Abstract
Cyanobacteria of the genus Lyngbya have proven to be prodigious producers of secondary metabolites. Many of these compounds are bioactive and show potential for therapeutic use. This review covers peptides and hybrid polyketide-non-ribosomal peptides isolated from Lyngbya species. The structures and bioactivities of 50 Lyngbya peptides which were reported since 2007 are presented.
Keywords: Lyngbya, cyanobacteria, peptide, secondary metabolites, bioactivity
1. Introduction
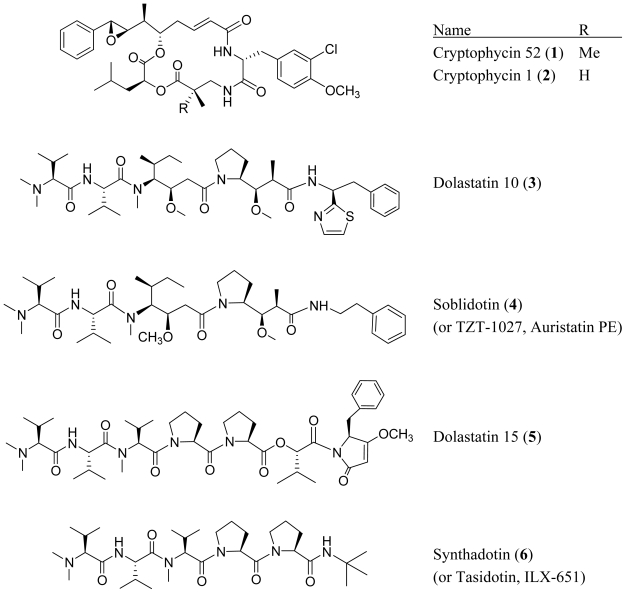
Cyanobacteria, also called blue-green algae, are ancient aquatic and photosynthetic prokaryotes. The oldest known fossils of cyanobacteria come from Archaean rocks of Western Australia, dated at 3.5 billion years [1]. Over 300 nitrogen-containing secondary metabolites from marine cyanobacteria have been reported in the literature [2]. It is possible that the ability to produce a wide range of defensive secondary metabolites has contributed to the high degree biological adaptation observed for cyanobacteria [3–6]. These secondary metabolites often enable cyanobacteria to compete effectively in a variety of environments, and many have been presented as lead compounds for further drug development. For instance, the synthetic analog cryptophycin 52 (1), which progressed to Phase II clinical trials for the treatment of patients with platinum-resistant ovarian cancer, is based on the cryptophycin 1 (2) which was isolated from terrestrial cyanobacteria [7,8]. Phase II clinical trials of dolastatin 10 failed to show significant anticancer activity. However, in vitro studies of soblidotin (or TZT-1027, auristatin PE) (4) a synthetic analog of dolastatin 10 (3), showed promising results against human colon adenocarcinomas and has progressed to phase II clinical trials [9,10]. Synthadotin (or ILX-651) (6), derived from dolastatin 15 (5) showed promising results in phase II clinical trials of inoperable, locally advanced or metastatic melanoma [9,11].
Cyanobacteria of toxicological or pharmacological significance include the genera Anabaena, Oscillatoria, Microcystis, Nodularia, Cylindrospermopsis and Lyngbya. Lyngbya sp. and Microcystis sp. are easily collected and cultured in the laboratory so that the isolation of compounds in the mg range is possible [12]. Lyngbya is a common and accessible genus of cyanobacteria, which is distributed worldwide throughout tropical and subtropical regions. The unbranched filaments of Lyngbya are cylindrical and usually wider than 6 μm. The straight, slightly wavy, or rarely coiled Lyngbya filaments usually form large, layered, leathery mats of varied thickness, and then form large benthic or surface blooms in freshwater and sea water. There are an increasing number of Lyngbya species which have been found to produce an impressive array of structurally varied compounds with diverse biological activities [4]. To date, the most important species of genus Lyngbya in terms of secondary metabolite production are L. majuscula, L. martensiana, L. aestuarii and L. wollei. Peptides or peptide containing substructures comprise a major group of cyanobacterial secondary metabolites. Many of these compounds were reviewed in 2006 [2]. This review covers new peptides reported from Lyngbya species after 2006, with an emphasis on their structures and biological activities. However, conflicting taxonomic identification of cyanobacteria, including the genus Lyngbya, is common [13]. In most instances, the taxonomic identification was made on the basis of morphological features. Less frequently, identification is made on the basis of rRNA gene sequences. Even when sequence data is available, existing databases are often not adequate for definitive identification even at the genus level. The majority of biological activities reported for the compounds fall into two major categories: cytotoxicity or protease inhibition.
2. Acyclic Peptides
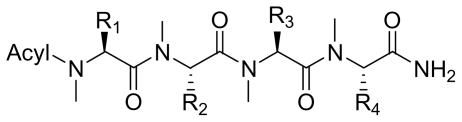
Six analogs of dragonamide A (7) [14], including carmabin A (8), dragomabin (9) and dragonamide B (10) [15], dragonamides C–D (11–12) [16] and E (13) [17], were isolated from the marine cyanobacteria Lyngbya majuscula and Lyngbya polychroa. This series possesses an 8 or 10-carbon long terminal alkynamide. To the best of our knowledge, carmabin A (8), dragomabin (9), and dragonamide A (7) were the only Lyngbya metabolites showing good antimalarial activity (IC50 = 4.3, 6.0, and 7.7 μM, respectively). Dragonamides A (7) and E (13) were also antileishmanial against Leishmania donovani with IC50 values of 6.5 and 5.1 μM. However, the nonaromatic analog, dragonamide B (10), was inactive against malaria or leishmaniasis. The lack of activity for dragonamide B (10) suggests that an aromatic amino acid at the carboxy terminus is necessary for antiparasitic activity in this series. Carmabin A (8) was more cytotoxic to Vero cells (IC50 = 9.8 μM) than dragomabin (5) (IC50 = 182.3 μM) or dragonamide A (7) (IC50 = 67.8 μM). Thus, dragomabin (9) possesses the best differential toxicity between parasite and mammalian cells. It appears that the longer and more branched alkynamide chain of carmabin A (8) leads to the increase in cytotoxicity over that of dragomabin (9). Dragonamides C–D (11–12) showed weak activity in cancer cell viability assays, with the 50% growth inhibition (GI50) values of 56 and 59 μM against U2OS osteosarcoma cells, 22 and 32 μM against HT29 colon adenocarcinoma cells, and 49 and 51 μM against IMR-32 neuroblastoma cells, respectively. These data are similar to cytotoxicity data reported for dragonamides A (7) and B (10) against other cell lines. The antiparasitic activity of dragonamides C–D (11–12) was not reported.
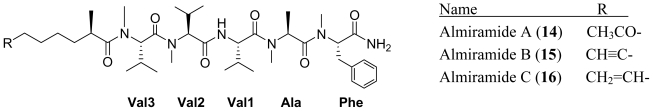
Screening of marine cyanobacteria from the Caribbean coast of Panama led to the identification of two antileishmanial lipopeptides, almiramides B–C (15–16) and their non-active analog, almiramide A (14) from Lyngbya majuscula [18]. Compared with the most closely related secondary metabolite dragonamide A (7), almiramides B–C (15–16) with an extra Ala residue, no methyl group on Val1 and the opposite configuration of the α-carbon of the lipophilic side chain, showed better antileishmanial potencies (IC50 = 2.4, and 1.9 μM, respectively), but no antimalarial activity up to 13.5 μM. The lack of antileishmanial activity for almiramide A (14) indicated that an unsaturated terminus on the lipophilic side chain played a critical role for antileishmanial activity in dragonamides and almiramides. Screening of a synthetic library of almiramide analogs with various modifications at the C- or N-terminus afforded several compounds with similar antileishmanial activity but improved selectivity.
