Abstract
IL-27 counters the effect of TGF-β+IL-6 on naïve CD4+ T cells, resulting in near complete inhibition of de novo Th17 development. In contrast, little is known about the effect of IL-27 on already differentiated Th17 cells. A better understanding of how IL-27 regulates these cells is needed to evaluate the therapeutic potential of IL-27 in Th17 cells-associated diseases.
Here we show that IL-27 had surprisingly little effect on committed Th17 cells, despite their expression of a functional IL-27R. Contrary to de novo differentiation of Th17 cells, IL-27 did not suppress expression of RORγt or RORα in committed Th17 cells. Consistent with this finding, the frequency of committed Th17 cells and their cytokine secretion remained unaffected by IL-27. Both memory Th17 cells (CD4+CD25−CD62Llow) that developed in vivo and encephalitogenic Th17 cells infiltrating the CNS of mice developing experimental autoimmune encephalomyelitis (EAE) produced similar amounts of IL-17A when reactivated with IL-23, in the absence and presence of exogenous IL-27. Finally, IL-27 failed to suppress encephalitogenicity of Th17 cells in an adoptive transfer of EAE. Analysis ex vivo of transferred Th17 cells in the spleen and CNS of recipient mice showed that cells retained similar phenotype irrespective of whether cells were treated or not with IL-27.
Our data demonstrate that in contrast to inhibition of de novo differentiation of Th17 cells, IL-27 has little or no effect on committed Th17 cells. These findings indicate that therapeutic applications of IL-27 might have a limited efficacy in inflammatory conditions where aggressive Th17 responses have already developed.
Keywords: IL-27, Th17 cells, IL-23, EAE
Introduction
To date, studies of mouse Th17 cells have focused mainly on regulation of their development from naïve CD4+ precursors. Th17 differentiation requires the presence of IL-6 and TGF-β and is further enhanced by IL-1β, TNF-α and IL-21 (1, 2). IL-23 is not required for initial Th17 commitment but is essential for Th17 cell function. Lack of IL-23R in Th17 cells prevented their terminal differentiation into functional effector cells in vivo. IL-23R-deficient Th17 cells were susceptible to apoptosis and maintained an immature phenotype with a failure to downregulate IL-2 and CD27 and to upregulate IL-7Rα (3, 4).
RORγt and RORα are lineage-specific transcription factors that direct Th17 development (5, 6). STAT3 is crucial to development of Th17 cells, and cytokines that promote differentiation of Th17 cells, IL-6, IL-21 and IL-23, all preferentially activate STAT3 (7–9). Commitment to the Th17 lineage is antagonized by Th1 and Th2 cytokines, and both IFN-γ and IL-4 suppress Th17 differentiation (10, 11). Th17 cells secrete a range of mediators including IL-17A, IL-17F, IL-21, and IL-22 (12). The postulated role of Th17 cells is in immunity to extracellular bacteria and fungi and the importance of Th17 cells in pathogenesis of certain autoimmune diseases is now widely accepted (13, 14). In light of reports of Th cells expressing both IL-17 and IFN-γ (15, 16), more attention has been directed to the biology of already committed Th17 cells, in particular to the stability and plasticity of their phenotype. Repeated stimulation of Th17 cells in the presence of IL-23 led to a decrease in IL-17A+ cells while the frequency of IL-17A− IFN-γ+ cells increased substantially (17). In contrast, continuous exposure to TGF-β, alone or in addition to either IL-23 or IL-6 during restimulation of Th17 cells, was required to avoid conversion to the Th1 lineage (17). IL-12 has a profound effect on committed Th17 cells by readily converting them into Th1 cells, even in the presence of IL-23, These findings demonstrate that Th17 cells have “unstable phenotype” and can transition into Th1 cells (17).
IL-27 is a heterodimeric cytokine consisting of Epstein-Barr virus-induced gene 3 (EBI3), and p28 (18). The main source of IL-27 appears to be activated antigen-presenting cells (APCs) (18, 19). IL-27 signals via its heterodimeric receptor (IL-27R), which consists of the receptor subunits gp130 and WSX-1 (20, 21). The two IL-27R subunits are expressed by a variety of immune cells including T cells, NK cells, mast cells, B cells and activated dendritic cells (DCs) (20–24). IL-27 promotes Th1 polarization by inducing expression of T-bet in a STAT1-dependent manner, resulting in IL-12Rβ2 expression on the surface of newly activated T cells and IFN-γ production (23, 25). IL-27 inhibits Th2 cell development as well as Th2 cytokine production from polarized Th2 cells by down-regulation of GATA-3 and up-regulation of T-bet expression simultaneously (26). IL-27 has also been shown to inhibit development of Foxp3+ inducible regulatory T cells (iTreg) (27, 28). These early reports have emphasized the pro-inflammatory functions of IL-27. However, subsequent studies showed a more complex role for IL-27, as it also exerts anti-inflammatory functions. Two such reports have shown increased CNS inflammation in WSX-1−/− (IL-27R-deficient) mice either with experimental autoimmune encephalomyelitis (EAE) or infected with T. gondi (29, 30). This enhanced inflammation was associated with increased numbers of Th17 cells in the CNS (29, 30). In addition, we have shown previously that delivery of exogenous IL-27, during the priming phase of anti-myelin response, ameliorates EAE, with evidence of suppression of both Th1 and Th17 responses (31). In vitro, IL-27 efficiently counters the effect of TGF-β+IL-6 on naïve CD4+ T cells, resulting in near complete inhibition of de novo Th17 development in a STAT1 dependent manner (29, 30). Further study of the mechanism of action of IL-27 on Th17 development has revealed that this cytokine inhibits the expression of RORγt (32). More recent findings showing the ability of IL-27 to induce IL-10 secretion from both CD4+ and CD8+ T cells provide a new mechanism that may explain the anti-inflammatory effects of IL-27. Accordingly, T cells from WSX-1−/− mice infected with T. gondi displayed a reduced capacity to produce IL-10 and to dampen excessive immune response (33). Similarly, IL-27-mediated inhibition of EAE was IL-10-dependent (34).
Overall, the findings presented above highlight the complex and pleiotropic role of IL-27 in immune responses. While IL-27 is one of the most potent inhibitors of Th17 differentiation, little is known about how IL-27 regulates committed Th17 cells. This aspect of effector/memory Th17 cell biology is crucial to understanding the mechanisms that regulate inflammation in peripheral tissues during the effector phase of an immune response. An assumption that IL-27 has similar effects on differentiated Th17 cells as on naïve CD4+ T cells might be incorrect. This view is supported by the finding that IL-27 augmented IFN-γ production by naïve T cells stimulated in non-polarizing conditions, while it suppressed IFN-γ secretion by activated CD4+ T cells (35). In addition, differentiated Th17 cells seem to acquire resistance to suppression by IL-4 and IFN-γ, two cytokines that, similarly to IL-27, have inhibitory effects on the initial development of Th17 cells (10). Thus, in order to assess the therapeutic potential of exogenous IL-27, it is essential to know whether IL-27 negatively regulates committed Th17 cells, given that in a clinical setting pathogenic Th17 cells have already developed before initiation of treatment.
We have previously shown that IL-27 suppressed encephalitogenic Th1 and Th17 responses (31). However, whether IL-27 influenced effector Th17 cells directly or indirectly have not been determined. In this study, using in vitro differentiated Th17 cells, we found that IL-27 does not affect an established Th17 phenotype. Even though committed Th17 cells retain expression of IL-27R and respond to IL-27 by phosphorylating both STAT1 and STAT3, IL-27 failed to suppress expression of RORγt, RORα and IL-23R, or to modify responsiveness of these cells to IL-23. Unlike in the case of developing Th17 cells, IL-27 did not upregulate expression of T-bet in committed Th17 cells, or converted their phenotype to Th1 lineage as IL-12 does. In addition, IL-27 did not suppress encephalitogenicity of Th17 cells in an adoptive EAE model. Taken together, our data clearly demonstrate that Th17 cells, depending on the stage of their development, exhibit a sharp difference in their susceptibility to IL-27, with differentiating Th17 cells being susceptible and committed Th17 cell being resistant to suppression by IL-27.
Material and Methods
Mice
C57BL/6 and T-bet-deficient mice were purchased from The Jackson Laboratory (Bar Harbor, ME). STAT1-deficient mice were purchased from Taconic (Hudson, NY). 2D2 mice were kindly provided by V.K Kuchroo (Harvard Medical School, Boston, MA). All experimental procedures were approved by the Institutional Animal Care and Use Committee of Thomas Jefferson University.
Reagents
Anti-CD3 (145-2C11) and anti-CD28 (37.51) antibodies were purchased from BD Biosciences (San Jose, CA). The following antibodies for flow cytometry were from BD Biosciences: anti-CD4 (RM4-5), anti-CD62L (MEL-14), anti-IFN-γ (XMG1.2), anti-IL-17 (TC11-18H10), and anti-CD16/32 antibody (2.4G2). Neutralizing antibodies against IFN-γ and IL-4 and all cytokines used were from R&D systems. Duoset ELISA kits used to quantify IL-17A, IL-17F, IL-21, IL-10 and a quantikine ELISA kit to measure IL-22 were from R&D systems (Minneapolis, MN).
