Abstract
Purpose
Protease-activated receptor-2 (PAR2) is highly expressed throughout the gut and regulates inflammatory, mitogenic, fibroproliferative, and nociceptive responses to injury. PAR2 is strikingly upregulated and exhibits increased activation in response to intestinal irradiation. We examined the mechanistic significance of in radiation enteropathy development by assessing the effect of exogenous PAR2 activation.
Methods and Materials
Rat small bowel was exposed to localized single-dose radiation (16.5 Gy). The PAR2 agonist (2-furoyl-LIGRLO-NH2) or vehicle was injected intraperitoneally daily for 3 days before irradiation (“before”), for 7 days after irradiation (“after”), or both 3 days before and 7 days after irradiation (“before-after”). Early and delayed radiation enteropathy was assessed 2 and 26 weeks after irradiation by quantitative histology, morphometry, and immuno-histochemical analysis.
Results
The PAR2 agonist did not elicit changes in unirradiated (shielded) intestine. In contrast, in irradiated intestine procured 2 weeks after irradiation, administration of PAR2 agonist was associated with more sever mucosal injury and increased intestinal wall thickness in all 3 treatment groups (p < 0.05) compared to vehicle-treated controls. The PAR2 agonist also exacerbated radiation injury score, serosal thickening, and mucosal inflammation (p < 0.05) in the before and the before-after groups. Short term exogenous activation of PAR2 did not impact radiation-induced intestinal injury at 26 weeks.
Conclusions
This study supports a role for PAR2 activation in the pathogenesis of early radiation-induced intestinal injury. Pharmacological PAR2 antagonists may have the potential to reduce intestinal side effects of radiation therapy and/or as countermeasures in radiological accidents or terrorism scenarios.
Keywords: Intestines, Radiation injury, PAR2 activating peptide, PAR2 agonist
Introduction
Despite advances in treatment delivery techniques, radiation therapy of cancer continues to be dose-limited by the tolerance of critical surrounding normal tissues. The intestine is an important dose-limiting organ during abdominal and pelvic radiotherapy. While many modifiers have been tested during the past 6 decades few are currently in clinical use. However, recent developments in the understanding of the mechanisms by which bowel toxicity occurs have provided an impetus for a more rational approach to identifying effective, non-toxic modulators of the intestinal radiation response.
The crypt epithelium is the main target cell responsible for intestinal radiation toxicity. However, the intestinal radiation response is also influenced by many additional factors, such as, endothelial dysfunction, intraluminal factors, and neuroimmune mechanisms. Recent work performed in our laboratory shows that radiation strongly upregulates and activates proteinase-activated receptor 2 (PAR2) in the rat intestine in vivo (1), suggesting a role in radiation enteropathy development.
PAR2 is one of a 4-member family of proteinase-activated receptors (PARs). PARs are G-protein coupled transmembrane receptors that are activated via specific serine proteases by a mechanism involving proteolytic cleavage of the receptor N terminus to expose a so-called “tethered ligand” sequence. The tethered ligand subsequently binds to the activation site and activates the receptor (2). PAR2 regulates many physiological processes, including inflammation and tissue remodeling (3). While specific PAR2 antagonists are not yet available, non-enzymatic PAR2 agonists provide an opportunity to directly investigate the involvement of PAR2 in the pathogenesis of various disease processes. In this study, an exogenous PAR2 agonist was used to address the role of PAR2 in intestinal radiation injury.
The present work provides clear evidence that PAR2 activation exacerbates early radiation-induced intestinal inflammation and structural injury. In contrast, short-term administration of PAR2 agonist did not appear to influence the severity of delayed intestinal radiation fibrosis.
Materials and Methods
Animals and Experimental Radiation Enteropathy Model
A total of 149 male Sprague-Dawley rats (200–250 g, Harlan, Indianapolis, IN) were housed in conventional cages in a pathogen-free environment with controlled humidity, temperature, and 12–12 light-dark cycle with free access to tap drinking water and chow (TD8640, Harlan Teklad, Madison, WI). The study was approved by the University of Arkansas for Medical Sciences Institutional Animal Care and Use Committee (IACUC).
The surgical model for localized small bowel irradiation was prepared as described previously (4). Briefly, rats underwent bilateral orchiectomy and a loop of distal ileum was sutured to the inside of the left part of the scrotum. The model creates a “scrotal hernia” that contains a 4-cm loop of small intestine that can be irradiated locally without additional surgery. The intestine remains technically within the abdominal cavity, and the surgical procedure does not cause appreciable long-term structural, functional, cellular, or molecular alterations. The model minimizes manipulation during irradiation and produces radiation-induced changes similar to those seen clinically. This model has been extensively used and validated in our laboratory.
After 3 weeks postoperative recovery, each rat was anesthetized with isoflurane inhalation and the bowel within the “scrotal hernia” was exposed to 16.5 Gy localized, single-dose radiation with a Seifert Isovolt 320 X-ray machine (Seifert X-Ray Corporation, Fairview Village, PA), operated at 250 kVp and 15 mA, with 3 mm Al added filtration (half-value layer 0.85 mm Cu, dose rate 4.49 Gy/min). Dosimetric considerations have been described previously. This radiation regimen was based on data from previous experiments and was designed to elicit moderate to severe radiation enteropathy.
Administration of PAR2 Agonist
The PAR2 agonist peptide 2-furoyl-LIGRLO-NH2 and control peptide (reverse-sequence peptide) 2-furoyl-OLRGIL-NH2 were synthesized by the Peptide Core Facility at the University of Calgary (Calgary, Alberta, Canada). All peptides were dissolved in 25 mM HEPES buffer and diluted to desired concentrations with 0.9% saline.