 | |||||
|---|---|---|---|---|---|
| Name | R1 | R2 | R3 | R4 | Acyl |
| Dragonamide A (7) | i-Pr | i-Pr | i-Pr | PhCH2- | 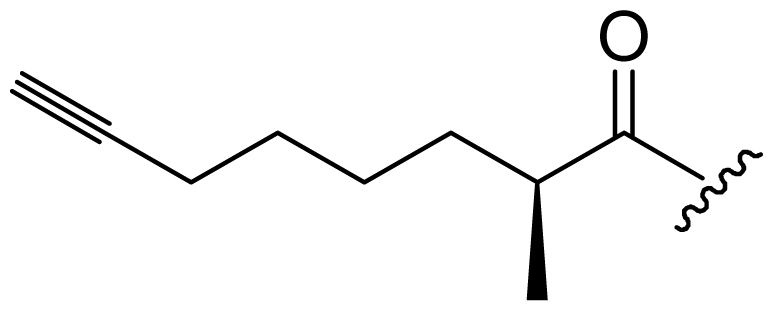 |
| Carmabin A (8) | Bn | Me | Me | 4-MeO-PhCH2- | 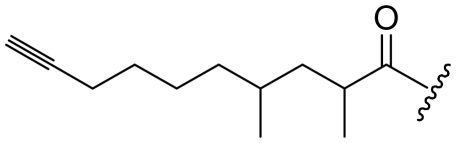 |
| Dragomabin (9) | Bn | Me | Me | 4-MeO-PhCH2- | 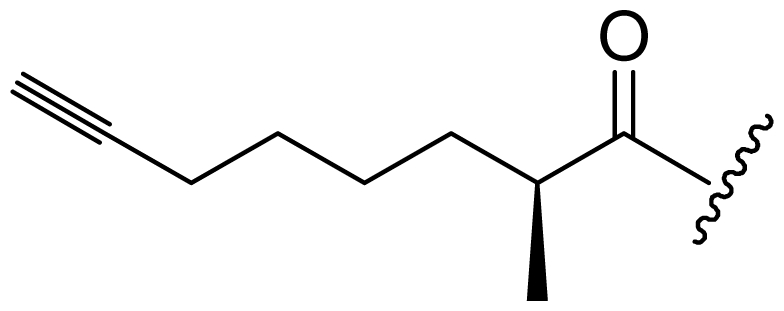 |
| Dragonamide B (10) | i-Pr | i-Pr | i-Pr | i-Pr | 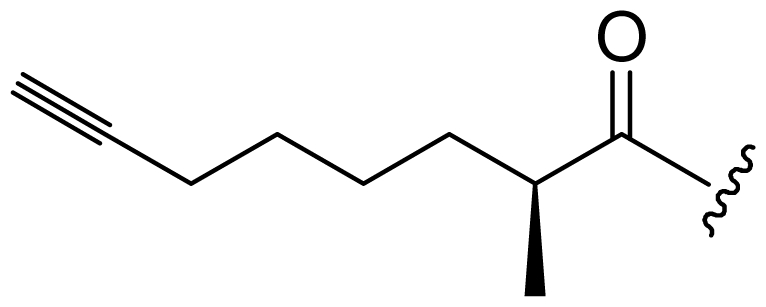 |
| Dragonamide C (11) | i-Pr | i-Pr | i-Pr | i-Pr | 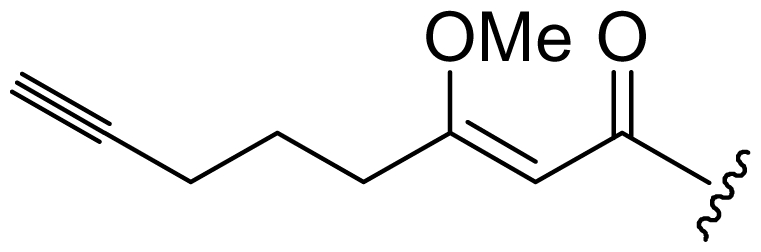 |
| Dragonamide D (12) | i-Pr | i-Pr | i-Pr | i-Pr | 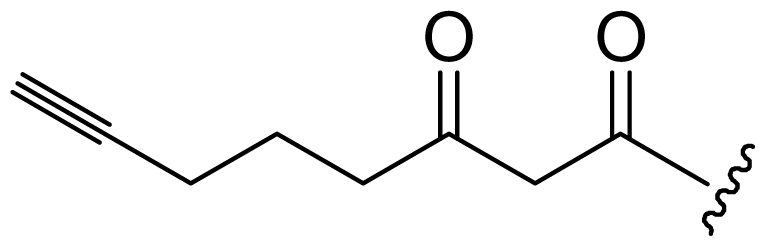 |
| Dragonamide E (13) | i-Pr | i-Pr | i-Pr | PhCH2- | 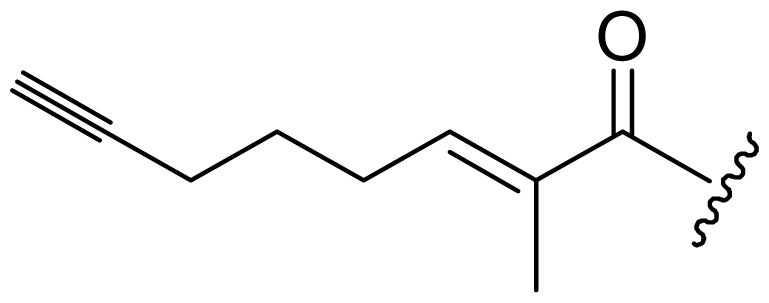 |
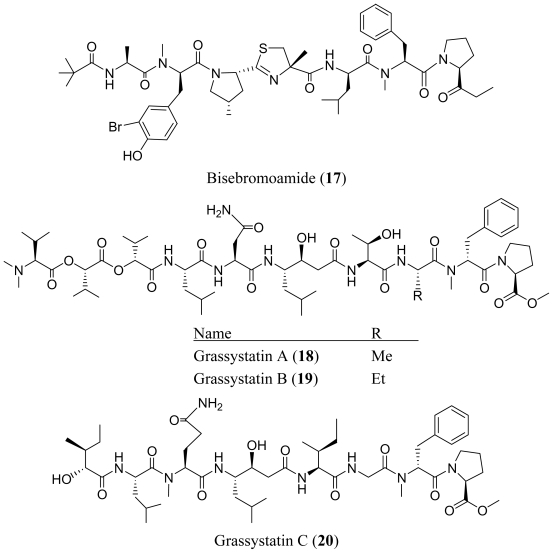
Efforts toward finding marine cyanobacterial metabolites with antitumor activity led to the isolation of bisebromoamide (17) from a Lyngbya sp. harvested in Okinawa Prefecture [19]. Bisebromoamide (17) is featured by four unusual structural units, the 2-substituted thiazoline-4-methyl-4-carboxylic acid unit fused to a methyl-proline, 2-(1-oxopropyl) pyrrolidine (Opp) residue, N-methyl-bromo-tyrosine and N-pivalamide moiety. Prior to the isolation of bisebromoamide (17), the Opp unit in had not been observed in any natural product. Bisebromoamide (17) showed cytotoxicity against HeLa S3 cells (IC50 = 0.04 μg/mL) and a panel of 39 human cancer cell lines (termed JFCR39) (the average GI50 = 40 nM). At 10 to 0.1 μM, bisebromoamide (17) selectively inhibited the phosphorylation of ERK (extracellular signal regulated protein kinase) in NRK (normal rat kidney) cells by PDGF (platelet-derived growth factor)-stimulation. However PKB (protein kinase B), PKD (protein kinase D), PLCγ1 (phospholipase Cγ1), or S6 ribosomal protein was not affected by bisebromoamide (17) at the same concentration range. Bisebromoamide (17) did not affect tubulin acylation as other tubulin modulators. It is possible that bisebromoamide (17) targets the ERK signal pathway which is activated in various cancers. Therefore, bisebromoamide (17) has potential as a lead for anticancer drugs.
Three statine unit (γ-amino-β-hydroxyacid)-containing linear peptides, termed grassystatins A–C (18–20), have been isolated from the marine cyanobacteria Lyngbya confervoides collected off Grassy Key in Florida [20]. Grassystatins A and B (18–19) showed similar potency and selectivity against cathepsin D (IC50 values of 26.5 nM and 7.27 nM, respectively) and E (IC50 values of 886 pM and 354 pM, respectively). The increased affinity for cathepsin E over cathepsin D may be interpreted by the interaction of the polar asparagine residue in grassystatins A and B (18–19) with glutamine-303 in cathepsin E, which corresponds to the nonpolar residue methionine-307 in cathepsin D. Grassystatin A (15) was observed to reduce antigen presentation by dendritic cells, a process thought to rely on cathepsin E. The truncated peptide analog grassystatin C (20), which consists of two fewer residues than grassystatins A and B (18–19), was less potent against both cathepsins D and E, but still selective for cathepsin E. The selectivity of grassystatins A–C (18–20) for cathepsin E over D (20–38-fold) suggests that these natural products may be useful tools to probe cathepsin E function. In addition, grassystatins A–C (18–20) were effective inhibitors of the metalloprotease TACE (tumor necrosis factor α converting enzyme) with IC50s of 1.23 μM, 2.23 μM and 28.6 μM respectively.
