Abstract
Gelatinases (MMP-2 and MMP-9) have been implicated in a number of pathological conditions, including cancer and cardiovascular disease. Hence, small molecule inhibitors of these enzymes are highly sought for use as potential therapeutic agents. 2-(4-Phenoxyphenylsulfonylmethyl)thiirane (SB-3CT) has previously been demonstrated to be a potent and selective inhibitor of gelatinases, however, it is rapidly metabolized because of oxidation at the para position of the phenoxy ring and at the α-position to the sulfonyl group. α-Methyl variants of SB-3CT were conceived to improve metabolic stability and as mechanistic probes. We describe herein the synthesis and evaluation of these structural variants as potent inhibitors of gelatinases. Two (compounds 5b and 5d) among the four synthetic stereoisomers were found to exhibit slow-binding inhibition of gelatinases and MMP-14 (MT1-MMP), which is a hallmark of the mechanism of this class of inhibitors. The ability of these compounds to inhibit MMP-2, MMP-9, and MMP-14 could target cancer tissues more effectively. Metabolism of the newly synthesized inhibitors showed that both oxidation at the α-position to the sulfonyl group and oxidation at the para position of the terminal phenyl ring were prevented. Instead oxidation on the thiirane sulfur is the only biotransformation pathway observed for these gelatinase inhibitors.
Keywords: enzyme inhibition, gelatinase, metabolism
Matrix metalloproteinases (MMPs) constitute a family of 26 zinc-dependent endopeptidases in humans, which are involved in restructuring of the extracellular matrix and processing of multiple bioactive proteins (1). The full scope of their functions is not understood, but they perform critical roles in development and tissue remodeling (2). To maintain tissue homeostasis, MMP activity is strictly regulated at multiple levels. However, deregulation of MMP activity is characteristic of a variety of pathological conditions (3,4). Gelatinases A and B, also known as MMP-2 and MMP-9, respectively, constitute a subclass of MMPs that have emerged as important targets for intervention in several pathological conditions, including cancer and neurological and cardiovascular diseases (5,6). Selective targeting of gelatinases over those of the other members of the MMP family has long been sought, but few such examples exist (7,8).
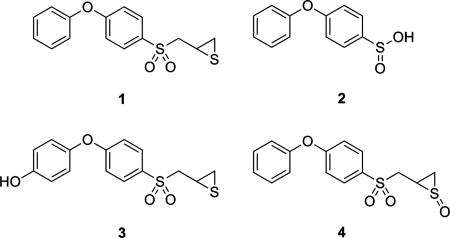
Previously, we described the design, synthesis, and biological effects of inhibition of gelatinases by 2-(4-phenoxyphenylsulfonylmethyl)thiirane (compound 1, also known as SB-3CT). This inhibitor exhibits high potency and selectivity in inhibition of gelatinases in vitro (9) and in vivo in animal models of cancer metastases (10–12) and stroke (13). An investigation of the metabolism of this compound revealed that it was extensively and rapidly metabolized (14,15), despite the fact that the compound exhibited in vivo activity. Compound 1 had low metabolic stability, with 0.5% of the compound remaining following a 15-min incubation with rat liver microsomes (16). Metabolic stability describes the rate and extent to which a molecule is metabolized. A compound that is rapidly and extensively metabolized is referred to as having a low degree of metabolic stability. Low metabolic stability can contribute to undesirable pharmaceutical properties, such as low oral efficacy and/or short duration of action, and can reduce the efficacy of an otherwise potent molecule. Therefore, the ability to evaluate metabolic stability of analogs early in the discovery process improves the odds of selecting a lead with good in vivo activity. The major metabolic pathways of 1 observed in vitro resulted from oxidation at the α-position to the sulfonyl group resulting in the formation of the sulfinic acid 2, oxidation at the para-position of the phenoxy group (compound 3) and oxidation at the thiirane ring sulfur (compound 4) (14). The hydroxylated metabolite 3 was, incidentally, a more potent gelatinase inhibitor than the parent compound 1 (14). Evaluation of the metabolic stability of 3 revealed that 30% of the compound remained after 15-min incubation with rat liver microsomes, with oxidation α to the sulfonyl as the primary pathway of metabolism.
We undertook in this study to prepare analogs of 1 (compounds 5) with the intention of minimizing metabolism. Reducing the degree of metabolism increases bioavailability and prolongs half-life, which in turn allows lower and less frequent dosing. Our approach was to methylate one of the P450-mediated oxidation sites of the molecule α to the sulfonyl moiety. As disclosed herein, blocking this position by methylation abrogated hydroxylation not only at that site, but also at the terminal phenyl ring. Instead, the only metabolic pathway observed for these compounds was S-oxidation of the thiirane moiety.
Methods and Materials
Chemicals and reagents
All organic reagents were purchased from either Sigma-Aldrich Chemical Company (St Louis, MO, USA) or Acros Organics (Geel, Belgium), unless otherwise stated. All reactions were performed under an atmosphere of nitrogen, unless noted otherwise. 1H and 13C NMR spectra were recorded on a Varian INOVA-500 (Varian Inc., Palo Alto, CA, USA) spectrometer. Thin-layer chromatography was performed with Whatman reagents 0.25 mm silica gel 60-F plates. Flash chromatography was carried out with silica gel 60, 230–400 mesh (0.040–0.063 mm particle size) purchased from EM Science (Gibbstown, NJ, USA). Human recombinant MMP-2 and MMP-9 were prepared as described previously (14). Human recombinant active MMP-7 and catalytic domains of MMP-3 and MMP-14 were purchased from EMD Biosciences (La Jolla, CA, USA). The catalytic domain of human recombinant MMP-1 was from Biomol International (Plymouth Meeting, PA, USA). Fluorogenic substrates were purchased from Peptides International (Louisville, KY, USA), (MOCAcPLGL(Dpa)AR-NH2, MOCAcRPKPVE(Nva)WRK(Dnp)-NH2), and (MOCAcKPLGL(Dpa)AR-NH2) were obtained from R&D Systems (Minneapolis, MN, USA). Inhibitor stock solutions (10 mm) were prepared in DMSO. The methodology for enzyme inhibition and assays was the same as reported previously (17). Substrate hydrolysis was measured with a Varian Cary Eclipse fluorescence spectrophotometer. Rat liver microsomes were purchased from BD Biosciences (Woburn, MA, USA).
Microsomal incubations
Incubations consisted of rat liver microsomes (0.5 mg), NADPH (0.5 mm), and 50 μm compound in potassium phosphate buffer (50 μm, pH 7.4) at 37 °C for 20 min in a volume of 500 μL. The reaction was terminated by addition of one volume of acetonitrile. The precipitated protein was centrifuged (3 min, 10 500× g) and the supernatant was analyzed by reversed-phase HPLC with UV detection and LC/MS/MS.
HPLC chromatography
The HPLC system consisted of a Perkin-Elmer series 200 quaternary pump (Perkin-Elmer Corp., Norwalk, CT, USA), a Perkin-Elmer UV detector series 200, and a Perkin-Elmer auto sampler series 200. Samples were analyzed on a YMC 5 μm basic 4.6 mm i.d. × 15 cm column (YMC Inc., Wilmington, NC, USA) connected to a YMC 5 μm basic 4.0 mm i.d. × 2 cm guard cartridge. The mobile phase consisted of elution at 1 mL/min with 90% A/10% B for 5 min, followed by a 20-min linear gradient to 10% A/90% B, then 10% A/90% B for 10 min (A = 0.1% TFA/water, B = 0.1% TFA/acetonitrile). Effluent was monitored by UV detection at 245 nm.
