Abstract
Anti-angiogenesis is a promising strategy for the treatment of cancer. Integrins, consisting of two noncovalently bound transmembrane α and β subunits, are an important molecular family involved in tumor angiogenesis. The blockade of integrin signaling has been demonstrated to be efficient to inhibit tumor growth, angiogenesis, and metastasis. Among all the integrins, αvβ3 seems to be the most important one during tumor angiogenesis. The inhibition of integrin αvβ3 signaling with antibodies, peptides, peptidomimetics, and other antagonists has great potential in the treatment of cancer. In addition, integrin αvβ3 is highly expressed on activated endothelial cells, new-born vessels as well as some tumor cells, but is not present in resting endothelial cells and most normal organ systems, making it a suitable target for anti-angiogenic therapy. In this article we will review the role of integrin αvβ3 in angiogenesis, present recent progress in the use of integrin αvβ3 antagonists and integrin-targeted delivery systems as potential cancer therapeutics, and discuss future perspectives.
Keywords: integrin αvβ3, angiogenesis, metastasis, anti-angiogenic therapy
INTRODUCTION
Despite positive initial responses to therapeutic drugs, many tumors become refractory to cytotoxic agents, leading to the failure of cancer treatment. Two major reasons have been found to be responsible for the therapeutic failures. First, the physiological barriers within the tumor impedes delivery of therapeutic agents at an effective concentration to tumor cells. Second, the drug resistance of the tumor reduces the effectiveness of available drugs [Jain, 2001]. To overcome or mitigate the problems, Folkman [1971] first introduced the concept that inhibition of angiogenesis (anti-angiogenesis) might be an effective strategy to treat human cancers. The intensive search for angiogenesis inducers and inhibitors has been ongoing ever since.
Tumor angiogenesis, or the sprouting of new vessels from preexisting vasculature, is well recognized as an essential mechanism for tumor growth and development of metastasis [Carmeliet and Jain, 2000; Folkman, 1995a, 2002]. Without the formation of neovasculature to provide oxygen and nutrients, tumors cannot grow beyond 1–2 mm in size [Folkman, 1995b; Sharma et al., 2001]. Once vascularized, previously dormant tumors begin to grow rapidly, invade surrounding tissues (invasion), and transfer to distant sites in the body (metastasis). The “angiogenic switch” depends on the balance between pro-angiogenic molecules such as vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), and epidermal growth factor (EGF), and anti-angiogenic molecules (e.g., angiostatin, endostatin, and thrombospondin) [Cai and Chen, 2006; Carmeliet and Jain, 2000].
As cancer cells become efficiently invasive and metastatic, partial degradation of the extracellular matrix (ECM) at the invasion front is required. Integrins, a family of cell adhesion molecules, are involved in a wide range of cell–ECM and cell–cell interactions [Brooks et al., 1994; Folkman, 2002; Jin and Varner, 2004; Kumar, 2003]. Although endothelial cells express many different integrins, αvβ3 appears to be the most important integrin for angiogenesis [Brooks et al., 1994; Kumar, 2003]. Integrin αvβ3 is highly expressed on activated endothelial cells and new-born vessels, but is absent in resting endothelial cells and most normal organ systems, making it a suitable target for anti-angiogenic cancer therapy. In addition, it is also expressed on some tumor cells, allowing for both tumor cell and tumor vasculature-targeted therapy. To date, numerous anti-angiogenic therapies based on integrin αvβ3 antagonism, including antibodies, peptides, small molecules, small interfering RNA (siRNA), combination therapy, and targeted delivery of anti-cancer agents, have been investigated. Our review will focus on the integrin αvβ3-targeted therapies of cancers, and address the most recent development.
INTEGRIN STRUCTURE AND SIGNALING
Integrins represent a subclass of cell adhesion molecules connecting the cytoskeleton with the extra-cellular matrix (ECM) or other cells. They consist of two genetically nonrelated subunits, α and β, which are noncovalently associated with each other. In mammals, there are 18 α and 8 β subunits capable of assembling at least 24 different functional heterodimers [Cai and Chen, 2006; Hynes, 2002] (Fig. 1). The alternative splicing of mRNA of some α- and β-subunits and posttranslational modifications of integrin subunits adds further diversity to the integrin family. Each individual integrin subunit consists of a large extra-cellular domain (~1,000 and ~750 residues), a single transmembrane domain, and a short cytoplasmic tail (~20 and ~50 residues, except for β4) [Alghisi and Raegg, 2006; Hood and Cheresh, 2002]. The assembled integrin heterodimer binds a specific set of endogenous ligands, including ligands in the ECM, soluble ligands, and ligands on other cell surfaces [Eble and Haier, 2006]. Upon ligand binding, the cytoplasmic tail contacts cytoskeletal filaments and proteins to initiate a signaling cascade, including a series of intracellular signaling events (both mechanical and chemical signals) that start from the recruitment and activation of Src kinases via phosphorylation of focal adhesion kinase (FAK) [Guo and Giancotti, 2004]. After signaling, the integrins play their roles not only in adhesion to ECM ligands or counter-receptors on adjacent cells, but also by initiating signaling that induces cell spreading, migration, survival, proliferation, and differentiation [Schwartz, 2001].
Fig. 1.
The integrin family: 24 heterodimers composed of 18 α and 8 β subunits.
INTEGRIN αvβ3 IN TUMOR ANGIOGENESIS
The integrin αvβ3, also known as the vitronectin receptor, consists of a 125-kDa αv subunit and a 105-kDa β3 subunit. Integrin αvβ3 binds a wide range of ECM molecules with an Arg-Gly-Asp (RGD) triple-peptide motif, including fibronectin, fibrinogen, von Willebrand factor, vitronectin, and proteolysed forms of collagen and laminin, whereas other integrins such as α5β1 can only selectively bind fibronectin [Hood and Cheresh, 2002; Hsu et al., 2007; Van der Flier and Sonnenberg, 2001]. Integrin αvβ3 has diverse roles in several distinct processes, such as osteoclast-mediated bone resorption, angiogenesis, and pathological neovascularization, and tumor metastasis [Wilder, 2002]. For angiogenesis, αvβ3 appears to be the most important one among all the integrins [Brooks et al., 1994; Kumar, 2003]. In addition to interacting with a number of ECM proteins, integrin αvβ3 has been shown to be associated with fibroblast growth factor-2 (FGF2), metalloproteinase MMP-2, activated PDGF, insulin, and VEGF receptors, facilitating the optimal activation of cell proliferation, invasion and preventing apoptosis [Kumar, 2003]. More importantly, the inhibition of αvβ3 integrin activity by monoclonal antibodies (mAbs), cyclic RGD peptide antagonists, and peptidomimetics has been shown to induce endothelial cell apoptosis [Meerovitch et al., 2003] and to inhibit angiogenesis [Kumar, 2003].
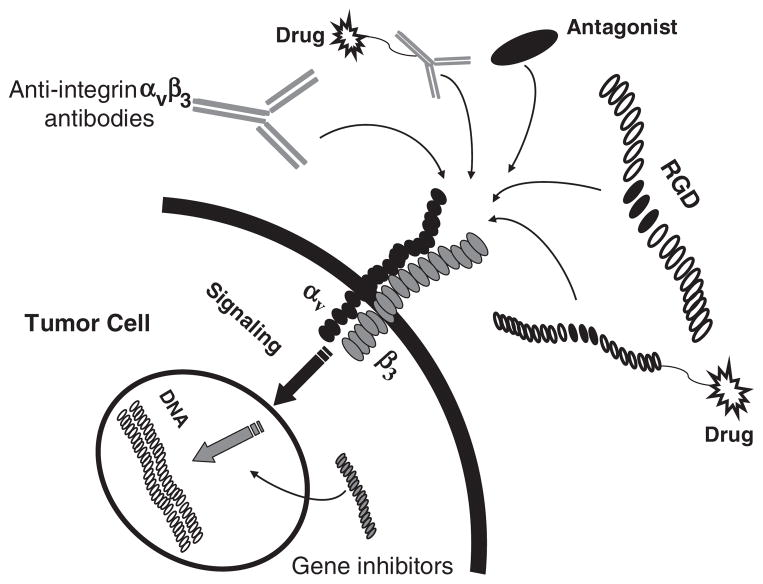
The crucial roles of integrin αvβ3 in tumor angiogenesis have led to a promising strategy to block its signaling by antagonists, as this would theoretically inhibit the tumor angiogenesis or enhance the efficacy of other tumor therapeutics. In addition, the high expression of integrin αvβ3 on tumor new-blood vessels and some tumor cells makes the integrin αvβ3 a suitable maker for cancer-targeted delivery of cytotoxic therapeutics (Fig. 2).
