Abstract
Purpose of review
Vitamin D is best known for its role in regulating calcium homeostasis and in strengthening bone. However, it has become increasingly clear that it also has important beneficial effects beyond the skeleton, including muscle. This review summarizes current knowledge about the role of vitamin D in skeletal muscle tissue and physical performance.
Recent findings
Molecular mechanisms of vitamin D action in muscle tissue include genomic and non-genomic effects via a receptor present in muscle cells. Knockout mouse models of the vitamin D receptor provide insight into understanding the direct effects of vitamin D on muscle tissue. Vitamin D status is positively associated with physical performance and inversely associated with risk of falling. Vitamin D supplementation has been shown to improve tests of muscle performance, reduce falls, and possibly impact on muscle fiber composition and morphology in vitamin D deficient older adults.
Summary
Further studies are needed to fully characterize the underlying mechanisms of vitamin D action in human muscle tissue, to understand how these actions translate into changes in muscle cell morphology and improvements in physical performance, and to define the 25-hydroxyvitamin D level at which to achieve these beneficial effects in muscle.
Keywords: Vitamin D, Skeletal muscle, Vitamin D receptor, Physical performance
Introduction
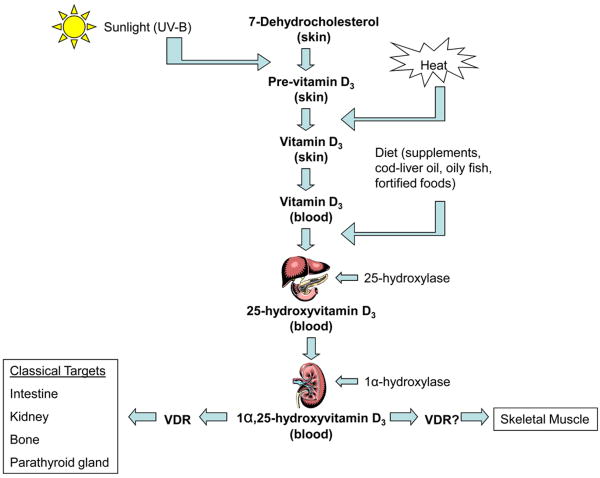
Vitamin D is involved in the regulation of calcium homeostasis and bone metabolism by exerting its actions on target tissues including the intestine, the kidney, and bone (Figure 1) [1]. Increasing evidence indicates that vitamin D plays an essential role in many other tissues including skeletal muscle. Early clinical descriptions of a myopathy associated with severe vitamin D deficiency recognized a potential association between vitamin D and muscle [2]. The myopathy has been characterized by proximal muscle weakness, muscle wasting, and a waddling gait [3]. In early studies, symptoms were found to be responsive to treatment with vitamin D suggesting that vitamin D played an etiological role; however, the underlying mechanisms remained undefined [4,5]. In the last several decades, a growing number of clinical studies of the muscular effects of vitamin D supplementation and research on the vitamin D receptor in muscle cells have helped to improve our understanding of the role and actions of vitamin D in muscle tissue and on physical performance. This review summarizes the potential underlying mechanisms of vitamin D activity in muscle tissue and the clinical evidence of an association between vitamin D status and muscle strength and performance.
Figure 1.
Synthesis of vitamin D3 occurs in the skin where 7-dehydrocholesterol is converted to pre-vitamin D3 in response to sunlight (ultraviolet B radiation) exposure. Vitamin D3 is produced from the isomerization of pre-vitamin D3 in the skin or intestinal absorption of natural and fortified foods and supplements. Vitamin D3 (bound to vitamin D-binding protein) circulates in the bloodstream, and is transported to the liver where it is hydroxylated by liver 25-hydroxylases. The resultant 25-hydroxyvitamin D3 is hydroxylated to the active secosteroid 1α,25(OH)2D3 in the kidney by 1α-hydroxylase. 1α,25(OH)2D3 acts on various target tissues via its receptor (VDR). 1α, 25(OH)2D3 appears to affect other nonclassical target tissues such as skeletal muscle possibly via the VDR.
Molecular Mechanisms of Vitamin D Activity
In its biologically active form, 1,25-dihydroxyvitamin D [1,25(OH)2D], exerts its actions by binding to a vitamin D receptor (VDR). Investigators have identified the VDR in both animal and human muscle tissues [6-9]. There are a well-described 1,25(OH)2D nuclear receptor and a less clearly defined cell membrane receptor which mediates the rapid nongenomic actions [10].
At the genomic level, 1,25(OH)2D binds to its nuclear receptor which results in changes in the gene transcription of mRNA and subsequent de novo protein synthesis [11]. The activation of VDR induces the heterodimerization between the active VDR and an orphan steroid receptor known as retinoic receptor (RXR) [11]. The formation of this heterodimer facilitates the interaction between the receptor's zinc finger region with DNA activating the protein transcription process [12]. The genomic pathway influences muscle calcium transport [13-20] and phospholipid metabolism [16,21,22].