Mass spectrometry
All mass spectrometric experiments were performed with a Micromass (Beverly, MA, USA) Quattro-LC triple quadrupole (Q1-q-Q3) instrument running MassLynx 4.0 software. The Quattro-LC was connected to a Waters (Milford, MA, USA) 2690 HPLC equipped with a Waters 2690 autosampler and a Waters 996 photodiode array detector. For LC/MS and LC/MS/MS experiments, the eluent flow rate of 1 mL/min was reduced by 50% prior to entering the ion source region with a custom-built flow splitter, which consisted of a zero dead volume PEEK tee with identical lengths and diameters of tubing on each outlet. The Quattro-LC Z-spray interface was operated in the ESI mode with the following parameters: capillary voltage = 2.8–3.0 kV, cone voltage = 20–35 V, source temperature = 125–150° C, desolvation temperature = 150–250 °C, desolvation gas (N2) flow rate = 430–550 L/h. For MS/MS experiments, the mass spectrometer tune was optimized by directly infusing a solution of compound 1. Collisionally activated dissociation was employed for all MS/MS experiments by admitting argon gas into the collision cell (q). For product ion scans, argon gas was admitted into q until the parent signal intensity was reduced by ~50% (i.e., single collision conditions), then collision energy (voltage offset between Q1 and Q3) was increased to 10–50 V. The resolving powers of both Q1 and Q3 were reduced from a setting of 15 to 12 to maximize signal intensities, and MS/MS spectra were acquired in the continuum mode of data acquisition at a rate of ≥400 a.m.u/seconds.
S-4-Phenoxyphenyl ethanethioate (6)
The procedure was the same as that reported by Lim et al. (18) except that acetyl chloride was used instead of epichlorohydrin. Purification of the crude product by column chromatography (hexanes/EtOAc = 8/1) afforded 6 in 66% yield. 1H NMR (500 MHz, CDCl3) δ 7.34–7.42 (m, 4H), 7.15–7.21 (m, 1H), 7.08 (d, J = 8.0 Hz, 2H), 7.03 (d, J = 8.4 Hz, 2H), 2.43 (s, 3H); 13C NMR (126 MHz, CDCl3) δ 194.8, 159.1, 156.2, 136.3, 130.1, 124.3, 121.4, 120.0, 119.0, 30.2; HRMS (FAB) calcd for C14H12O2S (M+) 244.0558, found 244.0544.
(S)-1-(But-3-yn-2-ylsulfonyl)-4-phenoxybenzene ((S)-9)
(R)-But-3-yn-2-yl methanesulfonate ((R)-7) was separately prepared from (R)-3-butyn-2-ol. To a cooled solution of (R)-3-butyn-2-ol (1.0 g, 14 mmol) in CH2Cl2 (30 mL) was added TEA (2.0 mL, 14 mmol). Mesyl chloride (1.0 mL, 14 mmol) was added dropwise and the resulting mixture was stirred under an atmosphere of nitrogen for 1 h in an ice-water bath. It was filtered over silica gel and the solvent was removed in vacuo. The mixture was diluted with ether and the solution was filtered through a layer of anhydrous MgSO4. The solvent was evaporated to afford (R)-7 in quantitative yield. This was used in the next step without further purification.
Compound 6 (1.7 g, 7.0 mmol) was dissolved in 1:1 MeCN/MeOH (30 mL). Potassium carbonate (1.5 g, 11 mmol) was added and the mixture was stirred under an atmosphere of nitrogen for 1 h at room temperature and then cooled in an ice-water bath. Compound (R)-7 (1.0 g, 6.8 mmol) – as prepared above – was added dropwise and the mixture was stirred for 3 h in the ice-water bath. The suspension was filtered and the solvent was evaporated. The concentrate was diluted with water and extracted with EtOAc. The combined organic extracts were dried over anhydrous MgSO4 and concentrated in vacuo. The product (i.e., (S)-8) was carried on to the next step without further purification.
The sample (S)-8 was dissolved in CH2Cl2 (30 mL) and cooled in an ice-water bath. It was treated with m-CPBA (3.3 g, 15 mmol) and the resulting white suspension was stirred at room temperature for 1 h. The mixture was filtered and the filtrate was washed with saturated Na2S2O3, followed by saturated NaHCO3. The organic layer was separated and the aqueous layer was extracted with CH2Cl2. The combined organic extracts were dried over anhydrous MgSO4 and concentrated in vacuo. Purification of the product by silica gel chromatography (hexanes/CH2Cl2 = 2/3) gave the title compound as a white solid in 67% yield (1.3 g). Enantiomeric excess was determined by NMR in the presence of a chiral shifting reagent, europium tris[3-(heptafluoropropylhydroxymethylene)-(+)-camphorate], Eu(hfc)3. The NMR sample was prepared by adding 1 mg of (S)-9 and 1 mg of Eu(hfc)3 in 0.7 mL CDCl3. >90% ee; (c 0.33, CHCl3); 1H NMR (500 MHz, CDCl3) δ 7.87–7.92 (m, 2H), 7.41–7.46 (m, 2H), 7.23–7.28 (m, 1H), 7.06–7.12 (m, 4H), 3.93 (qd, J = 2.5, 7.1 Hz, 1H), 2.41 (d, J = 2.4 Hz, 1H), 1.60 (d, J = 7.2 Hz, 3H); 13C NMR (126 MHz, CDCl3) δ 163.0, 154.7, 132.1, 130.2, 129.2, 125.2, 120.5, 117.1, 77.5, 75.7, 53.9, 15.1; HRMS (FAB) calcd for C16H15O3S (M + H+) 287.0742, found 287.0742.
(R)-1-(But-3-yn-2-ylsulfonyl)-4-phenoxybenzene ((R))-9)
The synthesis was carried out as described for (S)-9, using (S)-7. The 1H and 13C spectra were identical to those of (S)-9. >90% ee; (c 0.33, CHCl3); HRMS (ESI) calcd for C16H14NaO3S (M + Na+) 309.0556, found 309.0538.
(S)-1-(But-3-en-2-ylsulfonyl)-4-phenoxybenzene ((S)-10)
Compound (S)-9 (1.3 g, 4.6 mmol) was dissolved in EtOAc (30 mL), and was treated with 5% palladium–barium sulfate (0.13 g) and five drops of quinoline. The mixture was stirred under an atmosphere of hydrogen for 3.5 h at room temperature. It was filtered through a layer of Celite and the solution was concentrated under reduced pressure. Column chromatography on silica gel (hexanes/CH2Cl2 = 1/7) of the crude compound gave 1.2 g (92%) of the title compound. (c 0.33, CHCl3); 1H NMR (500 MHz, CDCl3) δ 7.74–7.79 (m, 2H), 7.39–7.45 (m, 2H), 7.21–7.27 (m, 1H), 7.02–7.10 (m, 4H), 5.83 (ddd, J = 7.8, 10.1, 17.4 Hz, 1H), 5.26–5.32 (m, 1H), 5.13 (dd, J = 1.1, 17.2 Hz, 1H), 3.70 (quin, 1H), 1.45 (d, 3H); 13C NMR (126 MHz, CDCl3) δ 162.7, 155.1, 131.8, 131.5, 130.4, 125.3, 121.9, 120.6, 117.4, 64.5, 13.3; HRMS (FAB) calcd for C16H17O3S (M + H+) 289.0898, found 289.0882.
(R)-1-(But-3-en-2-ylsulfonyl)-4-phenoxybenzene ((R)-10)
The synthesis was carried out as described for (S)-10, using (R)-9. The 1H and 13C spectra were identical to those of (S)-10. (c 0.35, CHCl3); HRMS (ESI) calcd for C16H16NaO3S (M + Na+) 311.0712, found 311.069.