Fig. 2.
Various strategies to inhibit integrin αvβ3 expression (including direct signaling inhibition and targeted therapeutics).
INTEGRIN αvβ3 INHIBITORS
Antibodies
LM609/Avastin
LM609 (Avastin, bevacizumab, Genentech) is a mouse anti-human integrin αvβ3 mAb, which cross-reacts with αvβ3 originated from rabbits, chicken, and hamsters but not from rodents [Cheresh, 1987]. The primary anti-angiogenic mechanism of LM609 was found to be its blocking of bFGF and tumor necrosis factor-α (TNF-α) induced angiogenesis [Friedlander et al., 1995]. Compared with other mAbs, which recognizes either the αv or β3, LM609 is more specific and effective. Intravenous (i.v.) injection of LM609 was reportedly capable of effectively reducing tumor growth and angiogenesis in a severe combined immunodeficient (SCID) mouse/human chimeric model [Brooks et al., 1995].
Based on the positive preclinical results from LM609, several humanized versions of the mAb were developed to reduce the possible HAMA (human anti-mouse antibody) reaction of LM609 and optimize its serum half-life. The humanized versions of LM609 retained the antigen and epitope specificity of the original murine mAb, but not the cross-reaction with integrin αIIbβ3 or αvβ5. The binding affinity of the humanized antibodies was also improved after optimization of the complementarity determining regions (CDRs) LCDR1, HCDR1, and HCDR3. After conversion into human IgG1 using a mammalian expression vector, the humanized mAb Vitaxin I (MEDI-523) showed significant inhibition of tumor growth in a Kaposi’s sarcoma mouse model and partially blocked the binding of human immunodeficiency virus (HIV-1) Tat protein to integrin αvβ3 [Rader et al., 2002]. In the subsequent Phase I clinical trial [Gutheil et al., 2000], Vitaxin I has been proven safe for therapeutic use, however, it had limited efficacy in patients with advanced malignancies. The affinity of this antibody was further increased using stepwise maturation with phage expression libraries [Wu et al., 1998]. The resulting antibody was named Abegrin (Vitaxin II, MEDI-522), which was later licensed to MedImmune, Inc. (Gaithersburg, MD). In a phase I study [Patel et al., 2001], 15 patients received Abegrin for a period of 6 months. Although the toxicity profile of Abegrin was quite satisfactory, it failed to show evidence of antitumor activity. In another clinical trial [Posey et al., 2001], patients with metastatic cancer who failed standard therapy received intravenous doses of 10, 50, or 200 mg in cohorts of three patients; again, no significant toxicity or immune responses were observed in the three different dose levels, nor were any anti-tumor responses noted. One potential limitation of Abegrin is that it blocks only αvβ3-mediated angiogenesis, not the angiogenic pathways of other integrin receptors. Other αv integrins, such as αvβ5, can also mediate tumor angiogenesis, and there is evidence that αvβ3 and αvβ5 promote angiogenesis via distinct pathways: αvβ3 through bFGF and TNF-α, and αvβ5 through VEGF and TGF-α [Friedlander et al., 1995]. Therefore, the combination of two or more angiogenic pathway inhibitors may be more effective than any single blocking agents alone for anti-angiogenic cancer therapy [Mullamitha et al., 2007].
CNTO 95
CNTO 95 (Centocor) is a fully humanized antibody that recognizes multiple αv integrins. It binds to purified human αvβ3 and αvβ5 with high affinity (~200 pM) and to αv integrin expressing human cells with a Kd of 1–24 nM [Trikha et al., 2004], but does not bind to murine angiogenic integrins. In vitro, CNTO 95 inhibited human melanoma cell adhesion, migration and invasion [Chen et al., 2008; Trikha et al., 2004]. In a rat aortic ring sprouting assay, CNTO 95 completely inhibited sprouting in a dose of ~70 nM. In an in vivo nude mouse model, CNTO 95 inhibited growth of human melanoma tumors by ~80% with a dose of 10 mg/kg (3 times/week) [Trikha et al., 2004]. In a phase I clinical trial [Mullamitha et al., 2007], CNTO 95 was generally safe and well tolerated. Among the 24 enrolled patients, six patients received extended therapy, including one patient with a prolonged response. More importantly, a partial response was observed in a patient whose tumor did not express αvβ3 integrin but did express αvβ1 integrin, which may be evidence that CNTO 95 could potentially be more effective for tumor therapy than the single-integrin inhibitors, due to its multiple integrin inhibition property.
c7E3
The c7E3 Fab (abciximab; ReoPro; [Centocor]) and m7E3 (Fab′)2 are the mouse–human chimeric and murine mAb fragments of the parent intact murine mAb 7E3 [Trikha et al., 2002; Varner et al., 1999]. C7E3 Fab not only binds to αIIbβ3 receptor on platelets, which is the major receptor involved in platelet aggregation, but also binds with equivalent affinity to the integrin αvβ3 and, more importantly, it can redistribute between the two receptors in vitro [Tam et al., 1998]. The c7E3 Fab was approved for use as adjunct therapy for the prevention of cardiac ischemic complications in patients undergoing percutaneous coronary intervention [Cohen et al., 2000]. In an in vitro angiogenesis assay, c7E3 Fab inhibited αvβ3-mediated human umbilical vein endothelial (HUVEC) and melanoma cell adhesion, migration, and invasion, and bFGF stimulated proliferation of HUVECs [Trikha et al., 2002; Varner et al., 1999]. In an animal study, m7E3 F(ab′)2 partially inhibited the growth of human melanoma tumors in nude mice and completely blocked the human melanoma tumor formation and growth in nude rats [Alghisi and Raegg, 2006; Trikha et al., 2002]. Overall, the results suggest that the combined blockade of αIIbβ3 and αvβ3 integrins would produce greater anti-angiogenic and anti-tumor benefits than monospecific αvβ3 antagonists alone, given that tumor growth and angiogenesis involve multiple integrin receptors.
17E6
The 17E6 antibody, which was raised against purified human αvβ3, can react with at least αvβ3, αvβ5, and αvβ1 [Mitjans et al., 1995]. 17E6 can strongly perturb cell attachment mediated by αv integrins and inhibit cell attachment to αv ligands such as vitronectin and fibronectin. Using a panel of six human melanomas and five carcinomas, 17E6 efficiently blocked the in vivo tumor growth of integrin αvβ3-positive xenografts but did not affect the αvβ3-negative xenografts [Mitjans et al., 2000; Mitjans et al., 1995]. Given that mAb 17E6 also does not recognize murine αvβ3, the anti-tumor effect is likely due to the direct anti-tumor activity, and not to the anti-host vasculature activity in the mouse model. In a recent study, it was reported that 17E6 can inhibit the HIV-1 infection of primary macrophages, and the effect of 17E6 on HIV-1 BaL replication in acutely infected macrophages was dose-dependent, suggesting that the αv subunit of integrin may be a pharmacological target for HIV infection in macrophages [Bosch et al., 2006].
