Abstract
Antimicrobial proteins constitute a phylogenetically ancient form of innate immunity that provides host defence at skin and mucosal surfaces. Although some components of this system are constitutively expressed, new evidence reviewed in this Progress article shows that the production of certain antimicrobial proteins by epithelial cells can also be regulated by cytokines of the innate and adaptive immune systems. In particular, the effector cytokines interleukin-17 and interleukin-22, which are produced by the T-helper-17-cell subset, are emerging as crucial regulators of antimicrobial-peptide production in the gut and the lungs. This suggests that this T-cell lineage and its cytokines have important roles in skin and mucosal immunity.
There are many classes of antimicrobial proteins, which have diverse structures and mechanisms of action. These include cationic antimicrobial peptides, S100 family proteins, peptidoglycan-recognition proteins (PGRPs in invertebrates, PGLYRPs in vertebrates), calcium-dependent lectins (C-type lectins) and iron metabolism proteins (FIG. 1; TABLE 1). The classical cationic antimicrobial peptides, which include defensins and cathelicidins, are the best characterized, and the finding that they are conserved across many phyla is testament to their importance as part of a primordial and highly effective system of host defence. They provide early, broad spectrum (and in invertebrates, sufficient) protection against invading microorganisms at epithelial-cell surfaces. In vertebrates, basal expression of antimicrobial proteins by gut epithelial cells also controls the overgrowth of commensal bacteria, thereby preventing bacterial invasion in the absence of a breach in the epithelial-cell barrier.
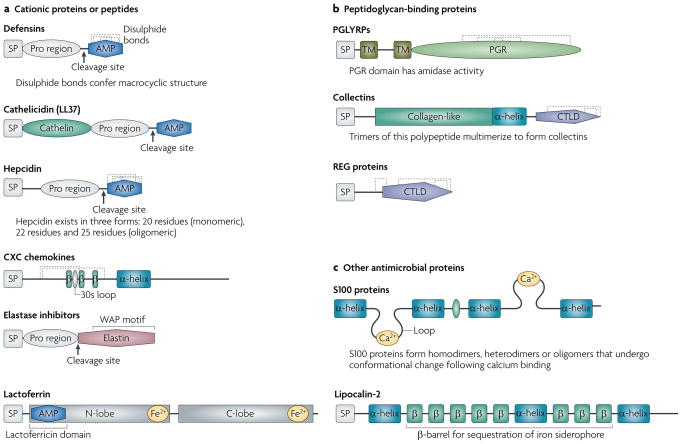
Figure 1. Antimicrobial protein structure and functional domains.
a | Defensins, cathelicidins and hepcidin are synthesized as pre-pro peptides that are cleaved to release mature cationic antimicrobial peptides (AMPs) that interact with negatively charged bacterial surface moieties. Hydrophobic regions in the peptide (not shown) then integrate into the bacterial membrane, leading to membrane permeabilization, cytoplasm leakage and ultimately complete membrane perforation. Based on their primary amino-acid structure, antimicrobial CXC chemokines are thought to have similar mechanisms of action. Elastase inhibitors are also synthesized in a pre-pro form with a carboxy (C)-terminal elastase inhibitor region that contains a conserved whey acidic protein (WAP) motif. The WAP motif binds bacterial peptidase and may have antibacterial activities that are independent of the elastase inhibitory activity. The pro form of the elastase inhibitor elafin has a cationic domain that probably kills bacteria by membrane permeabilization. Lactoferrin is a 75 kDa bilobed, bivalent iron-binding protein. The amino (N)- and C-lobes, which contain an iron-binding site, may be cleaved by proteolytic digestion. The cationic lactoferricin domain in the N-lobe is thought to confer its antimicrobial activity. b | These proteins bind the bacterial peptidoglycan layer and either hydrolyse the peptidoglycan or facilitate bacterial opsonization. Peptidoglycan-recognition proteins (PGLYRPs) comprise varying numbers of peptidoglycan-recognition motifs (PGR; only one is shown here) and all have a conserved C-terminal PGR that has homology with bacterial peptidase. Most vertebrate PGLYRPs are secreted and lack the transmembrane domains (TMs) that are shown in this figure. Collectins consist of polypeptides with an N-terminal cysteine-rich domain, a collagen-like region, an α-helical region and a C-type lectin domain (CTLD). Trimers of the shown polypeptide multimerize to form the collectins. Regenerating (REG) proteins have a single CTLD; their mechanism of antibacterial activity is unknown. c | S100 proteins have two helix–loop–helix calcium-binding motifs; their mechanism of antibacterial activity is unknown. Lipocalin-2 is a 25 kDa protein that binds bacterial siderophores, which are bacterial iron-scavenging proteins, and therefore deprives bacteria of their necessary nutrient, iron. β, β-strand; SP, signal peptide.
Table 1.
