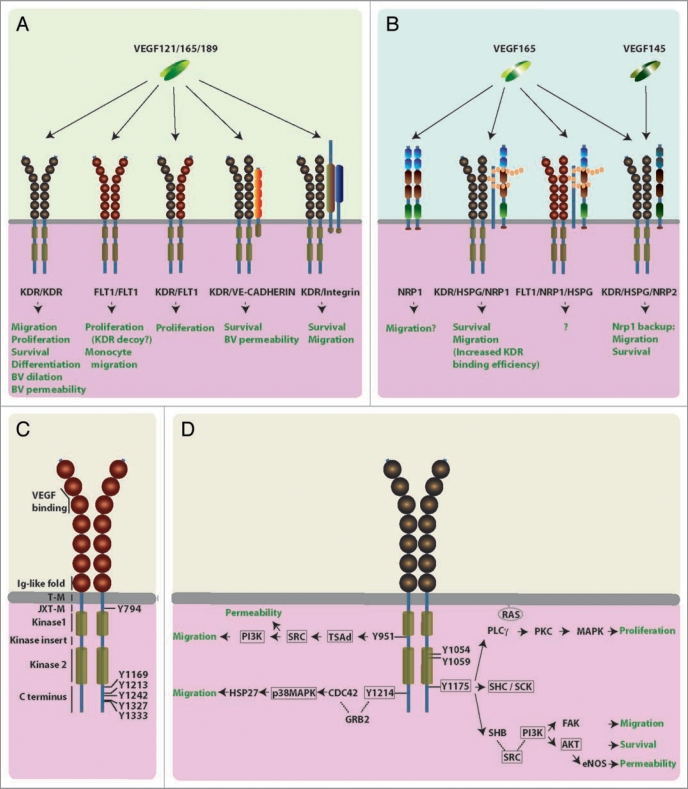
Figure 1.
Working models for VEGF receptor signaling. (AD) Schematic illustration of the different human VEGF receptors and their predicted physiological roles in endothelial cells, blood vessels and macrophages. (A) VEGF tyrosine kinase receptors: All VEGF isoforms (VEGF121, VEGF165, VEGF189) bind to homo or heterodimers of KDR and FLT1. KDR can form higher order complexes with VE-cadherin or integrins. (B) Isoform-specific VEGF receptors: VEGF165, but not VEGF121, binds receptor complexes containing NRP1 and HSPGs, or higher order complexes containing additionally FLT1 or KDR. VEGF165 and VEGF145 bind NRP2. The neuropilin CUB domains (a1 and a2) are shown in blue, the coagulation factor V/VIII homology domains (b1 and b2) are highlighted red, and the MAM domain is colored green. (C) FLT1 domain structure: The extracellular region consists of seven Ig-like folds (shown as spheres); they bind ligands and mediate receptor dimerisation; the cytoplasmic domain contains two kinase domains (light brown cylinders) interrupted by a kinase insert domain; a juxtamembrane domain is thought to inhibit autophosphorylation.