Abstract
Background
The Heschl gyrus and planum temporale have crucial roles in auditory perception and language processing. Our previous investigation using magnetic resonance imaging (MRI) indicated smaller gray matter volumes bilaterally in the Heschl gyrus and in left planum temporale in patients with first-episode schizophrenia but not in patients with first-episode affective psychosis. We sought to determine whether there are progressive decreases in anatomically defined MRI gray matter volumes of the Heschl gyrus and planum temporale in patients with first-episode schizophrenia and also in patients with first-episode affective psychosis.
Methods
At a private psychiatric hospital, we conducted a prospective high spatial resolution MRI study that included initial scans of 28 patients at their first hospitalization (13 with schizophrenia and 15 with affective psychosis, 13 of whom had a manic psychosis) and 22 healthy control subjects. Follow-up scans occurred, on average, 1.5 years after the initial scan.
Results
Patients with first-episode schizophrenia showed significant decreases in gray matter volume over time in the left Heschl gyrus (6.9%) and left planum temporale (7.2%) compared with patients with first-episode affective psychosis or control subjects.
Conclusions
These findings demonstrate a left-biased progressive volume reduction in the Heschl gyrus and planum temporale gray matter in patients with first-episode schizophrenia in contrast to patients with first-episode affective psychosis and control subjects. Schizophrenia but not affective psychosis seems to be characterized by a postonset progression of neocortical gray matter volume loss in the left superior temporal gyrus and thus may not be developmentally fixed.
The Heschl gyrus (HG) and the posteriorly adjacent planum temporale (PT) are located on the superior temporal gyrus (STG). The HG is primary auditory cortex (Brodmann area [BA] 41/42), playing a crucial role in auditory perception.1 The anterior portion of the PT is part of the unimodal auditory association cortex (part of BA22) that surrounds the HG, whereas the posterior portion adjacent to the temporoparietal junction (other portions of BA22 and part of BA39-40) is partially coextensive with the Wernicke area, consisting of heteromodal association cortex.2,3 The PT evinces the most prominent left-right asymmetry in the human brain,4 which is thought to reflect the critical role of PT in language processing.5
Abnormalities in auditory perception and language processing are among the core features of cognitive dysfunction in schizophrenia,6-8 prompting studies of structural and functional abnormalities of HG and PT. Rojas et al9 reported smaller HG volume (gray and white matter unsegmented) in male patients with paranoid schizophrenia than in control subjects, and our laboratory10 reported bilateral HG gray matter volume reduction in patients with first-episode schizophrenia compared with patients with first-episode affective psychosis and control subjects. However, other investigators have reported no reduction in HG surface area11,12 or gray matter volume13-15 in schizophrenia.
Functionally, mismatch negativity, an event-related potential representing early auditory processing, with a major generator in HG,16 is consistently reduced in chronic schizophrenia17-19 but, as recently reported, not in first-episode schizophrenia,19 suggesting the possibility of progressive cortical change. Wible et al20 revealed that functional magnetic resonance imaging (MRI) activation elicited by auditory mismatch stimuli was reduced bilaterally in HG and its vicinity in chronic schizophrenia. Dierks et al21 demonstrated an increase of functional MRI activation in left hemisphere HG during auditory hallucinations in right-handed patients with schizophrenia.
Kwon et al15 reported left PT gray matter volume reduction in chronic schizophrenia, and Hirayasu et al10 reported smaller left PT gray matter volumes in patients with first-episode schizophrenia compared with patients with first-episode affective psychosis and control subjects. However, others did not find such a difference.13,14,22 Evaluations of the PT surface area and length yielded some reports of loss or reversal of normal asymmetry of PT in schizophrenic patients,11,13,23 but others did not find these differences.12,22,24,25
The reduced left temporal amplitude of the auditory P300 event-related potential, which has one of its major generators in posterior STG26-28 (largely coextensive with PT), has been associated with smaller left posterior STG gray matter volume in both chronic29 and, recently, first-episode30 schizophrenia. Of relevance to progressive cortical change, cross-sectional studies on P300 demonstrated an abnormal age-related31 or duration-of-illness–related32 latency prolongation in schizophrenia. With respect to clinical correlates of PT, a reduced asymmetry has been associated with more severe thought disorder,11,23 and left posterior STG33 and PT13 gray matter volume reduction has been associated with the severity of thought disorder. Additionally, a recent functional MRI34 study has also demonstrated that severity of formal thought disorder was negatively correlated with activation changes in left BA22.
With respect to change over time, several investigators have reported a progressive decrease in MRI gray matter volumes of STG,35-38 although findings have been controversial. The retrospective study performed by Mathalon et al35 demonstrated that male patients with chronic schizophrenia exhibited greater volume decline than control subjects in bilateral posterior STG gray matter. Our laboratory36,37 reported smaller gray matter decline in left posterior STG in patients with first-episode schizophrenia compared with patients with first-episode affective psychosis and controls. By contrast, Keshavan et al38 reported a reversal of the initial gray matter STG volume reduction with neuroleptic treatment after 1-year follow-up in first-episode schizophrenia. However, to our knowledge, no study to date has evaluated progressive changes in HG and PT gray matter volume.
We report herein a prospective longitudinal study of HG and PT volumes. Our study used MRI scans at baseline (first hospitalization of patients with schizophrenia and affective psychosis) and a subsequent scan approximately 1½ years later, which revealed that schizophrenia, but not affective psychosis, is characterized by progressive gray matter volume reduction in left HG and PT compared with healthy control subjects.
METHODS
PARTICIPANTS
Twenty-two healthy control subjects (2 women) and 28 patients with first-episode psychosis, 13 with schizophrenia (3 women; 11 with paranoid schizophrenia, 1 with disorganized schizophrenia, and 1 with undifferentiated schizophrenia) and 15 with affective psychosis (1 woman; 13 with bipolar disorder and manic disorder and 2 with major depressive disorder), participated in this study (Table 1). Mood-incongruent features were present in 6 of 13 patients with manic psychosis, and all 6 had hallucinations and/or delusions. Mood-congruent features were present in 7 of 13, with 4 of 7 having psychotic features (hallucinations and/or delusions) other than grandiosity. Neither patient group differed significantly in age from the control group, and gender distribution, handedness (all right-handed39), and parental socioeconomic status (SES)40 did not differ among groups. Schizophrenic patients were significantly older than affective psychosis patients and had a significantly lower SES40 than affective psychosis patients and control subjects.
Table 1.