(2R)-2-(1R-(4-Phenoxyphenylsulfonyl)ethyl)oxirane (11a) and (2S)-2-(1S-(4-phenoxyphenylsulfonyl)ethyl)oxirane (11b)
A solution of compound (S)-10 (1.1 g, 3.8 mmol) in CH2Cl2 (40 mL) was cooled in an ice-water bath and was treated with m-CPBA (8.8 g, 39 mmol, 77%). The mixture was stirred for 1 h in the ice-water bath, warmed to room temperature and stirred for 6 days. The suspension was filtered over silica gel and the filtrate was washed with saturated Na2S2O3, followed by saturated NaHCO3. The organic layer was dried over anhydrous Na2SO4 and concentrated in vacuo. Purification by silica gel chromatography (hexanes/EtOAc = 5/1) gave 0.55 g of 11a and 0.26 g of 11b in 70% overall yield. Enantiomeric excess was determined by NMR in the presence of Eu(hfc)3. The NMR sample was prepared by adding 1mgof 11a and 1 mg of Eu(hfc)3 in 0.7 mL CDCl3. For 11a: >90% ee; (c 0.37, CHCl ); 1H NMR (500 MHz, CDCl3) δ 7.81–7.85 (m, 2H), 7.41–7.46 (m, 2H), 7.23–7.28 (m, 1H), 7.07–7.11 (m, 4H), 3.14 (ddd, J = 2.6, 3.9, 6.8 Hz, 1H), 2.84 (quin, 1H), 2.77–2.80 (m, 1H), 2.45 (dd, J = 2.6, 4.6 Hz, 1H), 1.43 (d, J = 7.0 Hz, 3H); 13C NMR (126 MHz, CDCl3) δ 162.9, 154.6, 131.2, 130.5, 130.2, 125.3, 120.5, 117.4, 62.9, 50.5, 46.6, 10.9; HRMS (FAB) calcd for C16H17O4S (M + H+) 305.0848, found 305.0850. For 11b: >90% ee; (c 0.33, CHCl3); 1H NMR (500 MHz, CDCl3) δ 7.84–7.88 (m, 2H), 7.40–7.45 (m, 2H), 7.22–7.26 (m, 1H), 7.07–7.11 (m, 4H), 3.24 (ddd, J = 2.6, 4.1, 7.0 Hz, 1H), 3.00 (quin, J = 7.2 Hz, 1H), 2.75–2.79 (m, 1H), 2.46 (dd, J = 2.6, 4.8 Hz, 1H), 1.37 (d, J = 7.2 Hz, 3H); 13C NMR (126 MHz, CDCl3) δ 162.8, 154.7, 131.2, 130.2, 125.2, 120.5, 117.4, 62.6, 50.0, 44.2, 9.3; HRMS (FAB) calcd for C16H17O4S (M + H+) 305.0848, found 305.0856.
(2S)-2-(1R-(4-Phenoxyphenylsulfonyl)ethyl)oxirane (11c) and (2R)-2-(1R-(4-phenoxyphenylsulfonyl)ethyl)oxirane (11d)
The synthesis was carried out as described for 11a and 11b, using (R)-10. The 1H and 13C spectra of 11c and 11d were identical to those of 11a and 11b, respectively. For 11c: >90% ee; (c 0.36, CHCl3); HRMS (ESI) calcd for C16H16NaO4S (M + Na+) 327.0662, found 327.0645. For 11d: >90% ee; (c 0.35, CHCl3); HRMS (ESI) calcd for C16H16NaO4S (M + Na+) 327.0662, found 327.0644.
(2S)-2-(1S-(4-Phenoxyphenylsulfonyl)ethyl)thiirane (5a)
To a solution of 11a (0.50 g, 1.6 mmol) in CH2Cl2 (5 mL) was added a mixture of thiourea (1.3 g, 16 mmol, 99%) in methanol (15 mL). The resulting mixture was stirred for 24 h at room temperature, after which the solvent was removed under reduced pressure. The residue was partitioned between ethyl ether and water. The organic layer was dried over anhydrous MgSO4, and the suspension was filtered. Evaporation of solvent gave the crude product, which was purified by silica gel chromatography (hexanes/EtOAc = 5/1). The desired product was recrystallized from 2-propanol as white crystals in 81% yield (0.43 g). Enantiomeric excess was determined by NMR in the presence of Eu(hfc)3. The NMR sample was prepared by adding 1 mg of 5a and 1 mg of Eu(hfc)3 in 0.7 mL CDCl3. >90% ee; (c 0.37, CHCl3); 1H NMR (500 MHz, CDCl3) δ 7.83–7.87 (m, 2H), 7.40–7.45 (m, 2H), 7.22–7.26 (m, 1H), 7.07–7.11 (m, 4H), 3.05–3.11 (m, 1H), 2.99 (quin, J = 7.1 Hz, 1H), 2.44 (dd, J = 1.7, 6.5 Hz, 1H), 2.08 (dd, J = 1.7, 5.3 Hz, 1H), 1.44 (d, J = 7.0 Hz, 3H); 13C NMR (126 MHz, CDCl3) δ 162.8, 154.8, 131.5, 130.5, 130.2, 125.1, 120.4, 117.6, 64.8, 31.4, 21.0, 11.2; HRMS (FAB) calcd for C16H17O3S2 (M + H+) 321.0619, found 321.0612.
(2R)-2-(1S-(4-Phenoxyphenylsulfonyl)ethyl)thiirane (5b)
The synthesis was carried out as described for 5a, using 11b, but the reaction mixture was stirred for 4 days at room temperature. The desired product was recrystallized from 2-propanol as white crystals in 72% yield. Enantiomeric excess was determined by NMR in the presence of Eu(hfc)3. The NMR sample was prepared by adding 1 mg of 5b and 1 mg of Eu(hfc)3 in 0.7 mL CDCl3. >90% ee; (c 0.37, CHCl3); 1H NMR (500 MHz, CDCl3) δ 7.82–7.86 (m, 2H), 7.42–7.47 (m, 2H), 7.24–7.29 (m, 1H), 7.087.12 (m, 4H), 2.96 (dt, J = 5.6, 9.5 Hz, 1H), 2.60 (dq, J = 6.9, 9.5 Hz, 1H), 2.55 (dd, J = 1.8, 6.0 Hz, 1H), 2.24 (dd, J = 1.9, 5.3 Hz, 1H), 1.51 (d, J = 7.0 Hz, 3H); 13C NMR (126 MHz, CDCl3) δ 163.2, 154.9, 131.6, 130.5, 125.5, 120.7, 117.7, 67.0, 34.0, 26.5, 14.9; HRMS (FAB) calcd for C16H17O3S2 (M + H+) 321.0619, found 321.0633.
(2R)-2-(1R-(4-Phenoxyphenylsulfonyl)ethyl)thiirane (5c)
The synthesis was carried out as described for 5a, using 11c. The 1H and 13C spectra of 5c were identical to those of 5a. >90% ee; (c 0.33, CHCl3); HRMS (ESI) calcd for C16H16NaO3S2 (M + Na+) 343.0433, found 343.0424.
(2S)-2-(1R-(4-Phenoxyphenylsulfonyl)ethyl)thiirane (5d)
The synthesis was carried out as described for 5b, using 11d. The 1H and 13C spectra of 5d were identical to those of 5b. >90% ee; (c 0.33, CHCl3); HRMS (ESI) calcd for C16H16NaO3S2 (M + Na+) 343.0433, found 343.0419.
(2R)-2-(1S-(4-Phenoxyphenylsulfonyl)ethyl)thiirane S-oxide (12)
A solution of compound 5b (31 mg, 97 μmol) in CH2Cl2 (3 mL) was cooled in an ice-water bath and treated with m-CPBA (22 mg, 98 μmol, 77%). After the mixture was stirred for 5 min, the solvent was evaporated. The mixture was purified by silica gel chromatography (EtOAc) to give 12 (5.0 mg, 15%). 1H NMR (500 MHz, CDCl3) δ 7.80–7.88 (m, 2H), 7.40–7.49 (m, 2H), 7.24–7.32 (m, 1H), 7.09–7.16 (m, 4H), 2.94–3.01 (m, 1H), 2.82–2.92 (m, 1H), 2.35–2.45 (m, 2H), 1.60 (d, J = 7.0 Hz, 3H); 13C NMR (126 MHz, CDCl3) δ 163.6, 131.6, 130.5, 129.9, 125.6, 120.8, 118.1, 58.6, 47.0, 37.8, 10.6; HRMS (FAB) calcd for C16H17O4S2 (M + H+) 337.0568, found 337.0579.
