Abstract
In our continuous interest to study the diversity of halogenated metabolites of Malaysian species of the red algal genus Laurencia, we examined the chemical composition of five populations of unrecorded Laurencia sp. A new brominated diterpene, 10-acetoxyangasiol (1), and four other known metabolites, aplysidiol (2), cupalaurenol (3), 1-methyl-2,3,5-tribromoindole (4), and chamigrane epoxide (5), were isolated and identified. Isolated metabolites exhibited potent antibacterial activities against clinical bacteria, Staphylococcus aureus, Staphylococcus sp., Streptococcus pyogenes, Salmonella sp. and Vibrio cholerae.
Keywords: Laurencia sp., halogenated metabolites, antibacterial activity
1. Introduction
Red algae of the genus Laurencia (Rhodomelaceae, Ceramiales) are known to be prolific sources of a wide variety of halogenated secondary metabolites, such as C15-acetogenins and C15-, C20-, and C30-terpenoids [1]. In the course of our chemical and biological investigation of Laurencia species from the coastal waters of Borneo (Malaysia), we reported the chemical composition of L. snackeyi (Weber-van Bosse) Masuda [2], L. similis Nam et Saito [3], and L. majuscula (Harvey) Lucas [4,5]. As part of the chemical analysis of the undescribed Laurencia species, we examined five populations of unrecorded Laurencia sp. collected from the coastal waters of North Borneo Island, Sabah. Each specimen contained one halogenated metabolite, a total of five halogenated metabolites were isolated and identified. These specimens yielded one new 10-acetoxyangasiol (1) and four known halogenated metabolites; aplysiadiol (2), cupalaurenol (3), 1-methyl-2,3,5-tribromoindole (4), and chamigrane epoxide (5). The structure of the new compound 10-acetoxyangasiol (1) was elucidated by spectral data. The structures of the known metabolites (2–5) were determined based on the comparison of spectral data to that of the published reports of Ojika et al. [6,7], Ichiba and Higa [8] and Carter and Rinehart [9]. In this paper, we describe the isolation and structure elucidation of these compounds and their antibacterial activities against clinical bacteria.
2. Results and Discussion
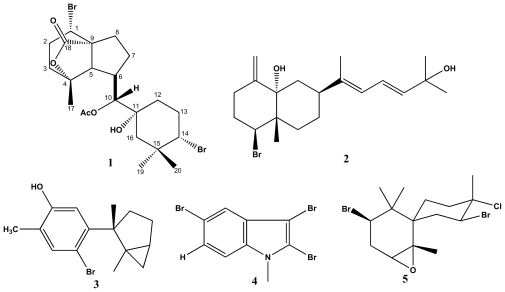
The partially dried specimens of algae Laurencia sp. were extracted in MeOH (1:1, v/v). The concentrated extracts were partitioned between H2O and EtOAc. The EtOAc soluble fraction was dehydrated over Na2SO4 anhydrous, filtered, concentrated and purified by a combination of silica gel column chromatography and High Performance Liquid Chromatography (HPLC) separation, to yield compounds 1–5 (Figure 1).
Figure 1.
The structure of compounds 1–5 from specimens of algae Laurencia sp.
Compound 1 was obtained as white powder, [α]25D +6.4 (c 0.9, CHCl3). The IR spectrum indicated the presence of OH group (3532 cm−1), γ-lactone carbonyl group (1773 cm−1) and acetoxy functionality (1734 and 1236 cm−1). The positive ESI-MS exhibited a characteristic molecular-ion cluster at m/z 535/537/539 in a ratio of 1:2:1, suggesting the presence of two bromine atoms. The molecular formula was determined to be C22H32Br2O5 by HR-ESI-TOFMS, indicating six degrees of unsaturation. The 13C NMR spectrum (Table 1) along with the DEPT experiments showed the presence of 22 carbons including four methyls, seven methylenes, five methines and six quaternary carbon atoms. In addition, the 1H and 13C NMR spectral data (Table 1) indicated the presence of a γ-lactone carbonyl group [δC 174.3 (s)] and an associated tertiary alkoxy group [δC 83.0 (s)], an acetoxy group [δC 169.4 (s), 20.2 (q); δH 1.58 (3H, s)], an acetoxymethine [δC 83.0 (d); δH 4.31 (1H, d, J = 8.9 Hz)], a tertiary alcohol [δC 73.7 (s)], two bromomethines [δC 64.4 (d); δH 3.52 (1H, dd, J = 13.1, 4.1 Hz) and δC 51.0 (d); δH 3.33 (1H, dd, J = 11.7, 6.2 Hz)] and three tertiary methyls [δH 1.25, 1.15 and 0.93 (each 3H, s)]. According to the molecular formula and the functionalities mentioned above, compound 1 is suggested to contain one γ-lactone and three carbocyclic rings. Furthermore, the 13C-NMR spectra of 1 closely resembled those of the known compound angasiol [10], except for the absence of one acetoxy group. It clearly suggested that 1 possesses the same skeleton and substituent.