DISINTEGRIN, PEPTIDES, AND OTHER ANTAGONISTS
Besides antibodies, there are many other types of antagonists that specifically bind to integrin αvβ3, such as disintegrins, peptides (mostly RGD-based) and nonpeptidic molecules. Many reports have been published on cancer therapy involving these integrin αvβ3 antagonists, and some have advanced to clinical trials (Table 1).
TABLE 1.
Integrin αvβ3 inhibitors in clinical trials for cancer therapy
| Name | Other identity | Targeted integrins | Clinical status | References | |
|---|---|---|---|---|---|
| Antibodies | Abegrin | Vitaxin II, MEDI-522 | αvβ3 | Phase II | [Delbaldo et al., 2008; Patel et al., 2001; Posey et al., 2001] |
| CNTO95 | αvβ3, αvβ5 | Phase I | [Mullamitha et al., 2007] | ||
| abciximab | C7E3 | áIIbâ3,αvβ3, αMβ2 | Phase III | [Cohen et al., 2000] | |
| Peptides | Cilengitide | EMD 121974 | αvβ3, αvβ5 | Phase II | [Beekman et al., 2006; Colevas et al., 2004; Hariharan et al., 2007; MacDonald et al., 2008; Nabors et al., 2007; Raguse et al., 2004] |
| ATN-161 | Ac-PHSCN-NH2 | α5β1, αvβ3 | Phase II | [Cianfrocca et al., 2006; Donate et al., 2008] |
Disintegrin
Disintegrin, a family of low molecular weight (47–84 amino acids) RGD containing cysteine-rich peptides derived from viper venoms, can bind specifically to RGD-dependent integrins (such as αvβ3 and αIIbβ3) and block its function [Huang, 1998]. The first reported disintegrin was the snake venom protein trigramin [Huang et al., 1987]. Since then, many more members of this protein family have been discovered and isolated, such as kistrin [Dennis et al., 1990], bitistatin [Shebuski et al., 1989], barbourin [Scarborough et al., 1991], echistatin [Gan et al., 1988], and contortrostatin [Swenson et al., 2004]. Because of their high integrin binding affinities and their inhibition efficacy, several disintegrins and other integrin inhibiting snake venom components have been evaluated in pre-clinical tests for their capacity to inhibit tumor growth and progression both in vitro and in vivo [Calvete et al., 2003; McLane et al., 2001; Zhou et al., 2004]. However, as proteins they would also elicit an immunological reaction in the patient. In addition, because they are naturally occurring proteins and more prone to enzymatic degradation, their potential clinical applications may be significantly limited. Swenson et al. [2004] reported an effective method for delivery of the snake venom disintegrin contortrostatin (CN) using liposomal system, in an orthotopic xenograft model of human breast cancer in mice. The liposomal formulation of CN (LCN) not only displayed a prolonged circulatory half-life, but also showed no platelet reactivity and no immune system response. Minea et al. [2005] also described a method amenable to large-scale production of a soluble form of recombinant CN (rCN) directly in the cytoplasm of an engineered bacterial system, and the liposomal formulation of the rCN demonstrated potent anti-tumor and anti-angiogenic activities in the MDA-MB-435 human breast cancer xenograft model. Therefore, modification and optimization of disintegrin are typically needed to make them more suitable for clinical translation.
Peptides
Synthetic peptide-based integrin inhibitors that mimic the structure of the natural ligands of integrins are another of the major aims in the development of integrin-targeting pharmaceuticals. Among the integrin inhibitors, the RGD mimetics are the best known. The RGD triple-peptide, however, is limited in vivo use because of its short circulation half-life. Fortunately, conformational restriction by ring closure of the peptides and further chemical modification, including the use of D-amino acids such as the c(RGDfV) (with f standing for D-phenylalanine) compound, not only could increase their αvβ3 binding affinity but also their bioavailability [Eble and Haier, 2006]. A head-to-tail cyclized RGD-containing pentapeptide, c(RGDf[NMe]V) (also known as Cilengitide or EMD 12, 1974, Merck KGaA, Darmstadt, Germany; Fig. 3), can bind to integrin αvβ3 (IC50 = 2.3 nM) and αvβ5 (IC50 = 37 nM) with high affinity [Ruoslahti and Pierschbacher, 1986]. Cilengitide has shown positive antiangiogenic effects in vitro [Nisato et al., 2003] and antitumor effects against melanoma, as well as head and neck cancer, breast cancer and brain tumor [Burke et al., 2002; Raguse et al., 2004; Smith, 2003; Taga et al., 2002]. Results of a phase I trial in 51 patients with recurrent malignant glioma demonstrated well tolerated doses of 2,400 mg/m2, and stable disease was observed in four patients [Nabors et al., 2007]. Cilengitide is currently in phase II clinical trial for the treatment of highly vascularized head and neck tumors [Raguse et al., 2004].
Fig. 3.
Schematic structures of a few representative integrin αvβ3 antagonists.
Peptidomimetics and Others
There is another class of integrin antagonists named peptidomimetics made either from chemical synthesis or screening from peptidomimetic library. Peptidomimetics are compounds containing nonpeptidic structural elements that are capable of mimicking the biological actions of a natural parent peptide. The important advantages of peptidomimetics are their ability to be amplified and screened from peptidomimetics libraries to identify the most potent antagonists. Also, because peptidomimetics lack peptide characteristics such as enzymatically scissile peptidic bonds, they are less sensitive to protease-mediated degradation and more suitable for oral availability.
Monsanto (St. Louis, MO) reported an orally bioavailable compound SC-68448 (Fig. 3), which was 100-fold more potent as a functional inhibitor of αvβ3 than αIIbβ3 [Carron et al., 2000]. In a preclinical model, SC-68448 inhibited Leydig tumor growth in a dose-dependent manner by up to 80% and completely blocked the development of hypercalcemia [Carron et al., 1998]. SCH221153 (Fig. 3), a compound screened from an RGD-based peptidomimetic library, is a dual inhibitor targeting both the αvβ3 and αvβ5 integrins. It showed high affinity to αvβ3 (IC50 = 3.2 nM) and αvβ5 (IC50 = 1.7 nM), and lower affinity to αIIbβ3 (IC50 = 1294 nM) and α5β1 (IC50 = 421 nM), and is thus highly selective for both of the two αv-dependent integrins [Kumar et al., 2001]. Dual antagonists of both αvβ3 and αvβ5 may have enhanced therapeutic advantages, because integrin αvβ5 also plays a critical role in angiogenesis [Friedlander et al., 1995].
GENE INHIBITORS AND OTHERS
The introduction of small interfering RNA (siRNA) has brought a new approach to the silencing of gene expression, with profound implications for the intervention of human diseases including cancer [Fuchs and Borkhardt, 2007; Lu and Woodle, 2008]. The siRNA oligonucleotides are associated with a multicomponent nuclease RNA-induced silencing complex, which targets and degrades mRNA complementary to the siRNA base sequence. It was reported that siRNA oligonucleotides targeted either the α or β subunit of the α6β4 integrin reduced cell surface expression of this integrin and reduced the invasion of MDA-MB-231 breast carcinoma cells, indicating that the siRNA silencing of integrin α6β4 could be effective in preventing carcinoma cell progression [Lipscomb et al., 2003]. We recently found that silencing αv integrin expression by siRNA can inhibit proliferation and induce apoptosis in MDA-MB-435 human breast cancer cells. Importantly, effective reduction of αv integrin expression with siRNA can significantly increase the radiosensitivity of tumor cells [Cao et al., 2008].
Recently, several groups have demonstrated that siRNA can also inhibit gene expression in vivo. For example, annealed 21-nucleotide siRNA for luciferase was administered into mice, in combination with a luciferase expression vector. At 72 h later, after giving D-luciferin intraperitoneally, mice receiving luciferase siRNA emitted significantly less light than reporter alone control; that is, siRNA significantly reduced luciferase expression in adult mice [McCaffrey et al., 2002]. The successful applications of siRNA in vivo indicate that RNAi may be a viable approach to treat human diseases including cancer, and siRNA against integrin αvβ3 may have a bright future as it takes effect by eliminating the source directly, rather than merely blocking its biological function.
