Abstract
Tendinopathy often involves inflammation and matrix degeneration. The inflammatory mediators such as prostaglandin E2 (PGE2) and leukotriene B4 (LTB4) are implicated in the development of tendinopathy. Therefore, the purpose of this study was to determine the effect of PGE2 and LTB4 on the proliferation of human patellar tendon fibroblasts (HPTFs), the gene expression of collagen type I, MMP-1 and MMP-3, as well as the protein secretion of these gene products by the cells. The results showed that LTB4 at low doses (0.1 and 1 nM) significantly increased cell proliferation compared to controls and LTB4 at 0.1 nM negated the PGE2-induced decrease in cell proliferation. In addition, PGE2 at 100 ng/ml significantly increased the expression of MMP-1 and MMP-3 at both mRNA and protein levels. These stimulatory effects were significantly diminished by co-treatment with LTB4 at 0.1 nM. Finally, neither PGE2 nor LTB4 treatment affected collagen type I gene expression. These results suggest that low levels of LTB4 counterbalance the negative effects mediated by PGE2 on tendon fibroblast proliferation and MMP production, which may lead to matrix degradation. Thus, our findings suggest that although LTB4 is generally thought to be pathogenic, low levels of LTB4 are actually beneficial in maintaining tendon tissue homeostasis.
Keywords: LTB4, PGE2, Collagen, MMPs, Tendon fibroblasts
1. Introduction
Tendinopathy generally describes a group of tendon disorders marked by tendon inflammation and degeneration (Maffulli et al., 1998). Tendon inflammation is marked by the presence of inflammatory mediators, including prostaglandin E2 (PGE2) and leukotriene B4 (LTB4) (Almekinders et al., 1993). Although the exact amounts of both PGE2 and LTB 4 in inflamed tendons are not known, their levels are markedly increased when tendons or tendon fibroblasts are subjected to repetitive mechanical loading conditions (Langberg et al., 1999; Wang et al., 2003; Li et al., 2004). These data suggest that PGE2 and LTB4 may play a role in tendon degeneration, the late stages of tendinopathy (Wang, 2005). Tendon degeneration refers to degenerative changes in tendon matrix and is carried out by matrix metalloproteinases (MMPs) (Riley, 2005a,b), which are a group of enzymes essential for matrix turnover (Birkedal-Hansen et al., 1993; Fingleton and Matrisian, 2001). MMPs are present in high concentrations in inflammatory regions (Birkedal-Hansen, 1993) and degrade specific connective tissue matrix components such as collagen. For example, MMP-1 can directly cleave native collagen type I (Pardo and Selman, 2005), which is the dominant form of collagen in tendons, while MMP-3 degrades aggrecan and collagen-associated small glycoproteins (Sternlicht and Werb, 2001).
Although MMPs are clearly involved in the pathogenesis of tendinopathy, the detailed molecular mechanisms of tendinopathy remain elusive. In vitro studies have shown that repetitive mechanical loading of tendon fibroblasts induces the release of PGE2 and LTB4 (Almekinders et al., 1995; Li et al., 2004). Both are also produced in substantial amounts at the sites of inflammation (Henderson, 1994; Lawrence et al., 2002). PGE2 and LTB4 are inflammatory mediators and have been implicated in the pathogenesis of several inflammatory diseases such as tendinopathy and rheumatoid arthritis (Davidson et al., 1983; Almekinders et al., 1993; Trebino et al., 2003). It has also been reported that the levels of both mediators are elevated in cases of tissue injury and dysfunctional joints as well as in inflamed tendons (Moreland et al., 1989; Quinn and Bazan, 1990). PGE2 is a potent inhibitor of type I collagen synthesis in several connective tissue cell types such as fibroblasts (Varga et al., 1987; Riquet et al., 2000). In addition, elevated PGE2 levels were found in the human tendon after repetitive mechanical loading (Langberg et al., 1999). Recently, we have shown that exogenous addition of PGE2 at a dose of 100 ng/ml decreases proliferation and collagen production in human patellar tendon fibroblasts (HPTFs) (Cilli et al., 2004). On the other hand, LTB4 at picomolar concentrations has been shown to stimulate DNA synthesis in cultured human epidermal keratinocytes and to induce proliferation of arterial smooth muscle cells in primary culture (Kragballe et al., 1985; Palmberg et al., 1989). Also, leukotrienes at low concentrations (0.1 to 1 nM) stimulate collagen synthesis of lung fibroblasts (Phan et al., 1987, 1988). Thus, although LTB4 has been implicated in the pathogenesis of various inflammatory diseases (Davidson et al., 1983; Sharon and Stenson, 1984; Konstan et al., 1993), its potential role in the tendon tissue homeostasis as well as pathogenesis remains to be explored.
Therefore, the purpose of this study was to determine the effect of LTB4 as well as its interactive effect with PGE2 on proliferation, gene expression and/or protein production of collagen type I and MMPs including MMP-1 and MMP-3 in HPTFs. Our results show that exogenous LTB4 at picomolar concentrations (≤100 pM) negated the decreased cell proliferation and the increased MMP-1 and MMP-3 gene expression and protein production that were induced by PGE2. However, higher concentrations of LTB4 (>1 nM) in combination with PGE2 further decreased cell proliferation. Our results highlight the important role of LTB4 in tendon homeostasis as well as in the pathogenesis of tendinopathy.