3. Cyclic Peptides
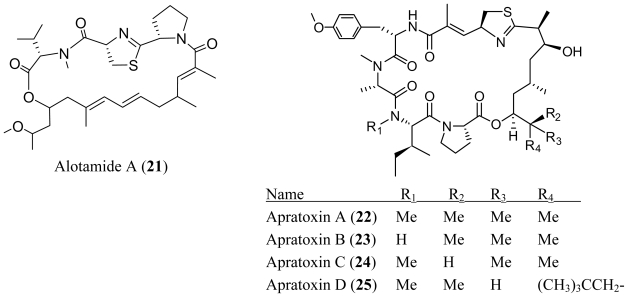
An assay-based screening program for new neuroactive compounds from cyanobacteria led to the discovery of alotamide A (21) [21]. Alotamide A (21) is a cyclic depsipeptide featuring three contiguous peptidic residues linked by a polyunsaturated dihydroxyheptaketide which has not been found in any other natural product. Alotamide A (21) displays an unusual calcium influx activation profile in murine cerebrocortical neurons with an EC50 of 4.18 μM. Although the molecular target and mechanism for the bioactivity of alotamide A (21) is still unclear, alotamide A (21) will attract considerable attention to further study of this new type of cyanobacterial neurotoxin.
Apratoxin D (25) was isolated from a Papua New Guinea derived strains of the marine cyanobacteria Lyngbya majuscula and Lyngbya sordida [22]. Apratoxin D (25) contains the same macrocycle as apratoxins A–C (22–24) but possesses the unprecedented 3,7-dihydroxy-2,5,8,10,10-pentamethylundecanoic acid as the polyketide moiety. Apratoxin D (25) showed potent in vitro cytotoxicity against H-460 human lung cancer cells with an IC50 value of 2.6 nM, which is nearly equal in potency to that of apratoxin A (22). The similar cytotoxicity of apratoxin A (22) and apratoxin D (25) indicates that the cytotoxicity of apratoxins is not strongly impacted by the larger lipopeptide tail. The isolation of apratoxin D (25) from two distinct species of the genus Lyngbya supports the hypothesis of genetic transfer of natural product biosynthetic pathways between marine cyanobacteria [23].
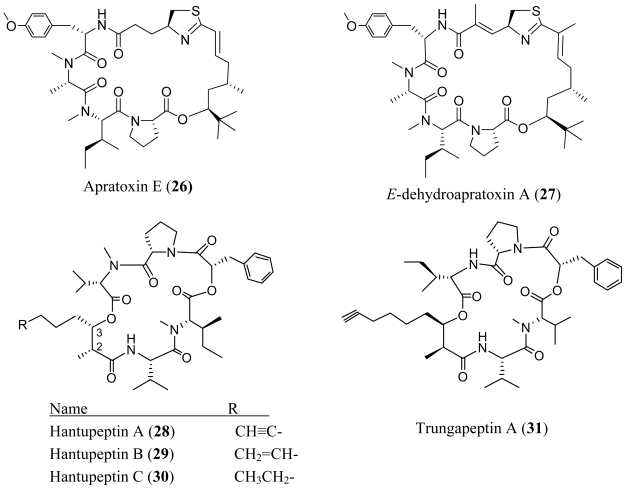
A collection of the marine cyanobacterium Lyngbya bouillonii from Guam afforded the cytotoxic apratoxin E (26) [24]. Apratoxin E (26) displayed stronger cytotoxicity than its closest analog, semisynthetic E-dehydroapratoxin A (27) against several cancer cell lines derived from colon, cervix, and bone, ranging from 21 to 72 nM, yet is 5- to 15-fold less active than apratoxin A (22). It was speculated that the conformational alteration to apratoxin E (26), which results from the dehydration in the polyketide chain, reduces its activity.
The marine Lyngbya majuscula from Pulau Hantu Besar, Singapore afforded a cyclodepsipeptide, hantupeptins A–C (28–30) [25,26]. Hantupeptin A (28), B (29) and C (30) all showed 100% brine shrimp mortality at 100 and 10 ppm. This was significantly higher than the activity reported for their closest analog trungapeptin A (31), which was only mildly toxic to brine shrimp [27]. Furthermore, in vitro cytotoxicity testing of hantupeptins A (28), B (29) and C (30) against the leukemia cell line MOLT-4, displayed IC50 values of 32 nM, 0.2 μM and 3.0 μM, respectively and 4.0 μM, 0.5 μM and 1.0 μM, respectively, against the breast cancer cell line MCF-7. In comparison, trungapeptin A (31), was reported to be inactive when tested at 10 μg/mL against KB or LoVo cells. It may be noteworthy to add that the hantupeptins have the 2R, 3S absolute configurations while the corresponding stereocenters in trungapeptin are 2S, 3R.
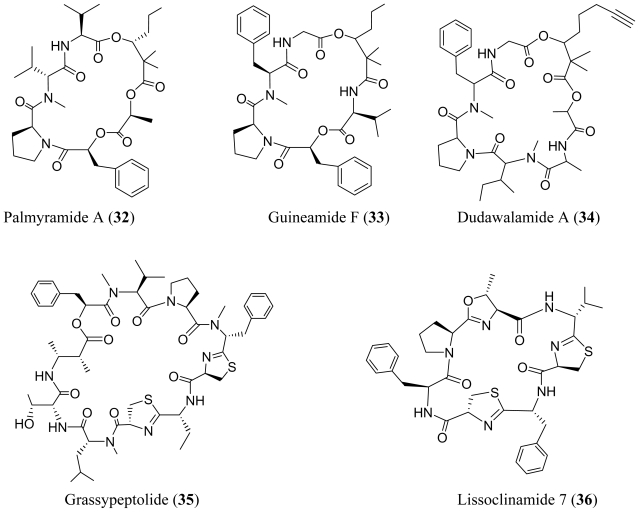
The marine Lyngbya majuscula collected from Palmyra Atoll produced palmyramide A (32), an unusual cyclic depsipeptide composed of three amino acids and three hydroxy acids [28]. The 2,2-dimethyl-3-hydroxyhexanoic acid unit (Dmhha) was also found in guineamide F (33) [29]. Palmyramide A (32) blocked the voltage gated sodium channel in neuro-2a cells (IC50 = 17.2 μM) and showed mild cytotoxicity against H-460 human lung carcinoma cells (IC50 = 39.7 μM). However, the cytotoxicity of guineamide F (33) was not reported. The planar structure of the 2,2-dimethyl-3-hydroxy-7-octynoic acid unit-containing dudawalamide A (34) was recently reported [30]. In an interesting correlation to the acyclic dragonamide A (7) and almiramides, it also exhibited anti-parasitic activity [31].
A collection of the cyanobacterium Lyngbya confervoides from the Florida Keys yielded grassypeptolide (35), a macrocyclic depsipeptide with an unusually high D-amino acid content, two thiazolines, and one β-amino acid [32]. Grassypeptolide (35) inhibited four cancer cell lines derived from human osteosarcoma (U2OS), cervical carcinoma (HeLa), colorectal adenocarcinoma (HT29), and neuroblastoma (IMR-32) with IC50 values from 1.0 to 4.2 μM. These data are within the range of IC50 values reported for lissoclinamide 7 (36) (53.7 nM to 21.5 μM), but in different cell lines [33,34]. Lissoclinamide 7 (36), the most cytotoxic of the lissoclinamide series, has two thiazoline rings with the same arrangement and stereoconfiguration as 35. It has been shown that the thiazolines of 36 are important to its cytotoxic activity [34]. If this is the case, then it is very possible that grassypeptolide (35) and lissoclinamide 7 (36) share a similar mechanism of action.
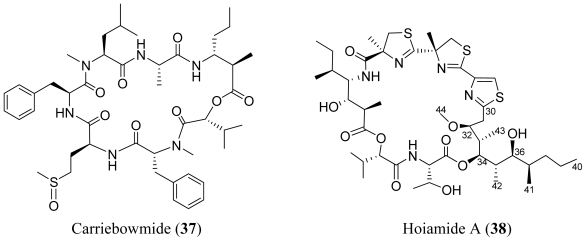
A cyclodepsipeptide, termed carriebowmide (37) was isolated from the lipophilic EtOAc-MeOH-soluble fraction of Lyngbya polychroa collected from Carrie Bow Cay, Belize [35]. Carriebowmide (37) was also isolated from the same strain of Lyngbya majuscula that produced two depsipeptides, itralamides A and B (53–54) [41]. Carriebowmide (37) contains two rare amino acids, 3-amino-2-methylhexanoic acid and methionine sulfoxide. The lipophilic EtOAc-MeOH-soluble fraction from Lyngbya polychroa significantly deterred feeding by a natural assemblage of reef fish. This is presumably due to the presence of carriebowmide (37), however the effectiveness of the purified compound as a feeding deterrent was not determined.