4-(But-3-en-2-ylsulfonyl)phenol (15)
A solution of 4-hydroxythiophenol (0.54 g, 4.3 mmol) in MeCN (20 mL) was treated with racemic 7 (0.70 g, 4.7 mmol). TEA (0.60 mL, 4.3 mmol) was added dropwise and the mixture was stirred under an atmosphere of nitrogen for 2 h at room temperature. After the solvent was removed, the crude compound was taken up into CH2Cl2 and water. Layers were separated and the aqueous layer was extracted with CH2Cl2. The combined organic extracts were dried over anhydrous MgSO4 and concentrated to dryness in vacuo. The resulting residue was carried on to the next reaction by dissolving it in CH2Cl2 (20 mL). m-CPBA (2.1 g, 9.4 mmol, 77%) was added at ice-water temperature and the mixture was stirred for 30 min. The suspension was filtered and the filtrate was purified by silica gel chromatography (hexanes/EtOAc = 1/1) to give 0.50 g (55%) of 4-(but-3-yn-2-ylsulfonyl)phenol as a solid. It was then dissolved in EtOAc (20 mL) and treated with 5% palladium–barium sul-fate (50 mg) and five drops of quinoline. The mixture was stirred under an atmosphere of hydrogen for 2 h at room temperature. The catalyst was filtered and the filtrate was evaporated to dryness. The residue was purified by silica gel chromatography (hexanes/EtOAc = 1/1) to give the title compound in 92% yield (0.46 g). 1H NMR (500 MHz, CDCl3) δ 7.65–7.75 (m, 2H), 6.91–7.01 (m, 2H), 5.81 (ddd, J = 7.9, 10.0, 17.4 Hz, 1H), 5.29 (d, J = 10.4 Hz, 1H), 5.13 (d, J = 17.1 Hz, 1H), 3.70 (quin, J = 7.1 Hz, 1H), 1.43 (d, J = 6.8 Hz, 3H); 13C NMR (126 MHz, CDCl3) δ 161.1, 131.9, 131.3, 127.8, 122.2, 115.9, 64.6, 13.3; HRMS (FAB) calcd for C10H12O3S (M + H+) 213.0585, found 213.0576.
(4-(4-(But-3-en-2-ylsulfonyl)phenoxy)phenoxy)(tert-butyl)dimethylsilane (16)
The procedure was adapted from that reported by Evans et al. (19). To a round bottom flask was added activated powdered 4 Å molecular sieves, compound 15 (0.20 g, 0.94 mmol), the boronic acid 14 (0.47 g, 1.9 mmol), Cu(OAc)2·H2O (0.19 g, 0.95 mmol), CH2Cl2 (15 mL), and TEA (0.40 mL, 2.8 mmol). The mixture was stirred under an atmosphere of nitrogen for 1 h. The solvent was evaporated and the product was purified by silica gel chromatography (hexanes/EtOAc = 5/1) to give 16 in 51% yield (0.20 g). 1H NMR (500 MHz, CDCl3) δ 7.73–7.77 (m, 2H), 6.98–7.02 (m, 2H), 6.93–6.97 (m, 2H), 6.86–6.90 (m, 2H), 5.79–5.88 (m, 1H), 5.29 (dt, J = 0.9, 10.3 Hz, 1H), 5.14 (dt, J = 1.0, 17.3 Hz, 1H), 3.66–3.74 (m, 1H), 1.44 (d, J = 7.0 Hz, 3H), 1.01 (s, 9H), 0.23 (s, 6H); 13C NMR (126 MHz, CDCl3) δ 163.5, 153.2, 148.6, 131.7, 131.6, 129.8, 121.9, 121.6, 116.6, 64.5, 25.9, 18.4, 13.3, -4.2; HRMS (FAB) calcd for C2H30O4SSi (M + H+) 419.1712, found 419.1714.
tert-Butyldimethyl(4-(oxiran-2-yl)ethylsulfonyl)phenoxy) phenoxy)silane (17a and 17b)
A solution of compound 16 (0.15 g, 0.36 mmol) in CH2Cl2 (15 mL) was cooled in an ice-water bath and was treated with m-CPBA (0.40 g, 1.8 mmol, 77%). The mixture was stirred for 1 h in an ice-water bath, warmed to room temperature and stirred for 6 days. The suspension was filtered over silica gel and the filtrate was washed with saturated Na2S2O3, followed by saturated NaHCO3. The organic layer was dried over anhydrous Na2SO4 and concentrated in vacuo. Purification by silica gel chromatography (hexanes/EtOAc = 5/1) gave 32 mg of 17a and 30 mg of 17b in 40% overall yield. For 17a: 1H NMR (500 MHz, CDCl3) δ 7.79–7.83 (m, 2H), 7.02–7.05 (m, 2H), 6.94–6.99 (m, 2H), 6.87–6.90 (m, 2H), 3.14 (ddd, J = 2.4, 4.0, 6.8 Hz, 1H), 2.77–2.86 (m, 2H), 2.46 (dd, J = 2.6, 4.6 Hz, 1H), 1.43 (d, J = 7.0 Hz, 3H), 1.01 (s, 9H), 0.23 (s, 6H); 13C NMR (126 MHz, CDCl3) δ 163.9, 153.3, 148.4, 131.4, 130.2, 122.0, 121.6, 117.0, 63.2, 50.8, 47.0, 25.8, 18.4, 11.2, -4.2; HRMS (FAB) calcd for C22H30O5SSi (M+) 434.1583, found 434.1588. For 17b: 1H NMR (500 MHz, CDCl3) δ 7.83–7.86 (m, 2H), 7.02–7.05 (m, 2H), 6.95–6.98 (m, 2H), 6.86–6.90 (m, 2H), 3.25 (dd, J = 2.3, 4.9 Hz, 1H), 3.01 (quin, J = 7.1 Hz, 1H), 2.77 (dd, J = 4.1, 4.7 Hz, 1H), 2.47 (dd, J = 2.6, 4.8 Hz, 1H), 1.37 (d, J = 7.2 Hz, 3H), 1.01 (s, 9H), 0.23 (s, 6H); 13C NMR (126 MHz, CDCl3) δ 163.8, 153.3, 148.5, 131.4, 130.9, 122.0, 121.6, 117.0, 62.8, 50.3, 44.4, 25.9, 18.4, 9.6, -4.2; HRMS (FAB) calcd for C22H30O5SSi 434.1583 (M+), found 434.1579.
tert-Butyldimethyl(4-(4-(S)-thiiran-2-yl)ethylsulfonyl)phenoxy) phenoxy)silane (18a)
To a solution of 17a (15 mg, 35 μmol) in CH2Cl2 (1 mL) was added a mixture of thiourea (14 mg, 0.18 mmol, 99%) in methanol (4 mL). The resulting mixture was stirred for 24 h at room temperature, after which, the solvent was removed under reduced pressure. The residue was partitioned between ethyl ether and water. The organic layer was dried over anhydrous MgSO4, and the suspension was filtered. Evaporation of solvent gave the crude product, which was purified by silica gel chromatography (hexanes/EtOAc = 6/1) to yield 12 mg (77%) of 18a. 1H NMR (500 MHz, CDCl3) δ 7.81–7.85 (m, 2H), 7.03–7.06 (m, 2H), 6.95–6.99 (m, 2H), 6.87–6.90 (m, 2H), 3.09 (td, J = 5.4, 6.9 Hz, 1H), 2.99 (quin, J = 7.1 Hz, 1H), 2.44 (dd, J = 1.7, 6.5 Hz, 1H), 2.08 (dd, J = 1.7, 5.3 Hz, 1H), 1.44 (d, J = 7.0 Hz, 3H), 1.01 (s, 9H), 0.23 (s, 6H); 13C NMR (126 MHz CDCl3) δ 163.8, 153.3, 148.6, 131.6, 130.2, 121.9, 121.6, 117.1, 65.0, 31.7, 25.9, 21.2, 18.4, 11.3, -4.2; HRMS (FAB) calcd for C22H30O4S2Si (M+) 450.1355, found 450.1331.