2. Materials and methods
2.1. Cell culture
Tendon samples were obtained from fresh surgical wastes of normal tendon grafts used for the reconstruction of the anterior cruciate ligament. The protocol for obtaining tendon samples was approved by the University of Pittsburgh Institutional Review Board. HPTFs were isolated and maintained in Dulbecco’s Modified Eagle Medium (DMEM) containing 10% FBS, penicillin (50 U/ml), and streptomycin (50 U/ml) as previously described (Wang et al., 2003). The fibroblasts were subcultured for 4–6 passages and were used for the experiments. After these passages, tendon fibroblasts maintained their morphology and doubling time. Cells from at least two donors were used in the experiments.
2.2. PGE2 and LTB4 treatments
For the proliferation experiments, HPTFs were plated in 6-well plates to attain roughly 50% confluence, which was equivalent to 4×104 cells per well. The cells were incubated at 37°C in a humidified atmosphere of 5% CO2 in growth medium to permit attachment and an even distribution. After 24 h, varying concentrations of LTB4 (Cayman Chemicals, Ann Arbor, Michigan), ranging from 10 pM to 10 nM, were added to the wells. PGE2 was added to the cell cultures at a dose of 100 ng/ml because our previous study showed that this PGE2 dose decreases HPTF proliferation and collagen production (Cilli et al., 2004). Fibroblasts treated with EtOH (0.1%), which is the vehicle for PGE2 or LTB4, were used as the control groups. The cells treated with both agents received LTB4 30 min prior to the addition of PGE2. All the cells in the experimental and control groups were incubated for an additional 72 h. To determine the number of viable cells in the cultures, an MTS assay was performed (see below).
For RNA and protein measurements, cells at near confluence (2×105/ml) were plated in triplicate in 6-well plates and incubated. After 24 h, LTB4 at 0.1 nM was added for 30 min. Then PGE2 at 100 ng/ml was added to the wells. In separate experiments, PGE2 at the doses of 100, 250, and 500 ng/ml alone were added to cell cultures to examine dose-dependent effect on collagen type I gene expression. EtOH (0.1%), which is the vehicle for PGE2 or LTB4, was added to some wells as the control groups. After an additional 48 h of incubation, the conditioned media were collected for MMP protein measurements. Using an RNeasy kit (Qiagen Inc., USA), total RNA was extracted from the cells for measuring gene expression.
2.3. Cell proliferation assay
This assay uses a soluble tetrazolium salt, MTS, and the electron coupling reagent, phenazine methosulfate (PMS), to measure the dehydrogenase enzyme activity in metabolically active cells. MTS is chemically reduced by cells to formazan, which is soluble in tissue culture media. MTS solutions were prepared according to the manufacturer’s instructions (Promega Corporation, Madison, WI). Stock PMS (Sigma Chemicals, St. Louis) was dissolved in PBS at a concentration of 0.92 mg/ml. These solutions were stored in light-protected tubes at −20 °C. MTS and PMS reagents were mixed at a ratio of 20:1 (MTS: PMS) immediately prior to addition to cell culture at a ratio of 1:5 (reagent:cell culture medium). After incubation for 1 h in a humidified atmosphere at 37 °C and 5% CO2, triplicate samples of 200 μl culture medium were aliquoted into 96-well plates and the absorbance was measured at 492 nm using a microplate reader. The absorbance value represented the number of viable cells in each sample since the production of formazan is proportional to the number of viable cells (Malich et al., 1997).
2.4. RT-PCR
Reverse transcription (RT) was carried out with 1 μg of total cellular RNA using the ThermoScript RT-PCR System (Invitrogen) for first strand cDNA synthesis in 20 μl of reaction volume. The sequences for the primers and annealing temperature optimized for each primer set are provided in Table 1. For all experiments, the conditions were determined to be in the linear range for the PCR amplification. Briefly, all samples were subjected to RT and subsequent amplification of the cDNA samples was performed by PCR at the same time. The cDNA samples were then assessed for GAPDH expression. The levels of GAPDH mRNA did not vary with time after the addition of LTB4 and PGE2 or their combination following 28 PCR cycles. Genomic DNA was included for the PCR to ensure that there was no genomic DNA contamination in the total RNA samples. The cDNA was amplified by PCR using 28 cycles at 95 °C for 30 s, 55~60 °C for 30 s, and 72 °C for 30 s in the presence of Taq polymerase (Invitrogen), 50 pmol of sense and antisense primers. PCR products were resolved on 1.5% agarose gels by electrophoresis and visualized by staining with ethidium bromide and UV transillumination. Integrated density values for the genes in question were normalized to the GAPDH values to yield a semi-quantitative assessment of gene expression levels.
Table 1.
Primer sequences for RT-PCR
| Gene, annealing temperature | Primer sequence, forward/reverse | PCR product size (bp) |
|---|---|---|
| MMP-1, 58 °C | 5′-CAACT CTGGAGTAAT GTCACA-3′ 5′-T ACATCAAAGC CCCGATATCA-3′ |
295 |
| MMP-3, 55 °C | 5′-TTT TGG CCA TCT CTT CCT TCA-3′ 5′-TGT GGA TGC CTC TTG GGT ATC-3′ |
138 |
| Collagen-I, 58 °C | 5′-GGT TAC TAC TGG ATT GAC C-3′ 5′-TTG CCA GTC TCC TCA TCC-3′ |
328 |
| GAPDH, 58 °C | 5′-AAATTCCATGGCAC CGTCAAGGCT-3′ 5′-CTCATGGTTCACACC CATGACGAA-3′ |
295 |
2.5. Assay for MMP-1 and MMP-3 protein production
Pro-MMP-1 and total MMP-3 (pro- and active MMP-3) concentrations in medium were determined using sandwich ELISA kits specific to human MMP-1 and MMP-3, respectively (R&D Systems, Minneapolis, MN, USA). The concentrations of MMP-1 and MMP-3 were determined using a standard curve in the range of 0.16–10 ng/ml and normalized to the cell number.
