Abstract
The reaction of [ReOCl3(PPh3)2] with N,N-bis(2-mercaptoethyl)benzylamine and 4-bromobenzenethiol allowed for the isolation of [ReO{η3-(SCH2CH2)2N(CH2C6H5)}-(η1-C6H4Br-4-S)] (1). The reaction of [ReOCl3(PPh3)2] with [(HSCH2CH2)2N(CH2C5H4N)] and the appropriate thiol in chloroform treated with triethylamine has led to the isolation of a series of neutral rhenium complexes of the type [ReO{η3-(SCH2CH2)2N(CH2C5H4N)}(η1-C6H4X-4-S)] (X = Br (2), Cl (3), F (4), and OCH3 (5)) and [ReO{η3-(SCH2CH2)2N(CH2C5H4N)}(η1-C6H4OCH3-4-CH2S)] (6). Likewise, under similar reaction conditions, the use of the related tridentate ligand, [(HSCH2CH2)2N(CH2CH2C5H4N)], has led to the isolation of a series of rhenium complexes of the type [ReO{η3-(SCH2CH2)2N(CH2CH2C5H4N)}(η1-C6H4X-4-S)] (X=Br (7), Cl (8), OCH3 (9)), as well as [ReO{η3-(SCH2CH2)2N(CH2CH2C5H4N)}(η1-C6H4Cl-4-CH2S)]·0.5CH3(CH2)4CH3 (10). These compounds are extensions of the ‘3+1’ approach to the synthesis of materials with the {MO}3+ core (M=Tc and Re), which have applications in nuclear medicine. The ligands chosen allow systematic exploration of the consequences of para-substitution on the monodentate thiolate ligand [S] and of derivatization of the substituent R on the tridentate aminodithiol ligand [SNS] of the type (HSCH2CH2)2NR. Such modifications can influence lipophilicity, charge, size and molecular weight of the complex and consequently the biodistribution.
Keywords: Crystal structure, Rhenium complexes, Oxo complexes, SNS-donor ligand complexes, S-donor ligand complexes
1. Introduction
The significant contemporary interest in the coordination chemistry of rhenium derives from its analogies to technetium chemistry, as well as its expanding role in therapeutic nuclear chemistry [1]. The radionuclides of Re, the Group 7 congener of technetium, are β-emitters with properties which make them suitable candidates for therapeutic applications. Rhenium-186 is particularly attractive [2–4] because of its half-life (90 h) and strong β-emission (βmax, 1070 keV), capable of delivering high radiation doses to tissues. Rhenium-188, also a β-emitting radionuclide (βmax, 2120 keV), with a half-life of 17 h and available from a 188W/188Re radionuclide generator at no carrier added levels [5], has become a logical choice for the development of radio-pharmaceuticals for use in the treatment of cancer [1].
The synthesis of novel compounds, which allow the systematic exploration of size, shape, molecular weight, and charge of the metal–thiolate complexes with respect to their biodistribution patterns, provides a driving force for advances in the radiopharmaceutical chemistry of rhenium. One approach to the systematic design of Re(V) complexes with the {ReO}3+ core exploits the ‘3+1’ concept of ligand addition to a Group 7 metal-oxo species [6–16]. The methodology is based on forming a tridentate ‘3’ and a monodentate ‘1’ mixed-thiolate core. The technique uses tridentate dithiolates with the (S–X–S)2− (X=NR, O, S) donor set and a −2 charge in combination with a monothiol ligand of −1 charge disposed about a Group 7 metal-oxo core, so as to occupy four coordination sites on the metal with a total formal charge of −3. The ‘3+1’ ligand combination preserves the metal-oxo core and the formal +5 oxidation state of the metal, thus conforming to the metal coordination and oxidation preferences.
Over the past decade, thiol based tridentate ligands have received significant attention in the design of new Group 7 metal-oxo complexes [8–11,17–31]. One such series of compounds belongs to the class of [SSS]/[S] ‘3+1’ mixed ligand complexes [10,11,17–21]. Numerous groups have synthesized neutral rhenium and technetium compounds containing 2-mercaptoethyl sulfide as the tridentate in combination with various monothiols, to produce complexes such as [ReO{(SCH2CH2)2-S}{SCH2C6H5}] [11], [ReO{(SCH2CH2)2S}{SCH2CH-(OH)CH(OH)CH2SH}] [17], [ReO{(SCH2CH2)2S}-{S(CH2)3N(CH3)(CH2)3C6H5}] [18] and [ReO{(SCH2C-H2)2S}{SCH2CH(CH2CH3)-4-HOC6H5}] [19]. Not only have such neutral compounds reported but also several cationic complexes such as [ReO{(SCH2CH2)2S}-{C5H4NH-2-S}][Br] [20].
Another subclass of compounds includes the class of [SOS]/[S] ‘3+1’ mixed ligand complexes [11,21,22]. In most instances of this family of compounds, the tridentate 2-mercaptoethylether seems to be the tridentate of choice, and has been used in the design of such neutral compounds as [ReO{(SCH2CH2)2O}{SC6H5}] [11] and [ReO{(SCH2CH2)2O}{SCH2-4-CH3OC6H5}] [22], a cationic species [ReO{(SCH2CH2)2O}{C5H4NH-2-S}] [22], and several binuclear complexes, of which [Tc2O2{(SC-H2CH2)2O}2{SCH2CH2OCH2CH2S}] [21] and [Re2-O2{(SCH2CH2)2O}2{SCH2CH2OCH2CH2S}] [22] are representatives.
A third series of ‘3+1’ complexes includes the [ONS]/[S] mixed ligand complexes [8,9,20,23–27]. One subclass of this family incorporates the tridentate Schiff base [HOC6H4C(H)NC6H4SH] and includes complexes such as [TcO{OC6H4C(H)NC6H4S}{SC6H5}] [9], [TcO-{OC6H4C(H)NC6H4S}{n-SC8H17}] [8], and a cationic species [ReO{OC6H4C(H)NC6H4S}{C5H4NH-2-S}][Br] [23]. Another tridentate ligand type found in the [ONS]/[S] series is represented by [C5H3N-2-CH2OH-6-CH2SH], which allows isolation of both neutral compounds, such as [ReO{C5H3N-2-CH2O-6-CH2S}{S-4-ClC6H4}] [27], and cationic species, [ReO{C5H3N-2-CH2O-6-CH2S}{C5H4NH-2-S}][Br] [20].
