Endurance exercise training is known to elicit metabolic adaptations in skeletal muscle by mechanisms requiring further elucidation. However, traditional endurance training regimes demand significant time commitment which may be incompatible with the current culture of time poverty. Furthermore, high-intensity interval training can be more effective than moderate intensity continuous training for improving cardiovascular health and clinical outcome in patients with metabolic syndrome (Tjønna et al. 2008). Endurance exercise training results in widespread physiological adaptations, one of the most prominent being an increase in skeletal muscle mitochondrial size and number leading to enhanced oxidative capacity (Holloszy, 1967). The role of mitochondria in energy production has fascinated physiologists for decades. Changes in the size, number and activity of mitochondria can be influenced by a variety of physiological stimuli. A complex regulatory network involving over 1000 genes and numerous cellular proteins controls mitochondrial biogenesis and function. Regulators such as nuclear respiratory factors (NRF)-1 and -2, peroxisome proliferator-activated receptor γ co-activator (PGC)-1α and mitochondrial transcription factor A (Tfam) are thought to play critical roles (Hock & Kralli, 2009). The NAD-dependent deacetylase sirtuin (silent mating type information regulation 2 homolog)-1 is also purported to have a key role in mitochondrial biogenesis via functional regulation of PGC-1α. The cellular mechanisms by which the above-mentioned regulators of mitochondrial biogenesis contribute to skeletal muscle adaptations to endurance training remain unclear. It is possible that differential mechanisms are involved in adaptation to low-volume high-intensity training as opposed to traditional endurance training programmes. Research in this field will advance our understanding of cellular oxidative processes in general: welcome progress in view of the escalating cost and prevalence of metabolic diseases such as obesity and diabetes.
In a recent issue of The Journal of Physiology, Little and colleagues (Little et al. 2010) describe a practical model of high-intensity interval training involving six sessions of 20–29 min over 2 weeks. Their low-volume cycle ergometer regime significantly improved performance by young, healthy, recreationally active males in 50 kJ and 750 kJ cycling time trials and mean power output during these both tests, whereas time trial familiarization alone had no effect on functional performance. The training sessions consisted of a 3 min warm-up at 30 W followed by 8–12 60 s efforts at the subject's peak power (from a 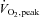 test) with 75 s recoveries at 30 W. To confirm the efficacy of their training intervention for enhancing skeletal muscle mitochondrial capacity, Little and colleagues (Little et al. 2010) examined the mitochondrial enzymes cytochrome C oxidase (COX) and citrate synthase (CS) in whole muscle homogenates from muscle biopsy tissue. Compared to pre-training values, a significant increase in both maximal activity, assessed by spectrophotometry, and protein content, assessed by Western blotting, for both COX and CS was found post training. Training intervention-induced changes in several proposed mediators of skeletal muscle mitochondrial biogenesis and metabolic adaptation were also investigated. Significant increases in whole muscle protein content of sirtuin 1 and Tfam but not NRF-1 were found post training. PGC-1α was elevated post-training in nuclear fractions but remained unchanged in whole muscle homogenates. In addition, training significantly increased total glucose transporter type 4 (GLUT4) protein content and resting muscle glycogen. The authors concluded that their low-volume high-intensity training model was effective for improving skeletal muscle mitochondrial capacity and functional exercise performance.
test) with 75 s recoveries at 30 W. To confirm the efficacy of their training intervention for enhancing skeletal muscle mitochondrial capacity, Little and colleagues (Little et al. 2010) examined the mitochondrial enzymes cytochrome C oxidase (COX) and citrate synthase (CS) in whole muscle homogenates from muscle biopsy tissue. Compared to pre-training values, a significant increase in both maximal activity, assessed by spectrophotometry, and protein content, assessed by Western blotting, for both COX and CS was found post training. Training intervention-induced changes in several proposed mediators of skeletal muscle mitochondrial biogenesis and metabolic adaptation were also investigated. Significant increases in whole muscle protein content of sirtuin 1 and Tfam but not NRF-1 were found post training. PGC-1α was elevated post-training in nuclear fractions but remained unchanged in whole muscle homogenates. In addition, training significantly increased total glucose transporter type 4 (GLUT4) protein content and resting muscle glycogen. The authors concluded that their low-volume high-intensity training model was effective for improving skeletal muscle mitochondrial capacity and functional exercise performance.