Cell preparation and culture
CD4+ T cells enriched from spleen mononuclear cells by magnetic microbead cell sorting (Miltenyi Biotec) or total splenocytes were cultured in RPMI 1640 supplemented with 10% FBS (Gibco BRL; Invitrogen), 2 mM L-glutamine, 1 mM Na-pyruvate, 1× non-essential amino acids, 100 µg/ml penicillin, 100 µg/ml streptomycin, and 0.5 µM 2-mercaptoethanol. Purified CD4+ T cells were stimulated with anti-CD3 (1 µg/ml) and anti-CD28 (1 µg/ml) antibodies in 48-well plates (1 ml of media containing 0.7×106 cells per well) in Th17 conditions (2 ng/ml TGF-β, 20 ng/ml IL-6) during 72h. Total splenocytes were stimulated in the same Th17 supporting conditions but in 24-well plates (2 ml of media containing 1.5×106 cells per well). Differentiated Th17 cells were then rested 2 days in the presence of IL-2 (2 ng/ml), washed and replated for a 2nd stimulation with anti-CD3 and anti-CD28 antibodies in the presence either of TGF-β+IL-6 (± IL-27), IL-23 (± IL-27) or medium (± IL-27) during 72h. When indicated, Th17 cells underwent a 3rd stimulation as described for the 2nd stimulation. Splenocytes from 2D2 mice were stimulated in the presence of MOG35–55 peptide (20 µg/ml) in 24-well plates (2 ml of media containing 3×106 cells per well) in Th17 conditions (2 ng/ml TGF-β, 20 ng/ml IL-6) during 72h. Differentiated Th17 cells were then rested 2 days in the presence of IL-2 (2 ng/ml), washed and replated for a 2nd stimulation with peptide in the presence either of TGF-β+IL-6 (± IL-27), IL-23 (± IL-27) or medium (± IL-27). Where indicated in figure legends, cultures were supplemented with anti-mouse IFN-γ (5 µg/ml), anti-mouse IL-4 (5 µg/ml), IL-23 (10 ng/ml) or IL-27 (10 ng/ml). After each stimulation period, cells were used for flow cytometric analysis or RNA extraction and supernatants were used for cytokine measurement by ELISA.
Induction of EAE and isolation of CNS infiltrating cells
Female 8- to 10-wk-old C57BL/6 mice were immunized s.c. with 150 µg of MOG35–55 in CFA containing 5 mg/ml Mycobacterium tuberculosis H37Ra (Difco), at two sites on the back. Mice were injected with 200 ng of pertussis toxin in PBS i.p. on days 0 and 2 and were scored daily for appearance of clinical signs of EAE. At the peak of disease (day 18 post immunization), mice were sacrificed and brains and spinal cords were removed and pooled after transcardial perfusion with PBS. Tissues were mechanically dissociated through a 100-µm strainer and washed with PBS. The resultant pellet was fractionated on a 60/30% Percoll gradient by centrifugation at 300 × g for 20 min. Infiltrating mononuclear cells were harvested from the interface, washed, counted, and cultured for 3 days in the presence of MOG35–55 peptide (20 µg/ml), IL-23 (± IL-27) and irradiated syngeneic splenocytes (3000 rad) in RPMI 1640 supplemented with 10% FBS (Gibco BRL; Invitrogen), 2 mM L-glutamine, 1 mM Na-pyruvate, 1× non-essential amino acids, 100 µg/ml penicillin, 100 µg/ml streptomycin, and 0.5 µM 2-mercaptoethanol. Cells were then stimulated during 4h with PMA (50 ng/ml), ionomycin (500 ng/ml), and GolgiPlug and analyzed by flow cytometry as described below.
Adoptive transfer of EAE
Splenocytes of 2D2 mice were stimulated in the presence of MOG35–55 peptide (20 µg/ml) in Th17 conditions for 72h as described above. Differentiated Th17 cells were then rested 2 days in the presence of IL-2 (2 ng/ml), washed and replated for a 2nd stimulation with MOG peptide in the presence of IL-23 (± IL-27). After 72h, CD4+ T cells were purified by magnetic microbead cell sorting (Miltenyi Biotec) and were injected (7×106 cells/mouse) into sublethally irradiated (400 rad) naive female 7- to 8-wk-old C57BL/6 mice via the tail vein. Mice were given 200 ng of pertussis toxin i.p. on days 0 and 2 post cell transfer.
EAE was clinically assessed by daily scoring using a scale from 0 to 5 as follows: partial limp tail, 0.5; full limp tail, 1; limp tail and waddling gait, 1.5; paralysis of one hind limb, 2; paralysis of one hind limb and partial paralysis of other hind limb, 2.5; paralysis of both hind limbs, 3; ascending paralysis, 3.5; paralysis of trunk, 4; moribund, 4.5; death, 5.
Flow cytometry
For all intracellular staining, cells were stimulated for 4 h with phorbol 12-myristate 13-acetate (50 ng/ml) and ionomycin (500 ng/ml (both from Sigma) and treated with GolgiPlug (1 µg per 1×106 cells; BD Pharmingen). In the staining procedure, Fc receptors on cells were first blocked with anti-CD16/32 antibody (2.4G2; BD Pharmingen) and surface and intracellular staining with antibodies was performed following manufacturers’ instructions for staining using Fix & Perm reagents (Caltag Laboratories). Data were acquired on a FACSAria (BD Biosciences) and analyzed with FlowJo software (Treestar).
Intracellular staining for phosphorylated STAT1 and STAT3
Purified CD4+ T cells (5×105) were stimulated with anti-CD3 (1 µg/ml) and anti-CD28 (1 µg/ml) antibodies in the presence of IL-6 (50 ng/ml) or IL-27 (50 ng/ml) for 30 min. Cells were then fixed for 10 min at 37 °C with 2% paraformaldehyde. After fixation, cells were permeabilized for 30 min on ice with 90% methanol, and were stained for phosphorylated STAT1 and STAT3. Antibodies to phosphorylated tyrosine residues of STAT1 (clone 4a) and STAT3 (clone 4/P-STAT3) were from BD Pharmingen.
Real-time PCR
Total RNA from T cells was isolated by TRIzol extraction (Invitrogen) according to the manufacturer’s instructions, and cDNA was synthesized with a reverse transcription kit (Applied Biosystems). Primer pairs for quantitative real-time PCR were from Applied Biosystems. Gene expression was analyzed by TaqMan real-time PCR (Applied Biosystems). Ribosomal 18S RNA was used as an endogenous control in all experiments. Error bars indicate SEM values calculated from −ΔΔCt values from triplicate PCR reactions, according to Applied Biosystems protocols.
Proliferation assay
Differentiated Th17 cells undergoing a 2nd round of stimulation as described above were pulsed for the last 18 h of culture with 1 µCi of [3H]thymidine. Thymidine incorporation was measured using a scintillation counter.
Statistics
An unpaired, two-tailed Student's t-test was used for statistical analysis. Differences with P values of less than 0.05 were considered significant.
Results
1. STAT1 but not T-bet is required for IL-27-mediated suppression of RORγt and RORα expression in developing Th17 cells
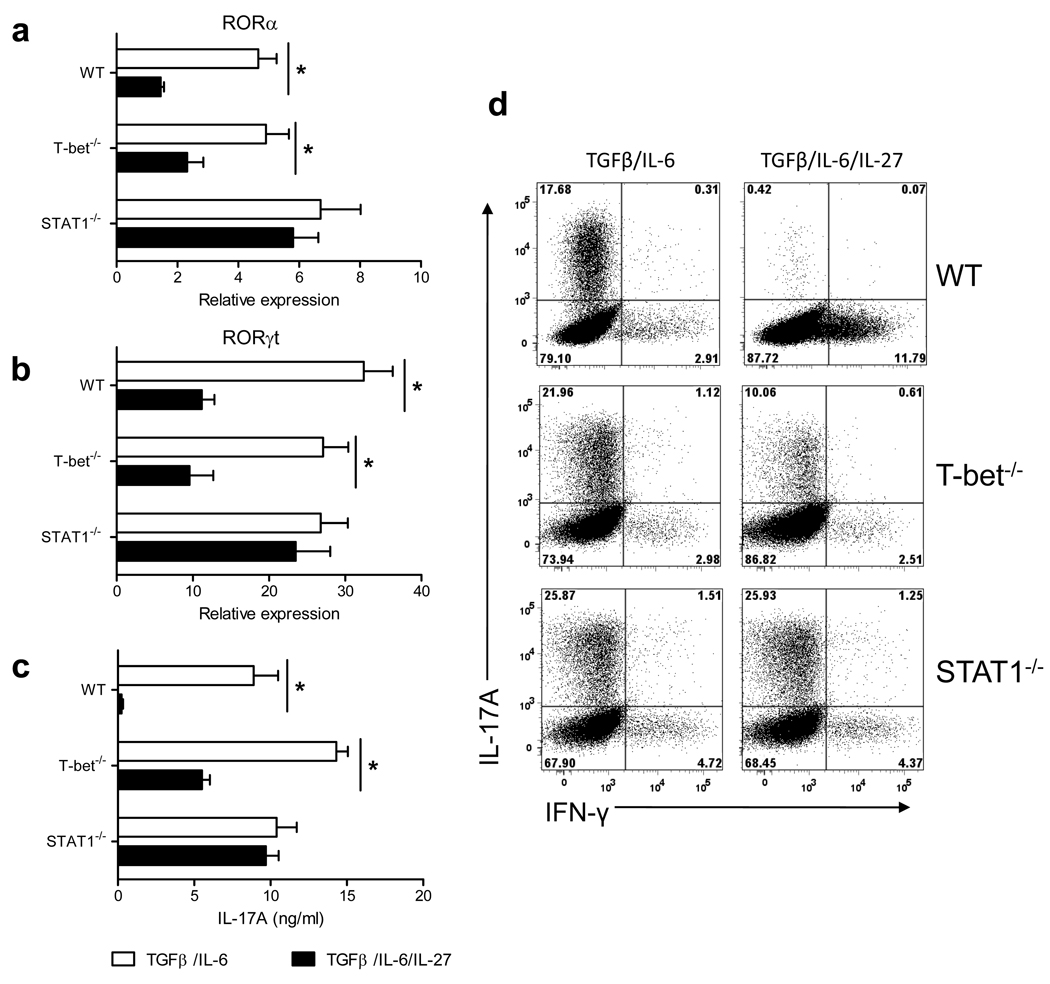
RORα is a transcription factor that in addition to RORγt directs Th17 lineage commitment (6). While downregulation of RORγt by IL-27 in differentiating Th17 cells has been reported (32), its effect on RORα expression has not been studied. We found that, like RORγt, RORα expression was suppressed by IL-27 in developing Th17 cells (Fig. 1a). The suppressive effect of IL-27 was STAT1-dependent and T-bet-independent (Fig. 1b). Flow cytometric analysis of IL-17A and IFN-γ expression in Th17 cells confirmed that IL-27 did not suppress development of STAT1−/− Th17 cells, while it suppressed Th17 differentiation of WT and T-bet−/− CD4+ T cells (Fig. 1d). Th17 differentiation of T-bet−/− CD4+ T cells was less potently suppressed by IL-27 when compared to WT cells, suggesting a contributing role of T-bet in suppression of Th17 development by IL-27 (Fig. 1d). In agreement with this observation, we found that T-bet was upregulated when IL-27 was added to the Th17 culture (supplementary Fig.1a). Measurement of IL-17A concentrations in cell culture supernatants confirmed a strong IL-27-mediated suppression of Th17 differentiation in WT CD4+ T cells, a less potent inhibition of T-bet−/− cells, and no effect on STAT1−/− cells (Fig. 1c). Taken together, these data demonstrate that IL-27 inhibits RORγt and RORα expression and prevents differentiation of Th17 cells in the STAT1-dependent pathway.
