Abstract
Mutations of voltage-gated ion channels cause several channelopathies of skeletal muscle, which present clinically with myotonia, periodic paralysis, or a combination of both. Expression studies have revealed both loss-of-function and gain-of-function defects for the currents passed by mutant channels. In many cases, these functional changes could be mechanistically linked to the defects of fibre excitability underlying myotonia or periodic paralysis. One remaining enigma was the basis for depolarization-induced weakness in hypokalaemic periodic paralysis (HypoPP) arising from mutations in either sodium or calcium channels. Curiously, 14 of 15 HypoPP mutations are at arginines in S4 voltage sensors, and recent observations show that these substitutions support an alternative pathway for ion conduction, the gating pore, that may be the source of the aberrant depolarization during an attack of paralysis.
Steve Cannon is professor and chair of neurology at UT Southwestern Medical Center in Dallas. He received a doctorate in medicine and graduate training in biomedical engineering at Johns Hopkins Medical School and completed his clinical neurology training at Massachusetts General Hospital. His research over the past two decades has focused on ion channel disorders of skeletal muscle and membrane biophysics.
 |
Derangements of ion channel function are firmly established as the cause of human disease and disability (Lehmann-Horn & Jurkat-Rott, 1999; Ashcroft, 2000). These disorders may be acquired, as with autoantibodies against the nicotinic acetylcholine receptor in myasthenia gravis, or inherited channelopathies such as susceptibility to arrhythmia in long QT Type 3 with mutation of the cardiac sodium channel NaV1.5. Mutations of genes encoding voltage-gated ion channels expressed in skeletal muscle disrupt the electrical excitability of the sarcolemma and produce two main phenotypes, myotonia and periodic paralysis (Cannon, 2006). Myotonia is a consequence of enhanced sarcolemmal excitability wherein sustained bursts of after-discharges persist for seconds beyond the termination of synaptic input at the neuromuscular junction. These after-discharges trigger sustained Ca2+ release from the sarcoplasmic reticulum to produce an involuntary after-contraction that patients describe as stiffness. Conversely, reduced sarcolemmal excitability, which may progress to a complete failure of spike initiation and propagation at the endplate, may cause weakness or a total loss of contraction in an attack of periodic paralysis. A characteristic feature for these channelopathies of skeletal muscle is that the symptoms fluctuate over time, and may be strongly influenced by environmental factors such as temperature, carbohydrate or salt content of food, stress, or prior level of physical activity (Lehmann-Horn et al. 2004).
Much has been learned about the pathophysiological basis for the derangements in sarcolemmal excitability that underlie myotonia and periodic paralysis (Lehmann-Horn et al. 2004; Cannon, 2006). Heterologous expression studies of mutant ion channels (Cannon & Strittmatter, 1993; Cummins et al. 1993; Yang et al. 1994), computational simulations of muscle excitability (Cannon et al. 1993), pharmacological and genetically engineered animal models (Hayward et al. 2008), and microelectrode studies in biopsied human fibres (Lehmann-Horn et al. 1981; Rüdel et al. 1984) have revealed several common mechanistic themes for these channelopathies of skeletal muscle. Susceptibility to myotonia occurs in two contexts. A marked reduction of the resting Cl− conductance (Adrian & Bryant, 1974) from loss-of-function mutations in the major sarcolemmal chloride channel ClC-1 (Koch et al. 1992; Kubisch et al. 1998; Pusch, 2002) impairs repolarization after a spike (ECl approximately equals Vrest) and increases the after-depolarization produced by the egress of myoplasmic K+ into the transverse tubule (loss of Cl− current to counteract the depolarized shift of EK). Both effects tend to depolarize the fibre and trigger after-discharges. The second mechanism for eliciting myotonia involves gain-of-function changes for the skeletal muscle sodium channel NaV1.4 (Lehmann-Horn et al. 1981) through either disruption of inactivation (Cannon et al. 1991) or a hyperpolarized shift of activation (Cummins et al. 1993), both of which increase the propensity for sustained bursts of discharges. Several variants of periodic paralysis have been delineated clinically, and all share the common mechanistic feature that during an attack the membrane is aberrantly depolarized and refractory from firing action potentials. In some cases the source of the depolarized shift of Vrest was readily apparent: loss of the inward rectifier Kir2.1 in Andersen-Tawil syndrome (Plaster et al. 2001; Davies et al. 2005) or persistent Na+ currents (Lehmann-Horn et al. 1981; Cannon et al. 1991) from impairment of fast- and slow-inactivation of NaV1.4 in hyperkalaemic periodic paralysis (HyperPP). One notable exception had previously defied explanation. In hypokalaemic periodic paralysis (HypoPP) caused by missense mutations in either CaV1.1 (Ptacek et al. 1994) or NaV1.4 (Bulman et al. 1999), the aberrant depolarization recorded from biopsied muscle fibres was not prevented by nitrendipine or TTX (Ruff, 1999), and therefore the origin of the depolarizing current remained unknown. The key to resolving this mystery came from the recognition that 14 of 15 HypoPP mutations occur at arginine residues in S4 voltage sensor domains (Matthews et al. 2009), coupled with the new observation from structure–function-based studies that mutations at positively charged arginines in S4 may create an alternative conduction pathway for small amplitude gating pore currents (Starace & Bezanilla, 2001), also referred to as the ω current (Tombola et al. 2005).