COMBINATION THERAPIES
It is conceivable that to be effective clinically, integrin antagonists will have to be used in combination with other antiangiogenic agents or standard anticancer regimens such as chemotherapy or radiotherapy. However, it is unclear which combinations will prove most effective. Previously, combining the anti-angiogenic therapy with other therapeutic modalities such as chemotherapy was considered paradoxical, because anti-angiogenic therapy was assumed to “starve” the tumor cells by destroying the tumor vasculature, thereby depriving the tumor of oxygen and nutrients, whereas chemotherapy needs the blood supply to deliver the drugs inside of the tumor. In recent years, there is emerging evidence to support an alternative hypothesis that certain anti-angiogenic agents can transiently normalize the abnormal structure and function of tumor blood vessel, leading to more efficient delivery of therapeutics and nutrients [Jain, 2001, 2005] (Fig. 4).
Fig. 4.

Scheme of changes in tumor vasculature during the course of anti-angiogenic therapy. Adapted with permission from ref. [Jain, 2005]. [Color figure can be viewed in the online version]
It has been reported in an experimental breast cancer mouse model that the administration of Cilengitide resulted in increased efficacy (53%) of radioimmunotherapy using yttrium-90 labeled antitumor antibody ChL6 as compared with mice treated with 90Y-ChL6 peptide only [Burke et al., 2002]. Abdollahi et al. [2005] recently showed that the combined treatment with S247, an RGD peptidomimetic antagonist of αvβ3 integrin (Fig. 3), and external beam radiotherapy, enhanced antiangiogenic and anti-tumor effects in glioma (U87MG), epidermoid (A431), and prostate cancer (PC3) xenograft models in nude mice. Other researchers also reported the successful combination of integrin αvβ3 antagonists with chemotherapies for tumor therapy, such as Cilengitide combined with anti-cancer drug gemcitabine for head and neck cancer therapy [Colomer, 2004]. After being treated with Cilengitide on day 1 and 4 in combination with gemcitabine administered days 1 and 8 every 3 weeks for five months, the patient could eat and smell better and remained stable for 12 months on Cilengitide maintenance therapy, with no tendency towards spontaneous bleeding. Abegrin was combined with dacarbazine (DTIC) for a Phase II study involving 112 patients with stage IV metastatic melanoma [Hersey, 2005]. The combined treatment had a 9.4-month median survival period, slightly longer than patients treated with DTIC alone, the current standard of care in advanced melanoma (7.9-month median survival time).
With the wider acceptance of the tumor vasculature normalization concept, more studies on combination therapy are expected to be initiated. The critical challenge will be to define the best combinations in term of drug association, timing, and schedule. In addition, combining the integrin inhibition agents with other antiangiogenic drugs is another highly promising approach. For instance, the combination of EMD270179 (cRGDf-ACHA, ACHA = α-amino cyclohexyl carboxylic acid), a cyclic RGD based integrin αv inhibitor, and SU5416 (a VEGFR-2 kinase inhibitor), showed significantly stronger inhibition of subcutaneous tumor growth and metastasis formation [Strieth et al., 2006]. As a result of the crosstalk and interactions of the different angiogenic molecules such as integrins, VEGF, FGF, EGFR, it will be important to test whether the inhibition of integrin may sensitize the tumor cells to other antiangiogenic drugs.
INTEGRIN αvβ3-TARGETED DRUG DELIVERY
To achieve an efficient concentration of therapeutics in the tumor, high dose of drugs are typically needed; this can lead to higher system toxicity in patients. For instance, targeted therapy using the specific binding ligand may significantly reduce the drug dose and hence the toxicity risk. In particular, overexpression of integrin αvβ3 on angiogenic tumor vasculature and certain tumor cells makes it an excellent vehicle for delivery of cancer therapeutics such as chemodugs, radioisotopes, and gene inhibitors.
We recently evaluated the antitumor activity of paclitaxel conjugated with a dimeric RGD peptide E[c(RGDyK)]2 (RGD2) [Cao et al., 2008; Minea et al., 2005]. Paclitaxel (PTX), a prototype of the taxane family of antitumor drugs, is now commonly used in the treatment of advanced metastatic breast cancer. The RGD2-PTX conjugate inhibited cell proliferation with activity comparable to that observed for paclitaxel, both of which are mediated by an arrest of G2/M phase of the cell cycle followed by apoptosis. In addition, when RGD2-PTX was labeled with 125I through the tyrosine residue on the RGD peptide, integrin-specific accumulation of 125I-RGD2-PTX in orthotopic MDA-MB-435 tumor was observed. In a followup study, we evaluated the in vivo anti-tumor effect of the RGD2-PTX conjugate [Cao et al., 2008]. The treatment efficacy of RGD2-PTX was confirmed by size measurement, in vivo PET imaging, and ex vivo histopathology. The tumor growth delay is related to tumor proliferation rather than tumor metabolism, as confirmed by 18F-FDG/PET and 18F-FLT/PET.
Burkhart et al. [2004] designed two RGD-based (RGD-4C and Cilengitide) conjugates with the prodrug of doxorubicin, another effective chemotherapeutic for the treatment of a variety of solid tumors including breast tumors. Both conjugates maintained a high affinity for αvβ3 (IC50 = 10 nM and 5 nM, respectively). The in vitro cytotoxicity studies revealed good growth inhibition of MDA-MB-435 breast cancer cells, with the IC50 at 50 nM and 90 nM for the two conjugates, respectively. No in vivo anti-tumor efficacy of the two conjugates was reported.
Tumor necrosis factor α (TNF-α) is a multi-functional cytokine playing a key role in apoptosis and cell survival, as well as in inflammation and immunity. TNF-α has been fused with peptides containing the NGR or the RGD motif by recombinant DNA technology to enhance the anti-tumor effect and reduce the system damage [Bertilaccio et al., 2008; Corti and Ponzoni, 2004; Sacchi et al., 2004; Van Laarhoven et al., 2006; Zarovni et al., 2004]. We recently developed an RGD4C-TNF fusion protein for tumor-specific delivery of TNF [Wang et al., 2008]. RGD4C-TNF was significantly more potent than TNF in inhibiting orthotopic MDA-MB-435 tumor growth. Intramuscular administration of plasmid DNA encoding RGD-4C-TNF also was found to inhibit the growth of melanomas and lymphomas implanted at sites distant from the plasmid injection site [Zarovni et al., 2004]. Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) is an apoptosis-inducing member of the TNF gene family, which acts as a homotrimer interacting with five cognate receptors: TRAIL-R1, TRAIL-R2, TRAIL-R3, TRAIL-R4, and osteoprotegerin (OPG). Unlike the other TNF family members TNF-α and CD95L, TRAIL has the unique ability to elicit apoptotic cell death in a variety of cancer cells, with minimal cytoxicity for normal cells and tissues. Cao et al. [Cao et al., 2008] reported that the fusion of TRAIL to the RGD-based peptide ACDCRGDCFC led to enhanced apoptosis inducing activity (caspase-3 and caspase-8 activation) in αvβ3 and αvβ5 integrin-positive cancer cells.