Key antimicrobial products in epithelial-cell defence
| Antimicrobial products | Sources | Location | Key functions | Refs |
|---|---|---|---|---|
| Antimicrobial peptides | ||||
| Defensins: α-defensins (HNP1, HNP2, HNP3, HNP4, HD5 and HD6) | Neutrophils, Paneth cells and female urogenital epithelium | Secreted |
|
42,43 |
| β-defensins (HBD1, HBD2, HBD3 and HBD4) | Various epithelial cells and macrophages | |||
| Cathelicidins: LL37 (humans) and CAMP (mice) | Various epithelial cells, monocytes, neutrophils, B cells, T cells and NK cells | Secreted |
|
44 |
| S100 proteins* | ||||
| S100A7 (psoriasin), S100A8 (calgranulin A), S100A9 (calgranulin B), S100A12 (calgranulin C) and S100A15 | Colon, keratinocytes, squamous and airway epithelium | Intracellular and extracellular |
|
45–48 |
| Elastase inhibitors | ||||
| Elafin | Neutrophils, macrophages and various epithelial cells | Secreted and associated with extracellular matrix components |
|
49–51 |
| SLPI | Mast cells, neutrophils, macrophages and various epithelial cells | |||
| PGLYRPs* | ||||
| PGLYRP1 (PGRP-s), PGLYRP2 (PGRP-L), PGLYRP3 (PGRP-Iα) and PGLYRP4 (PGRP-Iβ) | Widely distributed in leukocytes and various epithelial cells (except goblet and Paneth cells) | Transmembrane or secreted |
|
52 |
| C-type lectins | ||||
| Collectins: SP-A, SP-D, MBL, conglutinin, collectin-L1, collectin-P1 and collectin-K1 | Liver (MBL), lungs, upper airway and gut epithelium (SP-A and SP-D), and endothelial cells (collectin-P1) | Secreted locally and present in serum other than collectin-L1 (which is cytoplasmic) and collectin-P1 (which is membrane bound) |
|
1 |
| Reg proteins: Reg1, Reg2, Reg3 and Reg4 | Pancreas and gut epithelium | Secreted |
|
30,31 |
| Iron metabolism proteins | ||||
| Lactoferrin | Glandular epithelium and neutrophils | Secreted and present in neutrophil granules |
|
53 |
| Hepcidin | Liver | Secreted into serum and urine |
|
54 |
| Lipocalin-2 | Neutrophils, macrophages, various epithelial and endothelial cells, hepatocytes and renal tubular cells | Secreted and present in neutrophil granules |
|
55 |
| Chemokines | ||||
| CCL20, CXCL9, CXCL10 and CXCL11 | PBMCs | Secreted locally at sites of inflammation |
|
56 |
Alternative names are provided in parentheses. CAMP, cathelicidin antimicrobial peptide; CCR6, CC-chemokine receptor 6; C terminus, carboxyl terminus; HBD, human β-defensin; HD, human defensin; HNP, human neutrophil peptide; MBL, mannose-binding lectin; NK, natural killer; PGLYRP, peptidoglycan-recognition protein; PGRP, peptidoglycan-recognition protein; REG, regenerating; S100A, S100 calcium-binding protein A; SLPI, secretory leukocyte protease inhibitor; SP, surfactant protein.
CCL20, CC-chemokine ligand 20; CCR6, CC-chemokine receptor 6; CXCL, CXC-chemokine ligand; CXCR3, CXC-chemokine receptor; PBMCs, peripheral blood mononuclear cells.
In addition to functioning as direct antimicrobial compounds (for example, through bacterial-membrane permeabilization), antimicrobial proteins can function as opsonins1, chemokines2 and modulators of host-cell cytokine production that, in turn, regulate innate immune responses. For example, a recent study showed that the antimicrobial protein lactoferrin can induce the production of interleukin-18 (IL-18; an IL-1 family cytokine that is also known as IL-1F4), which increases natural killer (NK)-cell activity3. In addition, the human cathelicidin LL37 (also known as CAMP) can synergize with IL-1β to increase the production of cytokines, such as IL-6, IL-8 and IL-10, and chemokines, such as CC-chemokine ligand 2 (CCL2)4, as well as to increase the synthesis and release of α-defensins5. Cytokine and chemokine signalling then leads to the recruitment of macrophages and neutrophils and further increases the antimicrobial-protein response. High levels of antimicrobial proteins facilitate microbial clearance through their localized concentration, opsonization and further signalling. Finally, negative-feedback regulation by some antimicrobial proteins can limit inflammation; for example, α-defensins can block the secretion of IL-1β by lipopolysaccharide (LPS)-activated monocytes and therefore may be important for the resolution of inflammation6. Based on these observations, antimicrobial proteins are thought to provide a self-contained response to extracellular microbial infection that encompasses pathogen recognition (together with pattern-recognition receptors (PRRs)), inflammation, pathogen clearance and resolution of inflammation.
During invasion by pathogens or commensal flora, the local production of antimicrobial peptides can be increased by cytokines that are produced by cells of the innate immune system, such as dendritic cells and macrophages, and by epithelial cells. In addition, skin and mucosal T cells can produce cytokines that regulate the antimicrobial-protein response. In particular, two cytokines that are produced by the recently described T helper 17 (TH17)-cell lineage, IL-17 and IL-22, have been shown to be important regulators of skin and mucosal immunity by controlling the expression of antimicrobial proteins. This Progress article describes the cytokine-mediated regulation of anti microbial proteins and highlights the recent insights into immunity and homeostasis at skin and mucosal sites that have been gained from studying the function of IL-17 and IL-22.