Demographic and Clinical Characteristics of Study Groups
| Mean (SD) [Range] |
||||||
|---|---|---|---|---|---|---|
| Variable | Schizophrenic Patients (n = 13) |
Affective Psychosis Patients (n = 15) |
Control Subjects (n = 22) |
df* | F or t Test or χ2 Values |
P Value |
| Age, y† | 27.3 (8.5) [18-41] | 21.8 (2.9) [18-28] | 25.0 (4.3) [18-35] | 2,47 | 3.72 | .03 |
| Male/female | 10/3 | 14/1 | 20/2 | 2 | 2.09 | .35 |
| Handedness‡ | 0.8 (0.1) | 0.7 (0.2) | 0.8 (0.2) | 2,47 | 0.48 | .62 |
| SES§ | 3.7 (1.4) | 2.6 (1.3) | 2.1 (0.9) | 2,47 | 7.21 | .002 |
| Parental SES | 1.9 (0.6) | 1.5 (0.7) | 1.4 (0.6) | 2,47 | 3.18 | .051 |
| MMSE, baseline scan | 27.5 (3.1) | 29.1 (1.4) | 29.1 (1.2) | 2,45 | 3.14 | .053 |
| WAIS-R, baseline scan | ||||||
| Information, scaled | 11.3 (3.7) | 12.7 (2.9) | 13.0 (2.5) | 2,43 | 1.28 | .29 |
| Digits span, scaled | 9.8 (2.2) | 11.1 (2.8) | 11.0 (3.1) | 2,44 | 0.83 | .44 |
| Medication dose, baseline scan | 196.0 (126.3) | 205.6 (145.6) | NA | 23 | −0.16 | .88 |
| Age first medicated, y | 26.8 (8.6) [18-41] | 21.3 (3.1) [17-28] | NA | 26 | 2.32 | .02 |
| Median duration of medication use before baseline | 1 [0-24] | 0 [0-60] | NA | NA | NA | NA |
| Interscan interval, mo | 17.1 (11.0) [9-40] | 17.7 (8.1) [8-36] | 18.8 (8.9) [9-43] | 2,47 | 0.16 | .86 |
| GAS | ||||||
| Baseline scan | 38.5 (10.0) | 42.1 (9.2) | NA | 26 | −0.97 | .34 |
| Second scan | 50.9 (8.3) | 63.6 (14.7) | NA | 25 | −2.66 | .01 |
| Total BPRS | ||||||
| Baseline scan | 40.8 (12.7) | 33.4 (7.6) | NA | 26 | 1.89 | .07 |
| Second scan | 29.4 (5.7) | 23.5 (5.6) | NA | 20 | 2.59 | .02 |
Abbreviations: BPRS, Brief Psychiatric Rating Scale; GAS, Global Assessment Scale; MMSE, Mini-Mental State Examination; NA, data not applicable; SES, socioeconomic status; WAIS-R, Wechsler Adult Intelligence Scale–Revised.
The df differ among variables owing to unavailability of data in some participants.
The schizophrenic patients were significantly older than affective psychosis patients (Tukey honestly significant difference [HSD] tests, P = .03).
Right-handedness is above 0.
Higher scores indicate lower SES. The schizophrenic patients showed significantly lower SES than affective psychosis patients (Tukey HSD, P = .046) and control subjects (P = .001).
Patients were recruited from inpatient units at McLean Hospital, Belmont, Mass, a private psychiatric hospital affiliated with Harvard Medical School. Control subjects were recruited through newspaper advertisement. Our earlier study of HG and PT at baseline MRI scan10 included 66 participants (20, schizophrenia; 24, affective psychosis; 22, control). Thirty-one of these participants (12, schizophrenia; 8, affective psychosis; 11, control) agreed to participation in a second scan and were included in the present study. The remaining 19 participants were newly recruited. The rate of nonparticipation in the second scan (dropout rate) did not differ significantly among groups ( P=.20). Our local institutional review board approved this study. After a complete description of the study, written informed consent was obtained from all participants.
The protocols for diagnosis and clinical evaluations have been described in detail previously.10,41-45 Briefly, at both baseline and second scan, patients and control subjects met criteria for age (18-55 years), IQ above 75, right-handedness, and a history negative for the following: seizures, head trauma with loss of consciousness, neurologic disorder, and any lifetime history of alcohol or other drug dependence. No control subject had an Axis I or II psychiatric disorder or a first-degree relative with an Axis I psychiatric disorder with screening using the Structured Clinical Interview for DSM-III-R Non-Patient Edition (SCID-NP46) by trained interviewers (D.F.S. and M.E.S.), who also diagnosed patients based on the DSM-IV criteria, using the SCID interview47 and information from medical records. Diagnoses were confirmed at follow-up interview. Consistent with the literature,10,41-45,48,49 first episode was operationally defined as the first psychiatric hospitalization.
Median duration of psychotropic medication use before baseline scan was short (Table 1). At the time of the first scan, patients were variously receiving neuroleptics (typical [6, schizophrenia; 9, affective psychosis], atypical [2, schizophrenia; 5, affective psychosis], or both [3, schizophrenia; 0, affective psychosis]); mood stabilizers (lithium carbonate [1, schizophrenia; 5, affective psychosis], valproate sodium [3, schizophrenia; 5, affective psychosis]); or only drugs other than neuroleptics and mood stabilizers (1, schizophrenia; 1, affective psychosis). Typical or atypical neuroleptic status for 1 schizophrenic patient was unknown due to enrollment in a double-blinded olanzapine-haloperidol crossover protocol. Between scans, hospital records and self-reports indicated patients received neuroleptics (typical [1, schizophrenia; 0, affective psychosis], atypical [9, schizophrenia; 5, affective psychosis], or both [0, schizophrenia; 1, affective psychosis]); mood stabilizers exclusively (lithium [3, schizophrenia; 5, affective psychosis], valproate sodium [5, schizophrenia; 7, affective psychosis]); drugs other than neuroleptics or mood stabilizers (1, schizophrenia; 1, affective psychosis); or no drugs or discontinued use of medication (2, schizophrenia; 1, affective psychosis). The medication status for 1 affective psychosis patient was unknown.
Clinical evaluations at baseline and second scan included the Mini-Mental State Examination (MMSE),50 the information and digits span subscales of the Wechsler Adult Intelligence Scale–Revised (WAIS-R),51 the Global Assessment Scale (GAS),52 and each item and 4 syndrome factors53 of the Brief Psychiatric Rating Scale (BPRS).54 Of note, scores on the MMSE, WAIS-R subscales, GAS, and total BPRS at baseline scan were not significantly different between the 2 psychosis groups.
MRI ACQUISITION AND PROCESSING
The MRIs were acquired with a 1.5-T scanner (GE Medical Systems, Milwaukee, Wis) and the same acquisition protocol at baseline scan and second scan. The interscan interval was not significantly different among groups. The MRI acquisition protocol and the postprocessing of images have been described elsewhere in detail.55 Briefly, a 1.5-mm-thick coronal series of contiguous spoiled-gradient recalled acquisition (SPGR) images (repetition time, 35 milliseconds; echo time, 5 milliseconds; voxel dimensions, 0.9375×0.9375×1.5 mm) was used for delineating and measuring HG and PT. Then an axial series of contiguous double-echo (proton density and T2-weighted) images (repetition time, 3000 milliseconds; echo time, 30 and 80 milliseconds; voxel dimensions, 0.9375×0.9375×3.0 mm) was acquired. An anisotropic diffusion filter (k=13 for SPGR and 90 for proton and T2 images, iteration=3)56 was applied to the images to reduce noise before processing each set of scans. The intensity information from both the SPGR and T2 images was then used in a fully automated segmentation program to classify tissue into gray matter, white matter, and cerebrospinal fluid and to evaluate total intracranial content (ICC). An iterative expectation-maximization algorithm initially estimated image intensity inhomogeneities, applied intensity corrections based on these estimates, then classified tissue based on the same set of signal intensity parameters for all subjects.57 Images were aligned using the line between the anterior and posterior commissures and the sagittal sulcus to correct head tilt and were also resampled to make voxels isotropic (sides measured 0.9375 mm).10
REGIONS OF INTEREST
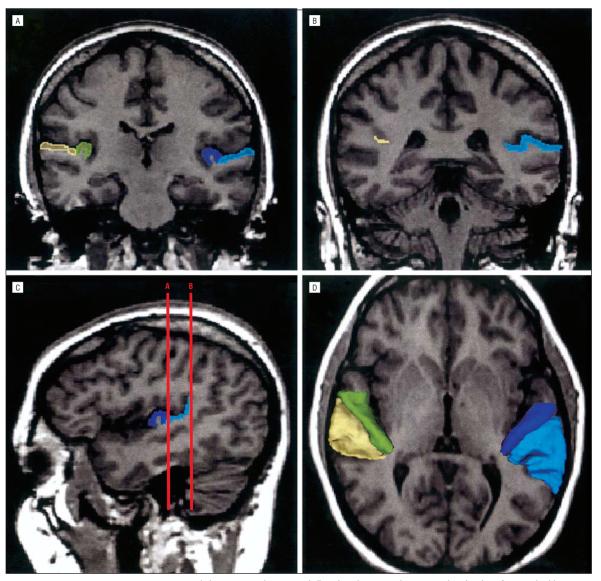
The HG and PT gray matter regions of interest (ROIs) were manually outlined without knowledge of diagnosis or time of scan using a software package for medical image analysis (3D Slicer; software available at http://www.slicer.org) on a workstation (Figure 1). The landmarks for delineating HG and PT gray matter have been previously described in detail,10 and there were no statistically significant group differences in HG morphologic features.
