Abstract
The secreted glycoprotein vascular endothelial growth factor A (VEGF or VEGFA) affects many different cell types and modifies a wide spectrum of cellular behaviors in tissue culture models, including proliferation, migration, differentiation and survival. The versatility of VEGF signaling is reflected in the complex composition of its cell surface receptors and their ability to activate a variety of different downstream signaling molecules. A major challenge for VEGF research is to determine which of the specific signaling pathways identified in vitro control development and homeostasis of tissues containing VEGF-responsive cell types in vivo.
Key words: VEGF, VEGFR1, VEGFR2, FLT1, KDR, FLK1, neuropilin, HSPG
Key Messages
VEGF is expressed in different isoforms,
VEGF isoforms bind different subsets of cell surface receptors,
VEGF receptors activate a plethora of downstream signaling pathways,
VEGF receptors mediate different cellular effects.
Introduction
Vascular Endothelial Growth Factor A (VEGF or VEGFA) is a critical organizer of vascular development due to its ability to regulate proliferation, migration, specialization and survival of endothelial cells (reviewed in ref. 1). VEGF also affects many other cell types in tissue culture models. For example, it is mitogenic for lymphocytes, retinal pigment epithelium and Schwann cells.2–4 It also stimulates the migration of hematopoietic precursors, monocytes/macrophages, neurons and vascular smooth muscle cells,5–11 and it promotes the survival of developing and mature neurons12 as well as chondrocytes.13,14
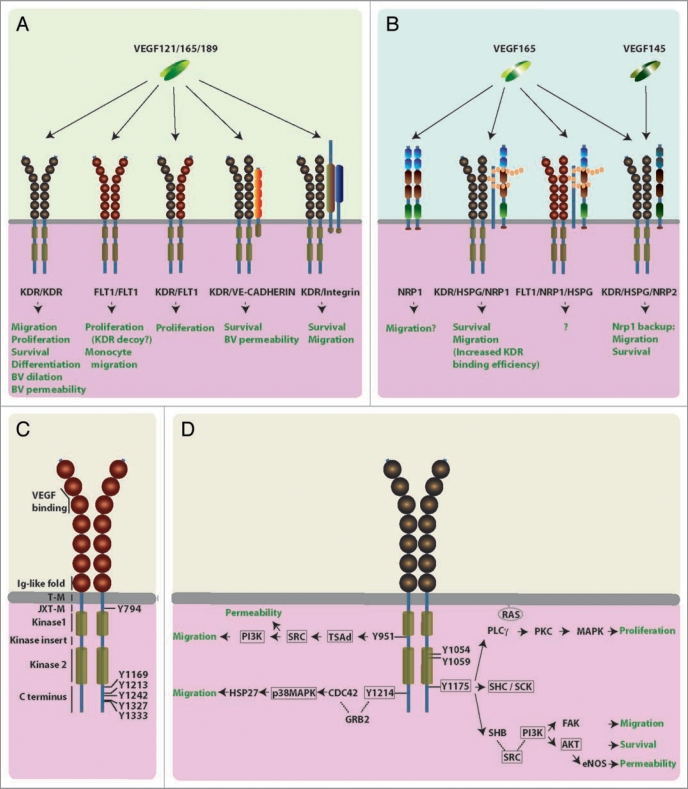
Differential splicing of the eight exons comprising the VEGF gene (Vegfa) gives rise to three main isoforms, termed VEGF121, VEGF165 and VEGF189 in humans and VEGF120, VEGF164 and VEGF188 in mice.1 All VEGF isoforms bind to two type III receptor tyrosine kinases, FLT1 (fms-related tyrosine kinase 1, also denominated VEGFR1) and KDR (kinase insert domain containing receptor, also known as FLK1 or VEGFR2) (Fig. 1A). In contrast, heparan sulphate proteoglycans (HSPGs) and the non-tyrosine kinase receptors neuropilin 1 (NRP1) and neuropilin 2 (NRP2) preferentially bind the VEGF isoforms containing the heparin-binding domains, encoded by exons 6 and 7 (Fig. 1B). In addition to the versatility provided by the existence of several different VEGF isoforms and VEGF receptors, VEGF signaling attains further plasticity from the association of VEGF receptors with other transmembrane proteins to form higher order signaling complexes (Fig. 1A). For example, KDR and FLT1 interact with integrins and vascular endothelial cadherin (VE-cadherin). In this chapter, we critically review current knowledge of the different VEGF signaling pathways and their interplay during development to expand on a more general recent review on VEGF receptors.15
Figure 1.
Working models for VEGF receptor signaling. (AD) Schematic illustration of the different human VEGF receptors and their predicted physiological roles in endothelial cells, blood vessels and macrophages. (A) VEGF tyrosine kinase receptors: All VEGF isoforms (VEGF121, VEGF165, VEGF189) bind to homo or heterodimers of KDR and FLT1. KDR can form higher order complexes with VE-cadherin or integrins. (B) Isoform-specific VEGF receptors: VEGF165, but not VEGF121, binds receptor complexes containing NRP1 and HSPGs, or higher order complexes containing additionally FLT1 or KDR. VEGF165 and VEGF145 bind NRP2. The neuropilin CUB domains (a1 and a2) are shown in blue, the coagulation factor V/VIII homology domains (b1 and b2) are highlighted red, and the MAM domain is colored green. (C) FLT1 domain structure: The extracellular region consists of seven Ig-like folds (shown as spheres); they bind ligands and mediate receptor dimerisation; the cytoplasmic domain contains two kinase domains (light brown cylinders) interrupted by a kinase insert domain; a juxtamembrane domain is thought to inhibit autophosphorylation.
Tyrosine Kinase Receptors for VEGF: FLT1 and KDR
Structure of FLT1 and KDR.
FLT1 and KDR are transmembrane glycoproteins of 180 and 200 kDa, respectively. They are closely related to other type III receptor tyrosine kinases, including FMS, KIT and PDGFR, and contain an extracellular domain composed of seven immunoglobulin (Ig)-like folds, a single transmembrane domain, a regulatory juxtamembrane domain and an intracellular tyrosine kinase domain (Fig. 1). The intracellular tyrosine kinase domain is interrupted by a kinase insert domain and contains several tyrosine residues that mediate the recruitment of downstream signaling molecules upon phosphorylation (Fig. 1C and D). Both KDR and FLT1 bind VEGF with high affinity. Mutation analysis of the extracellular domains of FLT1 and KDR revealed that the second and third Ig-like folds contain the high affinity ligand-binding domain for VEGF, while the first and fourth Ig-like folds regulate ligand binding and receptor dimerization, respectively (Fig. 1C and D).16–18 In addition to binding VEGF, FLT1 also acts as a receptor for VEGFB and PGF (previously known as PlGF), whilst KDR also binds the VEGF homologs VEGFC and VEGFD and the viral VEGFE.19 Binding of VEGF by tyrosine kinase receptors promotes their homophilic or heterophilic interaction to activate the kinase domain.20,21
Expression pattern of FLT1 and KDR.
KDR and FLT1 are expressed in endothelial cells in most, if not all tissues in mouse and human embryos. The expression level of FLT1 in vascular endothelium varies with gestational age. Between embryonic days (E) 8.5 and E14 in the mouse, the Flt1 gene is expressed at high levels in endothelial cells, but expression decreases thereafter.22 In newborn mice, Flt1 expression increases again, and it continues to be expressed in adults,22 consistent with the idea that it plays a role in the homeostasis of blood vessels. Flt1 gene expression is regulated by hypoxia, and a binding site for hypoxia-inducible factor (HIF1A) has been identified in the Flt1 promoter.23 Thus, Flt1 is upregulated in vascular smooth muscle cells experiencing hypoxic stress, perhaps to control vascular remodelling or tone.24 However, further studies are required to fully understand the physiological significance of the transcriptional regulation of FLT1 by hypoxia, and how it may complement the regulation of VEGF by hypoxia. In contrast to Vegfa and Flt1, Kdr has no HIF1A binding sites in its promoter region and is therefore not regulated by hypoxia.23 Kdr is already expressed in mesodermal progenitors of vascular endothelial cells in the yolk sac at E7 in the mouse, and its expression is often used as a marker for these progenitor cells.25–27 Kdr expression remains high on vascular endothelial cells during development, but it declines towards the end of gestation.28 Non-endothelial expression of KDR has been reported in neurons, osteoblasts, pancreatic ducts cells, retinal progenitor cells, platelets and megakaryocytes (reviewed in refs. 29–32). Due to its expression by adult neurons after brain injury, it has been suggested that KDR has a physiological, neuroprotective function. Like KDR, FLT1 is expressed in endothelial progenitor cells and osteoblasts, but additionally in hematopoietic stem cells, macrophages, osteoclasts, dendritic cells, pericytes, smooth muscle cells and placental trophoblasts.33–38
Functional requirements for FLT1.