Innate regulation of antimicrobial proteins
When pathogens cross the epithelial-cell barrier, they encounter a range of PRRs that are expressed by myeloid and non-myeloid cells. These PRRs induce a signalling cascade that leads to the production of innate inflammatory cytokines, such as the IL-1 family members IL-1α, IL-1β and IL-18 (FIG. 2), which are key inducers of antimicrobial-protein expression. The best known example of this is the induction of β-defensins in the lungs by IL-1β (REF. 7). IL-1β also induces the expression of lipocalin-2, an iron-sequestering antimicrobial protein, in the lungs8 and upregulates the transcription of genes that encode human β-defensin-2 (HBD2; also known as DEFB4), PGLYRPs, S100A7 (also known as psoriasin), calprotectin (a heterodimer of S100A8 and S100A9), secretory leukocyte protease inhibitor (SLPI) and lipocalin-2 in the skin, as shown by microarray analysis of cytokine-stimulated human keratinocytes9,10.
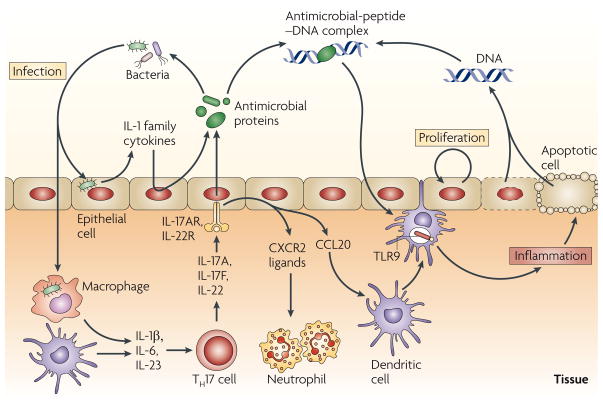
Figure 2. Cytokine networks and antimicrobial peptides at epithelial-cell surfaces.
In response to bacterial infection, the interleukin-1 (IL-1) family cytokines, such as IL-1β, potently induce the expression of antimicrobial proteins by the epithelium. IL-1β, together with IL-6 and IL-23, can also induce the differentiation of T helper 17 (TH17) cells, which produce IL-17A, IL-17F and IL-22. These cytokines further induce antimicrobial-protein expression by the epithelium. IL-17A can also induce the production of CC-chemokine ligand 20 (CCL20), which has antimicrobial activity, recruits dendritic cells and increases the production of CXC-chemokine receptor 2 (CXCR2) ligands that are important in neutrophil recruitment. This response is beneficial to the host during an acute infection. However, in autoimmune diseases (such as psoriasis) cationic antimicrobial peptides, which are present at high levels, can interact with negatively charged DNA that is released from dying cells (cell death occurs as a result of increased cell turnover during inflammation). Antimicrobial-peptide–DNA complexes can amplify inflammation in the skin by activating Toll-like receptor 9 (TLR9) signalling.
IL-18 is constitutively and inducibly expressed by many cell types and is associated with the clearance of several bacterial pathogens, including Escherichia coli and Burkholderia pseudomallei11,12. However, the mechanism of IL-18-mediated protection from these infections remains unclear. IL-18 has been shown to induce the expression of the antimicrobial proteins LL37 and α-defensins during Cryptosporidium parvum infection of intestinal epithelial cells, thereby providing a potential mechanism for the local control of infection13.
IL-1 family cytokines induce weak PGLYRP expression levels compared with those that are stimulated by the direct activation of the PRRs Toll-like receptor 2 (TLR2), TLR4, nucleotide-binding oligomerization domain protein 1 (NOD1) and NOD2 (REFs 14,15). This indicates that signalling through these PRRs, which activates the cleavage of pro-IL-1β into mature IL-1β, may increase antimicrobial-protein expression by the epithelium through both cytokine-dependent and cytokine-independent mechanisms. Furthermore, with the exception of TLR3, most TLRs and the IL-1 receptor (IL-1R) family signal through a common adaptor protein, myeloid differentiation primary-response gene 88 (MyD88), and this may provide another means of amplifying the antimicrobial response. However, determining the individual contributions of the TLR and IL-1R family members to the regulation of the antimicrobial protein response in vivo has been made difficult by this shared signalling pathway.