Demonstrating receptor activation and biological activity
Demonstration of receptor activation was performed by measuring calcium signaling in rat kidney KNRK cells transfected with either the PAR2-expressing construct (rPAR2 KNRK) or vector-alone (pcDNA3 KNRK) in response to PAR2 agonists as described in detail previously (5). The 2-furoyl-LIGRLO-NH2 (10 μM) was compared to that of the PAR2-activating enzyme, trypsin (1 U/ml; 2 nM). An agonist-mediated calcium signal observed in the rPAR2 KNRK cells, absent in receptor non-expressing pcDNA3 KNRK cells, indicates a selective activation of PAR2 by that agonist.
Temporary reduction of blood pressure is a prominent biological effect of PAR2 activation in vivo. On the other hand, the reverse sequence peptide should have no effect. A telemetry technique was used to measure blood pressure in conscious unrestrained rats (6). Male Sprague-Dawley rats were anesthetized with pentobarbital and atropine, and biotelemetry transmitters (PA-C40, Data Sciences International, St. Paul, MN) were implanted in the abdominal cavity with the pressure-sensing catheter in the descending aorta. The rats were placed in plastic cages above telemetry receivers and mean arterial blood pressure was recorded using the Dataquest IV software (Data Sciences International). Rats were infused with PAR2 agonist (N=4) or its reverse-sequence control peptide (N=4) through a jugular catheter.
Determining the effect of reverse-sequence peptide 2-furoyl-OLRGIL-NH2 on intestinal radiation injury
To ascertain that physiological saline was an appropriate vehicle for subsequent experiments, the effects of control reverse-sequence peptide 2-furoyl-OLRGIL-NH2 on radiation-induced intestinal injury were compared with those of 0.9% saline. Twenty rats were operated as described above to create the radiation model and randomly assigned to two groups: radiation (16.5 Gy) with reverse-sequence peptide 2-furoyl-OLRGIL-NH2 (0.3 mg/kg/day, N=10), or radiation with 0.9% saline (N=10). The reverse-sequence peptide or 0.9% saline were injected intraperitoneally once daily, starting 3 days before irradiation and continuing to 7 days after irradiation. The animals were euthanized 2 weeks after irradiation.
Effects of 2-furoyl-LIGRLO-NH2 on Radiation Injury
One hundred and twenty-one rats were randomly assigned to 4 groups: PAR2 agonist 2-furoyl-LIGRLO-NH2 (0.3 mg/kg intraperitoneally) injected once daily for 3 days before irradiation, followed by 7 daily injections with 0.9% saline (N=31); injections with 0.9% saline for 3 days before irradiation, followed by PAR2 agonist once daily for 7 days after irradiation (N=30); PAR2 agonist injected once daily for 3 days before and for 7 days after irradiation (N=30);, or 0.9% saline vehicle injected daily for 3 days before and 7 days after irradiation (N=30). The PAR2 agonist was injected no less than two hours after irradiation to avoid possible effects of blood pressure changes on radiation injury.
Assessment of the Intestinal Radiation Response
Groups of rats were euthanized 2 weeks and 26 weeks after irradiation (13–16 per treatment group and time point). These time points are representative of early and delayed radiation enteropathy in our model system. Specimens of irradiated and unirradiated intestine were procured and fixed in methanol-Carnoy’s solution for histological, morphometric, and immunohistochemical studies.
Quantitative histopathology and morphometry
Radiation injury score
The overall severity of structural radiation injury was assessed using the radiation injury score (RIS) scoring system. The RIS is a composite histopathological scoring system that provides a global measure of the severity of structural radiation injury and has been extensively used and validated (7). Briefly, seven histopathologic parameters of radiation injury (mucosal ulcerations, epithelial atypia, thickening of subserosa, vascular sclerosis, intestinal wall fibrosis, ileitis cystica profunda, and lymph congestion) were assessed and graded from 0–3. The sum of the scores for the individual alterations constitutes the RIS. All specimens were evaluated in a blinded fashion by two separate researchers.
Mucosal surface area
Radiation-induced decrease in the surface area of the intestinal mucosa is a sensitive parameter of small bowel radiation injury. Mucosal surface area was measured in vertical sections using a stereologic projection/cycloid method as described by Baddeley (8) and adapted by us for use with our model system (7). This technique does not require assumptions about the shape or orientation distribution of the specimens and thus circumvents problems associated with most other procedures for surface area measurement.
Thickness of the intestinal wall and subserosa
Intestinal wall thickening is a measure of both reactive intestinal wall fibrosis and intestinal smooth muscle cell hyperplasia, while subserosal thickening mainly reflects reactive fibrosis. Intestinal wall thickness (encompassing submucosa, muscularis externa, and subserosa) and subserosal thickness were measured with computer-assisted image analysis (Image-Pro Plus, Media Cybernetics, Silver Spring, MD) with a 10X objective. A total of 5 areas, 500 μm apart, were measured (3 measurements per area). The average of all 5 areas was used as a single value for statistical calculations.
Quantitative immunohistochemistry and image analysis
Immunohistochemistry and image analysis were used to assess 1) neutrophil infiltration; 2) proliferation rate of intestinal smooth muscle cells; 3) deposition of collagen types I and III; and 4) extracellular matrix-associated transforming growth factor-β (TGF-β) expression as described in detail and validated previously (9, 10).
