The contraction and relaxation of the beating heart is mediated by highly coordinated processes that allow rapid activation and deactivation of the myofilaments. The sarcomere is the fundamental contractile element and its shortening is the result of the myosin-containing thick filament sliding past the actin-containing thin filament. Fueled by ATP hydrolysis, myosin is the molecular motor that drives sarcomere shortening through a cyclical, ratchet-like interaction with actin. A critical component of cardiac contraction is the regulation of myosin-actin interactions, which are governed predominantly by the thin filament regulatory proteins, troponin and tropomyosin.
A change in myofilament isoform composition is one mechanism by which myocardial contractile performance may be affected in heart failure. The fast isoform of myosin heavy chain, α-MHC, normally accounts for approximately 10% fractional content in healthy human myocardium compared to 90% β-MHC, but is reduced to nearly undetectable concentrations during heart failure. 1 In animal models, a change in myofilament performance can be detected with MHC isoform shifting and the slight loss of α-MHC in human heart failure has been touted by some, 2 although not all, 3 as an underlying factor in the reduced cardiac function. Enhanced incorporation of the atrial isoform of myosin essential light chain in the failing human ventricle is observed and correlates with an increase in myofilament calcium sensitivity. 4 Finally, a shift in troponin-T isoform composition is reported in human heart failure 5 with likely modest effects on contractility. 6 In this issue Rajan et al. 7 identify a shift in tropomyosin isoform expression in human heart failure and investigate its possible functional consequences. As will be discussed, this study represents an important contribution to our understanding of how myofilament protein isoforms, through the cooperative activation of the thin filament, may influence cardiac performance in heart failure.
Tropomyosin is composed of two alpha helical chains coiled around each other in a parallel fashion. It lies in the groove of actin helical filament spanning 40 nm (i.e. 7 actin monomers), and in diastole, blocks the myosin binding sites on actin. For each tropomyosin molecule there is a paired troponin molecule. Troponin contains 3 subunits: the calcium binding troponin-C, the inhibitory protein troponin-I, and the tropomyosin binding subunit troponin-T. Troponin binds to tropomyosin and, in the absence of calcium, also binds to actin via troponin-I. The myofilament is activated by calcium binding to troponin-C, inducing a conformation change in troponin to result in decreased troponin-I affinity to actin. 8 This effectuates an azimuthal shift of tropomyosin on the actin filament, exposing myosin binding sites on actin, and through the cyclical binding of myosin to actin, sarcomere shortening is initiated.
Continuous and coordinated contraction of the myocardium is the result of rapid contraction and relaxation of the myofilament. This process requires that the myofilament activation be highly cooperative. By definition a cooperative biologic process is one where an action facilitates the same action in adjacent structures through an allosterically mediated change in protein conformation. 9 Perhaps one of the best studied cooperative processes is the oxygen-hemoglobin association curve, where at the level of the individual hemoglobin molecule the binding of one oxygen molecule greatly facilitates the occupancy of the remaining three oxygen binding sites. Cardiac muscle activation is highly cooperative and occurs at several levels. The binding of calcium to troponin results in the exposure of myosin binding sites along the 7 actin monomers blocked by the overlying tropomyosin molecule. However, the activation of the thin filament likely extends beyond the confines of this 7 actin/ tropomyosin/ troponin unit. As the N and C termini of adjacent tropomyosins overlap by 9 amino acids, 10 the azimuthal shift of tropomyosin extends to neighboring tropomyosin molecules. Moreover, we now know from elegant kinetic 11 and structural 12 studies that calcium activation of the thin filament only partially exposes the myosin binding site on the thin filament. The binding of one myosin molecule to the thin filament shifts tropomyosin further on actin fully exposing adjacent actin binding sites, accelerating the rate of subsequent myosin binding to the thin filament. Finally, myosin binding to the thin filament increases the calcium affinity of unsaturated troponin C, further accelerating the activation on the thin filament. 13
We are only beginning to understand the mechanisms underlying thin filament regulation. Through myofilament cooperativity, even seemingly minor translational or posttranslational modifications to the regulatory proteins can have dramatic effects on thin filament activation and contractility. Rajan et al. identifies an increase in protein expression of a recently discovered novel tropomyosin isoform (κ-tropomyosin) in human heart failure.7 In absolute terms, κ-tropomyosin expression is relatively small (3-5% of the total tropomyosin expressed) with α-tropomyosin being the predominantly expressed cardiac isoform (>90%). However, the authors demonstrate a doubling of the expression of κ-tropomyosin in the failing human heart. To define the functional implication of an increase in κ-tropomyosin expression an over expression transgenic model was developed using the well established α-MHC promoter. Human and mouse κ-tropomyosin are identical, adding credibility to this approach. Three separate transgenic lines with differing levels of κ-tropomyosin expression were established. The transgenic mice developed a dilated cardiomyopathy highlighted by chamber dilation and decreased ejection fraction, underscoring the global functional significance of tropomyosin isoform shifting in myocardial function. Mechanical assessment of myofilament function in demembranted myocardial strips demonstrated decreases in both calcium sensitive activation and myosin strong binding activation with no effect on maximal force development.
