Summary
Phosphodiesterases (PDEs) regulate the local concentration of 3′,5′ cyclic adenosine monophosphate (cAMP) within cells. cAMP activates the cAMP-dependent protein kinase (PKA). In patients, PDE inhibitors have been linked to heart failure and cardiac arrhythmias, although the mechanisms are not understood. We show that PDE4D gene inactivation in mice results in a progressive cardiomyopathy, accelerated heart failure after myocardial infarction, and cardiac arrhythmias. The phosphodiesterase 4D3 (PDE4D3) was found in the cardiac ryanodine receptor (RyR2)/calcium-release-channel complex (required for excitation-contraction [EC] coupling in heart muscle). PDE4D3 levels in the RyR2 complex were reduced in failing human hearts, contributing to PKA-hyperphosphorylated, “leaky” RyR2 channels that promote cardiac dysfunction and arrhythmias. Cardiac arrhythmias and dysfunction associated with PDE4 inhibition or deficiency were suppressed in mice harboring RyR2 that cannot be PKA phosphorylated. These data suggest that reduced PDE4D activity causes defective RyR2-channel function associated with heart failure and arrhythmias.
Introduction
Phosphodiesterases (PDEs) control the temporal and spatial dynamics of the second messenger 3′,5′ cyclic adenosine monophosphate (cAMP), allowing for highly localized cAMP gradients in cells (Zaccolo and Pozzan, 2002). Localization of PDEs in close proximity to cAMP-dependent protein kinase A (PKA) is thought to control access of cAMP to the regulatory kinase subunit (Conti et al., 2003; Houslay and Adams, 2003). PKA phosphorylation of proteins mediates a wide variety of signals, including those generated during activation of the sympathetic nervous system (SNS) as part of the “fight or flight” response. On the other hand, chronic activation of the SNS is a characteristic finding in heart failure, and acute stimulation of the SNS has been linked to triggered arrhythmias associated with sudden cardiac death.
In the heart, phosphodiesterase 4 (PDE4) contributes to the regulation of cAMP levels in cardiac myocytes. In particular, PDE4 cAMP-hydrolyzing activity has been localized to the transverse (T) tubule/sarcoplasmic reticulum (SR) junctional space that is involved in excitation-contraction coupling (Mongillo et al., 2004; Zaccolo and Pozzan, 2002). PDEs have been shown to be components of macromolecular signaling complexes via binding to targeting proteins including muscle A-kinase anchoring proteins (AKAPs) (Dodge et al., 2001). PDEs in cardiac muscle are complexed with proteins that mediate signals from SNS, including β-adrenergic receptors and β-arrestin (Mongillo et al., 2004; Perry et al., 2002; Xiang et al., 2005).
The PDE superfamily is subgrouped into 11 families that include at least 20 genes and 50 unique isoforms. Of these PDE families, only PDE4, PDE7, and PDE8 are cAMP specific (Conti et al., 2003). Through alternative splicing and the use of multiple promotors, the PDE4D gene encodes nine variants (PDE4D1–9) with identical catalytic domains and carboxyl termini and unique amino termini important for subcellular localization. For example, PDE4D3 binds to the targeting protein mAKAP via its unique N-terminal region, creating a mAKAP-PKA-PDE4D3 signaling module (Dodge et al., 2001; Tasken et al., 2001) in which PKA phosphorylation increases PDE4D3 activity approximately 2-fold (Carlisle Michel et al., 2004; Sette and Conti, 1996). Moreover, mAKAP colocalizes with the ryanodine receptor (RyR2)/calcium-release channel in cardiac muscle (Ruehr et al., 2003; Yang et al., 1998), where it is part of the RyR2 macromolecular signaling complex (Marx et al., 2000; Wehrens et al., 2003).
PKA-PDE signaling has been identified as a therapeutic target in several major diseases (Conti et al., 2003). Inhibitors of the PDE4 family are under development for asthma, chronic obstructive lung disease (COPD), cognitive disorders including Alzheimer's disease, and stroke (Gong et al., 2004; Gretarsdottir et al., 2003; Vignola, 2004). However, nonspecific PDE inhibition with theophylline, commonly used to treat asthma and COPD, and trials using PDE3 inhibition to treat heart failure have demonstrated increased mortality due to cardiac arrhythmias (Barnes, 2003; Packer et al., 1991).
We now show that PDE4D deficiency in mice is associated with a cardiac phenotype comprised of a progressive, age-related cardiomyopathy and exercise-induced arrhythmias, despite normal global cAMP signaling. Furthermore, PDE4D3 was found to be an integral component of the RyR2 macromolecular signaling complex. RyR2 located on the sarcoplasmic reticulum (SR) is the major Ca2+-release channel required for excitation-contraction coupling in heart muscle. RyR2 channels were PKA hyperphosphorylated and exhibited a “leaky” phenotype in PDE4D-deficient mice, similar to RyR2 defects observed in patients with heart failure and sudden cardiac death (SCD) (Marx et al., 2000; Wehrens et al., 2003). In failing human hearts, PDE4D3 levels were reduced in the RyR2 complex. Moreover, mice with PDE4D deficiency exhibited accelerated progression of heart failure following myocardial infarction associated with RyR2 channels that were PKA hyperphosphorylated and exhibited a “leaky” phenotype. Pharmacological PDE4 inhibition was associated with exercise-induced cardiac arrhythmias that were suppressed in mice harboring a mutation that prevents PKA phosphorylation of the RyR2 channel. Our data suggest that PDE4D deficiency may contribute to heart failure and arrhythmias by promoting defective regulation of the RyR2 channel.
Results
PDE4D Gene Inactivation Causes Age-Related Cardiomyopathy
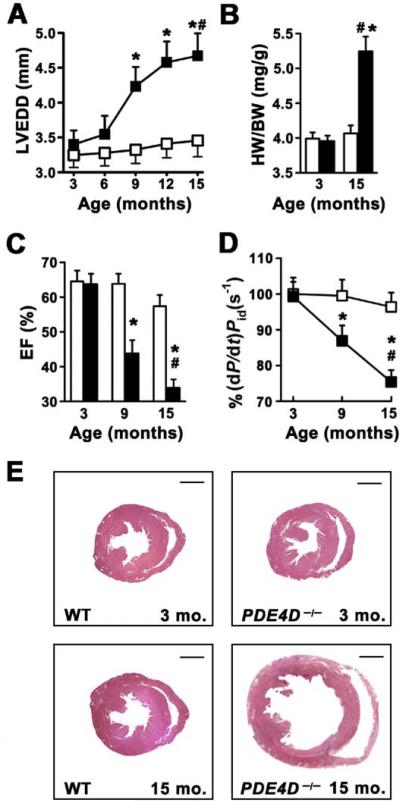
To explore the consequences of chronic PDE4D deficiency on cardiac function, we used a mouse model of PDE4D gene inactivation (Jin et al., 1999). Echocardiography of PDE4D−/− mice showed a progressive, age-dependent increase in left ventricular end-diastolic diameter (LVEDD), a hallmark of cardiac dysfunction (Figure 1A; n = 12 each for wild-type [wt] and PDE4D−/−). PDE4D−/− mice exhibited increased heart-weight-to-body-weight (HW/BW) ratios compared to wt controls (Figure 1B). PDE4D−/− mice had reduced ejection fractions (EF) and cardiac contractility (dP/dt)/Pid, documented by cardiac catheterization (Figures 1C and 1D). Histologic examination of PDE4D−/− hearts confirmed that the 15-month-old hearts were dilated, with no other structural abnormalities (Figure 1E). These data show that PDE4D deficiency is associated with progressive cardiac dysfunction consistent with a dilated cardiomyopathy similar to that seen in patients with chronic heart failure.
Figure 1. PDE4D Deficiency Promotes Age-Related Cardiomyopathy.
*p < 0.05 versus wt; #p < 0.05 versus PDE4D−/−.
(A) Echocardiography at 3 month intervals showing progressive increase in left ventricular end-diastolic diameter (LVEDD) in PDE4D−/− mice (open squares, wt; filled squares, PDE4D−/−; n = 12 each). Data in (A)–(D) are mean ± SEM.
(B) Age-dependent increase in heart-to-body-weight ratio (HW/BW) in PDE4D−/− mice (open bar, wt; filled bar, PDE4D−/−).
(C) Age-dependent decrease in ejection fraction (EF) in PDE4D−/− mice (open bar, wt; filled bar, PDE4D−/−).