Figure 1. Suppressive effect of IL-27 on RORγt and RORα expression is dependent of STAT1 but independent of T-bet.
CD4+ T cells from spleen of WT C57BL/6, T-bet−/−, and STAT1−/− mice were activated with anti-CD3 and anti-CD28 antibodies in the presence of TGF-β+IL-6 (± IL-27). 72 h after activation, cells were stimulated with PMA and ionomycin in the presence of Golgiplug for 4 h, stained and analyzed by flow cytometry for IL-17A and IFN-γ expression (d). mRNA was extracted from cells cultivated in (d) and analyzed by real-time PCR for RORα (a) and RORγt (b) expression. IL-17A levels were measured by ELISA in the supernatants of cells activated during 72 h as described above (c). *p < 0.001. Data are representative of 3 experiments. (error bars, s.e.m).
2. Committed Th17 cells are resistant to suppression by IL-27
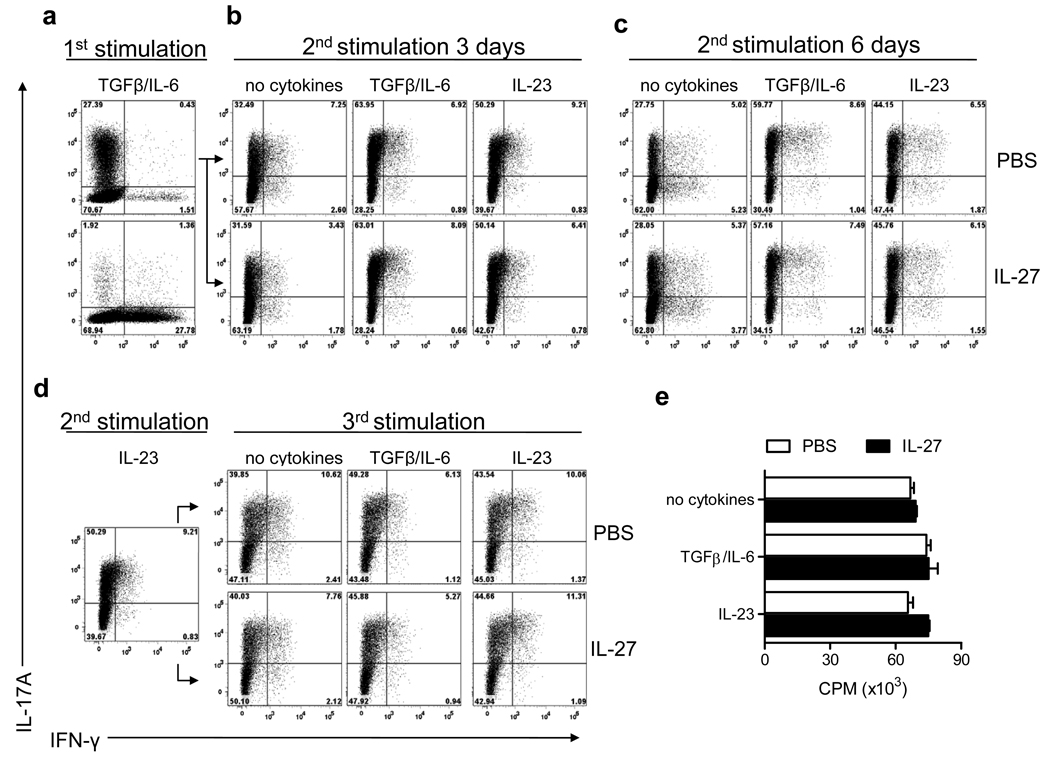
Although IL-27 has been shown to efficiently inhibit de novo generation of Th17 cells, its effects on already committed Th17 cells are poorly understood. To test if IL-27 suppresses committed Th17 cells, we stimulated purified CD4+ T cells in Th17 polarizing conditions (1st stimulation) and examined their phenotype during two rounds of reactivation in defined cytokine conditions. When added to Th17 cells restimulated (2nd stimulation) without exogenous cytokines, with TGF-β+IL-6 or IL-23 during three days, IL-27 did not affect the frequency of Th17 cells (Fig. 2b). We routinely used IL-27 at 10 ng/ml, and experiments performed with greater concentrations of IL-27 (up to 100 ng/ml) gave similar results (Supplementary Fig. 2), demonstrating that 10 ng/ml of IL-27 is sufficient for its maximum effect. It has been proposed that IL-27 suppresses IL-17A production by non-polarized activated T cells only after prolonged time (35). We extended the 2nd stimulation of Th17 cells to 6 days and again did not observe an effect of IL-27 on IL-17A expression (Fig. 2c). Th17 cells that underwent a 2nd stimulation in the presence of IL-23 were activated for a 3rd time. As shown in Figure 2d, the frequency of Th17 cells remained unaffected by IL-27. Similar results were obtained using Th17 cells that underwent a 2nd stimulation in the presence of TGF-β+IL-6 (Supplementary Fig. 3). It has been reported that repeated stimulation in vitro of Th17 cells led to an increase in cells producing both IL-17A and IFN-γ (17). In our experiments, addition of IL-27 to the Th17 polarized culture did not increase either the frequency of double positive IL-17A+ IFN-γ+ cells or of single IFN-γ+ cells (Fig. 2). In contrast, and as described previously (17), addition of IL-12 to committed Th17 cells rapidly induced production of IFN-γ. A minority of cells remained IL-17A+ (2.8%), while most cells became either IL-17A+ IFN-γ+ (17.9%) or IL-17A− IFN-γ+ (66.3%). Addition of IL-23 together with IL-12 did not prevent the loss of Th17 phenotype (Supplementary Fig. 4). As shown in Fig 2e, addition of IL-27 to Th17 cells during the 2nd stimulation did not suppress their proliferation. Taken together, these results clearly indicate that IL-27, while efficaciously suppressing Th17 cells development, does not readily alter committed Th17 phenotype.
Figure 2. IL-27 does not suppress committed Th17 cells.
Purified CD4+ T cells from spleens of C57BL/6 mice were activated with anti-CD3 and anti-CD28 antibodies in the presence of TGF-β+IL-6 (± IL-27), and anti-IFN-γ and anti-IL-4 antibodies (1st stimulation). Cells were activated 72 h later with PMA and ionomycin in the presence of GolgiPlug for 4 h and analyzed by flow cytometry for expression of IL-17A and IFN-γ (a). After the 1st stimulation, cells were rested 2 days in the presence of IL-2 and then reactivated with anti-CD3 and anti-CD28 antibodies (2nd stimulation) either during 3 days (b) or 6 days (c) in the presence of cytokine combinations indicated on each panel. Cells were then stimulated with PMA and ionomycin in the presence of GolgiPlug for the final 4 h, stained and analyzed by flow cytometry for IL-17A and IFN-γ expression. Th17 cells that underwent a 2nd stimulation in the presence of IL-23 as described in (b) were rested for 2 days in the presence of IL-2 and then restimulated (3rd stimulation) with anti-CD3 and anti-CD28 antibodies, in the presence of cytokine combinations indicated on each panel. After 72 h cells were stimulated with PMA and ionomycin in the presence of GolgiPlug for the final 4 h, stained and analyzed by flow cytometry for IL-17A and IFN-γ expression (d). Cells undergoing a 2nd stimulation as described in (b) were pulsed with 1 µCi of [3H]thymidine for the last 18 h of culture, and thymidine incorporation was measured using a scintillation counter (e). Data are representative of two experiments (c, d, e) or five experiments (a, b).