2.6. Data analysis
Data were expressed as mean±SD. The absorbance values obtained for cell proliferation data were normalized to the control. For the analysis of RT-PCR gene expression levels, the density values of the bands were normalized to the GAPDH values to yield a semi-quantitative assessment of gene expression. The amounts of MMP-1 and MMP-3 proteins were normalized to the respective cell number.
For statistical analysis of cell proliferation and collagen production, one-way ANOVA with a post hoc Bonferroni’s test was used to determine which two groups showed significant differences. A difference between two groups was considered statistically significant if the p value was less than 0.05.
3. Results
3.1. LTB4 regulated tendon fibroblast proliferation at low concentrations (<1 nM)
The effect of LTB4 at various concentrations on the proliferation of HPTFs was determined. At 0.01 nM, the fibroblast proliferation only slightly increased. At 0.1 nM, however, cell proliferation significantly increased by 14% compared to untreated fibroblasts (p=0.002). Also, at 1 nM of LTB4, fibroblast proliferation maintained a significant 14% increase (p=0.001). However, higher concentrations of LTB4 (>1 nM) did not induce any significant fibroblast proliferation (Fig. 1).
Fig. 1.
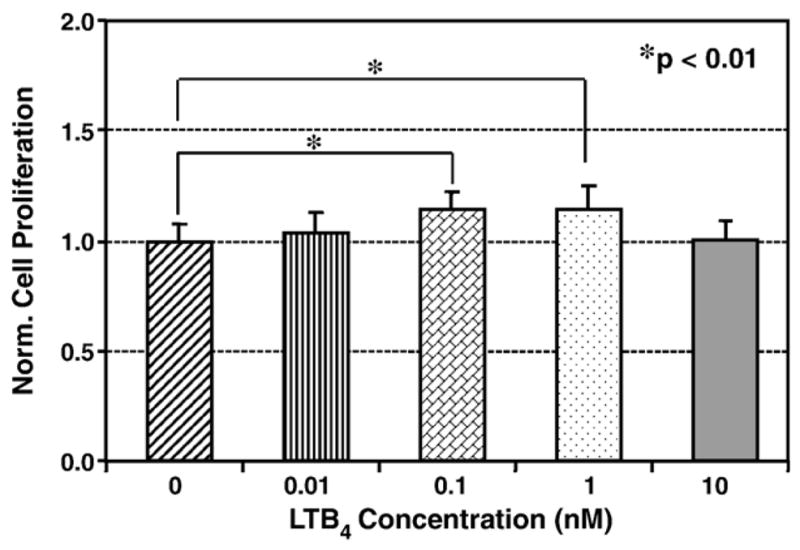
The effect of LTB4 on the proliferation of human patellar tendon fibroblasts. Low doses of LTB4 (0.1 and 1 nM) significantly increased fibroblast proliferation, but a higher dose (10 nM) did not have any effect. Six independent experiments were performed, with a total sample size ranging from 8 to 18 for each group.
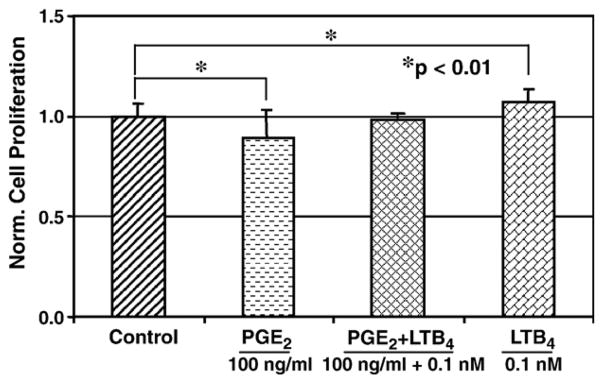
We further examined the effect of LTB4 and its combinations with PGE2 on fibroblast proliferation. At 100 ng/ml, PGE2 significantly decreased cell proliferation by 11% (p=0.02). However, when fibroblasts were treated with PGE2 and 0.1 nM LTB4 in combination, the effect of PGE2 on cell proliferation was negated and proliferation was increased close to the levels of control cells (Fig. 2). Cells which were treated with a higher concentration of LTB4 (10 nM) alone appeared to have no influence on the cell proliferation (Figs. 1 and 3). When this high concentration of LTB4 was added to the cells in combination with 100 ng/ml PGE2, LTB4 failed to reverse the anti-proliferative action mediated by PGE2. In fact, their combination further decreased fibroblast proliferation (p=0.001) compared to the effect of PGE2 alone (Fig. 3). It was noted that all doses of LTB4 and PGE2 used in this study did not cause apparent changes in the morphology of tendon fibroblasts in culture, which suggests that the possible toxic effects of these two agents on human tendon fibroblasts were minimal (data not shown).
Fig. 2.
The combined effect of PGE2 (100 ng/ml) and a low dose of LTB4 (100 pM) on human patellar tendon fibroblasts. The addition of PGE2 significantly decreased and the addition of LTB4 at 100 pM significantly increased the cell proliferation. However, the combined addition of both agents brought the proliferation level back to that of untreated cells. Four independent experiments were carried out; total sample size was 12 for each group.