Immunohistochemical staining was performed with avidin-biotin complex (ABC) technique, diaminobenzidine (DAB) chromogen, and hematoxylin counterstaining. Appropriate positive and negative controls were included. The primary antibodies, incubation times, dilutions, and sources were as follows: polyclonal anti-myeloperoxidase antibody (A0398, 2 hrs, 1:100, Dako, Carpinteria, CA); monoclonal antibody against proliferating cell nuclear antigen (PCNA, NA03, 2 hrs, 1:100, Calbiochem, Cambridge, MA); polyclonal antibodies against collagen types I and III (1310-01, 2 hrs, 1:100, and 1330-01, 2 hrs,1:100 dilution, Southern Biotechnology Associates, Birmingham, AL); and polyclonal antibody against TGF-β (AB-100-NA, 2 hrs, 1:300 dilution, R&D, Minneapolis, MN).
Myeloperoxidase
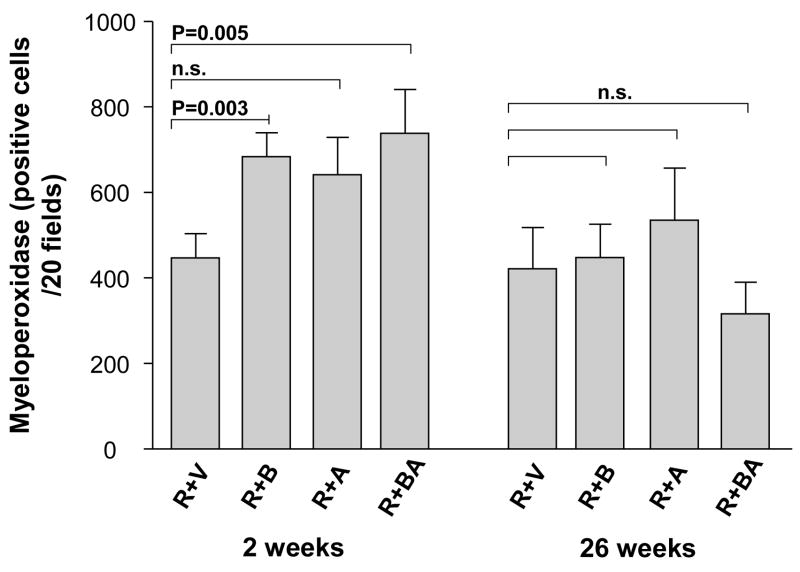
Myeloperoxidase is a well documented inflammation marker. Myeloperoxidase enzymatic activity in leukocytes correlates directly with neutrophil number (r = 0.99), and myeloperoxidase activity in tissue extract correlates directly with cellular infiltration assessed histologically (r = 0.94) (11). The number of myeloperoxidase-positive cells was determined by color thresholding and counted in twenty 40× fields per section, selected according to a predetermined grid pattern.
Smooth muscle cell proliferation
In the intestine, collagen is mainly produced by intestinal smooth muscle cells, rather than by fibroblasts. Intestinal smooth muscle cell proliferation rate is very low at baseline, but increases steeply after irradiation (12). Therefore, intestinal smooth muscle cell proliferation was assessed. The numbers of total smooth muscle cells and PCNA-positive smooth muscle cells were determined in twenty 40× fields using color thresholding and normalized to PCNA-positive smooth muscle cells per thousand smooth muscle cells.
Collagen deposition
Collagen accumulation is primarily a late endpoint after irradiation. In the irradiated intestine, however, significant accumulation of collagen type I and, even more markedly, collagen type III occurs as a reactive change after only 2 weeks. The relative areas positive for collagen type I and collagen type III were determined in 20 fields (40× magnification), according to procedures established by Raviv et al. (13) and modified by us (14).
Expression of TGF-β
TGF-β is overexpressed in many fibrotic conditions, including radiation fibrosis (15), and is mechanistically involved in radiation enteropathy (10). Relative areas positive for TGF-β were determined in 20 fields (40×) according to procedures described perviously (14).
Statistical Methods
Statistical analysis was performed with the software package NCSS2004 for Windows (NCSS, Kaysville, UT). Differences in endpoints among treatment groups were assessed by one-way analysis of variance (ANOVA). The Dunnett’s test was used for post hoc multiple comparison testing. Differences between two groups were assessed by the Mann-Whitney U test. P-values less than 0.05 were considered statistically significant.
Results
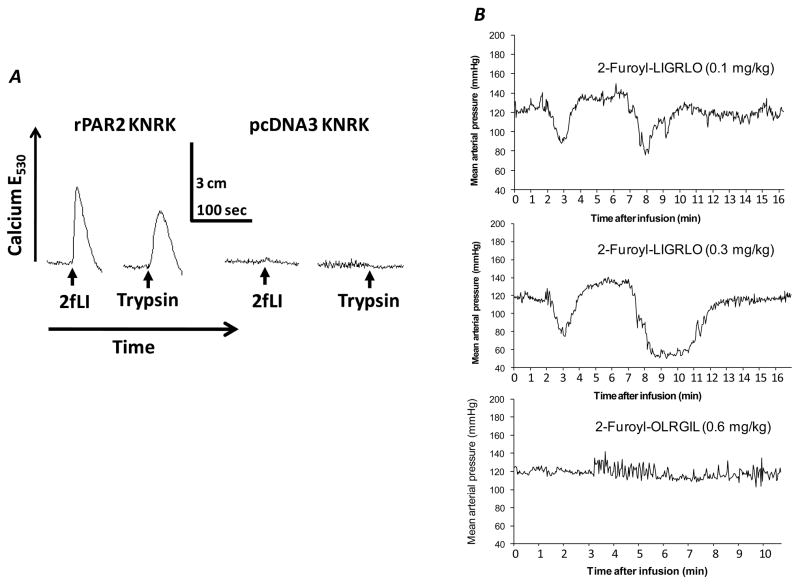
The PAR2 agonist demonstrated specific activation of PAR2 in vitro and the expected biological effect in vivo. A strong calcium signal was observed in the rPAR2 KNRK cells in reponse to both the PAR2 agonist and trypsin, while there was none in the receptor non-expressing pcDNA3 KNRK cells (Fig. 1A). The 2-furoyl-LIGRLO-NH2 induced a dose-dependent biphasic reduction in blood pressure in unrestrained rats; blood pressure was normalized after 12 minutes (Fig. 1B). The reverse-sequence peptide 2-furoyl-OLRGIL-NH2 had no impact on blood pressure.