Regulation by T-cell-derived cytokines
In addition to antimicrobial-protein regulation by cytokines of the innate immune system, cytokine products that are traditionally associated with T-cell immunity also regulate antimicrobial-protein expression at skin and mucosal surfaces. With the discovery of the TH1- and TH2-cell subsets of CD4+ T cells, it became clear that the differentiation of CD4+ T cells into mature T-cell subsets that have appropriate activities against the type of pathogen that is encountered is crucial for the maintenance of immunity at mucosal sites and elsewhere in the body. Indeed, patients with HIV-1 infection and a resultant acquired loss of CD4+ T cells suffer from more infections at epithelial-cell surfaces, such as the skin, gastrointestinal tract and lungs. The observation that HIV-1-infected individuals are more susceptible to infection with intracellular pathogens, such as human herpesvirus 8 (the aetiological agent of Kaposi’s sarcoma) and Mycobacterium tuberculosis, could be explained by their lack of TH1-cell-mediated immunity. However, deficiencies of TH1- and TH2-cell-mediated responses cannot fully explain the lack of skin and mucosal immunity against extracellular pathogens, such as Candida albicans or bacterial pneumonia. Recent studies indicate that IL-17 and IL-22, the cytokines that are produced by the newly described TH17-cell subset, might fill this void. In support of this suggestion, activation of PRRs, specifically TLRs and dectin-1, strongly induces IL-17 expression and provides protective immunity against bacterial and fungal infections16–19. This protection might be mediated by IL-17-driven upregulation of granulopoietic cytokines, which causes a marked increase in the number of neutrophils to effectively eliminate the extracellular pathogen20. However, it is becoming clear that TH17-cell-derived cytokines have an important role in upregulating humoral antimicrobial factors that act locally to limit infection and that drive inflammation by acting as chemokines or autocrine and/or paracrine signalling molecules. Indeed, data are accumulating to support a role for these TH17-cell-derived cytokines as key regulators of the antimicrobial-protein response in vitro and in vivo, and this may be an important mechanism by which they confer protection from infection.
In the lungs, IL-17A exerts its effects through its receptor, IL-17RA, which is expressed on the basolateral surface of the bronchial epithelium and induces the expression of HBD2 and CCL20 (REFS 21–23) (FIG. 2). This IL-17RA-mediated activity depends on the cytoplasmic adaptor protein actin-related gene 1 (ACT1), on the activation of phosphoinositide 3-kinase (PI3K) and on the nuclear translocation of nuclear factor-κB (NF-κB)23. IL-22, IL-17A and IL-17F have been shown to cooperate in the induction of antimicrobial-protein expression, such as HBD2, HBD3 (REF. 24) and calgranulin, by human skin keratinocytes and bronchial epithelial cells25–28 (FIG. 2). Furthermore, in mouse tracheal epithelial cells, IL-22 and IL-17 synergistically induce lipocalin-2 expression, and this induction is required for antimicrobial activity against the Gram-negative pathogen Klebsiella pneumoniae26. The mechanism of synergism between IL-17 and IL-22 is yet to be defined, but may be the result of a convergence of the STAT3 (signal transducer and activator of transcription 3) and NF-κB signalling pathways, which are induced downstream of the IL-22 and IL-17 receptors, respectively. Exactly how IL-22-induced STAT3 signalling converges with the ACT1–PI3K–NF-κB pathway to cooperate with IL-17 in antimicrobial-protein induction is unclear. However, it is possible this synergy occurs at the level of downstream kinases that have been implicated in both IL-22R and IL-17R signalling, including the mitogen-activated protein kinases and the JUN N-terminal kinases.
In the mouse gastrointestinal tract, IL-22 was recently shown to be required for the induction of expression of the C-type lectins regenerating protein 3β (REG3β) and REG3γ following challenge with Citrobacter rodentium29. Consistent with this, Zheng et al.29 also showed that IL-22-deficient mice are highly susceptible to infection with C. rodentium and can be rescued from an otherwise lethal challenge through the administration of recombinant human or mouse REG3γ. REG3γ is a soluble C-type lectin that is produced by Paneth cells and has direct antimicrobial activity against Gram-positive bacteria by interacting with bacterial peptidoglycan. REG3γ is not known to have direct antimicrobial activity against Gram-negative organisms, although commensal flora can induce its expression30. In addition, the study by Zheng et al. suggested that REG3γ has antimicrobial activity (albeit not directly microbicidal) against certain pathogenic Gram-negative bacteria29. Pathogenic Gram-positive bacteria, such as Listeria monocytogenes, can induce REG3γ production in a MyD88-dependent manner31, which indicates that TLR or IL-1R signalling might be involved in REG3γ expression and regulation. The preferred action of REG3γ against Gram-positive or commensal organisms presents an intriguing possibility: the balance of inflammation and tolerance against a constant presence of bacteria in the gut could be linked to differential cytokine-mediated regulation of antimicrobial proteins. The effects of IL-22 in this model imply that this cytokine has a role in inflammation, although this remains a controversial issue.
Balancing inflammation and homeostasis
In addition to being a crucial effector of mucosal immunity, IL-22 has been implicated in autoimmune inflammation and epithelial-cell proliferation. Whether IL-22 mediates inflammation and proliferation or has an anti-inflammatory protective effect may depend on the differential activation of STAT3 and the subsequent activation of either IL-21, which can further promote IL-17 production, or the suppression of cytokine signalling (SOCS) proteins, which inhibit cytokine-receptor signalling. In addition, similar to IL-17, IL-22 may have granulopoietic effects that lead to the proliferation of inflammatory cells, although this suggestion requires further investigation. On the one hand, IL-22 is known to induce the production of several acute-phase proteins (such as lipocalin-2), which are markers of inflammation. On the other hand, IL-22 has been shown to induce the production of high levels of LPS-binding protein (which neutralizes LPS), suggesting that IL-22 has a role in dampening inflammation32.
