Abstract
Voltage-gated sodium channels initiate action potentials in brain neurons, and sodium channel blockers are used in therapy of epilepsy. Mutations in sodium channels are responsible for genetic epilepsy syndromes with a wide range of severity, and the NaV1.1 channel encoded by the SCN1A gene is the most frequent target of mutations. Complete loss-of-function mutations in NaV1.1 cause severe myoclonic epilepsy of infancy (SMEI or Dravet's Syndrome), which includes severe, intractable epilepsy and comorbidities of ataxia and cognitive impairment. Mice with loss-of-function mutations in NaV1.1 channels have severely impaired sodium currents and action potential firing in hippocampal GABAergic inhibitory neurons without detectable effect on the excitatory pyramidal neurons, which would cause hyperexcitability and contribute to seizures in SMEI. Similarly, the sodium currents and action potential firing are also impaired in the GABAergic Purkinje neurons of the cerebellum, which is likely to contribute to ataxia. The imbalance between excitatory and inhibitory transmission in these mice can be partially corrected by compensatory loss-of-function mutations of NaV1.6 channels, and thermally induced seizures in these mice can be prevented by drug combinations that enhance GABAergic neurotransmission. Generalized epilepsy with febrile seizures plus (GEFS+) is caused by missense mutations in NaV1.1 channels, which have variable biophysical effects on sodium channels expressed in non-neuronal cells, but may primarily cause loss of function when expressed in mice. Familial febrile seizures is caused by mild loss-of-function mutations in NaV1.1 channels; mutations in these channels are implicated in febrile seizures associated with vaccination; and impaired alternative splicing of the mRNA encoding these channels may also predispose some children to febrile seizures. We propose a unified loss-of-function hypothesis for the spectrum of epilepsy syndromes caused by genetic changes in NaV1.1 channels, in which mild impairment predisposes to febrile seizures, intermediate impairment leads to GEFS+ epilepsy, and severe or complete loss of function leads to the intractable seizures and comorbidities of SMEI.
William Catterall (centre) is Professor and Chair of Pharmacology, John Oakley (right) is Acting Assistant Professor of Neurology, and Franck Kalume (left) is Acting Instructor of Pharmacology at the University of Washington School of Medicine in Seattle. Together with colleagues, they have produced and characterized a mouse model of severe myoclonic epilepsy of infancy (SMEI), which exhibits all of the characteristics of the human disease. Their work reveals that mutations of NaV1.1 channels in this disease cause selective loss of sodium current and electrical excitability of GABAergic inhibitory neurons, which is likely to be responsibility for both epilepsy and co-morbidities in SMEI.
 |
Introduction
The epilepsies are a heterogeneous group of conditions characterized by recurrent seizures. While many pathophysiological changes contribute to seizure susceptibility, recent work suggests that genetic factors are important. Polygenic inheritance patterns have been associated with febrile seizures and may be important in determining susceptibility to acquired epilepsy following brain injury. Monogenic inheritance patterns are seen in a number of epilepsies associated with mutations in ligand- or voltage-gated ion channels. The gene most frequently associated with epilepsy is SCN1A, which codes for the α subunit of the Nav1.1 sodium channel.
Voltage-gated sodium channels
Voltage-gated Na+ channels in the brain are complexes of a 260 kDa α subunit in association with auxiliary β subunits (β1–β4) of 33–36 kDa (Catterall, 2000). The α subunit contains the voltage sensors and the ion-conducting pore in four internally repeated domains (I–IV), which each consists of six α-helical transmembrane segments (S1–S6) and a pore loop connecting S5 and S6 (Catterall, 2000). The β subunits modify the kinetics and voltage dependence of gating and serve as cell adhesion molecules interacting with extracellular matrix, other cell adhesion molecules, and the cytoskeleton (Isom et al. 1995; Isom, 2002). The voltage-gated ion channels are encoded by one of the most ancient and conserved gene families, with sequence identity of >50% in the transmembrane domains of human sodium channel α subunits and those of the simplest multicellular eukaryotes. The mammalian genome contains nine functional voltage-gated sodium channel α subunits, which differ in patterns of tissue expression and biophysical properties. The NaV1.1, NaV1.2, NaV1.3 and NaV1.6 channel subtypes, encoded by the SCN1A, SCN2A, SCN3A and SCN8A genes, respectively, are the primary sodium channels in the central nervous system (Catterall, 2000; Goldin et al. 2000; Goldin, 2001; Trimmer & Rhodes, 2004). NaV1.1 and NaV1.3 channels are primarily localized in cell bodies (Westenbroek et al. 1989, 1992), NaV1.2 channels in unmyelinated or pre-myelinated axons and dendrites (Westenbroek et al. 1989, 1992), and NaV1.6 channels in myelinated axons and in dendrites (Caldwell et al. 2000; Krzemien et al. 2000; Jenkins & Bennett, 2001). These channels participate in generation of both somatodendritic and axonal action potentials (Stuart & Sakmann, 1994; Johnston et al. 1996; Callaway & Ross, 1997; Raman & Bean, 1999b; Khaliq & Raman, 2006). In rodents, NaV1.3 channels are highly expressed in the brain during embryonic life, and their expression declines after birth as NaV1.1 and NaV1.2 channels take over (Gordon et al. 1987; Beckh et al. 1989). NaV1.1 expression is first detectable at postnatal day 7 and increases steadily through young adulthood (Gordon et al. 1987; Beckh et al. 1989).
Sodium channels and inherited epilepsy
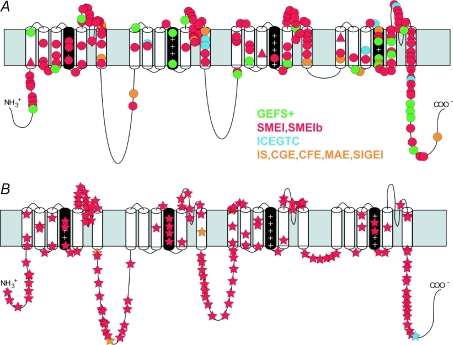
In spite of their amino acid sequence identity of >70%, knockout of any of the three sodium channel α subunit genes expressed primarily in adult brain (SCN1A, SCN2A and SCN8A) is lethal, demonstrating that each channel performs some non-redundant function. The NaV1.1 channel is remarkable for the number of mutations that cause inherited epilepsy. Screening of human patients with inherited epilepsy first led to the identification of mutations of NaV1.1 channels in two large families with the autosomal dominant epilepsy disorder GEFS+ (generalized epilepsy with febrile seizures plus, OMIM 604233) (Escayg et al. 2000). More than 20 different mutations were subsequently identified in GEFS+ patients, accounting for approximately 10% of cases (Fig. 1). Moreover, a mutation in the NaVβ1 subunit also causes GEFS+ epilepsy, very likely by impairing expression and function of NaV1.1 channels (Wallace et al. 1998). GEFS+ is caused by missense mutations that alter multiple biophysical properties of the channel expressed in non-neuronal cells (Meisler & Kearney, 2005; and see below).
Figure 1. Mutations in NaV1.1 channel patients with epilepsy.