The vast majority of the ‘3+1’ Group 7 metal-oxo complexes belong to the class [SN(R)S]/[S], where the −R group of the tridentate may be exploited to provide a variety of derivatives and secondary functionality [19,28–31]. Examples include [ReO{C5H3N(CH2S)2}{S-4-C6H4OH}] [19], [ReO{C2H5N(CH2CH2S)2}{S-4-Cl-C6H4}] [28], [ReO{C2H5SCH2CH2N(CH2CH2S)2}{S-4-CH3OC6H4}] [29], a binuclear complex [Re2-O2{C2H5N(CH2CH2S)2}3] [28] and a cationic species, [ReO{C5H3N(CH2S)2}{C5H4NH-2-S}][Cl] [27].
In an effort to expand the ‘3+1’ chemistry to the oxo-rhenium core, our work has concentrated on the reactions of the tridentate thiol, [(HSCH2CH2)2N-(CH2C6H5)], and the potentially tetradentate ligands-[(HSCH2CH2)2N(CH2C5H4N)] and [(HSCH2CH2)2-N(CH2CH2C5H4N)] with [ReOCl3(PPh3)2], so as to prepare families of compounds with mixed thiol ligands, and allowing the systematic exploration of the para-substituted benzenethiol and benzylmercaptan series. This study continues our development of the coordination chemistry of the Group 7 metal rhenium [17,20,22,23,27], in order to provide new materials for radiolabeling of chemotatic peptides [32]. We report the synthesis and structural characterization of a series of oxo-rhenium ‘3+1’ complexes, namely [ReO{η3-(SC-H2CH2)2N(CH2C6H5)}(η1-C6H4Br-4-S)] (1), [ReO{η3-(SCH2CH2)2N(CH2C5H4N)}(η1-C6H4X-4-S)] (X=Br (2), Cl (3), F (4), and OCH3 (5)), [ReO{η3-(SC-H2CH2)2N(CH2C5H4N)}(η1-C6H4OCH3-4-CH2S)] (6), [ReO{η3 - (SCH2CH2)2N(CH2CH2C5H4N)}(η1 - C6H4-X-4-S)] (X=Br (7), Cl (8), OCH3 (9)), and [ReO{η3-(SCH2CH2)2N(CH2CH2C5H4N)}(η1-C6H4Cl-4-CH2S)]· 0.5CH3(CH2)4CH3 (10).
2. Experimental
2.1. General considerations
NMR spectra were recorded on a Bruker DPX 300 (1H 300.10 MHz) spectrometer in CDCl3 (δ 7.27). IR spectra were recorded as KBr discs with a Perkin–Elmer series 1600 FT IR. Elemental analysis for carbon, hydrogen, and nitrogen were carried out by Oneida Research Services, Whitesboro, NY. Ammonium perrhenate (Aldrich), triethylamine (Aldrich), triphenylphosine (Aldrich), benzylamine (Aldrich), 2-(aminomethyl)pyridine (Aldrich), ethylene sulfide (Aldrich), 2-(2-aminoethyl)pyridine (Aldrich), 4-bromobenzenethiol (Aldrich), 4-chlorobenzenethiol (Aldrich), 4-fluorobenzenethiol (Aldrich), 4-methoxy-benzenethiol (Aldrich), 4-chlorobenzyl mercaptan (Aldrich), 4-methoxybenzyl mercaptan (Lancaster) were used as received without further purification. All other solvents and reagents were purchased from Aldrich and used as received, unless otherwise stated. The [ReOCl3(PPh3)2] [33] and N,N-bis(2-mercapto-ethyl)benzylamine [34] were synthesized according to published procedures.
2.2. Syntheses
2.2.1. [(HSCH2CH2)2N(CH2C5H4N)]
A similar procedure was employed as for the preparation of N,N-bis(2-mercaptoethyl)benzylamine. 1H NMR (CDCl3, ppm): 1.72 (s, 2H), 2.61 (m, 4H), 2.78 (m, 4H), 3.81 (s, 2H), 7.18 (m, 1H), 7.36, (d, 1H), 7.67 (m, 1H), 8.52 (m, 1H).
2.2.2. [(HSCH2CH2)2N(CH2CH2C5H4N)]
A similar procedure was employed as for the preparation of N,N-bis(2-mercaptoethyl)benzylamine. 1H NMR (CDCl3, ppm): 1.75 (s, 2H), 2.10–2.90 (m, 12H), 7.18 (m, 1H), 7.20, (d, 1H), 7.60 (t, 1H), 8.50 (d, 1H).
2.2.3. General synthetic procedure
To a solution of [ReOCl3(PPh3)2] (0.0822 g, 0.0986 mmol) in 1 ml of chloroform was added dropwise a solution of 1 equiv. (0.0986 mmol) of monothiol (the HSR donor ligand) and 1 equiv. of the dithiol [(HSCH2CH2)2NR, R=CH2C6H5, CH2C5H4N, (CH2)2C5H4N] (0.0986 mmol) in 1 ml of chloroform. The solution remained olive green until the addition of triethylamine (0.08 ml, 0.574 mmol) whereupon it immediately changed from olive to forest green. The solution was stirred and refluxed for an additional 30 min and then evaporated to dryness. Crystals for each of the compounds were grown as indicated.
2.2.3.1. Preparation of [ReO{η3-(SCH2CH2)2N(CH2 -C6H5)}(η1-C6H4Br -4 -S)] (1)
X-ray quality crystals were grown by slow diffusion of pentane in a solution of the compound in methylene chloride (yield: 0.021 g, 34.6%). Anal. Calc. for C17H19NOS3BrRe (mol. wt. 615.62): C, 33.2; H, 3.11; N, 2.28. Found: C, 32.5; H, 3.49; N, 2.16%.
2.2.3.2. Preparation of [ReO{η3-(SCH2CH2)2N(CH2 -C5H4N)}(η1-C6H4Br -4 -S)] (2)
X-ray quality crystals were grown by slow diffusion of pentane in a solution of the compound in methylene chloride (yield: 0.027 g, 44.4%). IR (KBr, cm−1): 1654 (m), 1560 (m), 1508 (w), 1267 (m), 1080 (m), 1008 (m), 948 (s), 813 (w). 1H NMR (CDCl3, ppm): 2.73 (m, 2H), 2.85 (m, 2H), 3.52 (m, 2H), 3.85 (m, 2H), 5.03 (s, 2H), 7.26 (m, 2H), 7.38 (m, 2H), 7.53 (m, 2H), 7.80 (m, 1H), 8.71 (m, 1H).
