Abstract
Vascular endothelial growth factor (VEGF, VEGFA) is critical for blood vessel growth in the developing and adult nervous system of vertebrates. Several recent studies demonstrate that VEGF also promotes neurogenesis, neuronal patterning, neuroprotection and glial growth. For example, VEGF treatment of cultured neurons enhances survival and neurite growth independently of blood vessels. Moreover, evidence is emerging that VEGF guides neuronal migration in the embryonic brain and supports axonal and arterial co-patterning in the developing skin. Even though further work is needed to understand the various roles of VEGF in the nervous system and to distinguish direct neuronal effects from indirect, vessel-mediated effects, VEGF can be considered a promising tool to promote neuronal health and nerve repair.
Key words: VEGF, neuron, neurogenesis, glia, endothelial cell, blood vessel, angiogenic niche
Key Messages
VEGF promotes neurogenesis.
VEGF has trophic effects on neurons and glia in the CNS and PNS.
VEGF supports neuronal migration in the developing CNS.
VEGF is essential for neuroprotection in adults.
VEGF may be of therapeutic value in the treatment of neural disorders.
Introduction
The cytokine vascular endothelial growth factor (VEGF) fulfils several critical functions in blood vessels, both in adult vertebrates and during their development.1,2 Accordingly, VEGF plays a central role in wound healing, tumor angiogenesis and retinopathies,3–5 and loss of VEGF or its tyrosine kinase receptors causes severe vascular defects and therefore early embryonic demise in the mouse.6–10 The role of VEGF in brain and retinal angiogenesis has been studied extensively. The most topical issue in VEGF biology, however, is the concept that VEGF has significant nonvascular functions in the nervous system. In this review, we will discuss recent data supporting the idea of neurotrophic and instructive roles for VEGF in the nervous system that extend beyond its vascular functions to complement several previous reviews (reviewed in refs.11–13).
VEGF Isoforms and VEGF Receptors in the Nervous System
VEGF is essential for mouse development, as it promotes the condensation of endothelial cells into blood vessel networks in a process termed vasculogenesis.8,9 Importantly, VEGF is normally made as a collection of three major isoforms that are produced by alternative splicing and are co-expressed in tissue-specific ratios.1 These isoforms consist of 121, 165 or 189 amino acids in humans and 120, 164 and 188 amino acids in mice. The VEGF188 isoform is retained in the extracellular matrix after secretion, due to its high affinity for heparan sulphate proteoglycans (HSPGs). VEGF120 and VEGF164 are diffusible, although VEGF164 is also able to bind HSPGs. Amazingly, retention of any one of the VEGF isoforms rescues many vascular defects of full VEGF knockouts, presumably because each isoform is sufficient to support endothelial cell formation and proliferation. In contrast, the VEGF isoforms play different roles during later stages of vascular development. Based on a differential affinity for HSPGs in the extracellular matrix, the isoforms cooperate to establish chemoattractive gradients around VEGF-secreting cells and attract blood vessel sprouts from pre-existing blood vessels in a process termed angiogenesis.14 In this fashion, VEGF isoform expression supports the formation of microvessel networks with optimal density. In addition, VEGF isoforms display a differential affinity for VEGF receptors. The tyrosine kinases KDR (also known as VEGFR2 or FLK1) and FLT1 (VEGFR1) have a high affinity for all VEGF isoforms. In contrast, the non-tyrosine kinase receptors NRP1 and NRP2 bind VEGF165, and possibly VEGF189, but not VEGF121.1
VEGF Controls Brain Angiogenesis
VEGF-induced blood vessel growth is essential for nervous tissue growth during embryonic development. This is demonstrated by the observation that loss of VEGF expression by central nervous system (CNS) neurons impairs vascularisation, curbs neuronal expansion and results in neuronal apoptosis in the developing brain.15,16 It was first hypothesized more than 20 years ago that VEGF is synthesized by rapidly growing neuronal precursors to form a chemoattractive gradient that recruits blood vessels from the perineural vascular plexus to the subventricular zone.17,18 This idea was corroborated by the expression pattern of VEGF and its receptors19 as well as genetic studies demonstrating the role of VEGF gradients in guiding sprouting vessels in the brain.14 VEGF gradients also guide blood vessels during retinal angiogenesis.20 In the adult, VEGF is upregulated following injury to the CNS, and the exogenous application of VEGF promotes CNS angiogenesis. VEGF also increases blood brain barrier permeability and may play a role in CNS inflammation.21–25 KDR is essential for the formation of blood vessels in the embryo, as it controls endothelial cell differentiation, endothelial cell assembly into vascular networks and blood vessel sprouting, whereas FLT1 is crucial for vascular development, because it modulates VEGF/KDR signaling.7,26,27 The requirement for KDR and FLT1 specifically in CNS vascularisation has not been examined due to the early lethality of full knockout mice. In contrast, NRP1 knockout mice survive long enough to form a multilayered brain and spinal cord, and it was demonstrated that this VEGF receptor is essential for vessel growth in the CNS.10,28 Endothelial cells express NRP1, and loss of NRP1 from endothelial cells impairs the vascularisation of the brain and spinal cord.29,30 The most popular hypothesis of NRP1 function in vessel growth suggests that VEGF164 binds to NRP1 on endothelial cells to potentiate KDR signaling.31
Neurotrophic Roles of VEGF during Development and in the Adult
The neurovascular connection.
During development, impaired CNS vascularisation inhibits neuronal proliferation and survival and thereby decreases cortical thickness in mice.15,16 Recent insights indicate that alterations or loss of vascular patterning factors also contribute to neurodegeneration in adults. For example, a reduced serum VEGF level, due to mutation of the hypoxia response element of the Vegfa promoter, renders mice unusually sensitive to transient spinal cord ischemia; they remain paralyzed after a minor ischemic insult, whereas wild type mice show only a transient clinical deficit.32 Moreover, mice with a reduced serum level of VEGF develop a condition akin to amyotrophic lateral sclerosis (ALS).33 ALS is a devastating disease characterized by progressive paralysis due to motor neuron degeneration, and it inevitably causes death. More than 90% of ALS patients are previously healthy with no family history, and the only molecular risk factor known to date is a reduced serum level of VEGF, due to a promoter polymorphism in the VEGF gene.32 Low levels of VEGF have also been found in another motor neuron disease, spinal bulbar muscular atrophy.34 Excitingly, VEGF treatment halts ALS-like motor neuron degeneration in mice.35 Endothelial cells are likely targets in this kind of VEGF therapy, but it is not yet known if other cell types in the nervous system also respond to the administered VEGF. This is an important question, because VEGF affects astrocytes, Schwann cells and motor neurons in cell culture models. In the following paragraphs, we will review evidence consistent with the idea that VEGF affects blood vessel endothelium, glia and neurons in neurodegenerative diseases.
