Abstract
Pregnancy is a normal physiological condition in which the maternal β-cell mass increases rapidly about two-fold to adapt to new metabolic challenges. We have used a lineage tracing of β-cells to analyse the origin of new β-cells during this rapid expansion in pregnancy. Double transgenic mice bearing a tamoxifen-dependent Cre-recombinase construct under the control of a rat insulin promoter, together with a reporter Z/AP gene, were generated. Then, in response to a pulse of tamoxifen before pregnancy, β-cells in these animals were marked irreversibly and heritably with the human placental alkaline phosphatase (HP AP). First, we conclude that the lineage tracing system was highly specific for β-cells. Secondly, we scored the proportion of the β-cells marked with HP AP during a subsequent chase period in pregnant and non-pregnant females. We observed a dilution in this labeling index in pregnant animal pancreata, compared to nonpregnant controls, during a single pregnancy in the chase period. To extend these observations we also analysed the labeling index in pancreata of animals during the second of two pregnancies in the chase period. The combined data revealed statistically-significant dilution during pregnancy, indicating a contribution to new beta cells from a non-β-cell source. Thus for the first time in a normal physiological condition, we have demonstrated not only β-cell duplication, but also the activation of a non-β-cell progenitor population. Further, there was no transdifferentiation of β-cells to other cell types in a two and half month period following labeling, including the period of pregnancy.
Key words: β-cells, pancreas, β-cell duplication, non-β-cell progenitor, pregnancy
Introduction
Essentially, all types of diabetes are characterized by inadequate numbers and function of β-cells, driving the need to develop effective therapies based on cell replacement. Islet transplantation is already under clinical investigation but for the foreseeable future will be very restricted in application due to the limited supplies of human cadaveric islets for transplantation. In consequence, there is intense interest in developing other sources of new β-cells, including differentiation of stem or precursor cell populations. Although clear evidence for such a means of β-cell development exists during ontogeny, maybe somewhat surprisingly, despite intensive study such a source of new β-cells has not yet been shown to operate under normal physiological conditions in vivo. In fact, most direct in vivo evidence to date suggests that normal β-cell maintenance and regeneration after surgery or targeted β-cell ablation depends on self-replication of differentiated existing β-cells. Clearly, identifying normal physiological models in which a non-β-cell source contributes substantially to β-cell formation in the adult could have major ramifications in the search for new ways of generating β-cells to treat diabetes.
During pregnancy, the maternal pancreas adapts in response to the increased insulin demand of the pregnant state. Pregnancy is associated with β-cell mass expansion which is quickly reversed post-partum.1–3 Thus although for the pancreas, by contrast with the liver, the organ size in non-pregnant adult animals is primarily dictated by the number of progenitor cells set aside in the developing pancreatic buds during embryonic development,4 the maternal β-cell mass is dynamically regulated during, and immediately after, pregnancy. In fact, β-cells exhibit remarkable plasticity during adult life with up to 10-fold increases in islet cell replication and doubling of β-cell mass in pregnancy,5 and marked increases in β-cell mass in response to obesity and particularly in some insulin resistant states;6 at least in part the common denominator here may be increased demands for insulin.
Other adaptations during pregnancy include a slight β-cell hypertrophy, and a decreased threshold of glucose-induced insulin secretion.2,3,5 Studies in pregnant rodents have shown that prolactin or placental lactogens increase β-cell mass.7 Lactogen signalling acts at least in part by repressing expression of the Men1 gene, which encodes a protein component of a histone methyltransferase, and the lactogen-dependent regulation of menin and its target genes in β-cells provides one mechanism for β-cell mass expansion in pregnancy.1
• In this report we address the origin of new β-cells during the mass expansion that occurs during pregnancy. This normal physiological process is one among many contexts where pancreas regeneration, and β-cell maintenance, renewal or adaptation have been analysed. Such contexts include: (1) normal early postnatal or adult growth; and the following experimental interventions, (2) dietary manipulation such as obesity, feeding soy beans, or the protein synthesis inhibitor ethionine, (3) pancreatic injury following partial pancreatectomy, duct ligation and cellophane wrapping, (4) streptozotocin- or alloxan-induced β-cell destruction, (5) rodent strains exhibiting features resembling human type 1 and type-2 diabetes, such as the NOD or Ob/Ob mouse respectively, and (6) transgenic manipulations including, autoimmune attack driven by expression of an interferon gamma transgene in β-cells, overexpression of growth factors, and conditional β-cell ablation in transgenic animals by activation of diphtheria toxin A chain gene, or c-Myc. From among this wide range of experimental manipulations it has been concluded that acinar cell destruction is followed by rapid extensive regeneration, surgical ablation by limited regeneration, and β-cell ablation by limited slow regeneration.8
It has long been suggested that β-cells can duplicate, and also that β-cells can arise from cells in the duct epithelium (reviewed in ref. 9). More recently, definitive conclusions have come from lineage tracing methods using a transgene driven by the rat insulin II gene promoter to heritably mark β-cells. This indicated that β-cell maintenance in adults, and regeneration following pancreatectomy or ablation by diphtheria toxin, are mainly accountable by β-cell proliferation.10,11 More recently, lineage tracing of ductal cells heritably marked using a transgene driven by the carbonic anhydrase II promoter, has led to the conclusion that ductal cells can give rise to β-cells in young mice shortly after birth, and also following ductal ligation in older mice.12 Further evidence has been adduced for Ngn3-positive, hormone-negative, multipotent progenitor cells located in the ductal lining,13 and for transdifferentiation of acinar cells to β-cells.14,15 Ngn3-positive endocrine progenitor cells associated with adult duct epithelium have recently been shown to drive alpha-cell neogenesis in glucagon deficiency, with the potential for subsequent transdifferentiation into beta-cells, under the control of Pax4.16 The presence of intraislet β-cell progenitors also can not be excluded.17–19 This literature has been reviewed comprehensively without providing a current clear reconciliation among the roles of the various potential, or putative, beta-cell sources that may be active in the different contexts.20–23 Importantly, lineage tracing has yet to be employed to investigate adaptive β-cell mass expansion in the adult.