1,25(OH)2D also has rapid non-transcriptional effects that cannot be explained by a slow genetic pathway. There is evidence supporting the presence of a cell surface receptor which mediates 1,25(OH)2D's rapid effects [23]. The characterization of this receptor remains somewhat controversial [16]. Thus far, it has been proposed that the initiation of the fast 1,25(OH)2D signal may involve binding to a novel membrane receptor [24] and/or the VDR itself which is translocated from the nucleus to the cell surface [25]. At the nongenomic level, 1,25(OH)2D activates several interacting second-messenger pathways that transmit the signal to the cytoplasm. These rapid effects also have been found to influence calcium transport and regulate intracellular calcium [26-33].
Other data indicate that 1,25(OH)2D promotes the fast activation of mitogen-activated protein kinase (MAPK) signaling pathways [34,35], which result in initiation of myogenesis, cell proliferation, differentiation, or apoptosis. In mammalian cells, the MAPK family has four different subgroups: extracellular signal-regulated kinases (ERKs 1/2), c-Jun N-terminal kinases (JNK), ERK5, and p38 MAPK [35]. When activated, these MAPKs regulate cell processes through phosphorylation of other kinases, proteins, and transcription factors. 1,25(OH)2D activates the ERK pathway through phosphorylation by several kinases, such as c-Src, Raf-1, Ras, and MAPKK [36,37]. Through these mechanisms, 1,25(OH)2D stimulates muscle cell proliferation and growth [36,38]. A recent in vivo study in rats suggested that vitamin D3 given over 8 weeks reduced exercise-induced apoptosis in gastrocnemius muscle [39].
VDR Knockout Mouse Model
VDR null mutant mice are characterized by growth retardation, osteomalacia, muscle impairment, and systemic metabolic changes such as secondary hyperparathyroidism and hypocalcemia [40]. VDR null mutant mice have muscle fiber diameters that are 20% smaller and more variable in size than those of wild type mice at 3 weeks of age (prior to weaning) [41]. By 8 weeks of age, these muscle fiber changes are more prominent in the VDR null mutant mice compared to the wild type suggesting either that these abnormalities progress over time or that as these mice age the metabolic alterations that occur contribute to the morphological changes [41]. The muscle fiber abnormalities are noted diffusely without any preference for type I or II fibers, differing from the human hypovitaminosis D myopathy with a predominance of type II fiber loss. At 3 weeks of age, VDR null mutant mice also demonstrate abnormally high expression of myogenic differentiation factors compared to wild type mice [41], thus suggesting alterations in muscle cell differentiation pathways resulting in abnormal muscle fiber development and maturation.
Other features of the VDR knockout phenotype include poor swimming ability (a well-known method to assess motor/balance functions in rodents) [42,43]. Initially this finding was attributed to muscular/motor impairments; however, a recent study by Minasyan et al. considers whether impaired vestibular function in the VDR null mutant mice may be a key factor [43]. Via immunohistochemical analysis, Minasyan et al. identified VDR-positive nuclei in epithelium of different structures in the vestibular system in wild-type mice and a significantly reduced expression of VDR in these structures in the VDR null mutant mice [43]. To further support the presence of a vestibular deficit in the VDR knockout, the VDR mutant mice had significantly greater abnormalities in postural control on balance tests such as the accelerating rotarod and tilting platform, compared to wild type mice [43]. These findings suggest another mechanism, loss of vestibular function, in the pathway to poor muscle performance and falls seen in humans with low 25(OH)D levels as discussed later in this review.
Effect of Vitamin D Status on Muscle Histology
Biopsies of skeletal muscle in adults with vitamin D deficiency have shown predominantly type II muscle fiber atrophy [3]. Type II muscle fibers are fast-twitch and are the first to be recruited to prevent a fall. Muscle tissue sections of vitamin D deficient individuals reveal enlarged interfibrillar spaces and infiltration of fat, fibrosis and glycogen granules [44]. Vitamin D supplementation may have an impact on muscle fiber composition. In a small uncontrolled study, Sorenson et al. [45] reported an increase in relative fiber composition and in fiber area of type IIa muscle fibers in muscle biopsies from elderly women after treatment with 1-α-hydroxyvitamin D and calcium for 3-6 months. A randomized, controlled study found that treatment of 48 elderly stroke survivors with 1000 IU of vitamin D2 daily significantly increased mean type II muscle fiber diameter and percentage of type II fibers over a 2 year period [46]. There was also a correlation between serum 25(OH)D level and type II muscle fiber diameter both at baseline and after two years of follow-up. It remains unclear, however, if the increase in type II muscle fiber number is caused by new formation of type II fibers or a transition of already existing fibers from type I to type II.
Effects of Vitamin D on Physical Performance
Multiple cross-sectional studies in community-dwelling older adults have found a direct association between vitamin D status and parameters of physical performance, especially when 25(OH)D levels are <75 nmol/l [47-52]. A recent cross-sectional analysis of the Longitudinal Study of Aging Amsterdam (LASA) reported a 25(OH)D threshold of 60 nmol/l for improvement in physical performance [51]. Whereas in an analysis of the NHANES III survey, elderly individuals with higher serum 25(OH)D levels up to 94 nmol/l showed better lower extremity muscle performance than subjects with lower levels [47], particularly in the subset with 25(OH)D levels <60 nmol/l.
