingly complex picture of the physiological mechanisms that regulate the cessation of body and organ growth in Drosophila. On the one hand, the cessation of growth in the imaginal discs is regulated by circulating hormones that coordinate differentiation across the whole organism.42 At the same time, however, discs also have an autonomous sense of how big they need to be, and do not overgrow even when provided with a growth-permissive environment.28 This apparent paradox is somewhat resolved by evidence that imaginal discs are able to influence the extrinsic hormonal mechanisms that regulate organismal growth. This requires a shift in how we view the physiological mechanisms that regulate and coordinate growth. There has been a tendency to view these physiological mechanisms as unidirectional hormonal cascades, with hormone release from “regulatory centers” such as the PG and brain regulating the duration (and rate) of organ growth. However, such uni-directionality means that coordinated growth can easily be disrupted through perturbation in the development of individual organs. The robustness of animal development has long been recognized, and is implicit to the regulatory feedback seen in developmental gene networks. The data described above indicate that these feedback mechanisms extend to developmental physiological pathways, such that organ growth regulates hormone release and vice versa. The physiological control of growth duration is therefore less of a hormone cascade and more of a hormone network.
Plasticity
Body and organ size in flies is affected by nutrition, temperature, the density of animals in their environment, oxygen levels, and genotype. With the exception of genotype, these are all examples of phenotypic plasticity, where variation is a consequence of variation in environmental factors.
Nutrition and size.
In all animals, variation in developmental nutrition causes variation in final body and organ size. In Drosophila, the growth response to nutrition is mediated through at least two inter-connected hormonal systems. The first is through the release of dILPs from the IPCs in the brain.36,43 The second is through the release of adipokinetic hormone (AKH) from the corpora cardiaca cells in the Drosophila ring gland.44
The Drosophila genome encodes seven insulin-like peptides: dILPs 3 and 5 are produced by the IPCs in the brain, dILP 2 by the IPCs and the imaginal discs, dILPs 4 and 6 are expressed in the gut, dILP 7 is expressed in the ventral nerve cord, and dILP 1 has apparently no detectable expression.36 In Drosophila the expression of only two of the three neurosecretory dILPs (dILPS 3 and 5) has been shown to be nutritionally regulated.45 How the neurosecretory cells respond to nutrient availability is unclear, but the response appears to involve regulation by the Drosophila short neuropeptide F (sNPF).46 In mammals, the expression of the homolog of sNPF (mammalian neuropeptide Y) is downregulated by starvation.47 Thus dietary restriction in Drosophila may lower the level of circulating dILPs by reducing the expression of sNPFs.
Dietary restriction also induces the release of AKH.48 AKH regulates haemolymph sugar homeostasis and larval lipolysis and so may influence organ growth directly by affecting the level of circulating nutrients available to dividing cells. There is also some evidence that AKH inhibits the release of dILPs from the IPC: dILP production is negatively regulated by the protein kinase A pathway,19 which is itself positively regulated by AKH.49
It is the IIS system that regulates cell growth and division in response to both dILPs and circulating nutrients. The IIS system comprise three pathways (Fig. 4): (1) the IIS pathway; (2) the Target of Ramapmycin (TOR) signaling pathway, and; (3) the AMP-dependent kinase (AMPK) pathway. These pathways are extremely conserved among all animals, and are essentially identical in Drosophila and vertebrates. However, in Drosophila the metabolic and mitogenic roles of IIS, which in vertebrates is separated into insulin-signaling and insulin-like growth-factor signaling respectively, are combined into a single pathway with a single insulin-receptor (Inr).18,43,50 Manipulation of different components of the IIS system have profound effects on body and organ size in Drosophila. A reduction in dILP production through ablation of the IPCs causes a reduction in body and organ size,43 while increasing the expression of dILPs in the IPCs causes an increase in body and organ size.19,46 Similarly, hypomorphic mutations of Inr, its substrate chico, TOR and other positively nutritionally-regulated components of the IIS system also decrease body and organ size, while increasing the expression (and presumably activity) of these components has the reverse effect.36,51–54 Importantly, these size effects are also seen organ autonomously. For example, it is possible to upregulate Inr expression in the developing eyes alone, using the GAL4-UAS method,55 resulting in an increase in relative and absolute eye size.36
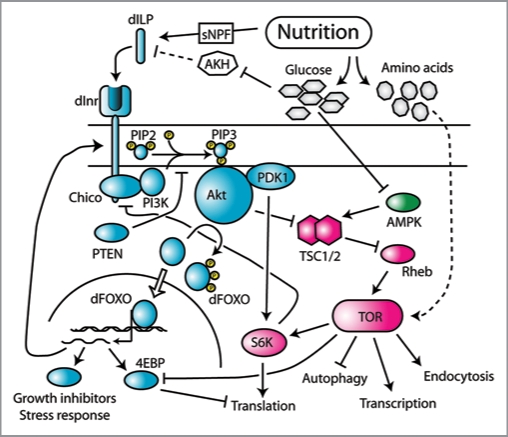
Figure 4.
The II S and TOR-signaling pathway in Drosophila. The II S pathway is shown in cyan, the TOR-signaling pathway is shown in magenta, and the AMPK signaling pathway is shown in green. Variation in nutrition influences the release of dILPs, possibly by the action of sNPFs and AKH. dILPs bind to Inr which initiates a signal transduction cascade involving the phosphorylation of multiple intermediate proteins. Downstream growth affectors include dFOXO, which is deactivated by II S via phosphorylation by AKT, and S6K, which is activated by II S via PDK1. S6K is also a target of TOR, which also restricts the effects of dFOXO by inhibiting one of dFOXO’s transcriptional targets, 4EBP. TOR is also regulated indirectly by II S via the action of AKT on TSC1/2. TOR also responds to amino acids, by an unkown mehchanism, and glucose, via the AMPK pathway. Both FOXO and TOR regulate the activity of multiple growth inhibitors and promoters, respectively. Data from references 37, 43 and 50. Dotted lines are putative relationships.
Nutrition influences signaling through the IIS system indirectly, by dILP binding to InR, and directly, through the cell autonomous response of TOR to cellular levels of amino acids and glucose, the latter via the AMPK pathway. There is also considerable crosstalk among the components of the IIS system (Fig. 4). For example, AKT, which is part of the IIS pathway, regulates the import of glucose into the cell,56 which in turn regulates TOR via AMPK.57 AKT also negatively regulates TSC1 and 2,58,59 which are negative regulators of TOR.60 The system also contains several negative feedback loops. For example FOXO, a transcription factor whose activity is negatively regulated by AKT,54,61,62 regulates transcription of Inr,63 while S6K, a target of TOR,64 negatively regulates expression of the Inr substrate IRS/chico, in mammals and likely Drosophila.65 These negative feedback loops probably serve as a homeostatic mechanism, dampening the cells response to changes in dILPs and nutrition when signaling is either very low or very high. Collectively, the components of the IIS system serve to regulate growth rate by affecting translational initiation (via 4EBP and S6K), ribosome biogenesis (via TIF-IA), transcription, autophagy and endocytosis, as well as the transcription of other growth suppressors and stress response proteins (via FOXO).66,67
Coordinating nutritional-regulated growth across the body.