A combination of 1H NMR and bioassay-guided screening for neuroactive compounds from cyanobacteria led to the discovery of hoiamide A (38), a bioactive cyclic depsipeptide [36]. The 15 carbon subunit (C30 to C44) appears to be derived from a polyketide pathway. The pattern of oxidation and placement of pendant methyl groups suggests that the eleven-carbon main chain of Dmetua (5,7-dihydroxy-3-methoxy-4,6,8-trimethylundecanoic acid) may arise from the condensation of four propionates and one acetate unit. Hoiamide A (38) acted as a partial agonist at site 2 of the voltage-gate sodium channel α subunit, inhibited batrachotoxin-induced elevation of [Na+]I in a concentration dependant manner, and exhibited modest cytotoxicity to cancer cells.
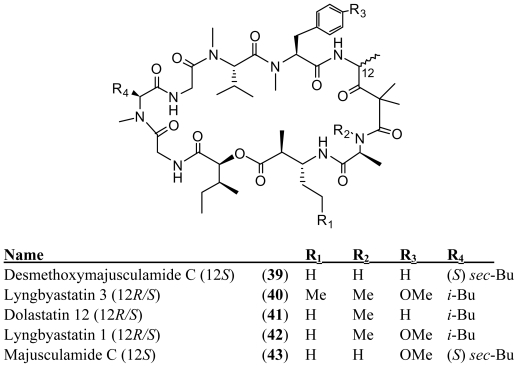
Desmethoxymajusculamide C (39) was isolated from Lyngbya majuscula collected in Fiji [37]. The closely related compounds, lyngbyastatin 3 (40), dolastatin 12 (41) and lyngbyastatin 1 (42), were determined to be mixtures of Ibu (4-amino-2,2-dimethyl-3-oxopentanoic acid units) epimers [R (major) and S (minor)] whereas the structurally related majusculamide C (43) is a single diastereomer with an S-Ibu unit. For 40–42, but not 43, significant NMR line broadening was observed. This was attributed to two factors: the presence of epimers and differing ratios of cis/trans conformers for individual epimers. Like majusculamide C (43) NMR line broadening was not observed for 39, suggesting the presence of a single Ibu epimer. Desmethoxymajusculamide C (39) and its ring-opened form (generated by breaking the ester bond) demonstrated equivalent efficacy and solid tumor selectivity against four cell lines, including HCT-116, H-460, MDA-MB-435 and Neuro-2A. HCT-116 was the most sensitive cell line, with IC50 values of 20 and 16 nM, for 39 and its ring-open analog, respectively. This finding was discordant with the common viewpoint that the cyclic form is the bioactive form of the many peptides.
4. Cyclic Peptides with One or More Amide or Ester Branches
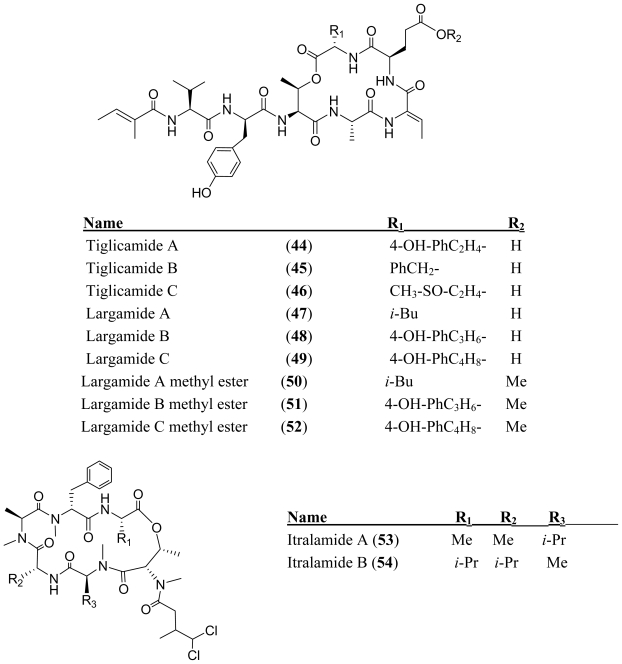
Tiglicamides A–C (44–46) were isolated from the Florida marine Lyngbya confervoides along with largamides A–C (47–49) [38,39]. The largamides and tiglicamides differ by only a single amino acid residue in the cyclic core. This single amino acid difference may result from assembly by an NRPS with adenylation domains having unusually relaxed specificity [40] or by a separate biosynthetic pathway. Both the largamides A–C (47–49) and the tiglicamides A–C (44–46) are serine protease inhibitors with selectivity for elastase over chymotrypsin and trypsin (Table 1). The carboxylic acid residue has little effect on the elastase inhibitory activity because the semi-synthetic largamide methyl esters 50–52 retained low-micromolar inhibitory activity.
Table 1.
Inhibition of serine proteases by peptides from the cyanobacterial genus Lyngbya (IC50, μM).
| Name | Elastase | Chymotrypsin | Trypsin | Reference |
|---|---|---|---|---|
| Tiglicamide A (44) | 2.14 | >50 | >50 | [38] |
| Tiglicamide B (45) | 6.99 | >50 | >50 | [38] |
| Tiglicamide C (46) | 7.28 | >50 | >50 | [38] |
| Largamide A (47) | 1.41 | >50 | >50 | [39] |
| Largamide B (48) | 0.53 | >50 | >50 | [39] |
| Largamide C (49) | 1.15 | >50 | >50 | [39] |
| Pompanopeptin A (55) | 2.4 | [42] | ||
| Lyngbyastatin 4 (59) | 0.0139 or 0.03 | 4.3 or 0.3 | >30 | [44,45] |
| Lyngbyastatin 5 (60) | 0.0032 | 2.8 | >30 | [45] |
| Lyngbyastatin 6 (61) | 0.0033 | 2.5 | >30 | [45] |
| Lyngbyastatin 8 (62) | 0.0083 or 0.047 | 2.5 | >30 | [45,46] |
| Lyngbyastatin 9 (63) | 0.123 | / | / | [46] |
| Lyngbyastatin 10 (64) | 0.210 | / | / | [46] |
| Lyngbyastatin 7 (65) | 0.120 | / | / | [46] |
| Somamide B (66) | 0.0095 | 4.2 | >30 | [47] |
| Kempopeptin A (67) | 0.32 | 2.6 | >67 | [48] |
| Kempopeptin B (68) | >67 | >67 | 8.4 | [48] |
| Scyptolin A (69) | 2.8 | / | >446 | [50] |
An eastern Caribbean collection of Lyngbya majuscula produced two depsipeptides, itralamides A and B (53–54) [41]. Itralamide B (54) displayed significant cytotoxicity in human embryonic kidney (HEK-293) cells (IC50 = 6 ± 1 μM). The closely related itralamide A (53) had a ten-fold lower potency than itralamide B (54). The difference in the cytotoxicity of itralamides A and B (53–54) demonstrates that biological activities can be dramatically altered by minor modifications of structure.