tert-Butyldimethyl(4-(4-(S-thiiran-2-yl)ethylsulfonyl) phenoxy)phenoxy)silane (18b)
The synthesis was carried out as described for 18a, using 17b, but the reaction mixture was stirred for 4 days at room temperature. Purification by silica gel chromatography (hexanes/EtOAc = 6/1) gave 18b in 64% yield. 1H NMR (500 MHz, CDCl3) δ 7.80–7.86 (m, 2H), 7.03–7.09 (m, 2H), 6.95–7.01 (m, 2H), 6.88–6.93 (m, 2H), 2.97 (dt, J = 5.4, 9.6 Hz, 1H), 2.54–2.65 (m, 2H), 2.25 (dd, J = 1.3, 5.3 Hz, 1H), 1.52 (d, J = 6.4 Hz, 3H), 1.03 (d, J = 0.8 Hz, 9H), 0.25 (d, J = 0.8 Hz, 6H); 13C NMR (126 MHz, CDCl3) δ 163.9, 153.4, 148.5, 131.5, 130.1, 121.9, 121.6, 117.1, 67.0, 34.0, 26.5, 25.9, 18.4, 15.0, -4.2. HRMS (FAB) calcd for C22H30O4S2Si (M+) 450.1355, found 450.1349.
4-(4-Thiiran-2-yl)ethylsulfonyl)phenoxy)phenol (13a)
To a solution of compound 18a (10 mg, 22 μmol) in methanol (5 mL) was added a few drops of conc. HCl. After the mixture was stirred for 2 h at room temperature, the solvent was evaporated in vacuo. The crude product was taken up into water and EtOAc. Layers were separated and the aqueous layer was extracted with EtOAc. The organic extracts were dried over anhydrous Na2SO4 and concentrated in vacuo. Purification by silica gel chromatography (hexanes/EtOAc = 1/1) gave 13a in 96% yield (7.2 mg). 1H NMR (500 MHz, CDCl3) δ 7.81–7.86 (m, 2H), 7.02–7.07 (m, 2H), 6.97–7.01 (m, 2H), 6.87–6.92 (m, 2H), 5.05 (s, 1H), 3.08 (td, J = 5.9, 6.6 Hz, 1H), 2.99 (t, J = 7.2 Hz, 1H), 2.45 (dd, J = 1.8, 6.4 Hz, 1H), 2.08 (dd, J = 1.8, 5.2 Hz, 1H), 1.44 (d, J = 7.0 Hz, 3H); 13C NMR (126 MHz, CDCl3) δ 163.9, 153.3, 148.2, 131.6, 130.2, 122.2, 117.0, 117.0, 65.1, 31.6, 21.2, 11.4; HRMS (FAB) calcd for C16H17O4S2 (M + H+) 337.0563, found 337.0564.
4-(4-Thiiran-2-yl)ethylsulfonyl)phenoxy)phenol (13b)
The synthesis was carried out as described for 13a, using 18b. Purification by silica gel chromatography (hexanes/EtOAc = 1/1) gave 13b in 96% yield. 1H NMR (500 MHz, CDCl3) δ 7.79–7.84 (m, 2 H), 7.03–7.07 (m, 2H), 6.96–7.01 (m, 2H), 6.88–6.92 (m, 2H), 4.99 (s, 1H), 2.95 (dt, J = 5.7, 9.6 Hz, 1H), 2.60 (dq, J = 7.0, 9.4 Hz, 1H), 2.54 (dd, J = 1.9, 6.1 Hz, 1H), 2.24 (dd, J = 1.9, 5.3 Hz, 1H), 1.50 (d, J = 6.8 Hz, 3H); 13C NMR (126 MHz, CDCl3) δ 163.9, 153.3, 148.1, 131.5, 130.1, 122.3, 117.0, 67.0, 34.0, 26.5, 14.9; HRMS (FAB) calcd for C16H17O4S2 (M + H+) 337.0563, found 337.0564.
Crystals growth and analysis
Crystals of suitable size for single-crystal X-ray diffraction analysis were obtained by diffusion of hexane into a diethyl ether solution of compound 11a at room temperature overnight. Crystals were examined under a light hydrocarbon oil. The datum crystal was cut from a larger needle. It was then affixed to a 0.2-mm Mitegen loop mounted atop a tapered copper mounting-pin and transferred to the 100 K nitrogen stream of a Bruker SMART Apex diffractometer (Bruker AXS Inc., Madison, WI, USA) equipped with an Oxford Cryosystems 700 (Oxford Cryosystems Ltd, Oxford, UK) series low-temperature apparatus. Cell parameters were determined using reflections harvested from three orthogonal sets of 30 0.5° φ scans. The orientation matrix derived from this was passed to COSMOa to determine the optimum data collection strategy. Minimum fourfold redundancy was achieved using a combination of ω and φ scan series. Data were measured to 0.81 Å. Cell parameters were refined using reflections with I ≥ 10ρ(I) harvested from the entire data collection. All data were corrected for Lorentz and polarization effects and runs were scaled using sadabsb. The structures were solved using direct methods. All non-hydrogen atoms were assigned after the initial solution. Hydrogens were placed at calculated geometries and allowed to ride on the position of the parent atom. Hydrogen thermal parameters were set to 1.2× the equivalent isotropic U of the parent atom, 1.5× for methyl hydrogens. Non-hydrogen atoms were refined with parameters for anisotropic thermal motion.
The same methodology was performed to elucidate the crystal structures of 11b, 5a, and 5b. An analysis of the crystal for compound 5a at 100 K showed a phase transition resulting in the destruction of the samples, thus data were collected at room temperature rather than at cryogenic temperatures. Crystallographic data (excluding structure factors) for compounds 5a, 5b, 11a, and 11b have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication numbers 737463, 737464, 737465, and 737466. Copies of the data can be obtained, free of charge, on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK [Fax: +44-1223-336033 or by deposit@ccdc.cam.ac.uk].
Results and Discussion
In our previous studies, SB3-CT was found to be a highly potent and selective inhibitor of gelatinases (9,20). However, we found that it is rapidly metabolized to the sulfinic acid (2) and to the p-hydroxy derivative (3) because of oxidation at the α-position to the sulfonyl group (and the attendant fragmentation) and at the para position of the terminal phenyl ring, respectively (14,15). We showed that p-hydroxylation results in a metabolite that is a more potent gelatinase inhibitor than the parent SB-3CT, however α-oxidation produces a metabolite that is not an inhibitor of gelatinases (14). In this study, we systematically α-methylated the sulfonyl group in an attempt to block oxidation at this position and thus maintain inhibitor potency. In addition, reducing the degree of metabolism has several advantages, including increasing bioavailability and prolonging half-life, thereby allowing lower and less frequent dosing.
Synthesis of isomers of 5
The four isomers of 5 were synthesized starting from optically pure 3-butyn-2-ol enantiomers. Basic methanolysis of thioacetate 6 yielded the corresponding thiolate, which was allowed to react with mesylate (R)-7 to give (S)-8 (Figure 1). This entailed a nucleophilic attack by the thiolate at the electrophilic carbon of the mesylate, leading to an inversion of configuration. After oxidation to the sulfone using m-CPBA, partial hydrogenation of alkyne to alkene over Pd–BaSO4 gave (S)-10. Standard epoxidation using m-CPBA produced the diastereomeric oxiranes 11a and 11b (Rf = 0.5 and 0.4 in hexanes/EtOAc = 2/1), which were separated by flash chromatography. Conversion of the isolated oxiranes to thiiranes 5a and 5b, by treatment with thiourea involved an inversion of configuration. Assignment of the stereochemistry of the separated oxiranes and thiiranes was carried out by X-ray crystallography (vide infra). The thiiranes 5c and 5d were accessed according to the same procedure, using (S)-7 as the starting mesylate.