Table 1.
1H-NMR and 13C-NMR spectral data of compound 1 (recorded at 600/150 MHz in CDCl3; δ in ppm, J in Hz).
| C | 13C (δ) | 1H (δ) | Multiplicity (J in Hz) |
|---|---|---|---|
| 1 | 51.0 | 3.33 | (dd, 11.7, 6.2) |
| 2 | 32.6 | 2.00 | m |
| 1.82 | m | ||
| 3 | 38.1 | 1.32–1.39 | m |
| 0.81 | (ddd, 13.7, 12.4, 5.5) | ||
| 4 | 83.0 | ||
| 5 | 60.1 | 1.34 | (d, 8.3) |
| 6 | 38.0 | 2.32 | (dddd, 11.0, 8.9, 8.3, 4.8) |
| 7 | 29.6 | 1.50–1.57 | m |
| 1.05 | (dddd, 14.5, 10.3, 4.8, 2.0) | ||
| 8 | 32.7 | 2.54 | (ddd, 13.1, 8.3, 2.0) |
| 1.43 | (ddd, 13.1, 10.3, 10.3) | ||
| 9 | 61.8 | ||
| 10 | 83.0 | 4.31 | (d, 8.9) |
| 11 | 73.7 | ||
| 12 | 34.3 | 0.90–0.95 | m |
| 0.56 | (ddd, 13.1, 13.1, 4.1) | ||
| 13 | 29.7 | 2.25 | (dddd, 13.1, 13.1, 13.1, 3.4) |
| 1.78 | (dddd, 13.1, 4.1, 4.1, 4.1) | ||
| 14 | 64.4 | 3.52 | (dd, 13.1, 4.1) |
| 15 | 36.1 | ||
| 16 | 47.2 | 1.29 | (dd, 13.7, 3.4) |
| 0.92 | (d, 13.7) | ||
| 17 | 21.9 | 1.15 | s |
| 18 | 174.3 | ||
| 19 | 22.7 | 1.25 | s |
| 20 | 32.3 | 0.93 | s |
| Ac | 169.4 | ||
| 20.2 | 1.58 | s |
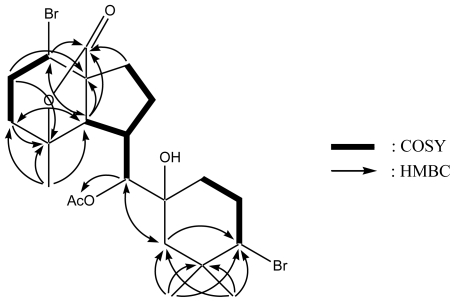
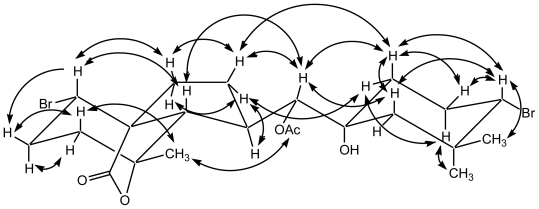
Assignments were carried out based on 1H-1H COSY, HSQC and HMBC spectra data. 1H-1H COSY experiments revealed the sequences of the correlations depicted by the bold lines in Figure 2. Key HMBC correlations as shown in Figure 2 were consistent with the proposed structure of 1. Furthermore, the relative stereochemistry of 1 was elucidated by NOESY experiments as well as the coupling constants in the 1H-NMR spectrum. The coupling constant (J6,10 = 8.9 Hz) indicated the anti conformation for the H-6/H-10. The configuration at C-10 was assigned as R* on the basis of the NOESY correlations observed between H-5/H-10, H-7β/H-10, H-10/Hax-12, H-10/Hax-16 and H3-17/H3-Ac. In addition, the remaining stereochemistry of 1 was determined by NOESY correlations as shown in Figure 3 and assigned to be the same as for angasiol and its related compounds, irieols A–G [10–12]. In consequence, the structure of 10-acetoxyangasiol (1) must be represented by structure 1.
Figure 2.
1H-1H COSY correlations (bold lines) and key HMBC correlations (H→C) of 1.
Figure 3.
NOESY correlations of 1.
All five metabolites were subjected to antibacterial bioassay against five species of clinical bacteria, and their antibacterial activities at 30 mg disc−1 are shown in Table 2. Compounds 1, 2 and 3 exhibited potent inhibition against three of the tested bacteria. The lowest MIC value was observed for compound 1 against Vibrio cholerae at 100 μg mL−1. Compounds 4 and 5 only exhibited weak inhibition against Staphylococcus sp. with a MIC value of 300 μg mL−1. Vancomycin, used as a positive control, exhibited a >23 mm inhibition zone against all the tested microbes at 30 mg disc−1. Based on our findings, these halogenated metabolites could be considered as possible candidates for further investigation against clinical microbes in our endeavor to combat the rise in antibiotic resistant microbes.