Fig. 1.
Panel A: KNRK cells that either do (rPAR2 KNRK) or do not (pcDNA3 KNRK) express functional PAR2 were exposed to either 2-furoyl-LIGRLO-NH2 (2fLI: 10 μM) or trypsin (1 U/ml; 2 nM) and the changes in intracellular calcium were monitored (upward deflection, E530). The scales for time (seconds) and calcium response (upward deflection in cm) are inset.
Panel B: Mean arterial blood pressure in conscious rats after infusion with 2-furoyl-LIGRLO-NH2 (recordings every 2 seconds). The 2-furoyl-LIGRLO-NH2 induced a biphasic dose-dependent reduction in blood pressure, while the reverse-sequence peptide had no effect.
There were no differences between control reverse-sequence peptide 2-furoyl-OLRGIL-NH2 and 0.9% saline in RIS, mucosal surface area, and the thickness of intestinal wall and serosa at 2 weeks after irradiation (p > 0.05 for all parameters, data not shown). Therefore, in subsequent experiments, 0.9% saline was used as vehicle control.
Radiation-induced histopathologic changes in this study were similar to those observed previously in our laboratory. Early alterations (2 weeks) consisted mainly of mucosal injury and ulcerations, reactive bowel wall thickening, and inflammatory cell infiltration. Vascular sclerosis, chronic mucosal ulcerations, and intestinal wall fibrosis predominated at the late observation time (26 weeks).
In unirradiated (shielded) intestine, administration of the PAR2 agonist did not affect intestinal mucosal surface area, thickness of intestinal wall and serosa, neutrophil infiltration, proliferation of intestinal smooth muscles, and deposition of collagen type III at 2 weeks and 26 weeks (p > 0.05 for all parameters, data not shown).
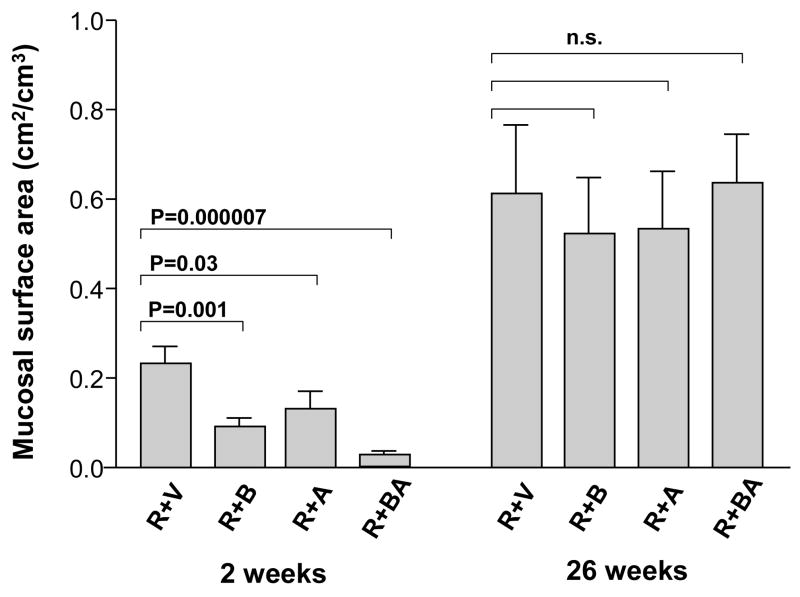
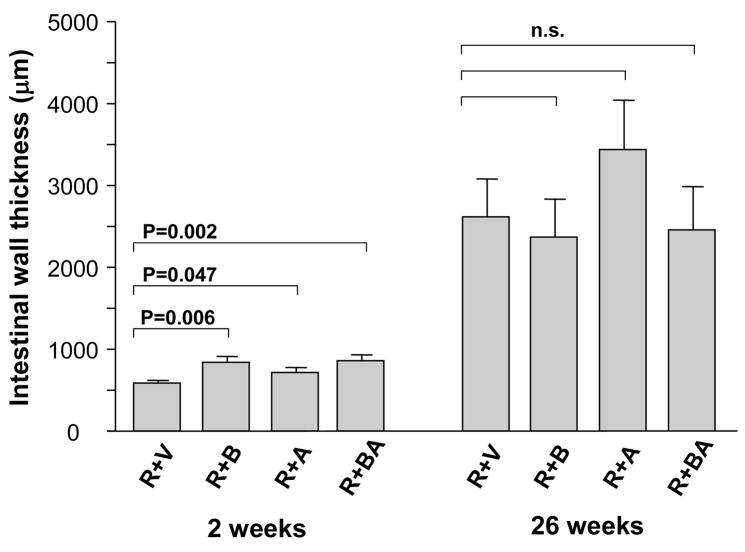
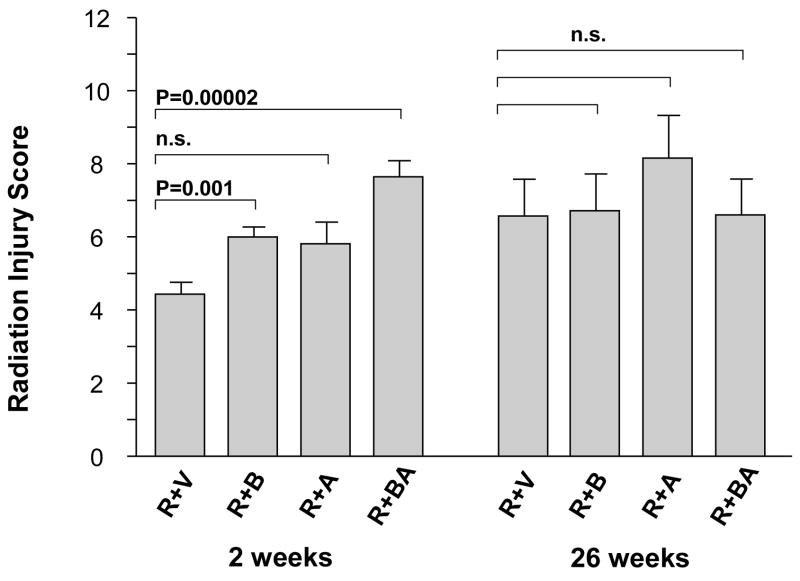
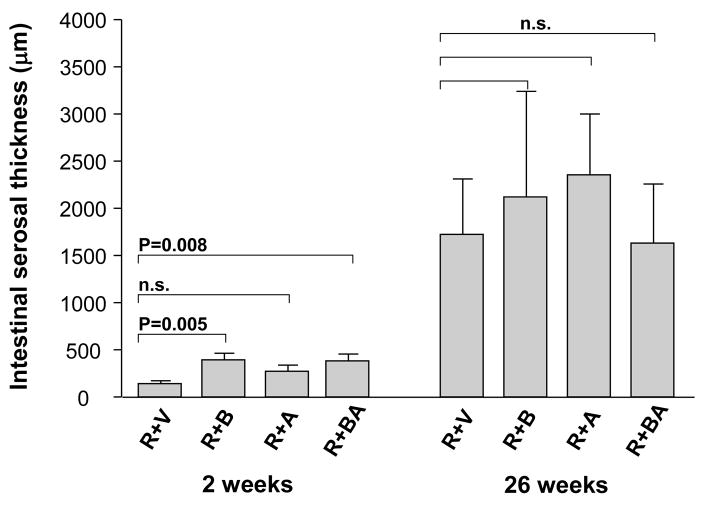