Figure 1.
Synthetic scheme for the preparation of compounds 5.
We add that alternative routes to the optically active thiiranes were explored prior to using the chiral synthetic scheme outlined in Figure 1, which lead to success. One initial approach was to generate racemic 10 from the direct reaction of 6 with 3-chlorobut-1-ene, but because of regioselectivity issues, we opted to prepare racemic 10 from 3-butyn-2-ol using the same methodology as in Figure 1. Racemic 10 could then be subjected to Sharpless asymmetric dihydroxylation reaction (21) and the resulting diol could be converted to the epoxide under Mitsunobu conditions (22). The installation of chirality by Sharpless dihydroxylation, however, did not give good ee values. Another approach was hydrolytic kinetic resolution of the racemic terminal epoxides 11 catalyzed by chiral (salen)CoIII complexes (23). Although this method afforded both the recovered unre-acted epoxide and the 1,2-diol product in enantioenriched forms, it was limited by low yield and the need for a long reaction time. At the end, careful considerations of yields, reaction times and stereo-selection prompted us to pursue the chemistry outlined in Figure 1.
The optical purities of the synthesized compounds were determined by NMR in the presence of the chiral shift reagent europium tris[3-(heptafluoropropylhydroxymethylene)-(+)-camphorate], Eu(hfc)3. 1H NMR invariably revealed the presence of only one enantiomer in each case, hence, we can indicate that the ee values for each isomer should be at the minimum 90%.
The absolute stereochemistry of the synthesized oxiranes and thiiranes was assigned by X-ray crystallography (Figure 2). The X-ray crystal structure of one TLC spot revealed it to be 11a (Figure 2B). The absolute stereochemistry was determined by comparison of the intensities of the Friedel pairs of reflections (Flack parameter = 0.054 (13); a value of 0 indicates the correct enantiomorph of the space group and chirality of the molecule) and by comparison of the known chirality of the mesylate precursor. The X-ray crystal structure of the other TLC spot was due to 11b [Figure 2C, Flack parameter = 0.041 (11)]. It is known that conversion of the oxiranes to the corresponding thiiranes using thiourea involves an inversion of stereochemistry (24,25). This was confirmed by obtaining the X-ray structures of the two thiiranes prepared from the two chromatographically separated oxiranes. The product of thiirane formation reaction from 11a proved to be 5a [Figure 2D, Flack parameters = 0.043 (19)]. The same inversion of stereochemistry at the thiirane was observed for the crystal structure of 5b [Figure 2E, Flack parameter = 0.044 (13)], which was prepared from 11b.
Figure 2.
The X-ray structures of the oxiranes 11a, 11b, and thiiranes 5a, 5b, in stereoview (left) and the ORTEP diagram (right) shown at 50% probability level (5a, shown at 30% probability level). Ar, 4-phenoxyphenyl group.
Inhibition of MMPs
Of the four synthetic diastereomers of 5, the 5b and 5d enantiomers showed slow-binding inhibition of gelatinases, with inhibition constants (Ki) that are in the nanomolar range for MMP-2 (0.18 ± 0.03 and 0.39 ± 0.03 μM, respectively) and in the low micromolar for MMP-9 (3.5 ± 0.2 and 3.3 ± 0.2 μM, respectively). The second-order rate constants for the onset of slow-binding inhibition (kon) are rapid (102 to 104 M/second) and the first-order rate constants for the reversal of the process (koff) are slow (10–4 to 10–3/second). For comparison, SB-3CT is an order of magnitude more potent against MMP-2 (Ki = 13.9 nM) and a five to sixfold better inhibitor of MMP-9 (Ki = 0.6 μM) (9). However, compounds 5b and 5d were >50-fold more metabolically stable than SB-3CT. As mentioned earlier, low metabolic stability can significantly reduce the efficacy of an otherwise potent compound, decreasing bioavailability and shortening the half-life, which in turns requires higher and more frequent dosing. Thus, by maximizing metabolic stability the likelihood of clinical success is improved. The slow-binding behavior appears to require anti arrangement of the hydrogen α to the sulfone and the thiirane sulfur of the inhibitor. On the contrary, compounds 5a and 5c were merely linear competitive inhibitors of gelatinases (with Ki values of 7.2 ± 0.1 and 4.1 ± 0.1 μM against MMP-2, respectively). Inhibition of MMPs by the enantiomers that exhibited the slow-binding behavior (also seen with SB-3CT) was investigated next. The kinetic parameters for the inhibition of MMPs are shown in Table 1. No significant activity against MMP-1, MMP-3, and MMP-7 was observed up to 60 μM. The two enantiomers inhibit each gelatinase equally well, although they are more potent in inhibition of MMP-2. However, we note that in addition to inhibition of gelatinases, the slow-binding kinetic profile was extended to include MMP-14, with Ki values of 0.74 ± 0.17 and 2.1 ± 0.2 μM for 5b and 5d, respectively. MMP-14 is known to activate MMP-2 (26), thus inhibition of MMP-14 will provide two levels of control on the activity of MMP-2. MMP-14 has been shown to be essential for invasion of cancer cells (27) and the gelatinases are known to be involved in cancer invasion and angiogenesis (28). Thus, compounds 5b and 5d could target the MMP-14/MMP-2/MMP-9 axis in cancer tissues in a more effective manner.
Table 1.
Kinetic parameters for inhibition of MMPs by the synthetic inhibitors
| 5b |
5d |
|||||
|---|---|---|---|---|---|---|
| Enzyme | 10–3 kon (M/second) | 104 koff (second) | Ki (μm) | 10–3 kon (M/second)) | 104 koff (second) | Ki (μm) |
| MMP-2 | 3.3 ± 0.1 | 5.9 ± 1.0 | 0.18 ± 0.03 | 2.1 ± 0.1 | 8.1 ± 0.6 | 0.39 ± 0.03 |
| MMP-9 | 0.26 ± 0.01 | 9.0 ± 0.6 | 3.5 ± 0.2 | 0.22 ± 0.01 | 7.2 ± 0.4 | 3.3 ± 0.2 |
| MMP-14cat | 1.2 ± 0.1 | 8.9 ± 2.1 | 0.74 ± 0.17 | 0.46 ± 0.01 | 9.5 ± 0.9 | 2.1 ± 0.2 |
| MMP-1cat | na | na | NI | na | na | NI |
| MMP-3cat | na | na | 8% Inhibition at 60 μm | na | na | 9% Inhibition at 60 μm |
| MMP-7 | na | na | NI | na | na | NI |
NI, not inhibiting up to 60 μm.
na, not applicable.
In vitro metabolism and metabolite identification
The in vitro metabolism of the active diastereomer 5b was investigated using rat liver microsomes. The HPLC chromatogram of 5b following incubation with rat liver microsomes showed one significant metabolite, designated as M, as well as the parent compound itself (Figure 3A). The two peaks flanking M were determined to be background peaks and not any metabolite. The presence of 5b in rat liver microsomes was confirmed by HPLC retention time comparison to the authentic synthetic standard (Figure 3B) and by product ion mass spectrometry.
Figure 3.
HPLC chromatogram of (A) compound 5b following 20-min incubation with rat liver microsomes (B) synthetic standards, and (C) selected ion chromatogram (SIC) using m/z = 337 (M + H+). HPLC conditions: YMC 5 μm basic 4.6 mm i.d. × 15 cm column, 1 mL/min, UV detection at 245 nm, 5-min 90% A/10% B, 20-min linear gradient to 10% A/90% B, 5-min 10% A/90% B (A = 0.1% TFA/water, B = 0.1% TFA/acetonitrile).