The IIS system ultimately regulates the rate of cell growth and proliferation in growing organs. However, to understand how nutritional changes in growth rate affect final size we must fit this within the context of Drosophila developmental physiology (Fig. 1). As discussed above, final body and organ size in Drosophila is controlled by the size of the body and imaginal discs at attainment of critical size, plus the amount of growth achieved during their subsequent TGPs, which is in turn determined by the duration of the TGP and the rate of growth during the TGP. Since the IIS primarily regulates growth rate, changes in nutrition and IIS should only affect final body and organ size during the body and organs’ TGPs. This is indeed the case: early studies in Drosophila found that final body size is only reduced in flies that are transiently starved after, but not before, critical size.16 Further, we have shown that changes in signaling through the IIS system only affect final body and organ size during their TGPs.18 Thus TGPs represent insulin-sensitive periods, during which changes in IIS affect final size through their effect on growth rate (Fig. 1B). This does not mean that changes in nutrition and IIS have no effect before attainment of critical size: as noted above, reduced nutrition and IIS early in development slows growth to, and delays attainment of, critical size and retards development.18
Because all growing organs are exposed to the same level of circulating dILPs and nutrients, the IIS system coordinates growth rate and hence final size across the organism as a whole. Thus a reduction in developmental nutrition causes a systemic reduction in IIS signaling, slowing growth of the body and the organs within it and resulting in a global reduction in size. However, not all organs share the same growth response to changes in nutrition. Some organs, for example the mammalian brain68–70 or the male genitalia of Drosophila,71 do not become substantially smaller in response to dietary restriction, that is they are nutritionally insensitive. This nutritional insensitivity may be viewed as a mechanism to protect the growth of key organs from dietary stress. In Drosophila, the nutritional insensitivity of the male genitalia reflects their reduced insulin-sensitivity: changes in IIS have much less of an effect on final genital size than on the size of other organs.18 This is due to the limited effect that a reduction in IIS has on the rate of cell proliferation in the developing genital imaginal disc (Shingleton AW, unpublished data, 2009). The genetic basis for the regulation of insulin-sensitivity is, however, unknown. Nevertheless, due to the evolutionary conservation of the IIS pathway and its importance in regulating body and organ size, it is possible that these mechanisms are generally utilized to regulate the sensitivity of animal organs to changes in nutrition.
The differential sensitivity of organ growth to changes in developmental nutrition and IIS is central to ensuring that correct scaling is maintained across a range of body sizes. In humans, smaller individuals have proportionally (but not absolutely) larger brains,72 and this is in part a consequence of the fact that the brain is less nutritionally sensitive than other organs in the body. Similarly, in Drosophila, male genitalia size is approximately constant across a range of body sizes,71 again because of their relative nutritional- and insulin-sensitivity. More generally, theoretical studies reveal that it is the relationship between an organ’s insulin-sensitivity and the duration of its insulin-sensitive period (TGP) that influences the extent to which changes in nutrition affect final organ size.11 This in turn affects how final organ size scales with final body size when both respond to variation in developmental nutrition.
Oxygen and size.
Body and organ size vary in response to changes in oxygen concentration, with hypoxia reducing size. As with nutrition, changes in oxygen concentration only affect final body size late in development, although it is unclear whether this switch also occurs at critical size.73 Interestingly, hypoxia continues to affect adult body size throughout pupation, and only stops having an effect once an adult has eclosed.73 This suggests that final body size is not necessarily a direct read-out of larval size at the cessation of feeding and that flies do not use all their stored nutrients during metamorphosis.
Considerable research has been directed at the molecular and physiological response to hypoxia in mammals, although only recently has this been extended to Drosophila.74 In mammals, the transcriptional response to hypoxia is mediated by the hypoxiainducible factors and (HIF1− and HIF1−).75 The Drosophila homologues of these factors are Similar (Sima) and Tango (Tgo) respectively.76 Overexpression of Sima protein genocopies hypoxia and causes an autonomous reduction in cell size.77 This appears to act in part through the IIS system: hypoxia has been shown to reduce TOR-pathway activity through the transcription of Scylla by HIF, which in turn activates TSC1/2, a suppressor of TOR signaling.78 Interestingly, HIF1-/Sima is also activated by PI3K-AKT,76 which imposes another negative-feedback loop in the IIS system. In mammals, HIF1 activates FOXO3a,79 and the same may be true for dFOXO in Drosophila. Thus the mechanisms that regulate body and organ size in response to oxygen concentration appear to converge on the mechanisms that regulate size in response to nutrition.
Temperature and size.
Variation in developmental temperature also affects body and organ size in Drosophila, with a decrease in size in flies reared at higher temperatures.71 As with nutrition the effects of temperature on final body and organ size vary during development.80 Temperature shifts affect final body size when applied before the middle of the third larval instar, but continue to affect final wing size almost until adult eclosion. Changes in wing size are largely through changes in cell area, although temperature also affects wing cell number before the beginning of pupariation, presumably when the wing imaginal cells are still undergoing cell division. The mechanisms by which temperature affects body and organ size are unknown, although in most ectothermic animals growth rate is extremely susceptible to changes in temperature. Canonically, this is thought to be a consequence of biochemical kinetics.81 Davidowitz and Nijhout82 have shown that in Manduca sexta the positive effect of increased rearing temperature on growth rate is insufficient to compensate for the dramatic shortening of the body’s TGP at higher temperatures, resulting in an overall decrease in body size. Thus the effect of temperature on body size may be a consequence of the effects of temperature on the rate of cell division and on the hormonal cascade that defines the TGP. Nevertheless, there must be additional mechanisms by which temperature affects final organ size, for two reasons. First, temperature affects Drosophila wing size after the cessation of cell division, by effecting cell size.80 Second, different organs show different sensitivities to changes in temperature.71 For example, the size of the male genitalia in Drosophila is relatively insensitive to changes in temperature compared to other organs. Since the TGP of the genital imaginal discs is regulated by the same hormonal events that regulate the TGP of other discs, either the rate of genital cell division is thermally insensitive, or genital cell size is not affected by temperature. Whatever the reason, Drosophila appear to be able to regulate the thermal response of individual tissues in their bodies. Elucidating the molecular mechanisms by which this regulation is achieved is an exciting area of future research.
Pattern
Perhaps one of the most remarkable, and overlooked, aspects of size regulation is how body and organ shape is maintained across a range of sizes. In humans, people with smaller hands do not have fewer fingers and toes. Hand shape is approximately constant irrespective of hand size. Similarly, in Drosophila the pattern of veins in the wing is more or less the same in small and large wings. Conceptually, organ size and shape are inter-related phenomena. Changes in organ shape reflects a change in the relative size of structures or dimensions in the organ, just as changes in body shape reflect a change in the relative size of organs within the body. Consequently, the mechanisms that regulate organ shape also have the capacity to regulate organ size.83
Organ shape in Drosophila and all animals is regulated by patterning genes: genes that regulate the ordered spatial arrangement of differentiated tissues.37 Patterning genes include short range paracrine signals (morphogens), and the signaling pathways that produce and respond to them. The signaling pathways that regulate pattern in the developing imaginal discs of Drosophila have been extensively studied and are well understood.84 These pathways define compartments within the disc, with all the cells within a compartment sharing a distinct cell lineage and affinity. For example, the wing imaginal discs is divided into dorsal and ventral compartments, and anterior and posterior compartments. In both cases, there is a stripe of cells at the compartment boundaries that secrete a morphogen: Decepentaplagic (Dpp) secreted by cells at the anterior-posterior (A-P) boundary85 and Wingless (Wg) secreted by the cells at the dorsal-ventral (D-V) boundary.86 These morphogens diffuse from the site of their production and produce a morphogen gradient that defines the area that will become the wing blade. Crucially, these morphogens also regulate cell proliferation.