2.2.3.3. Preparation of [ReO{η3-(SCH2CH2)2N(CH2 -C5H4N)}(η1-C6H4Cl -4 -S)] (3)
X-ray quality crystals were grown by slow diffusion of diethylether into a methylene chloride solution of 3 (yield: 0.019 g, 33.9%). IR (KBr, cm−1): 1654 (m), 1560 (m), 1508 (w), 1266 (m), 1085 (m), 1012 (m), 949 (s), 818 (w). 1H NMR (CDCl3, ppm): 2.72 (m, 2H), 2.83 (m, 2H), 3.52 (m, 2H), 3.86 (m, 2H), 5.03 (s, 2H), 7.35 (m, 2H), 7.41 (m, 2H), 7.73 (m, 2H), 7.82 (m, 1H), 8.71 (m, 1H). Anal. Calc. for C16H18N2OS3ClRe (mol. wt. 572.15): C, 33.6; H, 3.17; N, 4.90. Found: C, 34.1; H, 3.55; N, 4.07%.
2.2.3.4. Preparation of [ReO{η3-(SCH2CH2)2N(CH2 -C5H4N)}(η 1-C6H4F -4 -S)] (4)
X-ray quality crystals were grown by slow diffusion of pentane into a methylene chloride solution of 4 (yield: 0.025 g, 45.6%). IR (KBr, cm−1): 1654 (m), 1560 (w), 1508 (w), 1263 (s), 1154 (m), 1014 (m), 942 (s), 833 (w). 1H NMR (CDCl3, ppm): 2.69 (m, 2H), 2.83 (m, 2H), 3.53 (m, 2H), 3.85 (m, 2H), 5.03 (s, 2H), 7.09 (m, 2H), 7.39 (m, 2H), 7.63 (m, 2H), 7.80 (m, 1H), 8.71 (m, 1H).
2.2.3.5. Preparation of [ReO{η3-(SCH2CH2)2N(CH2 -C5H4N)}(η1-C6H4OCH3 -4 -S)] (5)
X-ray quality crystals were grown by slow diffusion of pentane into a solution of the compound in methylene chloride (yield: 0.023 g, 41.1%). IR (KBr, cm−1): 1654 (m), 1560 (w), 1508 (w), 1283 (s), 1172 (m), 1028 (m), 941 (s), 828 (w). 1H NMR (CDCl3, ppm): 2.71 (m, 2H), 2.84 (m, 2H), 3.55 (m, 2H), 3.82 (m, 2H), 3.88 (s, 3H), 5.07 (s, 2H), 6.95 (m, 2H), 7.37 (m, 2H), 7.61 (m, 2H), 7.79 (m, 1H), 8.72 (m, 1H).
2.2.3.6. Preparation of [ReO{η3-(SCH2CH2)2N(CH2 -C5H4N)}(η 1-C6H4OCH3 -4 -CH2S)] (6)
X-ray quality crystals were grown by slow diffusion of diethylether into a methylene chloride solution of 6 (yield: 0.027 g, 47.1%). IR (KBr, cm−1): 1654 (m), 1560 (w), 1509 (w), 1248 (s), 1104 (m), 1032 (m), 948 (s), 819 (w). 1H NMR (CDCl3, ppm): 2.61 (m, 2H), 3.01 (m, 2H), 3.53 (m, 2H), 3.81 (s, 3H), 3.87 (m, 2H), 4.89 (s, 2H), 5.00 (s, 2H), 6.84 (m, 2H), 7.34 (m, 2H), 7.41 (m, 2H), 7.76 (m, 1H), 8.71 (m, 1H). Anal. Calc. for C18H23N2O2S3Re (mol. wt. 581.76): C, 37.2; H, 3.98; N, 4.82. Found: C, 36.8; H, 3.73; N, 4.55%.
2.2.3.7. Preparation of [ReO{η3-(SCH2CH2)2N(CH2 -CH2C5H4N)}(η 1-C6H4Br -4 -S)] (7)
X-ray quality crystals were grown by slow diffusion of pentane into a methylene chloride solution of 7 (yield: 0.031 g, 49.9%). IR (KBr, cm−1): 1654 (m), 1578 (w), 1438 (w), 1273 (s), 1095 (m), 1029 (m), 943 (s), 846 (w). 1H NMR (CDCl3, ppm): 2.76 (m, 2H), 2.91 (m, 2H), 3.31 (t, 2H), 3.42 (m, 2H), 3.68 (m, 2H), 4.19 (m, 2H), 7.24 (m, 2H), 7.32 (m, 2H), 7.51 (m, 2H), 7.67 (m, 1H), 8.56 (m, 1H). Anal. Calc. for C17H20N2OS3BrRe (mol. wt. 630.64): C, 32.4; H, 3.20; N, 4.44. Found: C, 32.9; H, 3.36; N, 4.38%.
2.2.3.8. Preparation of [ReO{η3-(SCH2CH2)2N(CH2 -CH2C5H4N)}(η1-C6H4Cl -4 -S)] (8)
Crystals were grown by slow diffusion of pentane into a solution of the compound in methylene chloride (yield: 0.024 g, 41.5%). IR (KBr, cm−1): 1654 (m), 1574 (w), 1472 (w), 1268 (s), 1090 (m), 1009 (m), 947 (s), 816 (w). 1H NMR (CDCl3, ppm): 2.79 (m, 2H), 2.89 (m, 2H), 3.31 (t, 2H), 3.42 (m, 2H), 3.68 (m, 2H), 4.20 (m, 2H), 7.22 (m, 2H), 7.33 (m, 2H), 7.55 (m, 2H), 7.68 (m, 1H), 8.57 (m, 1H). Anal. Calc. for C17H20N2OS3ClRe (mol. wt. 586.19): C, 34.8; H, 3.44; N, 4.78. Found: C, 33.6; H, 3.21; N, 2.84%.
2.2.3.9. Preparation of [ReO{η3-(SCH2CH2)2N(CH2 -CH2C5H4N)}(η1-C6H4OCH3 -4 -S)] (9)
X-ray quality crystals were grown by slow diffusion of diethylether into a solution of the compound in methylene chloride (yield: 0.023 g, 40.1%). IR (KBr, cm−1): 1654 (m), 1577 (m), 1492 (w), 1283 (s), 1171 (m), 1024 (m), 938 (s), 826 (w). 1H NMR (CDCl3, ppm): 2.76 (m, 2H), 2.90 (m, 2H), 3.30 (t, 2H), 3.40 (m, 2H), 3.65 (m, 2H), 3.83 (s, 3H), 4.18 (m, 2H), 6.93 (m, 2H), 7.22 (m, 2H), 7.54 (m, 2H), 7.67 (m, 1H), 8.56 (m, 1H).
2.2.3.10. Preparation of [ReO{η3-(SCH2CH2)2N(CH2 -CH2C5H4N)}(η1-C6H4Cl -4 -CH2S)] · 0.5CH3(CH2)4CH3 (10)
X-ray quality crystals were grown by slow diffusion of hexane into a methylene chloride solution of 10 (yield: 0.028 g, 44.2%). IR (KBr, cm−1): 1650 (m), 1589 (w), 1487 (w), 1263 (s), 1090 (m), 1009 (m), 948 (s), 831 (w). 1H NMR (CDCl3, ppm): 2.71 (m, 2H), 3.01 (m, 2H), 3.30 (t, 2H), 3.43 (m, 2H), 3.69 (m, 2H), 4.15 (m, 2H), 4.83 (s, 2H), 7.19–7.27 (m, 4H), 7.33 (m, 2H), 7.65 (m, 2H), 8.56 (m, 1H).