(D) Reduced cardiac contractility (dP/dt/Pid) in PDE4D−/− mice at 3, 9, and 15 months of age (open squares, wt; filled squares, PDE4D−/−).
(E) Histology showing dilated cardiomyopathy in PDE4D-deficient mouse hearts.
Global cAMP Signaling Is Normal in PDE4D-Deficient Mice
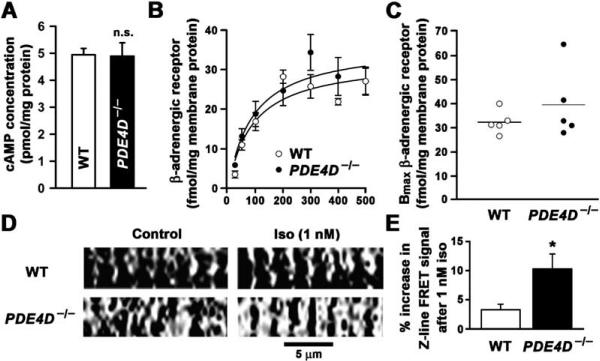
It is well established that chronic hyperadrenergic signaling is associated with heart failure. Therefore, we sought to determine whether the mechanism underlying the observed cardiac phenotype in PDE4D−/− mice was increased global cAMP signaling. However, there were no significant differences in global cAMP levels and β-adrenergic receptors in hearts from PDE4D−/− mice (Figures 2A–2C). Total cAMP-hydrolyzing activity of PDE in the heart was only slightly decreased in PDE4D−/− mice (data not shown), consistent with PDE4D activity representing only a fraction of total cytosolic PDE cAMP-hydrolyzing activity in the heart (Mongillo et al., 2004; Richter et al., 2005). However, rolipram-sensitive PDE4 activity in PDE4D−/− heart was reduced by ~50% (data not shown). Whereas global cAMP signaling was not perturbed in PDE4D-deficient mice, there was a significant increase in localized cAMP levels at the cardiomyocyte Z line (corresponding to the location of the RyR2 channel) in cardiomyocytes isolated from PDE4D−/− mice following a low dose (1 nM) of isoproterenol (Figures 2D and 2E). Thus, the abnormalities observed in cardiac function of PDE4D−/− mice must be explained by defects in localized cAMP-dependent signaling.
Figure 2. Normal cAMP and β-Adrenergic-Receptor Levels in PDE4D−/− Mouse Hearts and Increased cAMP Levels at the Z Lines Detected by FRET-PKA.
(A) cAMP concentrations were not significantly increased in hearts of 3- to 6-month-old PDE4D−/− mice (wt, n = 5; PDE4D−/−, n = 5; p = NS). Each heart was extracted and assayed separately in quadruplicate experiments. Data in (A), (B), and (E) are mean ± SD.
(B) β-adrenergic-receptor density was unchanged in PDE4D−/− mice (wt, n = 5; PDE4D−/−, n = 5; p = NS).
(C) Comparison of the Bmax values for β-adrenergic-receptor density calculated separately for each of the five wt or five PDE4D−/− knockout mice investigated.
(D) FRET-PKA showing increased cAMP-dependent signal over the Z lines (site of localization of RyR2) after low-dose isoproterenol stimulation in PDE4D−/− cardiomyocyte when compared to wild-type cardiomyocyte (white and black areas represent sensing of highest and lowest cAMP concentrations, respectively).
(E) Bar graph summarizes Z line intensity-profile analysis of 480 nm/545 nm intensity ratio from five wt and six PDE4D−/− cells (*p < 0.05). Dimensions as indicated.
PKA Phosphorylation of RyR2 in PDE4D−/− Mice
While many proteins in the heart are regulated by PKA phosphorylation and therefore can be affected by altered PDE4D gene expression, only a limited number of these PKA substrates are known to be dysregulated by PKA during heart failure. For example, it has been shown that PKA phosphorylation of phospholamban, a regulator of the SR Ca2+ uptake pump (SERCA2a), is decreased in heart failure. In contrast, of the other proteins known to be involved in regulating cardiac contractility, the SR Ca2+-release channel, RyR2, has been shown to be PKA hyperphosphorylated in heart failure (Antos et al., 2001; Marx et al., 2000; Reiken et al., 2003a, 2003b; Yano et al., 2000, 2003), although this finding has been challenged by others (Jiang et al., 2002).
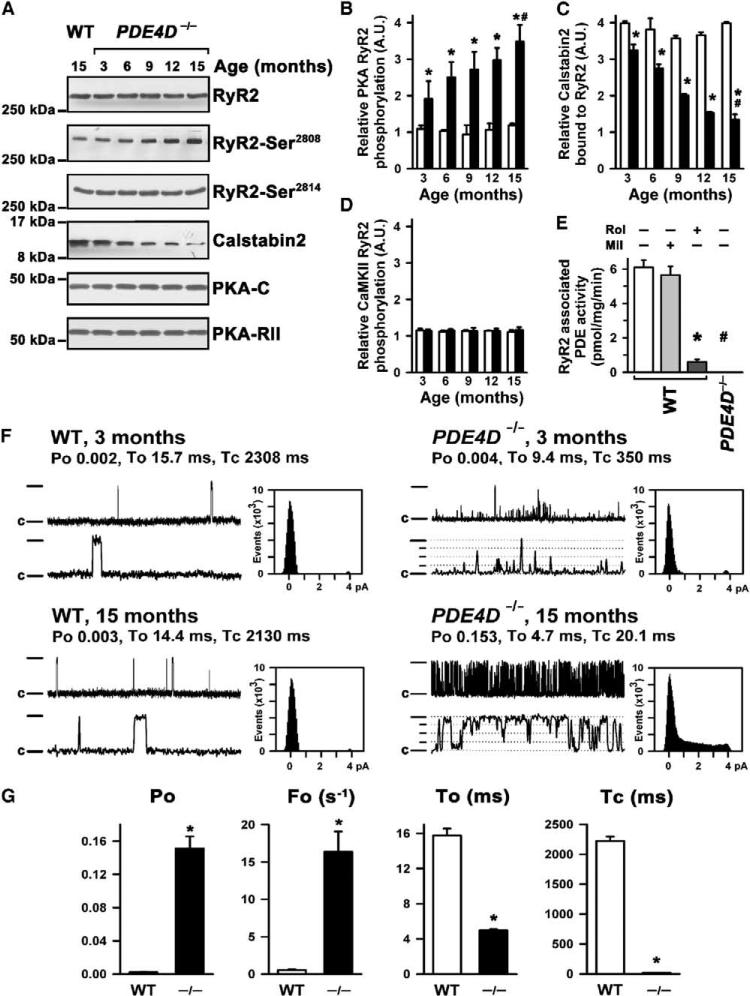
Given that RyR2 PKA hyperphosphorylation has been linked to cardiac dysfunction in humans and animal models and that cAMP concentrations are increased in the compartment of RyR2 Ca2+ release (Figures 2D and 2E), we sought to determine whether RyR2 PKA hyperphosphorylation might play a role in the observed cardiac phenotype in PDE4D−/− mice. Indeed, there was a progressive, age-dependent increase in PKA phosphorylation of RyR2 on Ser2808 (detected using a phosphoepitope-specific antibody) in PDE4D−/− mouse hearts (Figures 3A and 3B). PKA hyperphosphorylation of RyR2 in PDE4D−/− mouse hearts was associated with depletion of the RyR2-stabilizing protein calstabin2 (FKBP12.6) that prevents Ca2+ leak from the SR into the cytosol through RyR2 during diastole in the heart (Figure 3C) (Marx et al., 2000; Wehrens et al., 2003). Increased PKA phosphorylation of RyR2 was not caused by increased protein levels of PKA catalytic and regulatory subunits in the RyR2 complex (Figure 3A). Moreover, no changes in PP1 or PP2A phosphatase levels in the RyR2-complex levels were detected (data not shown). Phosphorylation of RyR2 by another kinase that phosphorylates the channel, Ca2+/calmodulin-dependent protein kinase A (CaMKII) (detected using a phosphoepitope-specific antibody), was not altered in PDE4D−/− mouse hearts (Figures 3A and 3D).
Figure 3. Age-Dependent Alterations in RyR2-Channel Complex and Function in PDE4D−/− Murine Heart.