Figure 1.
A and B, Delineation of the Heschl gyrus and planum temporale on coronal slices, based on magnetic resonance imaging data of a control subject. A, The rostral part of the regions of interset. B, The caudal portion of the planum temporale at the ascending ramus of the sylvian fissure. The gray matter of the Heschl gyrus is labeled dark blue on the subject's left and green on the subject's right. The gray matter of the planum temporale is light blue on the subject's left and yellow on the subject's right. C, Sagittal view of the Heschl gyrus and planum temporale in the left hemisphere. The coronal lines A and B correspond to the planes of panels A and B, respectively. D, Three-dimensional reconstruction of the Heschl gyrus and planum temporale gray matter superimposed on the axial plane. Each region is labeled using the same color as that in A, B, and C.
The initial identification of HG was based on an inspection of the retroinsular region in the axial plane.22,58-60 Moving from the superior to inferior slices, we continued to draw convolution(s) of HG. When the anterior boundary of HG was abruptly extended further anteriorly, we stopped drawing on axial slices. This is approximately the point where the transition of HG to anterior STG occurs. We then edited HG on coronal slices, with HG tracing proceeding from the most posterior coronal image with a mark to the most anterior. The first slice of HG in the coronal plane corresponds to the most anterior point of the appearance of the first transverse sulcus. The boundary of HG was finally confirmed in the sagittal plane to ensure that the delineated region was not extended to the anterior STG. In most cases, HG represented a single transverse convolution (left: 85% of schizophrenic patients, 87% of affective psychosis patients, 68% of control subjects; right: 38% of schizophrenic patients, 80% of affective psychosis patients, and 73% of control subjects). In the cases of more than one transverse convolution, we followed the literature definition22,58-60: when multiple convolutions originated medially from a common stem, all were defined as HG (the sulcus between these convolutions represents the sulcus intermedius of Beck) (left: 15% of schizophrenic patients, 13% of affective psychosis patients, 27% of control subjects; right: 31% of schizophrenic patients, 13% of affective psychosis patients, 23% of control subjects); when they originated separately from the retroinsular regions, only the most anterior gyrus was labeled as HG (left: 0% of schizophrenic patients, 0% of affective psychosis patients, 5% of control subjects; right: 31% of schizophrenic patients, 7% of affective psychosis patients, 5% of control subjects), and more posterior gyri were identified as PT. The prevalence of single HG, multiple transverse gyri from a common stem arising separately was not significantly different among groups (left HG: , P=.59; right HG: , P=.08). The posterior border of HG (Heschl sulcus) defined the anterior border of PT. Posteriorly, PT gray matter was traced on coronal images to the end of the sylvian fissure, and the gray matter of the ascending ramus of the sylvian fissure was also included. Thus, our definition of the PT included PT proper and its parietal extension. Once drawn, both HG and PT ROIs could be viewed in any plane and as a 3-dimensional object for any further editing.
For interrater reliability, raters (K.K., T.O., and Y.H.), blinded to group membership, independently drew ROIs. Ten cases were selected at random from the 3 diagnostic groups and from both baseline and second scans, and the raters drew ROIs on every slice. The intraclass correlation coefficient was 0.95/0.96 for left/right HG gray matter and 0.99/0.99 for left/right PT gray matter. Intrarater reliability, computed by using all of the slices from 1 randomly selected brain and measured by each of the 3 raters at 2 separate times (approximately 6 months apart), was 0.95 to 0.99 for all structures.
STATISTICAL ANALYSIS
HG and PT Volume at Baseline and Second Scan
We evaluated group differences in ROI (HG or PT) volume separately for baseline and second scan using a repeated-measures analysis of covariance (ANCOVA) with group (schizophrenic, affective psychosis, control) as the between-subjects factor, hemisphere (left, right) as the within-subjects factor, and age and parental SES as covariates. Relative volume ([absolute ROI volume/ICC]×100) was used as the dependent variable for statistical measures, although groups did not significantly differ in ICC volume at baseline or second scan (F2,45=1.19, P=.31; F2,45=1.53, P=.23, respectively). Of note, the statistical conclusions reported herein remained the same when we used ANCOVA with absolute volume as the dependent variable and ICC as the covariate.
HG and PT Volume Change Over Time
We evaluated change over time separately for HG and PT using a repeated-measures analysis of variance with group as the between-subjects factor and time of scan (baseline, second scan) and hemisphere as within-subjects factors. In the case of interactions with hemisphere, a follow-up repeated-measures analysis of variance (between-subjects factor: group; within-subjects factors: time) was performed for each hemisphere. The statistical significance level was P<.05. Finally, a paired t test was used to compare the volumes of each ROI between baseline and second scans in each group, with a Bonferroni-corrected post hoc t test level of significance set at P<.017 (.05 corrected for the 3 groups). The statistical conclusions reported herein remained the same when adopting ANCOVA with age as the covariate or when an age-matched subsample was tested (schizophrenia, n=12; affective psychosis, n=14; control, n=22). The statistical conclusions also remained the same when we used an ANCOVA with interscan interval as the covariate. For comparative purposes, we also used the Wilcoxon signed rank nonparametric test for change in volume for each ROI and group (P≤.0042 [.05/12 {3 groups, 4 ROIs}; Bonferroni correction]).
Correlational Analysis
In the case of significantly greater changes in a particular region and hemisphere in one group, exploratory analyses of the correlations between percent change score of the ROI volume (calculated by the formula 100×[{relative volume at second scan–relative volume at baseline scan}/relative volume at baseline scan]) and baseline or absolute change score of clinical measures were performed using the Spearman ρ. In addition, we computed and tested averaged scores across baseline and second scans as a potential compensation for early illness stage fluctuations in symptoms and functioning. Since we considered the analyses to be exploratory in nature, we used P<.05 as the cutoff for reporting statistical significance rather than using a correction for multiple correlations. The values reported herein are those for percent change score of the ROI volume, but all reported correlations were also significant at the P<.05 level for the absolute difference between baseline and second scan ROI relative volumes. In addition, for any ROI in which there was a significant group difference in change score, correlations between change score and interscan interval were also evaluated for each group.
RESULTS
Age, SES, parental SES, age of first medication, medication dose, duration of medication use before the first scan, and ICC volume at baseline or second scan did not correlate with any of the ROI volumes at baseline or second scan or with the change score of any of the ROI volumes in any group. Although numbers were too small for formal statistical analysis, there was no suggestion that mean change scores of left HG and PT volume differed according to the presence or absence of neuroleptic medication between scans in the patient populations (see the “Comment” section for details). Additionally, the statistical conclusions reported herein remained the same after the exclusion of the female participants and also when only the manic affective psychosis patients were included.
HG VOLUME AT BASELINE AND SECOND SCAN
At baseline scan, there was a significant main effect of group (F2,45=3.62, P=.04), with no group×hemisphere interaction (F2,45=0.12, P=.89). Left HG was larger than right HG for all groups (main effect of hemisphere: F1,45=5.91, P=.02). Post hoc tests revealed that total (left plus right) HG volume was significantly smaller in schizophrenic patients compared with affective psychosis patients and control subjects (Tukey honestly significant difference [HSD] tests: P = .008 and P = .007, respectively), but group differences for left and right HG did not reach significance by 1-factor ANCOVA (F2,45=1.86, P=.17 [20% reduction relative to controls]; F2,45=2.10, P=.14 [16%], respectively).