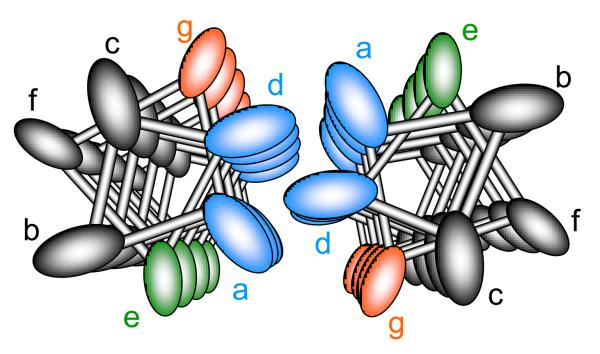
To appreciate the potential underlying effects of κ-tropomyosin over α-tropomyosin, we need to better understand the possible structural differences. While both tropomyosin isoforms are derived from the same gene (TMP1) the κ isoform differs from α by an exchange of exon 2 to the smooth muscle specific exon (exon 2a). As originally defined by Crick, the protein α helix is comprised of 7 repeating amino acid motifs (termed positions a, b, c, d, e, f, and g) for every 2 turns of the helix (Figure 1).14;15 Positions a and d are generally apolar residues which permit stabilizing hydrophobic interactions between the two alpha parallel helices of the coiled coil structure. Positions e and g are located on the outer surface of the tropomyosin molecule and stabilize the structure through salt bridges. Analysis of the primary sequence differences between exon 2b and 2a reveals substantial differences in these critical alpha helical positions (see supplement Table 5 of Rajan et al.).7 Based on this analysis, it would be predicted that κ-tropomyosin would be substantially less stable (and thus more compliant) than α-tropomyosin. This was in fact demonstrated by the authors with thermal perturbation /circular dichroism experiments.
Figure 1.
Helical relation of amino acid positions of tropomyosin's coiled coil as viewed from an axial perspective. Hydrophobic residues at position a and d stabilize interactions between α helices, where positions g and e are typically charged amino acids stabilizing tropomyosin through ionic interactions.
How could a change in tropomyosin stability lead to a decrease in thin filament activation by calcium and myosin strong binding ultimately to affect myocardial mechanical performance at the whole heart level? The answer likely lies in the critical contribution that tropomyosin plays in the cooperative activation of the myofilament. As indicated above, previous work indicates that through the overlap of adjacent tropomyosin molecules, the regional activation of thin filament may extended beyond the length of a single molecule. While the degree to which cooperative activation extends down the thin filament has generally been thought to be less than 80nm, i.e., approximately 2 tropomyosin molecules, mechanical assays that detect a single myosin molecule interacting with a regulated thin filament have indicated that the cooperative activation of the thin filament with myosin strong binding may extend beyond 100nm.16 Recent data by Li et al, demonstrate through electron microscopy and molecular dynamic simulation that tropomyosin is a far more rigid molecule than previously appreciated, raising the possibility that tropomyosin may be able to transmit cooperative activation as far as 1000nm, or 25 lengths of tropomyosin.17 Thus the relative compliance of the κ-tropomyosin isoform likely results in increased flexibility of tropomyosin, greatly shortening the cooperative distance that the thin filament is activated by either calcium or myosin binding. While the amount of κ-tropomyosin expressed in the human heart is relatively small, the data presented by Li et al. would suggest that even a 1:25 protein stoichiometry could potentially affect myofilament function.17
More studies are needed to better define the functional consequences of increased κ-tropomyosin expression in human heart failure. It is generally accepted that there is increased thin filament calcium sensitivity in failing humans hearts.18 While marked overexpression of κ-tropomyosin in a mouse model leads to cardiomyopathic changes, the smaller increase in κ-tropomyosin expression in human heart failure may represent a partial compensatory mechanism aimed at reducing the calcium sensitivity of the thin filament toward the non-failing state.
Acknowledgements
We would like to thank Kelly Begin for assisting in manuscript preparation
Funding
This work was funded by grants from the NIH, HL65586 to PVB and HL086902 to BMP.