(A) Immunoblots showing progressive increase in PKA phosphorylation of RyR2 at Ser2808 (second panel) but no change in CaMKII phosphorylation at Ser2814 (third panel) and progressive decrease in calstabin2 binding to the RyR2 complex (fourth panel). PKA catalytic (-C) and regulatory (-RII) subunits are not changed during heart failure (HF) development (fifth and six panels).
(B) Quantification of age-dependent increase in RyR2 PKA phosphorylation (open bars, wt; filled bars, PDE4D−/−; *p < 0.05 versus wt, #p < 0.05 versus PDE4D−/−). Data in (B)–(E) and (G) are mean ± SD.
(C) Quantification of age-dependent decrease in calstabin2 in the RyR2 complex (open bars, wt; filled bars, PDE4D−/−; *p < 0.05 versus wt, #p < 0.05 versus PDE4D−/−).
(D) Quantification of CaMKII-specific RyR2 phosphorylation showing no significant changes with aging.
(E) Immunoprecipitation of RyR2 showing complete absence of PDE activity in the RyR2 complex in PDE4D−/− mouse hearts. *p < 0.05 versus wt; #p < 0.05 versus untreated wt. Rol, rolipram; Mil, milrinone.
(F) Single-channel recordings of wt (left) and PDE4D−/− RyR2 (right) at 3 months of age (top) and 15 months of age (bottom). Current trace from 3-month-old PDE4D−/− heart shows slightly increased channel open probability (Po) with short openings consistent with increased PKA phosphorylation. At 15 months of age, RyR2 channels from PDE4D−/− hearts showed significantly increased Po and subconductance states (indicated by dotted lines), as evidenced by current-amplitude histograms. Upper traces represent 5 s; lower traces represent 500 ms. Channel openings are upward; full openings are 4 pA; closed state is indicated by “c.”
(G) Bar graphs summarizing Po, open frequency (Fo), average open time (To), and average closed time (Tc) at 15 months of age in wt and PDE4D−/− groups. *p < 0.001; n = 10 each.
In wt mice, PDE activity was associated with immunoprecipitated RyR2 channels (Figure 3E). RyR2-associated PDE activity was almost completely inhibited by the PDE4 antagonist rolipram but not by the PDE3 inhibitor milrinone. Moreover, PDE activity specifically associated with RyR2 channels was reduced to zero in the PDE4D−/− mice (Figure 3E). Taken together, these data suggested that one consequence of PDE4D deficiency is PKA hyperphosphorylation of RyR2, which has previously been associated with heart failure (Marx et al., 2000). Moreover, it appeared that the PDE activity associated with the RyR2 complex was encoded by the PDE4D gene.
Abnormal RyR2 Channels in PDE4D-Deficient Hearts
To ascertain the functional consequences of PKA hyperphosphorylation of RyR2 in hearts from PDE4D-deficient mice, we examined the single-channel properties of RyR2 in planar lipid bilayers. Compared to channels from wt mice, RyR2 from PDE4D−/− mice exhibited significantly increased open probability (Po) and frequency of openings (Fo) and decreased mean open and closed times when channels were examined under conditions that mimic diastole in the heart (cytosolic (cis) [Ca2+] 150 nM) (Figures 3F and 3G). Thus, PKA hyperphosphorylation of cardiac RyR2 in PDE4D-deficient mice was associated with the same defects in RyR2-channel function (“leaky” channels) previously linked to human heart failure (Marx et al., 2000) and genetically determined exercise-induced sudden cardiac death (Wehrens et al., 2003).
PDE4D3 Is a Component of the RyR2 Macromolecular Complex
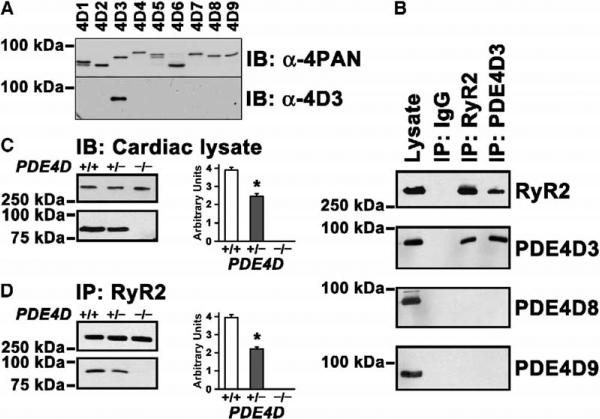
The finding that the PDE activity that coimmunoprecipitates with RyR2 was abrogated in channels from PDE4D−/− mouse hearts (Figure 3E) raised the possibility that a protein encoded by the PDE4D gene is an integral component of the RyR2 macromolecular signaling complex. Four genes constitute the type 4 phosphodiesterase family (PDE4A, PDE4B, PDE4C, and PDE4D), and all are expressed as multiple splice variants (Conti et al., 2003; Houslay and Adams, 2003). With the recent discovery of additional PDE4D variants (Gretarsdottir et al., 2003; Wang et al., 2003), a total of nine PDE4D splice variants (PDE4D1–9) are known (Richter et al., 2005). We generated an isoform-specific antibody against the unique N-terminal epitope of PDE4D3 (Figure 4A). PDE4D splice variants were identified by RT-PCR in heart (data not shown), and PDE4D3, PDE4D8, and PDE4D9 expression in the heart was demonstrated using isoform-specific antibodies (Figure 4B), confirming that these are the major PDE4D iso-forms expressed in heart muscle (Richter et al., 2005).
Figure 4. PDE4D3 Is a Component of the RyR2 Ca2+-Release-Channel Complex.
(A) PAN-PDE4 antibody against the conserved UCR2 domain (α-4PAN, top panel) and antibody raised against the N-terminal domain unique to PDE4D3 (α-4D3, bottom panel) were used to detect PDE4D isoforms in extracts of COS7 cells overexpressing recombinant PDE4D splice variants 1 to 9. Samples were size fractionated on 6% SDS-PAGE and blotted onto Immobilon-P membranes.
(B) Immunoblotting with splice-variant-specific anti-PDE4D3, anti-PDE4D8, and anti-PDE4D9 antibodies shows expression of all three major forms in the heart. However, immunoprecipitation of RyR2 followed by splice-variant-specific immunoblot demonstrates that only PDE4D3 is associated with RyR2. Reverse immunoprecipitation with a specific anti-PDE4D3 antibody confirms that PDE4D3 is physically associated with RyR2.
(C) Immunoblots of cardiac lysates showing amounts of RyR2 and PDE4D3 in wt, PDE4D+/−, and PDE4D−/− mice. Bar graph shows a 37% reduction of PDE4D3 in the RyR2 complex in cardiac lysates of PDE4D+/− mice relative to wt. *p < 0.05, n = 3 for each genotype. Data in (C) and (D) are mean ± SD.
(D) Coimmunoprecipitation using anti-RyR2 antibody, showing a 44% decrease of PDE4D3 bound to RyR2 in PDE4D+/− mouse hearts relative to wt. *p < 0.05, n = 3 for each genotype.
To examine the possibility that a specific PDE4D isoform is part of the cardiac RyR2 channel complex, immunoprecipitations were performed using human heart extracts. RyR2 channels were immunoprecipitated with anti-RyR2 antibody and assayed for coimmunoprecipitation of PDE4D3, PDE4D8, or PDE4D9 with RyR2 by immunoblotting. Using isoform-specific PDE4D antibodies, only PDE4D3 was detected in the RyR2 complex. In addition, anti-PDE4D3 antibody was used to coimmunoprecipitate RyR2 (Figure 4B). The interaction between PDE4D3 and RyR2 was specific because PDE4D3 was excluded from immunoprecipitates using control IgG (Figure 4B). Moreover, we used the PDE4D3-specific antibody to show that total PDE4D3 protein was decreased ~37% in haploinsufficient PDE4D+/− mouse hearts and by 100% in homozygous PDE4D−/− hearts (Figure 4C). Finally, PDE4D3 in the RyR2 macromolecular signaling complex was also decreased by ~44% in PDE4D+/− mouse hearts and by 100% in PDE4D−/− heart (Figure 4D). Taken together, these data show that PDE4D3 is an integral component of the RyR2 macromolecular complex in the heart and that PDE4D3 is the only PDE isoform in the RyR2 complex.