In the present study, we exploit a Cre/loxP lineage tracing system to efficiently label β-cells in non-pregnant female mice, and thereby during subsequent pregnancy in these animals to critically analyse the origin of new maternal β-cells after the β-cell mass has doubled. We demonstrate for the first time that pregnancy is associated not only with β-cell duplication but also with the activation of a non-β-cell progenitor population. These findings may have ramifications for other situations such as obesity and type 2 diabetes where β-cell mass is required to expand. Potentially this model also provides a physiological system in which to identify signals responsible for inducing a more embryonic mode of β-cell generation.
Results
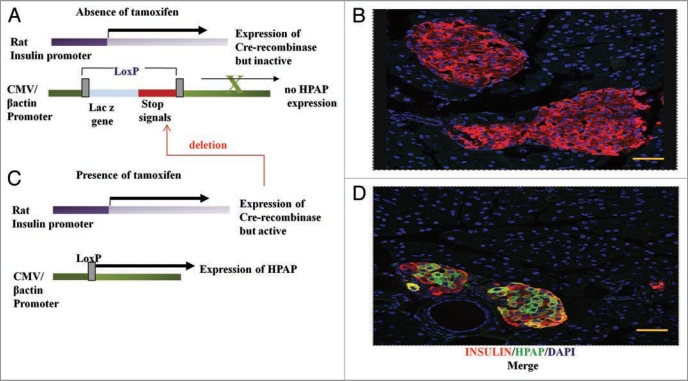
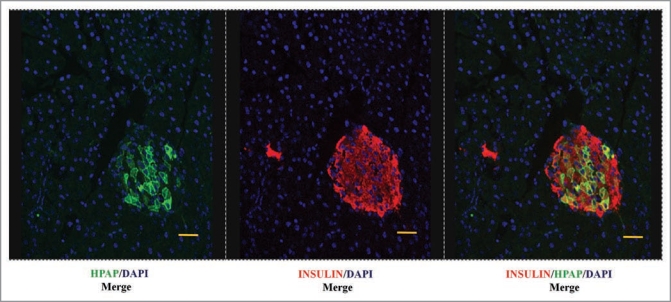
We have used the Cre/loxP-based direct lineage tracing of Dor and Melton11 to heritably mark β-cells and their progeny with human placental alkaline phosphatase (HPAP)24 in response to tamoxifen-induced activation of Cre-recombinase in β-cells (Fig. 1). Only those β-cells present at the time of tamoxifen injection (the ’pulse’) in the double transgenic mice will express the HPAP label, as well as any subsequent daughters of these cells arising during the “chase” period following the pulse. To maximise the potential of this lineage tracing technique for detecting any dilution of labeling during the chase period, we aimed to use a dose regime of tamoxifen that gave a relatively high proportion of labelled β-cells, in the range of 40–50% in control (nonpregnant) animals, without compromising subsequent fertility. Importantly, double staining for insulin and HPAP in the pancreata of mice immediately after the tamoxifen pulse confirmed that HPAP labeling is specific for β-cells (Fig. 1B and D).
Figure 1.
The Cre/loxP-based lineage tracing system. (A) Double transgenic mice, carry one transgene that consists of the rat insulin promoter driving expression of the tamoxifen-inducible Cre-recombinase (RIP-CreERTAM). The second transgene is a Z/AP reporter gene in which Cre-mediated recombination between loxP sites leads to expression of HP AP from a generally-active promoter. In the absence of tamoxifen, β-cells (red, B) are not marked by the HP AP reporter (green, D), but upon administration of tamoxifen the Cre-recombinase becomes active and mediates recombination at the loxP sites, hence causing deletion of transcriptional and translational stop signals upstream of the HP AP reporter gene (C). As a result the β-cells are irreversibly and heritably labelled for HP AP (D). Pancreatic sections were immunostained using anti-HP AP (green, B and D) and anti-insulin (red, B and D) antibodies. The pancreatic nuclei were stained using DAPI (blue, B and D). Bar scale corresponds to 40 µm.
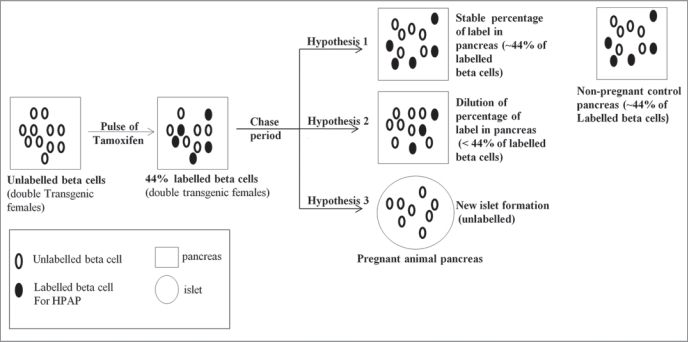
We exploited this lineage tracing system as a direct approach to analyse the origin of new β-cells during pregnancy, and we note that previous studies using such lineage tracing and BrdU labeling of DNA, in the context of experimental damage to the pancreas, have confirmed that HPAP labeling does not impair the replicative ability of labelled β-cells.10,11 If new β-cells arise exclusively from pre-existing β-cells during pregnancy, in a chase period following the tamoxifen pulse, the percentage of HPAP labelled β-cells should not change (Hypothesis 1—see Fig. 2 for a description of the different hypotheses). By contrast, dilution of the percentage of HPAP labelled cells during the chase period indicates a source of new β-cells other than pre-existing β-cells. For this purpose, age and weight-matched paired of female mice were pulsed with tamoxifen, at about 9 weeks of age, to heritably label their β-cells. Then animals were either mated for pregnancy, or were maintained as non-pregnant controls. Pregnant animals were killed at 15–19 days of pregnancy, and control non-pregnant animals were killed contemporaneously, for examination of their pancreata.