VEGF may affect neurodegeneration by acting on blood vessels.
Mice with reduced VEGF levels due to the mutation of the hypoxia response element in the Vegfa promoter show reduced neural tissue perfusion.33 This was originally thought to be caused by inefficient vessel growth in the CNS during development. However, these mice did not show reduced capillary densities in the CNS, even though VEGF levels were reduced by 25% in the CNS.33 Alternatively, reduced neural perfusion may be due to impaired vasoregulation, as VEGF affects vascular tone by controlling the release of the vasorelaxant nitric oxide by endothelial cells; accordingly, it has been hypothesised that VEGF is required for the preservation or the function of perivascular autonomic nerves, which regulate vascular tone.13 Perfusion deficits in other neurodegenerative disorders, including Alzheimer disease and Huntington disease, may precede the onset of clinical symptoms, suggesting that they also contribute to the pathogenesis of these disorders. However, whether low VEGF levels contribute to impaired neural perfusion in these disorders remains to be elucidated. Determining the precise role of VEGF in these situations will, however, be complicated by the fact that hypoxia, resulting from reduced perfusion, would likely lead to a secondary upregulation of VEGF.
Vessel independent trophic roles for VEGF in the nervous system.
In addition to its vascular roles in the nervous system, VEGF may be a trophic factor for neurons that acts independently of the blood circulation. In support of this idea, VEGF treatment enhances neuronal survival and neurite outgrowth in explanted brain cortex or substantia nigra.36,37 VEGF application also induces neurite outgrowth and enhances neuronal survival in cultured dorsal root ganglia.38,39 Moreover, VEGF protects cultured cerebral neurons or hippocampal neurons during hypoxia or serum withdrawal,40,41 and it also protects cultured hippocampal neurons against glutamate or NMDA toxicity.42,43 Finally, overexpression of KDR in motor neurons protects them from cell death by pathogenic SOD1 mutant protein in vivo.35 It is not yet clear if these neurotrophic effects of VEGF reflect a direct effect of VEGF on neurons or if they are mediated indirectly by glial cells. For example, VEGF promotes the survival of primary motor neurons on glial feeder layers following oxidative stress, an effect that is inhibited by antibodies that neutralize VEGF receptor activity.33 Moreover, one of the growth factors secreted by astrocytes and Schwann cells is VEGF itself. VEGF may therefore have a dual neurotrophic effect, an indirect effect that is mediated by glia and a direct effect on neurons independently of glia. Consistent with the idea that VEGF could be neurotrophic by acting through glia, VEGF is mitogenic for astroglia and Schwann cells,22,37,38,44–46 and both glial cell types produce a number of growth factors that support neuronal growth in explant cultures.47,48
Our own experiments support the idea of a direct neurotrophic role of VEGF on neurons. We found that VEGF promoted neurite extension in spinal cord explants (Fig. 1; Krum JM and Rosenstein JM, unpublished observations). This organotypic explant system was used to study VEGF’s effect on neurons in the context of three-dimensional tissue architecture, in order to maintain density-dependant regulatory mechanisms and tissue specific diffusion properties. However, as is the case when studying VEGF effects in the nervous system in vivo, this explant model did not distinguish direct effects of VEGF on neurons from indirect effects mediated by other VEGF-responsive cell types. For instance, VEGF application stimulated angiogenesis, which then supported astrocyte growth and thereby neuronal survival. That VEGF has a direct neurotrophic role for cultured neurons independently of glia was supported by the observation that VEGF treatment promoted neurite growth and maturation in cultures of primary cortical neurons lacking glia with similar efficiency to that in explant cultures containing glia36,49 (Fig. 2). Furthermore, these primary cell culture studies suggested that VEGF upregulates neuronspecific enolase (NSE) and microtubule associated protein 2 (MAP2), possibly by stabilizing them or by upregulating their expression.11,36 MAP2 and several other proteins are downregulated after neuronal injury, and VEGF application might help to raise the levels of some of these proteins again to contribute to neuroprotection. In the future, tissue-specific genetic model systems will advance our understanding of VEGF’s role on neurons, as they can distinguish VEGF’s cell type specific activities in an in vivo context.
Figure 1.
VEGF promotes neurite extension in spinal cord explants. Immunostaining of neurons in dorsal horn explants shows modest expression of MAP2 (A). When 50 ng/ml VEGF is added to lumbar spinal cord explants, MAP2 expression is upregulated in enlarged cell bodies as well as in elongated and branched neurites (B).
Figure 2.
VEGF promotes neurite extension from cortical neurons. In primary cultures of cortical neurons (>95% pure), neurofilament-positive neurites extend from neurons that are growing in clusters (A) or as single cells (C) in a serum-free environment. The addition of 50–100 ng/ml VEGF increases the diameter and length of neurites from clustered neurons significantly (B), with a stimulation of neurite outgrowth from the cell body by 30–40% (D).
VEGF signaling in neurons.
KDR has been implicated as a VEGF receptor in mature neurons in several culture models,33,39,50 and the alternative VEGF receptors FLT1, NRP1 and NRP2 have also been detected in several types of developing and adult neurons. For example, NRP1 is upregulated on neurons and endothelium after CNS ischemia,51 suggesting roles in brain injury. However, the functional requirements for these different VEGF receptors in neurons were initially difficult to determine, owing to the severe cardiovascular defects and early lethality caused by loss of these proteins in the embryo (see above). Fortunately, several novel genetic tools using Cre/Lox technology have recently been developed to facilitate the creation of neuron-specific mutations in VEGF receptors, which circumvents the embryonic lethality caused by cardiovascular defects. Surprisingly, the disruption of the Kdr gene in CNS neurons with a nestin-based recombination approach did not impair neuronal development or viability in the mouse.15 This finding suggested that KDR is not essential for neuronal growth and patterning. The genetic requirement for FLT1 as a VEGF receptor in neurons has not been examined so far, but we do know that FLT1’s tyrosine kinase activity is dispensable for both embryogenesis and postnatal survival.26 Therefore, any possible FLT1 function in neurons would be expected to involve its extracellular domain only. So far, it has been difficult to establish if neuropilins act as physiological VEGF receptors in neurons through loss of function studies, because they also transmit signals provided by neural guidance molecules of the class 3 semaphorin family, such as SEMA3A and SEMA3F.52 In fact, we presently know of only one neuronal cell type in which NRP1 acts predominantly as a VEGF164 rather than a semaphorin receptor: the neuropilin-expressing, but SEMA3A unresponsive facial branchiomotor neuron cell bodies in the mouse brainstem (see below).