PDE4D3 Is Decreased in the RyR2 Complex in Failing Human Hearts
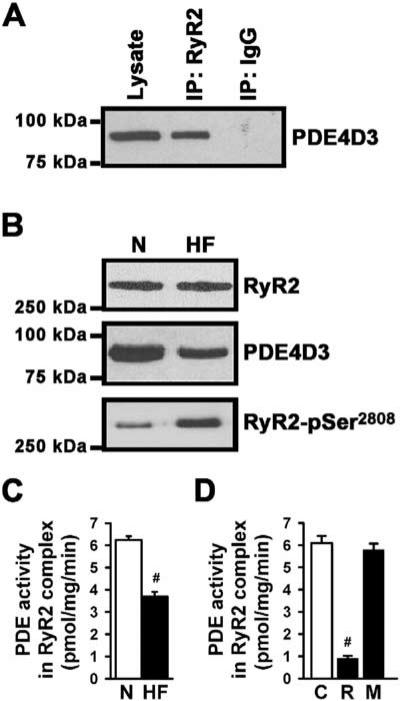
PDE4D3 was also associated with RyR2 from human hearts (Figure 5A). In human heart failure (HF), PDE4D3 levels in the RyR2 complex were decreased by 43% (normal, n = 6 versus HF, n = 9; p < 0.001), and the RyR2 channels in the human HF samples were PKA hyperphosphorylated (Figure 5B). We have previously shown that PKA hyperphosphorylation of RyR2 depletes calstabin2 from the RyR2 complex and significantly increases channel activity, consistent with a diastolic SR Ca2+ leak in human heart failure (Marx et al., 2000; Reiken et al., 2003a). The cAMP-hydrolyzing activity of RyR2 bound PDE4D3 was decreased by 42% in human HF samples (n = 6, each experiment was performed in triplicate; p < 0.001), providing a possible explanation for chronic RyR2-Ser2808 PKA hyperphosphorylation observed in failing human hearts (Figure 5C). This reduction in PDE4D3 activity in the human HF RyR2 complex was comparable to that observed in the RyR2 complexes in PDE4D+/− mice (Figure 4D). To explore the basis for the observed reduction in PDE4D3 amount and activity in the HF RyR2 complex, we examined PKA phosphorylation of PDE4D3, which has been shown to increase its activity (Sette and Conti, 1996) and binding to mAKAP (Carlisle Michel et al., 2004). We observed a ~40% reduction in PKA phosphorylation of PDE4D3 in the human HF RyR2 complexes compared to nonfailing controls (n = 3, p < 0.01), providing a possible explanation for the observed decrease in amount and activity of PDE4D3 in the HF RyR2 complexes (data not shown).
Figure 5. Reduced PDE4D3 in the RyR2 Complex in Human Heart Failure.
(A) PDE4D3 was detected in human cardiac lysate and in the immunoprecipitated RyR2 complex (IP:RyR2); IP:IgG, negative control.
(B) RyR2 was immunoprecipitated from cardiac homogenates of normal human (N) and heart failure (HF) samples. PDE4D3 binding to RyR2 was significantly decreased in human HF. Increased PKA phosphorylation was detected by a phosphoepitope-specific RyR2-Ser2808 antibody in HF samples.
(C) RyR2 bound PDE4D3 activity was significantly decreased in HF, as evidenced by close-proximity substrate cAMP catalysis (#p < 0.001). Data in (C) and (D) are mean ± SD.
(D) Rolipram (R), a PDE4-specific inhibitor, but not milrinone (M), a PDE3-specific inhibitor, decreased RyR2-associated PDE activity submaximally; C, control (#p < 0.001 versus untreated sample).
PDE4-specific inhibition with rolipram (10 μM) significantly decreased RyR2 bound PDE4D3 activity in normal human heart lysates (n = 3, p < 0.01), whereas the PDE3-specific inhibitor milrinone (10 μM) had no effect on RyR2-associated PDE activity (Figure 5D), confirming that the cAMP-hydrolyzing activity in the RyR2 complex is due to PDE4. Thus, PDE4D3 is part of the human RyR2 signaling complex, and reduction of PDE4D3 activity in heart failure may contribute to RyR2 PKA hyperphosphorylation and diastolic SR Ca2+ leak observed in failing hearts (Shannon et al., 2003).
Cardiac Arrhythmias due to PDE4 Inhibition Are Suppressed in Mice Harboring RyR2 that Cannot be PKA Phosphorylated
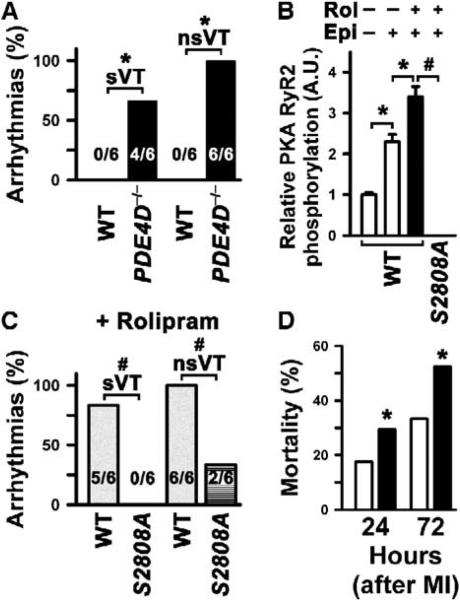
We have previously demonstrated a link between PKA hyperphosphorylation of RyR2, “leaky” RyR2 channels, and exercise-induced sudden cardiac death (Wehrens et al., 2003). Therefore, in the present study, we sought to determine whether PDE4D-deficient mice, which exhibit PKA-hyperphosphorylated RyR2, are more susceptible to exercise-induced cardiac arrhythmias. Resting heart rate in PDE4D−/− mice at 3–4 months of age was similar to wt, consistent with unchanged baseline sympathetic activity (wt 584 ± 22 bpm, PDE4D−/− 603 ± 32 bpm; p = NS). Since PDE inhibitors increase arrhythmogenic sudden cardiac death (Barnes, 2003; Packer et al., 1991), we tested the susceptibility of PDE4D−/− mice to cardiac arrhythmias during exercise followed by low-dose epinephrine injection (0.1 mg/kg) using a previously established protocol (Wehrens et al., 2003, 2004). Exercise-induced sustained (sVT) and nonsustained ventricular arrhythmias (nsVT) were observed in 66% and 100% of PDE4D−/− mice, respectively, but in none of the wt mice (Figure 6A; each n = 6, p < 0.01).
Figure 6. Cardiac Arrhythmias due to PDE4D3 Inhibition Are Suppressed in Mice Harboring Mutant RyR2 that Cannot be PKA Phosphorylated.
(A) Susceptibility to exercise-induced sustained ventricular arrhythmias (sVT) and nonsustained ventricular arrhythmias (nsVT) was significantly increased in PDE4D−/− compared to wt mice (each n = 6, *p < 0.05).
(B) Rolipram (0.3 mg/kg body weight) maximally increased RyR2-Ser2808 PKA phosphorylation during exercise in vivo. Treatment as indicated on top: Rol, rolipram; Epi, epinephrine (0.1 mg/kg); white bars, no rolipram; black bar, rolipram-treated mice; *p < 0.05 between treatments in wt mice; #p < 0.001 wt versus homozygous RyR2-S2808A knockin (S2808A+/+) mice. Control mice were treated with placebo (rolipram carrier, 0.5% DMSO). Data are mean ± SD.
(C) In rolipram-treated mice, susceptibility to exercise-induced sustained ventricular arrhythmias (sVT) and nonsustained ventricular arrhythmias (nsVT) was significantly decreased in homozygous RyR2-S2808A knockin (S2808A+/+) mice compared to wt mice (each n = 6, #p < 0.05).
(D) Mortality from sudden death was significantly increased in PDE4D+/− mice 24 and 72 hr after MI compared to wt mice. White bars, wt mice; black bars, PDE4D+/− mice. *p < 0.01 between wt and PDE4D+/− groups.
In order to investigate whether a diastolic SR Ca2+ leak due to PKA hyperphosphorylation of RyR2 directly contributes to a cardiac phenotype in PDE4D−/− mice, we treated wt mice with the PDE4 inhibitor rolipram. Rolipram (0.3 mg/kg) inhibited cAMP-hydrolyzing PDE activity in the RyR2 complex in wt mice (data not shown) and resulted in significantly increased RyR2 PKA phosphorylation during exercise (Figure 6B). Following exercise and epinephrine injection (0.1 mg/kg), ventricular arrhythmias or sudden death occurred in 100% of rolipram-treated wt mice (Figure 6C). Importantly, RyR2-S2808A knockin mice, which express a mutant RyR2 that cannot be PKA phosphorylated (Figure 6B), were protected against rolipram-induced exercise-triggered arrhythmias (Figure 6C). These findings indicate that the proarrhythmogenic effects of PDE4 inhibition are specifically due to PKA hyperphosphorylation of RyR2 at Ser2808. Thus, PKA phosphorylation of RyR2 at Ser2808 is necessary in order to generate triggered cardiac arrhythmias associated with PDE4 inhibition. Moreover, mortality due to sudden cardiac death at 24 and 72 hr following myocardial infarction (MI, induced by ligation of the left anterior descending artery) was significantly increased in PDE4D+/− compared to wt mice (Figure 6D), further suggesting that PDE deficiency in the RyR2-channel complex increases susceptibility to cardiac arrhythmias.