Figure 2.
Analysing the origin of new β-cells during pregnancy. Double transgenic female mice (Z/AP; RIP-CreERTAM) were pulsed with tamoxifen to label their β-cells. Then during the chase period, pregnancy was induced in only one group of mice while the other animals were assigned to the non-pregnant control group. After the chase period, if the percentage of β-cells positive for HP AP in pancreas of the pregnant animals is identical to the non-pregnant controls we conclude that new β-cells are the progeny of pre-existing β-cells (Hypothesis 1). However, if there is a dilution of the percentage of HP AP-labelled β-cells in the pregnant animals, we conclude that a non-β-cell source gives rise to new β-cells, possibly in combination with a contribution from pre-existing β-cells (Hypothesis 2). Finally, if the percentage of islets totally devoid of any label is higher in pregnant animals than in the controls, one can deduce that whole new islets arise from non-β-cell progenitors (Hypothesis 3).
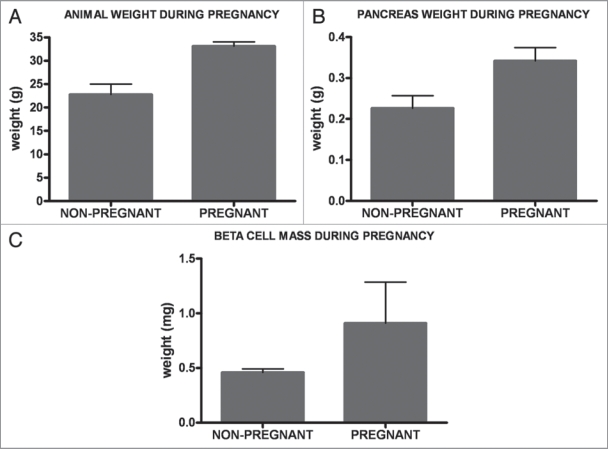
In our experimental system, consistent with previous reports, pregnancy was associated with a substantial increase in body mass,1 and also pancreas weight (Fig. 3A and B), compared with non-pregnant female age-matched control animals. At the time of analysis, we observed a 45% increase in the mean body mass and 51% increase in the mean pancreas weight. β-cell mass was also determined in these animals by morphometric analysis of pancreas sections. The mean increase in β-cell mass in the pregnant animals was 91% compared to the controls (Fig. 3C). This result corroborates data from previous studies1–3,25,26 indicating that new β-cells are formed during pregnancy, with little or none of the increase in β-cell mass being attributable to an increase in cell size.3,25
Figure 3.
Responses to pregnancy. During pregnancy, the mean body mass increased by 45% (A), and the mean pancreas mass by 51% (B), as compared with their age-matched non-pregnant control counterparts. Mean β-cell mass in pregnant animals increased by 91% (C).
Dilution of HPAP labelled β-cells during pregnancy.
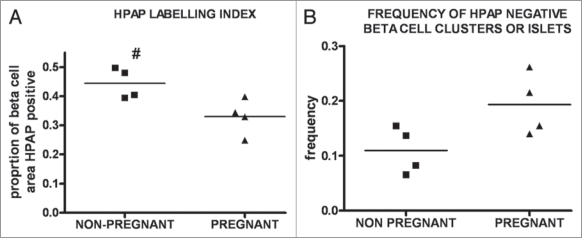
The proportion of β-cells labelled for HPAP was calculated as the total pixel number of β-cell cross-sectional area that is HPAP-positive, relative to the total pixel number of β-cell cross-sectional area (defined as insulin positive). The pixel numbers were determined for the whole of five microscopic sections in the given pancreas. Comparing the proportion of β-cells labelled for HPAP (labeling index) in the pancreata from pregnant versus non-pregnant groups (Fig. 4A), we observed that the mean labeling index in the nonpregnant animals was higher (0.44 ± 0.05) than in the pregnant mice (0.33 ± 0.06). For every pair, the labeling index was higher in the non-pregnant animal (Suppl. Information, Fig. S2) and the mean ratio of the HPAP labeling indices for the paired animals was 1.37. This difference between the mean labeling index for the two groups is statistically significant (p = 0.021, paired two-tailed t-test). The observed dilution of the HPAP label leads to rejection of Hypothesis 1, and indicates a contribution to new β-cells from a non-beta-cell source during β-cell mass expansion in pregnancy. The data are consistent with Hypothesis 2.
Figure 4.
Lineage tracing in pregnancy. (A) The HP AP labelling index in non-pregnant control and pregnant female animal pancreata was calculated by forming the ratio of total number of pixels of cross-sectional area immunostained for HP AP to total number of pixels cross-sectional area immunostained for insulin, using Image J. The mean labelling index in the pancreata of non-pregnant animals is higher (0.44 ± 0.05) than in the pregnant mice (0.33 ± 0.06). (B) The proportion of islet section completely negative for HP AP is calculated by forming the ratio of the HP AP-negative islet cross-sectional area to the total islet cross-sectional area in the whole of five sections for each pancreas. The mean proportion of islet sections HP AP negative in the pregnant group is (0.19 ± 0.06) is higher than in their age-matched, non-pregnant control counterparts (0.11 ± 0.04) as seen in the graph. # designates a p-value <0.05 by paired two-tailed student t-test.