2.3. X-ray crystallography
All data were collected on a Bruker SMART diffractometer system using graphite monochromated Mo Kα radiation (λ(Mo Kα)=.71073 Å). All the data collections were carried out at low temperature (87–93 K). The crystal parameters and other experimental details of the data collections are summarized in Table 1. A complete description of the details of the crystallographic methods is given in Supporting Information. Data was corrected for Lorentz and polarization effects, and absorption corrections were made using SAD-ABS [35]. The structures were solved by direct methods [36]. Neutral atom scattering factors were taken from Cromer and Waber [37] and anomalous dispersion corrections were taken from those of Creagh and McAuley [38]. All calculations were preformed using SHELXTL-96 [36]. Non-hydrogen atoms were refined anisotropically. No anomalies were encountered in the refinements of any of the structures. Atomic positional parameters for the structures have been deposited with the Cambridge Structural Database (Section 5).
Table 1.
Summary of the crystallographic data for the compounds (1–10)
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|
| Chemical formula | C17H19NOS3BrRe | C16H18N2OS3BrRe | C16H18N2OS3ClRe | C16H18N2OS3FRe | C17H21N2O2S3Re | C18H23N2O2S3Re | C17H20N2OS3BrRe | C18H23N2O2S3Re | C21H29N2OS3ClRe |
| a (Å) | 11.2989(6) | 9.3051(7) | 9.3247(4) | 8.9720(5) | 9.4042(4) | 8.3855(4) | 9.4161(4) | 11.6331(4) | 11.2621(7) |
| b (Å) | 13.1804(7) | 9.5482(7) | 9.4485(4) | 9.4770(5) | 9.4183(4) | 24.8281(12) | 9.5641(4) | 10.4400(4) | 12.0955(7) |
| c (Å) | 13.5621(8) | 11.4612(8) | 11.3743(5) | 11.6194(6) | 11.5443(5) | 9.7181(5) | 12.0349(5) | 16.5754(6) | 16.5589(10) |
| α (°) | 80.791(1) | 69.709(1) | 70.320 (1) | 72.876(1) | 111.975(1) | 90 | 109.8550(10) | 90 | 85.1660(10) |
| β (°) | 75.088(1) | 74.940(1) | 74.615(1) | 70.865(1) | 100.927(1) | 104.9880(10) | 104.4130(10) | 105.5100(10) | 74.3980(10) |
| γ (°) | 78.042(1) | 86.287(1) | 84.808(1) | 81.471(1) | 90.201(1) | 90 | 91.9330(10) | 90 | 85.7420(10) |
| V (Å3) | 1897.15(18) | 921.94(12) | 909.78(7) | 890.54(8) | 927.90(7) | 1954.44(17) | 978.80(7) | 1939.77(12) | 2161.7(2) |
| Z | 4 | 2 | 2 | 2 | 2 | 4 | 2 | 4 | 4 |
| Formula weight | 615.62 | 616.61 | 572.15 | 555.70 | 567.74 | 581.76 | 630.64 | 581.76 | 643.29 |
| Space group | P1̄ | P1̄ | P1̄ | P1̄ | P1̄ | P 21/n | P1̄ | P21/n | P1̄ |
| T (K) | 89(5) | 87(5) | 87(5) | 88(5) | 90(5) | 87(5) | 89(5) | 90(5) | 93(5) |
| λ (Å) | 0.71073 | 0.71073 | 0.71073 | 0.71073 | 0.71073 | 0.71073 | 0.71073 | 0.71073 | 0.71073 |
| Dcalc (g cm−1) | 2.155 | 2.221 | 2.089 | 2.072 | 2.032 | 1.977 | 2.140 | 1.992 | 1.977 |
| μ (mm−1) | 8.846 | 9.103 | 7.176 | 7.190 | 6.899 | 6.554 | 8.577 | 6.603 | 6.052 |
| Ra | 0.0439 | 0.0665 | 0.0289 | 0.0426 | 0.0315 | 0.0641 | 0.0345 | 0.0342 | 0.0560 |
| wR2b | 0.0832 | 0.1158 | 0.0710 | 0.1127 | 0.0710 | 0.0744 | 0.0753 | 0.0534 | 0.1238 |
Σ|Fo| − |Fc|/Σ|Fo|.
.
3. Results and discussion
The synthesis of N,N-bis(2-mercaptoethyl)benzylamine employs the route of Schröder and coworkers [34], in which 4 equiv. of ethylene sulfide are reacted with 1 equiv. of benylamine at 65°C for 48 h. A similar procedure was employed in the synthesis of [(HSCH2-CH2)2N(CH2C5H4N)] and [(HSCH2CH2)2N(CH2CH2-C5H4N)] starting from the appropriate amines, 2-(aminomethyl)pyridine and 2-(2-aminoethyl)pyridine, respectively.
The compounds of this study were all prepared readily from the reactions of the appropriate ligands with [ReOCl3(PPh3)2]. While [Bu4N][ReOBr4(OPPh3)] also proved to be an effective starting material, there was no advantage to its use in terms of yield or product purity. Since the synthesis of [Bu4N][ReOBr4(OPPh3)] is relatively involved and of low yield, the more easily prepared and purified starting material [ReOCl3(PPh3)2] was used exclusively for the synthesis of 1–10. The green solid is indefinitely air and moisture stable making it suitable for probing the reactivities of a variety of ligand types. Consequently, the compounds of this study were synthesized in a straightforward fashion from the reaction of [ReOCl3(PPh3)2] with one equivalent of the tridentate dithiol ligand (or ‘3’ ligand) and 1 equiv. of the monodentate thiol ligand (or ‘1’ ligand) in chloroform. An addition of excess triethylamine produces a forest green solution, which upon workup yields crystalline products 1–10.