Another factor that requires further investigation is the role of IL-22 binding protein (IL-22BP), which inhibits IL-22 from binding to IL-22R. The balance of IL-22 and IL-22BP levels, and the mechanism by which the expression of IL-22BP affects the binding of IL-22 to IL-22R in the setting of various inflammatory disorders, is currently unclear. A greater understanding of this balance could help to determine whether IL-22 has a primarily pro- or anti-inflammatory role.
The effects of IL-22 in inflammation are probably influenced by changes in the microenvironment that are yet to be determined, but may be associated with the baseline level of colonization by commensal bacteria. Antimicrobial proteins may be both mediators and ‘end-effectors’ in this cytokine-regulated commitment to inflammation and proliferation, as indicated by the paradigm of the crosstalk between innate immune cells and TH17 cells. More specifically, the persistent presence of commensal bacteria in the normal gut induces a constant range and level of antimicrobial-protein expression. As the innate immune system has developed a degree of tolerance to the presence of commensal bacteria, introduction of new pathogenic bacteria, or changes in the quantity or distribution of the commensal flora, may tip the balance towards TH17-cell development. This can subsequently modulate the antimicrobial-protein response accordingly. The differential recognition of bacterial species by antimicrobial proteins, such as REG3γ, also helps to ‘tag’ pathogens for recognition by the immune system and alert it to changes in the quantity and quality of the commensal milieu.
A role for IL-22-driven TH17-cell-mediated antimicrobial-protein expression in inflammatory disease is most apparent in the skin. Antimicrobial peptides are highly expressed in the skin of patients with psoriasis33, and IL-22 strongly induces both the proliferation of keratinocytes and the expression of antimicrobial proteins, such as S100A7 (rEFs 25,27,28,34). Moreover, neutralization of IL-22 can reduce cutaneous acanthosis (thickening of the skin) in models of psoriasis35. A role for TH17 cells in antimicrobial responses is also supported by the finding that patients with mutations in STAT3 that cause Job’s syndrome (hyper-IgE syndrome) and increased susceptibility to cutaneous infections with Staphylococcus aureus and C. albicans lack antigen-specific TH17 cells in the peripheral blood36. Curiously, these patients have an exaggerated TH2-cell-associated hyper-IgE syndrome. In individuals with atopic dermatitis37, the TH2-type cytokines IL-4 and IL-13 are highly expressed in the skin, where they seem to downregulate the expression of LL37; this may explain the frequent occurrence of S. aureus infections in patients with atopic dermatitis. IL-4 and IL-13 can activate STAT6, as well as SOCS1 and SOCS3, which then inhibit both tumour-necrosis factor (TNF)- and interferon-γ (IFNγ)-mediated induction of HBD2, and HBD3 expression by keratinocytes38. Further work is required to determine the role of IL-22 (or other activators of STAT3) in Job’s syndrome, and whether myeloid-cell or epithelial-cell expression of STAT3 contributes to the clinical phenotype of this syndrome, including the high IgE levels and susceptibility to S. aureus and C. albicans infections.
The role of TH2-type cytokines in pulmonary infection is less clear. IL-4 was recently shown to increase the transepithelial transport of the antimicrobial substrate thiocyanate in human bronchial epithelial cells39, which could enhance the activity of this innate immune defence mechanism in the lungs.
Finally, a less favourable potential effect of antimicrobial proteins is the perpetuation of inflammation. Recent studies have shown that certain antimicrobial proteins can directly activate PRRs and promote unrestrained inflammation. For example, LL37, which is overexpressed in psoriatic skin and is associated with epithelial-cell proliferation, can bind to self DNA (which can be released from dying keratinocytes) owing to its cationic charge. This DNA–LL37 complex can then activate TLR9 signalling, leading to the production of type I IFNs (such as IFNα). IFNα in turn promotes the maturation of dendritic cells and the subsequent activation of TH17 cells, which mediate unremitting inflammation through the production of their effector cytokines IL-17 and IL-22 (REF. 40). This could induce epithelial-cell proliferation and potentially the formation of further DNA–LL37 complexes, thereby resulting in a vicious cycle of inflammation. In this setting, the antimicrobial-protein response is both a target and a driver of autoimmune inflammation at mucosal surfaces. Whether similar mechanisms of inflammation are involved at other mucosal sites, such as the gut and lungs, needs to be determined. It is also possible that antimicrobial peptides, such as β-defensins, that are ligands for CC-chemokine receptor 6, which is expressed by TH17 cells41, could amplify inflammation by increasing the recruitment of TH17 cells.
Conclusions and outstanding questions
Until recently, it was generally thought that the expression of some antimicrobial proteins was ubiquitous and constitutive, whereas the expression of other antimicrobial proteins was inducible in response to changes in the local microenvironment. However, recent evidence now indicates that these proteins can be dynamically regulated through the interaction of commensal flora with pathogen sensors, such as the TLRs and NOD-like receptors, and through a complex network of cytokines that are produced by innate and adaptive immune cells. Cytokines that are produced by macrophages and lymphocytes allow immune effectors to modulate the antimicrobial-protein response to environmental stress or pathogen challenge. Components of this mucosal immune response, such as IL-22, can cause unwanted epithelial-cell proliferation and antimicrobial-protein production, and drive tissue inflammation. Moreover, if they are not appropriately regulated, some antimicrobial peptides might perpetuate tissue inflammation through their putative binding to chemokine receptors or through their formation of DNA-containing complexes that activate TLR9 (FIG. 2).