Integrin αvβ3-targeted delivery of radionuclide has also been reported. 90Y and 177Lu complexes (90Y-TA138 and 177Lu-TA138) of a DOTA-conjugated non-peptide integrin αvβ3 antagonist TA138 were prepared. 90Y-TA138 in the mouse model demonstrated a slowing of tumor growth at a dose of 15 mCi/m2, and a regression of tumors at a dose of 90 mCi/m2 [Harris et al., 2003]. Janssen et al. [2002] also reported 111In, 99mTc, and 90Y-labeled cyclic RGD dimer for tumor integrin-targeted imaging and therapy. Injection of 90Y-DOTA-E-[c(RGDfK)]2 induced a significant delay in tumor growth. Compared with RGD-based peptides, antibodies seem to be more efficient for tumor radioimmunotherapy (RIT) due to greater tumor uptake and longer tumor residence time. We have evaluated 90Y-labeled Abegrin in a U87MG glioblastoma xenograft model. 90Y-Abegrin showed partial tumor regression with a final fractional tumor volume (Vfinal/Vinitial) of 0.69, as compared with that of 3.76 for 90Y-hIgG and 5.43 for normal Abegrin controls, respectively [Veeravagu et al., in press].
Hood et al. [2002] reported the feasibility of selectively deliver inga gene of interest to angiogenic blood vessels in tumor-bearing mice by using a cationic nanoparticle (NP) coupled to an integrin αvβ3-targeting ligand. The therapeutic efficacy of this approach was tested by generating nanoparticles conjugated to a mutant Raf gene, which blocks endothelial signaling pathway and angiogenesis in response to multiple growth factors. Systemic injection of the nanoparticles into mice induced the apoptosis of the tumor-associated endothelium, ultimately leading to tumor cell apoptosis and sustained regression of established primary and metastatic tumors. Another integrin αvβ3-targeted gene delivery system was developed for siRNA delivery [Schiffelers et al., 2004]. Self-assembling nanoparticles with siRNA were constructed with polyethyleneimine (PEI) that is PEGylated with RGD peptide attached at the distal end of the polyethylene glycol (PEG). They were designed to target tumor neovasculature expressing integrins and deliver siRNA that could inhibit vascular endothelial growth factor receptor-2 (VEGFR2) expression and thereby tumor angiogenesis. Intravenous administration of the RGD-PEG-PEI/siRNA complex into tumor-bearing mice exhibited selective tumor uptake, siRNA sequence-specific inhibition of protein expression within the tumor, and inhibition of both tumor angiogenesis and growth rate. These experiments demonstrate the feasibility of tumor-targeted delivery of genes by exploiting the ability of integrins to limit intracellular uptake and reduce the blood stability of gene inhibitors.
CONCLUSIONS AND PERSPECTIVES
A growing body of preclinical and clinical evidence indicates that integrin αvβ3 is a promising target for antiangiogenic therapy. We summarized here the integrin αvβ3-targeted therapeutic approaches such as the direct signaling inhibition using antibodies, peptides, peptidomimetics, and other antagonists, as well as indirect tumor-targeted drug delivery systems.
Since integrin αvβ3 is not the only molecule involved in tumor angiogenesis, the combined inhibition of integrin αvβ3 with other angiogenic molecules such as VEGF, FGF, EGF, and other integrins is expected to generate significantly greater anti-angiogenic and anti-tumor benefits over monospecific αvβ3 antagonists. Additionally, the inhibition of integrin αvβ3 combined with other therapeutic modalities, such as chemotherapy and radiotherapy, should be much more effective than using integrin αvβ3 alone. However, the specific synergistic interactions among different modalities should be investigated and clearly understood first to make possible the personalized treatment protocols that will likely become the future of medicine.
Gene therapy is a fundamental way to treat cancer in so much as cancer is a genetic disease. The targeted delivery of siRNA and other gene inhibitors is and will continue to be a vitally important research area for anti-angiogenic cancer therapy in the future. To that end, novel integrin antagonists with high selectivity and affinity produced by chemical synthesis or biological production must be developed and evaluated, moving from preclinical studies to clinical trials. To advance the field, researchers must redouble their efforts to create more tailored agents with improved pharmacokinetics and pharmacodynamics, as well as establishing suitable routes of administration (e.g., oral available) for clinical applications.
Acknowledgments
Grant sponsor: National Cancer Institute; Grant number: P50 CA114747; R24 CA93862; R01 CA1; 19053; R21 CA121842; U54 CA1; 19367.
Z. Liu acknowledges the China Scholarship Council (CSC) for partial financial support during his visit to Stanford University.
References
- Abdollahi A, Griggs DW, Zieher H, Roth A, Lipson KE, Saffrich R, Gröne H-J, Hallahan DE, Reisfeld RA, Debus J, Niethammaer AG, Huber PE. Inhibition of alphavbeta3 integrin survival signaling enhances antiangiogenic and antitumor effects of radiotherapy. Clin Cancer Res. 2005;11:6270–6279. doi: 10.1158/1078-0432.CCR-04-1223. [DOI] [PubMed] [Google Scholar]
- Alghisi GC, Raegg C. Vascular integrins in tumor angiogenesis: mediators and therapeutic targets. Endothelium. 2006;13:113–135. doi: 10.1080/10623320600698037. [DOI] [PubMed] [Google Scholar]
- Beekman KW, Colevas AD, Cooney K, Dipaola R, Dunn RL, Gross M, Keller ET, Pienta KJ, Ryan CJ, Smith D, Hussain M. Phase II evaluations of cilengitide in asymptomatic patients with androgen-independent prostate cancer: scientific rationale and study design. Clin Genitourin Cancer. 2006;4:299–302. doi: 10.3816/CGC.2006.n.012. [DOI] [PubMed] [Google Scholar]
- Bertilaccio MTS, Grioni M, Sutherland BW, Degl’innocenti E, Freschi M, Jachetti E, Greenberg NM, Corti A, Bellone M. Vasculature-targeted tumor necrosis factor-alpha increases the therapeutic index of doxorubicin against prostate cancer. Prostate. 2008;68:1105–1115. doi: 10.1002/pros.20775. [DOI] [PubMed] [Google Scholar]
- Bosch B, Clotet-Codina I, Blanco J, Pauls E, Coma G, Cedeno S, Mitjans F, Llano A, Bofill M, Clotet B, Piulats J, Este JA. Inhibition of human immunodeficiency virus type 1 infection in macrophages by an alpha-v integrin blocking antibody. Antiviral Res. 2006;69:173–180. doi: 10.1016/j.antiviral.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- Brooks PC, Strömblad S, Klemke R, Visscher D, Sarkar FH, Cheresh DA. Antiintegrin alpha v beta 3 blocks human breast cancer growth and angiogenesis in human skin. J Clin Invest. 1995;96:1815–1822. doi: 10.1172/JCI118227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke PA, DeNardo SJ, Miers LA, Lamborn KR, Matzku S, DeNardo GL. Cilengitide targeting of alphavbeta3 integrin receptor synergizes with radioimmunotherapy to increase efficacy and apoptosis in breast cancer xenografts. Cancer Res. 2002;62:4263–4272. [PubMed] [Google Scholar]