The infrared spectrum of 2–10 are characterized by a series of ligand bands in the 1008–1654 cm−1 range and a strong band in the 938–949 cm−1 range attributed to ν(Re=O). The 1H NMR spectra of compounds 2–6 are unexceptional. All display a series of multiplets ranging from 6.84 to 8.72 ppm assignable to the eight aromatic protons, as well as four multiplets at 2.69, 2.87, 3.53, and 3.85 ppm assignable to each CH2 moiety comprising the backbone of the tridentate ligand (−SCH2CH2N(R)CH2CH2S−). All four of the multiplets integrate to two protons; however, individual assignments of the resonances are tenuous at best, due to the multiplicity of the peaks and the flexibility of the ligand in solution, as noted by others [6]. In addition, compounds 2–6 also displayed a single proton resonance integrating to two protons at 5.03 ppm corresponding to the methylene spacer of the tridentate backbone. Furthermore, [ReO{η3-(SCH2CH2)2N(CH2-C5H4N)}(η1-C6H4OCH3-4-S)] (5) and [ReO{η3-(SCH2-CH2)2N(CH2C5H4N)}(η1-C6H4OCH3-4-CH2S)] (6) exhibited an additional singlet at approximately 3.88 and 3.81 ppm, respectively, which is attributed to the three protons of the methoxy group. The monodentate benzylmercaptan of compound 6 displayed methylene protons at approximately 4.89 ppm as a single peak, as anticipated from Sadtler spectra [39].
Similarly, the 1H NMR spectra for compounds 7–10 contain multiplets at 2.76, 2.93, 3.42, and 3.68 ppm assignable to each CH2 moiety comprising the backbone of the tridentate ligand, as well as series of multiplets ranging from 6.93 to 8.57 ppm assignable to the eight aromatic protons. Compounds 7–10 also displayed a triplet at 3.31 ppm and a multiplet at 4.18 ppm each integrating to two protons and corresponding to the ethylene spacer of the tridentate ligand. In addition, the monodentate benzylmercaptan of compound 10 displayed methylene protons at approximately 4.83 ppm as a single peak, as anticipated from Sadtler spectra [38]. [ReO{η3-(SCH2CH2)2N(CH2CH2C5-H4N)}(η1-C6H4OCH3-4-S)] (9) exhibited an additional singlet at approximately 3.83 ppm, which is attributed to the three protons of the methoxy group. In no case does the 1H NMR data suggest the presence of syn/anti isomers with respect to the amine substituent R and the oxo-group orientations. The crystal structure data, likewise, confirm that all compounds adopt the syn geometry exclusively.
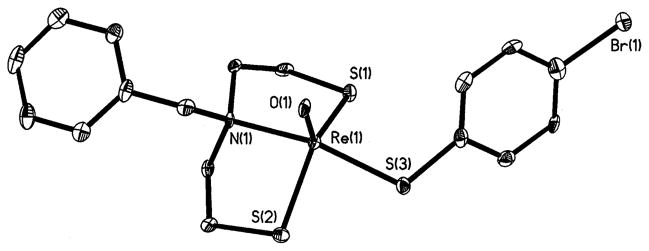
As shown in Fig. 1, the coordination geometry around the metal for [ReO{η3-(SCH2CH2)2-N(CH2C6H5)}(η1-C6H4Br-4-S)] (1) is distorted trigonal bipyramidal, with the oxo group and the sulfur atoms of the [SNS] ligand defining the equatorial plane and the axial positions occupied by the sulfur atom of the monothiol and the nitrogen atom of the [SNS] ligand. The angles between the atoms in the equatorial plane are close to the ideal 120° (Table 2), but a value of 157.91(15)° observed for the N(1)–Re(1)–S(3) angle indicates a distortion of the trigonal bipyramid, which can also be seen in the value of the trigonality index [40], τ=0.61. The bond distances in the coordination environment of the metal are characteristic for this type of complexation [31]. The Re=O bond length is found to be 1.699(5) Å well within the range observed for other {ReO}3+ complexes [24,31,41–52]. Likewise, the Re–N bond length [Re–N(1)= 2.208(6) Å] is typical for the Re–Namine single bond in which the nitrogen atom exhibits sp3 hybridization [24,31,41–52]. However, in the case of the metal–sulfur bond lengths, those in the equatorial plane are similar [Re–S(1)=2.2794(18), Re–S(2)=2.2824(18) Å], while for the Re–S axial bond there is an expected lengthening [Re–S(3)=2.3097(19) Å] [24,31,41–52]. The rhenium atom is situated 0.0817 Å, above the equatorial plane of the trigonal bipyramid in the direction of the monothiol. This distance is within the range reported for five-coordinate Re/Tc(V) complexes with similar distorted trigonal bipyramidal geometry [31].
Fig. 1.
A view of the structure of [ReO{η3-(SCH2CH2)2N(CH2C6-H5)}(η1-C6H4Br-4-S)] (1), showing the atom-labeling scheme and 50% thermal ellipsoids.
Table 2.
Comparison of selected bond angles a for the rhenium-mixed thiolate complexes of this study
| Angle | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|
| O(1)–Re–N(1) | 96.3(2) | 93.9(2) | 93.71(13) | 93.10(18) | 94.60(14) | 94.47(17) | 96.23(14) | 97.41(10) | 97.8(2) |
| O(1)–Re–S(2) | 118.23(17) | 121.6(2) | 122.19(11) | 118.37(14) | 117.24(12) | 119.65(13) | 118.12(11) | 117.78(9) | 117.36(16) |
| N(1)–Re–S(2) | 83.31(15) | 84.01(18) | 83.97(9) | 84.09(12) | 84.50(10) | 83.11(11) | 83.58(10) | 83.57(8) | 82.86(14) |
| O(1)–Re–S(1) | 120.07(17) | 118.6(2) | 118.14(11) | 122.05(14) | 122.55(12) | 117.31(13) | 118.15(11) | 117.55(9) | 117.34(16) |
| N(1)–Re–S(1) | 83.80(15) | 83.86(18) | 83.77(9) | 83.63(12) | 83.16(10) | 83.71(11) | 83.90(11) | 83.46(7) | 83.43(14) |
| S(2)–Re–S(1) | 121.23(7) | 119.05(8) | 118.94(4) | 118.74(5) | 119.60(4) | 122.21(5) | 123.26(4) | 124.27(3) | 124.84(6) |
| O(1)–Re–S(3) | 105.66(17) | 102.8(2) | 102.86(10) | 102.89(14) | 103.53(10) | 105.62(13) | 104.07(11) | 104.65(8) | 105.22(16) |
| N(1)–Re–S(3) | 157.91(15) | 163.35(17) | 163.43(9) | 164.00(12) | 161.85(10) | 159.56(12) | 159.42(10) | 157.78(7) | 156.50(14) |
| S(2)–Re–S(3) | 84.05(7) | 87.34(8) | 87.28(4) | 88.03(5) | 87.83(4) | 90.02(5) | 83.86(4) | 88.44(3) | 82.34(6) |
| S(1)–Re–S(3) | 87.37(7) | 87.97(8) | 88.21(4) | 88.03(5) | 86.39(4) | 83.75(5) | 89.45(4) | 84.03(3) | 90.19(6) |
Angles reported in degrees with e.s.d. values in parentheses.