To extend these observations, we reasoned that if a pregnancy led to dilution of the HPAP label, then two consecutive pregnancies might lead to greater dilution. Therefore, pairs of mice were set up according to the protocol already described, except that instead of one member of each pair being mated to induce a single pregnancy during the chase period, now they were mated for pregnancy, allowed to deliver pups, and then after a short period mated again to induce a second pregnancy during which the pancreas was examined as before (Suppl. information, Fig. S3). Again a dilution of the label was observed in pregnant animals compared to paired control animals. In fact the observed mean dilution during a second pregnancy was similar to that after a single pregnancy, not greater as we initially expected on simple assumptions (Suppl. information, Fig. S4). The mean ratio of the labeling indices for the pairs of animals for two pregnancies was 1.35. For present purposes, we pooled data for non-pregnant versus pregnant animals irrespective of whether pregnancy is a single or second one. The combined mean HPAP labeling index in the non-pregnant animals (0.44 ± 0.12) is higher than that in pregnant animals (0.33 ± 0.09), and the difference between the two groups is considered extremely significant (paired, two-sided student t-test, p-value 0.0007). The following data were obtained only from analysis of single pregnancies.
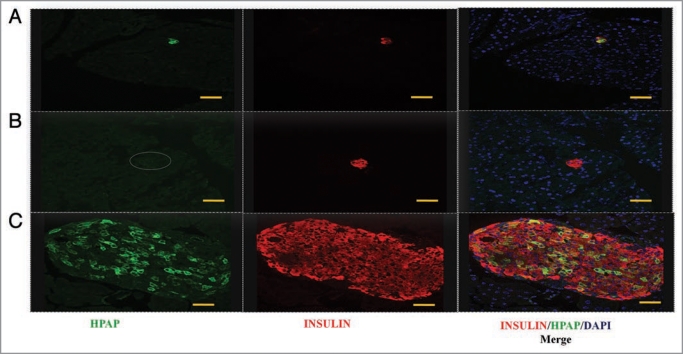
One potential mechanism for β-cell mass expansion is the creation of new islets. To investigate this, we focused on islet tissue presenting as small cross-sectional areas in pancreas sections. These ‘small-islet’ sections, could result either from islets of genuinely small mass, or from glancing sections of larger islets. Several authors have previously suggested that small islets are representative of newly-formed islets,11,27–30 such as might arise during β-cell mass expansion in pregnancy.26 We therefore scored islet sections that were completely HPAP-negative (such islet sections all presented as “small-islet” sections), and calculated the proportion of area presented by such islets by calculating the ratio of the cross-sectional area of these HPAP-negative islets to the total islet cross-sectional area. The ratio in the pregnant group (0.19 ± 0.06) was higher than in the non-pregnant group (0.11 ± 0.04) (Figs. 4B and 5). Although the difference was not significant (p = 0.093, paired two-tailed t-test), the trend may indicate some new, small, islet formation (Fig. 2 and i.e., a contribution according to Hypothesis 3). Alternatively, if the “small-islet” sections in fact mainly represent small glancing sections of larger islets, the observed increase in completely HPAP-negative sections is consistent with the reduction of labeling index evident in the previous analysis, because this dilution would increase the probability of small sections appearing as completely HPAP-negative.
β-cell clusters are associated with pancreatic ducts.
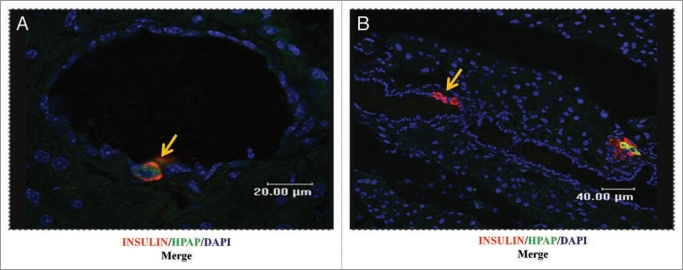
Previous studies have indicated that cells in the epithelium of pancreatic ducts can be a source for islet β-cell progenitors during regeneration in adult mice. We therefore examined the incidence of insulin-producing cells in the ductal epithelium in the pancreata of pregnant animals. Ducts were identified histologically by the presence of a ductal lumen. As observed in other studies31–34 we observed instances of insulin-positive cells in the duct epithelium itself, or immediately adjacent to ductal epithelium cells (Fig. 6). Because such insulin-positive ductal cells were relatively few in number, it was possible to directly count the number of cells that were HPAP positive or negative, to derive the labeling index. For comparison, we counted the numbers of HPAP-positive or HPAP-negative, insulin-positive, cells in several islets in the same sections, to give the labeling index for islets. The labeling indices were 0.21 (n = 57 cells) for ductal cells, and 0.31 (n = 7053 cells) for the islets, from pregnant animals. We note in passing that the labeling index (0.31) determined by cell counting for the islets, is closely similar to the value noted above (0.33) which was obtained by morphometric analysis based upon cell areas as determined by pixel numbers. The labeling indices of insulin-positive ductal cells and islets are not significantly dissimilar (2 × 2 contingency table, Fisher’s exact test, p = 0.15). The similar labeling indices of ductal cells and islets are not consistent with the cells associated with the duct, as defined by the presence of a ductal lumen, being a highly preferred source of new β-cells by comparison with islets.