In a prospective analysis of the LASA, older adults with lower serum 25(OH)D (<50 nmol/l) were found to be at increased risk of a decline in physical performance over three years compared to those with higher levels (≥75 nmol/l) [49,53]. In a prospective analysis of the Rancho Bernardo Study cohort, a population with higher baseline 25(OH)D levels, older women with 25(OH)D levels ≤80 nmol/l performed more poorly on lower extremity muscle performance tests compared to women with the highest 25(OH)D levels ≥115 nmol/l over a 2.5-year period [54]. Interestingly, the association was not seen in men [54]. A longitudinal survey of community-dwelling Japanese older women with impairments in physical function reported that higher baseline 25(OH)D levels (defined as >67.5 nmol) were associated with improvements in physical fitness after 3 months of an exercise program [55]. An observational study in older Italians, on the other hand, found a relationship between vitamin D status and measures of frailty in older men, but not women [50].
Two recent studies in adolescent girls [56,57] suggest that the effect of vitamin D on muscle performance may not be unique to older individuals. Ward et al. reported a direct relationship between 25(OH)D levels and muscle power, force, velocity, and jump height in 99 post-menarchal 12-14 year-old girls in the United Kingdom [57]. Of note, most of the girls had low 25(OH)D levels with a mean of 21.3 nmol/l and the analyses were not adjusted for physical activity [57]. The study by Foo et al. also found a similar positive relationship between 25(OH)D levels and handgrip strength after adjusting for physical activity in 301 adolescent girls with a mean age of 15 and serum 25(OH)D levels of 34 nmol/l [56]. Other studies combining younger and older women did not find a correlation between vitamin D status and handgrip strength [58] or other tests of physical performance [59].
Randomized clinical trials have examined the effect of vitamin D supplementation on tests of physical performance [46,60-62]. Specifically, vitamin D with calcium, compared to calcium alone, improved body sway by 9% in ambulatory elderly women with serum 25(OH)D levels <50 nmol/L within 8 weeks [60] and improved lower extremity muscle performance in institutionalized elders with serum 25(OH)D levels <50 nmol/L by 4-11% within 12 weeks [61]. Similarly, in a recent longer-term study among healthy older men and women with serum 25(OH)D levels <75 nmol/L, vitamin D3 800 IU and calcium 1000 mg daily, compared to calcium alone, significantly improved tests of physical performance over a 12-20-month period [62]. A randomized trial in 179 pre-menarchal girls, age 10-17, who received either oral vitamin D3 1,400 IU/week, vitamin D3 14,000 IU/week or placebo for 1 year, reported an increase in whole body lean mass (a surrogate marker of muscle mass) in supplemented girls [63].
Vitamin D and Falls
Given the relationship between 25(OH)D level and physical performance, one would expect a similar link when examining fall risk. In the LASA cohort, low 25(OH)D levels (<25 nmol/L) were associated with an increased risk of repeated falling over the subsequent year, particularly in persons under 75 years of age [64]. A similar association has been replicated in varied older populations [52,65-67]. In a randomized, controlled trial, Bischoff et al. showed that treatment with vitamin D3 and calcium (800 IU and 1200 mg per day) for 3 months reduced the risk of falls by 49% compared to calcium alone [61]. Similarly in an Australian study, treatment with vitamin D2 (initially 10,000 IU per week then 1000 IU per day) and calcium (600 mg per day) for 2 years reduced the risk of falls in the compliant group by 30% compared to calcium alone [68]. A recent large clinical trial in 242 healthy older seniors with 25(OH)D levels <75 nmol/l demonstrated that long-term supplementation with vitamin D3 and calcium (800 IU and 1000 mg per day), versus calcium alone, resulted in a 39% decrease in the number of subjects with first fall over a 20-month period [62]. In a meta-analysis of five randomized controlled trials, including over 1200 ambulatory and institutionalized subjects, vitamin D supplementation of 700 IU or greater lowered the risk of falling by 22% [69].
Other experimental studies using vitamin D in various doses did not observe significant effects on falls, but falls were not the primary outcomes in these studies, adherence to treatment was poor, and 25(OH)D levels achieved were suboptimal [70,71]. These two very large negative studies were pooled along with twelve other studies in a recent narrative review [72] and a recent Cochrane review [73], both examining the effect of vitamin D on falls. As a result, effects of vitamin D supplementation had a minimal to no benefit on falls [72,73].
VDR Polymorphisms and Muscle
Subtle variations in DNA sequence of the VDR gene, also known as VDR polymorphisms, are associated with a series of biological characteristics including muscle strength. For example, FokI is a polymorphism involving a T/C transition in exon 2 of the VDR gene [74]. Individuals with the C allele (“F”) have a shorter VDR than do those with the T (“f”) allele. The shorter VDR is associated with enhanced VDR transactivation capacity as a transcription factor [75], which would suggest a possible improvement in muscle strength in light of the clinical data reporting a positive association between vitamin D status and muscle strength. On the contrary, the C allele is associated with reduced fat-free mass and quadriceps strength in healthy elderly men [76] and elderly individuals with COPD [74].
BsmI, a restriction fragment length polymorphism at the 3′ end of the VDR gene, has also been associated with muscle performance. The 3′ end is known to play an important role in regulating gene expression. Young healthy women with the bb allele, which may be associated with higher VDR activity in combination with the C allele of FokI, were found to have lower fat-free mass and hamstring (but not quadriceps) strength compared to those with the BB allele [77]. In non-obese older women aged 70 and older, those with the bb genotype were found to have a 7% higher grip strength and a 23% higher quadriceps strength than those with BB genotype [78]. Why the allele associated with higher VDR activity would be found to have reduced muscle strength remains unclear.