Most of the observations that have been highlighted in this Progress article are based on in vitro studies with isolated cell populations. However, the role of cytokines, such as IL-22, in regulating antimicrobial proteins in vivo in response to infection is becoming clearer. Despite this, there are several unanswered questions. For example, which of the receptors that signal through MyD88 — the TLRs or the IL-1R family members — is more important for the cytokine-mediated regulation of antimicrobial peptides? Does IL-22 induce granulopoiesis (similarly to IL-17)? If so, how does this contribute to the local induction of epithelial-cell antimicrobial-protein production in vivo? Can these IL-17- or IL-22-mediated pathways be exploited to overcome defects in antimicrobial-protein expression in the setting of atopic dermatitis or to prevent infections in patients with hyper-IgE syndrome? Is STAT3 expression more important in myeloid cells or epithelial cells for maintaining mucosal immunity against the common commensal organisms S. aureus and C. albicans? Advances in cell- and tissue-specific gene targeting in mice and other model organisms, as well as advances in human genetics, will continue to determine the in vivo cellular requirements of PRRs (in myeloid or epithelial cells, for example) and the cellular source of cytokines that are crucial for regulating mucosal antimicrobial peptides.
Acknowledgments
This work was supported by grants from the National Institutes of Health (NIH), USA, to J.K.K (P50HL084932 and R01HL079142) and P.B.M. (P50HL61234), grants from the Roy J. Carver Charitable Trust to P.B.M. and a grant from the National Heart, Lung and Blood Institute (NHLBI) to Y.R.C. (K08HL089189).
Footnotes
DATABASES
Entrez Gene: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=gene
IL-1β|IL-4 | IL-13 | IL-17 | IL-22 | lipocalin-2 | LL37 | TNF
OMIM: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=OMIM
atopic dermatitis | Job’s syndrome | psoriasis
FURTHER INFORMATION
Jay K. Kolls’ homepage: http://www.gradbiomed.pitt.edu/immunology/faculty.asp?ID=86
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
Contributor Information
Jay K. Kolls, Department of Pediatrics, Children’s Hospital of Pittsburgh, University of Pittsburgh, 3705 Fifth Avenue, Pittsburgh, Pennsylvania 15213, USA
Paul B. McCray, Jr, Division of Allergy and Pulmonology, Department of Pediatrics, Carver College of Medicine, University of Iowa, Iowa City, Iowa 52242, USA.
Yvonne R. Chan, Division of Pulmonary, Allergy and Critical Care Medicine, University of Pittsburgh, 3459 Fifth Avenue, Pittsburgh, Pennsylvania 15213, USA
References
- 1.Zelensky AN, Gready JE. The C-type lectin-like domain superfamily. FEBS J. 2005;272:6179–6217. doi: 10.1111/j.1742-4658.2005.05031.x. [DOI] [PubMed] [Google Scholar]
- 2.Soruri A, Grigat J, Forssmann U, Riggert J, Zwirner J. β-defensins chemoattract macrophages and mast cells but not lymphocytes and dendritic cells: CCR6 is not involved. Eur J Immunol. 2007;37:2474–2486. doi: 10.1002/eji.200737292. [DOI] [PubMed] [Google Scholar]
- 3.Kuhara T, Yamauchi K, Tamura Y, Okamura H. Oral administration of lactoferrin increases NK cell activity in mice via increased production of IL-18 and type I IFN in the small intestine. J Interferon Cytokine Res. 2006;26:489–499. doi: 10.1089/jir.2006.26.489. [DOI] [PubMed] [Google Scholar]
- 4.Yu J, et al. Host defense peptide LL-37, in synergy with inflammatory mediator IL-1β, augments immune responses by multiple pathways. J Immunol. 2007;179:7684–7691. doi: 10.4049/jimmunol.179.11.7684. [DOI] [PubMed] [Google Scholar]
- 5.Zheng Y, et al. Cathelicidin LL-37 induces the generation of reactive oxygen species and release of human α-defensins from neutrophils. Br J Dermatol. 2007;157:1124–1131. doi: 10.1111/j.1365-2133.2007.08196.x. [DOI] [PubMed] [Google Scholar]
- 6.Shi J, et al. A novel role for defensins in intestinal homeostasis: regulation of IL-1β secretion. J Immunol. 2007;179:1245–1253. doi: 10.4049/jimmunol.179.2.1245. [DOI] [PubMed] [Google Scholar]
- 7.Singh PK, et al. Production of β-defensins by human airway epithelia. Proc Natl Acad Sci USA. 1998;95:14961–14966. doi: 10.1073/pnas.95.25.14961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cowland JB, Sorensen OE, Sehested M, Borregaard N. Neutrophil gelatinase-associated lipocalin is up-regulated in human epithelial cells by IL-1β, but not by TNF-α. J Immunol. 2003;171:6630–6639. doi: 10.4049/jimmunol.171.12.6630. [DOI] [PubMed] [Google Scholar]
- 9.Bando M, et al. Interleukin-1α regulates antimicrobial peptide expression in human keratinocytes. Immunol Cell Biol. 2007;85:532–537. doi: 10.1038/sj.icb.7100078. [DOI] [PubMed] [Google Scholar]