The importance of morphogen gradients in regulating organ size has recently been demonstrated in experiments that modify the diffusion of Dpp in the developing wing and haltere. Unlike almost all other insects, flies only have one pair of wings, the second pair of wings having been modified into small clublike balancing appendages, called halteres. Halteres are much smaller than wings, containing ∼fivefold fewer cells,87 and are defined during development by expression of the Hox gene Ultrabithorax (Ubx) in all of the cells of their imaginal disc.88 In contrast, there is no Ubx expression in the wing imaginal disc. Loss of Ubx expression in the haltere transforms the latter into a second pair of wings, producing the now famous four-winged bithorax fly created by Ed Lewis.89 Ubx restricts the growth of the haltere by downregulating transcription of Dpp and limiting its mobility by upregulating expression of the Dpp receptor, thickvein (tkv).90 Dpp-signaling regulates cell growth and cell cycle progression. Consequently limiting the diffusion of Dpp by Ubx limits its mitogenic effects and reduces haltere size.
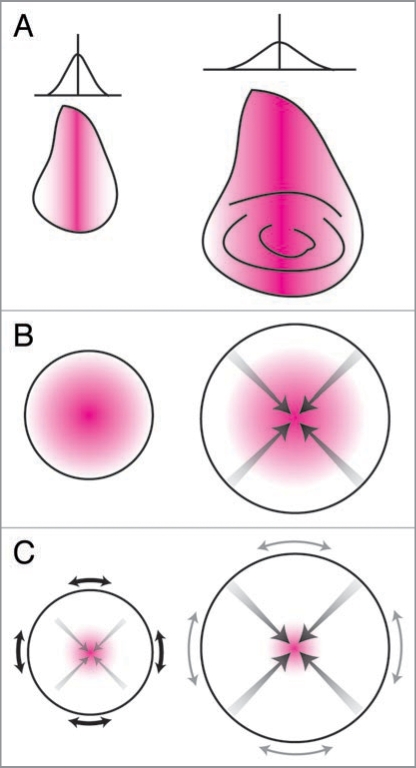
These and similar experiments (reviewed in ref. 91) have lead to the hypothesis that wing dimension is regulated by morphogen gradients, and that target final disc size is achieved when the discs have attained a particular pattern of morphogen expression or distribution. Nevertheless, the precise mechanism by which the morphogen gradient regulates disc size is still a matter of much discussion.92 One hypothesis proposed by Day and Lawrence,91 is that as the disc grows the morphogen gradient becomes flatter, with cells ceasing proliferation when the organ reaches a particular size and the gradient becomes sufficiently flat (Fig. 5A).
Figure 5.
The autonomous regulation of organ size. (A) Day and Lawrence Model.91 The expression of a morphogen (e.g., Dpp) along the axis of an organ (e.g., the wing) establishes a morphogen gradient perpendicular to the axis (left). Growth stops when the morphogen gradient becomes sufficiently flat (right). (B) Shraiman model.100,108 A growth factor at the center of an organ promotes growth. Growth at the center stops when the positive effects of the growth factors are matched by the negative effects of compression (gray arrows), and at the periphery when the tissue grows beyond the range of the growth factor. (C) Aegerter-Wilmsen Model:107 A growth factor at the center of the organ promotes growth, which causes stretch at the periphery (black arrows), which in turn promotes growth at the periphery. Peripheral growth does not completely remove stretch, causing compression at the center of the organ. Growth stops when compression at the center overcomes the effects of the growth factor, eliminating additional stretch at the periphery (gray arrows) and stopping growth there also. Magenta area indicates morphogen. Adapted from ref. 92.
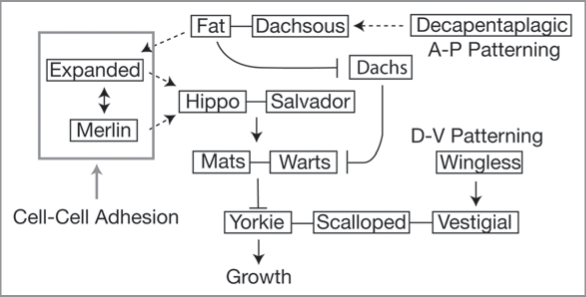
There is some evidence that cells are able to detect and show a proliferation response to a gradient of Dpp.93 This response appears to be mediated by the Fat/Hippo signaling pathway, a recent addition to the signaling pathways involved in size regulation.94,95 Fat encodes an atypical cadherin that acts as a transmembrane receptor for the signaling pathway. Fat ultimately regulates growth by activating Warts, which is a negative regulator of the growth-promoting transcription factor Yorkie (Yk) (Fig. 6). Fat does this either by (1) interacting with the adapter protein Expanded, an upstream regulator of Hippo, which is in turn a positive regulator of Warts, or; (2) by inhibiting Dachs, a negative regulator of Warts.96 The ligand of Fat is Dachsous (Ds).97 Interestingly, Ds is expressed as a gradient in the wing imaginal disc, while uniform expression of Ds inhibits cell proliferation.98 This suggests that it is difference in Ds levels between adjacent cells that inactivates Fat to promote growth. Further, this discontinuity is evident as polarized localization of Ds within cells along the A-P axis of the wing disc. This polarization is regulated by Dpp signaling.99 Thus a steep Dpp gradient may promote growth by maintaining the discontinuity of Ds levels.99 As the disc grows and the gradient flattens, polarized localization of Ds may be lost, activating Fat and causing the cessation of growth. However, the regulatory details of the Fat/Hippo signaling pathway have yet to be fully elucidated, so this mechanism remains hypothetical. Further, studies in the developing Drosophila wing show that morphogen gradients do not flatten with increasing disc size,100 while homogenous expression of Dpp across the disc does not halt cell division, as the model predicts.101
Figure 6.
A model of the Fat/Hippo regulation of growth. Dpp signaling may affect signaling through the pathway via its effects on Dachsous and Fat. Wingless signaling may affect the activity of Yorkie through Vestigial’s interaction with Scalloped, a Yorkie co-factor. How Vestigial affects the interaction between Yorkie and Scalloped is, however, unclear. Mechanical forces may affect the activity of Hippo through their effect on Merlin and Expanded. Solid lines indicate protein-protein binding, solid arrows indicate biochemical interactions, dotted arrows indicate genetic interactions, gray arrow indicates hypothetical interaction. Data from references 96, 99 and 103.
Nevertheless, the Fat/Hippo signaling pathway does appear to be an important component of the mechanism linking patterning genes with growth regulators. For example, Yorkie also interacts with the protein Scalloped (Sd), which is a downstream effector of the wingless signaling pathway and helps organize the D-V axis of the Drosophila wing.102 Sd forms a complex with Vestigal (Vg), and early evidence suggests that Yorkie works with the Sd-Vg complex to promote wing-tissue formation.103 Thus Yorkie may lie at the nexus of signaling pathways that regulate the growth of the A-P and D-V compartments.
There are also links between patterning signaling pathways and the IIS system. First, the mammalian homolog of Hippo, mammalian sterile twenty kinase 1 (MST1), can activate FOXO transcription factors.104 Second, there are cross-regulatory interactions between the wingless-signaling pathway and the IIS system at the level of Shaggy (Sgg), the Drosophila equivalent of glycogen synthase kinase 3,105 and at β-Catenin, an effector at wingless signaling.106 Finally, proliferation induced by activation of the Dpp signaling pathway is contingent on IIS.85 While it must be true that the mechanisms that regulate organ-size plasticity interact at some level with those that regulate organ pattern, we are only starting to elucidate how these interactions are mediated at a molecular level.