Conclusion
Vitamin D and its receptor are important for normal skeletal muscle development and in optimizing muscle strength and performance. Supplementation with various forms of vitamin D in older adults has mostly shown reduction in falls risk and improvements in tests of muscle performance. Despite these promising data, further research is needed to fully characterize the underlying mechanisms of vitamin D action on human muscle tissue, to understand how these actions translate into changes in muscle cell morphology and improvements in physical performance, and to define the 25-hydroxyvitamin D level at which to achieve these beneficial effects in muscle.
Acknowledgments
This material is based upon work supported by the U.S. Department of Agriculture, Agricultural Research Service, under agreement No. 58-1950-7-707. Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Dept of Agriculture.
The author is supported by “Boston OAIC: A Translational Approach to Function Promoting Anabolic Therapies” (P30 AG031679) and NIH T32 DK007651.
Footnotes
Disclosure Statement: The author has nothing to disclose.
References
- 1.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80:1689S–1696S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 2.Prineas JW, Mason AS, Henson RA. Myopathy in Metabolic Bone Disease. Br Med J. 1965;1:1034–1036. doi: 10.1136/bmj.1.5441.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boland R. Role of vitamin D in skeletal muscle function. Endocr Rev. 1986;7:434–448. doi: 10.1210/edrv-7-4-434. [DOI] [PubMed] [Google Scholar]
- 4.Smith R, Stern G. Myopathy, osteomalacia and hyperparathyroidism. Brain. 1967;90:593–602. doi: 10.1093/brain/90.3.593. [DOI] [PubMed] [Google Scholar]
- 5.Ekbom K, Hed R, Kirstein L, Astroem KE. Weakness of Proximal Limb Muscles, Probably Due to Myopathy after Partial Gastrectomy. Preliminary Report Acta Med Scand. 1964;176:493–496. [PubMed] [Google Scholar]
- 6.Simpson RU, Thomas GA, Arnold AJ. Identification of 1,25-dihydroxyvitamin D3 receptors and activities in muscle. J Biol Chem. 1985;260:8882–8891. [PubMed] [Google Scholar]
- 7.Boland R, Norman A, Ritz E, Hasselbach W. Presence of a 1,25-dihydroxy-vitamin D3 receptor in chick skeletal muscle myoblasts. Biochem Biophys Res Commun. 1985;128:305–311. doi: 10.1016/0006-291x(85)91679-1. [DOI] [PubMed] [Google Scholar]
- 8.Costa EM, Blau HM, Feldman D. 1,25-dihydroxyvitamin D3 receptors and hormonal responses in cloned human skeletal muscle cells. Endocrinology. 1986;119:2214–2220. doi: 10.1210/endo-119-5-2214. [DOI] [PubMed] [Google Scholar]
- 9.Bischoff HA, Borchers M, Gudat F, et al. In situ detection of 1,25-dihydroxyvitamin D3 receptor in human skeletal muscle tissue. Histochem J. 2001;33:19–24. doi: 10.1023/a:1017535728844. [DOI] [PubMed] [Google Scholar]
- 10.Norman AW. Receptors for 1alpha,25(OH)2D3: past, present, and future. J Bone Miner Res. 1998;13:1360–1369. doi: 10.1359/jbmr.1998.13.9.1360. [DOI] [PubMed] [Google Scholar]
- 11.Freedman LP. Transcriptional targets of the vitamin D3 receptor-mediating cell cycle arrest and differentiation. J Nutr. 1999;129:581S–586S. doi: 10.1093/jn/129.2.581S. [DOI] [PubMed] [Google Scholar]
- 12.McCary LC, Staun M, DeLuca HF. A characterization of vitamin D-independent intestinal calcium absorption in the osteopetrotic (op/op) mouse. Arch Biochem Biophys. 1999;368:249–256. doi: 10.1006/abbi.1999.1286. [DOI] [PubMed] [Google Scholar]
- 13.Brunner A, de Boland AR. 1,25-Dihydroxyvitamin D3 affects the synthesis, phosphorylation and in vitro calmodulin binding of myoblast cytoskeletal proteins. Z Naturforsch [C] 1990;45:1156–1160. doi: 10.1515/znc-1990-11-1212. [DOI] [PubMed] [Google Scholar]
- 14.Walters MR, Ilenchuk TT, Claycomb WC. 1,25-Dihydroxyvitamin D3 stimulates 45Ca2+ uptake by cultured adult rat ventricular cardiac muscle cells. J Biol Chem. 1987;262:2536–2541. [PubMed] [Google Scholar]
- 15.Ebashi S, Endo M. Calcium ion and muscle contraction. Prog Biophys Mol Biol. 1968;18:123–183. doi: 10.1016/0079-6107(68)90023-0. [DOI] [PubMed] [Google Scholar]
- 16.Boland R. Vitamin D and Muscle. In: Feldman D, Pike JW, Glorieux GH, editors. Vitamin D. 2nd. Elsevier Inc.; 2005. pp. 883–897. [Google Scholar]
- 17.Boland R, de Boland AR, Marinissen MJ, et al. Avian muscle cells as targets for the secosteroid hormone 1,25-dihydroxy-vitamin D3. Mol Cell Endocrinol. 1995;114:1–8. doi: 10.1016/0303-7207(95)03650-v. [DOI] [PubMed] [Google Scholar]
