Abstract
Background
Research on the physical and psychological late effects of treatment of childhood cancer has led to the identification of significant long-term neurocognitive deficits experienced by some survivors, particularly in the areas of memory and executive functioning. Despite indications of deficits based on cognitive assessment, the identification of specific mechanisms of neurocognitive deficits using neuroimaging techniques has yet to be adequately considered.
Procedure
This study used functional neuroimaging techniques to examine working memory and executive functioning deficits of survivors of childhood Acute Lymphocytic Leukemia (ALL), as compared to age and gender matched healthy controls.
Results
There was a trend for ALL survivors to perform more poorly on a working memory task in terms of overall accuracy. Additionally, survivors displayed significantly greater activation in areas underlying working memory (dorsolateral and ventrolateral prefrontal cortex) and error monitoring (dorsal and ventral anterior cingulate cortex).
Conclusions
These results support the theory of compensatory activation in necessary brain regions in order to complete tasks in pediatric ALL survivors, similar to that observed in multiple sclerosis patients. Concurrent examination of testing and brain imaging enables the connection of behavioral observations with underlying neurological characteristics of deficits in survivors and may help provide insight into mechanisms through which deficits appear.
Keywords: Children, Leukemia, Neuroimaging, Working Memory, Late Effects
Introduction
Research on the late effects of pediatric cancer has indicated that a subset of children treated for childhood acute lymphocytic leukemia (ALL) show some form of neurocognitive deficit [1, 2]. These children display a spectrum of neurocognitive changes that may occur as a result of treatment, which represents a moving target that varies with adjustments to treatment protocols. Most notably, neurocognitive changes are likely to become more subtle as protocols abandon the use of cranial radiation (CRT), which has well established neurotoxicity, in favor of intrathecal prophylactic chemotherapy for children diagnosed with standard-risk ALL. This indicates a need for new research methodology that is sensitive enough to examine these changes.
Research in the area of neurocognitive late effects has primarily focused on higher order functions necessary for advanced information processing. Recent meta-analyses have found that survivors of childhood ALL experience significant deficits in both global and specific domains of neurocognitive functions, including executive function, verbal and visuospatial memory, and attention [1, 2]. Among the higher order functions evidencing deficits is working memory, which involves the ability to retain, manipulate, and act on complex sets of information. Brain regions subserving working memory include the rostral, dorsolateral, and ventrolateral prefrontal cortex, and the dorsal anterior cingulate cortex [3]. These regions are among the last to myelinate, and their development continues through adolescence, leaving them potentially more susceptible to the introduction of intrathecal chemotherapy.
A necessary next step in understanding the nature of the deficits experienced by survivors of ALL involves the use of neuroimaging methods to identify structural and functional deficits within specified brain regions. Researchers have begun to examine the structural underpinnings of these executive function deficits, and have found that between 16-52% of those who have received treatment for ALL experience at least one brain abnormality [4, 5]. A subset of survivors of ALL have been found to have differences in white matter volume within the right frontal lobe [6], as well as defects in perfusion identified by SPECT in basal, temporal, and frontal regions [7]. However, no studies to date have used functional imaging to examine deficits in brain function related to the observed deficits in performance on standardized tests. Therefore, the current study examines functional working memory deficits in a prescribed set of brain regions in order to identify differences in activation patterns between survivors of ALL and healthy children.
Method
Participants
Eight survivors of pediatric ALL (4 boys) and 7 healthy children (4 boys) participated in this study; survivors constituted a subset of participants in a previous study on neurocognitive functioning of childhood cancer survivors who demonstrated the most significant deficits based on a neurocognitive assessment. Healthy children were matched for age and gender. Upon initial enrollment in the study, survivors were on average 14.07 years old (SD = 2.32) and healthy controls were 14.54 years old (SD = 2.47). On average, survivors were diagnosed at 4.94 years old with standard-risk precursor b-cell ALL, and were 6.46 years off-treatment. Survivors had received an average of 174.9 mg Methotrexate by intrathecal administration, requiring an average of 16.7 spinal taps, as well as an average of 70.0 mg Ara-C by intrathecal administration, requiring one spinal tap. Study exclusionary criteria included exposure to cranial or whole body irradiation, and a diagnosis of any genetic, cognitive or Autism Spectrum Disorder. All procedures were approved by the Institutional Review Board, and informed consent and assent were obtained from all participants.