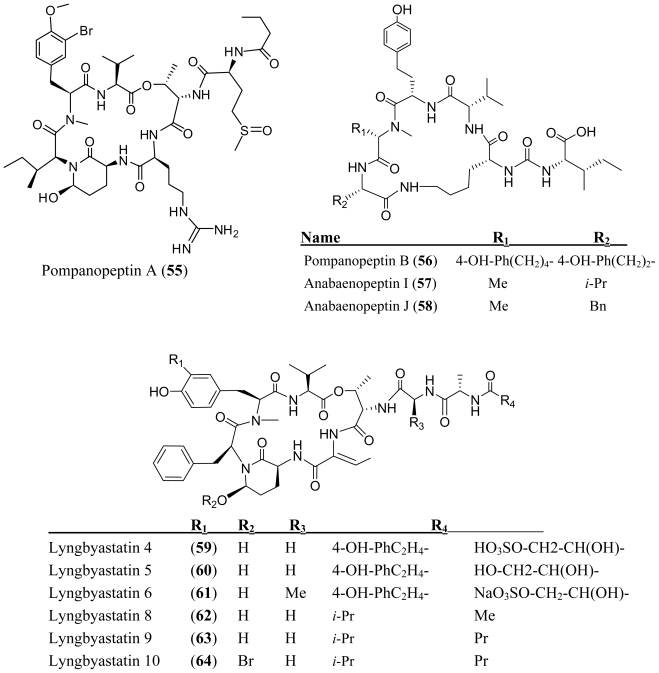
The investigation of the marine cyanobacterium Lyngbya confervoides collected from the southeastern coast of Florida led to the isolation of 3-amino-6-hydroxy-2-piperidone (Ahp) containing peptolide, pompanopeptin A (55), and a novel cyclic pentapeptide, pompanopeptin B (56) [42]. Pompanopeptin B (56) contains N-methyl-2-amino-6-(4′-hydroxyphenyl) hexanoic acid (N-Me-Ahpha) and is structurally related to carboxypeptidase-A inhibitors anabaenopeptins I (57) and J (58) which were isolated from the cyanobacterium Aphanizomenon flos-aquae. The L-Htyr and N-methyl-L-Ahpha, respectively in pompanopeptin B (56) were replaced by L-leucine/L-phenylalanine and N-methyl-L-alanine residues of 57 and 58 [43]. Pompanopeptin A (55) selectively inhibited trypsin over elastase and chymotrypsin, with an IC50 value of 2.4 μM (Table 1). The authors concluded that this selectivity is conferred by the arginine residue in the cyclic core. No activity data for 56 is reported.
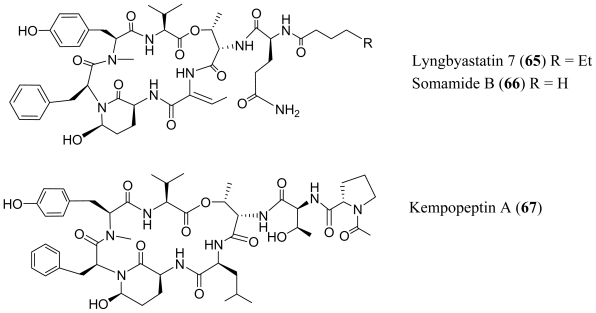
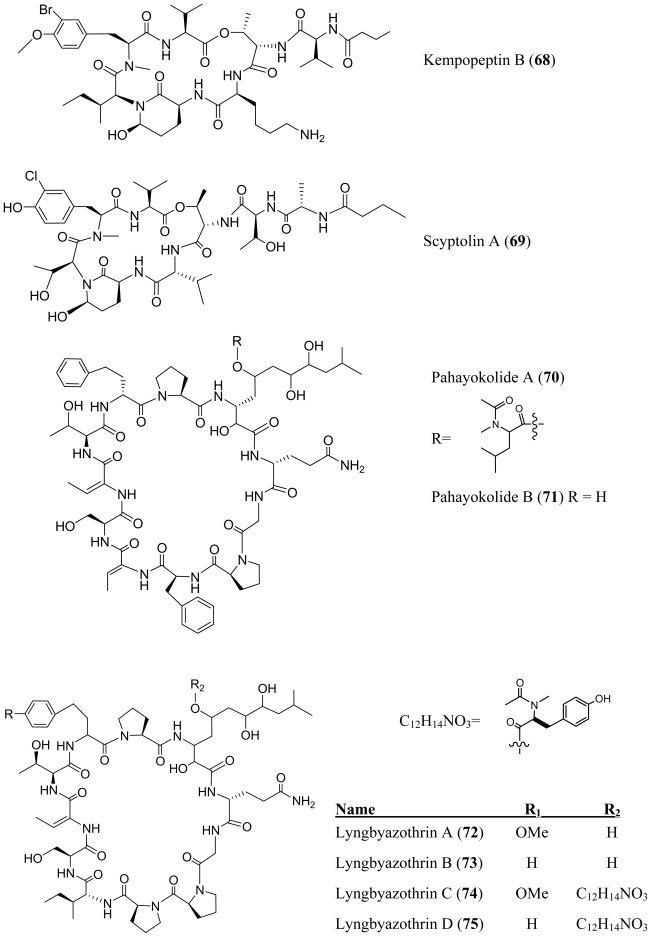
Several additional members of the Ahp (3-amino-6-hyroxy-piperidone) or Amp (3-amino-6-methoxy-piperidone) containing class of depsipeptides have been isolated and characterized from various species of Lyngbya. Lyngbyastatins 4–6 (59–61), were isolated from the marine cyanobacterium, Lyngbya confervoides which was collected off the Florida Atlantic coast [44,45]. Lyngbyastatins 8–10 (62–64) were isolated from the marine cyanobacterium Lyngbya semiplena, which was collected in Tumon Bay, Guam [46]. A Lyngbya sp., which was collected from a mangrove channel at Summerland Key in Florida, provided lyngbyastatin 7 (65) [45], somamide B (66) [47] and kempopeptins A and B (67–68) [48]. This class of compounds inhibits serine proteases, although the group exhibits a wide range of potency and varied selectivity (Table 1). When compared to the inhibitory activity of tiglicamides A–C (44–46), the Ahp containing depsipeptides are two to three orders of magnitudes more effective against porcine pancreatic elastase in vitro (Table 1). Like the tiglicamides (44–46) and the largamides (47–49), the Ahp containing serine proteases retain selectivity for elastase over chymostrysin or trypsin. Extensive structure activity studies as well as the crystal structures of the related desipeptide scyptolin A (69) bound to elastase [49,50] and cyanopeptolin A90720A bound to trypsin [51], revealed important interactions in the enzyme binding site and provided critical insights into the selectivity of this class of inhibitors. In the co-crystal structure of scyptolin-elastase, the threonine residue that forms ester bond occupies the S2 subsite of the protease. The valine which is C-terminal to this threonine binds the S1 subsite of the protease. The threonine and alanine of the pendant side-chain, bind subunits S3 and S4. The relative potencies of these new depsipeptides are consistent with earlier studies. Hydrophobic residues between the Thr that forms the ester bond of the depsipeptide and the Ahp residue impart selectivity for elastase as exemplified by the lynbyastatins (Abu), kempopeptin A (leucine) and scyptolin A (valine), whereas a basic residue at this position imparts selectivity for trypsin exemplified by kempopeptin B (lysine in 68) and pompanopeptin A (argenine in 55). In comparison with lynbyastatin 7 (65), lyngbyastatins 8–10 (62–64) exhibited weaker inhibition against porcine pancreatic elastase. Lyngbyastatins 8–10 (62–64) and lynbyastatin 7 (65) share the same depsipeptide core. Therefore, the reduced potency may be attributed to differences in the side chain residues. The exclusively hydrophobic residues in the pendant chain may form more favorable electrostatic interactions and hydrogen bonding with the enzyme. The fact that the protease-inhibitory activity is retained in the O-methylated (Amp) derivative, lyngbyastatin 6 (60), demonstrates that the hydroxyl group in the Ahp unit is not critical for the inhibition of elastase or chymotrypsin.