- 18.de Boland AR, Boland R. In vitro cellular muscle calcium metabolism. Characterization of effects of 1,25-dihydroxy-vitamin D3 and 25-hydroxy-vitamin D3. Z Naturforsch [C] 1985;40:102–108. doi: 10.1515/znc-1985-1-220. [DOI] [PubMed] [Google Scholar]
- 19.Zanello SB, Boland RL, Norman AW. cDNA sequence identity of a vitamin D-dependent calcium-binding protein in the chick to calbindin D-9K. Endocrinology. 1995;136:2784–2787. doi: 10.1210/endo.136.6.7750504. [DOI] [PubMed] [Google Scholar]
- 20.Drittanti L, de Boland AR, Boland R. Stimulation of calmodulin synthesis in proliferating myoblasts by 1,25-dihydroxy-vitamin D3. Mol Cell Endocrinol. 1990;74:143–153. doi: 10.1016/0303-7207(90)90116-p. [DOI] [PubMed] [Google Scholar]
- 21.Drittanti L, de Boland AR, Boland RL. Effects of 1,25-dihydroxyvitamin D-3 on phospholipid metabolism in chick myoblasts. Biochim Biophys Acta. 1988;962:1–7. doi: 10.1016/0005-2760(88)90088-4. [DOI] [PubMed] [Google Scholar]
- 22.Drittanti L, de Boland AR, Boland R. Changes in muscle lipid metabolism induced in vitro by 1,25-dihydroxy-vitamin D-3. Biochim Biophys Acta. 1987;918:83–92. doi: 10.1016/0005-2760(87)90012-9. [DOI] [PubMed] [Google Scholar]
- 23.Nemere I. 24,25-dihydroxyvitamin D3 suppresses the rapid actions of 1, 25-dihydroxyvitamin D3 and parathyroid hormone on calcium transport in chick intestine. J Bone Miner Res. 1999;14:1543–1549. doi: 10.1359/jbmr.1999.14.9.1543. [DOI] [PubMed] [Google Scholar]
- 24.Nemere I, Dormanen MC, Hammond MW, et al. Identification of a specific binding protein for 1 alpha,25-dihydroxyvitamin D3 in basal-lateral membranes of chick intestinal epithelium and relationship to transcaltachia. J Biol Chem. 1994;269:23750–23756. [PubMed] [Google Scholar]
- 25.Capiati D, Benassati S, Boland RL. 1,25(OH)2-vitamin D3 induces translocation of the vitamin D receptor (VDR) to the plasma membrane in skeletal muscle cells. J Cell Biochem. 2002;86:128–135. doi: 10.1002/jcb.10191. [DOI] [PubMed] [Google Scholar]
- 26.Capiati DA, Vazquez G, Tellez Inon MT, Boland RL. Role of protein kinase C in 1,25(OH)(2)-vitamin D(3) modulation of intracellular calcium during development of skeletal muscle cells in culture. J Cell Biochem. 2000;77:200–212. doi: 10.1002/(sici)1097-4644(20000501)77:2<200::aid-jcb4>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 27.Morelli S, Boland R, de Boland AR. 1,25(OH)2-vitamin D3 stimulation of phospholipases C and D in muscle cells involves extracellular calcium and a pertussis-sensitive G protein. Mol Cell Endocrinol. 1996;122:207–211. doi: 10.1016/0303-7207(96)03886-5. [DOI] [PubMed] [Google Scholar]
- 28.de Boland AR, Boland RL. Rapid changes in skeletal muscle calcium uptake induced in vitro by 1,25-dihydroxyvitamin D3 are suppressed by calcium channel blockers. Endocrinology. 1987;120:1858–1864. doi: 10.1210/endo-120-5-1858. [DOI] [PubMed] [Google Scholar]
- 29.Selles J, Boland R. Rapid stimulation of calcium uptake and protein phosphorylation in isolated cardiac muscle by 1,25-dihydroxyvitamin D3. Mol Cell Endocrinol. 1991;77:67–73. doi: 10.1016/0303-7207(91)90059-2. [DOI] [PubMed] [Google Scholar]
- 30.Vazquez G, Boland R, de Boland AR. Modulation by 1,25(OH)2-vitamin D3 of the adenylyl cyclase/cyclic AMP pathway in rat and chick myoblasts. Biochim Biophys Acta. 1995;1269:91–97. doi: 10.1016/0167-4889(95)00097-c. [DOI] [PubMed] [Google Scholar]
- 31.Vazquez G, de Boland AR. Involvement of protein kinase C in the modulation of 1alpha,25-dihydroxy-vitamin D3-induced 45Ca2+ uptake in rat and chick cultured myoblasts. Biochim Biophys Acta. 1996;1310:157–162. doi: 10.1016/0167-4889(95)00158-1. [DOI] [PubMed] [Google Scholar]
- 32.Vazquez G, de Boland AR, Boland RL. 1 alpha,25-(OH)2-vitamin D3 stimulates the adenylyl cyclase pathway in muscle cells by a GTP-dependent mechanism which presumably involves phosphorylation of G alpha i. Biochem Biophys Res Commun. 1997;234:125–128. doi: 10.1006/bbrc.1997.6590. [DOI] [PubMed] [Google Scholar]
- 33.Vazquez G, de Boland AR, Boland R. Stimulation of Ca2+ release-activated Ca2+ channels as a potential mechanism involved in non-genomic 1,25(OH)2-vitamin D3-induced Ca2+ entry in skeletal muscle cells. Biochem Biophys Res Commun. 1997;239:562–565. doi: 10.1006/bbrc.1997.7501. [DOI] [PubMed] [Google Scholar]
- 34.Wu Z, Woodring PJ, Bhakta KS, et al. p38 and extracellular signal-regulated kinases regulate the myogenic program at multiple steps. Mol Cell Biol. 2000;20:3951–3964. doi: 10.1128/mcb.20.11.3951-3964.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79:143–180. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- 36.Buitrago C, Boland R, de Boland AR. The tyrosine kinase c-Src is required for 1,25(OH)2-vitamin D3 signalling to the nucleus in muscle cells. Biochim Biophys Acta. 2001;1541:179–187. doi: 10.1016/s0167-4889(01)00142-2. [DOI] [PubMed] [Google Scholar]