Measures
Cognitive Assessment Phase
The Wechsler Intelligence Scale for Children – Fourth Edition (WISC-IV) [8] was administered to children to measure overall cognitive functioning, including general intelligence, working memory, and processing speed. The Wechsler intelligence scales have demonstrated excellent internal consistency (α = .97) and test-retest reliability (r = .93), and convergent and discriminant validity have been established. Subtests of the Delis-Kaplan Executive Function System (D-KEFS) [9], a comprehensive battery of tests that assess verbal and nonverbal executive functions, were also administered. These subtests included Color-Word Interference Test, the Tower Test, and the Sorting Test.
Neuroimaging Phase
A visual N-back task was selected to assess working memory. A letter version of the visual N-back task [10] was used, which involves sequences of uppercase consonants. In the 0-back condition, participants are instructed to respond to a single target (e.g., M). In the 1-back condition, participants are instructed to respond only when the consonant is identical to the one preceding it (e.g., M, M). In the 2-back condition, participants respond only when the consonant is identical to the one presented two trials prior (e.g., M, T, M), and in the 3-back condition, participants respond when the consonant is identical to the one presented three trials prior (e.g., M, T, F, M). Each condition is presented three times in order of increasing difficulty, for a total of 12 blocks. Participants responded by pressing a button with their right index finger via response keypad that was securely strapped to the right arm.
Data Analysis
Image Acquisition
Anatomic and functional MRI was performed on all participants. No structural abnormalities were identified in either the group of survivors or the control group. Images were acquired using a Philips Intera Achieva 3T MRI scanner. Imaging consisted of a 3-plane localizer (5 slices per plane, 22s scan time) from which 20 oblique axial slices (parallel to the AC-PC plane) were prescribed. High resolution 3D anatomical images were acquired using an inversion-prepared spoiling gradient recalled echo sequence (IR-SPGR), with an inversion time T1 of 400 ms, a TR of 15 ms, minimum TE (3ms), a matrix size 256 × 192 × 128 for a FOV of 200 × 200 × 154 mm3 with near isotropic resolution. All functional images were acquired with a gradient echo EPI pulse sequence, with TE 30mx (optimized for T2* at 3T), flip angle of 70°, TR 2s, 20 slices 5mm thick and 1mm skip, and a matrix size of 64 × 64 sampled at +/−62.5 kHz.
Statistical Analyses
All functional data were analyzed using BrainVoyager [11]. Images were first motion-corrected, and all data that exceed movement criteria (>2mm displacement, 0.5° rotation) were discarded. The functional data for each participant were registered to the participant’s high-resolution 3D anatomic data using first, a mathematical header-based alignment. Then any misalignment due to inter-session motion was manually adjusted by trained expert raters. Normalization was performed using a piecewise linear transformation to the Talairach and Tournoux atlas [12]. Statistical parametric maps were calculated using the general linear model (GLM) with a False Discovery Rate of p < .05, and a cluster threshold of 100 contiguous voxels. In addition, correlation activation maps were created by using contrast-weights determined by the participants’ performance and behavioral scores. These maps were used to identify clusters of activation in hypothesized regions of interest that remained significant under the statistical constraints mentioned above. Region of interest measurements were used to quantify signal change in these areas of significant activation.
Results
Demographics and Cognitive Assessment
Independent samples t-tests and chi square analyses revealed that survivors of ALL and healthy controls were of similar age (MALL = 14.07 years, SDALL = 2.23; MHC = 14.54 years, SDHC = 2.47) and race (χ2 = 2.02, p = .27). Confirmatory analyses of cognitive assessment data revealed that we successfully identified a subgroup of survivors of ALL who showed relative deficits on measures of working memory (WISC-IV WMI; t = −2.76, p = .02), processing speed (WISC-IV PSI; t = −3.30, p < .01), and overall cognitive functioning (WISC-IV FSIQ; t = −3.98, p < .01), as compared to healthy children. Survivors also performed more poorly on subtests of the D-KEFS, including Color Word Interference Test (t = −2.27, p = .04), and Sorting Test (t = −2.63, p = .02).
Neuroimaging
Task Performance
Independent samples t-tests revealed that groups performed similarly on the N-back task in terms of overall reaction time (t = 0.63, p = 0.54). In terms of accuracy, including correct responses, false-positives, and omissions, no statistically significant differences were found between groups. However, closer examination of effect sizes suggests that at high levels of difficulty, survivors may have been unable to perform at the same level as healthy children as indicated by the large effect sizes at the 2-back (d = 0.95; p = 0.10) and (d = 0.83; p = 0.14) 3-back level (the relatively small sample sizes of the groups restricted our ability to reach traditional levels of statistical significance). Similarly, although no significant between group differences were found for reaction times at each N-back level, medium to large effects were found for the 1-back (d = 0.79) and 2-back levels (d = 0.62), suggesting that survivors did not respond as quickly as healthy children at these levels; this is consistent with the lower performance of survivors on processing speed measures during cognitive assessment.