Footnotes
Disclosures
None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Reiser PJ, Portman MA, Ning XH, Schomisch MC. Human cardiac myosin heavy chain isoforms in fetal and failing adult atria and ventricles. Am J Physiol Heart Circ Physiol. 2001;280:H1814–H1820. doi: 10.1152/ajpheart.2001.280.4.H1814. [DOI] [PubMed] [Google Scholar]
- 2.Herron TJ, McDonald KS. Small amounts of alpha-myosin heavy chain isoform expression significantly increase power output of rat cardiac myocyte fragments. Circ Res. 2002;90:1150–1152. doi: 10.1161/01.res.0000022879.57270.11. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki T, Palmer BM, James J, Wang Y, Chen Z, VanBuren P, Maughan DW, Robbins J, LeWinter MM. Effects of cardiac myosin isoform variation on myofilament function and crossbridge kinetics in transgenic rabbits. Circ Heart Fail. 2009;2:334–341. doi: 10.1161/CIRCHEARTFAILURE.108.802298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morano I, Hadicke K, Haase H, Bohm M, Erdmann E, Schaub MC. Changes in essential myosin light chain isoform expression provide a molecular basis for isometric force regulation in the failing human heart. J Mol Cell Cardiol. 1997;29:1177–1187. doi: 10.1006/jmcc.1996.0353. [DOI] [PubMed] [Google Scholar]
- 5.Anderson PA, Malouf NN, Oakeley AE, Pagani ED, Allen PD. Troponin T isoform expression in the normal and failing human left ventricle: a correlation with myofibrillar ATPase activity. Basic Res Cardiol. 1992;87(Suppl 1):117–27. doi: 10.1007/978-3-642-72474-9_10. 117-127. [DOI] [PubMed] [Google Scholar]
- 6.Gomes AV, Guzman G, Zhao J, Potter JD. Cardiac troponin T isoforms affect the Ca2+ sensitivity and inhibition of force development. Insights into the role of troponin T isoforms in the heart. J Biol Chem. 2002;277:35341–35349. doi: 10.1074/jbc.M204118200. [DOI] [PubMed] [Google Scholar]
- 7.Rajan S, Jagatheesan G, Karam CN, Alves ML, Bodi I, Schwartz A, Bulcao CF, D'Souza KM, Akhter SA, Boivin GP, Dube DK, Petrashevskaya N, Herr AB, Hullin R, Liggett SB, Wolska BM, Solaro RJ, Wieczorek DF. Molecular and functional characterization of a novel cardiac specific human tropomyosin isoform. Circulation. 2009 doi: 10.1161/CIRCULATIONAHA.109.889725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takeda S, Yamashita A, Maeda K, Maeda Y. Structure of the core domain of human cardiac troponin in the Ca(2+)-saturated form. Nature. 2003;424:35–41. doi: 10.1038/nature01780. [DOI] [PubMed] [Google Scholar]
- 9.Monod J, Wyman J, Changeux JP. On the nature of allosteric transitions: a plausible model. J Mol Biol. 1965;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- 10.Phillips GNJ, Fillers JP, Cohen C. Tropomyosin crystal structure and muscle regulation. J Mol Biol. 1986;192:111–131. doi: 10.1016/0022-2836(86)90468-7. [DOI] [PubMed] [Google Scholar]
- 11.McKillop DF, Geeves MA. Regulation of the interaction between actin and myosin subfragment 1: evidence for three states of the thin filament. Biophys J. 1993;65:693–701. doi: 10.1016/S0006-3495(93)81110-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Craig R, Lehman W. Crossbridge and tropomyosin positions observed in native, interacting thick and thin filaments 13. J Mol Biol. 2001;311:1027–1036. doi: 10.1006/jmbi.2001.4897. [DOI] [PubMed] [Google Scholar]
- 13.Bremel RD, Weber A. Cooperation within actin filament in vertebrate skeletal muscle. Nat New Biol. 1972;238:97–101. doi: 10.1038/newbio238097a0. [DOI] [PubMed] [Google Scholar]
- 14.Crick FHC. The packing of alpha-helices: simple coiled-coils. Acta Cryst. 1953;6:698–697. [Google Scholar]
- 15.Parry DA, Fraser RD, Squire JM. Fifty years of coiled-coils and alpha-helical bundles: a close relationship between sequence and structure. J Struct Biol. 2008;163:258–269. doi: 10.1016/j.jsb.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 16.Kad NM, Kim S, Warshaw DM, VanBuren P, Baker JE. Single-myosin crossbridge interactions with actin filaments regulated by troponin-tropomyosin. Proc Natl Acad Sci U S A. 2005;102:16990–16995. doi: 10.1073/pnas.0506326102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li XE, Holmes KC, Lehman W, Jung H, Fischer S. The Shape and Flexibility of Tropomyosin Coiled-Coils: Implications for Actin Filament Assembly and Regulation. J Mol Biol. 2009 doi: 10.1016/j.jmb.2009.10.060. ePUB in press. [DOI] [PubMed] [Google Scholar]
- 18.Wolff MR, Buck SH, Stoker SW, Greaser ML, Mentzer RM. Myofibrillar calcium sensitivity of isometric tension is increased in human dilated cardiomyopathies: role of altered beta-adrenergically mediated protein phosphorylation. J Clin Invest. 1996;98:167–176. doi: 10.1172/JCI118762. [DOI] [PMC free article] [PubMed] [Google Scholar]