- 37.Buitrago CG, Pardo VG, de Boland AR, Boland R. Activation of RAF-1 through Ras and protein kinase Calpha mediates 1alpha,25(OH)2-vitamin D3 regulation of the mitogen-activated protein kinase pathway in muscle cells. J Biol Chem. 2003;278:2199–2205. doi: 10.1074/jbc.M205732200. [DOI] [PubMed] [Google Scholar]
- 38.Buitrago C, Gonzalez Pardo V, de Boland AR. Nongenomic action of 1 alpha,25(OH)(2)-vitamin D3. Activation of muscle cell PLC gamma through the tyrosine kinase c-Src and PtdIns 3-kinase. Eur J Biochem. 2002;269:2506–2515. doi: 10.1046/j.1432-1033.2002.02915.x. [DOI] [PubMed] [Google Scholar]
- 39.Kumar V, Mukhopadhyay S, Bedi PS, et al. A novel modulatory role of vitamin D3 in exercise-induced apoptosis of rat skeletal muscle. American Journal of Food Technology. 2008;3:361–372. [Google Scholar]
- 40.Burne TH, McGrath JJ, Eyles DW, Mackay-Sim A. Behavioural characterization of vitamin D receptor knockout mice. Behav Brain Res. 2005;157:299–308. doi: 10.1016/j.bbr.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 41.Endo I, Inoue D, Mitsui T, et al. Deletion of vitamin D receptor gene in mice results in abnormal skeletal muscle development with deregulated expression of myoregulatory transcription factors. Endocrinology. 2003;144:5138–5144. doi: 10.1210/en.2003-0502. [DOI] [PubMed] [Google Scholar]
- 42.Kalueff AV, Lou YR, Laaksi I, Tuohimaa P. Impaired motor performance in mice lacking neurosteroid vitamin D receptors. Brain Res Bull. 2004;64:25–29. doi: 10.1016/j.brainresbull.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 43.Minasyan A, Keisala T, Zou J, et al. Vestibular dysfunction in vitamin D receptor mutant mice. J Steroid Biochem Mol Biol. 2009;114:161–166. doi: 10.1016/j.jsbmb.2009.01.020. [DOI] [PubMed] [Google Scholar]; ** This study identified VDR expression in the vestibular system of wild type mice and decreased VDR expression in these tissues in a VDR knockout mouse model. VDR null mutant mice had poor swimming ability and performed poorly on balance tests compared to wild type mice, thus suggesting a vestibular deficit as an underlying cause.
- 44.Yoshikawa S, Nakamura T, Tanabe H, Imamura T. Osteomalacic myopathy. Endocrinol Jpn. 1979;26:65–72. doi: 10.1507/endocrj1954.26.supplement_65. [DOI] [PubMed] [Google Scholar]
- 45.Sorensen OH, Lund B, Saltin B, et al. Myopathy in bone loss of ageing: improvement by treatment with 1 alpha-hydroxycholecalciferol and calcium. Clin Sci (Lond) 1979;56:157–161. doi: 10.1042/cs0560157. [DOI] [PubMed] [Google Scholar]
- 46.Sato Y, Iwamoto J, Kanoko T, Satoh K. Low-dose vitamin D prevents muscular atrophy and reduces falls and hip fractures in women after stroke: a randomized controlled trial. Cerebrovasc Dis. 2005;20:187–192. doi: 10.1159/000087203. [DOI] [PubMed] [Google Scholar]
- 47.Bischoff-Ferrari HA, Dietrich T, Orav EJ, et al. Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged > or =60 y. Am J Clin Nutr. 2004;80:752–758. doi: 10.1093/ajcn/80.3.752. [DOI] [PubMed] [Google Scholar]
- 48.Gerdhem P, Ringsberg KA, Obrant KJ, Akesson K. Association between 25-hydroxy vitamin D levels, physical activity, muscle strength and fractures in the prospective population-based OPRA Study of Elderly Women. Osteoporos Int. 2005;16:1425–1431. doi: 10.1007/s00198-005-1860-1. [DOI] [PubMed] [Google Scholar]
- 49.Wicherts IS, van Schoor NM, Boeke AJ, et al. Vitamin D status predicts physical performance and its decline in older persons. J Clin Endocrinol Metab. 2007;92:2058–2065. doi: 10.1210/jc.2006-1525. [DOI] [PubMed] [Google Scholar]
- 50.Shardell M, Hicks GE, Miller RR, et al. Association of low vitamin D levels with the frailty syndrome in men and women. J Gerontol A Biol Sci Med Sci. 2009;64:69–75. doi: 10.1093/gerona/gln007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuchuk NO, Pluijm SM, van Schoor NM, et al. Relationships of serum 25-hydroxyvitamin D to bone mineral density and serum parathyroid hormone and markers of bone turnover in older persons. J Clin Endocrinol Metab. 2009;94:1244–1250. doi: 10.1210/jc.2008-1832. [DOI] [PubMed] [Google Scholar]; * A cross-sectional analysis of the LASA cohort showed a relationship between 25(OH)D levels and physical performance and a 25(OH)D threshold of 60 nmol/l.