- 10.Yano S, Banno T, Walsh R, Blumenberg M. Transcriptional responses of human epidermal keratinocytes to cytokine interleukin-1. J Cell Physiol. 2008;214:1–13. doi: 10.1002/jcp.21300. [DOI] [PubMed] [Google Scholar]
- 11.Weijer S, et al. Interleukin-18 facilitates the early antimicrobial host response to Escherichia coli peritonitis. Infect Immun. 2003;71:5488–5497. doi: 10.1128/IAI.71.10.5488-5497.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiersinga WJ, et al. Endogenous interleukin-18 improves the early antimicrobial host response in severe melioidosis. Infect Immun. 2007;75:3739–3746. doi: 10.1128/IAI.00080-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McDonald V, et al. A potential role for interleukin-18 in inhibition of the development of Cryptosporidium parvum. Clin Exp Immunol. 2006;145:555–562. doi: 10.1111/j.1365-2249.2006.03159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uehara A, et al. Chemically synthesized pathogen-associated molecular patterns increase the expression of peptidoglycan recognition proteins via toll-like receptors, NOD1 and NOD2 in human oral epithelial cells. Cell Microbiol. 2005;7:675–686. doi: 10.1111/j.1462-5822.2004.00500.x. [DOI] [PubMed] [Google Scholar]
- 15.Wang H, Gupta D, Li X, Dziarski R. Peptidoglycan recognition protein 2 (N-acetylmuramoyl-L-ala amidase) is induced in keratinocytes by bacteria through the p38 kinase pathway. Infect Immun. 2005;73:7216–7225. doi: 10.1128/IAI.73.11.7216-7225.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Happel KI, et al. Cutting Edge: roles of toll-like receptor 4 and IL-23 in IL-17 expression in response to Klebsiella pneumoniae infection. J Immunol. 2003;170:4432–4436. doi: 10.4049/jimmunol.170.9.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis. 2004;190:624–631. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- 18.Chen Z, Tato CM, Muul L, Laurence A, O’Shea JJ. Distinct regulation of interleukin-17 in human T helper lymphocytes. Arthritis Rheum. 2007;56:2936–2946. doi: 10.1002/art.22866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson NJ, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nature Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 20.Schwarzenberger P, et al. Requirement of endogenous stem cell factor and granulocyte-colony-stimulating factor for IL-17-mediated granulopoiesis. J Immunol. 2000;164:4783–4789. doi: 10.4049/jimmunol.164.9.4783. [DOI] [PubMed] [Google Scholar]
- 21.McAllister F, et al. Role of IL-17A, IL-17F, and the IL-17 receptor in regulating growth-related oncogene-α and granulocyte colony-stimulating factor in bronchial epithelium: implications for airway inflammation in cystic fibrosis. J Immunol. 2005;175:404–412. doi: 10.4049/jimmunol.175.1.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kao CY, et al. IL-17 markedly up-regulates β-defensin-2 expression in human airway epithelium via JAK and NF-κB signaling pathways. J Immunol. 2004;173:3482–3491. doi: 10.4049/jimmunol.173.5.3482. [DOI] [PubMed] [Google Scholar]
- 23.Huang F, et al. Requirement for both JAK-mediated PI3K signaling and ACT1/TRAF6/TAK1-dependent NF-κB activation by IL-17A in enhancing cytokine expression in human airway epithelial cells. J Immunol. 2007;179:6504–6513. doi: 10.4049/jimmunol.179.10.6504. [DOI] [PubMed] [Google Scholar]
- 24.Wolk K, et al. IL-22 increases the innate immunity of tissues. Immunity. 2004;21:241–254. doi: 10.1016/j.immuni.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 25.Liang SC, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aujla SJ, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nature Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boniface K, et al. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J Immunol. 2005;174:3695–3702. doi: 10.4049/jimmunol.174.6.3695. [DOI] [PubMed] [Google Scholar]
- 28.Wolk K, et al. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol. 2006;36:1309–1323. doi: 10.1002/eji.200535503. [DOI] [PubMed] [Google Scholar]
- 29.Zheng Y, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nature Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 30.Cash HL, Whitham CV, Behrendt CL, Hooper LV. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313:1126–1130. doi: 10.1126/science.1127119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brandl K, Plitas G, Schnabl B, DeMatteo RP, Pamer IE. MyD88-mediated signals induce the bactericidal lectin RegIIIγ and protect mice against intestinal Listeria monocytogenes infection. J Exp Med. 2007;204:1891–1900. doi: 10.1084/jem.20070563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolk K, et al. IL-22 induces lipopolysaccharide-binding protein in hepatocytes: a potential systemic role of IL-22 in Crohn’s disease. J Immunol. 2007;178:5973–5981. doi: 10.4049/jimmunol.178.9.5973. [DOI] [PubMed] [Google Scholar]