The statistical results at second scan were similar to those at baseline scan in terms of repeated-measures ANCOVA (main effect of group: F2,45=4.92, P=.01; main effect of hemisphere: F1,45 = 4.95, P = .03; group×hemisphere interaction: F2,45=0.35, P=.71) and post hoc tests (total HG volume was significantly smaller in schizophrenic patients compared with affective psychosis patients and control subjects [Tukey HSD: P=.003 and P=.002, respectively]). However, 1-factor ANCOVA separately for each hemisphere showed that group differences reached significance for the left hemisphere (F2,45=4.47, P=.02 [26% reduction relative to controls]) but not for the right hemisphere (F2,45= 1.75, P = .19 [15%]).
PT VOLUME AT BASELINE AND SECOND SCAN
At baseline scan, although the main effect of group did not reach statistical significance (F2,45=1.07, P=.35), there was a significant group × hemisphere interaction (F2,45=5.72, P=.006). One-factor ANCOVA showed that left PT differed among groups (F2,45=4.75, P=.01), with schizophrenic patients having significantly smaller volumes than affective psychosis and control groups (Tukey HSD: P=.01 and P=.01 [24% reduction relative to controls], respectively). However, right PT was not different among groups (F2,45=0.66, P=.52). Statistical conclusions were replicated for PT volumes at second scan (schizophrenic patients' left PT volume showed a 31% reduction relative to that of controls).
HG AND PT VOLUME CHANGES OVER TIME
For HG, groups were significantly different in gray matter volume (main effect of group: F2,47=7.21, P=.002), whereas total HG volume did not change over time (main effect of time: F1,47=2.33, P=.13). However, a significant group×time×hemisphere interaction (F2,47=10.5, P<.001) indicated that at least one group changed over time and that this change differed between the right and left hemispheres. We next compared right and left HGs separately. Subject groups were not significantly different in gray matter volume of right HG (main effect of group: F2,47=2.03, P=.14), which also did not change over time (main effect of time: F1,47=0.024, P=.88). There was no group×time interaction for right HG (F2,47=0.711, P=.50).
In contrast, subject groups were significantly different in gray matter volume of left HG (main effect of group: F2,47=6.58, P=.003), and the total gray matter volume for all groups of left HG showed a significant reduction over time (F1,47=5.92, P=.02). Moreover, the presence of a highly significant group × time interaction pointed to change in at least one group (F2,47= 9.62, P<.001). Separate within-group baseline–second scan comparisons revealed that it was the first-episode schizophrenic patients who showed a significant decrease in the gray matter volume of left HG over time (t12= 4.82, P<.001), whereas neither affective psychosis patients (t14=−0.734, P=.48) nor control subjects (t21=−0.377, P=.71) showed changes.
The statistical conclusions for PT were exactly the same as those for HG (main effect of group: F2,47=3.49, P = .04; main effect of time: F1,47 = 1.36, P = .25; group × time × hemisphere interaction: F2,47= 4.66, P=.01). For left PT, there was a significant main effect of group (F2,47 = 9.54, P<.001) and a significant group×time interaction (F2,47=15.4, P<.001). The post hoc paired t tests revealed that only schizophrenic patients showed a significant decrease (t12=5.11, P<.001), whereas neither affective psychosis patients (t14=−2.12, P=.052) nor control subjects (t21=−1.36, P=.19) showed changes. In contrast, for right PT, there was no significant main effect of group (F2,47=0.502, P=.61) or time (F1,47=0.001, P=.98) and no significant group×time interaction (F2,47=0.944, P=.40).
Additionally, the ICC volume did not significantly change from baseline to second scan (main effect of time: F1,45=3.57, P=.065), nor was this change different among groups (group×time interaction: F2,45=1.57, P=.22). Although the change of ICC over time was small (less than 1% for all groups) (Table 2), we conservatively tested a possibility of whether this trend-level effect of time might have led to a systematic error in ROI volume changes over time. However, ICC change scores (calculated by the following formula: 100×[(ICC volume at second scan–ICC volume at baseline scan)/ICC volume at baseline scan]) were not correlated with any of the change scores of ROIs (calculated by the following formula: 100×[(absolute volume at second scan–absolute volume at baseline scan)/absolute volume at baseline scan]) for any groups. Moreover, the statistical conclusions reported herein did not change when the percent change score of ICC was treated as a covariate.
Table 2.
Absolute and Relative Volumes of Regions of Interest at Baseline and Second Scan and Percent Change Over Time
| Mean (SD) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Schizophrenic Patients (n = 13) |
Affective Psychosis Patients (n = 15) |
Control Subjects (n = 22) |
|||||||
| Region | Baseline | Second Scan |
Percent Change* |
Baseline | Second Scan |
Percent Change* |
Baseline | Second Scan |
Percent Change* |
| Intracranial content, cm3 | 1498 (107) | 1486 (110) | −0.9 (1.0) | 1532 (116) | 1523 (123) | −0.7 (1.0) | 1566 (146) | 1561 (145) | −0.4 (1.2) |
| Left Heschl gyrus | |||||||||
| Absolute volume, cm3 | 1.48 (0.30) | 1.36‡ (0.25) | −7.7 (4.3) | 1.89 (0.33) | 1.89 (0.32) | 0.1 (3.4) | 1.93 (0.48) | 1.93 (0.47) | 0.2 (4.6) |
| Relative volume, %† | 0.099 (0.020) | 0.092‡ (0.015) | −6.9 (4.4) | 0.124 (0.023) | 0.125 (0.023) | 0.7 (3.5) | 0.124 (0.029) | 0.124 (0.029) | 0.5 (4.9) |
| Right Heschl gyrus | |||||||||
| Absolute | 1.29 (0.35) | 1.30 (0.35) | 1.1 (3.8) | 1.60 (0.26) | 1.58 (0.23) | −1.1 (4.6) | 1.58 (0.46) | 1.57 (0.44) | −0.3 (5.3) |
| Relative | 0.086 (0.023) | 0.087 (0.022) | 2.1 (4.6) | 0.105 (0.017) | 0.104 (0.015) | −0.5 (4.9) | 0.102 (0.031) | 0.102 (0.031) | 0.0 (5.5) |
| Left planum temporale | |||||||||
| Absolute | 1.90 (0.44) | 1.75‡ (0.41) | −8.0 (4.3) | 2.54 (0.54) | 2.57 (0.55) | 1.3 (3.4) | 2.61 (0.54) | 2.63 (0.54) | 0.9 (4.3) |
| Relative | 0.127 (0.027) | 0.117‡ (0.024) | −7.2 (4.2) | 0.166 (0.033) | 0.169 (0.033) | 2.0 (3.4) | 0.167 (0.035) | 0.170 (0.037) | 1.2 (4.4) |
| Right planum temporale | |||||||||
| Absolute | 2.05 (0.49) | 2.01 (0.43) | −1.7 (3.6) | 2.06 (0.39) | 2.04 (0.35) | −0.2 (5.3) | 1.99 (0.49) | 2.01 (0.49) | 1.0 (4.9) |
| Relative | 0.137 (0.029) | 0.135 (0.027) | −0.9 (3.4) | 0.135 (0.030) | 0.135 (0.028) | 0.5 (5.1) | 0.127 (0.028) | 0.128 (0.028) | 1.3 (5.1) |
Calculated by the following formula: 100 × ([volume at second scan–volume at baseline scan]/volumeat baseline scan). Negative value indicates decrease in volume.
Calculated by the following formula: (absolute region of interest volume/intracranial context) × 100
Significantly decreased over time (P<.001).