A, missense mutations (circles) and in-frame deletions (triangles). B, truncation mutations (stars). The clinical type of epilepsy is indicated by colour: GEFS+, generalized epilepsy with febrile seizures plus; SMEI, severe myoclonic epilepsy of infancy; SMEIb, borderline SMEI; ICEGTC, idiopathic childhood epilepsy with generalized tonic–clonic seizures; IS, infantile spasms; CGE, cryptogenic generalized epilepsy; CFE, cryptogenic focal epilepsy; MAE, myoclonic astatic epilepsy; SIGEI, severe idiopathic generalized epilepsy of infancy. Courtesy of M. Meisler and J. Kearney (Catterall et al. 2008).
Identification of these familial SCN1A mutations in GEFS+ epilepsy was followed by the surprising report of mutations in children with the sporadic epilepsy disorder SMEI (severe myoclonic epilepsy of infancy or Dravet's syndrome, OMIM 607208) (Claes et al. 2001). These children carry de novo mutations in one allele of the SCN1A gene, leading to haploinsufficiency of NaV1.1 channels (Claes et al. 2001; Ohmori et al. 2002; Sugawara et al. 2002; Claes et al. 2003; Fujiwara et al. 2003; Fukuma et al. 2004). More than 600 SCN1A mutations in the coding sequences of the SCN1A gene have been identified (Fig. 1), accounting for more than 70% of cases (Meisler & Kearney, 2005; http://www.molgen.ua.ac.be/SCN1AMutations/home/Default.cfm). Since only coding regions of the gene are sequenced, it is possible that many of the remaining 30% of SMEI patients harbour mutations in regulatory regions of the gene outside of the coding sequences that impair or prevent channel expression. In addition, duplications and deletions of segments of the SCN1A gene can also impair expression and/or function (Marini et al. 2009). Mutation hotspots, including several sites of CpG deamination, account for approximately 25% of new mutations (Kearney et al. 2006; Depienne et al. 2009). More than half of the SMEI mutations cause loss of function due to stop codons or deletions, demonstrating that haploinsufficiency of SCN1A is pathogenic. Missense mutations of NaV1.1 channels in patients with SMEI are concentrated in the transmembrane segments of the protein, where they may prevent channel expression or severely impair channel function (Fig. 1). In addition, recent studies show that homozygous loss-of-function mutations in the NaVβ1 subunits cause SMEI, probably by impairing expression of NaV1.1 channels on the cell surface (Patino et al. 2009). One practical result of the discovery that haploinsufficiency of NaV1.1 channels causes SMEI is avoidance of treatment with sodium channel blocking anti-epileptic drugs, which exacerbate symptoms in patients with reduced expression of SCN1A (Guerrini et al. 1998; Loscher, 2009).
Severe myoclonic epilepsy of infancy
SMEI begins during the first year of life, with seizures often associated with elevated body temperature due to fever or bathing, and progresses to prolonged, clustered, or continuous seizures and to status epilepticus (Dravet et al. 1992; Engel, 2001). After the second year of life, patients develop co-morbidities including psychomotor delay, ataxia and cognitive impairment. Medically refractory seizures including frequent and prolonged episodes of status epilepticus contribute to an unfavorable long-term outcome (Dravet et al. 1992; Oguni et al. 2001). It is a surprise that haploinsufficiency of a NaV channel causes epilepsy, because reduced sodium current should lead to hypoexcitability rather than hyperexcitability. To understand the mechanistic basis for hyperexcitability and co-morbidities in SMEI, an animal model was generated by targeted deletion or mutation of the Scn1a gene in mouse (Yu et al. 2006).
Selective loss of excitability of GABAergic interneurons and hyperexcitability in SMEI
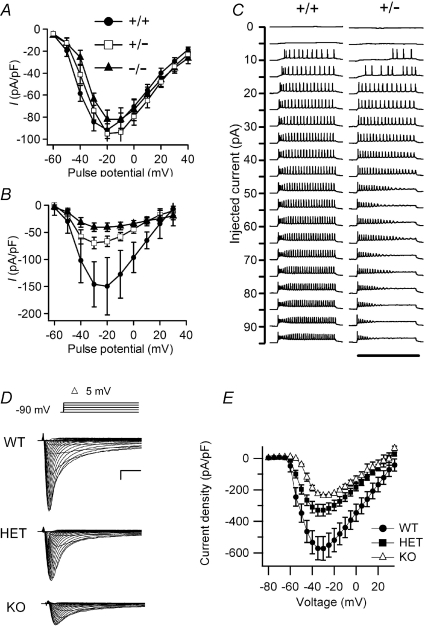
Homozygous null NaV1.1−/− mice developed ataxia and died on postnatal day (P)15, but could be sustained to P17.5 with manual feeding (Yu et al. 2006; Ogiwara et al. 2007). Heterozygous NaV1.1+/− mice exhibited spontaneous seizures and sporadic deaths beginning after P21, with a striking dependence on genetic background (Yu et al. 2006). Loss of NaV1.1 did not change voltage-dependent activation or inactivation of sodium channels in hippocampal neurons (Yu et al. 2006). However, the sodium current density was substantially reduced in inhibitory interneurons of NaV1.1+/− and NaV1.1−/− mice, but not in their excitatory pyramidal neurons (Fig. 2, Table 1). This reduction in sodium current caused a loss of sustained high-frequency firing of action potentials in hippocampal and cortical interneurons (Yu et al. 2006; Ogiwara et al. 2007), thereby impairing their in vivo inhibitory function that depends on generation of high-frequency bursts of action potentials. An immunocytochemical survey also revealed a specific up-regulation of NaV1.3 channels in a subset of hippocampal interneurons, but this up-regulation was insufficient to compensate for the loss of the sodium current of NaV1.1 channels (Yu et al. 2006). These results suggest that reduced sodium currents in GABAergic inhibitory interneurons in NaV1.1+/− heterozygotes may cause the hyperexcitability that leads to epilepsy in patients with SMEI. Loss of excitability of GABAergic inhibitory interneurons would allow hyperexcitability of dentate granule and pyramidal neurons, and this gain-of-function effect may cause epilepsy. Failure of firing of additional classes of interneurons in the cerebral cortex and thalamus may also contribute to this complex seizure phenotype.
Figure 2. Sodium currents from hippocampal neurons and cerebellar Purkinje cells in wild-type, heterozygous and null NaV1.1 mice.
A and B, current–voltage relationships of whole-cell sodium currents from hippocampal pyramidal (A) and bipolar inhibitory neurons (B) for wild-type (circle), heterozygous (square) and homozygous (triangle) mice (Yu et al. 2006). C, action potential traces recorded from wild-type (+/+) and heterozygous (+/−) interneurons during application of 800-ms injections of depolarizing current in +10 pA increments from a holding potential of −80 mV (Kalume et al. 2007). D, sodium currents in cerebellar Purkinje neurons of WT, HET, and KO mice evoked with a series of 50 ms depolarizations from a holding potential of −90 mV to potentials ranging from −80 to + 30 mV in 5-mV increments. Inset, diagram of stimulus protocol. Scale bars: 1 ms, 2 nA. E, current–voltage relationships for WT (filled circles), HET (filled squares) and KO (open triangles) mice.
Table 1.