Figure 6.
Insulin-positive cells associated with the ductal epithelium. Pancreas sections from pregnant female animals, stained for insulin (red), HP AP (green) and nuclei (blue, DAPI), show examples (yellow arrows) of HP AP-positive β-cells (A), or HP AP-negative β-cells (B), associated with duct epithelium. Bar scales correspond to 20 µm (A) and 40 µm (B).
β-cells do not transdifferentiate into other cell types.
An interesting aspect of cell plasticity is the ability to transdifferentiate into other cell types. Having confirmed that the HPAP- labeling was initially specific for β-cells (Fig. 1), we examined whether β-cells are able to transdifferentiate into other (insulin-negative) cells in the endocrine and exocrine components of the pancreas during pregnancy. We therefore looked for cells positive for HPAP and negative for insulin in the pancreata of pregnant and non-pregnant animals after the chase period (about 2.5 months after the last tamoxifen injection). We did not find any example of cells positive for HPAP and negative for insulin in the pancreatic sections of pregnant or non-pregnant female mice (Fig. 7). This analysis indicates that during this time window, β-cells do not fully lose differentiation or transdifferentiate into other cell types of either endocrine or exocrine tissue. This is similar to conclusions reached after induction of acute pancreatitis.35 However, we cannot exclude that transdifferentiation could take place during a much longer period following the tamoxifen pulse, or under different conditions, for example following pancreatic injury and regeneration.
Figure 7.
HP AP labeling remains restricted to β-cells after a chase period. Pancreatic sections from non-pregnant control females animals stained for insulin (red), HP AP (green) and nuclei (blue, DAPI), showing that the HP AP label is restricted to β-cells at 2.5 months following the pulse of tamoxifen. Bar scale corresponds to 40 µm.
Discussion
The lineage tracing data presented here lead us to conclude that: (1) the lineage tracing system was highly specific for β-cells in mice of the genetic constitution we employed; (2) there was a dilution of the labeling index associated with pregnancy; (3) although not statistically significant, pregnancy was associated with the appearance of more small-islet sections that were unlabelled by HPAP; and (4) there was no transdifferentiation of β-cells to other cell types, either within islets or in exocrine tissue, up to 2.5 months after labeling.
The dilution of the labeling index resulting from pregnancy is evident as a change in mean value of the labeling index from 0.44 to 0.33, corresponding to a 25% reduction and supporting Hypothesis 2, which implies a non-beta-cell source contributing to a substantial proportion of new β-cells. While it is not possible to give a precise estimate of the proportions of new β-cells arising from this source and the proportion arising from β-cell duplication, we note that a doubling of β-cell mass under Hypothesis 2 would be expected to give a 50% reduction in labeling index. Hence, the observed reduction implies approximately equal contributions from pre-existing β-cells and non-β-cells to the β-cell mass expansion that occurs in pregnancy. Thus for the first time in a normal physiological context, we have obtained evidence for a major role for non-β-cell progenitors of β-cells in adult animals.
Given that a non-β-cell source is active in β-cell mass expansion during pregnancy, how abundant are such progenitors, and how many divisions must they undergo? To account for about 25% of the expanded population of β-cells having arisen from a non-β-cell progenitor, we need postulate the existence of a relatively small number of progenitors with an abundance equivalent to only a few percent of the original β-cell numbers, and three or four cell divisions. Studies using serial labeling with two thymidine analogs, from days 4 to 18 of pregnancy, showed an increase in cell co-labeling (to a proportion of about 2% of β-cells) during pregnancy, indicating that two or more S-phases occurred in the lineage of an expanded population of β-cells.36 The serial labeling periods used in that study would make an underestimate of multiple S-phases from any progenitor cell population, and hence these co-labeling experiments are consistent with such an expansion from progenitors. The expansion of the new β-cells from progenitors is likely to be sensitive to overexpression of a Men1 transgene driven by the rat insulin promoter, and hence the reduction in β-cell mass expansion in such transgenic mice is not inconsistent with our conclusion.1
It is pertinent to ask where the non-β-cell progenitors of β-cells reside within the pancreas. It is currently difficult to exclude any of the several non-β-cell sources that have been suggested in previous studies in rodents, and indeed it is possible that more than one source is active. However, cells within or closely associated with the lining of the ducts, are a clear candidate. To analyse this, we examined the labeling index of these cells in pregnant animals, in comparison with the labeling index of nearby islets. The insulin-positive ductal cells were relatively rare, which precluded the analysis of a large number of cells. However if this was a source of new β-cells that is preferred or more active than islets themselves, we would expect to have seen a substantial dilution in labeling index for ductal cells, resulting in a labeling index that is lower than that for the nearby islets. Since this dilution was not evident, we can conclude that non-β-cells located in the lining of the duct are not a predominant source of new β-cells during pregnancy. However a limitation of this type of analysis is due to the histological identification of ducts. As shown by Bertelli et al.37 there is an extensive connection between islets and the ductal tree. In the rat they showed that at least 74% of the islets were in contact with ducts, with about 93.5% of such islets associated with centroacinar cells or small sized ducts. The latter are often overlooked by conventional histology, because they have no evident lumen.37–39 This suggests that the simple histological discrimination between intra-islet versus duct-associated insulinpositive cells is uncertain for many such cells. Therefore, we must leave open the possibility that progenitors associated with small ducts that are also closely associated with islets might contribute substantially to new beta-cells during pregnancy.