An essential role for FLT1 in development is highlighted by the fact that FLT1-deficient mice die in utero between E8 and E9, most likely due to a failure of endothelial cells to assemble into a vascular circuit. The primary defect underlying this phenotype appears to be an altered cell fate determination among mesenchymal cells, which increases hemangioblast numbers.39 The defect has been attributed to VEGF hyperactivity subsequent to the loss of endothelial FLT.40 Two different hypotheses have been put forward to explain the negative role of FLT1 in developmental angiogenesis. The most widely accepted hypothesis suggests that FLT1 functions as a cell surface-bound “decoy receptor” to sequester excess extracellular VEGF. In support of this idea, the FLT1 kinase domain is not normally active in endothelial cells, even though FLT1 has a tenfold higher affinity for VEGF compared to KDR; in fact, FLT1 activation in endothelial cells has only be achieved by overexpression of recombinant protein.41–43 Moreover, mice expressing a mutant form of FLT1 with an inactive tyrosine kinase domain (Flt1TK−/−) have no discernable defects in blood vessel formation, branching or remodelling, even though these mice show deficiencies in VEGF-induced macrophage migration.44 Finally, membrane tethering of FLT1 is essential for vascular development: 50% of mice expressing solely a soluble form of FLT1, which lacks the transmembrane and tyrosine kinase domains, died between E8.5 and E9.0 with a disorganized vascular network, similar to the full knockout.45 However, whilst 50% of mice expressing only a soluble form of FLT1 die, the other 50% of mice making only soluble FLT1 survive. A soluble form of FLT1 is produced endogenously by alternative splicing (sFLT1), raising the possibility that the soluble isoform normally cooperates with the membrane-tethered isoform to control vascular development. For example, it is conceivable that membrane bound FLT1 functions as a decoy receptor to limit VEGF availability to KDR, whilst sFLT1 sequesters soluble VEGF in the endothelial environment to sharpen VEGF gradients.46
Even though the FLT1 tyrosine kinase domain is dispensable for vascular development, FLT1 tyrosine kinase signaling significantly promotes pathological angiogenesis.47,48 Several different explanations have been put forward to explain this difference in developmental and pathological angiogenesis. Firstly, FLT1 upregulation might activate PGF and VEGF-responsive monocytes, which then release proangiogenic factors; in agreement with this idea, FLT1 tyrosine kinase signaling mediates chemotactic macrophage migration in response to PGF and VEGF,34,35,44,49 and PGF promotes macrophage survival during tumor angiogenesis.50 Alternatively, PGF may occupy FLT1 binding sites on endothelial cells, allowing VEGF to bind to KDR rather than FLT1; consistent with this suggestion, PGF potentiates mitogenic VEGF activity in endothelial cells in vitro, and it promotes VEGF-induced vascular permeability in vivo.51 It is also possible that PGF binding to FLT1 promotes the transphosphorylation of KDR by FLT1 in FLT1/KDR heterodimers to increase VEGF/KDR signaling.48 Lastly, PGF activation of FLT1 may stimulate vessel formation and maturation indirectly by acting on non-endothelial cell types, for example smooth muscle cells24,52 or bone marrow derived cells that are recruited to sites of neovascularization.25,53,54 It is presently debated whether proangiogenic bone marrow derived cells support tumor angiogenesis by differentiating into endothelial cells55 or by providing perivascular support cells.54 The recruited perivascular cells have monocyte/macrophage characteristics, such as expression of the integrin CD11b and the hematopoietic lineage marker CD45;54 this observation provides a link to the initial suggestion that PGF supports pathological angiogenesis by acting on cells in the monocyte/macrophage lineage. Importantly, the regulation of FLT1 by hypoxia (see above) might promote PGF responsiveness of both endothelial cells and macrophages during pathological angiogenesis.
FLT1-stimulated signaling pathways.
FLT1 contains several potential tyrosine autophosphorylation sites (Fig. 1C) (reviewed in ref. 56). Whereas a repressor element in the juxtamembrane domain of FLT1 inhibits autophosphorylation after VEGF binding, 57 this repression appears to be alleviated by an unknown mechanism in monocytes/macrophages. Biochemical assays suggest that the phosphorylated FLT1 can recruit several different proteins containing a SRC homology 2 (SH2) domain; this domain was first identified in the SRC protein kinase. In endothelial cells, phosphorylated KDR preferentially binds to and activates SRC, whereas phosphorylated FLT1 preferentially binds two other protein kinases that are closely related to SRC, namely FYN and YES.58 Mice lacking any one of the SRC family kinases do not suffer developmental defects, but the combined loss of SRC, FYN and YES results in embryonic lethality at E9.5.59 Lethality may be due to vascular insufficiency downstream of KDR rather than FLT1 signaling in endothelial cells (see below). The physiological role of the different SRC family kinases in VEGF/PGF mediated macrophage migration has not yet been examined, and the identity of the FLT1 and KDR phosphotyrosines involved in SRC kinase recruitment are also still unknown.
In addition to SRC kinase recruitment, tyrosine phosphorylation of FLT1 promotes recruitment of several other SH2 proteins, including phospholipase C gamma (PLCγ), SH2domain containing tyrosine phosphatase 2 (SHP2), the non-catalytic region of tyrosine kinase adaptor protein 1 (NCK1), the class IA phosphatidylinositol 3kinase (PI3K) and the cellular homolog of the viral oncogene v-crk (Fig. 1C). Phosphorylated Y1213, Y1333, Y794 and Y1169 all recruit PLCγ to activate protein kinase C (PKC). Phosphorylated Y1213 specifically binds SHP2 and NCK1. Phosphorylated Y1213 also activates PI3K, which then catalyses the production of the second messenger lipid PIP3 (Box 1). Y1333 binds CRK (the cellular homolog of v-crk) and NCK. Proteins that bind to phosphorylated Y1242 and Y1327 have so far remained elusive. Interestingly, VEGF and PGF appear to induce phosphorylation of a different subset of tyrosine residues.48 For example, PGF, but not VEGF binding to FLT1 results in Y1309 phosphorylation and activation of the AKT cell survival pathway (see below).
Box 1.
Role of class1A PI3 kinase in vascular growth. The lipid kinases of the PI3 kinase (PI3K) family produce the intracellular messenger PIP3 (phosphatidylinositol3,4,5-tri-phosphate); one of the major functions of PIP3 is activation of the serine/threonine kinase AKT to stimulate proliferation and prevent apoptosis. The PI3Ks have been grouped into three classes, with the class I family being further subdivided into IA and IB kinases. The class 1A PI3Ks signal downstream of receptor tyrosine kinases. A role for class IA PI3Ks in endothelial cells was initially demonstrated in tissue culture models, but has more recently been studied by genetic alteration of the genes encoding its different subunits. Interpretation of the null mutant phenotypes has, however, been complicated by the fact that ablation of any one of the PI3K subunits deregulates other subunits. For example, ablation of the regulatory subunits p85a, p55a or p50 also reduces expression of the p110 catalytic subunits. Conversely, ablation of the p110a subunit results in overexpression of the p85 regulatory subunit, which has a dominant negative effect on all class IA PI3K proteins. Perhaps the most resounding evidence so far in support of an essential role for class IA PI3Ks in vascular development comes from the endothelial cell-specific knockout of PTEN (phosphatase and tensin homolog), a lipid phosphatase that reverses PI3K signaling. In this mouse model, loss of PTEN results in an overstimulation of endothelial cell proliferation and migration, causing embryonic death at E11.5.141
Understanding the physiological significance of the different FLT1 signaling pathways has so far proven difficult. Firstly, SHP2, PI3K, NCK and PLCγ all play roles downstream of a variety of tyrosine kinases, and the analysis of null mutants for these genes therefore cannot identify specific requirements for signaling downstream of FLT1 or KDR. Secondly, no appropriate tissue culture model with a relevant readout has been identified to evaluate the physiological importance of the different phosphorylated tyrosine residues in FLT1.60 It would be particularly interesting to learn more about FLT1 signaling pathways in the monocyte/macrophage lineage.
Functional requirements for KDR.
Consistent with its expression in the mesodermal progenitors of blood islands in the yolk sac, Kdr is required for endothelial and hematopoietic cell differentiation and therefore vasculogenesis and hematopoiesis; thus, loss of KDR function results in embryonic death between E8.5 and E9.5.28 As KDR is tyrosine-phosphorylated more efficiently than FLT1 upon VEGF binding in endothelial cells (see above), KDR is thought to be principally responsible for VEGF signaling to stimulate the proliferation, chemotaxis, survival and differentiation of endothelial cells and to alter their morphology; moreover, KDR signaling is thought to stimulate vessel permeability and vessel dilation.41,61–63 However, owing to the early lethality of Kdr knockout mice, the requirement for KDR in specific stages of vascular development subsequent to vasculogenesis has not yet been formally demonstrated by knockout technology.
KDR-stimulated signaling pathways.
KDR functions similarly to most tyrosine kinase receptors: it dimerizes and is autophosphorylated on several cytoplasmic tyrosine residues upon ligand binding (Fig. 1D). Early experiments using recombinant KDR in bacteria and yeast demonstrated that several tyrosine residues are autophosphorylated upon VEGF binding to recruit SH2domain containing proteins. The following autophosphorylated tyrosine residues were subsequently identified in human endothelial cells: in the kinase insert domain, Y951 (corresponding to Y949 in the mouse); in the tyrosine kinase domain, Y1054 and Y1059 (corresponding to Y1053 and Y1057 in the mouse); and in the C-terminal domain, Y1175 and Y1214 (corresponding to Y1173 and Y1212 in the mouse).64 As observed in the case of FLT1, KDR phosphotyrosines are recognized by a number of different SH2domain containing proteins. For example, SRC kinases have been implicated in signaling pathways downstream of Y951 and Y1175 (Fig. 1D), and SRC kinases modulate endothelial proliferation and migration in tissue culture models65 and during neoangiogenesis in adults.66 To clarify the relative contribution of the different KDR phosphotyrosines to vascular development, we will discuss the phenotypes of mice that either lack single KDR tyrosine residues or the proteins predicted to bind to them following phosphorylation.