In contrast, in irradiated intestine procured 2 weeks after irradiation, the PAR2 agonist clearly exacerbated intestinal mucosa injury (p < 0.05) and increased intestinal wall thickness (p < 0.05) in all three PAR2 agonist groups, compared to vehicle-treated controls (Fig. 2 and Fig. 3). Moreover, the PAR2 agonist increased radiation injury score (p = 0.001 and p = 0.00002), intestinal serosal thickness (p = 0.005 and p = 0.008), and neutrophil infiltration (p = 0.003 and p = 0.005), when administered 3 days before irradiation as well as when administered both before and after irradiation, compared to the vehicle group (Fig. 4, Fig. 5, and Fig. 6). The PAR2 agonist did not affect the proliferation of intestinal smooth muscle and immunoreactivity of collagen type I, collagen type III, and TGF-β in either of the three PAR2 agonist groups (p > 0.05 for all three parameters, data not shown).
Fig. 2.
Intestinal mucosal surface area (mean, standard error of the mean [SEM]) in irradiated animals treated with vehicle and PAR2 agonist. The PAR2 agonist significantly exacerbated radiation-induced intestinal mucosal injury at 2 weeks but not at 26 weeks. The p-values refer to the difference between vehicle-treated and PAR2 agonist-treated animals. R + V = irradiation + vehicle; R + B = irradiation + PAR2 agonist (3 days before irradiation); R + A = irradiation + PAR2 agonist (7 days after irradiation); R + BA = irradiation + PAR2 agonist (3 days before and 7 days after irradiation).
Fig. 3.
Intestinal wall thickness (mean, SEM) in irradiated animals treated with vehicle and PAR2 agonist.
PAR2 agonist significantly exacerbated radiation-induced intestinal wall thickening at 2 weeks but not at 26 weeks. p-values and experimental group abbreviations as for Fig. 2.
Fig. 4.
Composite histopathologic intestinal radiation injury score (RIS) in irradiated animals treated with vehicle and PAR2 agonist (mean, SEM). PAR2 agonist significantly exacerbated radiation-induced intestinal histopathologic injury in the groups of R+B and R+BA at 2 weeks but not at 26 weeks. p-values and experimental group abbreviations as for Fig. 2.
Fig. 5.
Thickness of the intestinal subserosa (mean, SEM) in irradiated animals treated with vehicle and PAR2 agonist. PAR2 agonist significantly exacerbated radiation-induced intestinal subserosa thickening in the groups of R+B and R+BA at 2 weeks but not at 26 weeks. p-values and experimental group abbreviations as for Fig. 2.
Fig. 6.