It is pertinent to reiterate that the performance improvements and metabolic changes shown by Little and colleagues were induced by a training regime with a total exercise time commitment of only 2 h 25 min over 2 weeks. Although the authors state that the intensity of the intervals had been reduced from the repeated maximal Wingate cycling tests used in their previous work to better suit the general population and that their modified protocol was generally well tolerated, efforts at 100% maximal aerobic power still require a certain level of subject motivation. However, interval training at 90–95%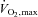 or maximum heart rate has successfully been carried out in a range of patient groups including individuals with metabolic syndrome (Tjønna et al. 2008). Another issue when considering this type of training as an effective health-enhancing strategy is the exercise modality used. Cycling requires a higher skill level than treadmill exercise and is less relevant to the demands of daily life of the general population.
or maximum heart rate has successfully been carried out in a range of patient groups including individuals with metabolic syndrome (Tjønna et al. 2008). Another issue when considering this type of training as an effective health-enhancing strategy is the exercise modality used. Cycling requires a higher skill level than treadmill exercise and is less relevant to the demands of daily life of the general population.
Aside from the improvement in exercise performance, the training-induced increase in nuclear but not cytosolic PGC-1α is arguably the most interesting finding of the study. This finding is consistent with previous research in Wistar rats showing that the initial phase of the adaptive increase in mitochondrial biogenesis is mediated by activation of PGC-1α and subcellular relocalization to the nucleus rather than an increase in PGC-1α protein expression (Wright et al. 2007). It is likely that had Little and colleagues prolonged the training period they would have observed a subsequent increase in PGC-1α protein, as was shown in rodents. Wright et al. proposed that this secondary phase of the adaptation sustains and enhances the increase in mitochondrial biogenesis which is initiated by activation of PGC-1α, the increase in PGC-1α nuclear abundance observed by Little and colleagues. Further investigation would be required to determine the time course and longevity of these effects in humans.
As well as probing the mechanisms behind exercise-induced mitochondrial biogenesis, future research could explore whether the type of training conducted in this study is an effective and indeed feasible strategy for improving metabolic health particularly in sedentary individuals or conditions of metabolic or indeed cardiovascular disease. This particular regime appears to be a potent stimulus to rapidly induce effects in skeletal muscle in healthy individuals, the novelty being in the low time commitment required. Naturally this Journal Club article focuses on the physiological adaptations investigated; one can only speculate on psychosocial variables which were not evaluated as part of the study. This type of training may be effective and suited to a society in the grip of time poverty; one wonders whether it also induces physiological changes in the brain that will have currently sedentary individuals coming back for more.
References
- Hock MB, Kralli A. Transcriptional control of mitochondrial biogenesis and function. Annu Rev Physiol. 2009;71:177–203. doi: 10.1146/annurev.physiol.010908.163119. [DOI] [PubMed] [Google Scholar]
- Holloszy JO. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem. 1967;242:2278–2282. [PubMed] [Google Scholar]
- Little JP, Safdar A, Wilkin GP, Tarnopolsky MA, Gibala MJ. A practical model of low-volume high-intensity interval training induces mitochondrial biogenesis in human skeletal muscle: potential mechanisms. J Physiol. 2010;588:1011–1022. doi: 10.1113/jphysiol.2009.181743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjønna AE, Lee SJ, Rognmo Ø, Stølen TO, Bye A, Haram PM, Loennechen JP, Al-Share QY, Skogvoll E, Slørdahl SA, Kemi OJ, Najjar SM, Wisløff U. Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome: a pilot study. Circulation. 2008;118:346–354. doi: 10.1161/CIRCULATIONAHA.108.772822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright DC, Han DH, Garcia-Roves PM, Geiger PC, Jones TE, Holloszy JO. Exercise-induced mitochondrial biogenesis begins before the increase in muscle PGC-1α expression. J Biol Chem. 2007;282:194–199. doi: 10.1074/jbc.M606116200. [DOI] [PubMed] [Google Scholar]