3. IL-27 does not alter cytokine production by committed Th17 cells
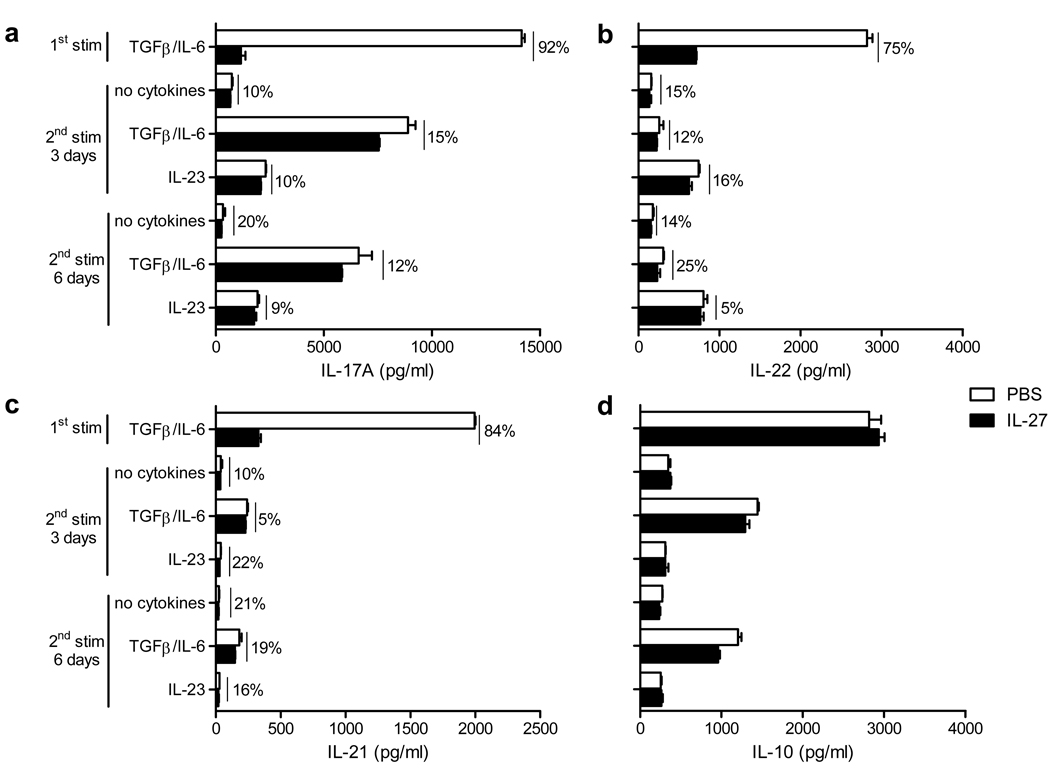
During Th17 differentiation, IL-27 efficiently inhibited IL-17A and IL-17F production (Fig. 3a and supplementary Fig. 1b). We next analyzed IL-17A and IL-17F production in Th17 cell cultures undergoing two rounds of stimulation. In agreement with our flow cytometry data (Fig. 2), IL-17A and IL-17F production by restimulated Th17 cells was either only slightly downregulated, by 10 to 20%, or not affected when compared to Th17 cells that had not been exposed to IL-27 (Fig. 3a and supplementary Fig. 1b). Prolonged exposure to IL-27 during 6 days did not additionally suppress IL-17A production (Fig. 3a). Similar results were obtained when Th17 cells were stimulated a 3rd time (data not shown).
Figure 3. IL-27 does not suppress cytokine production by committed Th17 cells.
Concentrations of IL-17A (a), IL-22 (b), IL-21 (c) and IL-10 (d) in 72 h supernatants collected from cell cultures after 1st and 2nd stimulations as described in Figure 2. Changes in cytokine concentrations (%) when IL-27 was added to the culture compared to PBS are indicated above the bars. Data are representative of three experiments (error bars, s.e.m).
Although IL-17A and IL-17F are hallmark Th17 cytokines, Th17 cells also secrete other cytokines including IL-22, IL-21 and IL-10 (12). IL-27 potently suppressed IL-22 and IL-21 secretion during Th17 differentiation (75 and 84% suppression respectively) but only modestly suppressed their secretion during the 2nd round of stimulation (from 5 to 25% suppression) (Fig. 3b and 3c). We and others have demonstrated that IL-27 induces production of IL-10 in both Th1 and Th2 cells, but not in developing Th17 cells (33, 34). As shown in Figure 3d, IL-27 did not upregulate IL-10 production in committed Th17 cells, and even slightly downregulated IL-10 production in the presence of TGF-β+IL-6 during the 2nd stimulation (Fig. 3d).
4. IL-27 does not downregulate RORγt, RORα and IL-23R expression in committed Th17 cells
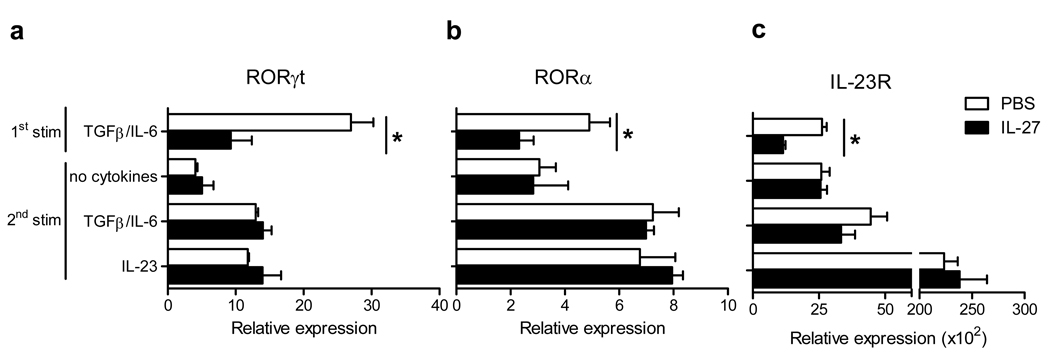
The development of Th17 cells is governed by transcription factors RORγt and RORα (5, 6). We analyzed an effect of IL-27 on their expression in Th17 cells during a 2nd round of activation, and found that RORγt and RORα expression was not affected by IL-27 (Fig. 4a and b). IL-23R is not expressed on naïve T cells, but once activated in Th17-polarizing conditions, T cells upregulate IL-23R, and become responsive to IL-23 (5, 6). We asked whether IL-27 affects IL-23R expression, which may result in diminished pathogenicity. IL-27 decreased IL-23R expression during Th17 differentiation but did not affect IL-23R expression in committed Th17 cells (Fig. 4c).
Figure 4. IL-27 does not suppress RORγt, RORα, and IL-23R expression in committed Th17 cells.
Real-time PCR analysis of RORγt (a), RORα (b), and IL-23R (c) mRNA expression in differentiating Th17 cells and in committed Th17 cells after the 2nd stimulation. *p < 0.001. Data are representative of 2 experiments. (error bars, s.e.m).
5. Committed Th17 cells express functional IL-27 receptor
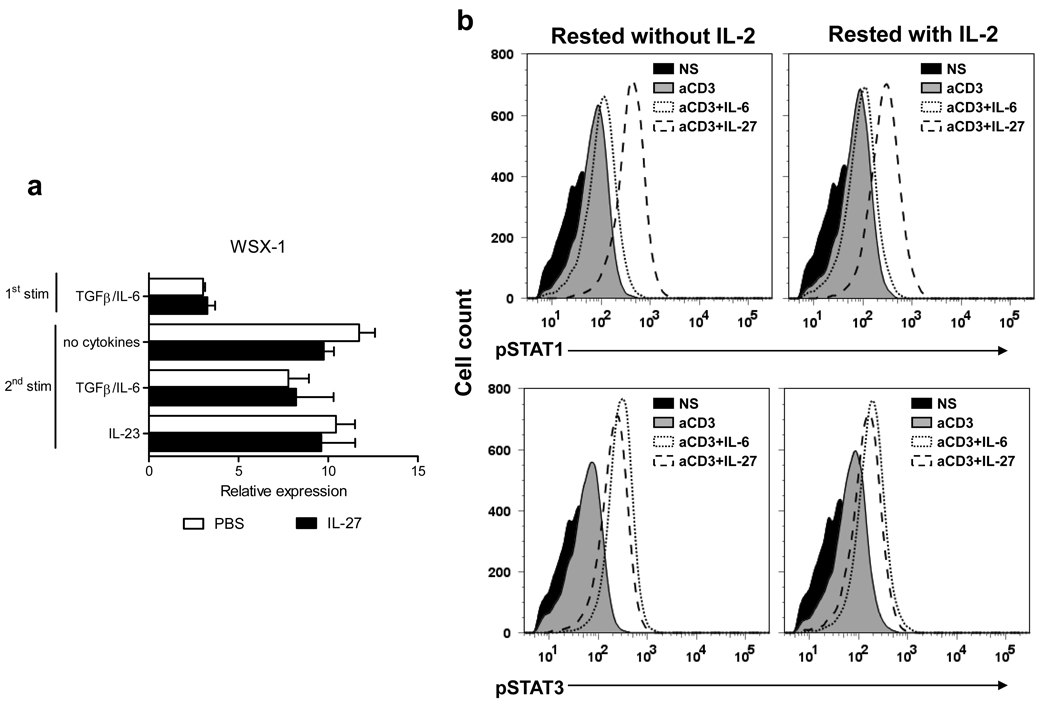
Considering that IL-27 had a minimal effect on committed Th17 cells we investigated whether they express functional IL-27R. Committed Th17 cells contained higher levels of mRNA for WSX-1 than recently activated naïve CD4+ T cells, and exposure to IL-27 did not alter its expression (Fig. 5a). To determine whether IL-27R expressed by committed Th17 cells is functional, we tested if IL-27 induces phosphorylation of STAT1 and STAT3, which are known to be activated by IL-27 signaling (35). To increase cell survival we routinely added 2 ng/ml of IL-2 to Th17 cells resting between stimulations. Since it has been described that IL-2 can affect expression of IL-27R in activated T cells (21), we tested in parallel samples that were rested with and without IL-2. Following the 1st stimulation committed Th17 cells were rested for 2 days (± IL-2) and then reactivated in the presence of either IL-6 or IL-27 during 30 minutes. As shown in Figure 5b, IL-27 induced phosphorylation of both STAT1 and STAT3, demonstrating that committed Th17 cells express functional IL-27R. A similar degree of phosphorylation of both STAT1 and STAT3 was found, irrespective of the presence of IL-2 during the resting period, indicating that responsiveness of Th17 cells to IL-27 was not modified by exogenous IL-2.