Functional impact of deletion of the NaV1.1 channel
| Functional effect | Heterozygous knockout | Homozygous knockout |
|---|---|---|
| Na+ current in hippocampal pyramidal cells (% WT) | 100 ± 5.1 | 96 ± 6.0 |
| Na+ current in hippocampal interneurons (% WT) | 47.0 ± 7.4 | 27.5 ± 5.4 |
| Na+ current in Purkinje neurons (% WT) | ||
| Peak | 57.6 ± 0.6 | 41.6 ± 0.5 |
| Persistent | 44.9 ± 4.1 | 41.0 ± 3.7 |
| Resurgent | 49.6 ± 5.5 | 31.2 ± 3.5 |
| Ataxia | Significant at P21 | Severe at P11–14 |
| Thermally induced seizures | First observed at P20 Increasing thereafter | Not tested |
| Spontaneous seizures | First observed at P21 Increasing thereafter | P11–14 |
| Premature death | Increasing premature death after P21 | Death at P15 |
Results from Yu et al. 2006; Kalume et al. 2007.
Loss of excitability of Purkinje neurons and ataxia in SMEI
Ataxia, spasticity and failure of motor coordination contribute substantially to the developmental delay and functional impairments of SMEI patients and are major determinants of their poor quality of life, burden of care, and premature deaths (Dravet et al. 2005). How might loss of NaV1.1 channels cause ataxia, spasticity and failure of motor coordination? Purkinje cells are GABAergic inhibitory neurons that serve as the output pathway for information on movement, coordination and balance from the cerebellar cortex. Degeneration of Purkinje neurons and abnormal expression of voltage-gated ion channels in them are associated with ataxia (Fletcher et al. 1996; Raman & Bean, 1997; Grusser-Cornehls & Baurle, 2001; Sausbier et al. 2004). Behavioural assessment indicated severe motor deficits in homozygous NaV1.1 knockout mice, including irregularity of stride length during locomotion, impaired motor reflexes in grasping, and mild tremor in limbs when immobile, consistent with cerebellar dysfunction (Yu et al. 2006; Kalume et al. 2007). A milder impairment of normal gait was observed in the heterozygotes after P21 (Kalume et al. 2007). Immunohistochemical studies showed that NaV1.1 and NaV1.6 channels are the primary sodium channel isoforms expressed in cerebellar Purkinje neurons (Kalume et al. 2007). The amplitudes of whole-cell peak, persistent and resurgent sodium currents in Purkinje neurons were reduced by 58–69%, without detectable change in the kinetics or voltage dependence of channel activation or inactivation (Table 1). Nonlinear loss of sodium current in Purkinje neurons from heterozygous and homozygous mutant animals suggested partial compensatory up-regulation of NaV1.6 channel activity (Table 1). Current-clamp recordings revealed that the firing rates of Purkinje neurons from mutant mice were substantially reduced, with no effect on threshold for action potential generation (Kalume et al. 2007). The results show that NaV1.1 channels play a crucial role in the excitability of cerebellar Purkinje neurons, with major contributions to peak, persistent and resurgent forms of sodium current and to sustained action potential firing. Loss of these channels in Purkinje neurons of mutant mice and SMEI patients may be sufficient to cause their ataxia and related functional deficits. These findings suggest the hypothesis that loss of sodium currents in different classes of GABAergic neurons may underlie the multiple co-morbidities in SMEI, including light hypersensitivity, altered circadian rhythms, and cognitive impairment.
Thermally induced seizures in a mouse model of SMEI
Children with SMEI frequently have seizures with elevated body temperature as their first symptom of the disease (Oguni et al. 2005). Experiments with a mouse model of SMEI demonstrated that haploinsufficiency of NaV1.1 channels is sufficient to allow induction of seizures by elevated body temperature (Oakley et al. 2009). P17–18 mice with SMEI did not have thermally induced seizures, but nearly all P20–22 and P30–46 mice with SMEI had myoclonic seizures followed by generalized seizures with elevated core body temperature. Spontaneous seizures were only observed in mice older than P21, indicating that mice with SMEI become susceptible to temperature-induced seizures before spontaneous seizures. Inter-ictal spike activity was seen at normal body temperature in most P30–46 mice with SMEI but not in P20–22 or P17–18 mice, suggesting that inter-ictal epileptic activity correlates with seizure susceptibility. These results define a critical developmental transition for susceptibility to seizures in a mouse model of SMEI and reveal a close correspondence between human and mouse SMEI in the striking temperature and age dependence of SMEI onset and progression.
Balancing excitation and inhibition with genetic compensation and drug treatment
The net electrophysiological properties of a neuron are the product of its total ion channel content, so inheritance of genetic variants of ion channels may contribute to polygenic inheritance or variable penetrance among family members. Since SMEI is apparently caused by loss of sodium current and failure of firing of GABAergic interneurons (Yu et al. 2006; Kalume et al. 2007), it may be compensated by mutations that reduce the sodium current and action potential firing of excitatory neurons and thereby re-balance excitation and inhibition in the brain. Such genetic compensation can be studied by mating mouse lines having different well-defined genetic deficiencies. NaV1.6 channels encoded by the Scn8a gene are highly expressed in excitatory neurons, and their functional properties are well suited to driving repetitive firing (Raman & Bean, 1997, 1999a; Chen et al. 2008). Double heterozygous mice with haploinsufficiency for both Scn1a and Scn8a did indeed have reduced susceptibility to drug-induced seizures and improved lifespan compared to NaV1.1 heterozygotes (Martin et al. 2007). These results support the concept that loss-of-function mutations in NaV1.1 channels in SMEI cause an imbalance of excitation over inhibition in the brain and that this imbalance can be partially compensated by a corresponding reduction in the activity of NaV1.6 channels.
In principle, the imbalance of excitation and inhibition can also be corrected by drug treatment. Unfortunately, there are no drugs that selectively inhibit NaV1.6 channels. However, an alternative approach to re-balance excitation and inhibition is to enhance GABAergic neurotransmission by drug treatment. The reduced frequency of action potentials in GABAergic inhibitory neurons in SMEI would decrease phasic release of GABA and impair inhibitory neurotransmission. Drugs such as tiagabine increase the concentration of GABA in the synaptic cleft by inhibiting its reuptake into nerve terminals and glia, and benzodiazepines such as clonazepam increase the response of the postsynaptic GABAA receptors to GABA. Using febrile seizures in a mouse model of SMEI (Oakley et al. 2009) as a test system, we found that combinations of tiagabine and clonazepam are effective in completely preventing thermally induced myoclonic and generalized tonic–clonic seizures (Oakley, Kalume, Scheuer, and Catterall, Dravet Syndrome International Workshop, Abstract, in press, 2010). These encouraging results suggest that similar combination drug therapies may be useful for children with SMEI.
Generalized epilepsy with febrile seizures plus
GEFS+ is usually a much milder epilepsy syndrome than SMEI. Seizures are typically well controlled by treatment with anti-epileptic drugs and no cognitive impairment is observed. The mutations that cause GEFS+ are usually single amino acid missense mutations (Meisler & Kearney, 2005; Fig. 1). Nevertheless, it has been difficult to determine the molecular mechanisms and genotype–phenotype correlations for GEFS+ epilepsy.