Table 2.
Diameter of inhibition zone (DIZ) and minimum inhibitory concentration (MIC) of halogenated metabolites from Laurencia sp against five bacteria.
| Tested Bacteria | Halogenated Metabolites |
||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| INHIBITION AT 30 mg/disc (mm) | |||||
| Staphylococcus aureus | 10 | 8 | 15 | - | - |
| Staphylococcus sp. | 12 | 10 | 15 | 9 | 9 |
| Streptococcus pyogenes | - | - | - | - | - |
| Salmonella sp. | - | 9 | 15 | - | - |
| Vibrio cholerae | 18 | - | 11 | - | - |
| MINIMUM INHIBITION CONCENTRATION (MIC) (μg/mL) | |||||
| Staphylococcus aureus | 250 | 200 | 125 | - | - |
| Staphylococcus sp. | 200 | 125 | 125 | 300 | 300 |
| Streptococcus pyogenes | - | - | - | - | - |
| Salmonella sp. | - | 250 | 125 | - | - |
| Vibrio cholerae | 100 | - | 200 | - | - |
Note: Bioassay were done in triplicate, SD <10%, and not shown.
3. Experimental Section
3.1. General
Optical rotations were measured on an AUTOPOL IV automatic polarimeter (Rudolph Research Analytical). 1H-NMR (600 MHz) and 13C-NMR (150 MHz) spectra were recorded with a JEOL ECA 600, with TMS as internal standard. HR-ESI-TOFMS spectrum was obtained with LCMS-IT-TOF (Shimadzu). Silica gel (Merck, Kieselgel 60, 70–230 mesh) was used for column chromatography. Separation of the eluted fraction were carried out using Shimadzu HPLC with Phenyl Hexyl (Phenomenex, USA) 10 × 250 mm eluted with 70% MeCN/H2O, and detected at 210 nm using SPD 20A Shimadzu UV-Vis detector. Analytical TLC was performed on Merck Kieselgel 60 F254. Spots were visualized by UV light or by spraying with a 5% phosphomolybdic acid-ethanol solution.
3.2. Algal material
Specimens of Laurencia sp. were collected from Lohok Butun (4°27’23”N, 118°41’12”E), Selakan Island (4°35’00”N, 118°42’04”E), Layangan Island (5°46’63”N, 115°53’39”E), and Gaya Island (6°00’47”N, 116°03’14”E). The specimens (voucher nos. 37604, 37610, 37665, 37767, 37774) are deposited in the Herbarium (BORH) of Institute for Tropical Biology and Conservation, Universiti Malaysia Sabah.
3.3. Extraction and isolation
The partially dried alga specimens (100 g) were extracted with MeOH. The MeOH solution was concentrated in vacuo and partitioned between EtOAc and H2O. The EtOAc fraction was washed with water, dried over anhydrous Na2SO4, and evaporated to leave dark green oil. The extract was then fractioned by Si gel column chromatography with a step gradient (hexane and EtOAc). The fractions eluted with hexanes-EtOAc (9:1) and hexanes-EtOAc (8:2) were further purified by HPLC to give 10-acetoxyangasiol (1) (2.4%) from specimen BORH 37610, aplysidiol (2) (3.2%) from specimen BORH 37665), cupalaurenol (3) (0.9%) from specimen BORH 37774, 1-methyl-2,3,5-tribromoindole (4) (2.8%) from specimen BORH 37767, and chamigrane epoxide (5) (3.6%) from specimen BORH 37774. Yield was calculated based on the extracted methanol crude.
3.4. Characterization of 10-acetoxyangasiol (1)
White powder; [α]25D +6.4 (c 0.9, CHCl3); HR-TOFMS m/z 535.0661 [M + H]+ (calculated for C22H33Br2O5, 535.0689); 1H-NMR and 13C-NMR spectral data: see Table 1.
3.5. Bioassay
The antibacterial bioassay for the isolated metabolite was carried out against 5 species of clinical pathogens obtained from Department of Pathology, Queen Elizabeth Hospital, Kota Kinabalu, Sabah, Malaysia. These bacteria are Staphylococcus aureus (UMS01-08), Staphylococcus sp. (UMS02-08), Streptococcus pyogenes(UMS03-08), Salmonella sp. (UMS04-08) and Vibrio cholerae(UMS05-08). One loopful of each organism was precultured in 20 mL of peptone water overnight. The turbidity of the culture was adjusted to an optical density (OD) of McFarland 0.5 [13]. Then 0.1 mL of the precultured bacterial suspension was used to seed Nutrient Agar plates. Paper discs (Whatman, 6 mm) impregnated with 30 mg disc−1 of the respective isolated compounds were placed on the seeded agar plates and the diameter of the inhibitory zones measured after incubation at 28 °C for 24 h. Antibacterial activity was evaluated by measuring the diameter of inhibition zone of the tested bacteria. Vancomycin (Sigma, Germany) was used as positive control. Minimum Inhibitory Concentration (MIC) determination for the positive inhibitions was carried out via microdilution broth method as described by Shan et al. (2008) with slight modifications [14].