Fig. 3.
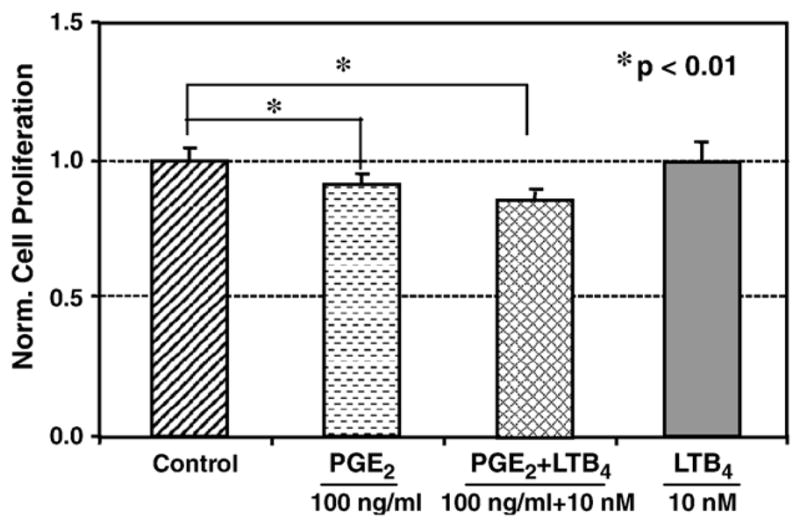
The combined effect of PGE2 (100 ng/ml) and a high dose of LTB4 (10 nM) on human patellar tendon fibroblasts. The addition of PGE2 decreased cell proliferation and the addition of LTB4 at 10 nM did not change the cell proliferation compared to that of untreated cells. When both agents were added in combination, cell proliferation further decreased compared to the already reduced level of cell proliferation brought by PGE2 alone. Two independent experiments were done, with a total sample size of 6 for each group.
3.2. LTB4 negated the MMP-1 and MMP-3 gene expression induced by PGE2
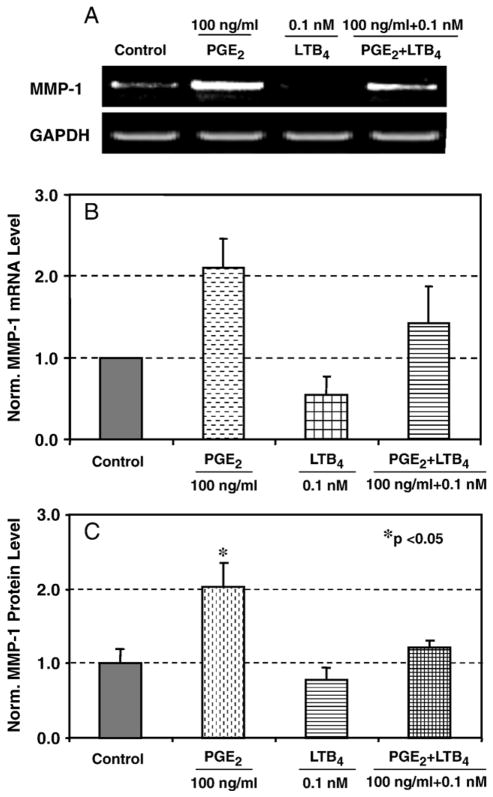
PGE2-mediated catabolic effect on matrix proteins has been well documented (Varga et al., 1987). We observed the counteraction of low concentration of LTB4 against PGE2 in cell proliferation. Next we examined the potential antagonistic effect of LTB4 on the catabolic action mediated by PGE2 by examining the expression and production of MMP-1 and MMP-3 in the presence of LTB4 alone and in combination with PGE2. Compared to the control level, treatment of HPTFs for 48 h with 100 ng/ml PGE2 increased MMP-1 gene expression whereas LTB4 at 0.1 nM decreased it. Interestingly, the addition of a low concentration of LTB4 (0.1 nM) significantly down-regulated 50% the expression of MMP-1 (Fig. 4A and B). Furthermore, the addition of LTB4 appeared to counteract the stimulatory action of PGE2 on MMP-1 expression, down-regulating close to the control level.
Fig. 4.
Effects of PGE2 and LTB4 on MMP-1 mRNA expression and protein production. A. Representative RT-PCR result from three independent experiments using cells from two different donors. B. The combined densitometric data from three independent experiments. The data show that PGE2 induced MMP-1 and LTB4 decreased it compared to the control; a combined treatment of PGE2 and LTB4 brought the MMP-1 mRNA level back close to the control. C. PGE2 treatment increased MMP-1 protein level whereas LTB4 reduced it compared to the control. Two independent experiments were performed with a total sample size of 6.
We further analyzed for MMP-1 protein production after stimulation with PGE2 alone or in combination with LTB4. As seen in mRNA level, MMP-1 protein production was correspondingly increased by 103% by 100 ng/ml PGE2 compared to the control whereas LTB4 at 0.1 nM slightly decreased the level by 20% compared to the control. A combined treatment with 0.1 nM LTB4 and 100 ng/ml PGE2 brought the MMP-1 level back to control (Fig. 4C). Collectively, our results suggest that the gene expression and the corresponding protein secretion of MMP-1 are regulated by PGE2 and LTB4 and low concentration of LTB4 can rescue PGE2-mediated stimulation of MMP-1 in HPTFs.