Brain area activation: Between group analyses
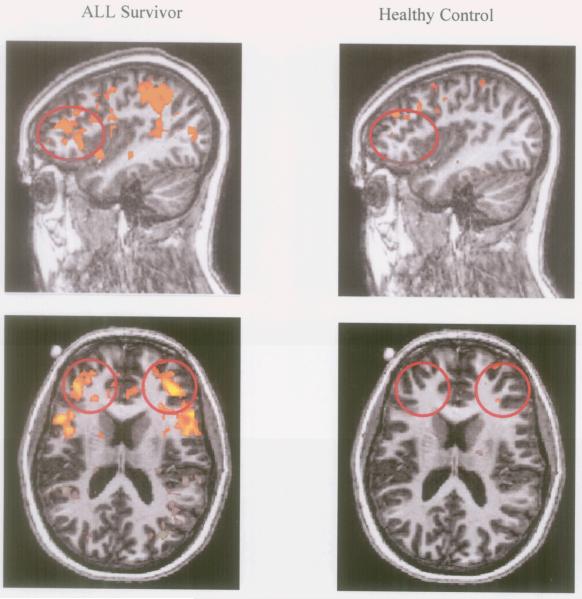
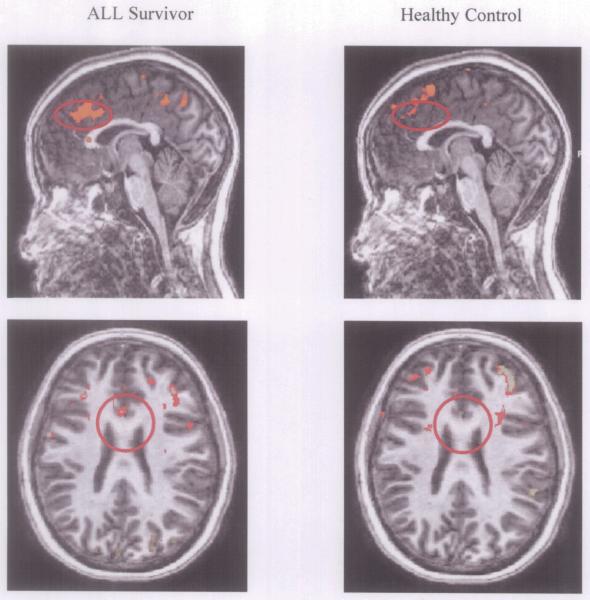
Between group analyses indicated that while survivors of ALL and healthy controls show similar levels of activation during low levels of the N-back, survivors show greater activation than controls in key regions of interest as the load level of the N-back task increased. In the DLPFC, survivors showed greater activation than controls during the 2-back condition relative to baseline (BA 9, t = 10.88, p < 0.01; BA 46, t = 9.21, p < 0.01), as well as the 3-back relative to the 1-back condition (BA 9, t = 9.39, p < 0.01). This indicates that at increased demand level, survivors recruited a greater amount of oxygenated blood to these areas than did healthy children, in order to complete the same task. Additionally, in the D-ACC (BA 32), survivors showed greater activation than controls during the 2-back condition relative to baseline (t = 9.44, p < 0.000001), as well as the 2-back and 3-back relative to the 1-back condition (t = 8.55, p < 0.01; t = 9.45, p < 0.01, respectively). This similarly indicates that at increased task demand level, survivors recruited a greater amount of oxygenated blood to the ACC. Mean levels of activation for ALL survivors and healthy controls are represented in sagittal views in Figure 1.
Figure 1.
Between Group Differences in DLPFC Activation on the 2 vs. 0 N-back Contrast During Neuroimaging. Differences in activation in the 2-back versus 0-back conditions for survivors of ALL (left column) and control subjects (right column). Yellow-to-orange clusters indicate areas of significantly greater activation in the 2-back condition relative to the 0-back conditions. Red circles identify the bilateral region of interest (right and left DLPFC).