- 33.Ong PY, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–1160. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 34.Sa SM, et al. The effects of IL-20 subfamily cytokines on reconstituted human epidermis suggest potential roles in cutaneous innate defense and pathogenic adaptive immunity in psoriasis. J Immunol. 2007;178:2229–2240. doi: 10.4049/jimmunol.178.4.2229. [DOI] [PubMed] [Google Scholar]
- 35.Zheng Y, et al. Interleukin-22, a TH17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 36.Ma CS, et al. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J Exp Med. 2008;205:1551–1557. doi: 10.1084/jem.20080218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nomura I, et al. Cytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genes. J Immunol. 2003;171:3262–3269. doi: 10.4049/jimmunol.171.6.3262. [DOI] [PubMed] [Google Scholar]
- 38.Albanesi C, et al. IL-4 and IL-13 negatively regulate TNF-α- and IFN-γ-induced β-defensin expression through STAT-6, suppressor of cytokine signaling (SOCS)-1, and SOCS-3. J Immunol. 2007;179:984–992. doi: 10.4049/jimmunol.179.2.984. [DOI] [PubMed] [Google Scholar]
- 39.Pedemonte N, et al. Thiocyanate transport in resting and IL-4-stimulated human bronchial epithelial cells: role of pendrin and anion channels. J Immunol. 2007;178:5144–5153. doi: 10.4049/jimmunol.178.8.5144. [DOI] [PubMed] [Google Scholar]
- 40.Lande R, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–569. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 41.Acosta-Rodriguez EV, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nature Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 42.Schneider JJ, Unholzer A, Schaller M, Schäfer-Korting M, Korting HC. Human defensins. J Mol Med. 2005;83:587–595. doi: 10.1007/s00109-005-0657-1. [DOI] [PubMed] [Google Scholar]
- 43.Lehrer RI. Primate defensins. Nature Rev Microbiol. 2004;2:727–738. doi: 10.1038/nrmicro976. [DOI] [PubMed] [Google Scholar]
- 44.Zanetti M. The role of cathelicidins in the innate host defenses of mammals. Curr Issues Mol Biol. 2005;7:179–196. [PubMed] [Google Scholar]
- 45.Glaser R, et al. Antimicrobial psoriasin (S100A7) protects human skin from Escherichia coli infection. Nature Immunol. 2005;6:157–164. doi: 10.1038/ni1142. [DOI] [PubMed] [Google Scholar]
- 46.Lee KC, Eckert RL. S100A7 (psoriasin) — mechanism of antibacterial action in wounds. J Invest Dermatol. 2007;127:945–957. doi: 10.1038/sj.jid.5700663. [DOI] [PubMed] [Google Scholar]
- 47.Buchau AS, et al. S100A15, an antimicrobial protein of the skin: regulation by E. coli through Toll-like receptor 4. J Invest Dermatol. 2007;127:2596–2604. doi: 10.1038/sj.jid.5700946. [DOI] [PubMed] [Google Scholar]
- 48.Gottsch JD, Eisinger SW, Liu SH, Scott AL. Calgranulin C has filariacidal and filariastatic activity. Infect Immun. 1999;67:6631–6636. doi: 10.1128/iai.67.12.6631-6636.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baranger K, Zani ML, Chandenier J, Dallet-Choisy S, Moreau T. The antibacterial and antifungal properties of trappin-2 (pre-elafin) do not depend on its protease inhibitory function. FEBS J. 2008;275:2008–2020. doi: 10.1111/j.1742-4658.2008.06355.x. [DOI] [PubMed] [Google Scholar]
- 50.Bellemare A, Vernoux N, Morisset D, Bourbonnais Y. Human pre-elafin inhibits a Pseudomonas aeruginosa-secreted peptidase and prevents its proliferation in complex media. Antimicrob Agents Chemother. 2008;52:483–490. doi: 10.1128/AAC.00585-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Williams SE, Brown TI, Roghanian A, Sallenave JM. SLPI and elafin: one glove, many fingers. Clin Sci (Lond) 2006;110:21–35. doi: 10.1042/CS20050115. [DOI] [PubMed] [Google Scholar]
- 52.Dziarski R, Gupta D. The peptidoglycan recognition proteins (PGRPs) Genome Biol. 2006;7:232. doi: 10.1186/gb-2006-7-8-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Legrand D, Elass E, Pierce A, Mazurier J. Lactoferrin and host defence: an overview of its immuno-modulating and anti-inflammatory properties. Biometals. 2004;17:225–229. doi: 10.1023/b:biom.0000027696.48707.42. [DOI] [PubMed] [Google Scholar]
- 54.Kemna EHJM, Tjalsma H, Willems HL, Swinkels DW. Hepcidin: from discovery to differential diagnosis. Haematologica. 2008;93:90–97. doi: 10.3324/haematol.11705. [DOI] [PubMed] [Google Scholar]
- 55.Borregaard N, Cowland J. Neutrophil gelatinase-associated lipocalin, a siderophore-binding eukaryotic protein. Biometals. 2006;19:211–215. doi: 10.1007/s10534-005-3251-7. [DOI] [PubMed] [Google Scholar]
- 56.Esche C, Stellato C, Beck LA. Chemokines: key players in innate and adaptive immunity. J Invest Dermatol. 2005;125:615–628. doi: 10.1111/j.0022-202X.2005.23841.x. [DOI] [PubMed] [Google Scholar]