The compounds [ReO{η3-(SCH2CH2)2N(CH2C5-H4N)}(η1-C6H4X-4-S)] (X = Br (2), Cl (3), F (4), OCH3 (5)), [ReO{η3-(SCH2CH2)2N(CH2C5H4N)}(η1-C6H4O-CH3-4-CH2S)] (6), [ReO{η3-(SCH2CH2)2N(CH2CH2-C5H4N)}(η1-C6H4X-4-S)] (X = Br (7), Cl (8), OCH3 (9)), and [ReO{η3-(SCH2CH2)2N(CH2CH2C5H4N)}(η1-C6H4Cl-4-CH2S)]·0.5CH3(CH2)4CH3 (10) are structurally similar to 1 with the exception of the para-substituent of the benzenethiol or benzylmercaptan, as well as the substitution on the nitrogen of the tridentate. The core geometry of the five-coordinate oxo-rhenium complexes containing the tridentate ligand, [(HSCH2CH2)2N(CH2C5H4N)], is shown in Fig. 2 for [ReO{η3-(SCH2CH2)2N(CH2C5H4N)}(η1-C6H4Br-4-S)] (2) and is seen to consist of distorted trigonal bipyramidal geometry with the oxo group and the sulfur atoms of the [SNS] ligand defining the equatorial plane and the axial positions occupied by the sulfur atom of the monothiol and the nitrogen atom of the [SNS] ligand. The angles between the atoms in the equatorial plane are close to the ideal 120° (Table 2), but a value of 163.35(17)° observed for the N(1)–Re(1)–S(3) angle indicates a distortion of the trigonal bipyramid, which can also be seen in the value of the trigonality index [51], τ=0.74. The Re=O bond length is found to be 1.695(6) Å and the Re–N bond length [Re–N(1)=2.221(7) Å] is typical for the Re–Namine single bond in which the nitrogen atom exhibits sp3 hybridization. The metal–sulfur bond lengths in the equatorial plane are unexceptional [Re–S(1)=2.270(2), Re–S(2)=2.281(2) Å], while the Re–S(3) axial bond length is 2.313(2) Å, a lengthening phenomena noted by others [24,31,41–52]. The rhenium atom is situated 0.0977 Å, above the equatorial plane of the trigonal bipyramid in the direction of the monothiol.
Fig. 2.
A view of the structure of [ReO{η3-(SCH2CH2)2-N(CH2C5H4N)}(η1-C6H4Br-4-S)] (2), showing the atom-labeling scheme and 50% thermal ellipsoids.
Similarly, the core geometry of the five-coordinate oxo-rhenium complexes containing the [(HSCH2-CH2)2N(CH2CH2C5H4N)] tridentate is shown in Fig. 3 for [ReO{η3-(SCH2CH2)2N(CH2CH2C5H4N)}(η1-C6-H4Br-4-S)] (7) and is also seen to consist of distorted trigonal bipyramidal geometry. The oxo group and the sulfur atoms of the [SNS] ligand define the equatorial plane, while the axial positions are occupied by the sulfur atom of the monothiol and the nitrogen atom of the [SNS] ligand. The angles between the atoms in the equatorial plane are close to the ideal 120° (Table 2), likewise, a value of 159.42(10)° observed for the N(1)–Re(1)–S(3) angle indicates a distortion of the trigonal bipyramid, which can also be seen in the value of the trigonality index [40], τ=0.60. The Re=O and Re–Namine bond lengths are typical for this particular class of compounds and are found to be 1.694(3) and 2.217(4) Å respectively [24,31,39–49,53]. As in the previous cases, the metal–sulfur bond lengths in the equatorial plane are unexceptional [Re–S(1)= 2.2758(12), Re–S(2)=2.2918(11) Å], while the Re–S(3) axial bond length is slightly lengthened to 2.3144(11) Å. The rhenium atom is situated 0.0811 Å above the equatorial plane of the trigonal bipyramid in the direction of the monothiol.
Fig. 3.
A view of the structure of [ReO{η3-(SCH2CH2)2-N(CH2CH2C5H4N)}(η1-C6H4Br-4-S)] (7), showing the atom-labeling scheme and 50% thermal ellipsoids.
In all the oxo-rhenium benzenthiolates and benzylmercaptans (compounds 2–7, 9, 10) the equatorial plane is defined by the two sulfur donors of the tridentate [SNS] ligand and the oxo group, while the sulfur atom of the monothiol and the nitrogen atom of the [SNS] ligand occupy the axial positions. The Addison τ indices for the rhenium mixed thiolate compounds of this study are listed in Table 3, revealing that compounds 1–7, 9 and 10 actually tend to lie closer to the distorted trigonal bipyramidal structure, a result that is similar to other compounds that are members of the oxo-rhenium ‘3+1’ [SNS]/[S] mixed-thiolate family [28–31].
Table 3.
The τ index for the five-coordinate rhenium complexes of this study, as a measure of geometric distortion from idealized geometries
| Compound | αa | βb | τc |
|---|---|---|---|
| [ReO{SN(R1)S}(η1-C6H4Br-4-S)] (1) | 121.23(7) | 157.91(15) | 0.61 |
| [ReO{SN(R2)S}(η1-C6H4Br-4-S)] (2) | 119.05(8) | 163.35(17) | 0.74 |
| [ReO{SN(R2)S}(η1-C6H4Cl-4-S)] (3) | 118.94(4) | 163.43(9) | 0.74 |
| [ReO{SN(R2)S}(η1-C6H4F-4-S)] (4) | 118.74(5) | 164.00(12) | 0.75 |
| [ReO{SN(R2)S}(η1-C6H4OCH3-4-S)] (5) | 119.60(4) | 161.85(10) | 0.70 |
| [ReO{SN(R2)S}(η1-C6H4OCH3-4-CH2S)] (6) | 122.21(5) | 159.56(12) | 0.62 |
| [ReO{SN(R3)S}(η1-C6H4Br-4-S)] (7) | 123.26(4) | 159.42(10) | 0.60 |
| [ReO{SN(R3)S}(η1-C6H4OCH3-4-S)] (9) | 124.27(3) | 157.78(7) | 0.56 |
| [ReO{SN(R3)S}(η1-C6H4Cl-4-CH2S)] (10) | 124.84(6) | 156.50(14) | 0.53 |
α ≡ the second largest basal angle.
β ≡ the largest basal angle.
τ ≡ (β−α)/60. SN(R)S=η3-{(SCH2CH2)2N(R)} (R1 = CH2C6H5; R2 = CH2C5H4N; R3 = (CH2)2C5H4N).