Functional effects of GEFS+ mutations expressed in non-neuronal cells
Functional effects of GEFS+ mutations were first studied by expression in non-neuronal cells and voltage clamp analysis. The initial study of two mutations inserted in rat NaV1.1 and expressed in Xenopus oocytes revealed that one was a gain-of-function mutation because of destabilized slow inactivation, whereas the second was a loss-of-function mutation because of enhanced slow inactivation (Spampanato et al. 2001). In contrast, the first three mutations inserted in human NaV1.1 and studied by expression in human somatic cells revealed a different functional effect – all three caused impaired inactivation and increased persistent sodium current, leading to the hypothesis that gain-of-function of mutant sodium channels due to loss of inactivation is responsible for GEFS+ epilepsy (Lossin et al. 2002). However, further studies of several GEFS+ mutations expressed in mammalian cells (Lossin et al. 2003; Kahlig et al. 2006) or Xenopus oocytes (Spampanato et al. 2003, 2004; Barela et al. 2006) revealed a mixture of loss-of-function and gain-of-function effects that were caused by several different changes in biophysical properties of NaV1.1 channels (Table 2). Moreover, Rusconi et al. (2007) found that loss-of-function of one GEFS+ mutation resulted from folding and/or trafficking defects that prevented channel expression in the absence of auxiliary β subunits and reduced expression even in the presence of β subunits (Rusconi et al. 2007, 2009). Remarkably, these two GEFS+ mutations can also be partially rescued by treatment with anti-epileptic drugs, which apparently stabilize the mutant channels by binding to them and contributing their binding energy to stabilization of the correctly folded channel protein (Rusconi et al. 2007, 2009). These results indicate that, at least as expressed in non-neuronal cells, mutations that cause GEFS+ epilepsy can have either gain-of-function or loss-of-function effects and these can result from changes in biophysical properties and/or defects in folding and cell surface expression.
Table 2.
GEFS+ Mutations
| Mutation | Functional effect in vitro | Mechanism | Functional effect in vivo | Mechanism | Reference |
|---|---|---|---|---|---|
| D188V | GoF | Impaired slow inactivation | Cossette et al. (2003) | ||
| R859C | LoF | Positive shift of activation; slowed recovery from slowed inactivation; reduced INa* | Barela et al. (2006) | ||
| T875M | LoF | Enhanced slow inactivation | Spamanato et al. (2001) | ||
| Increased persistent INa** | Lossin et al. (2002) | ||||
| W1204R | GoF | Increased persistent INa** | Lossin et al. (2002) | ||
| GoF | Negative shift of activation and inactivation; negative shift of window current* | Spampanato et al. (2003) | |||
| V1353L | LoF | ||||
| R1648H | GoF | Enhanced recovery from inactivation*; | Spampanato et al. (2001),Lossin et al. (2002) | ||
| Increased persistent INa** | Tang et al. (2009) | ||||
| LoF | Increased inactivation; impaired recovery from inactivation | Martin et al. (2010) | |||
| I1656M | LoF | Positive shift of activation | Lossin et al. (2003) | ||
| R1657C | LoF | Positive shift of activation; reduced expression | Lossin et al. (2003),Vanoye et al. (2005) | ||
| A1685V | LoF | Folding/trafficking defect** | |||
| M1841T | LoF | Folding/trafficking defect** | Rusconi et al. (2007) | ||
| D1866Y | GoF | Impairment of the effect of beta subunits to enhance inactivation* | Spampanato et al. (2004) | ||
| R1916G | LoF | Folding/trafficking defect** | Rusconi et al. (2009) |
Rat NaV1.4 expressed in Xenopus oocytes;
human NaV1.4 expressed in human embryonic kidney cells.
GEFS+ mutations in mouse genetic models
Considering the confusing picture from studies of the functional effects of GEFS+ mutations in transfected non-neuronal cells, it is important to determine the functional effects of these mutations in neurons in vivo. Toward this end, the mutation R1648H was incorporated into the mouse genome using a Bac transgene strategy, providing an animal model of GEFS+ that permits more detailed analysis of the effect of mutations on neuronal sodium currents in vivo. The transfected NaV1.1 channel contained both the GEFS+ mutation and an additional amino acid substitution that prevents block by the pore-blocker tetrodotoxin (TTX), thereby allowing selective block of endogenous sodium channels by TTX without effect on the transfected NaV1.1 channels (Tang et al. 2009). Mice expressing the transgene had increased sodium channel expression and reduced threshold for seizure induction by kainic acid. The level of transgene-induced sodium current was much greater in inhibitory neurons than in excitatory neurons, as expected from previous studies of NaV1.1 knockout mice showing selective expression in inhibitory neurons (Yu et al. 2006; Ogiwara et al. 2007). The R1648H channels showed reduced function in both excitatory and inhibitory neurons, but the biophysical mechanisms were different – reduced peak sodium currents and enhanced slow inactivation in inhibitory neurons versus negatively shifted voltage dependence of fast inactivation in excitatory neurons. These functional effects were predicted to have the net result of reduced excitability of inhibitory neurons. Thus, this GEFS+ mutation causes selective impairment of excitability of GABAergic inhibitory neurons in vivo (Tang et al. 2009), as previously demonstrated for SMEI mutations (Yu et al. 2006). More recent studies of a mouse model in which the R1648H mutation has been inserted into the mouse genome under the native promoter lead to similar conclusions (Martin et al. 2010). In light of these results, GEFS+ and SMEI may be caused by a continuum of mutational effects that selectively impair firing of GABAergic inhibitory neurons. According to this hypothesis, mild impairment of NaV1.1 channels and action potential firing of GABAergic neurons causes GEFS+ epilepsy, whereas complete (or nearly complete) loss of NaV1.1 channel function causes more severe impairment of action potential firing of GABAergic neurons and leads to SMEI.
Potential role of mutations in NaV1.1 channels in febrile seizures in childhood
Febrile seizures are common in childhood, but the basis for their prevalence is not known. Several lines of evidence now suggest that mild loss-of-function mutations or polymorphisms in NaV1.1 channels may cause a significant portion of febrile seizures. Mantegazza et al. (2005) characterized a mild loss-of-function mutation in NaV1.1 channels in a family with familial febrile seizures. This mutation caused reduction of peak sodium currents and positive shift in the voltage dependence of activation when expressed in non-neuronal cells. Therefore, this study provided the first evidence for association of mild loss of function of NaV1.1 channels with familial febrile seizures.
There has been considerable controversy regarding claims that routine childhood vaccination may be associated with the onset of both febrile and afebrile seizures and mental decline. Berkovic et al. (2006) studied 14 children with this diagnosis and identified SCN1A mutations in 11 of the children. Their observations indicate that vaccination and its associated fever may trigger the first seizure episode of an underlying genetic disorder, GEFS+ or SMEI, which often presents first as febrile seizures. These studies further implicate dysfunction of NaV1.1 channels in childhood febrile seizures.
Most recently, human genetic studies have also suggested an association of genetic alterations in NaV1.1 channels with non-familial febrile seizures (Schlachter et al. 2009). In normal development, the mRNAs encoding NaV1.1, NaV1.2 and NaV1.3 channels undergo a regulated change in alternative splicing of exon 5 (Sarao et al. 1991; Gazina et al. 2009), which has a striking effect on the voltage dependence of channel activation (Auld et al. 1990). Regulation of this alternative splicing process is disrupted by a single nucleotide polymorphism (SNP IVS5N+5 G>A; Tate et al. 2005, 2006). The presence of this SNP has been correlated with altered response to anti-epileptic drugs (Tate et al. 2005, 2006) and with risk of febrile seizures in a large cohort of epilepsy patients from Germany and Austria (Schlachter et al. 2009). On the other hand, correlation of this SNP with febrile seizures was not observed in a similar study of Australian epilepsy patients (Petrovski et al. 2009). Nevertheless, even considering these negative data, the combination of results from studies of familial febrile seizures (Mantegazza et al. 2005), vaccination-related seizures (Berkovic et al. 2006), responsiveness to anti-epileptic drugs (Tate et al. 2005, 2006), and febrile seizures in a German/Austrian cohort of patients (Schlachter et al. 2009) all point to a key role of NaV1.1 channels as molecular determinants of the risk of epilepsy in the general population and raise the possibility that a significant fraction of febrile seizures in children are caused by mild loss-of-function mutations or polymorphisms of NaV1.1 channels in combination with environmental precipitating factors.