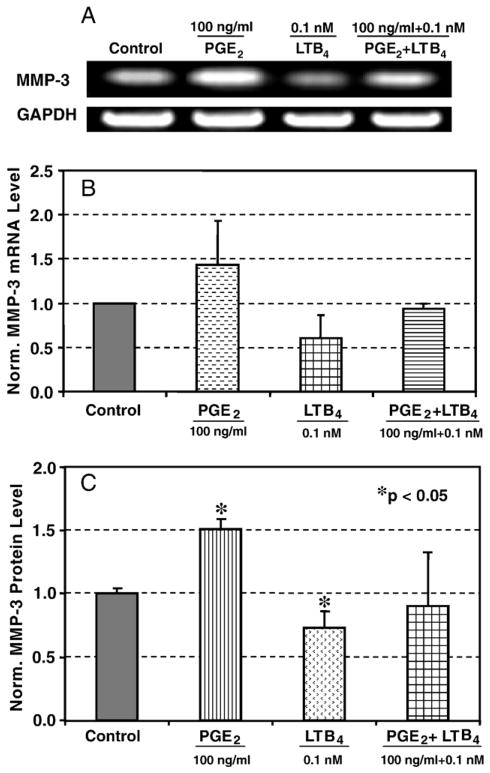
We also examined the effect of LTB4 and PGE2 on the gene expression and protein secretion of MMP-3. A similar pattern to MMP-1 in response to PGE2 and LTB4 treatments was observed (Fig. 5A, B, and C). Specifically, MMP-3 gene expression was increased by 43% with PGE2 treatment compared to the untreated, while LTB4 at 0.1 nM decreased the level by 40% compared to the control level. Again, pretreatment with LTB4 prior to PGE2 treatment brought the MMP-3 mRNA level back to the control levels (Fig. 5B). The level of MMP-3 protein production also was augmented by treatment with 100 ng/ml PGE2. Specifically, the MMP-3 level was increased by 50% in response to PGE2 while LTB4 at 0.1 nM decreased it by 30%. However, the combined treatment brought the MMP-3 protein production back close to the control level (Fig. 5C).
Fig. 5.
Effects of PGE2 and LTB4 on MMP-3 mRNA expression and protein production. A. A representative RT-PCR result from three independent experiments using cells from two different donors. B. The combined densitometric data from three independent experiments. The data show that MMP-3 mRNA level was markedly increased by PGE2 compared to the control while it was reduced by LTB4 compared to the control. A pretreatment with LTB4 for 30 min prior to PGE2 treatment brought the levels back to the control level. C. MMP-3 protein production also increased by PGE2 treatment and decreased by LTB4, while combined treatment brought the levels close to the control. Two independent experiments were performed with a total sample size of 6.
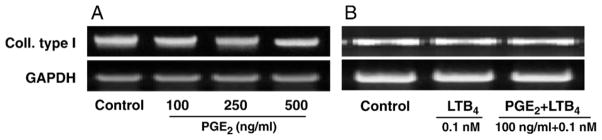
3.3. PGE2 and its combination with LTB4 did not affect collagen type I gene expression
The effect of increasing concentrations of PGE2 and the potential anabolic action of LTB4 at low concentrations on collagen I gene expression was determined. Treatment with 100 ng/ml PGE2, or higher doses (250 and 500 ng/ml), did not change collagen type I mRNA gene expression (Fig. 6A). LTB4 at 0.1 nM combined with 100 ng/ml PGE2 did not affect collagen type I mRNA gene expression either (Fig. 6B).
Fig. 6.
Effect of PGE2 and LTB4 on collagen type I gene expression in HPTFs. A. PGE2 did not affect collagen type I gene expression. B. The combined treatment with LTB4 did not affect collagen type I gene expression either. Three independent experiments were performed using cells from two different donors.
4. Discussion
This study showed that exogenous LTB4 affects HPTF proliferation in a dose-dependent manner. Specifically, low concentrations of LTB4 (≤1 nM) exerted a positive effect on the proliferation of HPTFS. Although there is no published study on the effects of LTB4 on human tendon fibroblasts, our results agree with previous reports obtained using other cell/tissue types. For example, LTB4 was shown to stimulate DNA synthesis in cultured human epidermal keratinocytes at picomolar concentrations (Kragballe et al., 1985). Also, in arterial smooth muscle cells, LTB4 at 10 pM was shown to stimulate cell proliferation and DNA synthesis (Palmberg et al., 1987, 1989).
Leukotrienes exert their effect primarily via their receptors, which elicit a cascade of signaling events (Denzlinger, 1996). The mechanism of LTB4-mediated proliferative response is thought to involve protein kinase C (PKC) activation and the stimulation of early response genes such as topoisomerase I and nuclear factor kappa-B (NFκ-B) (Mattern et al., 1990; Brach et al., 1992). In addition, there are interactions between growth stimulatory cytokines such as IL-6, which could potentially mediate LTB4-mediated proliferative effects (Brach et al., 1992; Rola-Pleszczynski and Stankova, 1992; Denzlinger, 1996). The optimal concentration for the leukotriene-induced cytokine production is shown to be in the sub-micromolar range (Rola-Pleszczynski and Stankova, 1992).
