Abstract
We have developed a method for the decellularization of whole rat livers by perfusion with increasing concentrations of detergents. This procedure resulted in an intact, decellularized organ with an intact liver capsule. These decellularized organs were analyzed by immunohistochemistry, and retained an appropriate distribution of extracellular matrix components. The laminin basement membranes of the liver vasculature also remain intact. These acellular vessel remnants were strong enough to be cannulated, providing a convenient means for the delivery of cells to areas deep within the decellularized organ. Cannulation of the extrahepatic vessel remnants allow for media to be circulated through the decellularized organ. These decellularized livers provide a natural matrix for research in the fields of bio-artificial livers and liver engineering.
Key words: bioartificial liver, liver transplant, liver matrix, liver progenitor cell, artificial liver, organ engineering, decellularization
Introduction
Liver pathologies, ranging from viral hepatitis to inborn metabolic disorders to injury resulting from alcohol abuse, often result in the need for liver transplantation. An insufficient supply of organs suitable for transplantation has limited our ability to cure many instances of these diseases. Approximately 6,500 liver transplants were performed in the United States during the year 2005.1 Over 17,000 Americans are currently waiting for a liver transplant.2 Unfortunately, many of these patients will succumb to their disease before a suitable organ becomes available. Patients who do receive liver transplant must contend with lifelong immunosuppression. Even with appropriate immunosuppression, the transplanted organ has a finite lifespan. These facts have driven research in fields that provide alternatives to orthotopic liver transplant.
Bioartificial livers (BAL) are not a permanent alternative to liver transplant. Instead, they would be used to sustain a critically ill patient until a suitable donor organ became available. BAL generally take the form of closed, ex vivo systems containing functional liver cells grown on a synthetic matrix.3–5 In theory, a patient’s blood could be passed through these systems, allowing for the detoxification of xenobiotics and ammonia as well as supplying plasma proteins and glucose. While this concept may seem straight forward, complications in maintaining functional hepatocytes in three-dimensional culture have limited the development of these systems.3–6 Synthetic matrices have proven to be sub-optimal in regards to both the extent of colonization as well as the long term maintenance of cell functionality and viability.
Ex Vivo engineering of a transplantable liver would be a permanent alternative to donor liver transplant. The discovery that liver stem cells may be derived from bone marrow open the possibility of colonizing a matrix scaffold with a patient’s own cells.7 We have developed a method for removing all of the cells from an intact liver. This process leaves behind the extracellular matrix and liver capsule, as well as the laminin basement membranes of the extrahepatic and extrahepatic vasculature. We feel that these intact decellularized livers (IDL) represent an exceptional tool for studies related to BAL and engineered livers.
Results
Following perfusion of the liver with PBS to clear the blood, Triton X-100 was used to solublize lipids (Fig. 1A and B). Analysis of sections from livers treated only with Triton X-100 indicated clearing of all cells. However, DAPI staining revealed intact nuclear cages containing DNA remained within the matrix (Data not shown). Subsequent perfusion of the acellular organ with solution containing SDS resulted in the clearance of all DNA (Fig. 1C). H&E staining of formalin fixed sections of IDL demonstrate a fine web of matrix remains within the IDL capsule (Fig. 2A). Immunohistochemical staining of the matrix indicated the presence of collagen IV within the matrix (Fig. 2B). Additionally, laminin was shown to be present within the remaining basement membrane of the vessels and surrounding the acellular remnants of the hepatic cords (Fig. 2C).
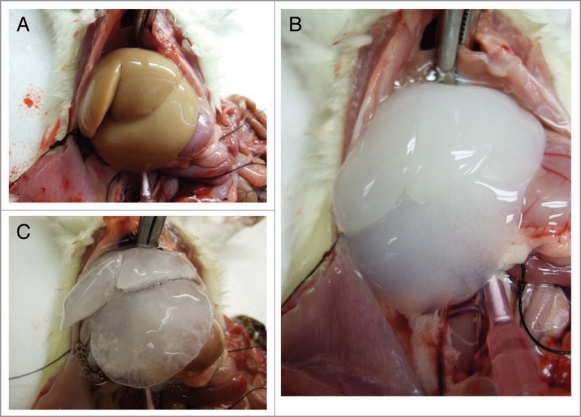
Figure 1.