VEGF and Neurogenesis
In the embryonic nervous system, neuronal progenitors proliferate, migrate and differentiate in a process termed neurogenesis to populate the growing brain and ganglia with neurons. Embryonic neurogenesis originates from neuroepithelial progenitors in the subventricular zone of the CNS, and from neural crest cell-derived progenitors in the peripheral nervous system. In the adult, neurogenesis occurs mainly in two regions of the brain; (a) in the subventricular zone lining the lateral ventricles to produce the rostral migratory stream that supplies interneurons to the olfactory bulb; and (b) in the dentate gyrus of the hippocampus, where new neurons are thought to participate in memory formation. Neurogenesis can also be induced in the adult brain in response to pathological situations, such as mechanical trauma, seizures and ischemia. It has been hypothesized that neurogenesis in the adult brain relies on neural stem cells. However, the origin of neural stem cells in the adult brain remains to be defined. Surprisingly, they have characteristics of fully differentiated glia, and it has therefore been proposed that adult neuronal stem cells are derived from a cell lineage that initially forms embryonic neuroepithelial stem cells, then radial glia with the potential to form astrocytes and neurons, and finally astrocyte-like adult stem cells. Several different mitogenic and/or trophic growth factors have been implicated in the process of neurogenesis. Fibroblast growth factor 2 (FGF2, also known as basic FGF) and epidermal growth factor (EGF) in particular are mitogens for neural progenitor and stem cells in vitro. In addition, neurotrophic factors such as brain-derived neurotrophic factor (BDNF) are also involved in neurogenesis. VEGF expression by neurons is prominent in the developing brain and during brain pathology, when it may play a dual role to promote neurogenesis, firstly by acting as a paracrine factor for endothelial cells to stimulate pro-neurogenic angiogenesis (see above) and secondly, as an autocrine factor for neuronal progenitors.50 Consistent with this idea, administration of VEGF to adult rat brain via an osmotic minipump stimulates neurogenesis, astrocyte production and endothelial cell growth in the hippocampus and the lateral subventricular zone.53 We will first review evidence supporting a role for VEGF-stimulated angiogenesis in promoting neurogenesis and then discuss data consistent with a direct neurogenic role of VEGF.
VEGF and the angiogenic niche of neurogenesis.
Adult neurogenesis in the residual germinal matrices of the brain occurs in parallel with the growth of new blood vessels, which are thought to provide an “angiogenic niche” for the neuronal progenitors. This concept was first proposed to explain the correlation of neuronal progenitor mitoses and endothelial cell growth in the pseudoglomerular structures of the dentate gyrus in the hippocampus.54 Blood vessels in the angiogenic niche of neurogenesis could potentially provide instructive neurotrophic factors that promote the differentiation or survival of neuronal progenitors. This idea is supported by findings in the adult songbird brain, where neurogenesis proceeds throughout life in the higher vocal centre. Here, testosterone upregulates VEGF and QUEK1 (bird KDR) to induce angiogenesis and stimulate BDNF release from blood vessel endothelium. BDNF then supports neuronal differentiation and migration of neurons derived from the ventricular zone of the higher vocal cord centre.55 Moreover, pigment epithelium-derived factor (PEDF) is produced by ependymal and endothelial cells to induce self-renewal of cultured neural stem cells.56 Alternatively, or additionally, blood vessels in the angiogenic niche of neurogenesis might provide a substrate for migrating neuronal progenitors during their journey from their germinal zone to the site of their differentiation; this substrate might be the vessel surface or the vessel-associated extracellular matrix. Surprisingly, experiments have not yet been performed that address how the angiogenic niche contributes to neurogenesis in the developing CNS.
VEGF signaling in neuronal progenitors during development.
Several findings support the idea that VEGF has direct effects on neuronal progenitors in the developing brain. VEGF164 stimulates the migration survival of a neuroectodermal progenitor cell line.57 Moreover, VEGF application enhances the proliferation of embryonic cortical neuronal progenitors in vitro, and the blockade of KDR signaling prevents their VEGF-induced proliferation.53 VEGF also affects the migration of primary neuronal progenitors: FGF stimulates the proliferation of neuronal progenitors derived from the newborn rat rostral subventricular zone concomitantly with increasing expression of KDR and FLT1, and the FGF2 stimulated neuronal progenitors become responsive to VEGF, which acts as a chemoattractant via KDR.58 The role of VEGF and its receptors in neurogenesis has also been studied in the developing retina. VEGF and KDR are expressed in the inner retina prior to its vascularisation and also in the avascular outer retina, and several different experimental approaches have raised the possibility that VEGF signaling plays a role in the development of the neural retina. For example, VEGF treatment promotes neurogenesis in the avascular chick retina, whilst KDR inhibition with a small interfering RNA blocks proliferation; similarly, inhibition of receptor tyrosine kinases, including KDR, blocks development of the inner mouse retina, which would normally contain Mueller and retinal ganglion cells.59–62 VEGF also stimulates photoreceptor development from retinal progenitors in vitro.63
VEGF signaling in neuronal stem cells and adult neuronal progenitors.