Figure 5. Committed Th17 cells express IL-27R and phosphorylate both STAT1 and STAT3 in response to IL-27.
(a) Real-time PCR analysis of WSX-1 expression in differentiating Th17 cells and in committed Th17 cells after the 2nd stimulation. (b) Committed Th17 cells rested 2 days in the absence or presence of IL-2 (2 ng/ml) were either not stimulated (NS) or stimulated with anti-CD3 (1 µg/ml) and anti-CD28 (1 µg/ml) antibodies (aCD3) in the presence of IL-6 (50 ng/ml) or IL-27 (50 ng/ml) for 30 min. Cells were then fixed, permeabilized and analyzed by flow cytometry for phosphorylated STAT1 (pSTAT1) and STAT3 (pSTAT3). *p < 0.001. Data are representative of 2 independent experiments. (error bars, s.e.m).
6. Accessory cells do not render committed Th17 cells susceptible to suppression by IL-27
IL-27 acts directly on CD4+ T cells to suppress Th17 differentiation (29). We have previously shown that IL-27 induces IL-10 production by Th1 cells and that this effect is enhanced by non-T cells (34). To determine whether accessory cells influence an effect of IL-27 on committed Th17 cells, we stimulated splenocytes with anti-CD3 and anti-CD28 Abs in Th17 polarizing conditions (1st stimulation) and reactivated them as described for isolated CD4+ T cells. In agreement with our findings with purified CD4+ T cells, IL-27 did not reduce either the percentage of IL-17A-producing cells or their IL-17A secretion (supplementary Figs 5a and b) Similar results were obtained when purified CD4+ T cells after the 1st stimulation were restimulated in the presence of T cell-depleted splenocytes (data not shown).
Antigen-specific activation of splenocytes from 2D2 mice with MOG35–55 showed that IL-27 strongly suppressed the Th17-polarizing effect of TGF-β+IL-6 in the 1st stimulation (data not shown). The addition of IL-27 during the 2nd stimulation did not reduce the percentage of 2D2 Th17 cells and had only a modest effect on IL-17A production (supplementary Figs. 5c and d). These findings in the model of antigen-specific activation reproduce and validate those made by mitogenic activation of purified CD4+ T cells and splenocytes.
7. Effector/memory Th17 cells that developed in vivo are resistant to suppression by IL-27
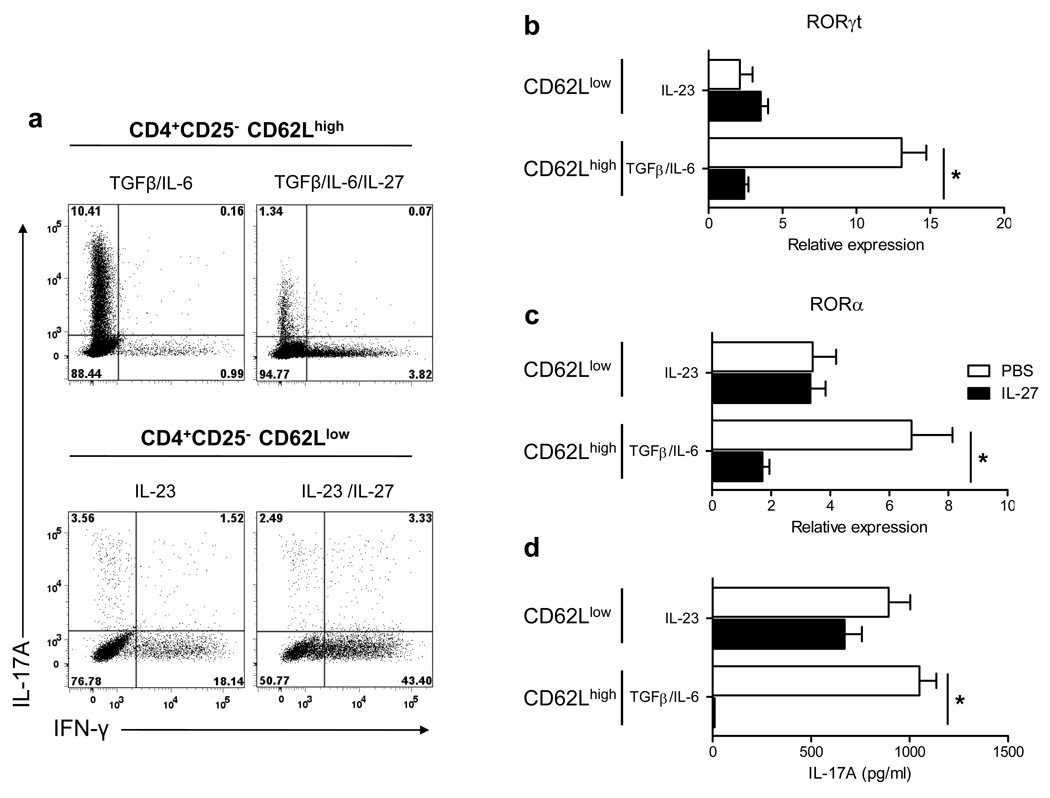
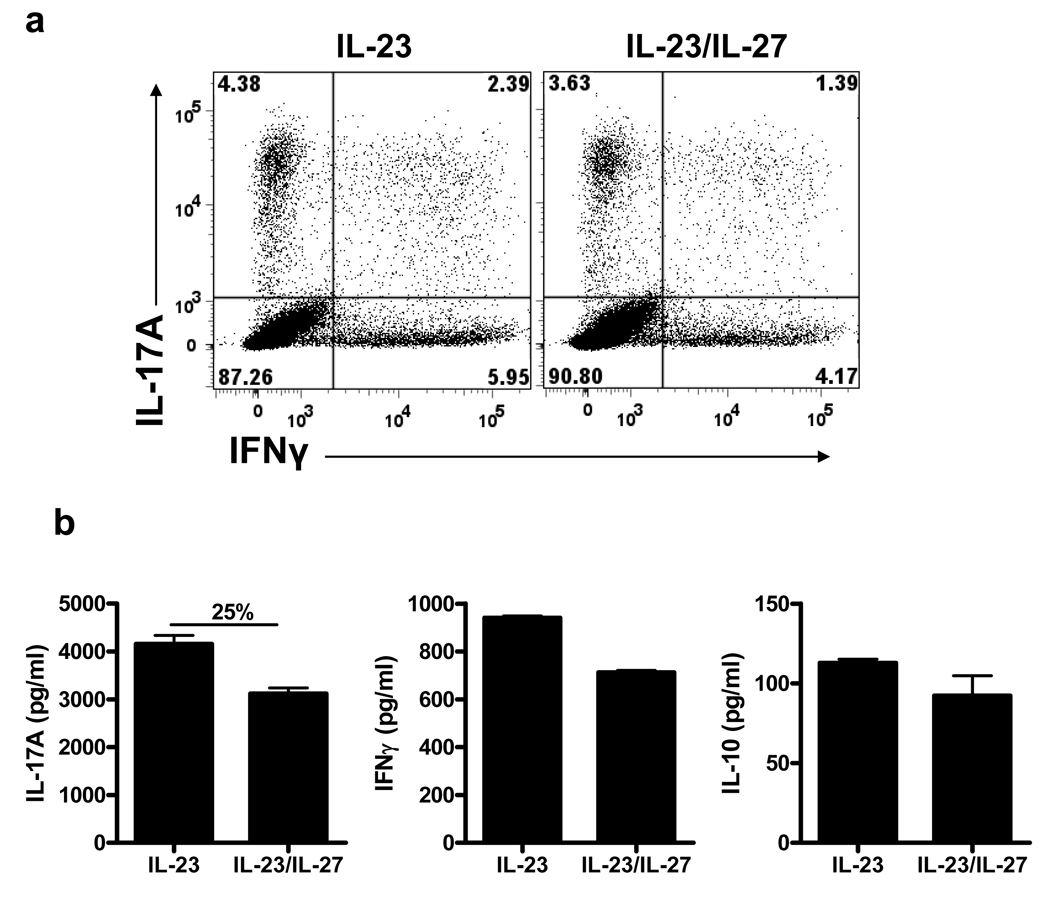
To test if Th17 cells that developed in vivo exhibit the same resistance to suppression by IL-27 as Th17 cells generated in vitro, we analyzed an effect of IL-27 on effector/memory Th17 cells from naïve mice and from mice that had been immunized for EAE induction. Sorted naive (CD4+CD25−CD62Lhigh) and memory (CD4+CD25−CD62Llow) T cells from naïve mice were activated with antibodies in the presence either of TGF-β+IL-6 for naive cells, or IL-23 for memory cells, with or without added IL-27. As expected, IL-27 potently inhibited differentiation of Th17 cells from naïve cells and prevented RORγt and RORα expression and IL-17A production (Fig. 6). When memory cells were restimulated with IL-23, RORγt and RORα expression was not affected by the addition of IL-27 and IL-17A production was only weakly decreased (Fig. 6). We next isolated mononuclear cells from the CNS of MOG35–55-immunized mice at the peak of EAE. Cells were stimulated with MOG35–55 in the presence of IL-23 or IL-23+IL-27. Analyses after 3 days of culturing showed that the frequency of IL-17A+ cells and IL-17A concentrations in supernatants was suppressed by IL-27 between 9 and 25% (Fig. 7 and data not shown), depending on the experiment. Similar data were obtained using mononuclear cells isolated from the CNS of SJL mice immunized with PLP139–151 (data not shown). In addition, we noticed in repeated experiments that IFN-γ secretion was also slightly suppressed by IL-27, while IL-10 secretion was not affected by the addition of IL-27 to cultures (Fig. 8b). These data are consistent with our results using in vitro polarized Th17 cells, showing no effect of IL-27 on committed Th17 cells.