Physical Force
Recently a third class of factors that regulate organ size in Drosophila has been proposed: physical forces. Several authors, dissatisfied with models of organ growth that utilize morphogen gradients, have argued that the effect of compression and tension on cells within growing imaginal discs regulate their proliferation.100,107,108 As an organ grows, cells located in its center will push against surrounding cells as a consequence of cell growth and division. In principle, these surrounding cells can inhibit cell growth and division as they push back against the cells at the center—think of a melon growing in a rigid box being constrained and shaped by the walls of that box. Thus cells may be negative regulators of tissue growth through mechanical compression. This model assumes that imaginal disc cells are not free to move around the disc, and that the disc acts as an elastic solid rather than a liquid. This is in contrast to models that explain the liquid-like tissue-spreading and cell segregation phenomenon in mammalian embryos.109
Two related but independently produced models of the effect of physical force on disc growth have been proposed. The first, by Shraiman,108 suggests that as an organ grows its central region becomes compressed, inhibiting growth. When combined with a morphogen gradient,100 organ growth stops when the positive growth-effects of the morphogen at the center of the organ are countered by the negative growth-effects of compression. At the same time, cells at the periphery of the organ stop division because they have grown beyond the edges of the morphogen gradient (Fig. 5B). This model is based on the observation that the Dpp mrophogen gradient does not change with an increase in disc size100 (reviewed in ref. 110). The model predicts that an increase in the range of the Dpp gradient will increase the size of the disc, which is indeed the case. As discussed above, altering the mobility of Dpp by changing the expression of tkv affects wing and haltere size,90 as does altering its mobility by changing the expression of the components of the proteoglycan matrix on the disc surface.111 Further, the model can also accommodate the growth effects of homogenous expression of Dpp across the wing blade.101
The second model, proposed by Aegeter-Wilmsen et al.107 also argues that growth at the center of an organ is promoted by morphogens and inhibited by compression. However, in this model growth at the periphery of the organ is not dependent on exposure to the edges of a morphogen gradient. Rather it is induced by stretch, imposed on the cells by growth at the center of the organ. Cell proliferation at the center stops when compression overcomes the growth effects of morphogens. This in turn reduces the stretch at the organ periphery, stopping growth there as well (Fig. 5C).
These models of a target disc size regulated by mechanical feedback are purely theoretical. They have yet to be tested empirically in vivo. Nevertheless there is ample evidence that mechanical force can affect cell division in vitro, in mammalian systems. For example, mechanical stretch has been shown to stimulate lung and skin cell proliferation,112,113 while shear stress has been shown to regulate antiapoptosis, cell cycle arrest and morphological remodeling in bovine vascular endothelial cells114 and human colon cancer cells.115 An essential component of the models is that cells adhere tightly to each other and that cellular mixing is limited. For cells adhering to an extracellular matrix or to each other, cellular force is transmitted through sites of adhesion.116 Adhesion is achieved through transmembrane cell adhesion proteins, for example cadherins and integrins. The extracellular domains of these proteins bind to ligands either on the surface of neighboring cells or in the extracellular matrix, while the intracellular domains bind to the cell’s cytoskeleton. It is therefore possible to alter the forces experienced by a cell by altering the expression or activity of these adhesion proteins.117
Importantly, cell adhesion proteins play not only a structural role but also a signaling role. There is a direct link between the binding of adhesion proteins and regulators of cell proliferation. For example, cadherin molecules bind to cadherins on adjacent cells, a process that also requires their binding to β-Catenin at their intracellular domain. β-Catenin, however, is also a key component of the wingless-signaling pathway.118 Thus binding of β-Catenins to cadherins sequesters the former from its role as a positive regulator of cell division through wingless signaling, potentially reducing growth. Intriguingly, work on Drosophila has shown that mechanical compression of the embryo affects the expression of twist, a gene involved in dorsal-ventral patterning, and that changes in twist expression are dependent on β-catenin activity in the nucleus.115,119 β-catenin may therefore be an important component of the mechanisms integrating mechanical force with gene expression.
There is also increasing evidence that the Fat/Hippos signaling pathway is involved in the contact-regulation of cell proliferation. Two cytoskeleton proteins, Merlin and Expanded, are involved in cell adhesion and structure and also control the activation of Hippo (Fig. 6). Merlin has been reported as binding to a number of factors, including β-Catenin, cytoskeletal actin, and phophatidylinositol (4,5)-bisphosphate (PIP2), the latter being a component of the IIS pathway.120,121 Further, mammalian cells lacking Merlin are resistant to contact inhibition of proliferation when grown in culture.122 Thus the Fat/Hippo signaling pathways may be important in transmitting physical forces experienced by cells through cell-cell interactions to the mechanisms that regulate cell growth and proliferation.
Conclusion: Integrating Regulators of Size
Final organ size is controlled by a combination of mechanisms: those involved in regulating physiology, plasticity, pattern and the response to physical force. Extensive research in Drosophila has led to the identification of key signaling pathways that transmit information about developmental time, environmental conditions, morphogen gradients and physical forces to growth regulators. However, it is very unclear how the mechanisms by which these different pieces of information are integrated to generate a functioning adult organ of a particular size. On the one hand, final organ size is regulated by the distribution of morphogens and/or physical forces across the developing organ, which is supported by evidence that organs have an autonomous target size. On the other hand, final organ size is plastic and depends on nutritional, thermal and oxic conditions. Thus, if organs do have a target size, this must be regulated by environmental factors. Consequently, there should be considerable crosstalk between the different growth regulatory pathways. At the same time, all of these processes must fit within the hormonal milieu that regulates the duration of organ growth.
In pulling together the different strands of size regulation, it is clear that a complete understanding of the mechanisms that regulate organ and body size, and the relationship between the two, will only come with an integrative approach. Future research must be directed at a deeper understanding of how different regulators of size interact. The probable result is that we will not see organ size regulation as a consequence of signaling through discrete pathways, but rather through regulatory networks. This is not surprising: organ size and shape is a complex phenotype and is likely controlled by an equally complex regulatory mechanism.
Regardless of the complexity of the task, there are some areas of size regulation that should be targeted for immediate research.
• The environmental regulation of patterning genes.
Despite varying by as much as 50% in size, the wings of malnourished and well-fed flies are essentially the same shape. The same is true for other organs. How pattern is maintained across a range of organ sizes is an unknown and understudied phenomenon, but an essential component of size regulation. Simply looking at the expression of patterning gene in different environmental contexts would be enormously useful. How does the Dpp morphogen gradient differ in wings from small and large flies? How does patterning gene expression changes with changes in nutrition and temperature? More generally, how are autonomous regulators of organ growth integrated with non-autonomous regulators of organ growth?
• The mechanical regulation of organ growth.
Several authors have recently proposed models of organ size regulation that incorporate physical forces. These models remain purely theoretical. There has been some progress in elucidating the role that tissue deformation plays in regulating gene expression in Drosophila embryos. These studies should be extended to look at tissue deformation and gene expression in the imaginal discs. How does stretch affect cell division in imaginal discs in vitro? By which pathways is mechanical information transmitted to more proximate regulators of cell growth and division?
• The hormonal mechanisms by which organs communicate their growth to the body as a whole.
There is considerable evidence that slow growing or damaged organs communicate their condition to other tissues around the body. How this is achieved is unknown, but may represent a novel size regulator mechanism. What aspects of organ growth must be disrupted in order for them to recognize themselves as damaged? What hormones do imaginal discs release that may communicate this information?
• The developmental regulation of scaling relationships.
A truism of animal growth is that larger adults have larger body parts, creating tightly correlated scaling relationships between final body size and organ size. Our theoretical work has indicated that nutrition-dependent IIS-regulated growth during the body and organs’ TGPs is sufficient to create such scaling relationships. Importantly, the different sensitivities of different organs to changes in circulating dILPs and nutrients is central to their maintaining correct scaling. How do organs regulate their specific response to changes in developmental nutrition? Are these mechanisms more generally utilized to regulate the extent of an organ’s size response to environmental plasticity?