Decellularization process. (A) Blood is removed from the organ by perfusion with PBS. (B) Triton X-100 is used to solublize cellular membranes. (C) SDSs used to clear the remaining nuclear cages and DNA from the matrix.
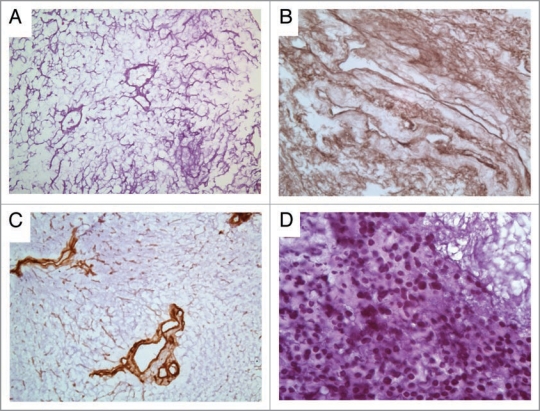
Figure 2.
H&E staining of IDL. (A) Extracellular matrix within the IDL. (B) Collagen IV staining of the IDL matrix. (C) Immunohistochemistry for laminin indicates intact basement membrane of the vessels as well as appropriate deposition of the matrix component between hepatic cords. (D) Cryosection of WB344 rat liver progenitor cells delivered to the center of the IDL through the cannulated IVC remnant.
Following decellularization, the acellular vessel remnants were strong enough to maintain cannulation. The rat liver progenitor cell line WB344 in RPMI medium was infused into the IDL through the cannulated IVC. H&E sections from within the center of the IDL contained cells indicating that the intrahepatic vasculature was able to traffic cells from the IVC to these areas (Fig. 2D).
Discussion
We have developed a simple method for removing the cellular component from intact rat livers. Triton X-100 was sufficient to clear the cells, but the nuclei remained. It is likely that Triton X-100 treatment spares the nuclear cage that is tethered to the matrix by the cytoskeleton. Perfusion with 0.1% SDS for 1 hour completely cleared all DNA from the IDL. Supplementation of all perfusion solutions with antibiotics/antimycotics prevented microbial growth, and the IDL could be stored at 4°C for several weeks. The extrahepatic vessel remnants maintained sufficient strength for cannulation. This should provide a continent means to deliver media to the internal regions of the IDL; supplying oxygen and nutrients for long term culture experiments.
The matrix within the IDL may be beneficial for inclusion in BAL studies. Because the distribution of proteins is maintained during decellularization, the resulting matrix should be quite close to that found in normal liver. The IDL matrix may also be useful in studies involving the epigenetic control of cell phenotype. For instance, IDL may be produced from livers containing experimentally induced tumors. Sections of IDL from these livers may be used to test the phenotype of tumor cells on tumor matrix as compared to normal adjacent matrix. It is also possible that IDL may be recellularized by mature or progenitor cells. This would likely involve the stepwise repopulation of the vascular basement membranes with endothelial cells followed by colonization of the hepatic cord remnants with hepatocytes. It is possible that equilibration of IDL with serum would allow rebinding of growth factors to the matrix. This may help to direct the proliferation and differentiation of progenitor or stem cells and result in a tissue architecture similar to normal liver.
Materials and Methods
Animals.
Fisher 344 rats ranging in age from 6 weeks to 8 months have been successfully used for the production of IDL. The animals are maintained in conventional housing on standard diet prior to treatment. All animal procedures are conducted under guidelines approved by the University of Florida IACUC.
Liver decellularization.
Rats received a lethal (100 mg/kg) dose of sodium pentobarbital. The inferior vena cava (IVC) was cannulated, the portal vein severed and the superior vena cava (SVC) was clamped as previously described.8 The liver was first perfused with 100 mL PBS (pH 7.4) to clear blood from the organ. The liver was then perfused with biological detergents to solublize cell membranes. Isotonic solutions (PBS) of 1, 2 and 3% (w/v) Triton X-100 (300 mL each) were perfused through the organ by peristaltic pump at a flow rate of 5 mL/minute. This was immediately followed by perfusion with 300 mL each of PBS containing 0.1% SDS (w/v). Detergent containing solutions were cleared from the liver by perfusion with 300 mL PBS. Finally, 10 mL fetal bovine serum was pumped into the organ. For experiments involving administration of cells to the IDL, 106 cells were delivered to the organ through the IVC. The IVC and SVC were then tied off and the liver was excised intact. All perfusion solutions (including FBS) contained 1% antibiotic/mycotic (Invitrogen, Carlsbad, CA).