Figure 6. IL-27 does not suppress effector/memory Th17 cells that developed in vivo.
Naive (CD4+CD25−CD62Lhigh) and memory T cells (CD4+CD25−CD62Llow) were sorted by flow cytometry and activated with anti-CD3 and anti-CD28 antibodies in the presence either of TGF-β+IL-6 (± IL-27) for naive cells, or IL-23 (± IL-27) for memory cells. All cultures were also supplemented with neutralizing anti-IFN-γ and anti-IL-4 antibodies. After 72 h, cells were stimulated with PMA and ionomycin in the presence of Golgiplug for 4 h, stained and analyzed by flow cytometry for IL-17A and IFN-γ expression (a). mRNA was extracted from cells cultivated in (a) and analyzed by real-time PCR for RORγt (b) and RORα (c) expression. IL-17A levels were measured by ELISA in the supernatants of cells activated for 72 h as described above (d). *p < 0.001. Data are representative of 2 experiments. (error bars, s.e.m).
Figure 7. Myelin-specific Th17 cells that develop in vivo are resistant to suppression by IL-27.
EAE was induced in C57BL/6 mice with MOG35–55 peptide. Brains and spinal cords were harvested at the peak of disease and mononuclear cells were isolated and stimulated for 3 days with MOG35–55 peptide in the presence of irradiated splenocytes and IL-23 (±IL-27). (a) Flow cytometry analysis of IL-17A and IFN-γ expression in CD4+ cells after stimulation with PMA, ionomycin, and GolgiPlug. (b) IL-17A, IFN-γ and IL-10 levels were measured by ELISA. Change in IL-17A concentration (%) when IL-27 was added to the culture is indicated above the bars.
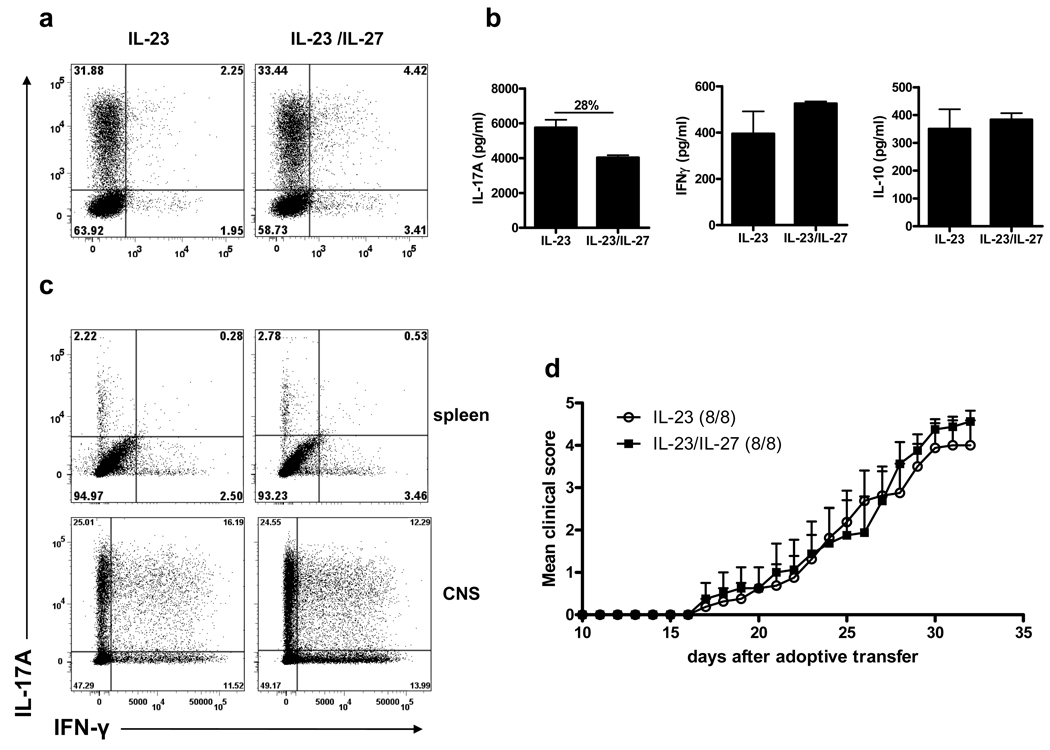
Figure 8. IL-27 does not suppress encephalitogenicity of 2D2 Th17 cells in adoptive EAE model.
2D2 Th17 were reactivated with MOG35–55 for 3 days in the presence of IL-23 (±IL-27). CD4+ T cells were enriched from culture and injected (7×106 cells/mouse) into 400 rad-irradiated C57BL/6 mice via the tail vein. Recipient mice received 200 ng of pertussis toxin on day 0 and 2 post-transfer. (a) Flow cytometry analysis of IL-17A and IFN-γ expression in CD4+ cells before transfer. (b) IL-17A, IFN-γ and IL-10 levels after the second stimulation of 2D2 cells were measured by ELISA. (d) Clinical scores of mice that received IL-23-stimulated or IL-23/IL-27-stimulated 2D2 Th17 cells. (c) Flow cytometry analysis of IL-17A and IFN-γ expression in CD4+Vβ11+ cells from spleens and isolated mononuclear cells from the CNS at day 32 post-transfer, after ex vivo stimulation with PMA, ionomycin, and GolgiPlug for 4 h. Change in IL-17A concentration (%) when IL-27 was added to the culture is indicated above the bars. Data are representative of two experiments. (error bars, s.e.m).
8. IL-27 does not suppress encephalitogenicity of myelin-reactive Th17 cells
To examine the effect of IL-27 on Th17 effector functions in vivo, we used an IL-23-driven adoptive EAE with Th17 cells differentiated from TCR transgenic MOG-specific 2D2 cells (36). 2D2 cells express a TCR composed of Vα3.2 and Vβ11 allowing tracking of these cells with anti-Vb11 Ab when injected into WT recipients. 2D2 Th17 cells were first differentiated in the presence of MOG35–55, TGF-β+IL-6, and then restimulated with IL-23 or IL-23+IL-27. As described earlier (supplementary Figs. 5c and d), addition of IL-27 into culture had no effect on frequency of 2D2 Th17 cells and IL-17A secretion was only modestly suppressed (Figure 8a and 8b). In repeated experiments, suppression of IL-17A secretion by IL-27 ranged between 13 and 28% (supplementary Fig. 5d; Fig. 8b and data not shown). In addition, 2D2 Th17 cell culture supernatants contained similar levels of IFN-γ and IL-10 irrespective of IL-27 (Fig. 8b). CD4+ cells were enriched by magnetic bead separation and 7×106 cells were injected into sublethally irradiated recipient mice. Clinical follow-up of the recipient mice showed that mice injected with IL-23-treated or IL-23+IL-27-treated Th17 2D2 cells developed indistinguishable EAE (Fig. 8d). We analyzed expression of IL-17A and IFN-γ in CD4+Vβ11+ (2D2) cells harvested from recipient mice 32 days post adoptive transfer. 2D2 cells constituted the majority among CD4+ cells and were readily detectable in splenocytes and mononuclear cells purified from the CNS. The vast majority of splenic 2D2 cells did not produce IL-17A or IFN-γ after being stimulated with PMA and ionomycin, irrespective of whether cells were treated or not with IL-27 before adoptive transfer (Fig. 8c). In contrast, 2D2 cells isolated from the CNS largely retained Th17 phenotype. Frequency of IL-17A+ positive and IL-17A+IFN-γ+ double positive cells were similar in both groups, demonstrating that IL-27 did not promote loss of Th17 phenotype and/or accelerated conversion in Th1 cells. All these findings strongly suggest that IL-27 does not affect effector functions of committed Th17 cells in vivo.
Discussion
IL-27 is possibly the most efficient suppressor of Th17 development. In stark contrast, already committed Th17 cells appear to be largely resistant to direct suppression by IL-27. We have followed several defining features of Th17 phenotype and failed to identify any significant alteration caused by IL-27. This includes expression of transcription factors RORγt and RORα, cytokine production, cell survival and proliferation. Most importantly, IL-27 did not affect effector functions of committed Th17 cells, as evidenced by their normal encephalitogenicity in an adoptive EAE model.
RORγt and RORα are transcription factors that drive development of the Th17 lineage. We confirmed previous findings that STAT1 is absolutely required for inhibition of Th17 development by IL-27 (29, 30). Although suppression of RORγt and RORα expression by IL-27 were independent of T-bet, its upregulation was necessary for full suppressive effect of IL-27 on Th17 differentiation, suggesting that T-bet participates in IL-27/STAT1-mediated suppression of Th17 development. Indeed, T-bet has been demonstrated to be a negative regulator of Th17 development and mice deficient in T-bet have increased numbers of Th17 cells (10, 11, 37).