The first decade of the 21st century has witnessed remarkable progress in elucidating the molecular and physiological regulators of organ size. The next decade should see integration of these mechanisms to understand size regulation not just at the level of the cell and organ, but at the level of complete organisms.
Acknowledgments
A.W.S. is supported by grants from Michigan State University and NSF grants 0845847 and 0919855.
Footnotes
Previously published online: www.landesbioscience.com/journals/organogenesis/article/10375
References
- 1.Nijhout HF. Physiological control of molting in insects. Am Zool. 1981;21:631–640. [Google Scholar]
- 2.Riddiford LM. Hormones and Drosophila development. In: Bate M, Martinez Arias A, editors. The Development of Drosophila melanogaster. Cold Spring Harbor: Cold Spring Harbor Press; 1993. pp. 899–939. [Google Scholar]
- 3.Truman JW, Riddiford LM. Physiology of insect rhythms 3. The temporal organization of the endocrine events underlying pupation of the tobacco hornworm. J Exp Biol. 1974;60:371–382. doi: 10.1242/jeb.60.2.371. [DOI] [PubMed] [Google Scholar]
- 4.Kawasaki H. Transition from larva to pupa: morphogenesis, cell proliferation and protein synthesis in Bombyx wing disc. Inv Repro Devel. 1998;34:101–108. [Google Scholar]
- 5.Ohtaki T, Yamanaka F, Sakurai S. Differential timing of pupal commitment in various tissues of the silkworm, Bombyx mori. J Insect Physiol. 1986;32:635. [Google Scholar]
- 6.Agui N, Granger NA, Gilbert LI, Bollenbacher WE. Cellular-localization of the insect prothoracicotropic hormone—Invitro assay of a single neurosecretory cell. Proc Natl Acad Sci USA. 1979;76:5694–5698. doi: 10.1073/pnas.76.11.5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sakurai S, Gilbert LI. Biosynthesis and sectration of ecdysteroids by the prothoracic glands. In: Ohnishi E, Ishizaki H, editors. Molting and Metamorophosis. Berlin: Springer-Verlag; 1990. [Google Scholar]
- 8.Truman JW, Riddiford LM, Safranek L. Temporal patterns of response to ecdysone and juvenile hormone in the epidermis of the tobacco hornworm, Manduca sexta. Dev Biol. 1974;39:247–262. doi: 10.1016/0012-1606(74)90238-3. [DOI] [PubMed] [Google Scholar]
- 9.Truman JW, Copenhaver PF. The larval eclosion hormone neurons in Manduca sexta—Identification of the brain-proctodeal neurosecretory-system. J Exp Biol. 1989;147:457–470. [Google Scholar]
- 10.Nijhout HF, Williams CM. Control of moulting and metamorphosis in the tobacco hornworm, Manduca sexta (L.): cessation of juvenile hormone secretion as a trigger for pupation. J Exp Biol. 1974;61:493–501. doi: 10.1242/jeb.61.2.493. [DOI] [PubMed] [Google Scholar]
- 11.Shingleton AW, Mirth CK, Bates PW. Developmental model of static allometry in holometabolous insects. Proc Roy Soc Lond B Biol Sci. 2008;275:1875–1885. doi: 10.1098/rspb.2008.0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stieper BC, Kupershtok M, Driscoll MV, Shingleton AW. Imaginal discs regulate developmental timing in Drosophila melanogaster. Dev Biol. 2008;321:18–26. doi: 10.1016/j.ydbio.2008.05.556. [DOI] [PubMed] [Google Scholar]
- 13.Shingleton AW, Frankino WA, Flatt T, Nijhout HF, Emlen DJ. Size and Shape: The developmental regulation of static allometry in insects. BioEssays. 2007;29:536–548. doi: 10.1002/bies.20584. [DOI] [PubMed] [Google Scholar]
- 14.Davidowitz G, D’Amico LJ, Nijhout HF. Critical weight in the development of insect body size. Evol Dev. 2003;5:188–197. doi: 10.1046/j.1525-142x.2003.03026.x. [DOI] [PubMed] [Google Scholar]
- 15.Nijhout HF, Davidowitz G, Roff DA. A quantitative analysis of the mechanism that controls body size in Manduca sexta. J Biol. 2006;5:161–165. doi: 10.1186/jbiol43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beadle G, Tatum E, Clancy C. Food level in relation to rate of development and eye pigmentation in Drosophila melanogaster. Biological Bulletin of the Marine Biology Laboratory, Woods Hole. 1938;75:447–462. [Google Scholar]
- 17.Nijhout HF, Williams CM. Control of moulting and metamorphosis in the tobacco hornworm, Manduca sexta (L.): growth of the last-instar larva and the decision to pupate. J Exp Biol. 1974;61:481–491. doi: 10.1242/jeb.61.2.481. [DOI] [PubMed] [Google Scholar]
- 18.Shingleton AW, Das J, Vinicius L, Stern DL. The temporal requirements for insulin signaling during development in Drosophila. PLoS Biol. 2005;3:289. doi: 10.1371/journal.pbio.0030289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walkiewicz M, Stern M. Increased insulin/insulin growth factor signaling advances the onset of metamorphosis in Drosophila. PLoS ONE. 2009;4:5072. doi: 10.1371/journal.pone.0005072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mirth C, Truman JW, Riddiford LM. The role of the prothoracic gland in determining critical weight for metamorphosis in Drosophila melanogaster. Curr Biol. 2005;15:1796–1807. doi: 10.1016/j.cub.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 21.Caldwell PE, Walkiewicz M, Stern M. Ras activity in the Drosophila prothoracic gland regulates body size and developmental rate via ecdysone release. Curr Biol. 2005;15:1785–1795. doi: 10.1016/j.cub.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 22.Colombani J, Bianchini L, Layalle S, Pondeville E, Dauphin-Villemant C, Antoniewski C, et al. Antagonistic actions of Ecdysone and Insulins determine final size in Drosophila. Science. 2005;310:667–670. doi: 10.1126/science.1119432. [DOI] [PubMed] [Google Scholar]
- 23.Mcbrayer Z, Ono H, Shimell M, Parvy J, Beckstead R, Warren J, et al. Prothoracicotropic hormone regulates developmental timing and body size in Drosophila. Dev Cell. 2007;13:857–871. doi: 10.1016/j.devcel.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rybczynski R, Bell SC, Gilbert LI. Activation of an extracellular signal-regulated kinase (ERK) by the insect prothoracicotropic hormone. Mol Cell Endocrinol. 2001;184:1–11. doi: 10.1016/s0303-7207(01)00664-5. [DOI] [PubMed] [Google Scholar]
- 25.Shingleton AW. Body-size regulation: combining genetics and physiology. Curr Biol. 2005;15:825–827. doi: 10.1016/j.cub.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 26.Russell M. Pattern formation in the imaginal discs of a temperature-sensitive cell-lethal mutant of Drosophila melanogaster. Dev Biol. 1974;40:24–39. doi: 10.1016/0012-1606(74)90104-3. [DOI] [PubMed] [Google Scholar]
- 27.Simpson P, Schneiderman HA. Isolation of temperature sensitive mutations blocking clone development in Drosophila melanogaster, and effects of a temperature sensitive cell lethal mutation on pattern formation in imaginal disks. Rouxs Arch Dev Biol. 1975;178:247–275. doi: 10.1007/BF00848432. [DOI] [PubMed] [Google Scholar]