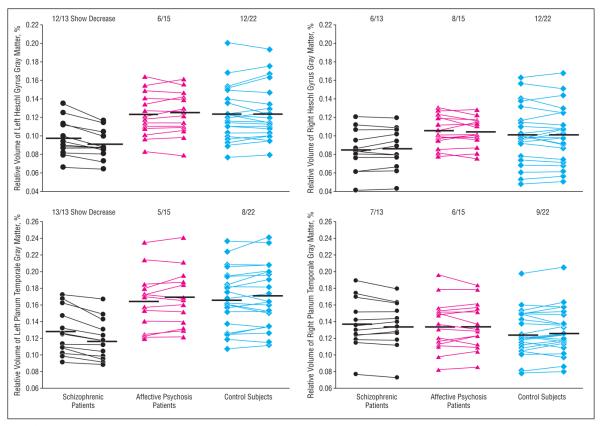
As an additional measure, a nonparametric Wilcoxon signed rank test confirmed these results; only left HG and left PT in the schizophrenia group showed a non-chance distribution of volume change (for left HG: z=−3.11, P=.002, 12 of 13 patients showed decrease; for left PT: z=−3.18, P=.001, 13 of 13 showed a decrease) (Figure 2). On average, schizophrenic patients showed 6.9% gray matter volume reduction for left HG and 7.2% for left PT (Table 2). In contrast, for the remaining ROIs, the distribution of volume change was within chance level for all of the groups (z=−1.76 to −0.227, P=.078 to P=.82).
Figure 2.
Relative volumes of the left and right Heschl gyrus and planum temporale gray matter at baseline and second scan in patients with first-episode schizophrenia (n=13), patients with first-episode affective psychosis (n=15), and healthy control subjects (n=22). Values of baseline and second scan in each subject are connected by lines. The numbers at the top of the scattergrams indicate the proportion of subjects who showed volume reduction over time (number of subjects/total number of subjects). Horizontal lines indicate means.
CORRELATIONS BETWEEN HG AND PT VOLUMES AND CLINICAL MEASURES
In schizophrenic patients, greater decreases of left HG and left PT volume over time were both significantly correlated with a more severe BPRS baseline score for the “conceptual disorganization” item (HG: ρ=−0.641, n=13, P=.02; PT: ρ=−0.581, n=13, P=.04). There was also a significant HG volume decrease correlation with the mean “conceptual disorganization” score (ρ=−0.669, n=13, P=.01) and the following items: (1) mean score for “suspiciousness” (ρ=−0.564, n=13, P=.045); (2) baseline score for “somatic concern” (ρ=−0.559, n=13, P=.047); and (4) baseline score for “anxiety-depression” factor (ρ=−0.573, n=13, P=.04). Thus, in all cases, the more pronounced the decrease, the worse the BPRS clinical measure. Additionally, left HG and left PT change score did not significantly correlate with the interscan interval in any groups (ρ=−0.421 to 0.227, P=.15 to P=.60).
COMMENT
To our knowledge, this is the first prospective study to demonstrate progressive gray matter volume reduction in the 1.5 years following first hospitalization in left HG and left PT in schizophrenic but not in affective psychosis patients. These results indicate the presence of a progressive process in the dominant temporal cortex, sub-serving auditory perception and language processing, a process that plays a role in the pathophysiology of schizophrenia but not of affective psychosis. We note that the smaller HG and PT gray matter volumes at baseline, although compatible with our prior results, are not a mere replication, since the patient groups only partly overlapped those in our earlier study.10 The principal finding of the present study, however, is the progression of further loss of gray matter volume during the 1.5-year period between scans.
The present prospective study's gray matter volume reduction during 1.5 years of 6.9% and 7.2%, respectively, for the left HG and PT in first-episode schizophrenia is close to our laboratory's earlier finding of 9% for the left posterior STG in first-episode schizophrenia36,37 but approximately 1.6 times that of the 3% per year for STG in patients with chronic disease found in the retrospective study by Mathalon et al.35 This suggestion of a more severe volume reduction early in the illness, however, can only be definitively tested through additional scans of the present cohort and additional subjects.
Our group's previous studies have found differences between first-episode samples of schizophrenic and affective psychoses in posterior STG gray matter41 and its overlapped subdivisions of HG and PT,10 as well as in prefrontal cortex,43 and in fusiform gyrus gray matter.45 Commonalities of the 2 psychoses at first episode included smaller gray matter volumes of left posterior amygdala-hippocampal complex (mostly hippocampus)41 and in the subgenual cingulate cortex42 (trend-level change in schizophrenia). These results suggest that, although smaller gray matter volumes in isocortical regions at first episode may be specific to schizophrenia, some of the abnormalities in limbic and paralimbic regions may be common to both psychoses. Also specific to schizophrenia is the observed progressive change in the isocortical region, and this may be associated with a different functional presentation and outcome between the 2 psychoses.61,62 These data also tend to favor the hypothesis that the 2 psychoses may represent manifestations of different disorders, an important but still unresolved question in psychiatry.
In the present study of first-episode schizophrenia, the baseline gray matter volume relative to controls was somewhat smaller in left HG (20% and not statistically significant) than in left PT (24% and statistically significant), findings compatible with the functional data of abnormal P30044 but normal mismatch negativity.19 However, the left HG and PT volume decreases in the subsequent 1.5 years were comparable (6.9% and 7.2%, respectively), which is compatible with the abnormal mismatch negativity seen later in schizophrenia.19
To obtain an unequivocal and reliable measure, the current study operationally defined first episode as the time of first hospitalization. However, since clinical symptoms of the disorder may have been present for months and even years before the first hospitalization, the present data can neither determine whether abnormalities of structural and functional indices at first hospitalization were consequences of neurodevelopmental deficits and/or peri-onset progressive processes nor address the question of whether the progressive volume decreases in left PT began earlier than those in left HG and/or whether left PT might have been more severely affected by a process during neurodevelopment. However, a finding of interest relative to the course of change was that an ANCOVA with interscan interval as the covariate did not affect the significance of the results in the schizophrenia group, compatible with much of the volume decline's having occurred in the months just after initial hospitalization, rather than being a linear decline over time. A shorter interscan interval will be of help in more accurately fixing the time course of decline.
The precise neurobiological mechanism that underlies this progressive, perhaps neurodegenerative, change in left HG and PT is unknown. However, there is a growing body of work implicating abnormal excitatory amino acid neurotransmission in schizophrenia, possibly mediated through a deficit in recurrent inhibition.63-65 Although controversial, this mechanism could be a possible cause of ongoing, use-dependent cellular damage through excitotoxic effects. Our study cannot answer the question of why the left hemisphere is the target for progressive volume reduction. However, left and right HG and PT have some differential cytoarchitectonic features,66-70 which may be important in the neurobiological mechanisms for lateralized progressive changes.
In terms of correlations between ROI volume change and clinical measures, the dual finding of association of both left HG and PT ROI volume change with conceptual disorganization in schizophrenia is highly compatible with our early findings of a formal thought disorder–posterior STG association in chronic schizophrenia33 and with the role of HG and PT in language and auditory processing. The association of HG volume change and suspiciousness may represent the effects of erroneous auditory sensory processing. In chronic schizophrenia, Mathalon et al35 reported that geometrically defined posterior temporal gray matter volume decline was related to greater BPRS total and negative symptom scores after a mean interscan interval of 4 years. However, we emphasize that our results should be regarded as tentative since the analyses were exploratory in nature and that confirmation in future planned studies will be needed.
In a follow-up MRI study of schizophrenia, the inclusion of medicated patients inevitably raises the question of whether progressive effects are possibly due to medication or the illness itself or whether medication ameliorates the pathologic condition under study. However, medication is almost impossible to control for in human clinical studies. This is also the case with the present naturalistic study, where it was not possible to control prescan or interscan medication type or dosage and where medication compliance was monitored through hospital records and patient accounts but not blood levels. Even so, the available data, because they are unique, may be useful to present. The percent change scores of left HG or PT volume between schizophrenic patients who received neuroleptics between scans (n=10) and those who did not (n=3) were not statistically different and visually showed no trends. The affective psychosis patients who received neuroleptics between scans (n=6) and those who did not (n=8) did not differ from control subjects in the percent change scores of left HG or left PT volume. Moreover, these 2 subgroups of affective psychosis were not different from each other in percent change scores of left HG or left PT volume. Exclusion of the 8 patients who received lithium also did not alter the statistical conclusions reported herein. The present limited data suggest that medication did not cause the gray matter volume change but are not suitable for drawing conclusions about the possible neuroprotective effects of antipsychotics.