Contrasting the strong suppressive effect of IL-27 during de novo differentiation of Th17 cells on RORγt and RORα expression, the latter not having been previously described, committed Th17 cells maintain similar RORγt and RORα levels regardless of IL-27 signaling. Similarly, while T-bet is upregulated by IL-27 during Th17 differentiation, only low levels of T-bet were detectable in committed Th17 cells after exposure to IL-27. Although underlying molecular mechanisms of differential responses to IL-27 signaling between developing and committed Th17 cells remain unknown, these findings provide evidence that these two cell populations respond to IL-27 differently. Thus, maintained expression of RORγt and RORα combined with the lack of T-bet upregulation provide a molecular basis for the stability of Th17 phenotype in spite of IL-27 signaling. This contrasts with the dramatic effects of IL-12 on committed Th17 cells, by readily inducing T-bet expression, suppressing expression of RORγt and efficaciously converting them into Th1 cells (17).
The observed lack of sensitivity of committed Th17 cells to IL-27 can potentially be due either to downregulation of IL-27R expression or modified IL-27R signaling. However, we demonstrate that differentiated Th17 cells expressed levels of WSX-1 that surpass those on recently activated naïve cells. In addition, IL-27 induced activation of both STAT1 and STAT3 in committed Th17 cells, demonstrating functionality of IL-27R. Thus, reduced susceptibility of committed Th17 cells to IL-27 cannot be explained by the absence of a functional IL-27R.
IL-27 suppresses IL-23R expression on developing Th17 cells (29), an observation that we confirmed in the present study. In contrast, IL-27 did not reduce expression of IL-23R on committed Th17 cells and in that way potentially affected their responsiveness to IL-23. Functionally, signaling of IL-23R was also not modified by IL-27 as demonstrated by similar IL-23-induced cytokine upregulation in Th17 cells treated or not by IL-27. These findings provide evidence that IL-27 does not affect responsiveness of Th17 cells to IL-23, a cytokine crucial for Th17 cells effector functions.
IL-27 had a strong inhibitory effect on the secretion of IL-17A, IL-17F, IL-21 and IL-22 during Th17 differentiation. However, we found a modest decrease, if any, in cytokine secretion by effector/memory Th17 cells, demonstrating that IL-27 has little effect on committed Th17 cells in this regard. Our findings are in agreement with reports of Yoshimura et al describing limited ability of IL-27 to downregulate IL-17A production in memory T cells (35) and recently published data by Kastelein’s group demonstrating that IL-27 was inefficient in suppressing IL-17A production by in vitro differentiated Th17 cells stimulated with IL-23 (38). Taken together our data demonstrate that phenotypic characteristics, most commonly used to define Th17 lineage, are not susceptible to change by IL-27 once these cells become committed.
IL-10 production induced by TGF-β and IL-6 during Th17 differentiation is not further augmented by IL-27 (33). Committed Th17 cells downregulate IL-10 production, unless they are re-stimulated with TGF-β and IL-6, while IL-23 does not have an IL-10-inducing effect on these cells (39). Diveu et al described that IL-27 up-regulated IL-10 in the presence of IL-23 in committed Th17 cell cultures (38). However, in our hands IL-27 did not induce IL-10 production by committed Th17 cells, as determined by intracellular staining (data not shown) and measurement of IL-10 in cell culture supernatants. The reasons for these contradictory findings are unclear. Nevertheless, Diveu et al. did not directly demonstrate intracellular co-expression of IL-10 and IL-17A in order to confirm that Th17 cells are the actual source of IL-27-induced IL-10.
We have described that administration of IL-27 to mice with EAE reduced disease severity (31). In that study, numbers of both encephalitogenic Th1 and Th17 cells were reduced in the inflamed CNS, making it difficult to infer whether IL-27 suppressed disease solely by acting on Th17 cells. Because IL-27 was administrated shortly after immunization, it likely suppressed EAE by inhibiting development of encephalitogenic Th17 cells rather than suppressing committed Th17 cells. This view is in accordance with published data showing that administration of IL-27 in the later phase of ongoing collagen-induced arthritis (CIA) does not suppress disease (40, 41). We made a similar observation in EAE, where IL-27 treatment post disease onset had no effect on disease course (our unpublished data).
To determine the effect of IL-27 on committed Th17 cell functions in vivo, we used highly polarized TCR transgenic Th17 cells specific for MOG35–55. IL-23+IL-27-treated Th17 cells were as encephalitogenic as IL-23-treated cells. These results are consistent with our in vitro findings showing no suppressive effect of IL-27 on differentiated Th17 cells. However, in a previous study, we have shown that IL-27 inhibited IL-23-driven adoptive transfer of EAE (31), which appears to contradict the findings presented here. One explanation for this discrepancy is the difference in composition of cells used for induction of adoptive EAE. In the previous study we injected total splenocytes, while in the present study we injected purified CD4+ T cells. Given that IL-27 impacts both T cells and non-T cells, the negative regulation of adoptive EAE by IL-27, which we reported previously, could be due to its action, during in vitro stimulation, on non-T cells that were subsequently transferred into recipient mice. Indeed, IL-27 was shown to directly inhibit APCs co-stimulatory functions and cytokine secretion (42, 43). In addition to directly affecting APCs, IL-27 could have also promoted a tolerogenic APC phenotype indirectly, by inducing IL-10 secretion from Th1 cells (33, 34, 44). IL-10 is well known to induce tolerogenic APCs (45). Hypothetically, these tolerogenic APCs, which were co-transferred with T cells, could have inhibited development of EAE in recipient mice (46). Additional investigation of the effects of IL-27 signaling in non-T cells will be essential to fully understand the role IL-27 plays in immune regulation.
Another explanation of the disparity with our previous study would be the polarization state of the cells used for adoptive EAE. Non-polarized EAE splenocytes contained a large proportion of Th1 cells, while in the present study, TGF-β+IL-6 used for the initial Th17 polarization efficiently inhibited development of Th1 cells, yielding highly enriched Th17 cells and few Th1 cells. Since EAE splenocytes contain both Th1 and Th17 cells, it is likely that IL-10 induced by IL-27 in Th1 cells suppressed Th17 subset both directly and indirectly via inhibition of APCs, as discussed above (47–49). This idea is supported by studies in our laboratory demonstrating that addition of IL-27 to EAE splenocytes concomitantly induced IL-10 and inhibited IL-17 production (34). Furthermore, IL-17 inhibition was significantly decreased when IL-10-deficient splenocytes were used, demonstrating that IL-10 produced by Th1 cells participated in the inhibition of Th17 cells by IL-27 (34). The concept that Th1 cells inhibit Th17 cells by secreting IL-10 is supported by a recent identification of Th1 cells as the principal source of IL-10 during flu infection. In the absence of IL-10, flu-specific T cell responses developed a stronger Th17 component suggesting that IL-10 produced by Th1 cells inhibits Th17 cells (50). A similar opinion that IL-27 influences committed Th17 cells by acting on their environment rather than directly on them was recently published by Kastelein’s group (38). All these data indicate that differences in the composition of cells used to study the effect of IL-27 on effector/memory Th17 cells are the likely reason for different findings.
In conclusion, we demonstrate here that effector/memory Th17 cells are unaffected by IL-27, contrasting its potent inhibitory effect on Th17 development. These findings that IL-27 does not suppress committed Th17 cells corroborate recently published reports (35, 38). These results, which show differential effects of a cytokine on naïve versus committed T cells, are in agreement with the already described T helper 1 and 2 paradigm, where the Th1 signature cytokine IFN-γ antagonizes the development of the Th2 subset and vice versa, but once differentiated, Th1 and Th2 cells acquire resistance to suppression by the opposite helper subset. In the case of Th17 cells, many specificities of their biology are still emerging and it is possible that IL-27 modifies some yet unknown features of committed Th17 cells that we have not studied here. Taking into account the complex and pleiotropic role of IL-27 in immune responses and our findings described here, IL-27 might not be a promising approach for treatment of established inflammatory diseases where Th17 cells are involved.
Supplementary Material
Acknowledgments
We are very grateful to K. Regan for editing the manuscript.