Human Y951, Y1175 and Y1214 have all been implicated in the control of endothelial cell proliferation or migration in culture models. Y951 is selectively phosphorylated in a subset of endothelial cells during development and binds to the T cell-specific adapter molecule (TSAd), which is thought to act upstream of SRC and PI3K (Fig. 1D). Even though TSAd is critical for actin reorganization in cell culture models, it is not essential for mouse development.67 No other protein has so far been identified that interacts functionally with Y951 in endothelial cells, and it is not known if Y951 is essential for vascular development.
Y1214 is embedded in a region of KDR that resembles the consensus binding sequence for the growth factor receptor bound protein 2 (GRB2) and has been implicated in the control of actin reorganization and cell migration through the activation of CDC42 and the mitogen activated protein kinase (MAPK) cascade68 (Fig. 1D). A mouse model for the tyrosine residues corresponding to human Y1214 has been created by replacing Y1212 with a phenylalanine residue; surprisingly, these mutants have no discernable defects.69
A mouse model for the tyrosine residue corresponding to human Y1175 has also been created by replacement of Y1173 with a phenylalanine residue. This mutation results in embryonic lethality between E8.5 and E9.5 with endothelial and hematopoietic defects, similar to those seen in complete KDR knockout mice.70 The essential Y1175 residue, located in the KDR C-terminal domain, interacts with a number of SH2 domain-containing proteins that are expressed in endothelial cells, including PLCγ and the adaptor proteins SHCA, SHCB (also called SCK) and SHB (Fig. 1D). Activation of PLCγ leads to the activation of PKC to control endothelial cell proliferation via the MAPK pathway in cultured endothelial cells (Fig. 1D). Several different MAPK are essential for embryogenesis, with p38 and ERK5 being required for vascular development; however, it is not clear if the effects on blood vessel growth reflect a requirement in endothelial cells or occur subsequent to defective placentation.71 SHCA KO mice suffer from embryonic lethality due to extensive vascular defects.72 However, SHCA also interacts with other tyrosine kinase receptors that may be involved in vasculogenesis and may therefore not be a specific downstream effector of KDR. SHCB is expressed in developing blood vessels, but SHCB KO mice have no vascular defects, possibly because it acts redundantly with other SHC family members such as SHCA.73 SHB controls endothelial cell migration through the focal adhesion kinase FAK in a pathway that involves PI3K activation (Fig. 1D). Even though SHB has not yet been knocked out in mice, it is essential for blood vessel growth in an embryonic stem cell model of angiogenesis.74
In addition to promoting the proliferation and migration of endothelial cells, VEGF also promotes their survival. Genetic mouse models suggest that VEGF supports endothelial cell survival in vivo by acting both in a paracrine fashion75 and in an autocrine loop.76 In vitro models have identified several different downstream signaling pathways that are activated by VEGF to promote endothelial survival. Paracrine survival signaling in cultured endothelial cells involves the interaction of KDR with cell adhesion molecules of the integrin family, which control cell survival in response to matrix signals in many cell types including endothelium,77 and the interaction of KDR with VE-cadherin, a component of endothelial cell adherens junctions78 (Fig. 1A). Mice lacking VE-cadherin die at 9.5 dpc due to vascular insufficiency, caused by defective blood vessel remodelling and maturation.79 These defects may be due to reduced activation of anti-apoptotic protein kinases such as AKT1, a protein that promotes endothelial cell survival in vitro and in vivo.80 AKT1 activation normally occurs downstream of VE-cadherin and VEGF/KDR in a process that requires SRC and PI3K66,81,82 (Fig. 1D). However, AKT1 is not essential for vascular development, possibly because it signals redundantly with closely related AKT1 and AKT3 proteins. Alternatively, or additionally, VE-cadherin/KDR interaction may impact on endothelial cell survival by controlling cell surface retention of KDR.83 It is not known which intracellular effectors play a role in autocrine VEGF survival signaling, as this pathway does not require VEGF secretion76 and therefore is likely to bypass KDR/VE-cadherin complexes on the cell surface.
Negative regulation of KDR signaling.
Whereas much effort has been directed at identifying the forward signaling pathways downstream of KDR, the molecular mechanisms that modulate KDR activity have received less attention. Presumably, KDR activation must be downregulated at some point to terminate signaling. The phosphatases SHP1 and SHP2 dephosphorylate the nonessential KDR Y1214 residue,84,85 and human cellular protein tyrosine phosphatase A (HCPTPA) inhibits VEGF signaling in tissue culture models, possibly by dephosphorylating KDR to inhibit MAPK activation.86 Unfortunately, the physiological significance of this pathway is unknown.
VEGF Isoform-Specific Receptors: Neuropilins and HSPGs
Identification of the neuropilins as VEGF receptors.
An isoform specific VEGF receptor that binds VEGF165, but not VEGF121, was first described in human umbilical vein-derived endothelial cells87 and subsequently in several tumor-derived cell lines that lack the expression of other VEGF receptors.88 This novel VEGF receptor was purified and identified as neuropilin 1 (NRP1), a 130 kDa type I transmembrane protein (Fig. 1B). NRP1 had previously been discovered as an axonal adhesion protein in the developing frog nervous system89,90 and as a receptor for secreted guidance molecules of the class 3 semaphorin family. 91,92 Besides NRP1, the neuropilin family includes NRP2.93 Even though NRP1 and NRP2 share only 44% homology at the amino acid level, each protein is highly conserved amongst different vertebrate species, including frog, chick, mouse and human. NRP1 and NRP2 bind a different subset of VEGF isoforms and semaphorins in vitro: whereas NRP1 preferentially binds VEGF165 and SEMA3A, NRP2 binds both VEGF165 and VEGF145 as well as SEMA3F.91–94
Structure of neuropilins.
NRP1 and NRP2 have an identical domain structure.93,95 Both contain a large N-terminal extracellular domain of approximately 850 amino acids, a short membrane spanning domain of approximately 24 amino acid residues and a small cytoplasmic domain of 40 residues. The extracellular domain contains two complement binding (CUB) domains (termed a1 and a2), two coagulation factor V/VIII homology domains (termed b1 and b2) and a meprin (MAM) domain (Fig. 1B). The a- and b-domains are crucial for ligand binding, whilst the MAM domain promotes dimerization and the interaction with other cell surface receptors.96 The cytoplasmic domain is short and was originally thought to lack signaling motifs, because its deletion did not impair axonal growth cone collapse in response to SEMA3A.97 Instead, neuropilins transduce semaphorin signals in neurons through a signaling coreceptor of the plexin family.98,99 In analogy, it was inferred that neuropilins recruit a coreceptor such as FLT1 and KDR to transmit VEGF signals in endothelial cells. In agreement with this idea, NRP1 potentiates the signaling of coexpressed KDR in porcine aortic endothelial cells, which surprisingly lack endogenous KDR expression.100 However, the relationship of NRP1 and KDR is different to that of NRP1 and plexins: whereas NRP1 is the compulsory ligand binding subunit in the semaphorin receptor, KDR does not require NRP1 to bind VEGF. Vice versa, recent evidence suggests that NRP1 can also signal independently of KDR in endothelial cells, suggesting that the cytoplasmic tail may have signaling activity after all (see below).
Functional requirements for neuropilins in vascular development.
Neuropilins are expressed by several types of embryonic neurons, and their targeted inactivation in the mouse impairs axon guidance and neuronal migration in response to semaphorins.101–105 In addition, loss of NRP1 disrupts neuronal migration in response to VEGF.11
In the vasculature, NRP1 is preferentially expressed on arterial and brain microvessel endothelium, whereas NRP2 is present on venous and lymphatic endothelium.106,107 Consistent with a role for NRP1 in vascular growth, overexpression of NRP1 in mice deregulates angiogenesis, causing embryonic lethality at E17.5; the mutant embryos exhibit excess capillaries and blood vessels, dilation of blood vessels, severe hemorrhage and malformed hearts.107 Mice lacking NRP1 die even earlier, at around E12.5, with impaired neural tube vascularization, agenesis or transposition of the aortic arches, persistent truncus arteriosus and insufficient development of the yolk sac vasculature.108 The physiological role of NRP1 during vascular development has also been addressed in zebrafish models. In this organism, knockdown of NRP1 impairs angiogenic sprouting from the major axial vessels and therefore formation of the intersomitic vessels.109 Others have shown that the knockdown of NRP1 in zebrafish disrupts even earlier stages of vascular development, including the formation of the dorsal longitudinal anastomosing vessels and the subintestinal vein.110
Consistent with its expression pattern, mice lacking NRP2 are deficient in the formation of small lymphatics and capillaries, but they show no other obvious cardiovascular abnormalities.111 Noteworthy, loss of both NRP1 and NRP2 in mice impairs vascular development more severely than loss of NRP1 alone, with death at E8.0 due to impaired yolk sac vascularization;112 these data suggest that both proteins can partially compensate for each other during the formation of arteriovenous circuits. However, the reason why NRP2 is able to compensate for NRP1 during vascular development is presently unclear. The observation that both proteins are expressed in a reciprocal pattern during the segregation of arteriovenous circuit in the chick106 raises the possibility that venous NRP2 function becomes essential only when arterial NRP1 expression is lost. Alternatively, NRP2 may be upregulated in NRP1-deficient vascular endothelial cells to compensate for NRP1. Consistent with this hypothesis, NRP2 is able to enhance KDR signaling in porcine aortic endothelial cells,113 which lack NRP1.100
The requirement for NRP1 in vascular growth is generally considered to reflect its essential role in promoting VEGF165 signaling in endothelial cells. In agreement with this idea, mice lacking NRP1 specifically in vascular endothelium show impaired microvessel growth in the brain.114 However, there are some striking differences in the vascular defects caused by loss of NRP1 or loss of its VEGF ligands in the developing trunk and central nervous system, with loss of NRP1 causing a more severe vascular deficiency particularly in the brain.108,115,116 These observations suggest that loss of VEGF isoform signaling through NRP1 is not entirely responsible for the vascular deficiency of NRP1 null mutants, and that NRP1 ligands other than VEGF165 may contribute to vessel patterning. The finding that SEMA3A inhibits endothelial cell migration in vitro by competing with VEGF165 for binding to NRP1/KDR complexes made it a candidate modulator of neuropilin-mediated vessel patterning in vivo.117 Yet, class 3 semaphorin signaling through neuropilins is not required for embryonic vascular development.114,118 Therefore, the nature of the hypothetical NRP1 ligand that cooperates with VEGF165 during vascular patterning remains elusive.