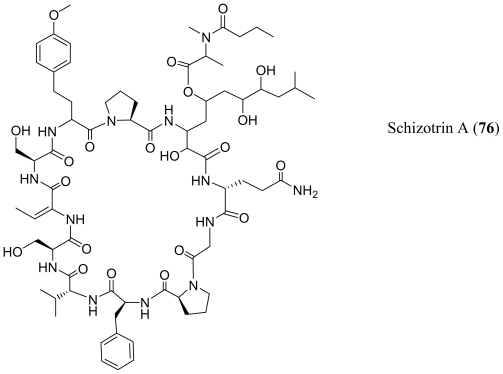
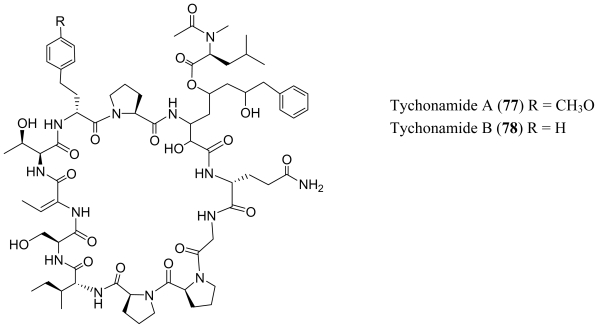
Pahayokolides A–B (70–71), lyngbyazothrins A–D (72–75) and schizotrin A (76) are remarkably similar in structure. Pahayokolides A–B (70–71) and lyngbyazothrins A–D (72–75) were produced by a freshwater Lyngbya sp. isolated from the Florida Everglades and the cultured Lyngbya sp. 36.91 SAG collected from Göttingen, Germany respectively [52–54]. On the other hand schizotrin A (76) and the tychonamides (77–78) were isolated from Schizotrix sp. [55] and Tchyonema sp. [56] respectively. Each of these cyclic undecapeptides contains the sequence (Val/Ile/Dhb)-Ser-Dhb-(Ser/Thr)-(homo-Phe/homo-Tyr)-Pro-X-Gln-Gly-Pro-(Pro/Phe), where X is an unusual, long-chain α,γ-hydroxy-β-amino acid. In the pahayokolides (70–71), the lyngbyazothrins (72–75) and schizotrin A (76) the α,γ-hydroxy-β-amino acid is 3-amino-2,5,7,8-tetrahydroxy-10-methylundecanoic acid (Athmu) and in tychonamides (77–78) it is a 3-amino-2,5,7,-trihydroxy-8-phenyloctanoic acid moiety (Atpoa). The γ-hydroxy group may or may not be decorated through an ester linkage to an N-acetyl-N-methyl leucine (pahayokolides and tychonamides) an N-butyroyl-N-methyl alanine (schizotrin A) or an N-acetyl-N-methyl tyrosine (lyngbyazothrins). To the best of our knowledge, these two long-chain α,γ-hydroxy-β-amino acids have not been observed elsewhere. It may be noteworthy that the pahayokolides, schizotrin, the lynbyazothrins and tychonamides, which are remarkably similar in structure, were all isolated from freshwater species. Pahayokolide A (70) inhibits a number of cancer cell lines over a range of concentrations (IC50 varied from 2.13 to 44.57 μM), is acutely toxic to zebrafish embryos (LC50 = 2.15 μM), and only marginally toxic against brine shrimp at the highest concentrations tested (1 mg/mL) [57]. The mixture of lyngbyazothrins A (72) and B (73) showed only low antimicrobial activity against Micrococcus flavus, whereas the mixture of lyngbyazothrins C (74) and D (75) was active against Bacillus subtilis, Escherichia coli, Pseudomonas aeruginosa, and Serratia marcescens. It seems that the acyl residue at C-5 of Athmu plays an important role in antimicrobial activity. This assumption was supported by the activity and structure of pahayokolides A–B (70–71).
Fifty new peptides isolated from Lyngbya species, reported since 2006 have been reviewed and their bioactivities discussed. All but six of these new compounds were derived from marine species of Lyngbya, although it is likely that this represents a sampling bias rather than a significant difference in biosynthetic capability between marine and freshwater species. The genus Lyngbya appears to be an emerging source of bioactive peptides. A search of Chemical Abstracts for original research articles using the keywords “Lyngbya” and “peptide” returns 105 citations from 1980 to the present, of which nearly 1/3 have been published during the time covered by this review. In a recent review, Welker described seven major and three minor structural classes which encompass just over 50% of all known cyanaobacterial peptides [12]. In contrast, only 11 of the 50 new peptides covered in this review fall within one of the identified classes: pompanopeptin A (55), lyngbyastatins 4–10 (59–64) and kempopeptins A and B (67–68) belong to the larger group of cyanopeptolins. While pompanopeptin B (56) falls within the anabaenopeptins. Lyngbya derived peptides share many features with other cyanobacterial peptides, such as N-methylation, the incorporation of D-amino acids, thiazolines or oxazolines and subunits of polyketide or mixed-polyketide and non-ribosomal peptide origin. Nonetheless, the majority of the peptides covered is unique to Lyngbya species and encompass one or only a few analogs. It is common for cyanobacteria, to produce more than one member of a certain structural class. This phenomenon is partially a result of relaxed specificity of biosynthetic enzymes [58]. As prokaryotic polyketide synthases (PKS) can show considerable substrate tolerance [59], so may adenylation domains of non-ribosomal peptide synthetase (NRPS) accept different amino acids within a group of polar or nonpolar residues [60]. Natural structural diversification may also be the result of slight genetic variation or a slightly changed environment. With the possible exception of the linear dragonamides and almiramides, it is likely that these peptides are made non-ribosomally. To date, five biosynthetic pathways for Lyngbya peptides have been identified; barbamide, curacin A, lyngbyatoxin, jamacamide, and hectochlorin [61–65]. These pathways serve to illustrate the tremendous biosynthetic diversity offered by this genus.
A number of the reviewed Lyngbya peptides, especially acyclic peptides (8–12 and 17) and cyclic peptides without branches (25–26, 28–30, 32, 35, 39, 53–54) exhibit cytotoxic activity against the various cancer cell lines. Only one cyclic Lyngbya peptide with branches, pahayokolide A inhibits a number of cancer cell lines over a range of concentrations. However, most of the reviewed cyclic branched-peptides (44–49, 55, 59–65, 67–68) inhibit proteases with a wide range of potency and varied selectivity. Bioactive Lyngbya peptides may be considered to be a valuable pool of lead compounds in structure-based drug design and discovery.
Acknowledgements
L. L. is grateful to the FIU Graduate School for a dissertation year fellowship.
References and Notes
- 1.Stanley SM. Earth System History. 2nd ed. WH Freeman & Co; New York, NY, USA: 2004. p. 263. [Google Scholar]
- 2.Tan LT. Bioactive natural products from marine cyanobacteria for drug discovery. Phytochemistry. 2007;68:954–979. doi: 10.1016/j.phytochem.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 3.Gerwick WH, Roberts MA, Proteau PJ, Chen JL. Screening cultured marine microalgae for anticancer-type activity. J. Appl. Phycol. 1994;6:143–149. [Google Scholar]
- 4.Gerwick WH, Tan LT, Siachitta N. The Alkaloids. Academic Press; San Diego, CA, USA: 2001. Nitrogen-containing metabolites from marine cyanobacteria; pp. 75–184. [DOI] [PubMed] [Google Scholar]
- 5.Namikoshi M, Rinehart KL. Bioactive compounds produced by cyanobacteria. J. Ind. Microbial. Biotechnol. 1996;17:373–384. [Google Scholar]
- 6.Mayer MS, Gustafson KR. Antitumor and cytotoxic compounds. Int. J. Cancer. 2003;105:291–299. doi: 10.1002/ijc.11080. [DOI] [PubMed] [Google Scholar]
- 7.Trimurtulu G, Ohtani I, Patterson GML, Moore RE, Corbett TH, Valeriote FA, Dechik L. Total structures of cryptophycins, potent antitumor depsipeptides from the blue-green alga Nostoc sp. strain GSV 224. J. Am. Chem. Soc. 1994;116:4729–4737. [Google Scholar]
- 8.D’Agostino G, del Campo J, Mellado B, Izquierdo MA, Minarik T, Cirri L, Marini L, Perez-Gracia JL, Scambia G. A multicenter phase II study of the cryptophycin analog LY355703 in patients with platinum-resistant ovarian cancer. Int. J. Gynecol. Cancer. 2006;16:71–76. doi: 10.1111/j.1525-1438.2006.00276.x. [DOI] [PubMed] [Google Scholar]
- 9.Simmons TL, Andrianasolo E, McPhail K, Flatt P, Gerwick WH. Marine natural products as anticancer drugs. Mol. Cancer Ther. 2005;4:333–342. [PubMed] [Google Scholar]
- 10.Shnyder SD, Cooper PA, Millington NJ, Pettit GR, Bibby MC. Auristatin PYE, a novel synthetic derivative of dolastatin 10, is highly effective in human colon tumour models. Int. J. Oncol. 2007;31:353–360. [PubMed] [Google Scholar]