Regarding the dropout rate, of the 66 subjects described in our earlier study at baseline scan,10 approximately half (n=31) underwent a second scan in the present study, and, importantly, the dropout rate did not differ significantly among groups. In addition, the dropout rate was not significantly different among schizophrenic subjects above or below the median value of left HG and left PT volume in the study by Hirayasu et al10 (Fisher exact test, P>.99). Additionally, the present findings of ROI volumes at baseline are in accord with our earlier study,10 confirming the specificity of smaller bilateral HG and left PT gray matter volume to schizophrenia at first episode, further suggesting the absence of selection bias in subjects undergoing 2 scans. In conclusion, the left HG and PT gray matter volume reduction over time in our sample of subjects with first-episode schizophrenia but not in those with first-episode affective psychosis suggests that a progressive change in the early stage of the illness in brain regions specialized for auditory perception and language processing may play a crucial role in the patho-physiology of schizophrenia.
Acknowledgments
This study was supported in part by the Department of Veterans Affairs Medical Research Service Merit Awards (Drs Shenton and McCarley) and Middleton Award (Dr McCarley) (Washington, DC); grants K02 MH 01110 and R01 MH 50747 (Dr Shenton), R01 MH 40799 (Dr McCarley), R01 RR 11747 (Dr Kikinis), and P41 PR13218 (Dr Jolesz) from the National Institutes of Health (Bethesda, Md); The MIND Institute (Dr McCarley) (Albuquerque, NM); and the Welfide Medicinal Research Foundation and the Uehara Memorial Foundation (Tokyo, Japan) (Dr Kasai).
We gratefully acknowledge the administrative support of Marie Fairbanks and the research assistant support of Margaret Fagan, AB. We also thank Paul G. Nestor, PhD, and Margaret A. Niznikiewicz, PhD, for their advice on data analysis.
REFERENCES
- 1.Zattore RJ, Binder JR. Functional and structural imaging of the human auditory system. In: Toga AW, Mazziotta JC, editors. Brain Mapping: The Systems. Academic Press; San Diego, Calif: 2000. pp. 365–402. [Google Scholar]
- 2.Pearlson GD. Superior temporal gyrus and planum temporale in schizophrenia: a selective review. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:1203–1229. doi: 10.1016/s0278-5846(97)00159-0. [DOI] [PubMed] [Google Scholar]
- 3.Mesulam MM. Behavioral neuroanatomy. In: Mesulam MM, editor. Principles of Behavioral and Cognitive Neurology. Oxford University Press; New York, NY: 2000. pp. 1–120. [Google Scholar]
- 4.Geschwind N, Levitsky W. Human brain: left-right asymmetries in temporal speech region. Science. 1968;161:186–187. doi: 10.1126/science.161.3837.186. [DOI] [PubMed] [Google Scholar]
- 5.Galaburda AM, Sanides F, Geschwind N. Human brain: cytoarchitectonic left-right asymmetries in the temporal speech region. Arch Neurol. 1978;35:812–817. doi: 10.1001/archneur.1978.00500360036007. [DOI] [PubMed] [Google Scholar]
- 6.Javitt DC. Psychophysiology of schizophrenia. Curr Opin Psychiatry. 1997;10:11–15. [Google Scholar]
- 7.Light GA, Braff DL. Human and animal studies of schizophrenia-related gating deficits. Curr Psychiatry Rep. 1999;1:31–40. doi: 10.1007/s11920-999-0008-y. [DOI] [PubMed] [Google Scholar]
- 8.McCarley RW, Niznikiewicz MA, Salisbury DF, Nestor PG, O'Donnell BF, Hirayasu Y, Grunze H, Greene RW, Shenton ME. Cognitive dysfunction in schizophrenia: unifying basic research and clinical aspects. Eur Arch Psychiatry Clin Neurosci. 1999;249(suppl 4):69–82. doi: 10.1007/pl00014188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rojas DC, Teale P, Sheeder J, Simon J, Reite M. Sex-specific expression of Heschl's gyrus functional and structural abnormalities in paranoid schizophrenia. Am J Psychiatry. 1997;154:1655–1662. doi: 10.1176/ajp.154.12.1655. [DOI] [PubMed] [Google Scholar]
- 10.Hirayasu Y, McCarley RW, Salisbury DF, Tanaka S, Kwon JS, Frumin M, Snyderman D, Yurgelun-Todd D, Kikinis R, Jolesz FA, Shenton ME. Planum temporale and Heschl gyrus volume reduction in schizophrenia. Arch Gen Psychiatry. 2000;57:692–699. doi: 10.1001/archpsyc.57.7.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petty RG, Barta PE, Pearlson GD, McGilchrist IK, Lewis RW, Tien AY, Pulver A, Vaughn DD, Casanova MF, Powers RE. Reversal of asymmetry of the planum temporale in schizophrenia. Am J Psychiatry. 1995;152:715–721. doi: 10.1176/ajp.152.5.715. [DOI] [PubMed] [Google Scholar]
- 12.Kulynych JJ, Vladar K, Fantie BD, Jones DW, Weinberger DR. Normal asymmetry of the planum temporale in patients with schizophrenia: three-dimensional cortical morphometry with MRI. Br J Psychiatry. 1995;166:742–749. doi: 10.1192/bjp.166.6.742. [DOI] [PubMed] [Google Scholar]
- 13.Barta PE, Pearlson GD, Brill LB, II, Royall R, McGilchrist IK, Pulver AE, Powers RE, Casanova MF, Tien AY, Frangou S, Petty RG. Planum temporale asymmetry reversal in schizophrenia: replication and relationship to gray matter abnormalities. Am J Psychiatry. 1997;154:661–667. doi: 10.1176/ajp.154.5.661. [DOI] [PubMed] [Google Scholar]
- 14.Frangou S, Sharma T, Sigmudsson T, Barta P, Pearlson G, Murray RM. The Maudsley family study 4: normal planum temporale asymmetry in familial schizophrenia: a volumetric MRI study. Br J Psychiatry. 1997;170:328–333. doi: 10.1192/bjp.170.4.328. [DOI] [PubMed] [Google Scholar]
- 15.Kwon JS, McCarley RW, Hirayasu Y, Anderson JE, Fischer IA, Kikinis R, Jolesz FA, Shenton ME. Left planum temporale volume reduction in schizophrenia. Arch Gen Psychiatry. 1999;56:142–148. doi: 10.1001/archpsyc.56.2.142. [DOI] [PubMed] [Google Scholar]
- 16.Kropotov JD, Naäaätaänen R, Sevostianov AV, Alho K, Reinikainen K, Kropotova OV. Mismatch negativity to auditory stimulus change recorded directly from the human temporal cortex. Psychophysiology. 1995;32:418–422. doi: 10.1111/j.1469-8986.1995.tb01226.x. [DOI] [PubMed] [Google Scholar]
- 17.Shelley AM, Ward PB, Catts SV, Michie PT, Andrews S, McConaghy N. Mismatch negativity: an index of a preattentive processing deficit in schizophrenia. Biol Psychiatry. 1991;30:1059–1062. doi: 10.1016/0006-3223(91)90126-7. [DOI] [PubMed] [Google Scholar]
- 18.Javitt DC, Doneshka P, Grochowski S, Ritter W. Impaired mismatch negativity generation reflects widespread dysfunction of working memory in schizophrenia. Arch Gen Psychiatry. 1995;52:550–558. doi: 10.1001/archpsyc.1995.03950190032005. [DOI] [PubMed] [Google Scholar]