A unified loss-of-function hypothesis for NaV1.1 genetic epilepsies
NaV1.1 channels are highly expressed in many GABAergic inhibitory neurons and are responsible for essentially all of the sodium current in the cell bodies of hippocampal interneurons (Yu et al. 2006). Loss of function of these sodium channels greatly impairs the ability of these inhibitory neurons to fire action potentials at high frequency and therefore would greatly reduce their phasic release of GABA (Yu et al. 2006). It is likely that this loss of action potential firing by inhibitory neurons leads to an imbalance of excitation and inhibition in the brain and consequently to febrile seizures and epilepsy. In embryonic and neonatal rodent brain, intracellular Cl− concentration is high, and activation of GABA-A receptors can depolarize neurons by conducting Cl− outward (Rivera et al. 1999; Blaesse et al. 2009). However, by postnatal day 22, when spontaneous seizures begin in mouse models of NaV1.1 epilepsies, intracellular concentration of Cl− is reduced owing to increased expression and function of cation–hloride co-transporters, and Cl− conductance resulting from activation of GABA-A receptors hyperpolarizes neurons and clamps the membrane potential near its resting level (Rivera et al. 1999; Blaesse et al. 2009).
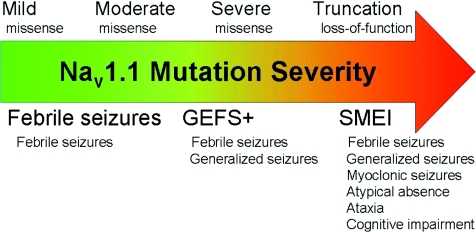
Although more work is needed to develop definite genotype–phenotype correlations for the NaV1.1 epilepsies, we extend a previous proposal (Ragsdale, 2008) and propose the unifying hypothesis that the spectrum of severity of the NaV1.1-associated forms of epilepsy results from a spectrum of increasing severity of loss-of-function mutations of NaV1.1 channels and increasing impairment of action potential firing in GABAergic inhibitory neurons (Fig. 3). Mild impairment of NaV1.1 channel function causes febrile seizures; moderate to severe impairment of NaV1.1 function by missense mutations and/or altered mRNA processing causes the range of phenotypes observed in GEFS+ epilepsy; and very severe to complete loss of function causes SMEI. The severity of phenotype in these genetic diseases is also influenced strongly by genetic background effects as illustrated by striking differences in phenotypes among GEFS+ patients with the same missense mutation (Scheffer et al. 2009), different severity of disease of SMEI patients with complete loss-of-function mutations (Mulley et al. 2005; Harkin et al. 2007), and dramatic differences in sensitivity among mouse strains to the same loss-of-function mutations (Yu et al. 2006). We hope that this unifying hypothesis and further analysis of the effects of mutations in mouse models will bring increasing clarity to our understanding of genotype-phenotype correlations in this family of epilepsy syndromes and will provide fresh insights into effective therapies.
Figure 3. The unified loss-of-function hypothesis for NaV1.1 genetic epilepsies.
Increasing severity of loss-of-function mutations of NaV1.1 channels, noted above the arrow, causes progressively more severe epilepsy syndromes from familial febrile seizures to GEFS+ and finally SMEI, noted below the arrow. Major symptoms of each syndrome are also listed.
References
- Auld VJ, Goldin AL, Krafte DS, Catterall WA, Lester HA, Davidson N, Dunn RJ. A neutral amino acid change in segment IIS4 dramatically alters the gating properties of the voltage-dependent sodium channel. Proc Natl Acad Sci U S A. 1990;87:323–327. doi: 10.1073/pnas.87.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barela AJ, Waddy SP, Lickfett JG, Hunter J, Anido A, Helmers SL, Goldin AL, Escayg A. An epilepsy mutation in the sodium channel SCN1A that decreases channel excitability. J Neurosci. 2006;26:2714–2723. doi: 10.1523/JNEUROSCI.2977-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckh S, Noda M, Lübbert H, Numa S. Differential regulation of three sodium channel messenger RNAs in the rat central nervous system during development. EMBO J. 1989;8:3611–3616. doi: 10.1002/j.1460-2075.1989.tb08534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkovic SF, Harkin L, McMahon JM, Pelekanos JT, Zuberi SM, Wirrell EC, Gill DS, Iona X, Mulley JC, Scheffer IE. De-novo mutations of the sodium channel gene SCN1A in alleged vaccine encephalopathy: a retrospective study. Lancet Neurol. 2006;5:488–492. doi: 10.1016/S1474-4422(06)70446-X. [DOI] [PubMed] [Google Scholar]
- Blaesse P, Airaksinen MS, Rivera C, Kaila K. Cation-chloride cotransporters and neuronal function. Neuron. 2009;61:820–838. doi: 10.1016/j.neuron.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Caldwell JH, Schaller KL, Lasher RS, Peles E, Levinson SR. Sodium channel Nav1.6 is localized at nodes of Ranvier, dendrites, and synapses. Proc Natl Acad Sci U S A. 2000;97:5616–5620. doi: 10.1073/pnas.090034797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway JC, Ross WN. Spatial distribution of synaptically activated sodium concentration changes in cerebellar Purkinje neurons. J Neurophysiol. 1997;77:145–152. doi: 10.1152/jn.1997.77.1.145. [DOI] [PubMed] [Google Scholar]
- Catterall WA. From ionic currents to molecular mechanisms: The structure and function of voltage-gated sodium channels. Neuron. 2000;26:13–25. doi: 10.1016/s0896-6273(00)81133-2. [DOI] [PubMed] [Google Scholar]
- Catterall WA, Dib-Hajj S, Meisler MH, Pietrobon D. Inherited neuronal ion channelopathies: new windows on complex neurological diseases. J Neurosci. 2008;28:11768–11777. doi: 10.1523/JNEUROSCI.3901-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Yu FH, Sharp EM, Beacham D, Scheuer T, Catterall WA. Functional properties and differential neuromodulation of Nav1.6 channels. Mol Cell Neurosci. 2008;38:607–615. doi: 10.1016/j.mcn.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claes L, Ceulemans B, Audenaert D, Smets K, Lofgren A, Del-Favero J, Ala-Mello S, Basel-Vanagaite L, Plecko B, Raskin S, Thiry P, Wolf NI, Van Broeckhoven C, De Jonghe P. De novo SCN1A mutations are a major cause of severe myoclonic epilepsy of infancy. Hum Mutat. 2003;21:615–621. doi: 10.1002/humu.10217. [DOI] [PubMed] [Google Scholar]