References
- 1.Bettelli E, Korn T, Kuchroo VK. Th17: the third member of the effector T cell trilogy. Current Opinion in Immunology. 2007;19:652–657. doi: 10.1016/j.coi.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Current Opinion in Immunology. 2007;19:281–286. doi: 10.1016/j.coi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, McClanahan TK, O'Shea JJ, Cua DJ. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10:314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 6.Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, Watowich SS, Tian Q, Jetten AM, Dong C. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007 doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 8.Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, Dong C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 9.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007 doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 10.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 11.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghilardi N, Ouyang W. Targeting the development and effector functions of TH17 cells. Seminars in Immunology. 2007;19:383–393. doi: 10.1016/j.smim.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 13.Cua DJ, Kastelein RA. TGF-beta, a 'double agent' in the immune pathology war. Nat Immunol. 2006;7:557–559. doi: 10.1038/ni0606-557. [DOI] [PubMed] [Google Scholar]
- 14.McKenzie BS, Kastelein RA, Cua DJ. Understanding the IL-23-IL-17 immune pathway. Trends Immunol. 2006;27:17–23. doi: 10.1016/j.it.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, Napolitani G. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 16.Suryani S, Sutton I. An interferon-gamma-producing Th1 subset is the major source of IL-17 in experimental autoimmune encephalitis. J Neuroimmunol. 2007;183:96–103. doi: 10.1016/j.jneuroim.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 17.Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, Hibbert L, Churakova T, Travis M, Vaisberg E, Blumenschein WM, Mattson JD, Wagner JL, To W, Zurawski S, McClanahan TK, Gorman DM, Bazan JF, de Waal Malefyt R, Rennick D, Kastelein RA. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16:779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 19.Wirtz S, Becker C, Fantini MC, Nieuwenhuis EE, Tubbe I, Galle PR, Schild HJ, Birkenbach M, Blumberg RS, Neurath MF. EBV-induced gene 3 transcription is induced by TLR signaling in primary dendritic cells via NF-kappa B activation. J Immunol. 2005;174:2814–2824. doi: 10.4049/jimmunol.174.5.2814. [DOI] [PubMed] [Google Scholar]
- 20.Pflanz S, Hibbert L, Mattson J, Rosales R, Vaisberg E, Bazan JF, Phillips JH, McClanahan TK, de Waal Malefyt R, Kastelein RA. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J Immunol. 2004;172:2225–2231. doi: 10.4049/jimmunol.172.4.2225. [DOI] [PubMed] [Google Scholar]
- 21.Villarino AV, Larkin J, 3rd, Saris CJ, Caton AJ, Lucas S, Wong T, de Sauvage FJ, Hunter CA. Positive and negative regulation of the IL-27 receptor during lymphoid cell activation. J Immunol. 2005;174:7684–7691. doi: 10.4049/jimmunol.174.12.7684. [DOI] [PubMed] [Google Scholar]
- 22.Larousserie F, Charlot P, Bardel E, Froger J, Kastelein RA, Devergne O. Differential effects of IL-27 on human B cell subsets. J Immunol. 2006;176:5890–5897. doi: 10.4049/jimmunol.176.10.5890. [DOI] [PubMed] [Google Scholar]
- 23.Lucas S, Ghilardi N, Li J, de Sauvage FJ. IL-27 regulates IL-12 responsiveness of naive CD4+ T cells through Stat1-dependent and -independent mechanisms. Proc Natl Acad Sci U S A. 2003;100:15047–15052. doi: 10.1073/pnas.2536517100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruckerl D, Hessmann M, Yoshimoto T, Ehlers S, Holscher C. Alternatively activated macrophages express the IL-27 receptor alpha chain WSX-1. Immunobiology. 2006;211:427–436. doi: 10.1016/j.imbio.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 25.Hibbert L, Pflanz S, De Waal Malefyt R, Kastelein RA. IL-27 and IFN-alpha signal via Stat1 and Stat3 and induce T-Bet and IL-12Rbeta2 in naive T cells. J Interferon Cytokine Res. 2003;23:513–522. doi: 10.1089/10799900360708632. [DOI] [PubMed] [Google Scholar]
- 26.Yoshimoto T, Yoshimoto T, Yasuda K, Mizuguchi J, Nakanishi K. IL-27 suppresses Th2 cell development and Th2 cytokines production from polarized Th2 cells: a novel therapeutic way for Th2-mediated allergic inflammation. J Immunol. 2007;179:4415–4423. doi: 10.4049/jimmunol.179.7.4415. [DOI] [PubMed] [Google Scholar]
- 27.Huber M, Steinwald V, Guralnik A, Brustle A, Kleemann P, Rosenplanter C, Decker T, Lohoff M. IL-27 inhibits the development of regulatory T cells via STAT3. Int Immunol. 2008;20:223–234. doi: 10.1093/intimm/dxm139. [DOI] [PubMed] [Google Scholar]
- 28.Neufert C, Becker C, Wirtz S, Fantini MC, Weigmann B, Galle PR, Neurath MF. IL-27 controls the development of inducible regulatory T cells and Th17 cells via differential effects on STAT1. Eur J Immunol. 2007;37:1809–1816. doi: 10.1002/eji.200636896. [DOI] [PubMed] [Google Scholar]
- 29.Batten M, Li J, Yi S, Kljavin MN, Danilenko DM, Lucas S, Lee J, de Sauvage FJ, Ghilardi N. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 30.Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D, Saris CJ, O'Shea JJ, Hennighausen L, Ernst M, Hunter CA. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 31.Fitzgerald DC, Ciric B, Touil T, Harle H, Grammatikopolou J, Das Sarma J, Gran B, Zhang GX, Rostami A. Suppressive effect of IL-27 on encephalitogenic Th17 cells and the effector phase of experimental autoimmune encephalomyelitis. J Immunol. 2007;179:3268–3275. doi: 10.4049/jimmunol.179.5.3268. [DOI] [PubMed] [Google Scholar]
- 32.Stumhofer JS, Silver J, Hunter CA. Negative regulation of Th17 responses. Semin Immunol. 2007;19:394–399. doi: 10.1016/j.smim.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, Ernst M, Saris CJ, O'Shea JJ, Hunter CA. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8:1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 34.Fitzgerald DC, Zhang GX, El-Behi M, Fonseca-Kelly Z, Li H, Yu S, Saris CJ, Gran B, Ciric B, Rostami A. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol. 2007;8:1372–1379. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 35.Yoshimura T, Takeda A, Hamano S, Miyazaki Y, Kinjyo I, Ishibashi T, Yoshimura A, Yoshida H. Two-sided roles of IL-27: induction of Th1 differentiation on naive CD4+ T cells versus suppression of proinflammatory cytokine production including IL-23-induced IL-17 on activated CD4+ T cells partially through STAT3-dependent mechanism. J Immunol. 2006;177:5377–5385. doi: 10.4049/jimmunol.177.8.5377. [DOI] [PubMed] [Google Scholar]
- 36.Bettelli E, Pagany M, Weiner HL, Linington C, Sobel RA, Kuchroo VK. Myelin oligodendrocyte glycoprotein-specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J Exp Med. 2003;197:1073–1081. doi: 10.1084/jem.20021603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mathur AN, Chang HC, Zisoulis DG, Kapur R, Belladonna ML, Kansas GS, Kaplan MH. T-bet is a critical determinant in the instability of the IL-17-secreting T-helper phenotype. Blood. 2006;108:1595–1601. doi: 10.1182/blood-2006-04-015016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Diveu C, McGeachy MJ, Boniface K, Stumhofer JS, Sathe M, Joyce-Shaikh B, Chen Y, Tato CM, McClanahan TK, de Waal Malefyt R, Hunter CA, Cua DJ, Kastelein RA. IL-27 blocks RORc expression to inhibit lineage commitment of Th17 cells. J Immunol. 2009;182:5748–5756. doi: 10.4049/jimmunol.0801162. [DOI] [PubMed] [Google Scholar]
- 39.McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 40.Niedbala W, Cai B, Wei X, Patakas A, Leung BP, McInnes IB, Liew FY. Interleukin 27 attenuates collagen-induced arthritis. Ann Rheum Dis. 2008;67:1474–1479. doi: 10.1136/ard.2007.083360. [DOI] [PubMed] [Google Scholar]
- 41.Niedbala W, Wei XQ, Cai B, Hueber AJ, Leung BP, McInnes IB, Liew FY. IL-35 is a novel cytokine with therapeutic effects against collagen-induced arthritis through the expansion of regulatory T cells and suppression of Th17 cells. Eur J Immunol. 2007;37:3021–3029. doi: 10.1002/eji.200737810. [DOI] [PubMed] [Google Scholar]
- 42.Holscher C, Holscher A, Ruckerl D, Yoshimoto T, Yoshida H, Mak T, Saris C, Ehlers S. The IL-27 receptor chain WSX-1 differentially regulates antibacterial immunity and survival during experimental tuberculosis. J Immunol. 2005;174:3534–3544. doi: 10.4049/jimmunol.174.6.3534. [DOI] [PubMed] [Google Scholar]
- 43.Wang S, Miyazaki Y, Shinozaki Y, Yoshida H. Augmentation of antigen-presenting and Th1-promoting functions of dendritic cells by WSX-1(IL-27R) deficiency. J Immunol. 2007;179:6421–6428. doi: 10.4049/jimmunol.179.10.6421. [DOI] [PubMed] [Google Scholar]
- 44.Batten M, Kljavin NM, Li J, Walter MJ, de Sauvage FJ, Ghilardi N. Cutting edge: IL-27 is a potent inducer of IL-10 but not FoxP3 in murine T cells. J Immunol. 2008;180:2752–2756. doi: 10.4049/jimmunol.180.5.2752. [DOI] [PubMed] [Google Scholar]
- 45.Adler HS, Steinbrink K. Tolerogenic dendritic cells in health and disease: friend and foe! Eur J Dermatol. 2007;17:476–491. doi: 10.1684/ejd.2007.0262. [DOI] [PubMed] [Google Scholar]
- 46.Muller G, Muller A, Tuting T, Steinbrink K, Saloga J, Szalma C, Knop J, Enk AH. Interleukin-10-treated dendritic cells modulate immune responses of naive and sensitized T cells in vivo. J Invest Dermatol. 2002;119:836–841. doi: 10.1046/j.1523-1747.2002.00496.x. [DOI] [PubMed] [Google Scholar]
- 47.D'Andrea A, Aste-Amezaga M, Valiante NM, Ma X, Kubin M, Trinchieri G. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J. Exp. Med. 1993;178:1041–1048. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J. Exp. Med. 1991;174:1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–3822. [PubMed] [Google Scholar]
- 50.McKinstry KK, Strutt TM, Buck A, Curtis JD, Dibble JP, Huston G, Tighe M, Hamada H, Sell S, Dutton RW, Swain SL. IL-10 deficiency unleashes an influenza-specific Th17 response and enhances survival against high-dose challenge. J Immunol. 2009;182:7353–7363. doi: 10.4049/jimmunol.0900657. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.