This study also showed that PGE2 at 100 ng/ml significantly decreased HPTF proliferation, which is consistent with the result of our previous report (Cilli et al., 2004). Furthermore, low concentrations (≤1 nM) of LTB4 negated the inhibitory effect of PGE2 on HPTF proliferation. However, LTB4 at higher concentrations (>1 nM) was not able to counteract the PGE2 effect; on the contrary, it appeared to further decrease the cell proliferation when combined with PGE2. These results may have important implications in tendon homeostasis as well as in the development of tendinopathy. The potential scenario would be that at low doses, LTB4 is beneficial in terms of negating the catabolic effect of PGE2 on fibroblast proliferation and thereby maintaining the tendon homeostasis in vivo. However, at high concentrations, LTB4 may worsen the catabolic effect of PGE2 on fibroblast proliferation. Consequently, there would not be enough tendon fibroblasts to maintain tendon matrix homeostasis. The small reduction in cell proliferation by PGE2 may also imply that the development of tendinopathy takes time, which is known to be an insidious process over a long period of time (Almekinders, 1998).
The current study showed that PGE2 significantly increased the gene expression and protein production of MMP-1 and MMP-3. Our results are consistent with the previous observation that exogenous PGE2 up-regulates MMPs in gingival fibroblasts (Sakaki et al., 2004). Other studies have reported that PGE2 mediates IL-1β-induced MMP-1 up-regulation in tendon and gingival fibroblasts (Domeij et al., 2002; Tsuzaki et al., 2003). It is noted that tendon consists predominantly of collagen type I with a triple helical structure that resists general proteolytic degradation but is susceptible to MMPs. MMPs are produced as inactive zymogens and their extracellular activation can be initiated by various factors such as plasminogens or other active MMPs (Visse and Nagase, 2003). Although MMP-3 does not degrade collagen type I, it degrades collagen-associated small proteoglycans, elastin, fibronectin, gelatin, and other types of collagens (Birkedal-Hansen, 1993). In addition, MMP-3 has been shown to activate MMP-1 (Murphy et al., 1987) and other collagen type I-degrading MMPs, including MMP-8 and MMP-13 as well as MMP-7, which is also involved in the activation of MMP-1 (Visse and Nagase, 2003). Therefore, the upregulated MMP-3 by PGE2 may enable MMP-1 and/or other collage-degrading enzymes to efficiently access collagen molecules for digestion in vivo.
It is interesting to note that neither PGE2 nor LTB4 nor their combination at the doses used in this study changed collagen gene expression (Fig. 6). These results suggest that PGE2-mediated inhibition of collagen production, as shown in our previous study (Cilli et al., 2004), is perhaps via the induction of collagen-degrading enzymes such as MMP-1 and MMP-3. Another possibility for the decrease in collagen production by PGE2 is that PGE2 affects collagen mRNA stability. Although it has been shown in human dermal fibroblasts that PGE2 did not alter the stability of procollagen I and III mRNA (Varga et al., 1987), the potential modulation of mRNA stability should be examined further, especially since PGE2 effects are highly cell and tissue specific (Serhan and Levy, 2003).
We are aware of a few limitations of this study. First, we did not determine the kinetic effect of LTB4 on HPTF proliferation and collagen synthesis. Also, we do not know if the decreased cell numbers mediated by either PGE2 alone or in combination with high concentrations of LTB4 are due to apoptosis or cell growth arrest. We did not look into the potential mechanistic cellular events by which LTB4 and PGE2 regulate collagen synthesis and the expression/production of MMPs. The molecular mechanisms by which the low concentration of LTB4 exerts anti-catabolic action against PGE2 and the potential molecular interplay between PGE2 and LTB4 will be of special interest and future studies are required. In addition, it is known that LTB4 and PGE2 actions are mediated via their receptors, e.g., BLT1 and BLT2 (Tager and Luster, 2003) and EP1, EP2, EP3, and EP4 (Negishi et al., 1995; Narumiya et al., 1999), respectively. Thus, it is important to investigate the expression and/or activation of their cognate receptors and the signaling pathways for better understanding the precise role of dose-dependent counter-activity of LTB4 on PGE2 in tendon homeostasis in future studies.
In conclusion, this study showed that LTB4 at sub-nanomolar concentrations increases tendon fibroblast proliferation and negates the catabolic effects of PGE2. The results suggest that although LTB4 is mainly pathogenic in nature, low levels are actually beneficial in maintaining tendon homeostasis by regulating tendon fibroblast proliferation and MMPs, which in turn control matrix turnover. The ability of LTB4 to counteract the catabolic action of PGE2 may thus play an important role in delaying the development of tendinopathy. A better understanding of the precise mechanisms by which LTB4 regulates the effects of PGE2 on tendon fibroblast proliferation and MMP expression will aid in developing effective therapeutic approaches for prevention as well as treatment of tendinopathy.
Acknowledgments
We are grateful to the funding support from the Arthritis Investigator Award, NIH grant AR049921 (JHW), and a grant from the Falk Foundation (HJI).