VEGF165/NRP1 signaling.
In analogy to the compulsory recruitment of plexins to transmit semaphorin signals, NRP1 was initially proposed to recruit a coreceptor such as FLT1 and KDR to transmit VEGF165 signals.100,119,120 In support of this idea, NRP1 does not promote the VEGF165-induced chemotaxis of KDR-negative cultured porcine aortic endothelial cells, but when coexpressed with KDR, it enhances chemotaxis more than KDR alone.100 Two alternative hypotheses have been proposed to explain the beneficial effect of NRP1 on KDR signaling: Complexes containing both KDR and NRP1 may bind VEGF165 with higher affinity than KDR or NRP1 alone,121 or NRP1 may promote KDR clustering to promote VEGF165 signaling.120
However, other tissue culture models suggest that NRP1 may also function in endothelial cells independently of its ability to enhance VEGF/KDR signaling. Firstly, when the extracellular domain of epidermal growth factor (EGF) receptor was fused to a NRP1 fragment comprised of its membrane spanning and cytoplasmic domain, the chimeric receptor promoted endothelial cell migration in response to EGF.122 Secondly, the last three amino acid residues of NRP1 (SEA-COOH) bind to the neuropilin interacting protein NIP (also known as GIPC or synectin),123 and this interaction contributes to vascular development in zebrafish and mice.113,124 One zebrafish study demonstrated that disruption of trunk vessel development by NRP1 knock down could be rescued by delivery of full length human NRP1, but not by human NRP1 lacking the NIP-binding SEA motif.113 Moreover, ectopic expression of NRP1 lacking the SEA motif or knockdown of NIP disrupted vessel growth in this study.113 Another zebrafish study found that knockdown of NIP affected vascular development at an even earlier stage by impairing dorsal aorta formation.124 In mice, loss of NIP leads to less severe cardiovascular defects than loss of NRP1.124 NIP-null mice are born at the expected Mendelian frequency; moreover, the brain and spinal chord are vascularized normally, even though these tissues are severely affected in NRP1-null mutants (Vieira JM, Ruhrberg C and Simons M, unpublished observations). However, NIPdeficient mice show a specific defect in arterial development and adult arteriogenesis, with reduced arterial density and branching in the retina, heart and kidney.124 These observations agree with those of other mouse studies, in which loss of VEGF165 signaling through NRP1 affected arterial patterning in the limb skin125 and in the retina.126
In summary, NRP1 is likely to play a dual role in vascular growth by enhancing VEGF164 signaling through KDR and by promoting VEGF164 signaling through its own intracellular domain.
Heparan sulphate proteoglycans.
Heparan sulphate proteoglycans (HSPGs) are abundant and highly conserved components of the cell surface and extracellular matrix. They play an important role in the formation and modulation of gradients of heparin-binding growth factors, morphogens and chemokines.127 Several reports have implicated HSPGs as modulators of VEGF signaling. Firstly, VEGF164 and VEGF188 bind heparin in vitro with different degrees of affinity, depending on the presence/ absence of the so-called heparin-binding domains; heparinbinding ability in vitro is thought to indicate HSPG binding in vivo.128 In support of this idea, loss of the heparin-binding VEGF isoforms affects VEGF distribution in the extracellular matrix during angiogenic sprouting in the brain and retina.115,129 Heparin also promotes VEGF165 binding to its receptors KDR130,131 and NRP1.88,100 Moreover, when heparan sulphate is enzymatically removed from endothelial cells, KDR phosphorylation is inhibited.132 The beneficial effect of heparin or heparan sulphate on VEGF signaling may additionally stem from a direct interaction with the VEGF receptors. Consistent with this suggestion, HSPG expression by perivascular smooth muscle cells transactivates endothelial KDR in an embryonic stem cell model of angiogenesis, and possibly facilitates the cross talk between both cell types during blood vessel formation in vivo.133 Finally, NRP1 may itself become a proteoglycan by posttranscriptional modification with glycosaminglycan side chains of the heparan sulphate or the chondroitin sulphate type, and this modification may enhance VEGF binding.134
Outlook
Initially, VEGF signaling pathways were characterized in tissue culture models of endothelium. More recently, the physiological relevance of the different pathways has been addressed with mouse mutants that carry point mutations in single KDR tyrosine phosphorylation sites or harbour null mutations in proteins that interact with these tyrosines. A more complete understanding of KDR signaling will, however, depend on the creation of further mouse mutants lacking other KDR tyrosine residues implicated in intracellular signaling, as well as the design of a novel strategy to study FLT1 tyrosine kinase signaling in vivo.
Despite the progress made in identifying intracellular adaptor molecules for KDR and FLT1, we still know very little about the intracellular trafficking of VEGF and its receptor complexes. For example, in some endothelial culture models the phosphorylation of tyrosine residues Y1054 and Y1059 controls internalization of the VEGF/KDR complex into clathrin-coated vesicles and endosomes prior to degradation,135 and KDR may also signal from endosomes to promote endothelial cell proliferation.83 In other endothelial tissue culture models, VEGF stimulates nuclear translocation of KDR.136–139 In addition, circumstantial evidence is emerging that autocrine VEGF signaling may be based on intracrine signaling; for example, autocrine VEGF survival signaling in endothelial cells does not require VEGF secretion.76 Further effort should therefore be directed at establishing the physiological significance of intracellular interactions between VEGF and its receptors during development or disease.
Owing to the absolute requirement for VEGF during embryogenesis, many previous studies focussed on elucidating the physiological requirement for VEGF signaling pathways in early vascular development. These studies also benefited from the fact that developmental angiogenesis produces a stereotypic pattern of hierarchical blood vessel networks in a well-defined tissue context. In contrast, adult angiogenesis occurs against a backdrop of environmental fluctuations and is influenced by the dynamic interaction of growing vessels with the immune system. Nevertheless, research into developmental VEGF signaling pathways has impacted on our understanding of neoangiogenesis in the adult, owing to the reactivation of VEGF signaling pathways in physiological processes such as wound healing and pathological conditions such as cancer, diabetic retinopathy and ischemic heart disease. Thus, the potential of novel anti-angiogenic therapies can be evaluated in the perinatal rodent eye before being tested in a disease model, because the rodent retina is vascularized only after birth and the eye is easily accessible for drug delivery.140
Finally, it will be necessary to extend the study of VEGF signaling pathways to include other VEGF-responsive cell types, most notably circulating progenitors cells, bone cell types and neuronal progenitors. It will be particularly interesting to elucidate if different VEGF-responsive cell types that grow in close spatiotemporal proximity activate distinct VEGF signaling pathways to coordinate their behavior. For example, VEGF signaling is likely to play a dual role in blood vessels and bone cell types during bone development, and it supports both blood vessel growth and neuronal growth in the angiogenic niche of neurogenesis. The identification of cell type-specific components in the VEGF signaling pathway might then provide the basis for the creation of selective tools to balance vascular effects of VEGF such as permeability against effects on non-endothelial cell types in novel pro and anti-angiogenic therapies.
Acknowledgements
We thank Dr. Patric Turowski for critical reading of the manuscript. J.M.V. holds a PhD studentship from the Fundação para a Ciência e Tecnologia (SFRH/BD/17812/2004). Q.S. is supported by an MRC project grant (G0600993).
Note
Previously published in VEGF in Development, edited by Christiana Ruhrberg. Landes Bioscience and Springer Science + Business Media 2008; 14–29.