- 11.Ebbinghaus S, Hersh E, Cunningham CC, O’Day S, McDermott D, Stephenson J, Richards DA, Eckardt J, Haider OL, Hammond LA. Phase II study of synthadotin (SYN-D; ILX651) administered daily for 5 consecutive days once every 3 weeks in patients with inoperable locally advanced or metastatic melanoma. Proceedings of the 2004 American Society of Clinical Oncology Annual Meeting; New Orleans, LA, USA. June 2004; Abstr. #7530. [Google Scholar]
- 12.Welker M, von Döhren H. Cyanobacterial peptides-Nature’s own combinatorial biosynthesis. FEMS Microiol. Rev. 2006;30:530–563. doi: 10.1111/j.1574-6976.2006.00022.x. [DOI] [PubMed] [Google Scholar]
- 13.Speziale BJ, Dyck LA. Lyngbya infestations: comparative taxonomy of Lyngbya wollei comb. nov. (Cyanobacteria) J. Phycol. 1992;28:693–706. [Google Scholar]
- 14.Jiménez JI, Scheuer PJ. New lipopeptides from the Caribbean cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2001;64:200–203. doi: 10.1021/np000462q. [DOI] [PubMed] [Google Scholar]
- 15.McPhail KL, Correa J, Linington RG, Gonzalez J, Ortega-Barría E, Capson TL, Gerwick WH. Antimalarial linear lipopeptides from a Panamanian strain of the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2007;70:984–988. doi: 10.1021/np0700772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gunasekera SP, Ross C, Paul VJ, Matthew S, Luesch H. Dragonamides C and D, linear lipopeptides from the marine cyanobacterium brown Lyngbya polychroa. J. Nat. Prod. 2008;71:887–890. doi: 10.1021/np0706769. [DOI] [PubMed] [Google Scholar]
- 17.Balunas MJ, Linington RG, Tidgewell K, Fenner AM, Ureña LD, Togna GD, Kyle DE, Gerwick WH. Dragonamide E, a modified linear lipopeptide from Lyngbya majuscula with antileishmanial activity. J. Nat. Prod. 2010;73:60–66. doi: 10.1021/np900622m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanchez LM, Lopez D, Vesely BA, Della Togna G, Gerwick WH, Kyle DE, Linington RG. Almiramides A–C: discovery and development of a new class of leishmaniasis lead compounds. J. Med. Chem. 2010 doi: 10.1021/jm100265s. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teruya T, Sasaki H, Fukazawa H, Suenaga K. Bisebromoamide, a potent cytotoxic peptide from the marine cyanobacterium Lyngbya sp.: isolation, stereostructure, and biological activity. Org. Lett. 2009;11:5062–5065. doi: 10.1021/ol9020546. [DOI] [PubMed] [Google Scholar]
- 20.Kwan JC, Eksioglu EA, Liu C, Paul VJ, Luesch H. Grassystatins A–C from marine cyanobacteria, potent cathepsin E inhibitors that reduce antigen presentation. J. Med. Chem. 2009;52:5732–5747. doi: 10.1021/jm9009394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soria-Mercado IE, Pereira A, Cao Z, Murray TF, Gerwick WH. Alotamide A, a novel neuropharmacological agent from the marine cyanobacterium Lyngbya bouillonii. Org. Lett. 2009;11:4704–4707. doi: 10.1021/ol901438b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gutiérrez M, Suyama TL, Engene N, Wingerd JS, Matainaho T, Gerwick WH. Apratoxin D, a potent cytotoxic cyclodepsipeptide from Papua New Guinea collections of the marine cyanobacteria Lyngbya majuscula and Lyngbya sordida. J. Nat. Prod. 2008;71:1099–1103. doi: 10.1021/np800121a. [DOI] [PubMed] [Google Scholar]
- 23.Simmons TL, Coates RC, Clark BR, Engene N, Gonzalez D, Esquenazi E, Dorrestein PC, Gerwick WH. Biosynthetic origin of natural products isolated from marine microorganism-invertebrate assemblages. Proc. Natl. Acad. Sci. USA. 2008;105:4587–4594. doi: 10.1073/pnas.0709851105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matthew S, Schupp PJ, Luesch H. Apratoxin E, a cytotoxic peptolide from a Guamanian collection of the marine cyanobacterium Lyngbya bouillonii. J. Nat. Prod. 2008;71:1113–1116. doi: 10.1021/np700717s. [DOI] [PubMed] [Google Scholar]
- 25.Tripathi A, Puddick J, Prinsep MR, Lee PPF, Tan LT. Hantupeptin A, a cytotoxic cyclic depsipeptide from a Singapore collection of Lyngbya majuscula. J. Nat. Prod. 2009;72:29–32. doi: 10.1021/np800448t. [DOI] [PubMed] [Google Scholar]
- 26.Tripathi A, Puddick J, Prinsep MR, Lee PPF, Tan LT. Hantupeptins B and C, cytotoxic cyclodepsipeptides from the marine cyanobacterium Lyngbya majuscula. Phytochemistry. 2009;72:29–32. doi: 10.1016/j.phytochem.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 27.Bunyajetpong S, Yoshida WY, Sitachitta N, Kaya K. Trungapeptins A–C, cyclodepsipeptides from the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2006;69:1539–1542. doi: 10.1021/np050485a. [DOI] [PubMed] [Google Scholar]
- 28.Taniguchi M, Nunnery JK, Engene N, Esquenazi E, Byrum T, Dorrestein PC, Gerwick WH. Palmyramide A, a cyclic depsipeptide from a Palmyra Atoll collection of the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2010;73:393–398. doi: 10.1021/np900428h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan LT, Sitachitta N, Gerwick WH. The guineamides, novel cyclic depsipeptides from a Papua New Guinea collection of the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2003;66:764–771. doi: 10.1021/np020492o. [DOI] [PubMed] [Google Scholar]
- 30.Liu WT, Ng J, Meluzzi D, Bandeira N, Gutierrez M, Simmons TL, Schultz AW, Linington RG, Moore BS, Gerwick WH, Pevzner PA, Dorrestein PC. Interpretation of tandem mass spectra obtained from cyclic nonribosomal peptides. Anal. Chem. 2009;81:4200–4209. doi: 10.1021/ac900114t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malloy K, Engene N, Pedler B, Clark BR, Gerwick WH. Isolation, structure elucidation, and SAR perspectives of cyanobacterial cyclic depsipeptides containing the unique Dhoya (3-hydroxy-2,2-dimethyl-7-octynoic acid) fragment and its derivatives. 42nd Western Regional Meeting of the American Chemical Society; Las Vegas, NV, USA. September 2008. [Google Scholar]
- 32.Kwan JC, Rocca JR, Abboud KA, Paul VJ, Luesch H. Total structure determination of grassypeptolide, a new marine cytotoxin. Org. Lett. 2008;10:789–792. doi: 10.1021/ol702946d. [DOI] [PubMed] [Google Scholar]
- 33.Hawkins CJ, Lavin MF, Marshall KA, van den Brenk AL, Watters DJ. Structure-activity relationships of the lissoclinamides: cytotoxic cyclic peptides from the ascidian Lissoclinum patella. J. Med. Chem. 1990;33:1634–1638. doi: 10.1021/jm00168a016. [DOI] [PubMed] [Google Scholar]
- 34.Wipf P, Fritch PC, Geib SJ, Sefler AM. Conformational studies and structure–activity analysis of lissoclinamide 7 and related cyclopeptide alkaloids. J. Am. Chem. Soc. 1998;120:4105–4112. [Google Scholar]
- 35.Gunasekera SP, Ritson-Williams R, Paul VJ. Carriebowmide, a new cyclodepsipeptide from the marine cyanobacterium Lyngbya polychroa. J. Nat. Prod. 2008;71:2060–2063. doi: 10.1021/np800453t. [DOI] [PubMed] [Google Scholar]
- 36.Pereira A, Cao Z, Murray TF, Gerwick WH. Hoiamide A, a sodium channel activator of unusual architecture from a consortium of two Pupa New guinea cyanobactiera. Chem. Biol. 2009;16:893–906. doi: 10.1016/j.chembiol.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simmons TL, Nogle LM, Media J, Valeriote FA, Mooberry SL, Gerwick WH. Desmethoxymajusculamide C, a cyanobacterial depsipeptide with potent cytotoxicity in both cyclic and ring-opened forms. J. Nat. Prod. 2009;72:1011–1016. doi: 10.1021/np9001674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matthew S, Paul VJ, Luesch H. Tiglicamides A–C, cyclodepsipeptides from the marine cyanobacterium Lyngbya confervoides. Phytochemistry. 2009;70:2058–2063. doi: 10.1016/j.phytochem.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matthew S, Paul VJ, Luesch H. Largamides A–C, tiglic acid-containing cyclodepsipeptides with elastase-inhibitory activity from the marine cyanobacterium Lyngbya confervoides. Planta Med. 2009;75:528–533. doi: 10.1055/s-0029-1185332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kleinkauf H, Von Döhren H. A nonribosomal system of peptide biosynthesis. Eur. J. Biochem. 1996;236:335–351. doi: 10.1111/j.1432-1033.1996.00335.x. [DOI] [PubMed] [Google Scholar]