VEGF/KDR signaling affects the fate of neuronal stem cells derived from the embryonic brain.64 On the one hand, VEGF promotes the survival of definitive neural stem cells, which form in the developing brain after 8.5 dpc and persist throughout adulthood. On the other hand, VEGF inhibits the survival of primitive neural stem cells, present in the embryonic brain up to 8.5 dpc. Interestingly, primitive neural stem cells would not normally be exposed to high VEGF levels until after 8.5 dpc, when VEGF is upregulated in the neural tube to attract blood vessels from the perineural vascular plexus. These observations raise the possibility that VEGF contributes to a developmental switch that affects neural stem cell behavior. KDR expression has also been reported in the proliferative zones of the adult rodent brain,50,53,65 although the specificity of the antibodies used was demonstrated in only one of these studies.65 When VEGF is administered to the brain at low concentrations, it stimulates the proliferation of KDR-expressing cells in the ventricular zone in vivo independently of its effect on blood vessels.65 VEGF application was also found to promote the survival of neural stem cells in vitro in a KDR-dependent fashion.65 The physiological significance of VEGF as an autocrine or paracrine signal for neuronal stem cells is not yet understood, as it has not been possible to ablate VEGF expression in the CNS without simultaneously affecting blood vessels.15,16 The specific deletion of VEGF receptors in the early neuronal lineage may therefore provide a more suitable approach to determine the contribution of VEGF signaling to neurogenesis. The ablation of KDR expression from neurons using nestin promoter-driven CRE-mediated recombination suggested that this VEGF receptor is not essential for embryonic neurogenesis.15 However, the impact of KDR loss from CNS neurons on neonatal and adult neurogenesis or neuronal survival has not yet been studied, and FLT1 and NRP1 have not yet been ablated specifically in the neuronal lineage. Further work on the role of VEGF and its receptors in the neuronal lineage has become pressing, given that reduced VEGF levels were found to cause motor neuron degeneration.
VEGF Signaling in Neurovascular Patterning
Several recent studies have explored the idea that endothelial cells and neurons share signaling pathways to control their growth and behavior. Accordingly, there is now evidence that axon guidance cues control blood vessel branching and, vice versa, that vascular patterning molecules can modify the migration of neurons and glia. For example, ephrin/EPH signals and netrins with their UNC and DCC receptors have been implicated both in neuronal and vascular patterning,66 and NRP1 plays a dual role as an isoform-specific VEGF receptor on endothelial cells31 and a neuronal cell surface receptor for semaphorins.67 The observation that VEGF165 and the semaphorin SEMA3A compete for NRP1 binding in cell culture models68,69 raised the possibility that shared transmembrane receptors serve to coordinate vascular and neuronal growth by integrating antagonistic signals.66 Several recent studies have begun to test if semaphorins control vascular development, if VEGF controls neuronal development, and if ligand sharing by NRP1 controls neuronal and vascular co-patterning in vivo. The outcome of these studies is discussed in the following paragraphs.
VEGF in neuronal patterning.
VEGF promotes neurite extension and neurite maturation in cell culture models (Figs. 1 and 2),36–39 and the VEGF164 isoform has been hypothesised to act as an axonal guidance cue by binding to NRP1 in competition with SEMA3A.70 Unfortunately, in vivo evidence that VEGF acts as an axonal patterning factor in NRP1 expressing neurons is so far lacking: In developing dorsal root ganglia, VEGF has no effect on neurons, even though it controls endothelial cell growth,71 and limb axons grow normally in the absence of VEGF164.72 VEGF164 does, however, pattern neuronal migration within the developing CNS, as it controls the NRP1-dependent cell body migration of facial branchiomotor neurons within the mouse brainstem.73 Strikingly, there is no competition of VEGF164 and SEMA3A during this process. Rather, both NRP1 ligands cooperate by regulating different aspects of neuronal behavior, with VEGF164 signaling being necessary only for the correct pathfinding of facial branchiomotor somata within the brainstem, and SEMA3A being involved solely in the guidance of their axons in the second branchial arch. Importantly, VEGF164 appears to pattern facial branchiomotor neurons independently of blood vessels, as the endothelial-specific NRP1 knockout displays vascular, but not neuronal migration defects. Similarly to facial branchiomotor axons, sensory and motor axons in the limb require SEMA3A, but not VEGF164 to control their growth. Whilst the developmental analysis of facial branchiomotor neurons has provided the first evidence that VEGF can directly control neuronal behavior independently of blood vessels, further work is required to examine if VEGF patterns other neuronal cell types and can therefore be considered a general neuronal patterning molecule like SEMA3A. In particular, it will be important to address if VEGF effects are restricted to the guidance of neuronal cell bodies, or if some types of axons also use VEGF as a migratory cue. Candidate neurons whose axons may use VEGF164 as a guidance molecule may be identified by their ability to express NRP1, even though they do not require SEMA3A signals for their patterning. The observation that VEGF promotes neurite maturation (see above) raises the possibility that VEGF might affect dendrite development.
A role for competition of VEGF164 and SEMA3A during neuronal and vascular patterning?
Tissue culture studies have shown that VEGF165 and SEMA3A compete for binding to the extracellular domain of NRP1.57,68 Moreover, in experiments with chick limbs carrying SEMA3A bead implants, vessels and nerves were both repelled by SEMA3A in a mechanism requiring NRP1.74 Based on these observations, it has been hypothesised that VEGF165 and SEMA3A coordinate vascular and neural development by competing for NRP1 and thereby contribute to the emergence of neurovascular congruence. However, the role of SEMA3A signaling in vascular development is presently controversial. SEMA3A has been implicated in vascular patterning in zebrafish, where one of two SEMA3A forms termed SEMA3AB (SEMA3A2) curbs intersegmental vessel branching.75 In addition, Serini and co-workers reported that loss of SEMA3A impairs head vessel remodelling, intersomitic vessel branching and formation of the anterior cardinal vein by modulating integrin signaling in the mouse and chick.76 In contrast, others and we have found that SEMA3A and semaphorin signaling through NRP1 are not required for microvessel patterning during mouse development.72,77 Moreover, we have found that there is no genetic interaction between VEGF164 and SEMA3A during vasculogenesis or angiogenic vessel growth and branching.72 The observation that there is no competition between VEGF164 and SEMA3A during vessel growth in vivo is consistent with the finding that VEGF164, but not SEMA3A controls the cell body migration of facial branchiomotor neurons, whilst SEMA3A, but not VEGF164 guides facial nerve and limb axons (see above). The emerging picture is therefore one of ligand cooperation rather than competition, with VEGF164 and SEMA3A being specialised to mediate distinct patterning events that occur in close spatiotemporal proximity.
VEGF mediates the co-patterning of nerves and arteries.
Whilst ligand competition between VEGF164 and SEMA3A does not appear to be critical for neurovascular development, VEGF does nevertheless play a role in neurovascular co-patterning independently of its relationship to SEMA3A: In the developing limb skin, VEGF is required for the alignment of nerves and arteries, because nerve-secreted VEGF acts on vascular NRP1 to promote arteriogenesis.78,79 Notably, VEGF is likely to cooperate with other neuronal-derived signals in the co-patterning of vessels and nerves in the limb, as it induces arterial character rather than mediating the recruitment of blood vessels to nerves. The factors that control the co-alignment of nerves and vessels have therefore remained elusive.