Exacerbation of Acute Heart Failure Associated with PDE4D3 Deficiency Is Attenuated in Mice Harboring RyR2 that Cannot be PKA Phosphorylated
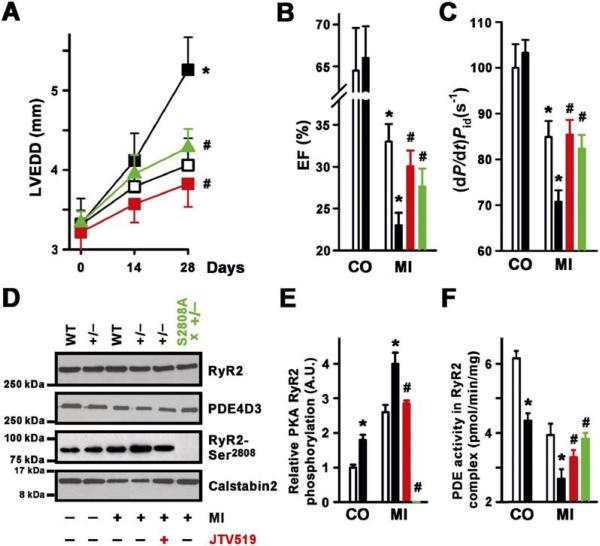
Since PDE4D3 protein levels and cAMP-hydrolyzing activity in the RyR2 complex were reduced by 42% and 43% in human heart failure, respectively, we examined whether a partial reduction of PDE4D in haploinsufficient PDE4D+/− mice affects progression of HF. Heterozygous PDE4D+/− and wt control mice were subjected to proximal left anterior descending (LAD) coronary artery ligation to induce myocardial infarction (MI), which results in progressive heart failure. Similar to human heart failure (Figure 5B), PDE4D+/− mice had a 44% reduction of PDE4D3 bound to the RyR2 complex as compared to control (Figure 4D) and developed significantly worse heart failure manifested by a larger increase in cardiac dimensions and more depressed cardiac contractility over a 28 day post-MI period (Figure 7). Cardiac dimensions (LVEDD) were 30% larger in PDE4D+/− hearts compared to wt 28 days post-MI (Figure 7A), consistent with more severe cardiomyopathy. Infarct sizes were not significantly different in 4- to 5-month-old wt (35.8% ± 3.1% LV, n = 11) and PDE4D+/− mice (37.2% ± 3.7% LV, n = 14). Cardiac function, measured by echocardiography and cardiac catheterization, was reduced in haploinsufficient PDE4D+/− mice compared with wt mice following MI (Figures 7B and 7C). Accelerated HF progression in PDE4D+/− mice was associated with enhanced RyR2 PKA hyperphosphorylation and reduced PDE4D3 protein levels in the RyR2 complex (Figures 7D and 7E) and significantly reduced PDE4D3 enzymatic activity in the RyR2 complex (Figure 7F). In the RyR2 complex, PKA catalytic and regulatory subunits, as well as the levels of the protein phosphatases PP1 and PP2A, were not significantly different between wt and PDE4D+/− hearts (data not shown). Thus, reduction of PDE4D3 activity in the RyR2 complex in haploinsufficient PDE4D+/− mice to levels similar to those observed in RyR2 complexes from failing human hearts results in accelerated progression of heart failure.
Figure 7. PDE4D3 Deficiency Promotes HF Progression.
(A) LVEDD increased in PDE4D+/− (black squares) compared to wt (open squares) mice before (control, CO) and 14 and 28 days after myocardial infarction (MI) (*p < 0.05 versus wt). Both treatment with the 1,4-benzothiazepine JTV-519 (red line), which enhances calstabin2 binding to RyR2, or crossing the PDE4D+/− mice with RyR2-S2808A mice that harbor RyR2 that cannot be PKA phosphorylated (green line) significantly reduced the remodeling of the left ventricle following MI (#p < 0.01 versus PDE4D+/−). Data in (A)–(C) are mean ± SEM. (B) Reduced cardiac EF in PDE4D+/− mice 28 days after MI. wt, open bars; PDE4D+/− mice, filled bars (*p < 0.05 versus CO same genotype). Both treatment with the 1,4-benzothiazepine JTV-519 (red bar), which enhances calstabin2 binding to RyR2, or crossing the PDE4D+/− mice with RyR2-S2808A mice that harbor RyR2 that cannot be PKA phosphorylated (green bar) significantly improved left ventricular EF following MI (#p < 0.01 versus PDE4D+/− MI).
(C) Left ventricular contractility (dP/dt)/Pid normalized to 100% of control in wt (open bars) and haploinsufficient PDE4D+/− mice (black bars) before (control, CO) and 28 days after MI (*p < 0.05 versus CO in same genotype). Treatment with JTV-519 (red bar) or crossing the PDE4D+/− mice with RyR2-S2808A mice (green bar) significantly improved contractility following MI (#p < 0.01 versus PDE4D+/− MI).
(D) Immunoblot showing levels of PDE4D3, calstabin2, and RyR2-Ser2808 PKA phosphorylation in the immunoprecipitated RyR2-channel complex. JTV-519 treatment (red) or crossing the PDE4D+/− mice with RyR2-S2808A mice (green) significantly increased the binding of calstabin2 to RyR2 in the PDE4D-deficient mice.
(E) Quantification of RyR2 PKA phosphorylation in wt and PDE4D+/− mice before (CO) and 28 days after MI, showing significantly more RyR2 PKA hyperphosphorylation in PDE4D+/− mice (*p < 0.05 versus wt at same time point). Treatment with JTV-519 (red bar) or crossing the PDE4D+/− mice with RyR2-S2808A mice (green bar) significantly reduced RyR2 PKA phosphorylation following MI (#p < 0.01 versus PDE4D+/− MI). Data in (E) and (F) are mean ± SD.
(F) Quantification of RyR2-associated PDE activity showing significant decrease in PDE4D+/− mice 28 days before (CO) and after MI compared to wt (*p < 0.05 versus wt). Treatment with JTV-519 (red bar) or crossing the PDE4D+/− mice with RyR2-S2808A mice (green bar) significantly increased RyR2-associated PDE activity following MI (#p < 0.01 versus PDE4D+/− MI).
We next set out to determine whether the detrimental effects of PDE4D deficiency in the heart were dependent on dysfunction of the RyR2-channel complex due to PKA hyperphosphorylation and reduced binding of calstabin2. Since recent studies have demonstrated that the 1,4-benzothiazepine JTV-519 increases the binding of calstabin2 to RyR2 in vivo, we treated PDE4D+/− mice subjected to myocardial infarction with JTV-519. Treatment with JTV-519 (symbols) significantly increased the amount of calstabin2 bound to RyR2 (Figure 7D) and was associated with improved cardiac function following MI (Figures 7B and 7C). We also crossed the PDE4D+/− mice with RyR2-S2808A mice to investigate the specific role of PKA hyperphosphorylation of RyR2 in the development of cardiac dysfunction in PDE4D+/− mice. RyR2-S2808A mice harbor RyR2 that cannot be PKA phosphorylated (Figure 7D). Prevention of PKA hyperphosphorylation improved cardiac function in PDE4D+/− mice subjected to MI (Figures 7A and 7C, green line and bars). Infarct sizes were not significantly different between PDE4D+/− mice, PDE4D+/− mice treated with JTV-519 (38.6% ± 3.9% LV, n = 12), or PDE4D+/− mice crossed with RyR2-S2808A mice (39.8% ± 4.3% LV, n = 11). These data demonstrate that normalizing RyR2 function, either by enhancing calstabin2 binding to RyR2 or by preventing PKA hyperphosphorylation of RyR2, improved cardiac function in PDE4D+/− mice following myocardial infarction. Taken together, these data suggest that the cardiac defects observed in PDE4D-deficient mice are due, at least in part, to defective RyR2 function.