- Burkhart DJ, Kalet BT, Coleman MP, Post GC, Koch TH. Doxorubicin-formaldehyde conjugates targeting alphavbeta3 integrin. Mol Cancer Ther. 2004;3:1593–1604. [PubMed] [Google Scholar]
- Cai W, Chen X. Anti-angiogenic cancer therapy based on integrin alphavbeta3 antagonism. Anticancer Agents Med Chem. 2006;6:407–428. doi: 10.2174/187152006778226530. [DOI] [PubMed] [Google Scholar]
- Calvete JJ, Moreno-Murciano MP, Theakston RDG, Kisiel DG, Marcinkiewicz C. Snake venom disintegrins: novel dimeric disintegrins and structural diversification by disulphide bond engineering. Biochem J. 2003;372:725–734. doi: 10.1042/BJ20021739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Du P, Jiang S-H, Jin G-H, Huang Q-L, Hua Z-C. Enhancement of antitumor properties of TRAIL by targeted delivery to the tumor neovasculature. Mol Cancer Ther. 2008;7:851–858. doi: 10.1158/1535-7163.MCT-07-0533. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- Carron CP, Meyer DM, Engleman VW, Rico JG, Ruminski PG, Ornberg RL, Westlin WF, Nickols GA. Peptidomimetic antagonists of alphavbeta3 inhibit bone resorption by inhibiting osteoclast bone resorptive activity, not osteoclast adhesion to bone. J Endocrinol. 2000;165:587–598. doi: 10.1677/joe.0.1650587. [DOI] [PubMed] [Google Scholar]
- Carron CP, Meyer DM, Pegg JA, Engleman VW, Nickols MA, Settle SL, Westlin WF, Ruminski PG, Nickols GA. A peptidomimetic antagonist of the integrin alphavbeta3 inhibits Leydig cell tumor growth and the development of hypercalcemia of malignancy. Cancer Res. 1998;58:1930–1955. [PubMed] [Google Scholar]
- Chen Q, Manning CD, Millar H, McCabe FL, Ferrante C, Sharp C, Shahied-Arruda L, Doshi P, Nakada MT, Anderson GM. CNTO 95, a fully human anti alphav integrin antibody, inhibits cell signaling, migration, invasion, and spontaneous metastasis of human breast cancer cells. Clin Exp Metastasis. 2008;25:139–148. doi: 10.1007/s10585-007-9132-4. [DOI] [PubMed] [Google Scholar]
- Cheresh DA. Human endothelial cells synthesize and express an Arg-Gly-Asp-directed adhesion receptor involved in attachment to fibrinogen and von Willebrand factor. Proc Natl Acad Sci USA. 1987;84:6471–6475. doi: 10.1073/pnas.84.18.6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianfrocca ME, Kimmel KA, Gallo J, Cardoso T, Brown MM, Hudes G, Lewis N, Weiner L, Lam GN, Brown SC, Shaw DE, Mazar AP, Cohen RB. Phase 1 trial of the antiangiogenic peptide ATN-161 (Ac-PHSCN-NH(2)), a beta integrin antagonist, in patients with solid tumours. Br J Cancer. 2006;94:1621–1626. doi: 10.1038/sj.bjc.6603171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SA, Trikha M, Mascelli MA. Potential future clinical applications for the GPIIb/IIIa antagonist, abciximab in thrombosis, vascular and oncological indications. Pathol Oncol Res. 2000;6:163–174. doi: 10.1007/BF03032368. [DOI] [PubMed] [Google Scholar]
- Colevas AD, Scharf O, Schoenfeldt M. Clinical trials referral resource. Current clinical trials of cilengitide, an alphav antagonist in clinical development as an anticancer agent. Oncology. 2004;18:1778–1781. [PubMed] [Google Scholar]
- Colomer R. Gemcitabine and paclitaxel in metastatic breast cancer: a review. Oncology. 2004;18(Suppl 12):8–12. [PubMed] [Google Scholar]
- Corti A, Ponzoni M. Tumor vascular targeting with tumor necrosis factor alpha and chemotherapeutic drugs. Ann NY Acad Sci. 2004;1028:104–112. doi: 10.1196/annals.1322.011. [DOI] [PubMed] [Google Scholar]
- Delbaldo C, Raymond E, Vera K, Hammershaimb L, Kaucic K, Lozahic S, Marty M, Faivre S. Phase I and pharmacokinetic study of etaracizumab (Abegrin), a humanized monoclonal antibody against alphavbeta3 integrin receptor, in patients with advanced solid tumors. Invest New Drugs. 2008;26:35–43. doi: 10.1007/s10637-007-9077-0. [DOI] [PubMed] [Google Scholar]
- Dennis MS, Henzel WJ, Pitti RM, Lipari MT, Napier MA, Deisher TA, Bunting S, Lazarus RA. Platelet glycoprotein IIb-IIIa protein antagonists from snake venoms: evidence for a family of platelet-aggregation inhibitors. Proc Natl Acad Sci USA. 1990;87:2471–2475. doi: 10.1073/pnas.87.7.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donate F, Parry GC, Shaked Y, Hensley H, Guan X, Beck I, Tel-Tsur Z, Plunkett ML, Manuia M, Shaw DE, Kerbel RS, Mazar AP. Pharmacology of the novel antiangiogenic Peptide ATN-161 (Ac-PHSCN-NH2): observation of a u-shaped dose-response curve in several preclinical models of angiogenesis and tumor growth. Clin Cancer Res. 2008;14:2137–2144. doi: 10.1158/1078-0432.CCR-07-4530. [DOI] [PubMed] [Google Scholar]
- Eble JA, Haier J. Integrins in cancer treatment. Curr Cancer Drug Targets. 2006;6:89–105. doi: 10.2174/156800906776056518. [DOI] [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nature Med. 1995a;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- Folkman J. Seminars in Medicine of the Beth Israel Hospital, Boston. Clinical applications of research on angiogenesis. N Engl J Med. 1995b;333:1757–1763. doi: 10.1056/NEJM199512283332608. [DOI] [PubMed] [Google Scholar]
- Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29(Suppl 16):15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- Friedlander M, Brooks PC, Shaffer RW, Kincaid CM, Varner JA, Cheresh DA. Definition of two angiogenic pathways by distinct alpha v integrins. Science. 1995;270:1500–1502. doi: 10.1126/science.270.5241.1500. [DOI] [PubMed] [Google Scholar]
- Fuchs U, Borkhardt A. The application of siRNA technology to cancer biology discovery. Adv Cancer Res. 2007;96:75–102. doi: 10.1016/S0065-230X(06)96004-7. [DOI] [PubMed] [Google Scholar]
- Gan ZR, Gould RJ, Jacobs JW, Friedman PA, Polokoff MA. Echistatin. A potent platelet aggregation inhibitor from the venom of the viper, Echis carinatus. J Biol Chem. 1988;263:19827–19832. [PubMed] [Google Scholar]
- Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat Rev Mol Cell Biol. 2004;5:816–826. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- Gutheil JC, Campbell TN, Pierce PR, Watkins JD, Huse WD, Bodkin DJ, Cheresh DA. Targeted antiangiogenic therapy for cancer using Vitaxin: a humanized monoclonal antibody to the integrin alphavbeta3. Clin Cancer Res. 2000;6:3056–3061. [PubMed] [Google Scholar]
- Hariharan S, Gustafson D, Holden S, McConkey D, Davis D, Morrow M, Basche M, Gore L, Zang C, O’Bryant CL, Baron A, Gallemann D, Colevas D, Eckhardt SG. Assessment of the biological and pharmacological effects of the alpha nu beta3 and alpha nu beta5 integrin receptor antagonist, cilengitide (EMD 12, 1974), in patients with advanced solid tumors. Ann Oncol. 2007;18:1400–1406. doi: 10.1093/annonc/mdm140. [DOI] [PubMed] [Google Scholar]