Outlook
VEGF production by CNS neurons early on in development is likely induced by hypoxia to match vessel growth to oxygen requirements and metabolic demand. In an analogous fashion, VEGF may be induced during nervous system damage and peripheral nerve regeneration to stimulate new vessel growth and enhance tissue repair. Consistent with this hypothesis, several studies suggest that VEGF treatment improves diabetic and peripheral neuropathy. Firstly, the intramuscular gene transfer of a plasmid encoding VEGF enhances motor and sensory functions in a rabbit model of ischemic peripheral neuropathy.45 Secondly, application of VEGF after stroke injury can decrease brain infarct size, likely by promoting angiogenesis and neurogenesis near the penumbral area.23,25,80,81 Thirdly, application of function-blocking VEGF-specific antibodies in a stab injury model increased lesion size and decreased angiogenic and astroglial activity in the striatum.82 In further support of a role for VEGF in damage control during brain injuries, application of VEGF to the contused spinal cord produced behavioral and cellular improvements,83,84 and VEGF significantly enhanced nerve regeneration when applied to matrigel implants into injured sciatic nerves.85
The studies described in this review demonstrate that VEGF plays multiple roles in the nervous system by acting on blood vessels, glia and neurons; a working model for the role of VEGF in peripheral nerves is presented in Figure 3. Because of its multiple effects, VEGF treatment may be beneficial for neurodegenerative and neuropathic conditions by enhancing both blood vessel and glial cell growth, whilst also providing direct neuroprotection. Whereas VEGF application after brain or spinal cord injury or in some neurodegenerative diseases is likely to aid neural and glial protection, VEGF may also have negative effects, as it can promote excessive macrophage infiltration, increase vascular permeability and disrupt the blood brain barrier.22–24,82,86 Whether VEGF treatment will ultimately be useful in the clinic to promote neoangiogenesis and neuronal survival may therefore depend on our ability to separate its effects on macrophages and vascular permeability from its angiogenic and neurotrophic effects. It will also be imperative to investigate if the use of anti-VEGF therapy to treat unwanted angiogenesis and vascular leakage in cancer and eye diseases could cause serious side effects in nervous tissue, as reduced VEGF levels may impair adult neurogenesis and neuroprotection. Investigating the contribution of VEGF and its signaling pathways to brain development is likely to provide critical clues that will help us understand VEGF’s physiological functions and therefore help to pioneer novel therapeutic strategies for nervous system repair.
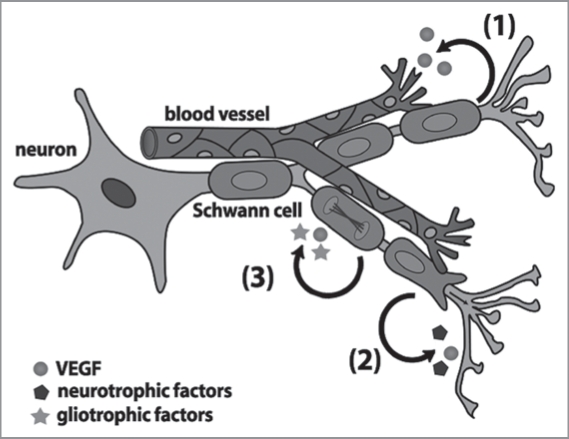
Figure 3.
Possible roles for Schwann cell-derived VEGF in peripheral nerves. Schwann cells secrete VEGF, but also several other gliotrophic and neurotrophic factors. VEGF stimulates the growth and patterning of blood vessels (1) and is likely to help protect motor and sensory neurons (2). In addition, VEGF is thought to protect Schwann cells and stimulate their proliferation and migration in an autocrine loop (3). It is not yet known if paracrine VEGF sources for Schwann cells exist in peripheral nerves.
Acknowledgements
We thank Drs. N. Mani and N. More for their expertise in tissue culture work and Dr. Q. Schwarz for (Fig. 3). J.M.K. and J.M.R. are supported by NS39282 and NS45189. C.R. is supported by an MRC Career Development Award.
Footnotes
Previously published online: www.landesbioscience.com/journals/organogenesis/article/11687
Note
Previously published in VEGF in Development, edited by Christiana Ruhrberg. Landes Bioscience and Springer Science + Business Media 2008; 91–103.
References
- 1.Ruhrberg C. Growing and shaping the vascular tree: Multiple roles for VEGF. BioEssays. 2003;25:1052–1060. doi: 10.1002/bies.10351. [DOI] [PubMed] [Google Scholar]
- 2.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:425. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 3.Dvorak HF, Brown LF, Detmar M, et al. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability and angiogenesis. Am J Pathol. 1995;146:1029–1039. [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrara N. VEGF: An update on biological and therapeutic aspects. Curr Opin Biotechnol. 2000;11:617–624. doi: 10.1016/s0958-1669(00)00153-1. [DOI] [PubMed] [Google Scholar]
- 5.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 6.Fong GH, Rossant J, Gertsenstein M, et al. Role of the Flt1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376:6535–6670. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- 7.Shalaby F, Rossant J, Yamaguchi TP, et al. Failure of blood island formation and vasculogenesis in Flk1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 8.Carmeliet P, Ferreira V, Breier G, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 9.Ferrara N, Carver-Moore K, Chen H, et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 10.Kawasaki T, Kitsukawa T, Bekku Y, et al. A requirement for neuropilin1 in embryonic vessel formation. Development. 1999;126:4895–4902. doi: 10.1242/dev.126.21.4895. [DOI] [PubMed] [Google Scholar]
- 11.Rosenstein JM, Krum JM. New roles for VEGF in nervous tissue beyond blood vessels. Exp Neurol. 2004;187:246–253. doi: 10.1016/j.expneurol.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 12.Carmeliet P, Storkebaum E. Vascular and neuronal effects of VEGF in the nervous system: Implications for neurological disorders. Semin Cell Dev Biol. 2002;13:39–53. doi: 10.1006/scdb.2001.0290. [DOI] [PubMed] [Google Scholar]