WB344 cell line.
The rat liver progenitor cell line WB344 was kindly provided by Dr. William Coleman (Dept. Pathology, University of North Carolina, Chapel Hill). These cells were cultured in RPMI 1640 (Cellgro, Herndon, VA) medium containing 10% FBS and 1% antibiotic/mycotic (Invitrogen, Carlsbad, CA). Cultures were maintained at 37°C under 5% CO2.
Histology and immunohistochemistry.
Hematoxylin and eosin staining was conducted by the standard method.9 Paraffin sections were fixed overnight in 10% neutral buffered formalin prior to embedding. Frozen sections were cut from O.C.T. blocks and fixed in 4°C acetone for two minutes. Primary antibodies were used at the following dilutions: laminin 1:100 (Dako); collagen IV 1:100 (Abcam) Secondary antibodies (1:200), ABC kit, and DAB peroxidase kit were purchased from Vector Laboratories (Burlingame, Ca).
Acknowledgements
Supported by PF-05-165-01 from the American Cancer Society.
Abbreviations
- BAL
bioartificial livers
- IDL
intact decellularized livers
- IVC
inferior vena cava
- SVC
superior vena cava
- FBS
fetal bovine serum
Footnotes
Previously published online: www.landesbioscience.com/journals/organogenesis/article/11546
References
- 1. http://www.liverfoundation.org/education/info/transplant/.
- 2.Grewal P, Martin P. Pretransplant management of the cirrhotic patient. Clin Liver Dis. 2007;11:431–449. doi: 10.1016/j.cld.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 3.Zeilinger K, Holland G, Sauer IM, et al. Time course of primary liver cell reorganization in three-dimensional highdensity bioreactors for extracorporeal liver support: an immunohistochemical and ultrastructural study. Tissue Eng. 2004;10:1113–1124. doi: 10.1089/ten.2004.10.1113. [DOI] [PubMed] [Google Scholar]
- 4.Riccalton-Banks L, Liew C, Bhandari L, Fry J, Shakesheff K. Long-term culture of functional liver tissue: three-dimensional coculture of primary hepatocytes and stellate cells. Tissue Eng. 2003;9:401–410. doi: 10.1089/107632703322066589. [DOI] [PubMed] [Google Scholar]
- 5.Gomez-Lechon MJ, Jover R, Donato T, et al. Longterm expression of differentiated functions in hepatocyte cultured in three-dimensional collagen matrix. J Cell Physiol. 1998;177:553–562. doi: 10.1002/(SICI)1097-4652(199812)177:4<553::AID-JCP6>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 6.Linke K, Schanz J, Hansmann J, Walles T, Brunner H, Mertsching H. Engineered liver-like tissue on a capillarized matrix for applied research. Tissue Eng. 2007;13:2699–2707. doi: 10.1089/ten.2006.0388. [DOI] [PubMed] [Google Scholar]
- 7.Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, et al. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168–1170. doi: 10.1126/science.284.5417.1168. [DOI] [PubMed] [Google Scholar]
- 8.Shupe TD, Piscaglia AC, Oh SH, Gasbarrini A, Petersen BE. Isolation and characterization of hepatic stem cells, or “oval cells,” from rat livers. Methods Mol Biol. 2009;482:387–405. doi: 10.1007/978-1-59745-060-7_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michalopoulos GK, Bowen WC, Zajac VF, Beer-Stolz D, Watkins S, Kostrubsky V, Strom SC. Morphogenetic events in mixed cultures of rat hepatocytes and nonparenchymal cells maintained in biological matrices in the presence of hepatocyte growth factor and epidermal growth factor. Hepatology. 1999;29:90–100. doi: 10.1002/hep.510290149. [DOI] [PubMed] [Google Scholar]