Pregnancy in mice results in an expansion in β-cell mass that is attractive for the further analysis of β-cell origins because it is natural, substantial, rapid, rapidly reversed and repeatable over several cycles of pregnancy in an individual animal. Our finding that non-β-cells contribute significantly to this expansion, prompts further analysis of expansion and reversal, to further understand the cells and mechanisms involved. In particular it will be very interesting to define the signals involved in this mode of β-cell generation. Our identification of such a model may be of great utility in the ongoing search for new sources of β-cells for the treatment of diabetes.
Materials and Methods
Animals.
The study was performed on 8 (RIP-CreERTAM; Z/AP) double transgenic female mice, weighing 16.4 ± 1.9 g and aged of 9 weeks ± 4 days at the time of their first pulse of tamoxifen. Ear punch biopsies were used to genotype the single transgenic RIP-CreERTAM,22 strain by PCR using the following set of primers (forward 5’ACG GCG CTA AGG ATG ACT CT3’ and reverse 5’CCA CCA GCT TGC ATG ATC TC3’), and the Z/AP strain23 by enzymatic staining using 5bromo-4chloro-3indolybetagalactopyranoside (X-gal, Sigma). The two single strains were maintained by crossing with CBAxC57BL/6 F1 background. The double transgenic mice were generated by crossing the two single transgenic mice aforementioned. Transgenic mice were housed under a 12 hour light/dark cycle and received food and water ad libitum until sacrifice. Animal care and experiments were carried out according to UK Home Office regulations.
Lineage tracing and pregnancy.
β-cell labeling with the human placental alkaline phosphatase (HPAP) reporter was carried out by injecting 5 doses of 4 mg of tamoxifen (Sigma), diluted in peanut oil, over a period of 2.5 weeks. Animals were age and weight matched, and injected in pairs with the same batch of tamoxifen suspension for each pair. As soon as the females were fertile again, that is 1.5 to 2.5 months after the last pulse of tamoxifen, one female of each pair was mated until visibly pregnant, the other female was retained as a non-pregnant virgin. Paired pregnant and virgin females were sacrificed at the same time. The mean weight of the pups at time of pregnant animal sacrifice, was 1.05 ± 0.22 g.
Tissue processing and immunohistochemistry.
Pancreata from non-pregnant control and pregnant females were dissected out and fixed in 4% paraformaldehyde for 2 hours at room temperature, then transferred to 30% sucrose overnight at 4°C before embedding in OCT mounting medium (Tissue Tek). One block for each pancreas was sectioned for immunohistochemistry. Five sections per pancreas, representing different parts of the organ and separated at least by 500 µm from each other, were immunostained with an insulin polyclonal guineapig anti-swine (DAKO), a polyclonal sheep anti-human placental alkaline phosphatase (ARP American Research Products, Inc.,) or a polyclonal rabbit anti-human placental alkaline phosphatase (Accurate Chemical & Scientific Corporation). Vectashield mounting medium with DAPI (Vector laboratories) was used to stain the nucleus. The secondary antibodies used to reveal the primary antibodies were Alexa 633-conjugated goat anti-guinea-pig (Invitrogen), FITC-conjugated donkey anti-sheep (Jackson ImmunoResearch laboratories) and FITC-conjugated goat anti-rabbit (Vector laboratories). All the antibodies were diluted in phosphate buffered saline (PBS) containing 0.1% Triton X-100 and 2% normal donkey serum (Jackson ImmunoResearch laboratories). The antibody working dilution was 1/100 for the insulin polyclonal guineapig anti-swine, 1/1,000 for the sheep and rabbit anti-human placental alkaline phosphatase, and 1/200 for the secondary antibodies. Briefly, the sections were stained sequentially with (1) the polyclonal sheep anti-human placental alkaline phosphatase overnight at 4°C, (2) the FITC labelled anti-sheep for 30 min at room temperature, (3) the polyclonal guinea-pig anti insulin for 1 hour at room temperature, (4) the Alexa 633 goat anti-guinea-pig for 30 min at room temperature. Then slides were mounted with Vectolab mounting medium with DAPI. The sections were washed 3 times with PBS containing 0.1% Triton X-100 before adding the next antibody.
Morphometric analysis and calculation of labeling index.
Images of pancreatic tissue sections, immunostained for DAPI, or for insulin and HPAP, were captured using a Leica confocal microscope. Slides were scored “blind” as to their experimental origin. Systematically, all single islets and β-cell clusters found in each section were photographed and 5 sections representing different regions of the pancreas were analysed. Image J was used to count the number of pixels of cross-sectional area stained for HPAP and insulin in each picture. Most of the time, these images showed only one islet. For the measurement of the all pancreatic tissue cross-sectional area positive for DAPI we first processed the pictures using the Gaussian blur option before proceeding with the count. The β-cell mass was calculated by forming the ratio of the total number of pixels of insulin cross-sectional area to the total number of pixels of the pancreatic tissue cross-sectional area in the five sections in each pancreas, multiplying by the pancreas weight of the given mouse. Similarly, the proportion of β-cell area positive for HPAP or the HPAP labeling index was calculated by forming the ratio of total number of pixels of cross-sectional area immunostained for HPAP to total number of pixels cross-sectional area immunostained for insulin, in all five sections in the given mouse.
Statistical analysis.
The results are presented as MEANS ± SD. Pregnant and non-pregnant animals were derived from pairs of age and weight-matched animals injected with tamoxifen at the same time with the same preparation of tamoxifen suspension. The paired design is relevant because experience suggests that substantial variation can be due to different preparations of tamoxifen suspension. The pairs of animals were killed contemporaneously, and their pancreata were processed and analysed in pairs. The paired 2-tailed student t test was used for statistical analysis using the GraphPad InStat3 software.
Figure 5.