Analysis of M by LC/MS indicated oxidation of the parent compound, as depicted by the protonated molecular ion at m/z 337, which was 16 a.m.u higher than that of 5b. The selected ion chromatogram (Figure 3C) showed only one peak, which suggested that only one compound with an m/z = 337 was produced as metabolite. This ruled out the possibility of sulfinic acid 2, the product resulting from the oxidation at the α-position to the sulfonyl group, as the metabolite. Hence, methylation α to the sulfone eliminated the possibility of this transformation, as was our intent. MS/MS analysis showed cleavage of the sulfonyl–methylthiirane linkage at m/z 233 and 87, with subsequent loss of SO from the ion at m/z 233 to give a fragment at m/z 185 (Figure 4A). Cleavage of the phenoxyphenyl–sulfonyl linkage resulted in an ion at m/z 169. These data indicated that the terminal phenyl ring and the middle phenyl ring were intact, and that oxidation of the sulfur in the thiirane ring was the most likely transformation to account for the increase in mass by 16 a.m.u. However, an increase of 16 a.m.u. for the fragment at m/z 87 was not observed. We had seen this previously for the sulfoxide metabolite of SB-3CT (compound 4) (14). As the fragment at m/z 185 could be obtained by hydroxylation of the terminal phenyl ring (m/z 169 + 16), we set out to prepare the authentic thiirane sulfoxide (compound 12) and p-hydroxy derivative of 5b (compound 13b) to confirm the identity of the metabolite.
Figure 4.
Product ion mass spectra of (A) metabolite M (B) synthetic standard 12, and (C) compound 13b.
The S-oxide 12 was produced after treatment of 5b with an equivalent of m-CPBA. Compound 12 should exist in two diastereomeric forms, as was seen in the 1H NMR spectrum of the crude product taken immediately after the reaction. However, only one was isolable; the other diastereomer decomposed to the corresponding alkene. Thiirane S-oxides are known to readily decompose to form olefins and sulfur monoxide (29,30). The synthesis of the p-hydroxy derivative 13b was initiated by copper-mediated arylation of racemic phenolic 15 using boronic acid 14 (18) to give 16 (Figure 5). Compound 15 was separately prepared by S-alkynylation of 4-hydroxythiophenol using racemic 7, and then oxidation to the sulfone using m-CPBA followed by partial catalytic hydrogenation. The succeeding steps involved epoxidation of the alkene to produce oxiranes 17a and 17b (which were separable by chromatography), conversion to the thiirane and desilylation of the phenolic alcohol to afford diastereomers 13a and 13b.
Figure 5.
Synthetic scheme for the preparation of p-hydroxy 13.
Comparison of HPLC retention times and MS/MS analyses of the authentic synthetic standards 12 and 13b (Figures 3 and 4) confirmed the identity of the metabolite. Compound 12 had the identical HPLC retention time (Figure 3B) and MS/MS spectrum (Figure 4B) as the metabolite. In contrast, the MS/MS spectrum of compound 13b showed cleavage of the sulfonyl–methylthiirane linkage at m/z 249 and 87, and cleavage of the phenoxyphenyl–sulfonyl bond at m/z 185 (Figure 4C). These data identified M as the sulfoxide of compound 5b (compound 12). It must be noted that only one diastereomer of 12 was observed as the metabolite in the chromatogram, and its retention time matched that of the isolated synthetic standard. Because the other diastereomer readily decomposes, as was observed in the synthetic preparation, the possibility that it could also form – but experiences decomposition – during metabolism could not be discounted.
The presence of the sulfoxide as the only metabolite of compound 5 indicated that α-methylation with suitable stereochemistry abrogated oxidation at both the α-position to the sulfonyl group as well as the para-position of the terminal phenyl ring. Interestingly, oxidation of the methyl group itself was not observed. The reason for the absence of hydroxylation at the para-position of the terminal ring of these methylated thiiranes is not immediately obvious. However, the most likely explanation for this outcome is that the methylated derivatives can no longer fit in the active site of the P450 enzyme that performs this transformation.
The S-oxide metabolite was tested for any inhibitory activities toward MMPs. When compared with the inhibition results of compound 5b (Table 1), compound 12 was shown to be a poorer inhibitor by at least three orders of magnitude (Ki of 20 μM against MMP-2, and MMP-9 and MMP-14 inhibition at a fixed 60 μM inhibitor concentration were 30% and 0%, respectively). The p-hydroxy derivative, compound 13b, was however found to exhibit a similar inhibition profile as 5b and 5d. The inhibition constants of 13b are in the nanomolar range for MMP-2 (Ki = 0.22 ± 0.09 μM) and in the low micromolar for MMP-9 and MMP-14 (Ki = 1.9 ± 0.7 and 2.1 ± 0.7 μM, respectively). Marginal to no inhibition against MMP-1, MMP-3, and MMP-7 was observed at inhibitor concentrations of up to 60 μM.
Conclusions
The metabolism of compound 5 was significantly less extensive than that of SB-3CT, a potent and selective gelatinase inhibitor. Introduction of a methyl group α to the sulfonyl moiety prevented oxidation at that position, as well as hydroxylation of the terminal phenyl ring. The ability of compounds 5b and 5d to inhibit both gelatinases and MMP-14 activities could result in effective targeting of the MMP-14/MMP-2/MMP-9 axis, which has been shown to be important for cancer progression. Compounds 5b and 5d would appear to be potent and selective for gelatinases and are poised for utilization in animal models of disease involving these enzymes.
Acknowledgments
We thank the Center for Environmental Science and Technology at the University of Notre Dame for allowing generous access to the Micromass Quattro LC mass spectrometer. This work was supported by grant CA122417 from the National Institutes of Health (to M.C and S.M) and CA100475 (to R.F). Major Gooyit is a Fellow of the Chemistry-Biochemistry-Biology Interface (CBBI) Program at the University of Notre Dame, supported by training grant T32GM075762 from the National Institute of General Medical Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the National Institutes of Health.
Abbreviations
- a.m.u.
atomic mass units
- CAD
collisionally activated dissociation
- m-CPBA
meta-chloroperoxybenzoic acid
- DMSO
dimethylsulfoxide
- ESI
electrospray ionization
- FAB
fast atom bombardment
- HPLC
high-performance liquid chromatography
- HRMS
high-resolution mass spectrometry
- LC
liquid chromatography
- MMP
matrix metalloproteinase
- MS
mass spectrometry
- MOCAc
(7-methoxycoumarin-4-yl)acetyl
- NADPH
reduced nicotinamide adenine dinucleotide phosphate
- NMR
nuclear magnetic resonance
- TEA
triethylamine
- TFA
trifluoroacetic acid
- UV
ultraviolet
Footnotes
COSMO (2004). Madison, WI: Bruker-AXS.
Sheldrick G.M. (2004) SADABS. Germany: University of Göttingen.