Abbreviations
- LTB4
Leukotriene B4
- PGE2
Prostaglandin E2
- MMP
Matrix Metalloproteinase
- HPTF
Human Patellar Tendon Fibroblast
- DMEM
Dulbecco’s Modified Eagle Medium
- PCR
Polymerase Chain Reaction
- EtOH
Ethanol
- PMS
Phenazine Methosulfate
- GAPDH
Glyceraldehyde Phosphate Dehydrogenase
- ELISA
Enzyme-linked Immunosorbent assay
- PKC
Protein Kinases C
- NF-kB
Nuclear Factor kappa-B
References
- Almekinders LC. Tendinitis and other chronic tendinopathies. J Am Acad Orthop Surg. 1998;6:157–164. doi: 10.5435/00124635-199805000-00003. [DOI] [PubMed] [Google Scholar]
- Almekinders LC, Banes AJ, Ballenger CA. Effects of repetitive motion on human fibroblasts. Med Sci Sports Exerc. 1993;25:603–607. [PubMed] [Google Scholar]
- Almekinders LC, Baynes AJ, Bracey LW. An in vitro investigation into the effects of repetitive motion and nonsteroidal antiinflammatory medication on human tendon fibroblasts. Am J Sports Med. 1995;23:119–123. doi: 10.1177/036354659502300120. [DOI] [PubMed] [Google Scholar]
- Birkedal-Hansen H. Role of matrix metalloproteinases in human periodontal diseases. J Periodontol. 1993;64:474–484. doi: 10.1902/jop.1993.64.5s.474. [DOI] [PubMed] [Google Scholar]
- Birkedal-Hansen H, et al. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4:197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- Brach MA, de Vos S, Arnold C, Gruss HJ, Mertelsmann R, Herrmann F. Leukotriene B4 transcriptionally activates interleukin-6 expression involving NK-chi B and NF-IL6. Eur J Immunol. 1992;22:2705–2711. doi: 10.1002/eji.1830221034. [DOI] [PubMed] [Google Scholar]
- Cilli F, Khan M, Fu F, Wang JH. Prostaglandin E2 affects proliferation and collagen synthesis by human patellar tendon fibroblasts. Clin J Sport Med. 2004;14:232–236. doi: 10.1097/00042752-200407000-00006. [DOI] [PubMed] [Google Scholar]
- Davidson EM, Rae SA, Smith MJ. Leukotriene B4, a mediator of inflammation present in synovial fluid in rheumatoid arthritis. Ann Rheum Dis. 1983;42:677–679. doi: 10.1136/ard.42.6.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denzlinger C. Biology and pathophysiology of leukotrienes. Crit Rev Oncol Hematol. 1996;23:167–223. doi: 10.1016/1040-8428(96)00205-3. [DOI] [PubMed] [Google Scholar]
- Domeij H, Yucel-Lindberg T, Modeer T. Signal pathways involved in the production of MMP-1 and MMP-3 in human gingival fibroblasts. Eur J Oral Sci. 2002;110:302–306. doi: 10.1034/j.1600-0722.2002.21247.x. [DOI] [PubMed] [Google Scholar]
- Fingleton B, Matrisian LM. Matrix metalloproteinases as targets for therapy in Kaposi sarcoma. Curr Opin Oncol. 2001;13:368–373. doi: 10.1097/00001622-200109000-00009. [DOI] [PubMed] [Google Scholar]
- Henderson WR., Jr The role of leukotrienes in inflammation. Ann Intern Med. 1994;121:684–697. doi: 10.7326/0003-4819-121-9-199411010-00010. [DOI] [PubMed] [Google Scholar]
- Konstan MW, Walenga RW, Hilliard KA, Hilliard JB. Leukotriene B4 markedly elevated in the epithelial lining fluid of patients with cystic fibrosis. Am Rev Respir Dis. 1993;148:896–901. doi: 10.1164/ajrccm/148.4_Pt_1.896. [DOI] [PubMed] [Google Scholar]
- Kragballe K, Desjarlais L, Voorhees JJ. Leukotrienes B4, C4 and D4 stimulate DNA synthesis in cultured human epidermal keratinocytes. Br J Dermatol. 1985;113:43–52. doi: 10.1111/j.1365-2133.1985.tb02043.x. [DOI] [PubMed] [Google Scholar]
- Langberg H, Skovgaard D, Karamouzis M, Bulow J, Kjaer M. Metabolism and inflammatory mediators in the peritendinous space measured by microdialysis during intermittent isometric exercise in humans. J Physiol. 1999;515 (Pt 3):919–927. doi: 10.1111/j.1469-7793.1999.919ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence T, Willoughby DA, Gilroy DW. Anti-inflammatory lipid mediators and insights into the resolution of inflammation. Nat Rev Immunol. 2002;2:787–795. doi: 10.1038/nri915. [DOI] [PubMed] [Google Scholar]
- Li Z, Yang G, Khan M, Stone D, Woo SL, Wang JH. Inflammatory response of human tendon fibroblasts to cyclic mechanical stretching. Am J Sports Med. 2004;32:435–440. doi: 10.1177/0095399703258680. [DOI] [PubMed] [Google Scholar]
- Maffulli N, Khan KM, Puddu G. Overuse tendon conditions: time to change a confusing terminology. Arthroscopy. 1998;14:840–843. doi: 10.1016/s0749-8063(98)70021-0. [DOI] [PubMed] [Google Scholar]
- Malich G, Markovic B, Winder C. The sensitivity and specificity of the MTS tetrazolium assay for detecting the in vitro cytotoxicity of 20 chemicals using human cell lines. Toxicology. 1997;124:179–192. doi: 10.1016/s0300-483x(97)00151-0. [DOI] [PubMed] [Google Scholar]
- Mattern MR, et al. Transient activation of topoisomerase I in leukotriene D4 signal transduction in human cells. Biochem J. 1990;265:101–107. doi: 10.1042/bj2650101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreland DB, Soloniuk DS, Feldman MJ. Leukotrienes in experimental spinal cord injury. Surg Neurol. 1989;31:277–280. doi: 10.1016/0090-3019(89)90052-9. [DOI] [PubMed] [Google Scholar]