- 41.Jiménez JI, Vansach T, Yoshida WY, Sakamoto B, Pörzgen P, Horgen FD. Halogenated fatty acid amides and cyclic depsipeptides from an eastern Caribbean collection of the cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2009;72:1573–1578. doi: 10.1021/np900173d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matthew S, Ross C, Paul VJ, Luesch H. Pompanopeptins A and B, new cyclic peptides from the marine cyanobacterium Lyngbya confervoides. Tetrahedron. 2008;64:4081–4089. [Google Scholar]
- 43.Murakami M, Suzuki S, Itou Y, Kodani S, Ishida K. New anabaenopeptins, potent carboxypeptidase-A inhibitors from the cyanobacterium Aphanizomenon flos-aquae. J. Nat. Prod. 2000;63:1280–1282. doi: 10.1021/np000120k. [DOI] [PubMed] [Google Scholar]
- 44.Matthew S, Ross C, Rocca JR, Paul VJ, Luesch H. Lyngbyastatin 4, a dolastatin 13 analog with elastase and chymotrypsin inhibitory activity from the marine cyanobacterium Lyngbya confervoides. J. Nat. Prod. 2007;70:124–127. doi: 10.1021/np060471k. [DOI] [PubMed] [Google Scholar]
- 45.Taori K, Matthew S, Rocca JR, Paul VJ, Luesch H. Lyngbyastatins 5–7, potent elastase inhibitors from Floridian marine cyanobacteria, Lyngbya spp. J. Nat. Prod. 2007;70:1593–1600. doi: 10.1021/np0702436. [DOI] [PubMed] [Google Scholar]
- 46.Kwan JC, Taori K, Paul VJ, Luesch H. Lyngbyastatins 8–10, elastase inhibitors with cyclic depsipeptide scaffolds isolated from the marine cyanobacterium Lyngbya semiplena. Mar. Drugs. 2009;7:528–538. doi: 10.3390/md7040528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nogle LM, Williamson RT, Gerwick WH. Somamides A and B, two new depsipeptide analogs of dolastatin 13 from a Fijian cyanobacterial assemblage of Lyngbya majuscula and Schizothrix species. J. Nat. Prod. 2001;64:716–719. doi: 10.1021/np000634j. [DOI] [PubMed] [Google Scholar]
- 48.Taori K, Paul VJ, Luesch H. Kempopeptins A and B, serine protease inhibitors with different selectivity profiles from a marine cyanobacterium, Lyngbya sp. J. Nat. Prod. 2008;71:1625–1629. doi: 10.1021/np8002172. [DOI] [PubMed] [Google Scholar]
- 49.Matern U, Schleberger C, Jelakovic S, Weckesser J, Schulz EG. Binding structure of elastase inhibitor scyptolin A. Chem. Biol. 2003;10:997–1001. doi: 10.1016/j.chembiol.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 50.Matern U, Oberer L, Falchetto RA, Erhard M, König WA, Herdman M, Weckesser J. Scyptolin A and B, cyclic depsipeptides from axenic cultures of Scytonema hofmanni PCC 7110. Phytochemistry. 2001;58:1087–1095. doi: 10.1016/s0031-9422(01)00400-9. [DOI] [PubMed] [Google Scholar]
- 51.Lee AY, Smitka TA, Bonjouklian R, Clardy J. Atomic structure of the trypsin-A90720A complex: a unified approach to structure and function. Chem. Biol. 1994;1:113–117. doi: 10.1016/1074-5521(94)90049-3. [DOI] [PubMed] [Google Scholar]
- 52.An T, Krishnaswamy T, Kumar S, Wang M, Liu L, Lay J, Jr, Liyanage R, Berry J, Gantar M, Marks V, Gawley RE, Rein KS. Structures of pahayokolides A and B, two cyclic peptides from a Lyngbya sp. J. Nat. Prod. 2007;70:730–735. doi: 10.1021/np060389p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zainuddin NE, Jansen R, Nimtz M, Wray V, Preisitsch M, Lalk M, Mundt S. Lyngbyazothrins A–D, antimicrobial cyclic undecapeptides from the cultured cyanobacterium Lyngbya sp. J. Nat. Prod. 2009;72:1373–1378. doi: 10.1021/np8007792. [DOI] [PubMed] [Google Scholar]
- 54.Zainuddin NE, Jansen R, Nimtz M, Wray V, Preisitsch M, Lalk M, Mundt S. Corrigendum to: Lyngbyazothrins A–D, antimicrobial cyclic undecapeptides from the cultured cyanobacterium Lyngbya sp. J. Nat. Prod. 2009;72:2080. doi: 10.1021/np8007792. The correct absolute configuration of the Gln residue is R as showed in the discussion. [DOI] [PubMed] [Google Scholar]
- 55.Pergament I, Carmeli S. Schizotrin A, a novel antimicrobial cyclic peptide from a cyanobacterium. Tetrahedron Lett. 1994;35:8473–8476. [Google Scholar]
- 56.Mehner C, Mueller D, Krick A, Kehraus S, Löeser R, Güetschow M, Maier A, Fiebig HF, Brun R, Köenig GM. A novel β-amino acid in cytotoxic peptides from the cyanobacterium Tychonema sp. Eur. J. Org. Chem. 2008;10:1732–1739. [Google Scholar]
- 57.Berry JP, Gantar M, Gawley RE, Wang M, Rein KS. Pharmacology and toxicology of pahayokolide A, a bioactive metabolite from a freshwater species of Lyngbya isolated from the Florida Everglades. Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol. 2004;139:231–238. doi: 10.1016/j.cca.2004.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Magarvey NA, Beck ZQ, Golakoti T, Ding Y, Huber U, Hemscheidt TK, Abelson D, Moore RE, Sherman DH. Biosynthetic characterization and chemoenzymatic assembly of the cryptophycins. Potent anticancer agents from cyanobionts. ACS Chem. Biol. 2006;1:766–779. doi: 10.1021/cb6004307. [DOI] [PubMed] [Google Scholar]
- 59.Watts RE, Tse ML, Khosla C. Substrate tolerance of module 6 of the epothilone synthetase. Biochemistry. 2007;46:3385–3393. doi: 10.1021/bi0616448. [DOI] [PubMed] [Google Scholar]
- 60.Challis GL, Ravel J, Townsend CA. Predictive, structure-based model of amino acid recognition by nonribosomal peptide synthetase adenylation domains. Chem. Biol. 2000;7:211–224. doi: 10.1016/s1074-5521(00)00091-0. [DOI] [PubMed] [Google Scholar]
- 61.Chang Z, Flatt P, Gerwick WH, Nguyen VA, Willis CL, Sherman DH. The barbamide biosynthetic gene cluster: a novel marine cyanobacterial system of mixed polyketide synthase (PKS)-non-ribosomal peptide synthetase (NRPS) origin involving an unusual trichloroleucyl starter unit. Gene. 2002;296:235–247. doi: 10.1016/s0378-1119(02)00860-0. [DOI] [PubMed] [Google Scholar]
- 62.Chang Z, Sitachitta N, Rossi JV, Roberts MA, Flatt PM, Jia J, Sherman DH, Gerwick WH. Biosynthetic pathway and gene cluster analysis of curacin A, an antitubulin natural product from the tropical marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2004;67:1356–1367. doi: 10.1021/np0499261. [DOI] [PubMed] [Google Scholar]
- 63.Edwards DJ, Gerwick WH. Lyngbyatoxin biosynthesis: sequence of biosynthetic gene cluster and identification of a novel aromatic prenyltransferase. J. Am. Chem. Soc. 2004;126:11432–11433. doi: 10.1021/ja047876g. [DOI] [PubMed] [Google Scholar]
- 64.Edwards DJ, Marquez BL, Nogle LM, McPhail K, Goeger DE, Roberts MA, Gerwick WH. Structure and biosynthesis of the jamaicamides, new mixed polyketide-peptide neurotoxins from the marine cyanobacterium Lyngbya majuscula. Chem. Biol. 2004;11:817–833. doi: 10.1016/j.chembiol.2004.03.030. [DOI] [PubMed] [Google Scholar]
- 65.Ramaswamy AV, Sorrels CM, Gerwick WH. Cloning and biochemical characterization of the hectochlorin biosynthetic gene cluster from the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2007;70:1977–1986. doi: 10.1021/np0704250. [DOI] [PubMed] [Google Scholar]