References
- 1.Stamenkovic I. Extracellular matrix remodeling: the role of matrix metalloproteinases. J Pathol. 2003;200:448–464. doi: 10.1002/path.1400. [DOI] [PubMed] [Google Scholar]
- 2.Kleiner DD, Stetler-Stevenson WG. Matrix metalloproteinases and metastasis. Cancer Chemother Pharmacol. 1999;43:S42–S51. doi: 10.1007/s002800051097. [DOI] [PubMed] [Google Scholar]
- 3.Bergers G, Benjamin LE. Angiogenesis: tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 4.Steeg PS. Angiogenesis inhibitors: motivators of metastasis? Nat Med. 2003;9:822–823. doi: 10.1038/nm0703-822. [DOI] [PubMed] [Google Scholar]
- 5.Moses MA. The regulation of neovascularization by matrix metalloproteinases and their inhibitors. Stem Cells. 1997;15:180–189. doi: 10.1002/stem.150180. [DOI] [PubMed] [Google Scholar]
- 6.Nhuyen M, Arkell J, Jackson C. Human endothelial gelatinases and angiogenesis. Int J Biochem Cell Biol. 2001;33:960–970. doi: 10.1016/s1357-2725(01)00007-3. [DOI] [PubMed] [Google Scholar]
- 7.Fisher J, Mobashery S. Recent advances in MMP inhibitor design. Cancer Metastasis Rev. 2006;25:115–136. doi: 10.1007/s10555-006-7894-9. [DOI] [PubMed] [Google Scholar]
- 8.Bernardo MM, Brown S, Li ZH, Fridman R, Mobashery S. Design, synthesis, and characterization of potent, slow-binding inhibitors that are selective for gelatinases. J Biol Chem. 2002;277:11201–11207. doi: 10.1074/jbc.M111021200. [DOI] [PubMed] [Google Scholar]
- 9.Brown S, Bernardo M, Li ZH, Kotra LP, Tanaka Y, Fridman R, Mobashery M. Potent and selective mechanism-based inhibition of gelatinases. J Am Chem Soc. 2000;122:6799–6800. [Google Scholar]
- 10.Kruger A, Arlt MJE, Gerg M, Kopitz C, Bernardo MM, Chang M, Mobashery S, Fridman R. Antimetastatic activity of a novel mechanism of a gelatinase inhibitor. Cancer Res. 2005;65:3523–3526. doi: 10.1158/0008-5472.CAN-04-3570. [DOI] [PubMed] [Google Scholar]
- 11.Bonfil RD, Sabbota A, Nabha S, Bernardo MM, Dong Z, Meng H, Yamamoto H, Chinni SR, Lim IT, Chang M, Filetti LC, Mobashery S, Cher ML, Fridman R. Inhibition of human prostate cancer growth, osteolysis and angiogenesis in a bone metastasis model by a novel mechanism-based selective gelatinase inhibitor. Int J Cancer. 2006;118:2721–2726. doi: 10.1002/ijc.21645. [DOI] [PubMed] [Google Scholar]
- 12.Martin MD, Carter KJ, Jena-Philippe SR, Chang M, Mobashery S, Thiolloy S, Lynch CC, Matrisian LM, Fingleton B. Effect of ablation or inhibition of stromal matrix metalloproteinase-9 on lung metastasis in a breast cancer model is dependent on genetic background. Cancer Res. 2008;68:6251–6259. doi: 10.1158/0008-5472.CAN-08-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gu Z, Cui J, Brown S, Fridman R, Mobashery S, Strongin AY, Lipton SA. A highly specific inhibitor of matrix metalloproteinase-9 rescues laminin from proteolysis and neurons from apoptosis in transient focal cerebral ischemia. J Neurosci. 2005;25:6401–6408. doi: 10.1523/JNEUROSCI.1563-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee M, Villegas-Estrada A, Celenza G, Boggess B, Toth M, Kreitinger G, Forbes C, Fridman R, Mobashery S, Chang M. Metabolism of a highly selective gelatinase inhibitor generates active metabolite. Chem Biol Drug Des. 2007;70:371–382. doi: 10.1111/j.1747-0285.2007.00577.x. [DOI] [PubMed] [Google Scholar]
- 15.Celenza G, Villegas-Estrada A, Lee M, Boggess B, Forbes C, Wolter WR, Suckow MA, Mobashery S, Chang M. Metabolism of (4-phenoxyphenylsulfonyl)methylthiirane, a selective gelatinase inhibitor. Chem Biol Drug Des. 2008;71:187–196. doi: 10.1111/j.1747-0285.2008.00632.x. [DOI] [PubMed] [Google Scholar]
- 16.Lee M, Celenza G, Boggess B, Blasé J, Shi Q, Toth M, Bernardo MM, Wolter WR, Suckow M, Hesek D, Noll BC, Fridman R, Mobashery S, Chang M. A potent gelatinase inhibitor with anti-tumor-invasive activity and its metabolic disposition. Chem Biol Drug Des. 2009;73:189–202. doi: 10.1111/j.1747-0285.2008.00750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikejiri M, Bernardo MM, Bonfil RD, Toth M, Chang M, Fridman R, Mobashery S. Potent mechanism-based inhibitors for matrix metalloproteinases. J Biol Chem. 2005;280:33992–34002. doi: 10.1074/jbc.M504303200. [DOI] [PubMed] [Google Scholar]
- 18.Lim IT, Brown S, Mobashery S. A convenient synthesis of a selective gelatinase inhibitor as an antimetastatic agent. J Org Chem. 2004;69:3572–3573. doi: 10.1021/jo049857v. [DOI] [PubMed] [Google Scholar]
- 19.Evans DA, Katz JL, West TH. Synthesis of diaryl ethers through the copper-promoted arylation of phenols with arylboronic acids. An expedient synthesis of thyroxine. Tetrahedron Lett. 1998;39:2937–2940. [Google Scholar]
- 20.Lee M, Bernardo MM, Meroueh SO, Brown S, Fridman R, Mobashery M. Synthesis of chiral 2-(4-phenoxyphenylsulfonylmethyl)thiiranes as selective gelatinase inhibitors. Org Lett. 2005;7:4463–4465. doi: 10.1021/ol0517269. [DOI] [PubMed] [Google Scholar]
- 21.Guzman-Perez A, Noe MC, Corey EJ. The application of a mechanistic model leads to the extension of the Sharpless asymmetric dihydroxylation to allylic 4-methoxybenzoates and conformationally related amine and homoallylic alcohol derivatives. J Am Chem Soc. 1995;117:10805–10816. [Google Scholar]
- 22.Garcia-Delgado N, Riera A, Verdaguer X. Phosphine-dependent stereoselectivity in the Mitsunobu cyclodehydration of 1,2-diols: stereodivergent approach to triaryl-substituted epoxides. Org Lett. 2007;9:635–638. doi: 10.1021/ol0629420. [DOI] [PubMed] [Google Scholar]
- 23.Schaus SE, Brandes BD, Larrow JF, Tokunaga M, Hansen KB, Gould AE, Furrow ME, Jacobsen EN. Highly selective hydrolytic kinetic resolution of terminal epoxides catalyzed by chiral (salen)CoIII complexes. Practical synthesis of enantioenriched terminal epoxides and 1,2-diols. J Am Chem Soc. 2002;124:1307–1315. doi: 10.1021/ja016737l. [DOI] [PubMed] [Google Scholar]
- 24.Sander M. Thiiranes. Chem Rev. 1966;66:297–339. [Google Scholar]
- 25.Hauptman E, Fagan PJ, Marshall W. Synthesis of novel (P,S) ligands based on chiral nonracemic episulfides. Use in asymmetric hydrogenation. Organometallics. 1999;18:2061–2073. [Google Scholar]
- 26.Bonfil RD, Dong Z, Trindade Filho JC, Sabbota A, Osenkowski P, Nabha S, Yamamoto H, Chinni Sreenivasa R, Zhao H, Mobashery S, Vessella Robert L, Fridman R, Cher ML. Prostate cancer-associated membrane type 1-matrix metalloproteinase: a pivotal role in bone response and intraosseous tumor growth. Am J Pathol. 2007;170:2100–2111. doi: 10.2353/ajpath.2007.060720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hotary KB, Allen ED, Brooks PC, Datta NS, Long MW, Weiss SJ. Membrane type matrix metalloproteinase I usurps tumor growth control imposed by the three-dimensional extracellular matrix. Cell. 2003;114:33–45. doi: 10.1016/s0092-8674(03)00513-0. [DOI] [PubMed] [Google Scholar]
- 28.Taniwaki K, Fukamachi H, Komori K, Ohtake Y, Nonaka T, Sakamoto T, Shiomi T, Okada Y, Itoh T, Itohara S, Seiki M, Yana I. Stroma-derived matrix metalloproteinase (MMP)-2 promotes membrane type 1-MMP-dependent tumor growth in mice. Cancer Res. 2007;67:4311–4319. doi: 10.1158/0008-5472.CAN-06-4761. [DOI] [PubMed] [Google Scholar]
- 29.Hartzell GE, Paige JN. Ethylene episulfoxide. J Am Chem Soc. 1967;88:2616–2617. [Google Scholar]
- 30.Hartzell GE, Paige JN. Stereochemistry of the decomposition of 2-butene episulfoxides. J Org Chem. 1966;32:459–460. [Google Scholar]