- Harris TD, Kalogeropoulos S, Nguyen T, Liu S, Bartis J, Ellars C, Edwards S, Onthank D, Silva P, Yalamanchili P, Robinson S, Lazewatsky J, Barrett J, Bozarth J. Design, synthesis, and evaluation of radiolabeled integrin alpha v beta 3 receptor antagonists for tumor imaging and radiotherapy. Cancer Biother Radiopharm. 2003;18:627–641. doi: 10.1089/108497803322287727. [DOI] [PubMed] [Google Scholar]
- Hersey PS, O’Day S, Richards J, Bedikian A, Gonzalez R, Sharfman W, Weber R, Logan T, Lawson D, Zhang J, Hammershaimb L, Kirkwood JM. A Phase II, randomized, open-label study evaluating the antitumor activity of MEDI-522, a humanized monoclonal antibody directed against the human alpha v beta 3 (αvβ3) integrin, +/−dacarbazine (DTIC) in patients with metastatic melanoma (MM) J Immunother. 2005;28:643–644. [Google Scholar]
- Hood JD, Bednarski M, Frausto R, Guccione S, Reisfeld RA, Xiang R, Cheresh DA. Tumor regression by targeted gene delivery to the neovasculature. Science. 2002;296:2404–2407. doi: 10.1126/science.1070200. [DOI] [PubMed] [Google Scholar]
- Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2:91–100. doi: 10.1038/nrc727. [DOI] [PubMed] [Google Scholar]
- Hsu AR, Veeravagu A, Cai W, Hou LC, Tse V, Chen X. Integrin alphavbeta3 antagonists for anti-angiogenic cancer treatment. Recent Patents Anticancer Drug Discov. 2007;2:143–158. doi: 10.2174/157489207780832469. [DOI] [PubMed] [Google Scholar]
- Huang TF. What have snakes taught us about integrins? Cell Mol Life Sci. 1998;54:527–540. doi: 10.1007/s000180050181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TF, Holt JC, Lukasiewicz H, Niewiarowski S. Trigramin. A low molecular weight peptide inhibiting fibrinogen interaction with platelet receptors expressed on glycoprotein IIb–IIIa complex. J Biol Chem. 1987;262:16157–16163. [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med. 2001;7:987–989. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- Janssen ML, Oyen WJ, Dijkgraaf I, Massuger LF, Frielink C, Edwards DS, Rajopadhye M, Boonstra H, Corstens FH, Boerman OC. Tumor targeting with radiolabeled alphavbeta3 integrin binding peptides in a nude mouse model. Cancer Res. 2002;62:6146–6151. [PubMed] [Google Scholar]
- Jin H, Varner J. Integrins: roles in cancer development and as treatment targets. Br J Cancer. 2004;90:561–565. doi: 10.1038/sj.bjc.6601576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar CC. Integrin alpha v beta 3 as a therapeutic target for blocking tumor-induced angiogenesis. Curr Drug Targets. 2003;4:123–131. doi: 10.2174/1389450033346830. [DOI] [PubMed] [Google Scholar]
- Kumar CC, Malkowski M, Yin Z, Tanghetti E, Yaremko B, Nechuta T, Varner J, Liu M, Smith EM, Neustadt B, Presta M, Armstrong L. Inhibition of angiogenesis and tumor growth by SCH221153, a dual alphavbeta3 and alphavbeta5 integrin receptor antagonist. Cancer Res. 2001;61:2232–2238. [PubMed] [Google Scholar]
- Lipscomb EA, Dugan AS, Rabinovitz I, Mercurio AM. Use of RNA interference to inhibit integrin (alpha6beta4)-mediated invasion and migration of breast carcinoma cells. Clin Exp Metastasis. 2003;20:569–576. doi: 10.1023/a:1025819521707. [DOI] [PubMed] [Google Scholar]
- Lu PY, Woodle MC. Delivering small interfering RNA for novel therapeutics. Methods Mol Biol. 2008;437:93–107. doi: 10.1007/978-1-59745-210-6_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald TJ, Stewart CF, Kocak M, Goldman S, Ellenbogen RG, Phillips P, Lafond D, Poussaint TY, Kieran MW, Boyett JM, Kun LE. Phase I clinical trial of cilengitide in children with refractory brain tumors: Pediatric Brain Tumor Consortium Study PBTC-012. J Clin Oncol. 2008;26:919–924. doi: 10.1200/JCO.2007.14.1812. [DOI] [PubMed] [Google Scholar]
- McCaffrey AP, Meuse L, Pham T-TT, Conklin DS, Hannon GJ, Kay MA. RNA interference in adult mice. Nature. 2002;418:38–39. doi: 10.1038/418038a. [DOI] [PubMed] [Google Scholar]
- McLane MA, Kuchar MA, Brando C, Santoli D, Paquette-Straub CA, Miele ME. New insights on disintegrin-receptor interactions: eristostatin and melanoma cells. Haemostasis. 2001;31:177–182. doi: 10.1159/000048061. [DOI] [PubMed] [Google Scholar]
- Meerovitch K, Bergeron F, Leblond L, Grouix B, Poirier C, Bubenik M, Chan L, Gourdeau H, Bowlin T, Attardo G. A novel RGD antagonist that targets both alphavbeta3 and alpha5beta1 induces apoptosis of angiogenic endothelial cells on type I collagen. Vascul Pharmacol. 2003;40:77–89. doi: 10.1016/s1537-1891(02)00339-7. [DOI] [PubMed] [Google Scholar]
- Minea R, Swenson S, Costa F, Chen TC, Markland FS. Development of a novel recombinant disintegrin, contortrostatin, as an effective anti-tumor and anti-angiogenic agent. Pathophysiol Haemost Thromb. 2005;34:177–183. doi: 10.1159/000092419. [DOI] [PubMed] [Google Scholar]
- Mitjans F, Meyer T, Fittschen C, Goodman S, Jonczyk A, Marshall JF, Reyes G, Piulats J. In vivo therapy of malignant melanoma by means of antagonists of alphav integrins. Int J Cancer. 2000;87:716–723. [PubMed] [Google Scholar]
- Mitjans F, Sander D, Adán J, Sutter A, Martinez JM, Jäggle CS, Moyano JM, Kreysch HG, Piulats J, Goodman SL. An anti-alpha v-integrin antibody that blocks integrin function inhibits the development of a human melanoma in nude mice. J Cell Sci. 1995;108:2825–2838. doi: 10.1242/jcs.108.8.2825. [DOI] [PubMed] [Google Scholar]
- Mullamitha SA, Ton NC, Parker GJM, Jackson A, Julyan PJ, Roberts C, Buonaccorsi GA, Watson Y, Davies K, Cheung S, Hope L, Valle JW, Radford JA, Lawrance J, Saunders MP, Munteanu MC, Nakada MT, Nemeth JA, Davis HM, Kiao Q, Prabhakar U, Lang Z, Corringham RB, Beckman RA, Jayson GC. Phase I evaluation of a fully human anti-alphav integrin monoclonal antibody (CNTO 95) in patients with advanced solid tumors. Clin Cancer Res. 2007;13:2128–2135. doi: 10.1158/1078-0432.CCR-06-2779. [DOI] [PubMed] [Google Scholar]
- Nabors LB, Mikkelsen T, Rosenfeld SS, Hochberg F, Akella NS, Fisher JD, Cloud GA, Zhang Y, Carson K, Wittemer SM, Colevas AD, Grossman SA. Phase I and correlative biology study of cilengitide in patients with recurrent malignant glioma. J Clin Oncol. 2007;25:1651–1657. doi: 10.1200/JCO.2006.06.6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisato RE, Tille J-C, Jonczyk A, Goodman SL, Pepper MS. alphav beta 3 and alphav beta 5 integrin antagonists inhibit angiogenesis in vitro. Angiogenesis. 2003;6:105–119. doi: 10.1023/B:AGEN.0000011801.98187.f2. [DOI] [PubMed] [Google Scholar]