- 13.Storkebaum E, Carmeliet P. VEGF: A critical player in neurodegeneration. J Clin Invest. 2004;113:14–18. doi: 10.1172/JCI200420682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruhrberg C, Gerhardt H, Golding M, et al. Spatially restricted patterning cues provided by heparin-binding VEGFA control blood vessel branching morphogenesis. Genes Dev. 2002;16:2684–2698. doi: 10.1101/gad.242002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haigh JJ, Morelli PI, Gerhardt H, et al. Cortical and retinal defects caused by dosage-dependent reductions in VEGFA paracrine signaling. Dev Biol. 2003;262:225–241. doi: 10.1016/s0012-1606(03)00356-7. [DOI] [PubMed] [Google Scholar]
- 16.Raab S, Beck H, Gaumann A, et al. Impaired brain angiogenesis and neuronal apoptosis induced by conditional homozygous inactivation of vascular endothelial growth factor. Thromb Haemost. 2004;91:595–605. doi: 10.1160/TH03-09-0582. [DOI] [PubMed] [Google Scholar]
- 17.Bar T. Patterns of vascularization in the developing cerebral cortex. Ciba Found Symp. 1983;100:20–36. doi: 10.1002/9780470720813.ch3. [DOI] [PubMed] [Google Scholar]
- 18.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 19.Breier G, Albrecht U, Sterrer S, et al. Expression of vascular endothelial growth factor during embryonic angiogenesis and endothelial cell differentiation. Development. 1992;114:521–532. doi: 10.1242/dev.114.2.521. [DOI] [PubMed] [Google Scholar]
- 20.Gerhardt H, Golding M, Fruttiger M, et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenstein JM, Mani N, Silverman WF, et al. Patterns of brain angiogenesis after vascular endothelial growth factor administration in vitro and in vivo. Proc Natl Acad Sci USA. 1998;95:7086–7091. doi: 10.1073/pnas.95.12.7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krum JM, Mani N, Rosenstein JM. Angiogenic and astroglial responses to vascular endothelial growth factor administration in adult rat brain. Neuroscience. 2002;110:589–604. doi: 10.1016/s0306-4522(01)00615-7. [DOI] [PubMed] [Google Scholar]
- 23.Zhang ZG, Zhang L, Jiang Q, et al. VEGF enhances angiogenesis and promotes blood brain barrier leakage in the ischemic brain. J Clin Invest. 2000;106:829–838. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Proescholdt MA, Heiss JD, Walbridge S, et al. Vascular endothelial growth factor (VEGF) modulates vascular permeability and inflammation in rat brain. J Neuropathol Exp Neurol. 1999;58:613–627. doi: 10.1097/00005072-199906000-00006. [DOI] [PubMed] [Google Scholar]
- 25.van Bruggen N, Thibodeaux H, Palmer JT, et al. VEGF antagonism reduces edema formation and tissue damage after ischemia/reperfusion injury in the mouse brain. J Clin Invest. 1999;104:1613–1620. doi: 10.1172/JCI8218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hiratsuka S, Minowa O, Kuno J, et al. Flt1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proc Natl Acad Sci USA. 1998;95:9349–9354. doi: 10.1073/pnas.95.16.9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kearney JB, Kappas NC, Ellerstrom C, et al. The VEGF receptor flt1 (VEGFR1) is a positive modulator of vascular sprout formation and branching morphogenesis. Blood. 2004;103:4527–4535. doi: 10.1182/blood-2003-07-2315. [DOI] [PubMed] [Google Scholar]
- 28.Gerhardt H, Ruhrberg C, Abramsson A, et al. Neuropilin1 is required for endothelial tip cell guidance in the developing central nervous system. Dev Dyn. 2004;231:503–509. doi: 10.1002/dvdy.20148. [DOI] [PubMed] [Google Scholar]
- 29.Kitsukawa T, Shimono A, Kawakami A, et al. Overexpression of a membrane protein, neuropilin, in chimeric mice causes anomalies in the cardiovascular system, nervous system and limbs. Development. 1995;121:4309–4318. doi: 10.1242/dev.121.12.4309. [DOI] [PubMed] [Google Scholar]
- 30.Gu C, Rodriguez ER, Reimert DV, et al. Neuropilin1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev Cell. 2003;5:45–57. doi: 10.1016/s1534-5807(03)00169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soker S, Takashima S, Miao HQ, et al. Neuropilin1 is expressed by endothelial and tumor cells as an isoform specific receptor for vascular endothelial growth factor. Cell. 1998;92:735–745. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- 32.Lambrechts D, Storkebaum E, Morimoto M, et al. VEGF is a modifier of amyotrophic lateral sclerosis in mice and humans and protects motoneurons against ischemic death. Nat Gen. 2003;34:383–393. doi: 10.1038/ng1211. [DOI] [PubMed] [Google Scholar]
- 33.Oosthuyse B, Moons L, Storkebaum E, et al. Deletion of the hypoxia response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nat Gene. 2001;28:131–138. doi: 10.1038/88842. [DOI] [PubMed] [Google Scholar]
- 34.Sopher BL, Thomas PS, Jr, LaFevre-Bernt MA, et al. Androgen receptor YAC transgenic mice recapitulate SBMA motor neuronopathy and implicate VEGF164 in the motor neuron degeneration. Neuron. 2004;41:687–699. doi: 10.1016/s0896-6273(04)00082-0. [DOI] [PubMed] [Google Scholar]
- 35.Storkebaum E, Lambrechts D, Dewerchin M, et al. Treatment of motoneuron degeneration by intra-cerebroventricular delivery of VEGF in a rat model of ALS. Nat Neurosci. 2005;8:85–92. doi: 10.1038/nn1360. [DOI] [PubMed] [Google Scholar]
- 36.Rosenstein JM, Mani N, Khaibullina A, et al. Neurotrophic effects of vascular endothelial growth factor on organotypic cortical explants and primary cortical neurons. J Neurosci. 2003;23:11036–11044. doi: 10.1523/JNEUROSCI.23-35-11036.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silverman WF, Krum JM, Mani N, et al. Vascular, glial and neuronal effects of vascular endothelial growth factor in mesencephalic explant cultures. Neuroscience. 1999;90:1529–1541. doi: 10.1016/s0306-4522(98)00540-5. [DOI] [PubMed] [Google Scholar]