Footnotes
Previously published online: www.landesbioscience.com/journals/organogenesis/article/11686
References
- 1.Ruhrberg C. Growing and shaping the vascular tree: Multiple roles for VEGF. Bioessays. 2003;25:1052–1060. doi: 10.1002/bies.10351. [DOI] [PubMed] [Google Scholar]
- 2.Praloran V. Structure, biosynthesis and biological roles of monocyte-macrophage colony stimulating factor (CSF1 or MCSF) Nouv Rev Fr Hematol. 1991;33:323–333. [PubMed] [Google Scholar]
- 3.Guerrin M, Moukadiri H, Chollet P, et al. Vasculotropin/vascular endothelial growth factor is an autocrine growth factor for human retinal pigment epithelial cells cultured in vitro. J Cell Physiol. 1995;164:385–394. doi: 10.1002/jcp.1041640219. [DOI] [PubMed] [Google Scholar]
- 4.Sondell M, Lundborg G, Kanje M. Vascular endothelial growth factor has neurotrophic activity and stimulates axonal outgrowth, enhancing cell survival and Schwann cell proliferation in the peripheral nervous system. J Neurosci. 1999;19:5731–5740. doi: 10.1523/JNEUROSCI.19-14-05731.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grosskreutz CL, Anand-Apte B, Duplaa C, et al. Vascular endothelial growth factor-induced migration of vascular smooth muscle cells in vitro. Microvasc Res. 1999;58:128–136. doi: 10.1006/mvre.1999.2171. [DOI] [PubMed] [Google Scholar]
- 6.Wang H, Keiser JA. Vascular endothelial growth factor upregulates the expression of matrix metalloproteinases in vascular smooth muscle cells: Role of flt1. Circ Res. 1998;83:832–840. doi: 10.1161/01.res.83.8.832. [DOI] [PubMed] [Google Scholar]
- 7.Clauss M, Gerlach M, Gerlach H, et al. Vascular permeability factor: A tumor-derived polypeptide that induces endothelial cell and monocyte pro-coagulant activity, and promotes monocyte migration. J Exp Med. 1990;172:1535–1545. doi: 10.1084/jem.172.6.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Senger DR, Ledbetter SR, Claffey KP, et al. Stimulation of endothelial cell migration by vascular permeability factor/vascular endothelial growth factor through cooperative mechanisms involving the alphavbeta3 integrin, osteopontin and thrombin. Am J Pathol. 1996;149:293–305. [PMC free article] [PubMed] [Google Scholar]
- 9.Cleaver O, Krieg PA. VEGF mediates angioblast migration during development of the dorsal aorta in Xenopus. Development. 1998;125:3905–3914. doi: 10.1242/dev.125.19.3905. [DOI] [PubMed] [Google Scholar]
- 10.Shalaby F, Ho J, Stanford WL, et al. A requirement for Flk1 in primitive and definitive hematopoiesis and vasculogenesis. Cell. 1997;89:981–990. doi: 10.1016/s0092-8674(00)80283-4. [DOI] [PubMed] [Google Scholar]
- 11.Schwarz Q, Gu C, Fujisawa H, et al. Vascular endothelial growth factor controls neuronal migration and cooperates with Sema3A to pattern distinct compartments of the facial nerve. Genes Dev. 2004;18:2822–2834. doi: 10.1101/gad.322904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silverman WF, Krum JM, Mani N, et al. Vascular, glial and neuronal effects of vascular endothelial growth factor in mesencephalic explant cultures. Neuroscience. 1999;90:1529–1541. doi: 10.1016/s0306-4522(98)00540-5. [DOI] [PubMed] [Google Scholar]
- 13.Zelzer E, Mamluk R, Ferrara N, et al. VEGFA is necessary for chondrocyte survival during bone development. Development. 2004;131:2161–2171. doi: 10.1242/dev.01053. [DOI] [PubMed] [Google Scholar]
- 14.Maes C, Stockmans I, Moermans K, et al. Soluble VEGF isoforms are essential for establishing epiphyseal vascularization and regulating chondrocyte development and survival. J Clin Invest. 2004;113:188–199. doi: 10.1172/JCI19383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olsson AK, Dimberg A, Kreuger J, et al. VEGF receptor signaling—In control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 16.Davis-Smyth T, Presta LG, Ferrara N. Mapping the charged residues in the second immunoglobulin-like domain of the vascular endothelial growth factor/placenta growth factor receptor Flt1 required for binding and structural stability. J Biol Chem. 1998;273:3216–3222. doi: 10.1074/jbc.273.6.3216. [DOI] [PubMed] [Google Scholar]
- 17.Fuh G, Li B, Crowley C, et al. Requirements for binding and signaling of the kinase domain receptor for vascular endothelial growth factor. J Biol Chem. 1998;273:11197–11204. doi: 10.1074/jbc.273.18.11197. [DOI] [PubMed] [Google Scholar]
- 18.Shinkai A, Ito M, Anazawa H, et al. Mapping of the sites involved in ligand association and dissociation at the extracellular domain of the kinase insert domain-containing receptor for vascular endothelial growth factor. J Biol Chem. 1998;273:31283–31288. doi: 10.1074/jbc.273.47.31283. [DOI] [PubMed] [Google Scholar]
- 19.Cebe-Suarez S, Zehnder-Fjallman A, Ballmer-Hofer K. The role of VEGF receptors in angiogenesis; complex partnerships. Cell Mol Life Sci. 2006;63:601–615. doi: 10.1007/s00018-005-5426-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kendall RL, Wang G, DiSalvo J, et al. Specificity of vascular endothelial cell growth factor receptor ligand binding domains. Biochem Biophys Res Commun. 1994;201:326–330. doi: 10.1006/bbrc.1994.1705. [DOI] [PubMed] [Google Scholar]
- 21.Huang K, Andersson C, Roomans GM, et al. Signaling properties of VEGF receptor1 and 2 homo and heterodimers. Int J Biochem Cell Biol. 2001;33:315–324. doi: 10.1016/s1357-2725(01)00019-x. [DOI] [PubMed] [Google Scholar]
- 22.Peters KG, De Vries C, Williams LT. Vascular endothelial growth factor receptor expression during embryogenesis and tissue repair suggests a role in endothelial differentiation and blood vessel growth. Proc Natl Acad Sci USA. 1993;90:8915–8919. doi: 10.1073/pnas.90.19.8915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerber HP, Condorelli F, Park J, et al. Differential transcriptional regulation of the two vascular endothelial growth factor receptor genes. Flt1, but not Flk1/KDR, is upregulated by hypoxia. J Biol Chem. 1997;272:23659–23667. doi: 10.1074/jbc.272.38.23659. [DOI] [PubMed] [Google Scholar]
- 24.Bellik L, Vinci MC, Filippi S, et al. Intracellular pathways triggered by the selective FLT1agonist placental growth factor in vascular smooth muscle cells exposed to hypoxia. Br J Pharmacol. 2005;146:568–575. doi: 10.1038/sj.bjp.0706347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hattori K, Heissig B, Wu Y, et al. Placental growth factor reconstitutes hematopoiesis by recruiting VEGFR1(+) stem cells from bone marrow microenvironment. Nat Med. 2002;8:841–849. doi: 10.1038/nm740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bellamy WT. Vascular endothelial growth factor as a target opportunity in hematological malignancies. Curr Opin Oncol. 2002;14:649–656. doi: 10.1097/00001622-200211000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Ishida A, Murray J, Saito Y, et al. Expression of vascular endothelial growth factor receptors in smooth muscle cells. J Cell Physiol. 2001;188:359–368. doi: 10.1002/jcp.1121. [DOI] [PubMed] [Google Scholar]
- 28.Shalaby F, Rossant J, Yamaguchi TP, et al. Failure of blood island formation and vasculogenesis in Flk1 deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 29.Ogunshola OO, Antic A, Donoghue MJ, et al. Paracrine and autocrine functions of neuronal vascular endothelial growth factor (VEGF) in the central nervous system. J Biol Chem. 2002;277:11410–11415. doi: 10.1074/jbc.M111085200. [DOI] [PubMed] [Google Scholar]
- 30.Yang X, Cepko CL. Flk1, a receptor for vascular endothelial growth factor (VEGF), is expressed by retinal progenitor cells. J Neurosci. 1996;16:6089–6099. doi: 10.1523/JNEUROSCI.16-19-06089.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Casella I, Feccia T, Chelucci C, et al. Autocrineparacrine VEGF loops potentiate the maturation of megakaryocytic precursors through Flt1 receptor. Blood. 2003;101:1316–1323. doi: 10.1182/blood-2002-07-2184. [DOI] [PubMed] [Google Scholar]
- 32.Selheim F, Holmsen H, Vassbotn FS. Identification of functional VEGF receptors on human platelets. FEBS Lett. 2002;512:107–110. doi: 10.1016/s0014-5793(02)02232-9. [DOI] [PubMed] [Google Scholar]
- 33.Sawano A, Iwai S, Sakurai Y, et al. Flt1, vascular endothelial growth factor receptor 1, is a novel cell surface marker for the lineage of monocyte-macrophages in humans. Blood. 2001;97:785–791. doi: 10.1182/blood.v97.3.785. [DOI] [PubMed] [Google Scholar]
- 34.Clauss M, Weich H, Breier G, et al. The vascular endothelial growth factor receptor Flt1 mediates biological activities. Implications for a functional role of placenta growth factor in monocyte activation and chemotaxis. J Biol Chem. 1996;271:17629–17634. doi: 10.1074/jbc.271.30.17629. [DOI] [PubMed] [Google Scholar]