Discussion
Cognitive development during childhood and adolescence is characterized in part by the acquisition and maturation of skills necessary to process and act on increasingly complex information [13, 14]. These skills enable individuals to effectively regulate their attention and emotions, and engage in complex problem solving. Working memory is a central component of many of these behaviors, and involves cognitively holding information and manipulating it online. Deficits in this process may result in an inability to engage in some of these age-appropriate and essential behaviors, and may be a factor in psychosocial adjustment [15, 16]. Survivors of ALL have been found to display deficits in a variety of executive functions, including working memory [1]. These deficits have been hypothesized to develop as a result of chemotherapeutic and/or corticosteroid agents that are administered during prophylactic treatment.
This study examined patterns of brain activation during a working memory task of healthy children and survivors of childhood ALL who had shown evidence of cognitive deficits in several aspects of executive functioning during testing. The primary brain regions underlying working memory include regions of the prefrontal cortex and the anterior cingulate cortex (ACC), which has been identified as an area involved in incorporating task demands with an evaluative component, performance monitoring, detection of errors and error processing, detection of response conflict, and attention focusing [17, 18].
Between-group analyses of the magnitude of brain activation revealed that although similar regions were activated in working memory processing, survivors of ALL recruited greater amounts of oxygenated blood to the DLPFC and ACC during the task, specifically at greater levels of task difficulty. This suggests that, although these regions may still be functional in survivors, greater energy is required to perform to the same level as peers. We have conducted separate analyses of a test of behavioral inhibition [19] indicating a similar pattern of activation within another set of brain regions relevant to that task, suggesting that these are not overall changes in hemodynamic response after treatment, but rather tied to particular functions. These findings are consistent with theories of inefficient functioning or compensatory activation, which hypothesize that individuals with deficits in executive functions may compensate through recruitment of a greater supply of resources (i.e., oxygen) to facilitate task completion [20, 21, 22]. Staffen and colleagues suggested that this compensation indicates cerebral pathology, and serves to preserve functioning by connecting integrated parts of the systems of error processing and response inhibition [23]. Functional connectivity MRI may be useful for examining the extent of reliance on and the connections between multiple cortical areas by adolescents with potential deficits, and would allow for examination of a greater number of brain regions than in this region-of-interest analysis.
This study provides the first evidence of differences in brain function in survivors of pediatric ALL as compared with healthy controls and establishes the feasibility and acceptability of this method with this important population, as ALL survivors were able to tolerate a lengthy neuroimaging protocol. The application of these findings to the broader population of survivors of ALL is limited by the fact that we pre-screened our sample of survivors to include those who did show evidence of cognitive deficits. Because this is one of the first studies to use functional imaging methods to examine children who have undergone treatment for ALL, limited information is known about the neurological underpinnings of deficits, and we therefore focused on examining those with the most notable deficits in cognitive function. Research has suggested that these survivors may constitute a subgroup of ALL survivors [24]. Applications of these methods to a broader, more representative population of survivors along the entire spectrum of functioning may provide additional information in the larger context of survivorship and could allow for examination of the role of the specifics of treatment protocols in acquired deficits [25]. Future research utilizing diffusion tensor imaging is necessary to examine specific effects on various aspects of brain development (i.e., the effects of chemotherapy on glial cells and myelination), and may be a useful tool in examining differences in white matter densities between groups. Exposure to the toxic agents used in cancer treatment may be especially problematic when they are administered while children’s brains are still undergoing active development.
In summary, the findings of the present study suggest that fMRI offers an important tool for understanding the mechanisms that may underlie the cognitive deficits that have been observed in survivors of pediatric ALL [1, 2]. Additional research involving more difficult cognitive tasks during neuroimaging, a more diverse sample of survivors, and potentially a larger sample to facilitate group comparisons on assessment and questionnaire data, would be useful in informing the medical care of long-term survivors. Late-effects clinics for survivors of pediatric cancer are becoming more prevalent in healthcare settings as survival rates increase, and the provision of more substantial evidence to suggest the resources needed by survivors is essential. By providing survivors with optimal levels of medical and psychosocial care, keeping in mind the varying levels of functioning individuals may be experiencing, long term psychological consequences of pediatric cancer can be reduced and quality of life improved. Finally, the identification of sources of individual differences in susceptibility to neurotoxic effects, including genetic differences in the metabolism of methotrexate and other treatment agents, is needed.
Figure 2.
Between Group Differences in D-ACC Activation on the 3 vs. 1 N-back Contrast During Neuroimaging. Differences in activation in the 3-back versus 1-back conditions for survivors of ALL (left column) and control subjects (right column). Yellow-to-orange clusters indicate areas of significantly greater activation in the 3-back condition relative to the 1-back conditions. Red circles identify the region of interest (D-ACC).