4. Conclusions
This is our first report on the composition of halogenated metabolites and their activities in unrecorded Laurencia sp. from the coastal waters of North Borneo Island of Sabah, Malaysia. Our initiative pertaining to the isolation and identification of halogenated secondary metabolites from Borneon Laurencia continues to excite us, with the isolation of a wide diversity of structurally interesting metabolites. To date, we have isolated a total of 42 halogenated metabolites from L. snackeyii, L. majuscula, L. similis, L. nangii and these five species of undescribed Laurencia spp.
Acknowledgements
We are grateful to the Sabah Parks for the support and assistance during field survey. This study was funded by the “International Foundation for Science (IFS)” and Committee for the Prohibition of Chemical Weapon, Hague, The Netherlands (IFS), Grant No: AF/4286-2). The authors would also like to acknowledge Hiroshi Matsuura for his kind assistance during data comparison.
Footnotes
Samples Availability: Available from the authors.
References
- 1.Erickson KL. In: Marine Natural Products: Chemical and Biological Perspectives. Scheuer PJ, editor. V. Academic Press; New York, NY, USA: 1983. pp. 131–257. [Google Scholar]
- 2.Vairappan CS, Tan KL. Halogenated secondary metabolites from sea hare Aplysia dactylomela. Malaysian J. Sci. 2004;24:17–22. [Google Scholar]
- 3.Vairappan CS, Ang MY, Ong CY, Phang SM. Biologically active polybrominated indoles in the red algae Laurencia similis from the coastal waters of Sabah (Rhodomelaceae, Ceramiales) Malaysian J. Sci. 2004;23:119–126. [Google Scholar]
- 4.Vairappan CS. Potent antibacterial activity of Malaysian red algal halogenated metabolites against human pathogenic bacteria. Biomol. Eng. 2003;20:255–259. doi: 10.1016/s1389-0344(03)00067-4. [DOI] [PubMed] [Google Scholar]
- 5.Vairappan CS, Phang SM. Morphology and halochamigrene metabolite content of Laurencia majuscula (Rhodomelaceae, Ceramiales) from the Spratly Islands. Malaysian J. Sci. 2005;24(2):29–36. [Google Scholar]
- 6.Ojika M, Shizuri Y, Yamada K. A halogenated chamigrane epoxide and six related halogen containing sesquiterpenes from the red alga Laurencia okamurai. Phytochemistry. 1982;21:2410–2411. [Google Scholar]
- 7.Ojika M, Yoshida Y, Okumura M, Ieda S, Yamada K. Alysiadiol, a new brominated diterpene from the marine mollusc Aplysia kurodai. J. Nat. Prod. 1990;53:1619–1622. [Google Scholar]
- 8.Ichiba T, Higa T. New cuparene derived sesquiterpenes with unprecedented oxygenation patterns from the sea hare Aplysia dactylomela. J. Org. Chem. 1986;51:3364–3366. [Google Scholar]
- 9.Carter GT, Rinehart KL, Jr, Li LH, Kuentzel SL, Connor JL. Brominated indoles from Laurencia brongniartii. Tetrahedron Lett. 1978;46:4479–4482. [Google Scholar]
- 10.Pettit GR, Herald CL, Einck JJ, Vanell LD, Brown P, Gust D. Isolation and structure of angasiol. J. Org. Chem. 1978;43:4685–4686. [Google Scholar]
- 11.Howard BM, Fenical W. Structures of irieols, new dibromoditerpenoids of a unique skeletal class from the marine red alga Laurencia irieii. J. Org. Chem. 1978;43:4401–4408. [Google Scholar]
- 12.Ji NY, Li XM, Cui CM, Wang BG. Terpenes and polybromoindoles from the marine red alga Laurencia decumbens (Rhodomelaceae) Helv. Chim. Acta. 2007;90:1731–1736. [Google Scholar]
- 13.Vairappan CS, Suzuki M, Ishii T, Okino T, Abe T, Masuda M. Antibacterial activity of halogenated sesquiterpenes from Malaysian Laurencia spp. Phytochemistry. 2008;69:2490–2494. doi: 10.1016/j.phytochem.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 14.Shan B, Cai YZ, Brooks JD, Corke H. Antibacterial properties of Polygonum cuspidatum roots and their major bioactive constituents. Food Chem. 2008;109:503–537. [Google Scholar]