- 35.Barleon B, Sozzani S, Zhou D, et al. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt1. Blood. 1996;87:3336–3343. [PubMed] [Google Scholar]
- 36.Dikov MM, Ohm JE, Ray N, et al. Differential roles of vascular endothelial growth factor receptors 1 and 2 in dendritic cell differentiation. J Immunol. 2005;174:215–222. doi: 10.4049/jimmunol.174.1.215. [DOI] [PubMed] [Google Scholar]
- 37.Nomura M, Yamagishi S, Harada S, et al. Possible participation of autocrine and paracrine vascular endothelial growth factors in hypoxiainduced proliferation of endothelial cells and pericytes. J Biol Chem. 1995;270:28316–28324. doi: 10.1074/jbc.270.47.28316. [DOI] [PubMed] [Google Scholar]
- 38.Kaipainen A, Korhonen J, Pajusola K, et al. The related FLT4, FLT1 and KDR receptor tyrosine kinases show distinct expression patterns in human fetal endothelial cells. J Exp Med. 1993;178:2077–2088. doi: 10.1084/jem.178.6.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fong GH, Rossant J, Gertsenstein M, et al. Role of the Flt1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376:66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- 40.Fong GH, Zhang L, Bryce DM, et al. Increased hemangioblast commitment, not vascular disorganization, is the primary defect in flt1 knockout mice. Development. 1999;126:3015–3025. doi: 10.1242/dev.126.13.3015. [DOI] [PubMed] [Google Scholar]
- 41.Waltenberger J, Claesson-Welsh L, Siegbahn A, et al. Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J Biol Chem. 1994;269:26988–26995. [PubMed] [Google Scholar]
- 42.Olofsson B, Korpelainen E, Pepper MS, et al. Vascular endothelial growth factor B (VEGFB) binds to VEGF receptor1 and regulates plasminogen activator activity in endothelial cells. Proc Natl Acad Sci USA. 1998;95:11709–11714. doi: 10.1073/pnas.95.20.11709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gille H, Kowalski J, Li B, et al. Analysis of biological effects and signaling properties of Flt1 (VEGFR1) and KDR (VEGFR2): A reassessment using novel receptor-specific vascular endothelial growth factor mutants. J Biol Chem. 2001;276:3222–3230. doi: 10.1074/jbc.M002016200. [DOI] [PubMed] [Google Scholar]
- 44.Hiratsuka S, Minowa O, Kuno J, et al. Flt1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proc Natl Acad Sci USA. 1998;95:9349–9354. doi: 10.1073/pnas.95.16.9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kearney JB, Kappas NC, Ellerstrom C, et al. The VEGF receptor flt-1 (VEGFR-1) is a positive modulator of vascular sprout formation and branching morphogenesis. Blood. 2004;103:4527–4535. doi: 10.1182/blood-2003-07-2315. [DOI] [PubMed] [Google Scholar]
- 47.Hiratsuka S, Maru Y, Okada A, et al. Involvement of Flt-1 tyrosine kinase (vascular endothelial growth factor receptor-1) in pathological angiogenesis. Cancer Res. 2001;61:1207–1213. [PubMed] [Google Scholar]
- 48.Autiero M, Waltenberger J, Communi D, et al. Role of PlGF in the intra- and intermolecular cross talk between the VEGF receptors Flt1 and Flk1. Nat Med. 2003;9:936–943. doi: 10.1038/nm884. [DOI] [PubMed] [Google Scholar]
- 49.Selvaraj SK, Giri RK, Perelman N, et al. Mechanism of monocyte activation and expression of pro-inflammatory cytochemokines by placenta growth factor. Blood. 2003;102:1515–1524. doi: 10.1182/blood-2002-11-3423. [DOI] [PubMed] [Google Scholar]
- 50.Adini A, Kornaga T, Firoozbakht F, et al. Placental growth factor is a survival factor for tumor endothelial cells and macrophages. Cancer Res. 2002;62:2749–2752. [PubMed] [Google Scholar]
- 51.Park JE, Chen HH, Winer J, et al. Placenta growth factor. Potentiation of vascular endothelial growth factor bioactivity, in vitro and in vivo, and high affinity binding to Flt1 but not to Flk1/KDR. J Biol Chem. 1994;269:25646–25654. [PubMed] [Google Scholar]
- 52.Parenti A, Brogelli L, Filippi S, et al. Effect of hypoxia and endothelial loss on vascular smooth muscle cell responsiveness to VEGFA: Role of flt1/VEGFreceptor1. Cardiovasc Res. 2002;55:201–212. doi: 10.1016/s0008-6363(02)00326-7. [DOI] [PubMed] [Google Scholar]
- 53.Luttun A, Tjwa M, Moons L, et al. Revascularization of ischemic tissues by PlGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by antiFlt1. Nat Med. 2002;8:831–840. doi: 10.1038/nm731. [DOI] [PubMed] [Google Scholar]
- 54.Grunewald M, Avraham I, Dor Y, et al. VEGF-induced adult neovascularization: Recruitment, retention and role of accessory cells. Cell. 2006;124:175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 55.Lyden D, Hattori K, Dias S, et al. Impaired recruitment of bone marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- 56.Matsumoto T, Claesson-Welsh L. VEGF receptor signal transduction. Sci STKE. 2001;2001:21. doi: 10.1126/stke.2001.112.re21. [DOI] [PubMed] [Google Scholar]
- 57.Gille H, Kowalski J, Yu L, et al. A repressor sequence in the juxtamembrane domain of Flt1 (VEGFR1) constitutively inhibits vascular endothelial growth factor-dependent phosphatidylinositol 3’kinase activation and endothelial cell migration. EMBO J. 2000;19:4064–4073. doi: 10.1093/emboj/19.15.4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chou MT, Wang J, Fujita DJ. Src kinase becomes preferentially associated with the VEGFR, KDR/Flk1, following VEGF stimulation of vascular endothelial cells. BMC Biochem. 2002;3:32. doi: 10.1186/1471-2091-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stein PL, Vogel H, Soriano P. Combined deficiencies of Src, Fyn and Yes tyrosine kinases in mutant mice. Genes Dev. 1994;8:1999–2007. doi: 10.1101/gad.8.17.1999. [DOI] [PubMed] [Google Scholar]
- 60.Ito N, Huang K, ClaessonWelsh L. Signal transduction by VEGF receptor1 wild type and mutant proteins. Cell Signal. 2001;13:849–854. doi: 10.1016/s0898-6568(01)00209-1. [DOI] [PubMed] [Google Scholar]
- 61.Terman BI, Dougher-Vermazen M, Carrion ME, et al. Identification of the KDR tyrosine kinase as a receptor for vascular endothelial cell growth factor. Biochem Biophys Res Commun. 1992;187:1579–1586. doi: 10.1016/0006-291x(92)90483-2. [DOI] [PubMed] [Google Scholar]
- 62.Quinn TP, Peters KG, De Vries C, et al. Fetal liver kinase 1 is a receptor for vascular endothelial growth factor and is selectively expressed in vascular endothelium. Proc Natl Acad Sci USA. 1993;90:7533–7537. doi: 10.1073/pnas.90.16.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bernatchez PN, Soker S, Sirois MG. Vascular endothelial growth factor effect on endothelial cell proliferation, migration and platelet-activating factor synthesis is Flk1dependent. J Biol Chem. 1999;274:31047–31054. doi: 10.1074/jbc.274.43.31047. [DOI] [PubMed] [Google Scholar]
- 64.Takahashi T, Yamaguchi S, Chida K, et al. A single autophosphorylation site on KDR/Flk1 is essential for VEGFA-dependent activation of PLC-gamma and DNA synthesis in vascular endothelial cells. EMBO J. 2001;20:2768–2778. doi: 10.1093/emboj/20.11.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Werdich XQ, Penn JS. Src, Fyn and Yes play differential roles in VEGF-mediated endothelial cell events. Angiogenesis. 2005;8:315–326. doi: 10.1007/s10456-005-9021-x. [DOI] [PubMed] [Google Scholar]
- 66.Eliceiri BP, Paul R, Schwartzberg PL, et al. Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol Cell. 1999;4:915–924. doi: 10.1016/s1097-2765(00)80221-x. [DOI] [PubMed] [Google Scholar]
- 67.Rajagopal K, Sommers CL, Decker DC, et al. RIBP, a novel Rlk/Txk and itk-binding adaptor protein that regulates T cell activation. J Exp Med. 1999;190:1657–1668. doi: 10.1084/jem.190.11.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lamalice L, Houle F, Jourdan G, et al. Phosphorylation of tyrosine 1214 on VEGFR2 is required for VEGF-induced activation of Cdc42 upstream of SAPK2/p38. Oncogene. 2004;23:434–445. doi: 10.1038/sj.onc.1207034. [DOI] [PubMed] [Google Scholar]
- 69.Holmqvist K, Cross MJ, Rolny C, et al. The adaptor protein shb binds to tyrosine 1175 in vascular endothelial growth factor (VEGF) receptor2 and regulates VEGF-dependent cellular migration. J Biol Chem. 2004;279:22267–22275. doi: 10.1074/jbc.M312729200. [DOI] [PubMed] [Google Scholar]
- 70.Sakurai Y, Ohgimoto K, Kataoka Y, et al. Essential role of Flk1 (VEGF receptor 2) tyrosine residue 1173 in vasculogenesis in mice. Proc Natl Acad Sci USA. 2005;102:1076–1081. doi: 10.1073/pnas.0404984102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aouadi M, Binetruy B, Caron L, et al. Role of MAPKs in development and differentiation: Lessons from knockout mice. Biochimie. 2006;88:1091–1098. doi: 10.1016/j.biochi.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 72.Lai KM, Pawson T. The ShcA phosphotyrosine docking protein sensitizes cardiovascular signaling in the mouse embryo. Genes Dev. 2000;14:1132–1145. [PMC free article] [PubMed] [Google Scholar]