- 19.Salisbury DF, Shenton ME, Griggs CB, Bonner-Jackson A, McCarley RW. Mismatch negativity in chronic schizophrenia and first-episode schizophrenia. Arch Gen Psychiatry. 2002;59:686–694. doi: 10.1001/archpsyc.59.8.686. [DOI] [PubMed] [Google Scholar]
- 20.Wible CG, Kubicki M, Yoo SS, Kacher DF, Salisbury DF, Anderson MC, Shenton ME, Hirayasu Y, Kikinis R, Jolesz FA, McCarley RW. A functional magnetic resonance imaging study of auditory mismatch in schizophrenia. Am J Psychiatry. 2001;158:938–943. doi: 10.1176/appi.ajp.158.6.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dierks T, Linden DEJ, Jandl M, Formisano E, Goebel R, Lanfermann H, Singer W. Activation of Heschl's gyrus during auditory hallucinations. Neuron. 1999;22:615–622. doi: 10.1016/s0896-6273(00)80715-1. [DOI] [PubMed] [Google Scholar]
- 22.Shapleske J, Rossell SL, Simmons A, David AS, Woodruff PWR. Are auditory hallucinations the consequence of abnormal cerebral lateralization? a morphometric MRI study of the sylvian fissure and planum temporale. Biol Psychiatry. 2001;49:685–693. doi: 10.1016/s0006-3223(00)01006-4. [DOI] [PubMed] [Google Scholar]
- 23.Rossi A, Serio A, Stratta P, Petruzzi C, Schiazza G, Mancini F, Casacchia M. Planum temporale asymmetry and thought disorder in schizophrenia. Schizophr Res. 1994;12:1–7. doi: 10.1016/0920-9964(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 24.DeLisi LE, Hoff AL, Neale C, Kushner M. Asymmetries in the superior temporal lobe in male and female first-episode schizophrenic patients: measures of the planum temporale and superior temporal gyrus by MRI. Schizophr Res. 1994;12:19–28. doi: 10.1016/0920-9964(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 25.Kleinschmidt A, Falkai P, Huang Y, Schneider T, Furst G, Steinmetz H. In vivo morphometry of planum temporale asymmetry in first-episode schizophrenia. Schizophr Res. 1994;12:9–18. doi: 10.1016/0920-9964(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 26.Knight RT, Scabini D, Woods DL, Clayworth CC. Contributions of temporal-parietal junction to the human auditory P3. Brain Res. 1989;502:109–116. doi: 10.1016/0006-8993(89)90466-6. [DOI] [PubMed] [Google Scholar]
- 27.Ford JM. Schizophrenia: the broken P300 and beyond. Psychophysiology. 1999;36:667–682. [PubMed] [Google Scholar]
- 28.O'Donnell BF, McCarley RW, Potts GF, Salisbury DF, Nestor PG, Hirayasu Y, Niznikiewicz MA, Barnard J, Shen ZJ, Weinstein DM, Bookstein FL, Shenton ME. Identification of neural circuits underlying P300 abnormalities in schizophrenia. Psychophysiology. 1999;36:388–398. doi: 10.1017/s0048577299971688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCarley RW, Shenton ME, O'Donnell BF, Faux SF, Kikinis R, Nestor PG, Jolesz FA. Auditory P300 abnormalities and left posterior temporal gyrus volume reduction in schizophrenia. Arch Gen Psychiatry. 1993;50:190–197. doi: 10.1001/archpsyc.1993.01820150036003. [DOI] [PubMed] [Google Scholar]
- 30.McCarley RW, Salisbury DF, Hirayasu Y, Yurgelun-Todd DA, Tohen M, Zarate C, Kikinis R, Jolesz FA, Shenton ME. Association between smaller left posterior superior temporal gyrus MRI volume and smaller left temporal P300 amplitude in first episode schizophrenia. Arch Gen Psychiatry. 2002;59:321–331. doi: 10.1001/archpsyc.59.4.321. [DOI] [PubMed] [Google Scholar]
- 31.O'Donnell BF, Faux SF, McCarley RW, Kimble MO, Salisbury DF, Nestor PG, Kikinis R, Jolesz FA, Shenton ME. Increased rate of P300 latency prolongation with age in schizophrenia. Arch Gen Psychiatry. 1995;52:544–549. doi: 10.1001/archpsyc.1995.03950190026004. [DOI] [PubMed] [Google Scholar]
- 32.Mathalon DH, Ford JM, Rosenbloom M, Pfefferbaum A. P300 reduction and prolongation with illness duration in schizophrenia. Biol Psychiatry. 2000;47:413–427. doi: 10.1016/s0006-3223(99)00151-1. [DOI] [PubMed] [Google Scholar]
- 33.Shenton ME, Kikinis R, Jolesz FA, Pollak SD, LeMay M, Wible CG, Hokama H, Martin J, Metcalf D, Coleman M, McCarley RW. Abnormalities of the left temporal lobe and thought disorder in schizophrenia: a quantitative magnetic resonance imaging study. N Engl J Med. 1992;327:604–612. doi: 10.1056/NEJM199208273270905. [DOI] [PubMed] [Google Scholar]
- 34.Kircher TTJ, Liddle PF, Brammer MJ, Williams S, Murray RM, McGuire PK. Neural correlates of formal thought disorder in schizophrenia: preliminary findings from a functional magnetic resonance imaging study. Arch Gen Psychiatry. 2001;58:769–774. doi: 10.1001/archpsyc.58.8.769. [DOI] [PubMed] [Google Scholar]
- 35.Mathalon DH, Sullivan EV, Lim KO, Pfefferbaum A. Progressive brain volume changes and the clinical course of schizophrenia in men: a longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 2001;58:148–157. doi: 10.1001/archpsyc.58.2.148. [DOI] [PubMed] [Google Scholar]
- 36.Hirayasu Y, Shenton ME, Salisbury DF, Frumin M, Fischer IA, Farrell D, Yurgelun-Todd DA, Zarate C, McCarley RW. Progressive change in posterior superior temporal gyrus in schizophrenia. Biol Psychiatry. 1999;45:117S. [Google Scholar]
- 37.Kasai K, Shenton ME, Salisbury DF, Hirayasu Y, Lee CU, Ciszewski AA, Yurgelun-Todd DA, Kikinis R, Jolesz FA, McCarley RW. Progressive decrease of left superior temporal gyrus gray matter volume in patients with first-episode schizophrenia. Am J Psychiatry. 2003;160:156–164. doi: 10.1176/appi.ajp.160.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keshavan MS, Haas GL, Kahn CE, Aguilar E, Dick EL, Schooler NR, Sweeney JA, Pettegrew JW. Superior temporal gyrus and the course of early schizophrenia: progressive, static, or reversible? J Psychiatr Res. 1998;32:161–167. doi: 10.1016/s0022-3956(97)00038-1. [DOI] [PubMed] [Google Scholar]
- 39.Oldfield RC. The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 40.Hollingshead AB, New Haven. Two Factor Index of Social Position. Yale University Press; Conn: 1965. [Google Scholar]
- 41.Hirayasu Y, Shenton ME, Salisbury DF, Dickey CC, Fisher IA, Mazzoni P, Kisler T, Aarakaki H, Kwon JS, Anderson JE, Yurgelun-Todd D, Tohen M, McCarley RW. Lower left temporal lobe MR volumes in patients with first-episode schizophrenia compared with psychotic patients with first-episode affective disorder and normal subjects. Am J Psychiatry. 1998;155:1384–1391. doi: 10.1176/ajp.155.10.1384. [DOI] [PubMed] [Google Scholar]