- Murphy G, Cockett MI, Stephens PE, Smith BJ, Docherty AJ. Stromelysin is an activator of procollagenase. A study with natural and recombinant enzymes. Biochem J. 1987;248:265–268. doi: 10.1042/bj2480265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- Negishi M, Sugimoto Y, Ichikawa A. Molecular mechanisms of diverse actions of prostanoid receptors. Biochim Biophys Acta. 1995;1259:109–119. doi: 10.1016/0005-2760(95)00146-4. [DOI] [PubMed] [Google Scholar]
- Palmberg L, Claesson HE, Thyberg J. Leukotrienes stimulate initiation of DNA synthesis in cultured arterial smooth muscle cells. J Cell Sci. 1987;88 (Pt 2):151–159. doi: 10.1242/jcs.88.2.151. [DOI] [PubMed] [Google Scholar]
- Palmberg L, Claesson HE, Thyberg J. Effects of leukotrienes on phenotypic properties and growth of arterial smooth muscle cells in primary culture. J Cell Sci. 1989;93 (Pt 3):403–408. doi: 10.1242/jcs.93.3.403. [DOI] [PubMed] [Google Scholar]
- Pardo A, Selman M. MMP-1: the elder of the family. Int J Biochem Cell Biol. 2005;37:283–288. doi: 10.1016/j.biocel.2004.06.017. [DOI] [PubMed] [Google Scholar]
- Phan SH, McGarry BM, Loeffler KM, Kunkel SL. Regulation of macrophage-derived fibroblast growth factor release by arachidonate metabolites. J Leukoc Biol. 1987;42:106–113. doi: 10.1002/jlb.42.2.106. [DOI] [PubMed] [Google Scholar]
- Phan SH, McGarry BM, Loeffler KM, Kunkel SL. Binding of leukotriene C4 to rat lung fibroblasts and stimulation of collagen synthesis in vitro. Biochemistry. 1988;27:2846–2853. doi: 10.1021/bi00408a028. [DOI] [PubMed] [Google Scholar]
- Quinn JH, Bazan NG. Identification of prostaglandin E2 and leukotriene B4 in the synovial fluid of painful, dysfunctional temporomandibular joints. J Oral Maxillofac Surg. 1990;48:968–971. doi: 10.1016/0278-2391(90)90011-p. [DOI] [PubMed] [Google Scholar]
- Riley G. Chronic tendon pathology: molecular basis and therapeutic implications. Expert Rev Mol Med. 2005a;7:1–25. doi: 10.1017/S1462399405008963. [DOI] [PubMed] [Google Scholar]
- Riley GP. Gene expression and matrix turnover in overused and damaged tendons. Scand J Med Sci Sports. 2005b;15:241–251. doi: 10.1111/j.1600-0838.2005.00456.x. [DOI] [PubMed] [Google Scholar]
- Riquet FB, Lai WF, Birkhead JR, Suen LF, Karsenty G, Goldring MB. Suppression of type I collagen gene expression by prostaglandins in fibroblasts is mediated at the transcriptional level. Mol Med. 2000;6:705–719. [PMC free article] [PubMed] [Google Scholar]
- Rola-Pleszczynski M, Stankova J. Leukotriene B4 enhances interleukin-6 (IL-6) production and IL-6 messenger RNA accumulation in human monocytes in vitro: transcriptional and posttranscriptional mechanisms. Blood. 1992;80:1004–1011. [PubMed] [Google Scholar]
- Sakaki H, et al. Interleukin-1beta induces matrix metalloproteinase-1 expression in cultured human gingival fibroblasts: role of cyclooxygenase-2 and prostaglandin E2. Oral Dis. 2004;10:87–93. doi: 10.1046/j.1354-523x.2003.00982.x. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Levy B. Success of prostaglandin E2 in structure-function is a challenge for structure-based therapeutics. Proc Natl Acad Sci U S A. 2003;100:8609–8611. doi: 10.1073/pnas.1733589100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon P, Stenson WF. Enhanced synthesis of leukotriene B4 by colonic mucosa in inflammatory bowel disease. Gastroenterology. 1984;86:453–460. [PubMed] [Google Scholar]
- Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tager AM, Luster AD. BLT1 and BLT2: the leukotriene B(4) receptors. Prostaglandins Leukot Essent Fat Acids. 2003;69:123–134. doi: 10.1016/s0952-3278(03)00073-5. [DOI] [PubMed] [Google Scholar]
- Trebino CE, et al. Impaired inflammatory and pain responses in mice lacking an inducible prostaglandin E synthase. Proc Natl Acad Sci U S A. 2003;100:9044–9049. doi: 10.1073/pnas.1332766100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuzaki M, et al. IL-1 beta induces COX2, MMP-1, -3 and -13, ADAMTS-4, IL-1 beta and IL-6 in human tendon cells. J Orthop Res. 2003;21:256–264. doi: 10.1016/S0736-0266(02)00141-9. [DOI] [PubMed] [Google Scholar]
- Varga J, Diaz-Perez A, Rosenbloom J, Jimenez SA. PGE2 causes a coordinate decrease in the steady state levels of fibronectin and types I and III procollagen mRNAs in normal human dermal fibroblasts. Biochem Biophys Res Commun. 1987;147:1282–1288. doi: 10.1016/s0006-291x(87)80209-7. [DOI] [PubMed] [Google Scholar]
- Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- Wang JH. Mechanobiology of tendon. J Biomech. 2005 doi: 10.1016/j.jbiomech.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Wang JH, et al. Cyclic mechanical stretching of human tendon fibroblasts increases the production of prostaglandin E2 and levels of cyclooxygenase expression: a novel in vitro model study. Connect Tissue Res. 2003;44:128–133. doi: 10.1080/03008200390223909. [DOI] [PubMed] [Google Scholar]