- 73.Sakai R, Henderson JT, O’Bryan JP, et al. The mammalian ShcB and ShcC phosphotyrosine docking proteins function in the maturation of sensory and sympathetic neurons. Neuron. 2000;28:819–833. doi: 10.1016/s0896-6273(00)00156-2. [DOI] [PubMed] [Google Scholar]
- 74.Kriz V, Agren N, Lindholm CK, et al. The SHB adapter protein is required for normal maturation of mesoderm during in vitro differentiation of embryonic stem cells. J Biol Chem. 2006;281:34484–34491. doi: 10.1074/jbc.M604084200. [DOI] [PubMed] [Google Scholar]
- 75.Haigh JJ, Morelli PI, Gerhardt H, et al. Cortical and retinal defects caused by dosage-dependent reductions in VEGFA paracrine signaling. Dev Biol. 2003;262:225–241. doi: 10.1016/s0012-1606(03)00356-7. [DOI] [PubMed] [Google Scholar]
- 76.Lee S, Chen TT, Barber CL, et al. Autocrine VEGF signaling is required for vascular homeostasis. Cell. 2007;130:691–703. doi: 10.1016/j.cell.2007.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Serini G, Valdembri D, Bussolino F. Integrins and angiogenesis: A sticky business. Exp Cell Res. 2006;312:651–658. doi: 10.1016/j.yexcr.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 78.Carmeliet P, Collen D. Molecular basis of angiogenesis: Role of VEGF and VE-cadherin. Ann NY Acad Sci. 2000;902:249–262. doi: 10.1111/j.1749-6632.2000.tb06320.x. [DOI] [PubMed] [Google Scholar]
- 79.Carmeliet P, Lampugnani MG, Moons L, et al. Targeted deficiency or cytosolic truncation of the VEcadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell. 1999;98:147–157. doi: 10.1016/s0092-8674(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 80.Sun JF, Phung T, Shiojima I, et al. Microvascular patterning is controlled by fine-tuning the Akt signal. Proc Natl Acad Sci USA. 2005;102:128–133. doi: 10.1073/pnas.0403198102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gerber HP, McMurtrey A, Kowalski J, et al. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′kinase/Akt signal transduction pathway. Requirement for Flk1/ KDR activation. J Biol Chem. 1998;273:30336–30343. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- 82.Thakker GD, Hajjar DP, Muller WA, et al. The role of phosphatidylinositol 3kinase in vascular endothelial growth factor signaling. J Biol Chem. 1999;274:10002–10007. doi: 10.1074/jbc.274.15.10002. [DOI] [PubMed] [Google Scholar]
- 83.Lampugnani MG, Orsenigo F, Gagliani MC, et al. Vascular endothelial cadherin controls VEGFR2 internalization and signaling from intracellular compartments. J Cell Biol. 2006;174:593–604. doi: 10.1083/jcb.200602080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guo DQ, Wu LW, Dunbar JD, et al. Tumor necrosis factor employs a proteintyrosine phosphatase to inhibit activation of KDR and vascular endothelial cell growth factor-induced endothelial cell proliferation. J Biol Chem. 2000;275:11216–11221. doi: 10.1074/jbc.275.15.11216. [DOI] [PubMed] [Google Scholar]
- 85.Gallicchio M, Mitola S, Valdembri D, et al. Inhibition of vascular endothelial growth factor receptor 2-mediated endothelial cell activation by Axl tyrosine kinase receptor. Blood. 2005;105:1970–1976. doi: 10.1182/blood-2004-04-1469. [DOI] [PubMed] [Google Scholar]
- 86.Huang L, Sankar S, Lin C, et al. HCPTPA, a protein tyrosine phosphatase that regulates vascular endothelial growth factor receptor-mediated signal transduction and biological activity. J Biol Chem. 1999;274:38183–38188. doi: 10.1074/jbc.274.53.38183. [DOI] [PubMed] [Google Scholar]
- 87.Gitay-Goren H, Cohen T, Tessler S, et al. Selective binding of VEGF121 to one of the three vascular endothelial growth factor receptors of vascular endothelial cells. J Biol Chem. 1996;271:5519–5523. doi: 10.1074/jbc.271.10.5519. [DOI] [PubMed] [Google Scholar]
- 88.Soker S, Fidder H, Neufeld G, et al. Characterization of novel vascular endothelial growth factor (VEGF) receptors on tumor cells that bind VEGF165 via its exon 7encoded domain. J Biol Chem. 1996;271:5761–5767. doi: 10.1074/jbc.271.10.5761. [DOI] [PubMed] [Google Scholar]
- 89.Takagi S, Tsuji T, Amagai T, et al. Specific cell surface labels in the visual centers of Xenopus laevis tadpole identified using monoclonal antibodies. Dev Biol. 1987;122:90–100. doi: 10.1016/0012-1606(87)90335-6. [DOI] [PubMed] [Google Scholar]
- 90.Fujisawa H, Ohtsuki T, Takagi S, et al. An aberrant retinal pathway and visual centers in Xenopus tadpoles share a common cell surface molecule, A5 antigen. Dev Biol. 1989;135:231–240. doi: 10.1016/0012-1606(89)90175-9. [DOI] [PubMed] [Google Scholar]
- 91.He Z, Tessier-Lavigne M. Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell. 1997;90:739–751. doi: 10.1016/s0092-8674(00)80534-6. [DOI] [PubMed] [Google Scholar]
- 92.Kolodkin AL, Levengood DV, Rowe EG, et al. Neuropilin is a semaphorin III receptor. Cell. 1997;90:753–762. doi: 10.1016/s0092-8674(00)80535-8. [DOI] [PubMed] [Google Scholar]
- 93.Chen H, Chedotal A, He Z, et al. Neuropilin2, a novel member of the neuropilin family, is a high affinity receptor for the semaphorins Sema E and Sema IV but not Sema III. Neuron. 1997;19:547–559. doi: 10.1016/s0896-6273(00)80371-2. [DOI] [PubMed] [Google Scholar]
- 94.Gluzman-Poltorak Z, Cohen T, Herzog Y, et al. Neuropilin2 is a receptor for the vascular endothelial growth factor (VEGF) forms VEGF145 and VEGF165 [corrected] J Biol Chem. 2000;275:18040–18045. doi: 10.1074/jbc.M909259199. [DOI] [PubMed] [Google Scholar]
- 95.Takagi S, Hirata T, Agata K, et al. The A5 antigen, a candidate for the neuronal recognition molecule, has homologies to complement components and coagulation factors. Neuron. 1991;7:295–307. doi: 10.1016/0896-6273(91)90268-5. [DOI] [PubMed] [Google Scholar]
- 96.Lee CC, Kreusch A, McMullan D, et al. Crystal structure of the human neuropilin1 b1 domain. Structure. 2003;11:99–108. doi: 10.1016/s0969-2126(02)00941-3. [DOI] [PubMed] [Google Scholar]
- 97.Nakamura F, Tanaka M, Takahashi T, et al. Neuropilin1 extracellular domains mediate semaphorin D/IIIinduced growth cone collapse. Neuron. 1998;21:1093–1100. doi: 10.1016/s0896-6273(00)80626-1. [DOI] [PubMed] [Google Scholar]
- 98.Rohm B, Ottemeyer A, Lohrum M, et al. Plexin/neuropilin complexes mediate repulsion by the axonal guidance signal semaphorin 3A. Mech Dev. 2000;93:95–104. doi: 10.1016/s0925-4773(00)00269-0. [DOI] [PubMed] [Google Scholar]
- 99.Tamagnone L, Artigiani S, Chen H, et al. Plexins are a large family of receptors for transmembrane, secreted and GPI-anchored semaphorins in vertebrates. Cell. 1999;99:71–80. doi: 10.1016/s0092-8674(00)80063-x. [DOI] [PubMed] [Google Scholar]
- 100.Soker S, Takashima S, Miao HQ, et al. Neuropilin1 is expressed by endothelial and tumor cells as an isoformspecific receptor for vascular endothelial growth factor. Cell. 1998;92:735–745. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- 101.Kitsukawa T, Shimizu M, Sanbo M, et al. Neuropilin-semaphorin III/D-mediated chemorepulsive signals play a crucial role in peripheral nerve projection in mice. Neuron. 1997;19:995–1005. doi: 10.1016/s0896-6273(00)80392-x. [DOI] [PubMed] [Google Scholar]
- 102.Kawasaki T, Bekku Y, Suto F, et al. Requirement of neuropilin 1mediated Sema3A signals in patterning of the sympathetic nervous system. Development. 2002;129:671–680. doi: 10.1242/dev.129.3.671. [DOI] [PubMed] [Google Scholar]
- 103.Marin O, Yaron A, Bagri A, et al. Sorting of striatal and cortical interneurons regulated by semaphorinneuropilin interactions. Science. 2001;293:872–875. doi: 10.1126/science.1061891. [DOI] [PubMed] [Google Scholar]
- 104.Giger RJ, Cloutier JF, Sahay A, et al. Neuropilin2 is required in vivo for selective axon guidance responses to secreted semaphorins. Neuron. 2000;25:29–41. doi: 10.1016/s0896-6273(00)80869-7. [DOI] [PubMed] [Google Scholar]
- 105.Chen H, Bagri A, Zupicich JA, et al. Neuropilin2 regulates the development of selective cranial and sensory nerves and hippocampal mossy fiber projections. Neuron. 2000;25:43–56. doi: 10.1016/s0896-6273(00)80870-3. [DOI] [PubMed] [Google Scholar]
- 106.Herzog Y, Kalcheim C, Kahane N, et al. Differential expression of neuropilin1 and neuropilin2 in arteries and veins. Mech Dev. 2001;109:115–119. doi: 10.1016/s0925-4773(01)00518-4. [DOI] [PubMed] [Google Scholar]