- 52.Suzuki T, Kwon J, Kim H, et al. Low serum 25-hydroxyvitamin D levels associated with falls among Japanese community-dwelling elderly. J Bone Miner Res. 2008;23:1309–1317. doi: 10.1359/jbmr.080328. [DOI] [PubMed] [Google Scholar]
- 53.Visser M, Deeg DJ, Lips P. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the Longitudinal Aging Study Amsterdam. J Clin Endocrinol Metab. 2003;88:5766–5772. doi: 10.1210/jc.2003-030604. [DOI] [PubMed] [Google Scholar]
- 54.Dam TT, von Muhlen D, Barrett-Connor EL. Sex-specific association of serum vitamin D levels with physical function in older adults. Osteoporos Int. 2009;20:751–760. doi: 10.1007/s00198-008-0749-1. [DOI] [PMC free article] [PubMed] [Google Scholar]; A prospective study in the Rancho Bernardo cohort with higher 25(OH)D levels showed a decline in physical performance in women with lower 25(OH)D levels, but not men.
- 55.Okuno J, Tomura S, Yabushita N, et al. Effects of serum 25-hydroxyvitamin D(3) levels on physical fitness in community-dwelling frail women. Arch Gerontol Geriatr. 2009 doi: 10.1016/j.archger.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 56.Foo LH, Zhang Q, Zhu K, et al. Low vitamin D status has an adverse influence on bone mass, bone turnover, and muscle strength in Chinese adolescent girls. J Nutr. 2009;139:1002–1007. doi: 10.3945/jn.108.102053. [DOI] [PubMed] [Google Scholar]; * A cross-sectional study in adolescent Chinese girls with low 25(OH)D levels found a direct association between 25(OH) level and hand grip strength.
- 57.Ward KA, Das G, Berry JL, et al. Vitamin D status and muscle function in post-menarchal adolescent girls. J Clin Endocrinol Metab. 2009;94:559–563. doi: 10.1210/jc.2008-1284. [DOI] [PubMed] [Google Scholar]; ** A cross-sectional study in 99 post-menarchal girls with low 25(OH)D levels found a direct association between 25(OH) level and muscle power, force, velocity and jump height.
- 58.Allali F, El Aichaoui S, Khazani H, et al. High prevalence of hypovitaminosis D in Morocco: relationship to lifestyle, physical performance, bone markers, and bone mineral density. Semin Arthritis Rheum. 2009;38:444–451. doi: 10.1016/j.semarthrit.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 59.Garnero P, Munoz F, Sornay-Rendu E, Delmas PD. Associations of vitamin D status with bone mineral density, bone turnover, bone loss and fracture risk in healthy postmenopausal women. The OFELY study Bone. 2007;40:716–722. doi: 10.1016/j.bone.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 60.Pfeifer M, Begerow B, Minne HW, et al. C: Effects of a short-term vitamin D and calcium supplementation on body sway and secondary hyperparathyroidism in elderly women. J Bone Miner Res. 2000;15:1113–1118. doi: 10.1359/jbmr.2000.15.6.1113. [DOI] [PubMed] [Google Scholar]
- 61.Bischoff HA, Stahelin HB, Dick W, et al. Effects of vitamin D and calcium supplementation on falls: a randomized controlled trial. J Bone Miner Res. 2003;18:343–351. doi: 10.1359/jbmr.2003.18.2.343. [DOI] [PubMed] [Google Scholar]
- 62.Pfeifer M, Begerow B, Minne HW, et al. Effects of a long-term vitamin D and calcium supplementation on falls and parameters of muscle function in community-dwelling older individuals. Osteoporos Int. 2009;20:315–322. doi: 10.1007/s00198-008-0662-7. [DOI] [PubMed] [Google Scholar]; ** A randomized, controlled trial in 242 community-dwelling older men and womed with 25(OH)D levels <75 nmol/l reported that vitamin D3 800 IU and calcium 1000 mg daily, versus calcium alone, led to a 39% decrease in number of subjects with a first fall and an improvement in physical performance over a 20 month period.