- Claes L, Del-Favero J, Ceulemans B, Lagae L, Van Broeckhoven C, De Jonghe P. De novo mutations in the sodium-channel gene SCN1A cause severe myoclonic epilepsy of infancy. Am J Hum Genet. 2001;68:1327–1332. doi: 10.1086/320609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossette P, Loukas A, Lafreniere RG, Rochefort D, Harvey-Girard E, Ragsdale DS, Dunn RJ, Rouleau GA. Functional characterization of the D188V mutation in neuronal voltage-gated sodium channel causing generalized epilepsy with febrile seizures plus (GEFS) Epilepsy Res. 2003;53:107–117. doi: 10.1016/s0920-1211(02)00259-0. [DOI] [PubMed] [Google Scholar]
- Depienne C, Trouillard O, Saint-Martin C, Gourfinkel-An I, Bouteiller D, Carpentier W, Keren B, Abert B, Gautier A, Baulac S, Arzimanoglou A, Cazeneuve C, Nabbout R, LeGuern E. Spectrum of SCN1A gene mutations associated with Dravet syndrome: analysis of 333 patients. J Med Genet. 2009;46:183–191. doi: 10.1136/jmg.2008.062323. [DOI] [PubMed] [Google Scholar]
- Dravet C, Bureau M, Guerrini R, Giraud N, Roger J. Severe myoclonic epilepsy in infants. In: Roger J, Dravet C, Bureau M, Dreifus FE, Perret A, Wolf P, editors. Epileptic Syndromes in Infancy, Childhood and Adolescence. 2nd edn. London: John Libbey; 1992. pp. 75–102. [Google Scholar]
- Dravet C, Bureau M, Oguni H, Fukuyama Y, Cokar O. Severe myoclonic epilepsy in infancy: Dravet syndrome. Adv Neurol. 2005;95:71–102. [PubMed] [Google Scholar]
- Engel J., Jr A proposed diagnostic scheme for people with epileptic seizures and with epilepsy: report of the ILAE Task Force on Classification and Terminology. Epilepsia. 2001;42:796–803. doi: 10.1046/j.1528-1157.2001.10401.x. [DOI] [PubMed] [Google Scholar]
- Escayg A, MacDonald BT, Meisler MH, Baulac S, Huberfeld G, An-Gourfinkel I, Brice A, LeGuern E, Moulard B, Chaigne D, Buresi C, Malafosse A. Mutations of SCN1A, encoding a neuronal sodium channel, in two families with GEFS+2. Nat Genet. 2000;24:343–345. doi: 10.1038/74159. [DOI] [PubMed] [Google Scholar]
- Fletcher CF, Lutz CM, O'Sullivan TN, Shaughnessy JD, Jr, Hawkes R, Frankel WN, Copeland NG, Jenkins NA. Absence epilepsy in tottering mutant mice is associated with calcium channel defects. Cell. 1996;87:607–617. doi: 10.1016/s0092-8674(00)81381-1. [DOI] [PubMed] [Google Scholar]
- Fujiwara T, Sugawara T, Mazaki-Miyazaki E, Takahashi Y, Fukushima K, Watanabe M, Hara K, Morikawa T, Yagi K, Yamakawa K, Inoue Y. Mutations of sodium channel α subunit type 1 (SCN1A) in intractable childhood epilepsies with frequent generalized tonic-clonic seizures. Brain. 2003;126:531–546. doi: 10.1093/brain/awg053. [DOI] [PubMed] [Google Scholar]
- Fukuma G, Oguni H, Shirasaka Y, Watanabe K, Miyajima T, Yasumoto S, Ohfu M, Inoue T, Watanachai A, Kira R, Matsuo M, Muranaka H, Sofue F, Zhang B, Kaneko S, Mitsudome A, Hirose S. Mutations of neuronal voltage-gated Na+ channel α1 subunit gene SCN1A in core severe myoclonic epilepsy in infancy (SMEI) and in borderline SMEI (SMEB) Epilepsia. 2004;45:140–148. doi: 10.1111/j.0013-9580.2004.15103.x. [DOI] [PubMed] [Google Scholar]
- Gazina EV, Richards KL, Mokhtar MB, Thomas EA, Reid CA, Petrou S. Differential expression of exon 5 splice variants of sodium channel α subunit mRNAs in the developing mouse brain. Neuroscience. 2009;166:195–200. doi: 10.1016/j.neuroscience.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Goldin AL. Resurgence of sodium channel research. Annu Rev Physiol. 2001;63:871–894. doi: 10.1146/annurev.physiol.63.1.871. [DOI] [PubMed] [Google Scholar]
- Goldin AL, Barchi RL, Caldwell JH, Hofmann F, Howe JR, Hunter JC, Kallen RG, Mandel G, Meisler MH, Netter YB, Noda M, Tamkun MM, Waxman SG, Wood JN, Catterall WA. Nomenclature of voltage-gated sodium channels. Neuron. 2000;28:365–368. doi: 10.1016/s0896-6273(00)00116-1. [DOI] [PubMed] [Google Scholar]
- Gordon D, Merrick D, Auld V, Dunn R, Goldin AL, Davidson N, Catterall WA. Tissue-specific expression of the RI and RII sodium channel subtypes. Proc Natl Acad Sci U S A. 1987;84:8682–8686. doi: 10.1073/pnas.84.23.8682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grusser-Cornehls U, Baurle J. Mutant mice as a model for cerebellar ataxia. Prog Neurobiol. 2001;63:489–540. doi: 10.1016/s0301-0082(00)00024-1. [DOI] [PubMed] [Google Scholar]
- Guerrini R, Dravet C, Genton P, Belmonte A, Kaminska A, Dulac O. Lamotrigine and seizure aggravation in severe myoclonic epilepsy. Epilepsia. 1998;39:508–512. doi: 10.1111/j.1528-1157.1998.tb01413.x. [DOI] [PubMed] [Google Scholar]
- Harkin LA, McMahon JM, Iona X, Dibbens L, Pelekanos JT, Zuberi SM, Sadleir LG, Andermann E, Gill D, Farrell K, Connolly M, Stanley T, Harbord M, Andermann F, Wang J, Batish SD, Jones JG, Seltzer WK, Gardner A, Sutherland G, Berkovic SF, Mulley JC, Scheffer IE. The spectrum of SCN1A-related infantile epileptic encephalopathies. Brain. 2007;130:843–852. doi: 10.1093/brain/awm002. [DOI] [PubMed] [Google Scholar]
- Isom LL. The role of sodium channels in cell adhesion. Front Biosci. 2002;7:12–23. doi: 10.2741/isom. [DOI] [PubMed] [Google Scholar]
- Isom LL, Ragsdale DS, De Jongh KS, Westenbroek RE, Reber BFX, Scheuer T, Catterall WA. Structure and function of the beta-2 subunit of brain sodium channels, a transmembrane glycoprotein with a CAM-motif. Cell. 1995;83:433–442. doi: 10.1016/0092-8674(95)90121-3. [DOI] [PubMed] [Google Scholar]
- Jenkins SM, Bennett V. Ankyrin-G coordinates assembly of the spectrin-based membrane skeleton, voltage-gated sodium channels, and L1 CAMs at Purkinje neuron initial segments. J Cell Biol. 2001;155:739–746. doi: 10.1083/jcb.200109026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston D, Magee JC, Colbert CM, Christie BR. Active properties of neuronal dendrites. Annu Rev Neurosci. 1996;19:165–186. doi: 10.1146/annurev.ne.19.030196.001121. [DOI] [PubMed] [Google Scholar]