Table I.
Treatment Information for Survivors of Childhood ALL
| ALL Participant Information |
Age at Testing |
Age at Diagnosis |
Gender | Protocol |
|---|---|---|---|---|
| 1 | 14.47 | 2.90 | F | COG 1922 |
| 2 | 13.58 | 6.95 | F | COG 1952 |
| 3 | 15.84 | 5.41 | M | COG 1922 |
| 4 | 11.75 | 3.17 | F | COG 1952 |
| 5 | 15.74 | 4.96 | F | COG 1922 |
| 6 | 16.90 | 5.54 | M | COG 1922 |
| 7 | 13.92 | 3.74 | M | COG 1952 |
| 8 | 10.20 | 6.71 | M | COG 1991 |
Table II.
Significant Between-Group Differences in BOLD fMRI Responses, by Contrast and Region, for Survivors and Controls
| Talairach Coordinatesa | ||||||||
|---|---|---|---|---|---|---|---|---|
| Region | BA | x | y | z | t | p | # Voxels | |
| ALL > Control | ||||||||
| 2-0 | DLPFC | 9 | - | - | - | 10.879 | <.00001 | 533 |
| D-ACC | 32 | −4.7 | 31 | 25 | 9.442 | <.000001 | 247 | |
| DLPFC | 46 | −39 | 35 | 21 | 9.211 | <.000001 | 103 | |
| 2-1 | D-ACC | 32 | −1 | 48 | 7.9 | 8.552 | <.000001 | 148 |
| 3-1 | DLPFC | 9 | 11 | 42 | 20 | 9.386 | <.000001 | 389 |
| D-ACC | 32 | −3.4 | 18 | 32 | 9.450 | <.000001 | 226 | |
Note. DLPFC = Dorsolateral Prefrontal Cortex; VLPFC = Ventrolateral Prefrontal Cortex; V-ACC = Ventral Anterior Cingulate Cortex; D-ACC = Dorsal Anterior Cingulate Cortex; IFG = Inferior Frontal Gyrus.
Talairach coordinates are provided for brain regions in which only one cluster was significantly activated. For brain regions with multiple active clusters, a single center of gravity was not identified.
Acknowledgements
This research was supported by a grant from the Vanderbilt Ingram Cancer Center National Cancer Institute Core grant (CA068485) and a gift from Patricia and Rodes Hart. The authors are grateful to Drs. Frances Niarhos and Debbie Van Slyke for their contributions to this work.
Footnotes
Conflict of Interest The authors have no conflicts of interest to declare.
References
- 1.Campbell LK, Scaduto M, Sharp W, et al. A meta-analysis of the neurocognitive sequelae of treatment for childhood acute lymphocytic leukemia. Pediatr Blood Cancer. 2007;49:65–73. doi: 10.1002/pbc.20860. [DOI] [PubMed] [Google Scholar]
- 2.Peterson CC, Johnson CE, Ramirez LY, et al. A meta-analysis of the neuropsychological sequelae of chemotherapy-only treatment for pediatric acute lymphoblastic leukemia. Pediatr Blood Cancer. 2008;51:99–104. doi: 10.1002/pbc.21544. [DOI] [PubMed] [Google Scholar]
- 3.Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hertzberg H, Huk WJ, Ueberall MA, et al. CNS late effects after ALL therapy in childhood 1: Neuroradiological findings in long-term survivors of childhood ALL: An evaluation of the interferences between morphology and neuropsychological performance. Med Pediatr Oncol Suppl. 1997;28:387–400. doi: 10.1002/(sici)1096-911x(199706)28:6<387::aid-mpo1>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 5.Porto L, Kieslich M, Schwabe D, et al. Central nervous system imaging in childhood leukaemia. Eur J Cancer. 2004;40:2082–2090. doi: 10.1016/j.ejca.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 6.Carey ME, Haut MW, Reminger SL, et al. Reduced frontal white matter volume in long-term childhood leukemia survivors: A voxel-based morphomentry study. Am J Neuroradiol. 2008;29:792–797. doi: 10.3174/ajnr.A0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paakko E, Lehtinen S, Harila-Saari A, et al. Perfusion MRI and SPECT of brain after treatment for childhood acute lymphoblastic leukemia. Med Pediatr Oncol. 2003;40:88–92. doi: 10.1002/mpo.10210. [DOI] [PubMed] [Google Scholar]
- 8.Wechsler D. Wechsler Intelligence Scale for Children – Fourth Edition: Technical and interpretive manual. The Psychological Corporation; San Antonio: 2003. [Google Scholar]
- 9.Delis DC, Kaplan E, Kramer JH. Examiner’s manual for the Delis-Kaplan Executive Function System. The Psychological Corporation; San Antonio: 2001. [Google Scholar]
- 10.Barch DM, Sheline YI, Csernansky JG, Snyder AZ. Working memory and prefrontal cortex dysfunction: Specificity to schizophrenia compared with major depression. Biol Psychiatry. 2003;53:376–384. doi: 10.1016/s0006-3223(02)01674-8. [DOI] [PubMed] [Google Scholar]
- 11.Goebel R, Esposito F, Formisano E. Analysis of functional image analysis contest (FIAC) data with Brainvoyager QX: From single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Hum Brain Mapp. 2006;27:392–401. doi: 10.1002/hbm.20249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Talairach TJ, Tournoux P. Co-planar stereotaxic atlas of the human brain. 3-Dimensional proportional system: An approach to cerebral imaging. 1988. Thime.