In all cases, one equatorial position is occupied by a terminal oxo-group with a typical Re=O distance ranging from approximately 1.69–1.71 Å. The two other equatorial positions are occupied by the two sulfur donors of the [SNS] tridentate. In the complexes of this study, the displacement of Re site from the equatorial plane in the direction of the monothiol group is approximately 0.0753–0.1087 Å as shown in Table 4. The Re–Namine distances range from 2.21 to 2.23 Å while the sulfur distances are all fairly unexceptional, ranging from 2.27 to 2.32 Å well within the range of those reported previously [52–54]. Tables 2 and 4 compare the relevant bond angles and lengths for compounds 1–7, 9 and 10, respectively.
Table 4.
Comparison of selected bond lengths a for rhenium-mixed thiolate complexes
| Complex | Re=O | η1-Thiolate |
η3-Donor [S(1)–N(1)–S(2)] |
ΔReb | ||
|---|---|---|---|---|---|---|
| Re–S(3) | Re–S(1) | Re–N(1) | Re–S(2) | |||
| 1 | 1.699(5) | 2.3097(19) | 2.2794(18) | 2.208(6) | 2.2824(18) | 0.0817 |
| 2 | 1.695(6) | 2.313(2) | 2.270(2) | 2.221(7) | 2.281(2) | 0.0977 |
| 3 | 1.702(3) | 2.3087(10) | 2.2821(10) | 2.229(3) | 2.2757(10) | 0.1011 |
| 4 | 1.705(4) | 2.3074(14) | 2.2793(13) | 2.231(5) | 2.2772(14) | 0.1087 |
| 5 | 1.697(3) | 2.3119(11) | 2.2876(11) | 2.234(4) | 2.2704(11) | 0.0926 |
| 6 | 1.707(4) | 2.2983(15) | 2.2766(14) | 2.215(5) | 2.2929(14) | 0.1084 |
| 7 | 1.694(3) | 2.3144(11) | 2.758(12) | 2.217(4) | 2.2918(11) | 0.0811 |
| 9 | 1.703(2) | 2.3189(8) | 2.2889(9) | 2.220(3) | 2.3189(8) | 0.0753 |
| 10 | 1.688(5) | 2.2954(15) | 2.2875(15) | 2.226(5) | 2.2962(16) | 0.0815 |
Distances reported in Å with e.s.d. values in parentheses.
ΔRe, distance of the Re from the least square plane of the equatorial donors.
4. Conclusions
Exploitation of the thiolate chemistry of the oxo-rhenium(V) core has allowed the preparation of a series of mononuclear oxo-rhenium-mixed thiolate complexes. We have demonstrated that the action of the tridentate [SN(R)S] aminodithiol ligand on a suitable {ReO}3+ precursor in the presence of a monodentate ligand gives rise to stable, neutral oxo-rhenium complexes of the ‘3+1’ type. This route appears to be a quite general, facile method for moderate yield synthesis of materials with the [ReO{SN(R)S}(S)] core. The geometry and charge of the monodentate thiol ligand may be manipulated easily to produce complexes of various size, shape and charges for clinical development.
Acknowledgments
This work was supported by a grant from the Department of Energy, Office of Health and Environmental Research (D2-FG02-99ER62791).
Footnotes
5. Supplementary material
All atomic and thermal parameters and all interatomic angles are available from the authors upon request. Crystallographic data (excluding structure factors) for the structures reported in this paper have been deposited with the Cambridge Crystallographic Data Centre as publication Nos. CCDC 139416–139424. Copies of the data can be obtained free of charge on application to The Director, CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (fax: +44-1223-336033; deposit@ccdc.cam.ac.uk or www:http://www.ccdc.cam.ac.uk).
References
- 1.Schubiger PA, Alberto R, Smith A. Bioconjug Chem. 1996;7:165. doi: 10.1021/bc950097s. [DOI] [PubMed] [Google Scholar]
- 2.Weininger J, Ketring AR, Deutsch E, Maxon HR, Goeckler WR. J Nucl Med. 1983;24:125. [Google Scholar]
- 3.Wessels BW, Rogus RD. Med Phys. 1984;11:638. doi: 10.1118/1.595559. [DOI] [PubMed] [Google Scholar]
- 4.Yorke ED, Beaumier P, Wessels B, Fritzberg A, Morgan A. Nucl Med Biol. 1991;18:827. doi: 10.1016/0883-2897(91)90090-8. [DOI] [PubMed] [Google Scholar]
- 5.Vanderheyden JL, Fritzberg AR, Rao TN, Kasina S, Srinivasan A, Reno JM, Morgan AC. J Nucl Med. 1987;28:656. [Google Scholar]
- 6.Pietzsch HJ, Spies H, Hoffman S, Stach J. Inorg Chim Acta. 1989;161:15. [Google Scholar]
- 7.Spies H, Pietzsch HJ, Sayhe R, Hoffman S. Isotopenpraxis. 1990;26:159. [Google Scholar]
- 8.Pietzsch HJ, Spies H, Hoffman S, Scheller D. Appl Radiat Isot. 1990;41:185. [Google Scholar]
- 9.Bandoli G, Mazzi U, Pietzsch HJ, Spies H. Acta Crystallogr, Sect C. 1992;48:1422. [Google Scholar]
- 10.Fietz T, Spies H, Pietzsch HJ, Leibnitz P. Inorg Chim Acta. 1995;231:233. [Google Scholar]
- 11.Spies H, Fietz T, Pietzsch HJ, Johannsen B, Leibnitz P, Reck G, Scheller D, Klostermann K. J Chem Soc, Dalton Trans. 1995:2277. [Google Scholar]
- 12.Pelecanou M, Pirmettis IC, Nock BA, Papadopoulous MS, Chiotellis E, Stassinopoulou CI. Inorg Chim Acta. 1998;281:148. [Google Scholar]
- 13.Megalla S, Plossl K, Kung MP, Chumpradit S, Stevenson DA, Frederick D. Bioconjug Chem. 1996;7:421. doi: 10.1021/bc960029l. [DOI] [PubMed] [Google Scholar]
- 14.Megalla S, Plossl K, Kung MP, Liable-Sands LM, Rheingold AL. J Am Chem Soc. 1995;117:11037. [Google Scholar]