- 42.Hirayasu Y, Shenton ME, Salisbury DF, Kwon JS, Wible CG, Fischer IA, Yurgelun-Todd D, Zarate C, Kikinis R, Jolesz FA, McCarley RW. Subgenual cingulate cortex volume in first-episode psychosis. Am J Psychiatry. 1999;156:1091–1093. doi: 10.1176/ajp.156.7.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hirayasu Y, Tanaka S, Shenton ME, Salisbury DF, DeSantis MA, Levitt JJ, Wible C, Yurgelun-Todd D, Kikinis R, Jolesz FA, McCarley RW. Prefrontal gray matter volume reduction in first-episode schizophrenia. Cereb Cortex. 2001;11:374–381. doi: 10.1093/cercor/11.4.374. [DOI] [PubMed] [Google Scholar]
- 44.Salisbury DF, Shenton ME, Sherwood AR, Fischer IA, Yurgelun-Todd DA, Tohen M, McCarley RW. First-episode schizophrenic psychosis differs from first-episode affective psychosis and controls in P300 amplitude over left temporal lobe. Arch Gen Psychiatry. 1998;55:173–180. doi: 10.1001/archpsyc.55.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee CU, Shenton ME, Salisbury DF, Kasai K, Onitsuka T, Dickey CC, Yurgelun-Todd D, Kikinis R, Jolesz FA, McCarley RW. Fusiform gyrus volume reduction in first-episode schizophrenia: an MRI study. Arch Gen Psychiatry. 2002;59:775–781. doi: 10.1001/archpsyc.59.9.775. [DOI] [PubMed] [Google Scholar]
- 46.Spitzer RL, Williams JBW, Gibbson M, First M. The Structured Clinical Interview for DSM-III-R Non-Patient Edition (SCID-NP) American Psychiatric Association; Washington, DC: 1990. [Google Scholar]
- 47.Spitzer RL, Williams JBW, Gibbson M, First M. The Structured Clinical Interview for DSM-III-R (SCID) American Psychiatric Association; Washington, DC: 1990. [Google Scholar]
- 48.DeLisi LE, Sakuma M, Tew W, Kushner M, Hoff AL, Grimson R. Schizophrenia as a chronic active brain process: a study of progressive brain structural change subsequent to the onset of schizophrenia. Psychiatry Res. 1997;74:129–140. doi: 10.1016/s0925-4927(97)00012-7. [DOI] [PubMed] [Google Scholar]
- 49.Lieberman JA, Chakos M, Wu H, Alvir J, Hoffman E, Robinson D, Bilder R. Longitudinal study of brain morphology in first episode schizophrenia. Biol Psychiatry. 2001;49:487–499. doi: 10.1016/s0006-3223(01)01067-8. [DOI] [PubMed] [Google Scholar]
- 50.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 51.Wechsler D. Wechsler Adult Intelligence Scale–Revised. Harcourt Brace Jovanovich Inc; New York, NY: 1981. [Google Scholar]
- 52.Endicott J, Spitzer RL, Fleiss JL, Cohen J. The global assessment scale: a procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry. 1976;33:766–771. doi: 10.1001/archpsyc.1976.01770060086012. [DOI] [PubMed] [Google Scholar]
- 53.Overall JE, Hollister LE, Pichot P. Major psychiatric disorders: a four-dimensional model. Arch Gen Psychiatry. 1967;16:146–151. doi: 10.1001/archpsyc.1967.01730200014003. [DOI] [PubMed] [Google Scholar]
- 54.Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- 55.Wible CG, Shenton ME, Hokama H, Kikinis R, Jolesz FA, Metcalf D, McCarley RW. Prefrontal cortex and schizophrenia: a quantitative magnetic resonance imaging study. Arch Gen Psychiatry. 1995;52:279–288. doi: 10.1001/archpsyc.1995.03950160029007. [DOI] [PubMed] [Google Scholar]
- 56.Gerig G, Kubler O, Kikinis R, Jolesz FA. Nonlinear anisotropic filtering of MRI data. IEEE Trans Med Imaging. 1992;11:221–232. doi: 10.1109/42.141646. [DOI] [PubMed] [Google Scholar]
- 57.Wells WM, III, Grimson WEL, Kikinis R, Jolesz FA. Adaptive segmentation of MRI data. IEEE Trans Med Imaging. 1996;15:429–442. doi: 10.1109/42.511747. [DOI] [PubMed] [Google Scholar]
- 58.Steinmetz H, Rademacher J, Huang Y, Hefter H, Zilles K, Thron A, Freund HJ. Cerebral asymmetry: MR planimetry of the human planum temporale. J Comput Assist Tomogr. 1989;13:996–1005. [PubMed] [Google Scholar]
- 59.Barta PE, Petty RG, McGilchrist I, Lewis RW, Jerram M, Casanova MF, Powers RE, Brill LB, II, Pearlson GD. Asymmetry of the planum temporale: methodological considerations and clinical associations. Psychiatry Res. 1995;61:137–150. doi: 10.1016/0925-4927(95)02650-m. [DOI] [PubMed] [Google Scholar]
- 60.Honeycutt NA, Musick A, Barta PE, Pearlson GD. Measurement of the planum temporale (PT) on magnetic resonance imaging scans: temporal PT alone and with parietal extension. Psychiatry Res. 2000;98:103–116. doi: 10.1016/s0925-4927(00)00043-3. [DOI] [PubMed] [Google Scholar]
- 61.Mojtabai R, Bromet EJ, Harvey P, Carlson GA, Craig TJ, Fennig S. Neuropsychological differences between first-admission schizophrenia and psychotic affective disorders. Am J Psychiatry. 2000;157:1453–1460. doi: 10.1176/appi.ajp.157.9.1453. [DOI] [PubMed] [Google Scholar]
- 62.Seidman LJ, Kremen WS, Koren D, Faraone SV, Goldstein JM, Tsuang MT. A comparative profile analysis of neuropsychological functioning in patients with schizophrenia and bipolar psychoses. Schizophr Res. 2002;53:31–44. doi: 10.1016/s0920-9964(01)00162-1. [DOI] [PubMed] [Google Scholar]
- 63.Coyle JT. The glutamatergic dysfunction hypothesis for schizophrenia. Harv Rev Psychiatry. 1996;3:241–253. doi: 10.3109/10673229609017192. [DOI] [PubMed] [Google Scholar]
- 64.McCarley RW, Hsiao JK, Freedman R, Pfefferbaum A, Donchin E. Neuroimaging and the cognitive neuroscience of schizophrenia. Schizophr Bull. 1996;22:703–725. doi: 10.1093/schbul/22.4.703. [DOI] [PubMed] [Google Scholar]
- 65.McCarley RW, Wible CG, Frumin M, Hirayasu Y, Levitt JJ, Fischer IA, Shenton ME. MRI anatomy of schizophrenia. Biol Psychiatry. 1999;45:1099–1119. doi: 10.1016/s0006-3223(99)00018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seldon H. Structure of human auditory cortex, I: cytoarchitectonics and dendritic distributions. Brain Res. 1981;229:277–294. doi: 10.1016/0006-8993(81)90994-x. [DOI] [PubMed] [Google Scholar]
- 67.Seldon H. Structure of human auditory cortex, II: axon distributions and morphological correlates of speech perception. Brain Res. 1981;229:295–310. doi: 10.1016/0006-8993(81)90995-1. [DOI] [PubMed] [Google Scholar]
- 68.Seldon H. Structure of human auditory cortex, III: statistical analysis of dendritic trees. Brain Res. 1982;249:211–221. doi: 10.1016/0006-8993(82)90055-5. [DOI] [PubMed] [Google Scholar]
- 69.Hutsler J, Gazzaniga M. Acetylcholinesterase staining in human auditory and language cortices: regional variation of structural features. Cereb Cortex. 1996;6:260–270. doi: 10.1093/cercor/6.2.260. [DOI] [PubMed] [Google Scholar]
- 70.Hollinger DP, Galaburda AM, Harrison PJ. Cerebral asymmetry. In: Harrison PJ, Roberts GW, editors. The Neuropathology of Schizophrenia. Oxford University Press; New York, NY: 2000. pp. 151–171. [Google Scholar]