Photomicrographs of pancreatic sections from pregnant animals stained for insulin (red), HP AP (green), and nuclei (blue, DAPI). Small islet sections are either partly or wholly HP AP-positive (A), or completely HP AP-negative (dashed circle, B). Small islet sections represent either genuinely small islets or glancing sections of larger islets (C). Bar scale corresponds to 40 µm.
Acknowledgements
This project was supported by the BBSRC, EPSRC and the Wellcome Trust. We would like to thank the animal unit at the University of Warwick and L.C. Murtaugh for providing the RIP-CreERTAM and Z/AP transgenic mice. Our thanks go to N. Ahmad, L. Zhou and L.Y. Cheung.
Footnotes
Previously published online: www.landesbioscience.com/journals/organogenesis/article/10374
Supplementary Material
References
- 1.Karnick SK, Chen H, McLean GW, Heit JJ, Gu X, Zhang AY, et al. Menin controls growth of pancreatic β-cells in pregnant mice and promotes gestational diabetes mellitus. Science. 2007;318:806–809. doi: 10.1126/science.1146812. [DOI] [PubMed] [Google Scholar]
- 2.Parsons JA, Bartke A, Sorenson RL. Number and size of islets of Langerhans in pregnant, human growth hormone-expressing transgenic, and pituitary dwarf mice: effect of lactogenic hormones. Endocrinology. 1995;136:2013–2021. doi: 10.1210/endo.136.5.7720649. [DOI] [PubMed] [Google Scholar]
- 3.Scaglia L, Smith FE, Bonner-Weir S. Apoptosis contributes to the involution of β-cell mass in the post partum rat pancreas. Endocrinology. 1995;136:5461–5468. doi: 10.1210/endo.136.12.7588296. [DOI] [PubMed] [Google Scholar]
- 4.Stanger BZ, Tanaka AJ, Melton DA. Organ size is limited by the number of embryonic progenitor cells in the pancreas but not the liver. Nature. 2007;445:886–891. doi: 10.1038/nature05537. [DOI] [PubMed] [Google Scholar]
- 5.Parsons JA, Brelje TC, Sorenson RL. Adaptation of islets of Langerhans to pregnancy: increased islet cell proliferation and insulin secretion correlates with the onset of placental lactogen secretion. Endocrinology. 1992;130:1459–1466. doi: 10.1210/endo.130.3.1537300. [DOI] [PubMed] [Google Scholar]
- 6.Bruning JC, Winnay J, Bonner-Weir S, Taylor SI, Accili D, Kahn CR. Development of a novel polygenic model of NIDDM in mice heterozygous for IR and IRS-1 null alleles. Cell. 1997;88:561–572. doi: 10.1016/s0092-8674(00)81896-6. [DOI] [PubMed] [Google Scholar]
- 7.Sorenson RL, Brelje TC. Adaptation of islets of Langerhans to pregnancy: β-cell growth, enhanced insulin secretion and the role of lactogenic hormones. Horm Metab Res. 1997;29:301–307. doi: 10.1055/s-2007-979040. [DOI] [PubMed] [Google Scholar]
- 8.Slack JM. Developmental biology of the pancreas. Development. 1995;121:1569–1580. doi: 10.1242/dev.121.6.1569. [DOI] [PubMed] [Google Scholar]
- 9.Limbert C, Path G, Jakob F, Seufert J. Beta-cell replacement and regeneration: Strategies of cell-based therapy for type 1 diabetes mellitus. Diabetes Res Clin Pract. 2008;79:389–399. doi: 10.1016/j.diabres.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 10.Nir T, Melton DA, Dor Y. Recovery from diabetes in mice by β-cell regeneration. J Clin Invest. 2007;117:2553–2561. doi: 10.1172/JCI32959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 12.Inada A, Nienaber C, Katsuta H, Fujitani Y, Levine J, Morita R, et al. Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc Natl Acad Sci USA. 2008;105:19915–19919. doi: 10.1073/pnas.0805803105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu X, D’Hoker J, Stange G, Bonne S, De Leu N, Xiao X, et al. β-cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132:197–207. doi: 10.1016/j.cell.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 14.Minami K, Okuno M, Miyawaki K, Okumachi A, Ishizaki K, Oyama K, et al. Lineage tracing and characterization of insulin-secreting cells generated from adult pancreatic acinar cells. Proc Natl Acad Sci USA. 2005;102:15116–15121. doi: 10.1073/pnas.0507567102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to β-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collombat P, Xu X, Ravassard P, Sosa-Pineda B, Dussaud S, Billestrup N, et al. The ectopic expression of Pax4 in the mouse pancreas converst progenitor cells into α and subsequently β cells. Cell 2. 2009;138:449–462. doi: 10.1016/j.cell.2009.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernandes A, King LC, Guz Y, Stein R, Wright CV, Teitelman G. Differentiation of new insulin-producing cells is induced by injury in adult pancreatic islets. Endocrinology. 1997;138:1750–1762. doi: 10.1210/endo.138.4.5049. [DOI] [PubMed] [Google Scholar]
- 18.Guz Y, Nasir I, Teitelman G. Regeneration of pancreatic β-cells from intra-islet precursor cells in an experimental model of diabetes. Endocrinology. 2001;142:4956–4968. doi: 10.1210/endo.142.11.8501. [DOI] [PubMed] [Google Scholar]