- Patel SR, Jenkins J, Papadopolous N, Burgess MA, Plager C, Gutterman J, Benjamin RS. Pilot study of vitaxin—an angiogenesis inhibitor-in patients with advanced leiomyosarcomas. Cancer. 2001;92:1347–1348. doi: 10.1002/1097-0142(20010901)92:5<1347::aid-cncr1456>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Posey JA, Khazaeli MB, DelGrosso A, Saleh MN, Lin CY, Huse W, LoBuglio AF. A pilot trial of Vitaxin, a humanized anti-vitronectin receptor (anti alpha v beta 3) antibody in patients with metastatic cancer. Cancer Biother Radiopharm. 2001;16:125–132. doi: 10.1089/108497801300189218. [DOI] [PubMed] [Google Scholar]
- Rader C, Popkov M, Neves JA, Barbas CF. Integrin alphavbeta3 targeted therapy for Kaposi’s sarcoma with an in vitro evolved antibody. FASEB J. 2002;16:2000–2002. doi: 10.1096/fj.02-0281fje. [DOI] [PubMed] [Google Scholar]
- Raguse J-D, Gath HJ, Bier J, Riess H, Oettle H. Cilengitide (EMD 12, 1974) arrests the growth of a heavily pretreated highly vascularised head and neck tumour. Oral Oncol. 2004;40:228–230. doi: 10.1016/j.oraloncology.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E, Pierschbacher MD. Arg-Gly-Asp: a versatile cell recognition signal. Cell. 1986;44:517–518. doi: 10.1016/0092-8674(86)90259-x. [DOI] [PubMed] [Google Scholar]
- Sacchi A, Gasparri A, Curnis F, Bellone M, Corti A. Crucial role for interferon gamma in the synergism between tumor vasculature-targeted tumor necrosis factor alpha (NGR-TNF) and doxorubicin. Cancer Res. 2004;64:7150–7155. doi: 10.1158/0008-5472.CAN-04-1445. [DOI] [PubMed] [Google Scholar]
- Scarborough RM, Rose JW, Hsu MA, Phillips DR, Fried VA, Campbell AM, Nannizzi L, Charo IF. Barbourin. A GPIIb-IIIa-specific integrin antagonist from the venom of Sistrurus m. barbouri. J Biol Chem. 1991;266:9359–9362. [PubMed] [Google Scholar]
- Schiffelers RM, Ansari A, Xu J, Zhou Q, Tang Q, Storm G, Molema G, Lu PY, Scaria PV, Woodle MC. Cancer siRNA therapy by tumor selective delivery with ligand-targeted sterically stabilized nanoparticle. Nucleic Acids Res. 2004;32:e149. doi: 10.1093/nar/gnh140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MA. Integrin signaling revisited. Trends Cell Biol. 2001;11:466–470. doi: 10.1016/s0962-8924(01)02152-3. [DOI] [PubMed] [Google Scholar]
- Sharma RA, Harris AL, Dalgleish AG, Steward WP, O’Byrne KJ. Angiogenesis as a biomarker and target in cancer chemoprevention. Lancet Oncol. 2001;2:726–732. doi: 10.1016/S1470-2045(01)00586-1. [DOI] [PubMed] [Google Scholar]
- Shebuski RJ, Ramjit DR, Bencen GH, Polokoff MA. Characterization and platelet inhibitory activity of bitistatin, a potent arginine-glycine-aspartic acid-containing peptide from the venom of the viper Bitis arietans. J Biol Chem. 1989;264:21550–21556. [PubMed] [Google Scholar]
- Smith JW. Cilengitide Merck. Curr Opin Invest Drugs. 2003;4:741–745. [PubMed] [Google Scholar]
- Strieth S, Eichhorn ME, Sutter A, Jonczyk A, Berghaus A, Dellian M. Antiangiogenic combination tumor therapy blocking alphav-integrins and VEGF-receptor-2 increases therapeutic effects in vivo. Int J Cancer. 2006;119:423–431. doi: 10.1002/ijc.21838. [DOI] [PubMed] [Google Scholar]
- Swenson S, Costa F, Minea R, Sherwin RP, Ernst W, Fujii G, Yang D, Markland FS. Intravenous liposomal delivery of the snake venom disintegrin contortrostatin limits breast cancer progression. Mol Cancer Ther. 2004;3:499–511. [PubMed] [Google Scholar]
- Taga T, Suzuki A, Gonzalez-Gomez I, Gilles FH, Stins M, Shimada H, Barsky L, Weinberg KI, Laug WE. alpha v-Integrin antagonist EMD 12, 1974 induces apoptosis in brain tumor cells growing on vitronectin and tenascin. Int J Cancer. 2002;98:690–697. doi: 10.1002/ijc.10265. [DOI] [PubMed] [Google Scholar]
- Tam SH, Sassoli PM, Jordan RE, Nakada MT. Abciximab (ReoPro, chimeric 7E3 Fab) demonstrates equivalent affinity and functional blockade of glycoprotein IIb/IIIa and alphavbeta3 integrins. Circulation. 1998;98:1085–1091. doi: 10.1161/01.cir.98.11.1085. [DOI] [PubMed] [Google Scholar]
- Trikha M, Zhou Z, Nemeth JA, Chen Q, Sharp C, Emmell E, Giles-Komar J, Nakada MT. CNTO 95, a fully human monoclonal antibody that inhibits alphav integrins, has antitumor and antiangiogenic activity in vivo. Int J Cancer. 2004;110:326–335. doi: 10.1002/ijc.20116. [DOI] [PubMed] [Google Scholar]
- Trikha M, Zhou Z, Timar J, Raso E, Kennel M, Emmell E, Nakada MT. Multiple roles for platelet GPIIb/IIIa and alphavbeta3 integrins in tumor growth, angiogenesis, and metastasis. Cancer Res. 2002;62:2824–2833. [PubMed] [Google Scholar]
- Van der Flier A, Sonnenberg A. Function and interactions of integrins. Cell Tissue Res. 2001;305:285–298. doi: 10.1007/s004410100417. [DOI] [PubMed] [Google Scholar]
- Van Laarhoven HWM, Gambarota G, Heerschap A, Lok J, Verhagen I, Corti A, Toma S, Gallo Stampino C, van der Kogel A, Punt CJA. Effects of the tumor vasculature targeting agent NGR-TNF on the tumor microenvironment in murine lymphomas. Invest New Drugs. 2006;24:27–36. doi: 10.1007/s10637-005-4540-2. [DOI] [PubMed] [Google Scholar]
- Varner JA, Nakada MT, Jordan RE, Coller BS. Inhibition of angiogenesis and tumor growth by murine 7E3, the parent antibody of c7E3 Fab (abciximab; ReoPro) Angiogenesis. 1999;3:53–60. doi: 10.1023/a:1009019223744. [DOI] [PubMed] [Google Scholar]
- Veeravagu A, Liu Z, Niu G, Chen K, Jia B, Cai W, Jin C, Hsu AR, Connolly AJ, Tse V, Wang IF, Chen X. Integrin alphavbeta3-targeted radioimmunotherapy of glioblastoma multiforme. Clin Cancer Res. 2008 doi: 10.1158/1078-0432.CCR-08-0797. (in press) [DOI] [PubMed] [Google Scholar]
- Wang H, Chen K, Cai W, Li Z, He L, Kashefi A, Chen X. Integrin-targeted imaging and therapy with RGD4C-TNF fusion protein. Mol Cancer Ther. 2008;7:1044–1053. doi: 10.1158/1535-7163.MCT-07-2084. [DOI] [PubMed] [Google Scholar]
- Wilder RL. Integrin alpha V beta 3 as a target for treatment of rheumatoid arthritis and related rheumatic diseases. Ann Rheum Dis. 2002;61(Suppl 2):96–99. doi: 10.1136/ard.61.suppl_2.ii96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Beuerlein G, Nie Y, Smith H, Lee BA, Hensler M, Huse WD, Watkins JD. Stepwise in vitro affinity maturation of Vitaxin, an alphav beta3-specific humanized mAb. Proc Natl Acad Sci USA. 1998;95:6037–6042. doi: 10.1073/pnas.95.11.6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarovni N, Monaco L, Corti A. Inhibition of tumor growth by intramuscular injection of cDNA encoding tumor necrosis factor alpha coupled to NGR and RGD tumor-homing peptides. Hum Gene Ther. 2004;15:373–382. doi: 10.1089/104303404322959524. [DOI] [PubMed] [Google Scholar]
- Zhou X-D, Jin Y, Chen R-Q, Lu Q-M, Wu J-B, Wang W-Y, Xiong Y-L. Purification, cloning and biological characterization of a novel disintegrin from Trimeresurus jerdonii venom. Toxicon. 2004;43:69–75. doi: 10.1016/j.toxicon.2003.10.023. [DOI] [PubMed] [Google Scholar]