- 38.Sondell M, Lundborg G, Kanje M. Vascular endothelial growth factor has neurotrophic activity and stimulates axonal outgrowth, enhancing cell survival and Schwann cell proliferation in the peripheral nervous system. Neuroscience. 1999;19:5731–5740. doi: 10.1523/JNEUROSCI.19-14-05731.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sondell M, Sundler F, Kanje M. Vascular endothelial growth factor is a neurotrophic factor which stimulates axonal outgrowth through the flk1 receptor. Eur J Neurosci. 2000;12:4243–4254. doi: 10.1046/j.0953-816x.2000.01326.x. [DOI] [PubMed] [Google Scholar]
- 40.Jin KL, Mao XO, Nagayama T, et al. Induction of vascular endothelial growth factor and hypoxiainducible factor1alpha by global ischemia in rat brain. Neuroscience. 2000;99:577–585. doi: 10.1016/s0306-4522(00)00207-4. [DOI] [PubMed] [Google Scholar]
- 41.Jin KL, Mao XO, Greenberg DA. Vascular endothelial growth factor: Direct neuroprotective effect in in vitro ischemia. Proc Natl Acad Sci USA. 2000;97:10242–10247. doi: 10.1073/pnas.97.18.10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matsuzaki H, Tamatani M, Yamaguchi A, et al. Vascular endothelial growth factor rescues hippocampal neurons from glutamate-induced toxicity: Signal transduction cascades. FASEB J. 2001;15:1218–1220. [PubMed] [Google Scholar]
- 43.Svensson B, Peters M, Konig HG, et al. Vascular endothelial growth factor protects cultured rat hippocampal neurons against hypoxic injury via an antiexcitotoxic, caspase-independent mechanism. J Cereb Blood Flow Metab. 2002;22:1170–1175. doi: 10.1097/01.wcb.0000037988.07114.98. [DOI] [PubMed] [Google Scholar]
- 44.Sondell M, Lundborg G, Kanje M. Vascular endothelial growth factor stimulates Schwann cell invasion and neovascularization of acellular nerve grafts. Brain Res. 1999;846:219–228. doi: 10.1016/s0006-8993(99)02056-9. [DOI] [PubMed] [Google Scholar]
- 45.Schratzberger P, Schratzberger G, Silver M, et al. Favorable effect of VEGF gene transfer on ischemic peripheral neuropathy. Nat Med. 2000;6:405–413. doi: 10.1038/74664. [DOI] [PubMed] [Google Scholar]
- 46.Mani N, Khaibullina A, Krum JM, et al. Astrocyte growth effects of vascular endothelial growth factor (VEGF) application to perinatal neocortical explants: Receptor mediation and signal transduction pathways. Exp Neurol. 2005;192:394–406. doi: 10.1016/j.expneurol.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 47.Eddleston M, Mucke L. Molecular profile of reactive astrocytes—implications for their role in neurologic disease. Neuroscience. 1993;54:15–36. doi: 10.1016/0306-4522(93)90380-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krum JM, Rosenstein JM. VEGF mRNA and its receptor flt1 are expressed in reactive astrocytes following neural grafting and tumor cell implantation in the adult CNS. Exp Neurol. 1998;154:57–65. doi: 10.1006/exnr.1998.6930. [DOI] [PubMed] [Google Scholar]
- 49.Khaibullina AA, Rosenstein JM, Krum JM. Vascular endothelial growth factor promotes neurite maturation in primary CNS neuronal cultures. Brain Research. Dev Brain Res. 2004;148:59–68. doi: 10.1016/j.devbrainres.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 50.Ogunshola OO, Antic A, Donoghue MJ, et al. Paracrine and autocrine functions of neuronal vascular endothelial growth factor (VEGF) in the central nervous system. J Biol Chem. 2002;277:11410–11415. doi: 10.1074/jbc.M111085200. [DOI] [PubMed] [Google Scholar]
- 51.Zhang ZG, Tsang W, Zhang L, et al. Upregulation of neuropilin1 in neovasculature after focal cerebral ischemia in the adult rat. J Cereb Blood Flow Metab. 2001;21:541–549. doi: 10.1097/00004647-200105000-00008. [DOI] [PubMed] [Google Scholar]
- 52.Raper JA. Semaphorins and their receptors in vertebrates and invertebrates. Curr Opin Neurobiol. 2000;10:88–94. doi: 10.1016/s0959-4388(99)00057-4. [DOI] [PubMed] [Google Scholar]
- 53.Jin K, Zhu Y, Sun Y, et al. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci USA. 2002;99:11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 55.Louissaint A, Jr, Rao S, Leventhal C, et al. Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron. 2002;34:945–960. doi: 10.1016/s0896-6273(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 56.Ramirez-Castillejo C, Sanchez-Sanchez F, Andreu-Agullo C, et al. Pigment epithelium-derived factor is a niche signal for neural stem cell renewal. Nat Neurosci. 2006;9:331–339. doi: 10.1038/nn1657. [DOI] [PubMed] [Google Scholar]
- 57.Bagnard D, Vaillant C, Khuth ST, et al. Semaphorin 3Avascular endothelial growth factor165 balance mediates migration and apoptosis of neural progenitor cells by the recruitment of shared receptor. J Neurosci. 2001;21:3332–3341. doi: 10.1523/JNEUROSCI.21-10-03332.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang H, Vutskits L, Pepper MS, et al. VEGF is a chemoattractant for FGF2-stimulated neural progenitors. J Cell Biol. 2003;163:1375–1384. doi: 10.1083/jcb.200308040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang K, Cepko CL. Flk1, a receptor for vascular endothelial growth factor (VEGF), is expressed by retinal progenitor cells. J Neurosci. 1996;16:6089–6099. doi: 10.1523/JNEUROSCI.16-19-06089.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Robinson GS, Ju M, Shih SC, et al. Nonvascular role for VEGF: VEGFR1, 2 activity is critical for neural retinal development. FASEB J. 2001;15:1215–1217. doi: 10.1096/fj.00-0598fje. [DOI] [PubMed] [Google Scholar]