- Kahlig KM, Misra SN, George AL., Jr Impaired inactivation gate stabilization predicts increased persistent current for an epilepsy-associated SCN1A mutation. J Neurosci. 2006;26:10958–10966. doi: 10.1523/JNEUROSCI.3378-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalume F, Yu FH, Westenbroek RE, Scheuer T, Catterall WA. Reduced sodium current in Purkinje neurons from Nav1.1 mutant mice: implications for ataxia in severe myoclonic epilepsy in infancy. J Neurosci. 2007;27:11065–11074. doi: 10.1523/JNEUROSCI.2162-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney JA, Wiste AK, Stephani U, Trudeau MM, Siegel A, RamachandranNair R, Elterman RD, Muhle H, Reinsdorf J, Shields WD, Meisler MH, Escayg A. Recurrent de novo mutations of SCN1A in severe myoclonic epilepsy of infancy. Pediatr Neurol. 2006;34:116–120. doi: 10.1016/j.pediatrneurol.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Khaliq ZM, Raman IM. Relative contributions of axonal and somatic Na channels to action potential initiation in cerebellar Purkinje neurons. J Neurosci. 2006;26:1935–1944. doi: 10.1523/JNEUROSCI.4664-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzemien DM, Schaller KL, Levinson SR, Caldwell JH. Immunolocalization of sodium channel isoform NaCh6 in the nervous system. J Comp Neurol. 2000;420:70–83. [PubMed] [Google Scholar]
- Loscher W. Preclinical assessment of proconvulsant drug activity and its relevance for predicting adverse events in humans. Eur J Pharmacol. 2009;610:1–11. doi: 10.1016/j.ejphar.2009.03.025. [DOI] [PubMed] [Google Scholar]
- Lossin C, Rhodes TH, Desai RR, Vanoye CG, Wang D, Carniciu S, Devinsky O, George AL., Jr Epilepsy-associated dysfunction in the voltage-gated neuronal sodium channel SCN1A. J Neurosci. 2003;23:11289–11295. doi: 10.1523/JNEUROSCI.23-36-11289.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lossin C, Wang DW, Rhodes TH, Vanoye CG, George AL., Jr Molecular basis of an inherited epilepsy. Neuron. 2002;34:877–884. doi: 10.1016/s0896-6273(02)00714-6. [DOI] [PubMed] [Google Scholar]
- Mantegazza M, Gambardella A, Rusconi R, Schiavon E, Annesi F, Cassulini RR, Labate A, Carrideo S, Chifari R, Canevini MP, Canger R, Franceschetti S, Annesi G, Wanke E, Quattrone A. Identification of an Nav1.1 sodium channel (SCN1A) loss-of-function mutation associated with familial simple febrile seizures. Proc Natl Acad Sci U S A. 2005;102:18177–18182. doi: 10.1073/pnas.0506818102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini C, Scheffer IE, Nabbout R, Mei D, Cox K, Dibbens LM, McMahon JM, Iona X, Carpintero RS, Elia M, Cilio MR, Specchio N, Giordano L, Striano P, Gennaro E, Cross JH, Kivity S, Neufeld MY, Afawi Z, Andermann E, Keene D, Dulac O, Zara F, Berkovic SF, Guerrini R, Mulley JC. SCN1A duplications and deletions detected in Dravet syndrome: Implications for molecular diagnosis. Epilepsia. 2009;50:1670–1678. doi: 10.1111/j.1528-1167.2009.02013.x. [DOI] [PubMed] [Google Scholar]
- Martin MS, Tang B, Papale LA, Yu FH, Catterall WA, Escayg A. The voltage-gated sodium channel Scn8a is a genetic modifier of severe myoclonic epilepsy of infancy. Hum Mol Genet. 2007;16:2892–2899. doi: 10.1093/hmg/ddm248. [DOI] [PubMed] [Google Scholar]
- Martin MS, Dutt K, Papale LA, Dub CM, Dutton SB, de Haan G, Shankar A, Tufik S, Meisler MH, Baram TZ, Goldin AL, Escayg A. Altered function of the SCN1A voltage-gated sodium channel leads to gamma-aminobutyric acid-ergic (GABAergic) interneuron abnormalities. J Biol Chem. 2010;285:9823–9834. doi: 10.1074/jbc.M109.078568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisler MH, Kearney JA. Sodium channel mutations in epilepsy and other neurological disorders. J Clin Invest. 2005;115:2010–2017. doi: 10.1172/JCI25466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulley JC, Scheffer IE, Petrou S, Dibbens LM, Berkovic SF, Harkin LA. SCN1A mutations and epilepsy. Hum Mutat. 2005;25:535–542. doi: 10.1002/humu.20178. [DOI] [PubMed] [Google Scholar]
- Oakley JC, Kalume F, Yu FH, Scheuer T, Catterall WA. Temperature- and age-dependent seizures in a mouse model of severe myoclonic epilepsy in infancy. Proc Natl Acad Sci U S A. 2009;106:3994–3999. doi: 10.1073/pnas.0813330106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogiwara I, Miyamoto H, Morita N, Atapour N, Mazaki E, Inoue I, Takeuchi T, Itohara S, Yanagawa Y, Obata K, Furuichi T, Hensch TK, Yamakawa K. Nav1.1 localizes to axons of parvalbumin-positive inhibitory interneurons: a circuit basis for epileptic seizures in mice carrying an Scn1a gene mutation. J Neurosci. 2007;27:5903–5914. doi: 10.1523/JNEUROSCI.5270-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguni H, Hayashi K, Awaya Y, Fukuyama Y, Osawa M. Severe myoclonic epilepsy in infants: a review based on the Tokyo Women's Medical University series of 84 cases. Brain Dev. 2001;23:736–748. doi: 10.1016/s0387-7604(01)00276-5. [DOI] [PubMed] [Google Scholar]
- Oguni H, Hayashi K, Osawa M, Awaya Y, Fukuyama Y, Fukuma G, Hirose S, Mitsudome A, Kaneko S. Severe myoclonic epilepsy in infancy: clinical analysis and relation to SCN1A mutations in a Japanese cohort. Adv Neurol. 2005;95:103–117. [PubMed] [Google Scholar]
- Ohmori I, Ouchida M, Ohtsuka Y, Oka E, Shimizu K. Significant correlation of the SCN1A mutations and severe myoclonic epilepsy in infancy. Biochem Biophys Res Commun. 2002;295:17–23. doi: 10.1016/s0006-291x(02)00617-4. [DOI] [PubMed] [Google Scholar]
- Patino GA, Claes LR, Lopez-Santiago LF, Slat EA, Dondeti RS, Chen C, O’Malley HA, Gray CB, Miyazaki H, Nukina N, Oyama F, De Jonghe P, Isom LL. A functional null mutation of SCN1B in a patient with Dravet syndrome. J Neurosci. 2009;29:10764–10778. doi: 10.1523/JNEUROSCI.2475-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovski S, Scheffer IE, Sisodiya SM, O’Brien TJ, Berkovic SF. Lack of replication of association between scn1a SNP and febrile seizures. Neurology. 2009;73:1928–1930. doi: 10.1212/WNL.0b013e3181c3fd6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragsdale DS. How do mutant Nav1.1 sodium channels cause epilepsy? Brain Res Rev. 2008;58:149–159. doi: 10.1016/j.brainresrev.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Raman IM, Bean BP. Resurgent sodium current and action potential formation in dissociated cerebellar Purkinje neurons. J Neurosci. 1997;17:4517–4526. doi: 10.1523/JNEUROSCI.17-12-04517.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman IM, Bean BP. Ionic currents underlying spontaneous action potentials in isolated cerebellar Purkinje neurons. J Neurosci. 1999a;19:1664–1674. doi: 10.1523/JNEUROSCI.19-05-01663.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman IM, Bean BP. Properties of sodium currents and action potential firing in isolated cerebellar Purkinje neurons. Ann N Y Acad Sci. 1999b;868:93–96. doi: 10.1111/j.1749-6632.1999.tb11279.x. [DOI] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- Rusconi R, Combi R, Cestele S, Grioni D, Franceschetti S, Dalpra L, Mantegazza M. A rescuable folding defective Nav1.1 (SCN1A) sodium channel mutant causes GEFS+: common mechanism in Nav1.1 related epilepsies? Hum Mutat. 2009;30:E747–760. doi: 10.1002/humu.21041. [DOI] [PubMed] [Google Scholar]