- 19.Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–2457. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- 20.Murtaugh LC, Melton DA. Genes, signals and lineages in pancreas development. Annu Rev Cell Dev Biol. 2003;19:71–89. doi: 10.1146/annurev.cellbio.19.111301.144752. [DOI] [PubMed] [Google Scholar]
- 21.Bouwens L, Rooman I. Regulation of pancreatic β-cell mass. Physiol Rev. 2005;85:1255–1270. doi: 10.1152/physrev.00025.2004. [DOI] [PubMed] [Google Scholar]
- 22.Heit JJ, Karnik SK, Kim SK. Intrinsic regulators of pancreatic β-cell proliferation. Ann Rev Cell Dev Biol. 2006;22:311–338. doi: 10.1146/annurev.cellbio.22.010305.104425. [DOI] [PubMed] [Google Scholar]
- 23.Hanley NA, Hanley KP, Miettinen PJ, Otonkoski T. Weighing up β-cell mass in mice and humans: Self-renewal, progenitors or stem cells? Mol Cell Endocrinol. 2008;288:79–85. doi: 10.1016/j.mce.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Lobe CG, Koop KE, Kreppner W, Lomeli H, Gertsenstein M, Nagy A. Z/AP, a double reporter for cre-mediated recombination. Dev Biol. 1999;208:281–292. doi: 10.1006/dbio.1999.9209. [DOI] [PubMed] [Google Scholar]
- 25.Avril I, Blondeau B, Duchene B, Czernichow P, Breant B. Decreased beta-cell proliferation impairs the adaptation to pregnancy in rats malnourished during perinatal life. J Endocrinol. 2002;174:215–223. doi: 10.1677/joe.0.1740215. [DOI] [PubMed] [Google Scholar]
- 26.Aerts L, Vercruysse L, Van Assche FA. The endocrine pancreas in virgin and pregnant offspring of diabetic pregnant rats. Diabetes Res Clin Pract. 1997;38:9–19. doi: 10.1016/s0168-8227(97)00080-6. [DOI] [PubMed] [Google Scholar]
- 27.Lipsett M, Finegood DT. Beta-cell neogenesis during prolonged hyperglycemia in rats. Diabetes. 2002;51:1834–1841. doi: 10.2337/diabetes.51.6.1834. [DOI] [PubMed] [Google Scholar]
- 28.Yamamoto K, Miyagawa J, Waguri M, Sasada R, Igarashi K, Li M, et al. Recombinant human betacellulin promotes the neogenesis of beta-cells and ameliorates glucose intolerance in mice with diabetes induced by selective alloxan perfusion. Diabetes. 2000;49:2021–2027. doi: 10.2337/diabetes.49.12.2021. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto K, Miyagawa J, Waguri M, Itoh N, Imagawa A, Kuwajima M, et al. Proliferation and differentiation of pancreatic beta-cells: ultrastructural analysis of the pancreas in diabetic mice induced by selective alloxan perfusion. Med Electron Microsc. 1997;30:170–175. [Google Scholar]
- 30.Wang RN, Kloppel G, Bouwens L. Duct-to islet-cell differentiation and islet growth in the pancreas of ductligated adult rats. Diabetologia. 1995;38:1405–1411. doi: 10.1007/BF00400600. [DOI] [PubMed] [Google Scholar]
- 31.Gu D, Sarvetnick N. Epithelial cell proliferation and islet neogenesis in IFNg transgenic mice. Development. 1993;118:33–46. doi: 10.1242/dev.118.1.33. [DOI] [PubMed] [Google Scholar]
- 32.Gu D, Lee MS, Krahl T, Sarvetnick N. Transitional cells in the regenerating pancreas. Development. 1994;120:1873–1881. doi: 10.1242/dev.120.7.1873. [DOI] [PubMed] [Google Scholar]
- 33.Bonner-Weir S, Toschi E, Inada A, Reitz P, Fonseca SY, Aye T, Sharma A. The pancreatic ductal epithelium serves as a potential pool of progenitor cells. Pediatr Diabetes. 2004;5:16–22. doi: 10.1111/j.1399-543X.2004.00075.x. [DOI] [PubMed] [Google Scholar]
- 34.Holland AM, Gonez LJ, Naselli G, Macdonald RJ, Harrison LC. Conditional expression demonstrates the role of the homeodomain transcription factor Pdx1 in maintenance and regeneration of beta-cells in the adult pancreas. Diabetes. 2005;54:2586–2595. doi: 10.2337/diabetes.54.9.2586. [DOI] [PubMed] [Google Scholar]
- 35.Strobel O, Dor Y, Stirman A, Trainor A, Fernandez-del-Castillo C, Warshaw AL, et al. b-cell transdifferentiation does not contribute to preneoplastic/metaplastic ductal lesions of the pancreas by genetic lineage tracing in vivo. Proc Natl Acad Sci USA. 2007;104:4419–4424. doi: 10.1073/pnas.0605248104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teta M, Rankin MM, Long SY, Stein GM, Kushner JA. Growth and regeneration of adult β-cells does not involve specialized progenitors. Dev Cell. 2007;12:817–826. doi: 10.1016/j.devcel.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 37.Bertelli E, Regoli M, Orazioli D, Bendayan M. Association between islets of Langerhans and pancreatic ductal system in adult rat. Where endocrine and exocrine meet together? Diabetologia. 2001;44:575–584. doi: 10.1007/s001250051663. [DOI] [PubMed] [Google Scholar]
- 38.Bertelli E, Bendayan M. Association between endocrine pancreas and ductal system. More than an epiphenomenon of endocrine differentiation and development? J Histochem Cytochem. 2005;53:1071–1086. doi: 10.1369/jhc.5R6640.2005. [DOI] [PubMed] [Google Scholar]
- 39.Bouwens L. Cytokeratins and cell differentiation in the pancreas. J Pathol. 1998;184:234–239. doi: 10.1002/(SICI)1096-9896(199803)184:3<234::AID-PATH28>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.