- 107.Kitsukawa T, Shimono A, Kawakami A, et al. Overexpression of a membrane protein, neuropilin, in chimeric mice causes anomalies in the cardiovascular system, nervous system and limbs. Development. 1995;121:4309–4318. doi: 10.1242/dev.121.12.4309. [DOI] [PubMed] [Google Scholar]
- 108.Kawasaki T, Kitsukawa T, Bekku Y, et al. A requirement for neuropilin1 in embryonic vessel formation. Development. 1999;126:4895–4902. doi: 10.1242/dev.126.21.4895. [DOI] [PubMed] [Google Scholar]
- 109.Lee P, Goishi K, Davidson AJ, et al. Neuropilin1 is required for vascular development and is a mediator of VEGF-dependent angiogenesis in zebrafish. Proc Natl Acad Sci USA. 2002;99:10470–10475. doi: 10.1073/pnas.162366299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang L, Mukhopadhyay D, Xu X. C terminus of RGS-GAIP-interacting protein conveys neuropilin 1-mediated signaling during angiogenesis. FASEB J. 2006;20:1513–1515. doi: 10.1096/fj.05-5504fje. [DOI] [PubMed] [Google Scholar]
- 111.Yuan L, Moyon D, Pardanaud L, et al. Abnormal lymphatic vessel development in neuropilin 2 mutant mice. Development. 2002;129:4797–4806. doi: 10.1242/dev.129.20.4797. [DOI] [PubMed] [Google Scholar]
- 112.Takashima S, Kitakaze M, Asakura M, et al. Targeting of both mouse neuropilin1 and neuropilin2 genes severely impairs developmental yolk sac and embryonic angiogenesis. Proc Natl Acad Sci USA. 2002;99:3657–3662. doi: 10.1073/pnas.022017899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Favier B, Alam A, Barron P, et al. Neuropilin2 interacts with VEGFR2 and VEGFR3 and promotes human endothelial cell survival and migration. Blood. 2006;108:1243–1250. doi: 10.1182/blood-2005-11-4447. [DOI] [PubMed] [Google Scholar]
- 114.Gu C, Rodriguez ER, Reimert DV, et al. Neuropilin1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev Cell. 2003;5:45–57. doi: 10.1016/s1534-5807(03)00169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ruhrberg C, Gerhardt H, Golding M, et al. Spatially restricted patterning cues provided by heparin-binding VEGFA control blood vessel branching morphogenesis. Genes Dev. 2002;16:2684–2698. doi: 10.1101/gad.242002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gerhardt H, Ruhrberg C, Abramsson A, et al. Neuropilin1 is required for endothelial tip cell guidance in the developing central nervous system. Dev Dyn. 2004;231:503–509. doi: 10.1002/dvdy.20148. [DOI] [PubMed] [Google Scholar]
- 117.Miao HQ, Soker S, Feiner L, et al. Neuropilin1 mediates collapsin1/semaphorin III inhibition of endothelial cell motility: Functional competition of collapsin1 and vascular endothelial growth factor165. J Cell Biol. 1999;146:233–242. doi: 10.1083/jcb.146.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vieira JM, Schwarz Q, Ruhrberg C. Selective requirements for NRP1 ligands during neurovascular patterning. Development. 2007;134:1833–1843. doi: 10.1242/dev.002402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fuh G, Garcia KC, de Vos AM. The interaction of neuropilin1 with vascular endothelial growth factor and its receptor flt1. J Biol Chem. 2000;275:26690–26695. doi: 10.1074/jbc.M003955200. [DOI] [PubMed] [Google Scholar]
- 120.Whitaker GB, Limberg BJ, Rosenbaum JS. Vascular endothelial growth factor receptor2 and neuropilin1 form a receptor complex that is responsible for the differential signaling potency of VEGF(165) and VEGF(121) J Biol Chem. 2001;276:25520–25531. doi: 10.1074/jbc.M102315200. [DOI] [PubMed] [Google Scholar]
- 121.Soker S, Miao HQ, Nomi M, et al. VEGF165 mediates formation of complexes containing VEGFR2 and neuropilin1 that enhance VEGF165 receptor binding. J Cell Biochem. 2002;85:357–368. doi: 10.1002/jcb.10140. [DOI] [PubMed] [Google Scholar]
- 122.Wang L, Zeng H, Wang P, et al. Neuropilin 1 mediated vascular permeability factor/vascular endothelial growth factordependent endothelial cell migration. J Biol Chem. 2003;278:48848–48860. doi: 10.1074/jbc.M310047200. [DOI] [PubMed] [Google Scholar]
- 123.Cai H, Reed RR. Cloning and characterization of neuropilin1interacting protein: A PSD95/Dlg/ZO1 domaincontaining protein that interacts with the cytoplasmic domain of neuropilin1. J Neurosci. 1999;19:6519–6527. doi: 10.1523/JNEUROSCI.19-15-06519.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chittenden TW, Claes F, Lanahan AA, et al. Selective regulation of arterial branching morphogenesis by synectin. Dev Cell. 2006;10:783–795. doi: 10.1016/j.devcel.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 125.Mukouyama YS, Gerber HP, Ferrara N, et al. Peripheral nerve-derived VEGF promotes arterial differentiation via neuropilin 1mediated positive feedback. Development. 2005;132:941–952. doi: 10.1242/dev.01675. [DOI] [PubMed] [Google Scholar]
- 126.Stalmans I, Ng YS, Rohan R, et al. Arteriolar and venular patterning in retinas of mice selectively expressing VEGF isoforms. J Clin Invest. 2002;109:327–336. doi: 10.1172/JCI14362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bernfield M, Kokenyesi R, Kato M, et al. Biology of the syndecans: A family of transmembrane heparan sulfate proteoglycans. Annu Rev Cell Biol. 1992;8:365–393. doi: 10.1146/annurev.cb.08.110192.002053. [DOI] [PubMed] [Google Scholar]
- 128.Park JE, Keller GA, Ferrara N. The vascular endothelial growth factor (VEGF) isoforms: Differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Mol Biol Cell. 1993;4:1317–1326. doi: 10.1091/mbc.4.12.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gerhardt H, Golding M, Fruttiger M, et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tessler S, Rockwell P, Hicklin D, et al. Heparin modulates the interaction of VEGF165 with soluble and cell associated flk1 receptors. J Biol Chem. 1994;269:12456–12461. [PubMed] [Google Scholar]
- 131.Terman B, Khandke L, Dougher-Vermazan M, et al. VEGF receptor subtypes KDR and FLT1 show different sensitivities to heparin and placenta growth factor. Growth Factors. 1994;11:187–195. doi: 10.3109/08977199409046916. [DOI] [PubMed] [Google Scholar]
- 132.AshikariHada S, Habuchi H, Kariya Y, et al. Heparin regulates vascular endothelial growth factor165 dependent mitogenic activity, tube formation, and its receptor phosphorylation of human endothelial cells: Comparison of the effects of heparin and modified heparins. J Biol Chem. 2005;280:31508–31515. doi: 10.1074/jbc.M414581200. [DOI] [PubMed] [Google Scholar]
- 133.Jakobsson L, Kreuger J, Holmborn K, et al. Heparan sulfate in trans potentiates VEGFR-mediated angiogenesis. Dev Cell. 2006;10:625–634. doi: 10.1016/j.devcel.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 134.Shintani Y, Takashima S, Asano Y, et al. Glycosaminoglycan modification of neuropilin1 modulates VEGFR2 signaling. EMBO J. 2006;25:3045–3055. doi: 10.1038/sj.emboj.7601188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dougher M, Terman BI. Autophosphorylation of KDR in the kinase domain is required for maximal VEGF-stimulated kinase activity and receptor internalization. Oncogene. 1999;18:1619–1627. doi: 10.1038/sj.onc.1202478. [DOI] [PubMed] [Google Scholar]
- 136.Feng Y, Venema VJ, Venema RC, et al. VEGF induces nuclear translocation of Flk1/KDR, endothelial nitric oxide synthase, and caveolin1 in vascular endothelial cells. Biochem Biophys Res Commun. 1999;256:192–197. doi: 10.1006/bbrc.1998.9790. [DOI] [PubMed] [Google Scholar]
- 137.Li W, Keller G. VEGF nuclear accumulation correlates with phenotypical changes in endothelial cells. J Cell Sci. 2000;113:1525–1534. doi: 10.1242/jcs.113.9.1525. [DOI] [PubMed] [Google Scholar]
- 138.Ilan N, Tucker A, Madri JA. Vascular endothelial growth factor expression, beta-catenin tyrosine phosphorylation, and endothelial proliferative behavior: A pathway for transformation? Lab Invest. 2003;83:1105–1115. doi: 10.1097/01.lab.0000083531.84403.8b. [DOI] [PubMed] [Google Scholar]
- 139.Santos SC, Miguel C, Domingues I, et al. VEGF and VEGFR2 (KDR) internalization is required for endothelial recovery during wound healing. Exp Cell Res. 2007;313:1561–1574. doi: 10.1016/j.yexcr.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 140.Pan Q, Chanthery Y, Liang WC, et al. Blocking neuropilin1 function has an additive effect with anti-VEGF to inhibit tumor growth. Cancer Cell. 2007;11:53–67. doi: 10.1016/j.ccr.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 141.Hamada K, Sasaki T, Koni PA, et al. The PTEN/PI3K pathway governs normal vascular development and tumor angiogenesis. Genes Dev. 2005;19:2054–2065. doi: 10.1101/gad.1308805. [DOI] [PMC free article] [PubMed] [Google Scholar]