Discussion
The present study shows that phosphodiesterase (PDE4D) deficiency is associated with a severe cardiac phenotype consisting of heart failure and lethal cardiac arrhythmias. The importance of this cardiac phenotype in PDE4D-deficient mice is underscored by the fact that it is similar to that observed in humans with heart failure: decreased cardiac function and increased susceptibility to cardiac arrhythmias. Moreover, PDE inhibition has been associated with increased mortality in patients with heart failure (Packer et al., 1991), arrhythmias, and sudden cardiac death (Bittar and Friedman, 1991; Suissa et al., 1996), although the mechanism has been unknown. Finally, since PDE4 inhibitors are being tested in clinical trials to treat common chronic diseases including Alzheimer's disease (Gong et al., 2004), asthma, and COPD (Giembycz, 2002), it is important to understand the consequences of long-term inhibition of PDE4 activity in the heart, where PDE4 activity plays a major role in regulating cAMP-dependent signals (Perry et al., 2002; Verde et al., 1999; Xiang et al., 2005).
Since the PDE4D deficiency caused no detectable alteration in global cAMP levels or β-adrenergic signaling in the heart, the cardiac phenotype in PDE4D−/− mice must be due to abnormalities in localized signaling, i.e., altered microdomains of cAMP, which is supported by our FRET imaging data showing increased cAMP concentrations at the Z lines of PDE4D-deficient cardiomyocytes (where RyR2 is present) after physiologic stimulation of β-adrenergic receptors. Indeed, the concept that localized signaling regulates cAMP in the heart is supported by previous findings showing that PDE4 is a localized regulator of β-adrenergic receptor (β2-AR) signaling in cardiomyocytes (Baillie et al., 2003; Mongillo et al., 2004; Perry et al., 2002; Xiang et al., 2005). Moreover, it has been shown that PDE4D3 can be targeted to specific compartments including the cardiomyocyte Z line via the targeting protein mAKAP (Carlisle Michel et al., 2004; Dodge et al., 2001; Sette and Conti, 1996; Yang et al., 1998). Our experiments were carefully designed to examine differences in cAMP levels at the Z line in cardiomyocytes from wt versus PDE4D-deficient mice using low-dose β-adrenergic stimulation (1 nM isoproterenol). Others have shown that nonspecific pharmacologic PDE inhibition using maximal β-adrenergic stimulation causes cAMP spillover into different compartments (Zaccolo and Pozzan, 2002).
There are likely many changes in cAMP-dependent signaling in the hearts of PDE4D-deficient mice. For example, receptor-stimulated β-arrestin-mediated recruitment of PDE4 regulates G protein switching by the β2-AR in cardiomyocytes (Baillie et al., 2003). Moreover, PDE4D is an integral component of the β2-AR signaling complex (Xiang et al., 2005). Loss of other PDE4D isoforms (e.g., PDE4D8 and PDE4D9) likely also contributes to localized alterations in cAMP levels in PDE4D−/− cardiomyocytes (Richter et al., 2005). Our work shows that PKA phosphorylation of RyR2, which occurs at an early stage, before any structural or functional abnormalities were observed in PDE4D−/− mouse hearts (Figure 3), is a critical event since crossing the PDE4D-deficient mice with RyR2-S2808A mice inhibits the development of the cardiac phenotype (Figures 6 and 7).
Indeed, there are at least two lines of evidence that strongly suggest that the cardiac phenotype in PDE4D-deficient mice is due to defects related to PKA hyperphosphorylation of RyR2 and the resulting abnormal regulation of this channel required for EC coupling in the heart. First, the levels of PDE4D3 in RyR2-channel complexes from failing human hearts are reduced to the same degree as in RyR2 complexes in the hearts of PDE4D+/− mice, which develop accelerated heart failure following myocardial infarction. This suggests that PDE4D deficiency in the RyR2 complex may play a role in PKA hyperphosphorylation of the channel and the associated cardiomyopathy. Second, and more importantly, sustained cardiac arrhythmias associated with pharmacologic PDE4 inhibition and accelerated progression of heart failure following MI were not observed in RyR2-S2808A mice harboring a RyR2 that cannot be PKA phosphorylated (Figures 6 and 7). Thus, the present study indicates that the cardiac phenotype in PDE4D-deficient mice is directly related to defective regulation of RyR2 and provides support for the model whereby PKA hyperphosphorylation of RyR2 causes a diastolic SR Ca2+ leak that (1) depletes SR Ca2+, contributing to decreased cardiac function (Marx et al., 2000), and (2) may provide a trigger for fatal cardiac arrhythmias (Wehrens et al., 2003).
Since the role of PKA hyperphosphorylation of RyR2 in heart failure (Jiang et al., 2002) has been challenged, it is important to establish a mechanism underlying the PKA hyperphosphorylation of RyR2 and to show that this defect can specifically account for the observed cardiac phenotype. We now show that PDE4D3 deficiency in the RyR2 complex contributes to PKA hyperphosphorylation of RyR2 in human and animal heart failure. Furthermore, a mutant RyR2 that cannot be PKA phosphorylated (RyR2-S2808A) protects against the cardiac effects of PDE4D3 deficiency in the RyR2 complex in vivo. Indeed, the finding that PDE4D3 is decreased in RyR2 complexes in human heart failure helps address one of the controversial issues in this field, the mechanism whereby RyR2 become PKA hyperphosphorylated (Marx et al., 2000) despite decreases in global cAMP levels in failing human hearts (Regitz-Zagrosek et al., 1994).
The current findings suggest a novel function of PDE4D3 in the regulation of RyR2, the major intracellular Ca2+-release channel in the heart. PDE4D3 activity provides an important negative-feedback mechanism to limit β-AR-dependent PKA phosphorylation of RyR2-Ser2808. Under physiologic conditions, PDE4D3 may regulate local PKA activity and channel activation via phosphorylation of RyR2-Ser2808, thereby preventing excess accumulation of cAMP (Zaccolo and Pozzan, 2002) and uncontrolled PKA-mediated activation of the channel. In human heart failure, loss of negative feedback due to PDE4D3 deficiency in the RyR2 complex likely contributes to RyR2 PKA hyperphosphorylation; calstabin2 depletion; and hyperactive, “leaky” RyR2 channels (Marx et al., 2000; Pieske et al., 1999). Taken together, our data suggest that PDE4D3 plays a protective role in the heart against heart failure and arrhythmias.
These data further suggest that chronic pharmacologic PDE4 inhibition could contribute to a cardiac phenotype including cardiac dysfunction and arrhythmias, particularly in individuals with underlying cardiac disease. In addition, other signaling systems may be affected by reduced PDE4D activity, e.g., β-arrestin targeting of PDE4D3 activity may be important for β2-AR desensitization (Perry et al., 2002). Importantly, PDE4D3 deficiency and pharmacologic PDE4 inhibition with rolipram was associated with stress-induced cardiac arrhythmias, which did not occur in mice lacking the RyR2 PKA phosphorylation site at Ser2808. These findings suggest that PDE4 inhibitors could increase the risk of cardiac arrhythmias due to “leaky” RyR2 channels as observed in individuals with genetic forms of sudden cardiac death linked to RyR2 mutations (Lehnart et al., 2004; Wehrens et al., 2003) and in patients with heart failure.
Experimental Procedures
PDE4D–/– Mice and RyR2-S2808A Knockin Mice
PDE4D–/– mice were generated and genotyped as described (Jin et al., 1999). RyR2-S2808A knockin mice (see the Supplemental Data available with this article online for details), generated using homologous recombination, exhibited normal cardiac structure and function, and no PKA phosphorylation of RyR2 was detected using a kinasing reaction with [γ-32P]ATP or with a phosphoepitope-specific antibody that detects PKA-phosphorylated RyR2.
Transthoracic Echocardiography and In Vivo Hemodynamic Analyses on Mice
Transthoracic 2D echocardiography and in vivo hemodynamic analyses on mice were performed as previously described (Wehrens et al., 2005) (see Supplemental Data for details). All animal studies were performed according to protocols approved by the Institutional Animal Care and Use Committee of Columbia University and according to NIH guidelines.
Myocardial Infarct Model
PDE4D–/– and age-and-litter-matched wild-type mice (4 to 5 months old) were anesthetized with 1.5% isoflurane and ventilated with a small-rodent respirator (Harvard Apparatus). A left thoracotomy was performed, and the left anterior descending artery (LAD) was ligated proximally with an 8-0 suture as described (Wehrens et al., 2005).