- 61.Gariano RF, Hu D, Helms J. Expression of angiogenesis- related genes during retinal development. Gene Expr Patterns. 2006;6:187–192. doi: 10.1016/j.modgep.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 62.Hashimoto T, Zhang XM, Chen BY, et al. VEGF activates divergent intracellular signaling components to regulate retinal progenitor cell proliferation and neuronal differentiation. Development. 2006;133:2201–2210. doi: 10.1242/dev.02385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yourey PA, Gohari S, Su JL, et al. Vascular endothelial cell growth factors promote the in vitro development of rat photoreceptor cells. J Neurosci. 2000;20:6781–6788. doi: 10.1523/JNEUROSCI.20-18-06781.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wada T, Haigh JJ, Ema M, et al. Vascular endothelial growth factor directly inhibits primitive neural stem cell survival but promotes definitive neural stem cell survival. J Neurosci. 2006;26:6803–6812. doi: 10.1523/JNEUROSCI.0526-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schanzer A, Wachs FP, Wilhelm D, et al. Direct stimulation of adult neural stem cells in vitro and neurogenesis in vivo by vascular endothelial growth factor. Brain Pathol. 2004;14:237–248. doi: 10.1111/j.1750-3639.2004.tb00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eichmann A, Makinen T, Alitalo K. Neural guidance molecules regulate vascular remodeling and vessel navigation. Genes Dev. 2005;19:1013–1021. doi: 10.1101/gad.1305405. [DOI] [PubMed] [Google Scholar]
- 67.Kolodkin AL, Ginty DD. Steering clear of semaphorins: Neuropilins sound the retreat. Neuron. 1997;19:1159–1162. doi: 10.1016/s0896-6273(00)80408-0. [DOI] [PubMed] [Google Scholar]
- 68.Miao HQ, Soker S, Feiner L, et al. Neuropilin1 mediates collapsin1/semaphorin III inhibition of endothelial cell motility: Functional competition of collapsin1 and vascular endothelial growth factor165. J Cell Biol. 1999;146:233–242. doi: 10.1083/jcb.146.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bagnard D, Lohrum M, Uziel D, et al. Semaphorins act as attractive and repulsive guidance signals during the development of cortical projections. Development. 1998;125:5043–5053. doi: 10.1242/dev.125.24.5043. [DOI] [PubMed] [Google Scholar]
- 70.Carmeliet P. Blood vessels and nerves: Common signals, pathways and diseases. Nat Rev Genet. 2003;4:710–720. doi: 10.1038/nrg1158. [DOI] [PubMed] [Google Scholar]
- 71.Kutcher ME, Klagsbrun M, Mamluk R. VEGF is required for the maintenance of dorsal root ganglia blood vessels but not neurons during development. FASEB J. 2004;18:1952–1954. doi: 10.1096/fj.04-2320fje. [DOI] [PubMed] [Google Scholar]
- 72.Vieira JM, Schwarz Q, Ruhrberg C. Selective requirements for neuropilin ligands in neurovascular development. Development. 2007;134:1833–1843. doi: 10.1242/dev.002402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schwarz Q, Gu C, Fujisawa H, et al. Vascular endothelial growth factor controls neuronal migration and cooperates with Sema3A to pattern distinct compartments of the facial nerve. Genes Dev. 2004;18:2822–2834. doi: 10.1101/gad.322904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bates D, Taylor GI, Minichiello J, et al. Neurovascular congruence results from a shared patterning mechanism that utilizes Semaphorin3A and Neuropilin1. Dev Biol. 2003;255:77–98. doi: 10.1016/s0012-1606(02)00045-3. [DOI] [PubMed] [Google Scholar]
- 75.Torres-Vazquez J, Gitler AD, Fraser SD, et al. Semaphorin-plexin signaling guides patterning of the developing vasculature. Dev Cell. 2004;7:117–123. doi: 10.1016/j.devcel.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 76.Serini G, Valdembri D, Zanivan S, et al. Class 3 semaphorins control vascular morphogenesis by inhibiting integrin function. Nature. 2003;424:391–397. doi: 10.1038/nature01784. [DOI] [PubMed] [Google Scholar]
- 77.Gu C, Yoshida Y, Livet J, et al. Semaphorin 3E and plexinD1 control vascular pattern independently of neuropilins. Science. 2005;307:265–268. doi: 10.1126/science.1105416. [DOI] [PubMed] [Google Scholar]
- 78.Mukouyama YS, Gerber HP, Ferrara N, et al. Peripheral nerve-derived VEGF promotes arterial differentiation via neuropilin 1mediated positive feedback. Development. 2005;132:941–952. doi: 10.1242/dev.01675. [DOI] [PubMed] [Google Scholar]
- 79.Mukouyama YS, Shin D, Britsch S, et al. Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell. 2002;109:693–705. doi: 10.1016/s0092-8674(02)00757-2. [DOI] [PubMed] [Google Scholar]
- 80.Sun Y, Jin K, Xie L, et al. VEGF-induced neuroprotection, neurogenesis and angiogenesis after focal cerebral ischemia. J Clin Invest. 2003;111:1843–1851. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hayashi T, Abe K, Itoyama Y. Reduction of ischemic damage by application of vascular endothelial growth factor in rat brain after transient ischemia. J Cereb Blood Flow Metab. 1998;18:887–895. doi: 10.1097/00004647-199808000-00009. [DOI] [PubMed] [Google Scholar]
- 82.Krum JM, Khaibullina A. Inhibition of endogenous VEGF impedes revascularization and astroglial proliferation: Roles for VEGF in brain repair. Exp Neurol. 2003;181:241–257. doi: 10.1016/s0014-4886(03)00039-6. [DOI] [PubMed] [Google Scholar]
- 83.Facchiano F, Fernandez E, Mancarella S, et al. Promotion of regeneration of corticospinal tract axons in rats with recombinant vascular endothelial growth factor alone and combined with adenovirus coding for this factor. J Neurosurg. 2002;97:161–168. doi: 10.3171/jns.2002.97.1.0161. [DOI] [PubMed] [Google Scholar]
- 84.Widenfalk J, Lipson A, Jubran M, et al. Vascular endothelial growth factor improves functional outcome and decreases secondary degeneration in experimental spinal cord contusion injury. Neuroscience. 2003;120:951–960. doi: 10.1016/s0306-4522(03)00399-3. [DOI] [PubMed] [Google Scholar]
- 85.Hobson MI, Green CJ, Terenghi G. VEGF enhances intraneural angiogenesis and improves nerve regeneration after axotomy. J Anat. 2000;197:591–605. doi: 10.1046/j.1469-7580.2000.19740591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kalaria RN, Cohen DL, Premkumar DR, et al. Vascular endothelial growth factor in Alzheimer disease and experimental cerebral ischemia. Brain Res Mol Brain Res. 1998;62:101–105. doi: 10.1016/s0169-328x(98)00190-9. [DOI] [PubMed] [Google Scholar]