- 28.Simpson P, Berreur P, Berreurbonnenfant J. The initiation of pupariation in Drosophila—Dependence on growth of the imaginal disks. J Embryol Exp Morph. 1980;57:155–165. [PubMed] [Google Scholar]
- 29.Poodry CA, Woods DF. Control of the developmental timer for Drosophila pupariation. Rouxs Arch Dev Biol. 1990;199:219–227. doi: 10.1007/BF01682081. [DOI] [PubMed] [Google Scholar]
- 30.French V, Bryant PJ, Bryant SV. Pattern regulation in epimorphic fields. Science. 1976;193:969–981. doi: 10.1126/science.948762. [DOI] [PubMed] [Google Scholar]
- 31.Smith-Bolton RK, Worley MI, Kanda H, Hariharan IK. Regenerative growth in Drosophila imaginal discs is regulated by Wingless and Myc. Dev Cell. 2009;16:797–809. doi: 10.1016/j.devcel.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simpson P, Morata G. Differential mitotic rates and patterns of growth in compartments in the Drosophila wing. Dev Biol. 1981;85:299–308. doi: 10.1016/0012-1606(81)90261-x. [DOI] [PubMed] [Google Scholar]
- 33.Morata G, Ripoll P. Minutes: mutants of drosophila autonomously affecting cell division rate. Dev Biol. 1975;42:211–221. doi: 10.1016/0012-1606(75)90330-9. [DOI] [PubMed] [Google Scholar]
- 34.Martin FA, Morata G. Compartments and the control of growth in the Drosophila wing imaginal disc. Development. 2006;133:4421–4426. doi: 10.1242/dev.02618. [DOI] [PubMed] [Google Scholar]
- 35.Saebøe-Larssen S, Lyamouri M, Merriam J, Oksvold MP, Lambertsson A. Ribosomal protein insufficiency and the minute syndrome in Drosophila: a dose-response relationship. Genetics. 1998;148:1215–1224. doi: 10.1093/genetics/148.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brogiolo W, Stocker H, Ikeya T, Rintelen F, Fernandez R, Hafen E. An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr Biol. 2001;11:213–221. doi: 10.1016/s0960-9822(01)00068-9. [DOI] [PubMed] [Google Scholar]
- 37.Edgar B. How flies get their size: genetics meets physiology. Nat Rev Genet. 2006;7:907–916. doi: 10.1038/nrg1989. [DOI] [PubMed] [Google Scholar]
- 38.Bryant PJ, Simpson P. Intrinsic and extrinsic control of growth in developing organs. Q Rev Biol. 1984;59:387–415. doi: 10.1086/414040. [DOI] [PubMed] [Google Scholar]
- 39.Bryant PJ, Levinson P. Intrinsic growth control in the imaginal primordia of Drosophila, and the autonomous action of a lethal mutation causing overgrowth. Dev Biol. 1985;107:355–363. doi: 10.1016/0012-1606(85)90317-3. [DOI] [PubMed] [Google Scholar]
- 40.Dittmer JE, Goss RJ, Dinsmore CE. The growth of infant hearts grafted to young and adult rats. Am J Anat. 1974;141:155–160. doi: 10.1002/aja.1001410112. [DOI] [PubMed] [Google Scholar]
- 41.Silber SJ. Growth of baby kidneys transplanted into adults. Arch Surg. 1976;111:75–77. doi: 10.1001/archsurg.1976.01360190077014. [DOI] [PubMed] [Google Scholar]
- 42.Mirth C, Riddiford L. Size assessment and growth control: how adult size is determined in insects. Bioessays. 2007;29:344–355. doi: 10.1002/bies.20552. [DOI] [PubMed] [Google Scholar]
- 43.Rulifson EJ, Kim SK, Nusse R. Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science. 2002;296:1118–1120. doi: 10.1126/science.1070058. [DOI] [PubMed] [Google Scholar]
- 44.Kim SK, Rulifson EJ. Conserved mechanisms of glucose sensing and regulation by Drosophila corpora cardiaca cells. Nature. 2004;431:316–320. doi: 10.1038/nature02897. [DOI] [PubMed] [Google Scholar]
- 45.Ikeya T, Galic M, Belawat P, Nairz K, Hafen E. Nutrient-dependent expression of insulin-like peptides from neuroendocrine cells in the CNS contributes to growth regulation in Drosophila. Curr Biol. 2002;12:1293–1300. doi: 10.1016/s0960-9822(02)01043-6. [DOI] [PubMed] [Google Scholar]
- 46.Lee K, Kwon O, Lee J, Kwon K, Min K, Jung S, et al. Drosophila short neuropeptide F signalling regulates growth by ERK-mediated insulin signalling. Nat Cell Biol. 2008;10:468–475. doi: 10.1038/ncb1710. [DOI] [PubMed] [Google Scholar]
- 47.Baranowska B, Chmielowska M, Wolinska-Witort E, Roguski K, Wasilewska-Dziubinska E. The relationship between neuropeptides and hormones in starvation. Neuro Endocrinol Lett. 2001;22:349–355. [PubMed] [Google Scholar]
- 48.Lee G, Park JH. Hemolymph sugar homeostasis and starvation-induced hyperactivity affected by genetic manipulations of the adipokinetic hormone-encoding gene in Drosophila melanogaster. Genetics. 2004;167:311–323. doi: 10.1534/genetics.167.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gade G, Auerswald L. Mode of action of neuropeptides from the adipokinetic hormone family. Gen Comp Endocrinol. 2003;132:10–20. doi: 10.1016/s0016-6480(03)00159-x. [DOI] [PubMed] [Google Scholar]
- 50.Britton JS, Lockwood WK, Li L, Cohen S, Edgar B. Drosophila’s insulin/PI3-kinase pathway coordinates cellular metabolism with nutritional conditions. Dev Cell. 2002;2:239–249. doi: 10.1016/s1534-5807(02)00117-x. [DOI] [PubMed] [Google Scholar]
- 51.Oldham S, Stocker H, Laffargue M, Wittwer F, Wymann M, Hafen E. The Drosophila insulin/IGF receptor controls growth and size by modulating PtdInsP(3) levels. Development. 2002;129:4103–4109. doi: 10.1242/dev.129.17.4103. [DOI] [PubMed] [Google Scholar]
- 52.Bohni R, Riesgo-Escovar J, Oldham S, Brogiolo W, Stocker H, Andruss BF, et al. Autonomous control of cell and organ size by CHICO, a Drosophila homolog of vertebrate IRS1-4. Cell. 1999;97:865–875. doi: 10.1016/s0092-8674(00)80799-0. [DOI] [PubMed] [Google Scholar]
- 53.Goberdhan DC, Paricio N, Goodman EC, Mlodzik M, Wilson C. Drosophila tumor suppressor PTEN controls cell size and number by antagonizing the Chico/PI3-kinase signaling pathway. Genes Dev. 1999;13:3244–3258. doi: 10.1101/gad.13.24.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Puig O, Marr MT, Ruhf ML, Tjian R. Control of cell number by Drosophila FOXO: downstream and feedback regulation of the insulin receptor pathway. Genes Dev. 2003;17:2006–2020. doi: 10.1101/gad.1098703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Duffy JB. GAL4 system in Drosophila: A fly geneticist’s Swiss army knife. Genesis. 2002;34:1–15. doi: 10.1002/gene.10150. [DOI] [PubMed] [Google Scholar]
- 56.Wang Q, Somwar R, Bilan PJ, Liu Z, Jin J, Woodgett JR, et al. Protein kinase B/Akt participates in GLUT4 translocation by insulin in L6 myoblasts. Mol Cell Biol. 1999;19:4008–4018. doi: 10.1128/mcb.19.6.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gao X, Pan D. TSC1 and TSC2 tumor suppressors antagonize insulin signaling in cell growth. Genes Dev. 2001;15:1383–1392. doi: 10.1101/gad.901101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 60.Gao X, Zhang Y, Arrazola P, Hino O, Kobayashi T, Yeung RS, et al. Tsc tumour suppressor proteins antagonize amino-acid-TOR signalling. Nat Cell Biol. 2002;4:699–704. doi: 10.1038/ncb847. [DOI] [PubMed] [Google Scholar]