Number of myeloperoxidase-positive cells (mean, SEM) in the intestinal wall of irradiated animals treated with vehicle and PAR2 agonist. PAR2 agonist significantly increased the accumulation of myeloperoxidase-positive cells in the groups of R+B and R+BA at 2 weeks but not at 26 weeks. p-values and experimental group abbreviations as for Fig. 2.
Despite the prominent changes seen at 2 weeks, at 26 weeks after irradiation, no statistically significant differences among the treatment groups were found in RIS, mucosal surface area, thickness of intestinal wall and serosa (Fig. 2–6) or for smooth muscle proliferation, neutrophil infiltration, and immunoreactivity of collagen type I, collagen type III, and TGF-β (p > 0.05 for all parameters, data not shown).
Discussion
Our results demonstrate the following: First, PAR2 activation exerts a detrimental effect on early structural injury and mucositis development in the irradiated intestine. Second, the observation that PAR2 activation before or before and after irradiation had a more pronounced effect than PAR2 activation only after irradiation has implications for the likely relative roles of two major physiological PAR2 activators, pancreatic trypsin and mast cell proteases, in the radiation response. Furthermore, the potential translational implication of this finding is that, during a course of fractionated irradiation, PAR2 activation that takes place during the early part of the treatment course may be particularly detrimental.
The primary focus of this study was on localized irradiation as during radiation therapy of cancer. However, we used single dose radiation to be able to unequivocally distinguish between the effects of pre-and post-radiation administration of the PAR2 agonist. The use of local (instead of total abdominal or total body) irradiation, besides being clinically more relevant, provided a shielded intestinal control segment in each animal, thus increasing statistical power and reducing the number of animals required.
Nonenzymatic activation of PARs can be achieved by direct binding of synthetic peptides based on the tethered ligand sequence (16). PAR2 peptide agonists have proven useful in delineating the effects of activating PAR2 in more complex biological systems in vitro and in vivo (2, 16). The PAR2 peptide 2-furoyl-LIGRLO-NH2 is the most potent among known PAR2 agonists, in part because it is relatively resistant to degradation by aminopeptidases and also because it is highly specific for the activation of PAR2, i.e., it does not activate PAR1 and PAR4 (17).
Previous work in our laboratory has shown that PAR2 is upregulated and activated in response to irradiation and that elimination of the major physiological PAR2 activators ameliorate intestinal radiation injury (1). The present study shows that early, short-term exogenous PAR2 activation either before or after irradiation exacerbates overall early radiation-induced structural damage, mucosal injury, and intestinal inflammation. Taken together, these data strongly support a role for PAR2 in the regulation of early radiation enteropathy.
The observation that PAR2 activation exacerbates intestinal radiation injury is consistent with evidence from other studies. First, PAR2 regulates several physiological processes that are relevant to intestinal radiation injury, including ion transport (18); motility (19); proliferation of endothelial cells, myocytes, and fibroblasts (20, 21); vascular tone (22); vascular and epithelial barrier permeability (23, 24); and inflammation (25). Second, PAR2 activation promotes endothelial dysfunction by inducing tissue factor (TF) and by causing the release of von Willebrand factor (26). PAR2 activation also contributes to several early events in inflammation, including leukocyte rolling, adherence, and recruitment (27).
Despite the fairly strong evidence cited above, the roles of PAR2 as a regulator of intestinal injury and inflammation in vivo is complex. Some investigators have reported that PAR2 activation exacerbates intestinal injury in non-radiation models (28, 29) and that PAR2 deficient mice sustain less injury than wild-type controls (30, 31). On the other hand, others have found that PAR2 activation is protective (32, 33). These apparent inconsistencies may be due to a) differences between local versus systemic administration of the activating peptide, b) to the presence of other proteases (e.g., elastase and cathepsin G) that may disarm PAR2 and thus prevent activation (34), c) or to differences among disease models.
Trypsin, a serine endopeptidase present in high concentration in secretions from the exocrine pancreas, is the major biologically relevant activator of PAR2. Intraluminal instillation of trypsin induces colonic inflammation, bacterial translocation, and upregulates PAR2 (35). Previous studies from our laboratory show that blockade of exocrine pancreatic secretions not only confers protection against radiation mucositis, but also blunts post-radiation upregulation and activation of PAR2 (1). Taken together, these observations suggest that trypsin/PAR2 interactions may be involved in the pathogenesis of radiation enteropathy.
Mast cell proteases are the other major class of PAR2 activating proteinases, and it has now become apparent that tryptase and similar mast cell proteases mediate many of their inflammatory and fibroproliferative properties through activation of PAR2. Mucosal mast cells of the intestine are highly radiation sensitive (36), and irradiation causes rapid mast cell degranulation (37). Our studies in mast cell-deficient rats have firmly established a role for mast cells in radiation enteropathy and also point to PAR2 activation as a potential pathogenic mechanism (1, 12).
In contrast to the robust link between PAR2 and early radiation enteropathy, administration of a PAR2 agonist during the peri-irradiation period did not appreciably influence development of delayed intestinal radiation fibrosis. This observation may reflect differences in pathogenetic mechanisms underlying early versus delayed injury. Another possibility relates to the duration of exogenous versus endogenous stimulation of PAR2 activation. The half life of peptide PAR2 agonists is relatively short and (patho-)physiological effects are predominantly due to downstream events. On the other hand, endogenous activators, such as, mast cell proteases or pancreatic trypsin may have more sustained effect beyond a 7-day post-radiation period.