- Rusconi R, Scalmani P, Cassulini RR, Giunti G, Gambardella A, Franceschetti S, Annesi G, Wanke E, Mantegazza M. Modulatory proteins can rescue a trafficking defective epileptogenic Nav1.1 Na+ channel mutant. J Neurosci. 2007;27:11037–11046. doi: 10.1523/JNEUROSCI.3515-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarao R, Gupta SK, Auld VJ, Dunn RJ. Developmentally regulated alternative RNA splicing of rat brain sodium channel mRNAs. Nucleic Acids Res. 1991;19:5673–5679. doi: 10.1093/nar/19.20.5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sausbier M, Hu H, Arntz C, Feil S, Kamm S, Adelsberger H, Sausbier U, Sailer CA, Feil R, Hofmann F, Korth M, Shipston MJ, Knaus HG, Wolfer DP, Pedroarena CM, Storm JF, Ruth P. Cerebellar ataxia and Purkinje cell dysfunction caused by Ca2+-activated K+ channel deficiency. Proc Natl Acad Sci U S A. 2004;101:9474–9478. doi: 10.1073/pnas.0401702101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffer IE, Zhang YH, Jansen FE, Dibbens L. Dravet syndrome or genetic (generalized) epilepsy with febrile seizures plus? Brain Dev. 2009;31:394–400. doi: 10.1016/j.braindev.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Schlachter K, Gruber-Sedlmayr U, Stogmann E, Lausecker M, Hotzy C, Balzar J, Schuh E, Baumgartner C, Mueller JC, Illig T, Wichmann HE, Lichtner P, Meitinger T, Strom TM, Zimprich A, Zimprich F. A splice site variant in the sodium channel gene SCN1A confers risk of febrile seizures. Neurology. 2009;72:974–978. doi: 10.1212/01.wnl.0000344401.02915.00. [DOI] [PubMed] [Google Scholar]
- Spampanato J, Escayg A, Meisler MH, Goldin AL. Functional effects of two voltage-gated sodium channel mutations that cause generalized epilepsy with febrile seizures plus type 2. J Neurosci. 2001;21:7481–7490. doi: 10.1523/JNEUROSCI.21-19-07481.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spampanato J, Escayg A, Meisler MH, Goldin AL. Generalized epilepsy with febrile seizures plus type 2 mutation W1204R alters voltage-dependent gating of Nav1.1 sodium channels. Neuroscience. 2003;116:37–48. doi: 10.1016/s0306-4522(02)00698-x. [DOI] [PubMed] [Google Scholar]
- Spampanato J, Kearney JA, de Haan G, McEwen DP, Escayg A, Aradi I, MacDonald BT, Levin SI, Soltesz I, Benna P, Montalenti E, Isom LL, Goldin AL, Meisler MH. A novel epilepsy mutation in the sodium channel SCN1A identifies a cytoplasmic domain for beta subunit interaction. J Neurosci. 2004;24:10022–10034. doi: 10.1523/JNEUROSCI.2034-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart GJ, Sakmann B. Active propagation of somatic action potentials into neocortical pyramidal cell dendrites. Nature. 1994;367:69–72. doi: 10.1038/367069a0. [DOI] [PubMed] [Google Scholar]
- Sugawara T, Mazaki-Miyazaki E, Fukushima K, Shimomura J, Fujiwara T, Hamano S, Inoue Y, Yamakawa K. Frequent mutations of SCN1A in severe myoclonic epilepsy in infancy. Neurology. 2002;58:1122–1124. doi: 10.1212/wnl.58.7.1122. [DOI] [PubMed] [Google Scholar]
- Tang B, Dutt K, Papale L, Rusconi R, Shankar A, Hunter J, Tufik S, Yu FH, Catterall WA, Mantegazza M, Goldin AL, Escayg A. A BAC transgenic mouse model reveals neuron subtype-specific effects of a Generalized Epilepsy with Febrile Seizures Plus (GEFS+) mutation. Neurobiol Dis. 2009;35:91–102. doi: 10.1016/j.nbd.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate SK, Depondt C, Sisodiya SM, Cavalleri GL, Schorge S, Soranzo N, Thom M, Sen A, Shorvon SD, Sander JW, Wood NW, Goldstein DB. Genetic predictors of the maximum doses patients receive during clinical use of the anti-epileptic drugs carbamazepine and phenytoin. Proc Natl Acad Sci U S A. 2005;102:5507–5512. doi: 10.1073/pnas.0407346102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate SK, Singh R, Hung CC, Tai JJ, Depondt C, Cavalleri GL, Sisodiya SM, Goldstein DB, Liou HH. A common polymorphism in the SCN1A gene associates with phenytoin serum levels at maintenance dose. Pharmacogenet Genomics. 2006;16:721–726. doi: 10.1097/01.fpc.0000230114.41828.73. [DOI] [PubMed] [Google Scholar]
- Trimmer JS, Rhodes KJ. Localization of voltage-gated ion channels in mammalian brain. Annu Rev Physiol. 2004;66:477–519. doi: 10.1146/annurev.physiol.66.032102.113328. [DOI] [PubMed] [Google Scholar]
- Vanoye CG, Lossin C, Rhodes TH, George AL., Jr Single-channel properties of human Nav1.1 and mechanism of channel dysfunction in SCN1A-associated epilepsy. J Gen Physiol. 2006;127:1–14. doi: 10.1085/jgp.200509373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace RH, Wang DW, Singh R, Scheffer IE, George JAL, Phillips HA, Saar K, Reis A, Johnson EW, Sutherland GR, Berkovic SF, Mulley JC. Febrile seizures and generalized epilepsy associated with a mutation in the sodium channel b1 subunit gene SCN1B. Nat Genet. 1998;19:366–370. doi: 10.1038/1252. [DOI] [PubMed] [Google Scholar]
- Westenbroek RE, Merrick DK, Catterall WA. Differential subcellular localization of the RI and RII Na+ channel subtypes in central neurons. Neuron. 1989;3:695–704. doi: 10.1016/0896-6273(89)90238-9. [DOI] [PubMed] [Google Scholar]
- Westenbroek RE, Noebels JL, Catterall WA. Elevated expression of type II Na+ channels in hypomyelinated axons of shiverer mouse brain. J Neurosci. 1992;12:2259–2267. doi: 10.1523/JNEUROSCI.12-06-02259.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FH, Mantegazza M, Westenbroek RE, Robbins CA, Kalume F, Burton KA, Spain WJ, McKnight GS, Scheuer T, Catterall WA. Reduced sodium current in GABAergic interneurons in a mouse model of severe myoclonic epilepsy in infancy. Nat Neurosci. 2006;9:1142–1149. doi: 10.1038/nn1754. [DOI] [PubMed] [Google Scholar]