- 61.Junger MA, Rintelen F, Stocker H, Wasserman JD, Vegh M, Radimerski T, et al. The Drosophila Forkhead transcription factor FOXO mediates the reduction in cell number associated with reduced insulin signaling. J Biol. 2003;2:20. doi: 10.1186/1475-4924-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kramer JM, Davidge JT, Lockyer JM, Staveley BE. Expression of Drosophila FOXO regulates growth and can phenocopy starvation. BMC Dev Biol. 2003;3:5. doi: 10.1186/1471-213X-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Puig O, Tjian R. Transcriptional feedback control of insulin receptor by dFOXO/FOXO1. Gene Dev. 2005;19:2435–2446. doi: 10.1101/gad.1340505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miron M, Lasko P, Sonenberg N. Signaling from Akt to FRAP/TOR targets both 4E-BP and S6K in Drosophila melanogaster. Mol Cell Biol. 2003;23:9117–9126. doi: 10.1128/MCB.23.24.9117-9126.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Harrington LS, Findlay GM, Gray A, Tolkacheva T, Wigfield S, Rebholz H, et al. The TSC1-2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J Cell Biol. 2004;166:213–223. doi: 10.1083/jcb.200403069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 67.Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7:85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- 68.Brown ML, Guthrie HA. Effect of severe undernutrition in early life upon body and organ weights in adult rats. Growth. 1968;32:143–151. [PubMed] [Google Scholar]
- 69.Guthrie HA, Brown ML. Effect of severe undernutrition in early life on growth, brain size and composition in adult rats. J Nutr. 1968;94:419–426. doi: 10.1093/jn/94.4.419. [DOI] [PubMed] [Google Scholar]
- 70.Kind KL, Roberts CT, Sohlstrom AI, Katsman A, Clifton PM, Robinson JS, et al. Chronic maternal feed restriction impairs growth but increases adiposity of the fetal guinea pig. Am J Physiol Regul Integr Comp Physiol. 2005;288:119–126. doi: 10.1152/ajpregu.00360.2004. [DOI] [PubMed] [Google Scholar]
- 71.Shingleton AW, Estep CM, Driscoll MV, Dworkin I. Many ways to be small: different environmental regulators of size generate distinct scaling relationships in Drosophila melanogaster. Proc Roy Soc Lond B Biol Sci. 2009;276:2625–2633. doi: 10.1098/rspb.2008.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Koh I, Lee MS, Lee NJ, Park KW, Kim KH, Kim H, et al. Body size effect on brain volume in Korean youth. Neuroreport. 2005;16:2029–2032. doi: 10.1097/00001756-200512190-00012. [DOI] [PubMed] [Google Scholar]
- 73.Peck LS, Maddrell SH. Limitation of size by hypoxia in the fruit fly Drosophila melanogaster. J Exp Zool Comp Exp Biol. 2005;303:968–975. doi: 10.1002/jez.a.211. [DOI] [PubMed] [Google Scholar]
- 74.Arquier N, Vigne P, Duplan E, Hsu T, Therond PP, Frelin C, et al. Analysis of the hypoxia-sensing pathway in Drosophila melanogaster. Biochem J. 2006;393:471–480. doi: 10.1042/BJ20050675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Déry MA, Michaud MD, Richard DE. Hypoxiainducible factor 1: regulation by hypoxic and nonhypoxic activators. Int J Biochem Cell Biol. 2005;37:535–540. doi: 10.1016/j.biocel.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 76.Dekanty A, Lavista-Llanos S, Irisarri M, Oldham S, Wappner P. The insulin-PI3K/TOR pathway induces a HIF-dependent transcriptional response in Drosophila by promoting nuclear localization of HIF-alpha/Sima. J Cell Sci. 2005;118:5431–5441. doi: 10.1242/jcs.02648. [DOI] [PubMed] [Google Scholar]
- 77.Centanin L, Ratcliffe PJ, Wappner P. Reversion of lethality and growth defects in Fatiga oxygen-sensor mutant flies by loss of hypoxia-inducible factor-alpha/Sima. EMBO Rep. 2005;6:1070–1075. doi: 10.1038/sj.embor.7400528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reiling JH, Hafen E. The hypoxia-induced paralogs Scylla and Charybdis inhibit growth by downregulating S6K activity upstream of TSC in Drosophila. Genes Dev. 2004;18:2879–2892. doi: 10.1101/gad.322704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bakker WJ, Harris IS, Mak T. FOXO3a is activated in response to hypoxic stress and inhibits HIF1-induced apoptosis via regulation of CITED2. Mol Cell. 2007;28:941–953. doi: 10.1016/j.molcel.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 80.French V, Feast M, Partridge L. Body size and cell size in Drosophila: the developmental response to temperature. J Insect Physiol. 1998;44:1081–1089. doi: 10.1016/s0022-1910(98)00061-4. [DOI] [PubMed] [Google Scholar]
- 81.Gillooly JF, Charnov EL, West GB, Savage VM, Brown JH. Effects of size and temperature on developmental time. Nature. 2002;417:70–73. doi: 10.1038/417070a. [DOI] [PubMed] [Google Scholar]
- 82.Davidowitz G, Nijhout HF. The physiological basis of reaction norms: The interaction among growth rate, the duration of growth and body size. Integr Comp Biol. 2004;44:443–449. doi: 10.1093/icb/44.6.443. [DOI] [PubMed] [Google Scholar]
- 83.Lecuit T, Le Goff L. Orchestrating size and shape during morphogenesis. Nature. 2007;450:189–192. doi: 10.1038/nature06304. [DOI] [PubMed] [Google Scholar]
- 84.Held LI. Imaginal discs: the genetic and cellular logic of pattern formation. Cambridge: Cambridge University Press; 2002. [Google Scholar]
- 85.Martín-Castellanos C, Edgar B. A characterization of the effects of Dpp signaling on cell growth and proliferation in the Drosophila wing. Development. 2002;129:1003–1013. doi: 10.1242/dev.129.4.1003. [DOI] [PubMed] [Google Scholar]
- 86.Neumann CJ, Cohen SM. Distinct mitogenic and cell fate specification functions of wingless in different regions of the wing. Development. 1996;122:1781–1789. doi: 10.1242/dev.122.6.1781. [DOI] [PubMed] [Google Scholar]
- 87.Martin PF. Direct determination of the growth rate of Drosophila Imaginal Discs. J Exp Zool. 1982;222:97–102. [Google Scholar]
- 88.Lewis EB. A gene complex controlling segmentation in Drosophila. Nature. 1978;276:565–570. doi: 10.1038/276565a0. [DOI] [PubMed] [Google Scholar]
- 89.Lawrence PA. The making of the fly: the genetics of animal design. Oxford and Boston: Wiley-Blackwell; 1992. [Google Scholar]
- 90.Crickmore MA, Mann RS. Hox control of organ size by regulation of morphogen production and mobility. Science. 2006;313:63–68. doi: 10.1126/science.1128650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Day SJ, Lawrence PA. Measuring dimensions: the regulation of size and shape. Development. 2000;127:2977–2987. doi: 10.1242/dev.127.14.2977. [DOI] [PubMed] [Google Scholar]
- 92.Affolter M, Basler K. The Decapentaplegic morphogen gradient: from pattern formation to growth regulation. Nat Rev Genet. 2007;8:663–674. doi: 10.1038/nrg2166. [DOI] [PubMed] [Google Scholar]