In conclusion, or findings point to PAR2 as a potential target for pharmacological interventions aimed at preventing or treating early intestinal radiation toxicity in the clinic. While the findings reported here are primarily relevant to adverse effects in cancer patients, they also suggest that PAR2 antagonists could be developed as a countermeasure against radiological and nuclear accidents and terrorism. Further studies should be performed to assess PAR2 involvement in the pathogenesis of delayed radiation-induced intestinal injury and to investigate whether PAR2 antagonists could be used to reduce structural or functional injury to the bowel after exposure to total body irradiation.
Acknowledgments
We are grateful to Dr. Mahmoud Saifeddine, University of Calgary, for conducting the calcium signaling experiments.
Financial Support: National Institutes of Health (Grants CA-72382, CA-83719, and IA-67798 to MH-J).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wang J, Zheng H, Hollenberg MD, et al. Upregulation and activation of proteinase-activated receptor-2 (PAR-2) in early and delayed radiation injury in the rat intestine: influence of biological PAR-2 activators. Radiat Res. 2003;160:524–535. doi: 10.1667/rr3080. [DOI] [PubMed] [Google Scholar]
- 2.Hollenberg MD, Compton SJ. International Union of Pharmacology. XXVIII. Proteinase-activated receptors. Pharmacol Rev. 2002;54:203–217. doi: 10.1124/pr.54.2.203. [DOI] [PubMed] [Google Scholar]
- 3.Vergnolle N, Wallace JL, Bunnett NW, et al. Protease-activated receptors in inflammation, neuronal signaling and pain. Trends Pharmacol Sci. 2001;22:146–152. doi: 10.1016/s0165-6147(00)01634-5. [DOI] [PubMed] [Google Scholar]
- 4.Hauer-Jensen M, Poulakos L, Osborne JW. Effects of accelerated fractionation on radiation injury of the small intestine: a new rat model. Int J Radiat Oncol Biol Phys. 1988;14:1205–1212. doi: 10.1016/0360-3016(88)90399-9. [DOI] [PubMed] [Google Scholar]
- 5.Al-Ani B, Saifeddine M, Kawabata A, et al. Proteinase-activated receptor 2 (PAR2): development of a ligand-binding assay correlating with activation of PAR2 by PAR1 and PAR2-derived peptide ligands. J Pharmacol Exp Ther. 1999;290:753–760. [PubMed] [Google Scholar]
- 6.Kennedy RH, Melchert RB, Joseph J. Cardiovascular effects of hyperhomocysteinemia in conscious unrestrained rats. Am J Hypertens. 2006;19:94–97. doi: 10.1016/j.amjhyper.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 7.Langberg CW, Sauer T, Reitan JB, et al. Relationship between intestinal fibrosis and histopathologic and morphometric changes in consequential and late radiation enteropathy. Acta Oncol. 1996;35:81–87. doi: 10.3109/02841869609098484. [DOI] [PubMed] [Google Scholar]
- 8.Baddeley AJ, Gundersen HJ, Cruz-Orive LM. Estimation of surface area from vertical sections. J Microsc. 1986;142:259–276. doi: 10.1111/j.1365-2818.1986.tb04282.x. [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Zheng H, Ou X, et al. Deficiency of microvascular thrombomodulin and up-regulation of protease-activated receptor-1 in irradiated rat intestine: possible link between endothelial dysfunction and chronic radiation fibrosis. Am J Pathol. 2002;160:2063–2072. doi: 10.1016/S0002-9440(10)61156-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng H, Wang JR, Koteliansky VE, et al. Recombinant soluble transforming growth factor beta type II receptor ameliorates radiation enteropathy in mice. Gastroenterology. 2000;119:1286–1296. doi: 10.1053/gast.2000.19282. [DOI] [PubMed] [Google Scholar]
- 11.Boughton-Smith NK, Wallace JL, Whittle, et al. Relationship between arachidonic acid metabolism, myeloperoxidase activity and leukocyte infiltration in a rat model of inflammatory bowel disease. Agents Actions. 1988;25:115–123. doi: 10.1007/BF01969102. [DOI] [PubMed] [Google Scholar]
- 12.Zheng H, Wang J, Hauer-Jensen M. Role of mast cells in early and delayed radiation injury in rat intestine. Radiat Res. 2000;153:533–539. doi: 10.1667/0033-7587(2000)153[0533:romcie]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 13.Raviv G, Kiss R, Vanegas JP, et al. Objective measurement of the different collagen types in the corpus cavernosum of potent and impotent men: an immunohistochemical staining with computerized-image analysis. World J Urol. 1997;15:50–55. doi: 10.1007/BF01275157. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Zheng H, Hauer-Jensen M. Influence of short-term octreotide administration on chronic tissue injury, transforming growth factor β (TGF-β) overexpression, and collagen accumulation in irradiated rat intestine. J Pharmacol Exp Ther. 2001;297:35–42. [PubMed] [Google Scholar]
- 15.Richter KK, Langberg CW, Sung CC, et al. Association of transforming growth factor beta Immunoreactivity with specific histopathologic lesions in subacute and chronic experimental radiation enteropathy. Radiother Oncol. 1996;39:243–251. doi: 10.1016/0167-8140(95)01735-6. [DOI] [PubMed] [Google Scholar]
- 16.MacFarlane SR, Seatter MJ, Kanke T, et al. Proteinase-activated receptors. Pharmacol Rev. 2001;53:245–282. [PubMed] [Google Scholar]