- 13.Luna B, Garver KE, Urban TA, et al. Maturation of cognitive processes from late childhood to adulthood. Child Dev. 2004;75:1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- 14.Luna B, Sweeney JA. The emergence of collaborative brain function: fMRI studies of the development of response inhibition. Ann N Y Acad Sci. 2004;1021:296–309. doi: 10.1196/annals.1308.035. [DOI] [PubMed] [Google Scholar]
- 15.Eisenberg N, Valiente C, Fabes RA, et al. The relations of effortful control and ego-control to children’s resiliency and social functioning. Dev Psychol. 2003;39:761–776. doi: 10.1037/0012-1649.39.4.761. [DOI] [PubMed] [Google Scholar]
- 16.Rothbart MK, Bates JE. Temperament. In: Damon W, Eisenberg N, editors. Handbook of child psychology: Social, emotional, and personality development. Wiley; New York: 1998. pp. 105–176. [Google Scholar]
- 17.Cacioppo JT, Amaral DG, Blanchard JJ, et al. Social neuroscience: Progress and implications for mental health. Perspectives on Psychological Science. 2007;2:99–123. doi: 10.1111/j.1745-6916.2007.00032.x. [DOI] [PubMed] [Google Scholar]
- 18.Staffen W, Zauner H, Mair A, et al. Magnetic resonance spectroscopy of memory and frontal brain region in early multiple sclerosis. J Neuropsychiatry Clin Neurosci. 2005;17:357–363. doi: 10.1176/jnp.17.3.357. [DOI] [PubMed] [Google Scholar]
- 19.Livesay KL, Robinson KE, Campbell LK, et al. Neurocognitive effects in childhood survivors of acute lymphocytic leukemia: A functional neuroimaging study of inhibitory interference control. 2009. Manuscript submitted for publication.
- 20.Audoin B, Ibarrola D, Ranjeva JP, et al. Compensatory cortical activation observed by fMRI during a cognitive task at the earliest stage of MS. Hum Brain Mapp. 2003;20:51–58. doi: 10.1002/hbm.10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sweet LH, Rao SM, Primeau M, et al. Functional magnetic resonance imaging response to increased verbal working memory demands among patients with multiple sclerosis. Hum Brain Mapp. 2006;27:28–36. doi: 10.1002/hbm.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zou P, Mulhern RK, Butler RW, et al. BOLD responses to visual stimulation in survivors of childhood cancer. NeuroImage. 2005;24:61–69. doi: 10.1016/j.neuroimage.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 23.Staffen W, Mair A, Zauner H, et al. Cognitive function and fMRI in patients with multiple sclerosis: Evidence for compensatory cortical activation during an attention task. Brain. 2002;125:1275–1282. doi: 10.1093/brain/awf125. [DOI] [PubMed] [Google Scholar]
- 24.Robison LL, Green DM, Hudson M, et al. Longer-term outcomes of adult survivors of childhood cancer: Results from the Childhood Cancer Survivor Study. Cancer. 2005;104:2557–2564. doi: 10.1002/cncr.21249. [DOI] [PubMed] [Google Scholar]
- 25.Compas BE. Psychobiological processes of stress and coping: Implications for resilience in childhood and adolescence. Ann N Y Acad Sci. 2006;1094:226–234. doi: 10.1196/annals.1376.024. [DOI] [PubMed] [Google Scholar]