- 15.Mastrostamatis SG, Papadopoulos MS, Pirmettis IC, Paschali E, Varvarigou AD, Stassinopoulou CI, Raptopoulou CP, Terzis A, Chiotellis E. J Med Chem. 1994;37:3212. doi: 10.1021/jm00046a004. [DOI] [PubMed] [Google Scholar]
- 16.Pirmettis IC, Papdopoulous MS, Chiotellis E. J Med Chem. 1997;40:2539. doi: 10.1021/jm960273y. [DOI] [PubMed] [Google Scholar]
- 17.Maresca KP, Bonavia GH, Babich JW, Zubieta J. Inorg Chim Acta. 1999;284:252. [Google Scholar]
- 18.Johannsen B, Scheunemann M, Spies H, Brust P, Wober J, Syhre R, Pietzsch HJ. Nucl Med Biol. 1996;23:429. doi: 10.1016/0969-8051(96)00015-7. [DOI] [PubMed] [Google Scholar]
- 19.Skaddan MB, Katzenellenbogen JA. Bioconjug Chem. 1999;10:119. doi: 10.1021/bc980094q. [DOI] [PubMed] [Google Scholar]
- 20.Maresca KP, Femia FJ, Bonavia GH, Babich JW, Zubieta J. Inorg Chim Acta. 2000;297:98. [Google Scholar]
- 21.Pietzsch HJ, Spies H, Hoffmann S. Inorg Chim Acta. 1989;165:163. [Google Scholar]
- 22.Maresca KP, Femia FJ, Babich JW, Zubieta J. Inorg Chem Commun. 1998;1:209. [Google Scholar]
- 23.Femia FJ, Chen X, Babich JW, Zubieta J. Inorg Chim Acta. 2000;300–302:517. doi: 10.1016/S0020-1693(99)00586-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tisato F, Refosco F, Mazzi U, Bandoli G, Nicolini M. J Chem Soc, Dalton Trans. 1987:1693. [Google Scholar]
- 25.Bandoli G, Mazzi U, Wilcox B, Jurisson S, Deutsch E. Inorg Chim Acta. 1984;95:217. [Google Scholar]
- 26.Duatti A, Marchi A, Rossi R, Magon L, Deutsch E, Bertoalsi V, Bellucci F. Inorg Chem. 1988;27:4208. [Google Scholar]
- 27.Chen X, Femia FJ, Babich JW, Zubieta J. Inorg Chim Acta. 2000;306:113–116. doi: 10.1016/S0020-1693(00)00146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Papadopoulos M, Pirmettis I, Tsoukalas C, Nock B, Maina T, Raptopoulou CP, Pietzsch HJ, Friebe M, Spies H, Johannsen D, Chiotellis E. Inorg Chim Acta. 1999;295:1. [Google Scholar]
- 29.Pelecanou M, Pirmettis IC, Papadopoulos MS, Raptopoulou CP, Terzis A, Chiotellis E, Stassinopoulou CI. Inorg Chim Acta. 1999;287:142. [Google Scholar]
- 30.Pelecanou M, Pirmettis IC, Nock BA, Papadopoulos M, Chiotellis E, Stassinopoulou CI. Inorg Chim Acta. 1998;281:148. [Google Scholar]
- 31.Papadopoulous M, Tsoukalas C, Pirmettis I, Nock B, Maina T, Abedin Z, Raptopoulou CP, Terzis A, Chiotellis E. Inorg Chim Acta. 1999;285:97. [Google Scholar]
- 32.Babich JW, Ferill K, Rose DJ, Graham W, Maresca KP, Zubieta J, Fischman AJ. 43rd Meeting of the Society of Nuclear Medicine; Denver, CO. 3–6 June 1996; abstract. [Google Scholar]
- 33.Chatt J, Rowe GA. J Chem Soc. 1962:4019. [Google Scholar]
- 34.Spencer DJE, Blake AJ, Parsons S, Schröder M. J Chem Soc, Dalton Trans. 1999:1041. [Google Scholar]
- 35.Sheldrick GM. SADABS, Program for Empirical Absorption Corrections. University of Gottingen; Germany: 1996. [Google Scholar]
- 36.Sheldrick GM. SHELXL96, Program for Refinement of Crystal Structures. University of Gottingen; Germany: 1996. [Google Scholar]
- 37.Cromer DT, Waber JT. International Tables for X-ray Crystallography. IV. Kynoch; Birmingham, UK: 1974. [Google Scholar]
- 38.Creagh DC, McAuley JWJ. International Tables for X-ray Crystallography, vol C, Table 4. Kluwer Academic; Boston: 1992. [Google Scholar]
- 39.The Sadtler standard spectra. Sadtler Research Laboratories; Philadelphia, PA: 1991. [Google Scholar]
- 40.Addison AW, Rao TN, Reedijk K, Verschoor GC. J Chem Soc, Dalton Trans. 1984:1349. [Google Scholar]
- 41.Papadopoulos M, Pirmettis I, Pelecanou M. Inorg Chem. 1996;35:7377. doi: 10.1021/ic9604167. [DOI] [PubMed] [Google Scholar]
- 42.Lever SZ, Baidoo KE, Mahmood A. Inorg Chim Acta. 1990;176:183. [Google Scholar]
- 43.Davison A, Orvig C, Trop HS, Sohn M, DePamphilis BV, Jones AG. Inorg Chem. 1980;19:1988. [Google Scholar]
- 44.Bryson N, Dewan JC, Lister-James J, Davison A. Inorg Chem. 1988;27:2154. [Google Scholar]
- 45.deVries N, Jones AG, Davison A. Inorg Chem. 1991;30:2662. [Google Scholar]
- 46.Blower PJ, Dilworth JR, Hutchison JP, Nicholson T. J Zubieta, J Chem Soc, Dalton Trans. 1986:1339. [Google Scholar]
- 47.Davison A, Dephamphilis BV, Faggiani R, Jones AG, Lock CJL, Orvig C. Can J Chem. 1985;63:319. [Google Scholar]
- 48.Bandoli G, Gerber TIA. Inorg Chim Acta. 1987;126:205. [Google Scholar]
- 49.Glegg W, Boyde S, Garner CD. Acta Cyrstallogr, Sect C. 1988;44:172. [Google Scholar]
- 50.Jones AG, Davison A, LaTegola MR, Brodak JW, Orvig C, Sohn M, Toothaker AK, Lock CJL, Franklin KJ, Costello CE, Carr SA, Biemann K, Kaplan ML. J Nucl Med. 1982;23:809. [PubMed] [Google Scholar]
- 51.Smith JE, Byrne EF, Cotton FA, Sekutowski JC. J Am Chem Soc. 1978;112:5571. [Google Scholar]
- 52.Francesconi LC, Graczyk G, Wehrl S, Shaikh SN, Mc-Clinton D, Liu S, Zubieta J, Kung HF. Inorg Chem. 1993;32:3114. [Google Scholar]
- 53.Chryssou K, Pelecanou M, Papadopoulos MS, Raptopoulou CP, Pirmettis IC, Chiotellis E, Stassinopoulou CI. Inorg Chim Acta. 1998;268:169. [Google Scholar]
- 54.Rose DJ, Chang YD, Chen Q, Kettler PB, Zubieta J. Inorg Chem. 1995;34:3973. [Google Scholar]