- 93.Rogulja D, Irvine KD. Regulation of cell proliferation by a morphogen gradient. Cell. 2005;123:449–461. doi: 10.1016/j.cell.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 94.Pan D. Hippo signaling in organ size control. Genes Dev. 2007;21:886–897. doi: 10.1101/gad.1536007. [DOI] [PubMed] [Google Scholar]
- 95.Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kango-Singh M, Singh A. Regulation of organ size: insights from the Drosophila Hippo signaling pathway. Dev Dyn. 2009;238:1627–1637. doi: 10.1002/dvdy.21996. [DOI] [PubMed] [Google Scholar]
- 97.Sopko R, Silva E, Clayton L, Gardano L, Barrios-Rodiles M, Wrana J, et al. Phosphorylation of the tumor suppressor fat is regulated by its ligand Dachsous and the kinase discs overgrown. Curr Biol. 2009;19:1112–1117. doi: 10.1016/j.cub.2009.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Willecke M, Hamaratoglu F, Sansores-Garcia L, Tao C, Halder G. Boundaries of Dachsous Cadherin activity modulate the Hippo signaling pathway to induce cell proliferation. Proc Natl Acad Sci USA. 2008;105:14897–14902. doi: 10.1073/pnas.0805201105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rogulja D, Rauskolb C, Irvine KD. Morphogen control of wing growth through the Fat signaling pathway. Dev Cell. 2008;15:309–321. doi: 10.1016/j.devcel.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hufnagel L, Teleman AA, Rouault H, Cohen SM, Shraiman BI. On the mechanism of wing size determination in fly development. Proc Natl Acad Sci USA. 2007;104:3835–3840. doi: 10.1073/pnas.0607134104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nellen D, Burke R, Struhl G, Basler K. Direct and long-range action of a DPP morphogen gradient. Cell. 1996;85:357–368. doi: 10.1016/s0092-8674(00)81114-9. [DOI] [PubMed] [Google Scholar]
- 102.Neumann CJ, Cohen SM. A hierarchy of cross-regulation involving Notch, wingless, vestigial and cut organizes the dorsal/ventral axis of the Drosophila wing. Development. 1996;122:3477–3485. doi: 10.1242/dev.122.11.3477. [DOI] [PubMed] [Google Scholar]
- 103.Goulev Y, Fauny JD, Gonzalez-Marti B, Flagiello D, Silber J, Zider A. SCALLOPED interacts with YORKIE, the nuclear effector of the hippo tumor-suppressor pathway in Drosophila. Curr Biol. 2008;18:435–441. doi: 10.1016/j.cub.2008.02.034. [DOI] [PubMed] [Google Scholar]
- 104.Lehtinen MK, Yuan Z, Boag PR, Yang Y, Villen J, Becker EB, et al. A conserved MST-FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell. 2006;125:987–1001. doi: 10.1016/j.cell.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 105.Papadopoulou D, Bianchi MW, Bourouis M. Functional studies of shaggy/glycogen synthase kinase 3 phosphorylation sites in Drosophila melanogaster. Mol Cell Biol. 2004;24:4909–4919. doi: 10.1128/MCB.24.11.4909-4919.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hoogeboom D, Essers MA, Polderman PE, Voets E, Smits LM, Burgering BM. Interaction of FOXO with beta-catenin inhibits beta-catenin/T cell factor activity. J Biol Chem. 2008;283:9224–9230. doi: 10.1074/jbc.M706638200. [DOI] [PubMed] [Google Scholar]
- 107.Aegerter-Wilmsen T, Aegerter CM, Hafen E, Basler K. Model for the regulation of size in the wing imaginal disc of Drosophila. Mech Dev. 2007;124:318–326. doi: 10.1016/j.mod.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 108.Shraiman BI. Mechanical feedback as a possible regulator of tissue growth. Proc Natl Acad Sci USA. 2005;102:3318–3323. doi: 10.1073/pnas.0404782102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Steinberg MS. Differential adhesion in morphogenesis: a modern view. Curr Opin Genes Devel. 2007;17:281–286. doi: 10.1016/j.gde.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 110.Teleman AA, Cohen SM. Dpp gradient formation in the Drosophila wing imaginal disc. Cell. 2000;103:971–980. doi: 10.1016/s0092-8674(00)00199-9. [DOI] [PubMed] [Google Scholar]
- 111.Belenkaya TY, Han C, Yan D, Opoka RJ, Khodoun M, Liu H, et al. Drosophila Dpp morphogen movement is independent of dynamin-mediated endocytosis but regulated by the glypican members of heparan sulfate proteoglycans. Cell. 2004;119:231–244. doi: 10.1016/j.cell.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 112.Liu M, Skinner SJ, Xu J, Han RN, Tanswell AK, Post M. Stimulation of fetal rat lung cell proliferation in vitro by mechanical stretch. Am J Physiol. 1992;263:376–383. doi: 10.1152/ajplung.1992.263.3.L376. [DOI] [PubMed] [Google Scholar]
- 113.Kippenberger S, Loitsch S, Guschel M, Muller J, Knies Y, Kaufmann R, et al. Mechanical stretch stimulates protein kinase B/Akt phosphorylation in epidermal cells via angiotensin II type 1 receptor and epidermal growth factor receptor. J Biol Chem. 2005;280:3060–3067. doi: 10.1074/jbc.M409590200. [DOI] [PubMed] [Google Scholar]
- 114.Shyy JY, Chien S. Role of integrins in cellular responses to mechanical stress and adhesion. Curr Opin Cell Biol. 1997;9:707–913. doi: 10.1016/s0955-0674(97)80125-1. [DOI] [PubMed] [Google Scholar]
- 115.Avvisato CL, Yang X, Shah S, Hoxter B, Li W, Gaynor R, et al. Mechanical force modulates global gene expression and beta-catenin signaling in colon cancer cells. J Cell Sci. 2007;120:2672–2682. doi: 10.1242/jcs.03476. [DOI] [PubMed] [Google Scholar]
- 116.Balaban NQ, Schwarz US, Riveline D, Goichberg P, Tzur G, Sabanay I, et al. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat Cell Biol. 2001;3:466–472. doi: 10.1038/35074532. [DOI] [PubMed] [Google Scholar]
- 117.Hayashi T, Carthew RW. Surface mechanics mediate pattern formation in the developing retina. Nature. 2004;431:647–652. doi: 10.1038/nature02952. [DOI] [PubMed] [Google Scholar]
- 118.Sanson B, White P, Vincent JP. Uncoupling cadherinbased adhesion from wingless signalling in Drosophila. Nature. 1996;383:627–630. doi: 10.1038/383627a0. [DOI] [PubMed] [Google Scholar]
- 119.Farge E. Mechanical induction of Twist in the Drosophila foregut/stomodeal primordium. Curr Biol. 2003;13:1365–1377. doi: 10.1016/s0960-9822(03)00576-1. [DOI] [PubMed] [Google Scholar]
- 120.Sun CX, Robb VA, Gutmann DH. Protein 4.1 tumor suppressors: getting a FERM grip on growth regulation. J Cell Sci. 2002;115:3991–4000. doi: 10.1242/jcs.00094. [DOI] [PubMed] [Google Scholar]
- 121.McClatchey AI, Giovannini M. Membrane organization and tumorigenesis—the NF2 tumor suppressor, Merlin. Genes Dev. 2005;19:2265–2277. doi: 10.1101/gad.1335605. [DOI] [PubMed] [Google Scholar]
- 122.Lallemand D, Curto M, Saotome I, Giovannini M, McClatchey AI. NF2 deficiency promotes tumorigenesis and metastasis by destabilizing adherens junctions. Genes Dev. 2003;17:1090–1100. doi: 10.1101/gad.1054603. [DOI] [PMC free article] [PubMed] [Google Scholar]