Exercise Testing and Mouse ECG Recording
Ambulatory ECG recordings were performed using implantable radiotelemetry transmitters (DSI) as described (Wehrens et al., 2004). For the pharmacological experiments performed in wt and RyR2-S2808A knockin mice, animals were pretreated for 30 min by intraperitoneal injection with the PDE4 inhibitor rolipram (0.3 mg/kg) or placebo (DMSO 0.5% as carrier) followed by the exercise protocol described above.
β-Adrenergic-Receptor Measurements
β-adrenergic-receptor levels were assessed as previously described (Reiken et al., 2003b) (see Supplemental Data for details).
Immunoprecipitation and Immunoblot Analysis
RyR2 channels were immunoprecipitated and immunoblotted as previously described (Marx et al., 2000) (see Supplemental Data for details).
Phosphodiesterase Activity Assay
Phosphodiesterase (PDE) activity was measured using selective binding of 5′-AMP to yttrium silicate beads with embedded scintillant. Immunoprecipitated RyR2 complexes were incubated with 50 nM 3H-cyclic nucleotide (Amersham, 5–60 Ci/mM) in 50 mM TrisHCl (pH 7.5), 8.3 mM MgCl2, 1.7 mM EGTA, BSA 0.01% at 30°C for 30 min. The reaction was terminated by adding one-third of 5 mg/ml yttrium silicate beads in 18 mM Zn acetate/Zn sulfate solution (3:1). After 30 min, hydrolysis was quantified by a scintillation counter (Wallac 1409, PerkinElmer).
FRET-PKA Assay
Local intracellular cAMP concentrations were determined in murine cardiomyocytes using fluorescence resonance energy transfer (FRET) between the cyan (CFP) and yellow (YFP) variants of green fluorescent protein as described (Zaccolo and Pozzan, 2002) (see Supplemental Data for details).
Back Phosphorylation of PDE4D3
PKA phosphorylation of PDE4D3 was assessed using a kinasing reaction on PDE4D3 immunoprecipitated from 100 μg of human cardiac homogenates (see Supplemental Data for details).
Single-Channel Recordings
RyR2 single channels were recorded in planar lipid bilayers as previously described (Marx et al., 2000). Symmetrical solutions used were (in mM) trans-HEPES 250 and Ca(OH)2 53 (pH 7.35) and cis-HEPES 250, Tris 125, EGTA 1.0, and CaCl2 0.5 (pH 7.35). Free Ca2+ concentrations were calculated by CHELATOR software. At the conclusion of each experiment, ryanodine (5 μM) or ruthenium red (20 μM) was applied to confirm RyR2-channel identity.
PKA Phosphorylation of Cardiac Ryanodine Receptors
PKA phosphorylation of RyR2 was assessed as previously described (Marx et al., 2000) (see Supplemental Data for details).
Statistical Analysis
Data are reported as mean ± SEM for in vivo experiments and mean ± SD for biochemical studies. Differences between multiple experimental groups were compared by analysis of variance (ANOVA) followed by Tukey's multiple comparison test. Analysis between two groups was performed by t test (paired or unpaired as appropriate). Serial studies were tested by repeated-measure ANOVA. A value of p < 0.05 was considered significant.
Supplementary Material
Acknowledgments
This work was supported by grants to A.R.M. from the NHLBI. A.R.M. is the Doris Duke Charitable Foundation Distinguished Clinical Scientist. S.E.L. and X.H.T.W. are supported by AHA Scientist Development grants. M.C. was supported by NIH-RO1-HD20788. R.D.H. was supported by NIH-RO1-HL68170. We thank Dr. Richard Axel for providing mouse ES cells for the generation of the RyR2-S2808A mice. M.C. is a consultant for Pfizer.
Footnotes
Supplemental Data Supplemental Data include Supplemental Experimental Procedures and Supplemental References and can be found with this article online at http://www.cell.com/cgi/content/full/123/1/25/DC1/.
References
- Antos CL, Frey N, Marx SO, Reiken S, Gaburjakova M, Richardson JA, Marks AR, Olson EN. Dilated cardiomyopathy and sudden death resulting from constitutive activation of protein kinase a. Circ. Res. 2001;89:997–1004. doi: 10.1161/hh2301.100003. [DOI] [PubMed] [Google Scholar]
- Baillie GS, Sood A, McPhee I, Gall I, Perry SJ, Lefkowitz RJ, Houslay MD. beta-Arrestin-mediated PDE4 cAMP phosphodiesterase recruitment regulates beta-adrenoceptor switching from Gs to Gi. Proc. Natl. Acad. Sci. USA. 2003;100:940–945. doi: 10.1073/pnas.262787199. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Barnes PJ. Theophylline: new perspectives for an old drug. Am. J. Respir. Crit. Care Med. 2003;167:813–818. doi: 10.1164/rccm.200210-1142PP. [DOI] [PubMed] [Google Scholar]
- Bittar G, Friedman HS. The arrhythmogenicity of theophylline. A multivariate analysis of clinical determinants. Chest. 1991;99:1415–1420. doi: 10.1378/chest.99.6.1415. [DOI] [PubMed] [Google Scholar]
- Carlisle Michel JJ, Dodge KL, Wong W, Mayer NC, Langeberg LK, Scott JD. PKA-phosphorylation of PDE4D3 facilitates recruitment of the mAKAP signalling complex. Biochem. J. 2004;381:587–592. doi: 10.1042/BJ20040846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti M, Richter W, Mehats C, Livera G, Park JY, Jin C. Cyclic AMP-specific PDE4 phosphodiesterases as critical components of cyclic AMP signaling. J. Biol. Chem. 2003;278:5493–5496. doi: 10.1074/jbc.R200029200. [DOI] [PubMed] [Google Scholar]
- Dodge KL, Khouangsathiene S, Kapiloff MS, Mouton R, Hill EV, Houslay MD, Langeberg LK, Scott JD. mAKAP assembles a protein kinase A/PDE4 phosphodiesterase cAMP signaling module. EMBO J. 2001;20:1921–1930. doi: 10.1093/emboj/20.8.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giembycz MA. Development status of second generation PDE4 inhibitors for asthma and COPD: the story so far. Monaldi Arch. Chest Dis. 2002;57:48–64. [PubMed] [Google Scholar]
- Gong B, Vitolo OV, Trinchese F, Liu S, Shelanski M, Arancio O. Persistent improvement in synaptic and cognitive functions in an Alzheimer mouse model after rolipram treatment. J. Clin. Invest. 2004;114:1624–1634. doi: 10.1172/JCI22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gretarsdottir S, Thorleifsson G, Reynisdottir ST, Manolescu A, Jonsdottir S, Jonsdottir T, Gudmundsdottir T, Bjarnadottir SM, Einarsson OB, Gudjonsdottir HM, et al. The gene encoding phosphodiesterase 4D confers risk of ischemic stroke. Nat. Genet. 2003;35:131–138. doi: 10.1038/ng1245. [DOI] [PubMed] [Google Scholar]
- Houslay MD, Adams DR. PDE4 cAMP phosphodiesterases: modular enzymes that orchestrate signalling cross-talk, desensitization and compartmentalization. Biochem. J. 2003;370:1–18. doi: 10.1042/BJ20021698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang MT, Lokuta AJ, Farrell EF, Wolff MR, Haworth RA, Valdivia HH. Abnormal Ca2+ release, but normal ryanodine receptors, in canine and human heart failure. Circ. Res. 2002;91:1015–1022. doi: 10.1161/01.res.0000043663.08689.05. [DOI] [PubMed] [Google Scholar]
- Jin SL, Richard FJ, Kuo WP, D'Ercole AJ, Conti M. Impaired growth and fertility of cAMP-specific phosphodiesterase PDE4D-deficient mice. Proc. Natl. Acad. Sci. USA. 1999;96:11998–12003. doi: 10.1073/pnas.96.21.11998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnart SE, Wehrens XH, Laitinen PJ, Reiken SR, Deng SX, Cheng Z, Landry DW, Kontula K, Swan H, Marks AR. Sudden death in familial polymorphic ventricular tachycardia associated with calcium release channel (ryanodine receptor) leak. Circulation. 2004;109:3208–3214. doi: 10.1161/01.CIR.0000132472.98675.EC. [DOI] [PubMed] [Google Scholar]
- Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- Mongillo M, McSorley T, Evellin S, Sood A, Lissandron V, Terrin A, Huston E, Hannawacker A, Lohse MJ, Pozzan T, et al. Fluorescence resonance energy transfer-based analysis of cAMP dynamics in live neonatal rat cardiac myocytes reveals distinct functions of compartmentalized phosphodiesterases. Circ. Res. 2004;95:67–75. doi: 10.1161/01.RES.0000134629.84732.11. [DOI] [PubMed] [Google Scholar]
- Packer M, Carver JR, Rodeheffer RJ, Ivanhoe RJ, DiBianco R, Zeldis SM, Hendrix GH, Bommer WJ, Elkayam U, Kukin ML, et al. Effect of oral milrinone on mortality in severe chronic heart failure. The PROMISE Study Research Group. N. Engl. J. Med. 1991;325:1468–1475. doi: 10.1056/NEJM199111213252103. [DOI] [PubMed] [Google Scholar]
- Perry SJ, Baillie GS, Kohout TA, McPhee I, Magiera MM, Ang KL, Miller WE, McLean AJ, Conti M, Houslay MD, Lefkowitz RJ. Targeting of cyclic AMP degradation to beta 2-adrenergic receptors by beta-arrestins. Science. 2002;298:834–836. doi: 10.1126/science.1074683. [DOI] [PubMed] [Google Scholar]
- Pieske B, Maier LS, Bers DM, Hasenfuss G. Ca2+ handling and sarcoplasmic reticulum Ca2+ content in isolated failing and nonfailing human myocardium. Circ. Res. 1999;85:38–46. doi: 10.1161/01.res.85.1.38. [DOI] [PubMed] [Google Scholar]
- Regitz-Zagrosek V, Hertrampf R, Steffen C, Hildebrandt A, Fleck E. Myocardial cyclic AMP and norepinephrine content in human heart failure. Eur. Heart J. 1994;15(Suppl. D):7–13. doi: 10.1093/eurheartj/15.suppl_d.7. [DOI] [PubMed] [Google Scholar]
- Reiken S, Gaburjakova M, Guatimosim S, Gomez AM, D'Armiento J, Burkhoff D, Wang J, Vassort G, Lederer WJ, Marks AR. Protein kinase A phosphorylation of the cardiac calcium release channel (ryanodine receptor) in normal and failing hearts. Role of phosphatases and response to isoproterenol. J. Biol. Chem. 2003a;278:444–453. doi: 10.1074/jbc.M207028200. [DOI] [PubMed] [Google Scholar]
- Reiken S, Wehrens XH, Vest JA, Barbone A, Klotz S, Mancini D, Burkhoff D, Marks AR. Beta-blockers restore calcium release channel function and improve cardiac muscle performance in human heart failure. Circulation. 2003b;107:2459–2466. doi: 10.1161/01.CIR.0000068316.53218.49. [DOI] [PubMed] [Google Scholar]
- Richter W, Jin SL, Conti M. Splice variants of the cyclic nucleotide phosphodiesterase PDE4D are differentially expressed and regulated in rat tissue. Biochem. J. 2005;388:803–811. doi: 10.1042/BJ20050030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruehr ML, Russell MA, Ferguson DG, Bhat M, Ma J, Damron DS, Scott JD, Bond M. Targeting of protein kinase A by muscle A kinase-anchoring protein (mAKAP) regulates phosphorylation and function of the skeletal muscle ryanodine receptor. J. Biol. Chem. 2003;278:24831–24836. doi: 10.1074/jbc.M213279200. [DOI] [PubMed] [Google Scholar]
- Sette C, Conti M. Phosphorylation and activation of a cAMP-specific phosphodiesterase by the cAMP-dependent protein kinase. Involvement of serine 54 in the enzyme activation. J. Biol. Chem. 1996;271:16526–16534. doi: 10.1074/jbc.271.28.16526. [DOI] [PubMed] [Google Scholar]
- Shannon TR, Pogwizd SM, Bers DM. Elevated sarcoplasmic reticulum Ca2+ leak in intact ventricular myocytes from rabbits in heart failure. Circ. Res. 2003;93:592–594. doi: 10.1161/01.RES.0000093399.11734.B3. [DOI] [PubMed] [Google Scholar]
- Suissa S, Hemmelgarn B, Blais L, Ernst P. Bronchodilators and acute cardiac death. Am. J. Respir. Crit. Care Med. 1996;154:1598–1602. doi: 10.1164/ajrccm.154.6.8970341. [DOI] [PubMed] [Google Scholar]
- Tasken KA, Collas P, Kemmner WA, Witczak O, Conti M, Tasken K. Phosphodiesterase 4D and protein kinase a type II constitute a signaling unit in the centrosomal area. J. Biol. Chem. 2001;276:21999–22002. doi: 10.1074/jbc.C000911200. [DOI] [PubMed] [Google Scholar]
- Verde I, Vandecasteele G, Lezoualc'h F, Fischmeister R. Characterization of the cyclic nucleotide phosphodiesterase subtypes involved in the regulation of the L-type Ca2+ current in rat ventricular myocytes. Br. J. Pharmacol. 1999;127:65–74. doi: 10.1038/sj.bjp.0702506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignola AM. PDE4 inhibitors in COPD—a more selective approach to treatment. Respir. Med. 2004;98:495–503. doi: 10.1016/j.rmed.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Wang D, Deng C, Bugaj-Gaweda B, Kwan M, Gunwaldsen C, Leonard C, Xin X, Hu Y, Unterbeck A, De Vivo M. Cloning and characterization of novel PDE4D isoforms PDE4D6 and PDE4D7. Cell. Signal. 2003;15:883–891. doi: 10.1016/s0898-6568(03)00042-1. [DOI] [PubMed] [Google Scholar]
- Wehrens XH, Lehnart SE, Huang F, Vest JA, Reiken SR, Mohler PJ, Sun J, Guatimosim S, Song LS, Rosemblit N, et al. FKBP12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death. Cell. 2003;113:829–840. doi: 10.1016/s0092-8674(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Wehrens XH, Lehnart SE, Reiken SR, Deng SX, Vest JA, Cervantes D, Coromilas J, Landry DW, Marks AR. Protection from cardiac arrhythmia through ryanodine receptor-stabilizing protein calstabin2. Science. 2004;304:292–296. doi: 10.1126/science.1094301. [DOI] [PubMed] [Google Scholar]
- Wehrens XH, Lehnart SE, Reiken S, van der Nagel R, Morales R, Sun J, Cheng Z, Deng SX, de Windt LJ, Landry DW, Marks AR. Enhancing calstabin binding to ryanodine receptors improves cardiac and skeletal muscle function in heart failure. Proc. Natl. Acad. Sci. USA. 2005;102:9607–9612. doi: 10.1073/pnas.0500353102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y, Naro F, Zoudilova M, Jin SL, Conti M, Kobilka B. Phosphodiesterase 4D is required for beta2 adrenoceptor subtype-specific signaling in cardiac myocytes. Proc. Natl. Acad. Sci. USA. 2005;102:909–914. doi: 10.1073/pnas.0405263102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Drazba JA, Ferguson DG, Bond M. A-kinase anchoring protein 100 (AKAP100) is localized in multiple subcellular compartments in the adult rat heart. J. Cell Biol. 1998;142:511–522. doi: 10.1083/jcb.142.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano M, Ono K, Ohkusa T, Suetsugu M, Kohno M, Hisaoka T, Kobayashi S, Hisamatsu Y, Yamamoto T, Noguchi N, et al. Altered stoichiometry of FKBP12.6 versus ryanodine receptor as a cause of abnormal Ca(2+) leak through ryanodine receptor in heart failure. Circulation. 2000;102:2131–2136. doi: 10.1161/01.cir.102.17.2131. [DOI] [PubMed] [Google Scholar]
- Yano M, Kobayashi S, Kohno M, Doi M, Tokuhisa T, Okuda S, Suetsugu M, Hisaoka T, Obayashi M, Ohkusa T, Matsuzaki M. FKBP12.6-mediated stabilization of calcium-release channel (ryanodine receptor) as a novel therapeutic strategy against heart failure. Circulation. 2003;107:477–484. doi: 10.1161/01.cir.0000044917.74408.be. [DOI] [PubMed] [Google Scholar]
- Zaccolo M, Pozzan T. Discrete microdomains with high concentration of cAMP in stimulated rat neonatal cardiac myocytes. Science. 2002;295:1711–1715. doi: 10.1126/science.1069982. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.