- 63.El-Hajj Fuleihan G, Nabulsi M, Tamim H, et al. Effect of vitamin D replacement on musculoskeletal parameters in school children: a randomized controlled trial. J Clin Endocrinol Metab. 2006;91:405–412. doi: 10.1210/jc.2005-1436. [DOI] [PubMed] [Google Scholar]
- 64.Snijder MB, van Schoor NM, Pluijm SM, et al. Vitamin D status in relation to one-year risk of recurrent falling in older men and women. J Clin Endocrinol Metab. 2006;91:2980–2985. doi: 10.1210/jc.2006-0510. [DOI] [PubMed] [Google Scholar]
- 65.Dhesi JK, Bearne LM, Moniz C, et al. Neuromuscular and psychomotor function in elderly subjects who fall and the relationship with vitamin D status. J Bone Miner Res. 2002;17:891–897. doi: 10.1359/jbmr.2002.17.5.891. [DOI] [PubMed] [Google Scholar]
- 66.Stein MS, Wark JD, Scherer SC, et al. Falls relate to vitamin D and parathyroid hormone in an Australian nursing home and hostel. J Am Geriatr Soc. 1999;47:1195–1201. doi: 10.1111/j.1532-5415.1999.tb05199.x. [DOI] [PubMed] [Google Scholar]
- 67.LeBoff MS, Hawkes WG, Glowacki J, et al. Vitamin D-deficiency and post-fracture changes in lower extremity function and falls in women with hip fractures. Osteoporos Int. 2008;19:1283–1290. doi: 10.1007/s00198-008-0582-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Flicker L, MacInnis RJ, Stein MS, et al. Should older people in residential care receive vitamin D to prevent falls? Results of a randomized trial J Am Geriatr Soc. 2005;53:1881–1888. doi: 10.1111/j.1532-5415.2005.00468.x. [DOI] [PubMed] [Google Scholar]
- 69.Bischoff-Ferrari HA, Dawson-Hughes B, Willett WC, et al. Effect of Vitamin D on falls: a meta-analysis. Jama. 2004;291:1999–2006. doi: 10.1001/jama.291.16.1999. [DOI] [PubMed] [Google Scholar]
- 70.Porthouse J, Cockayne S, King C, et al. Randomised controlled trial of calcium and supplementation with cholecalciferol (vitamin D3) for prevention of fractures in primary care. Bmj. 2005;330:1003. doi: 10.1136/bmj.330.7498.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grant AM, Avenell A, Campbell MK, et al. Oral vitamin D3 and calcium for secondary prevention of low-trauma fractures in elderly people (Randomised Evaluation of Calcium Or vitamin D, RECORD): a randomised placebo-controlled trial. Lancet. 2005;365:1621–1628. doi: 10.1016/S0140-6736(05)63013-9. [DOI] [PubMed] [Google Scholar]
- 72.Cranney A, Weiler HA, O'Donnell S, Puil L. Summary of evidence-based review on vitamin D efficacy and safety in relation to bone health. Am J Clin Nutr. 2008;88:513S–519S. doi: 10.1093/ajcn/88.2.513S. [DOI] [PubMed] [Google Scholar]; * This narrative review examined 14 randomized trials on the effect of vitamin D supplementation on falls risk and found a small benefit.
- 73.Gillespie LDRM, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database of Systematic Reviews. 2009 doi: 10.1002/14651858.CD007146.pub2. [DOI] [PubMed] [Google Scholar]; * This narrative review examined 13 randomized trials on the effect of vitamin D supplementation on falls risk and found no benefit.
- 74.Hopkinson NS, Li KW, Kehoe A, et al. Vitamin D receptor genotypes influence quadriceps strength in chronic obstructive pulmonary disease. Am J Clin Nutr. 2008;87:385–390. doi: 10.1093/ajcn/87.2.385. [DOI] [PubMed] [Google Scholar]; * This case-control study evaluated whether quadriceps weakness in patients with COPD is influenced by common vitamin D receptor polymorphisms. Patients and control subjects who were homozygous for the C allele of the FokI polymorphism had less quadriceps strength than did those with the T allele.
- 75.Whitfield GK, Remus LS, Jurutka PW, et al. Functionally relevant polymorphisms in the human nuclear vitamin D receptor gene. Mol Cell Endocrinol. 2001;177:145–159. doi: 10.1016/s0303-7207(01)00406-3. [DOI] [PubMed] [Google Scholar]
- 76.Roth SM, Zmuda JM, Cauley JA, et al. Vitamin D receptor genotype is associated with fat-free mass and sarcopenia in elderly men. J Gerontol A Biol Sci Med Sci. 2004;59:10–15. doi: 10.1093/gerona/59.1.b10. [DOI] [PubMed] [Google Scholar]
- 77.Grundberg E, Brandstrom H, Ribom EL, et al. Genetic variation in the human vitamin D receptor is associated with muscle strength, fat mass and body weight in Swedish women. Eur J Endocrinol. 2004;150:323–328. doi: 10.1530/eje.0.1500323. [DOI] [PubMed] [Google Scholar]
- 78.Geusens P, Vandevyver C, Vanhoof J, et al. Quadriceps and grip strength are related to vitamin D receptor genotype in elderly nonobese women. J Bone Miner Res. 1997;12:2082–2088. doi: 10.1359/jbmr.1997.12.12.2082. [DOI] [PubMed] [Google Scholar]