- 17.McGuire JJ, Saifeddine M, Triggle CR, et al. 2-furoyl-LIGRLO-amide: a potent and selective proteinase-activated receptor 2 agonist. J Pharmacol Exp Ther. 2004;309:1124–1131. doi: 10.1124/jpet.103.064584. [DOI] [PubMed] [Google Scholar]
- 18.Vergnolle N, MacNaughton WK, Al-Ani B, et al. Proteinase-activated receptor 2 (PAR2)-activating peptides: identification of a receptor distinct from PAR2 that regulates intestinal transport. Proc Natl Acad Sci USA. 1998;95:7766–7771. doi: 10.1073/pnas.95.13.7766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corvera CU, Déry O, McConalogue K, et al. Mast cell tryptase regulates rat colonic myocytes through proteinase-activated receptor 2. J Clin Invest. 1997;100:1383–1393. doi: 10.1172/JCI119658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mirza H, Yatsula V, Bahou WF. The proteinase activated receptor-2 (PAR-2) mediates mitogenic responses in human vascular endothelial cells. J Clin Invest. 1996;97:1705–1714. doi: 10.1172/JCI118597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akers IA, Parsons M, Hill MR, et al. Mast cell tryptase stimulates human lung fibroblast proliferation via protease-activated receptor-2. Am J Physiol. 2000;278:L193–L201. doi: 10.1152/ajplung.2000.278.1.L193. [DOI] [PubMed] [Google Scholar]
- 22.Emilsson K, Wahlestedt C, Sun MK, et al. Vascular effects of proteinase-activated receptor 2 agonist peptide. J Vasc Res. 1997;34:267–272. doi: 10.1159/000159233. [DOI] [PubMed] [Google Scholar]
- 23.Kawabata A, Kuroda R, Minami T, et al. Increased vascular permeability by a specific agonist of protease-activated receptor-2 in rat hindpaw. Br J Pharmacol. 1998;125:419–422. doi: 10.1038/sj.bjp.0702063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bueno L, Fioramonti J. Protease-activated receptor 2 and gut permeability. Neurogastroenterology & Motility. 2008;20:580–587. doi: 10.1111/j.1365-2982.2008.01139.x. [DOI] [PubMed] [Google Scholar]
- 25.Fiorucci S, Distrutti E. Role of PAR2 in pain and inflammation. Trends Pharmacol Sci. 2002;23:153–155. doi: 10.1016/s0165-6147(00)01932-5. [DOI] [PubMed] [Google Scholar]
- 26.Storck J, Kusters B, Vahland M, et al. Trypsin induced von Willebrand factor release from human endothelial cells is mediated by PAR-2 activation. Thromb Res. 1996;84:463–473. doi: 10.1016/s0049-3848(96)00214-9. [DOI] [PubMed] [Google Scholar]
- 27.Vergnolle N. Proteinase-activated receptor-2-activating peptides induce leukocyte rolling, adhesion, and extravasation in vivo. J Immunol. 1999;163:5064–5069. [PubMed] [Google Scholar]
- 28.Jacob C, Yang PC, Darmoul D, et al. Mast cell tryptase controls paracellular permeability of the intestine. Role of protease-activated receptor 2 and beta-arrestins. J Biol Chem. 2005;280:31936–31948. doi: 10.1074/jbc.M506338200. [DOI] [PubMed] [Google Scholar]
- 29.Cenac N, Coelho AM, Nguyen C, et al. Induction of intestinal inflammation in mouse by activation of proteinase-activated receptor-2. Am J Pathol. 2002;161:1903–1915. doi: 10.1016/S0002-9440(10)64466-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cottrell GS, Amadesi S, Pikios S, et al. Protease-activated receptor 2, dipeptidyl peptidase I, and proteases mediate Clostridium difficile toxin A enteritis. Gastroenterology. 2007;132:2422–2437. doi: 10.1053/j.gastro.2007.03.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hyun E, Andrade-Gordon P, Steinhoff M, et al. Protease-activated receptor-2 activation: a major actor in intestinal inflammation. Gut. 2008;57:1222–1229. doi: 10.1136/gut.2008.150722. [DOI] [PubMed] [Google Scholar]
- 32.Cataruzza F, Cenac N, Barocelli E, et al. Protective effect of proteinase-activated receptor 2 activation on motility impairment and tissue damage induced by intestinal ischemia/reperfusion in rodents. Am J Pathol. 2006;169:177–188. doi: 10.2353/ajpath.2006.051098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fiorucci S, Mencarelli A, Palazzetti B, et al. Proteinase-activated receptor 2 is an anti-inflammatory signal for colonic lamina propria lymphocytes in a mouse model of colitis. Proc Natl Acad Sci USA. 2001;98:13936–13941. doi: 10.1073/pnas.241377298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dulon S, Cande C, Bunnett NW, et al. Proteinase-Activated Receptor-2 and Human Lung Epithelial Cells: Disarming by Neutrophil Serine Proteinases. Am J Respir Cell Mol Biol. 2003;28:339–346. doi: 10.1165/rcmb.4908. [DOI] [PubMed] [Google Scholar]
- 35.Cenac N, Coelho AM, Nguyen C, et al. Induction of intestinal inflammation in mouse by activation of proteinase-activated receptor 2. Am J Pathol. 2002;161:1903–1913. doi: 10.1016/S0002-9440(10)64466-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fukuzumi T, Waki N, Kanakura Y, et al. Differences in irradiation susceptibility and turnover between mucosal and connective tissue-type mast cells of mice. Exp Hematol. 1990;18:843–847. [PubMed] [Google Scholar]
- 37.Albrecht M, Muller K, Kohn FM, et al. Ionizing radiation induces degranulation of human mast cells and release of tryptase. Int J Radiat Biol. 2007;83:535–541. doi: 10.1080/09553000701444657. [DOI] [PubMed] [Google Scholar]