Channelopathies often disrupt gating
Now two decades into the molecular era of studying channelopathies, we have learned that a surprisingly large number of mutant alleles do code for functional channels, either as heteromeric protein complexes with wild-type subunits or as monomeric pore-forming proteins (Lehmann-Horn & Jurkat-Rott, 1999). Moreover, the gating behaviour of mutant channels is commonly affected, whereas isolated defects of permeation are exceedingly rare and have not been described for skeletal muscle channelopathies. The other commonly observed defects for disease-associated mutations are failure to form functional homomeric mutant channels or dominant-negative suppression observed from co-expression with wild-type. The full range of channel defects encompasses several other mechanisms, many of which are not optimally assessed by voltage-clamp recoding in artificial heterologous systems. For example, modulation of channel activity, targeting to specialized membrane compartments, and surface expression level may not be faithfully reproduced in heterologous expression systems.
Gating defects have been identified for every NaV1.4 mutant channel associated with myotonia or HyperPP (over 35 tested, see Cannon (2006) for review). In most cases, NaV1.4 mutations produce gain-of-function defects, characterized by impaired inactivation (slowed rate of onset, enhanced rate of recovery, less complete entry, or depolarized shift in voltage dependence) or an enhancement of activation (hyperpolarized shift in voltage dependence or slower rate of deactivation). Channel mutations that alter steady-state gating properties, and thereby cause aberrant persistent inward Na+ currents, predispose to prolonged attacks of depolarization-induced paralysis. On the other hand, defects in gating kinetics alone, such as a slower rate of inactivation, produce a dynamic instability in membrane potential with susceptibility to myotonia but do not cause paralysis. Many of the NaV1.4 mutations associated with gating defects are located in channel domains that have established contributions to gating, including S4 segments, the domain III–IV linker thought to form the inactivation gate, and the cytoplasmic ends of S5 or S6 segments which may form the receptor for the inactivation gate.
The S4 voltage sensor is a common site of missense mutations
Transmembrane segments and specialized domains within cytoplasmic loops or termini are common sites for disease-associated missense mutations in voltage-gated ion channels. The consensus is that this association occurs because these areas are critical for channel function, whereas amino acid substitutions or in-frame deletions are better tolerated in hydrophilic segments that extend into the aqueous phase and therefore are more likely to be benign polymorphisms. This pattern is distinctly different from the spatial distribution of mutations in disorders for which nonsense or splice site mutations destroy the coding potential of the channel protein. For example, the NaV1.1 nonsense/stop mutations in epilepsy syndromes have a nearly uniform distribution in the primary sequence (114 of over 300 mutations reported; Lossin, 2009). From a total of 53 missense mutations reported in NaV1.4, 21 are in S4 voltage sensors, whereas 6 of 7 mutations in CaV1.1 are in S4 segments. Missense mutations within the pore-loops have not been reported for NaV1.4 or CaV1.1, which is consistent with the experimental observation that gating, rather than permeation, is commonly affected by disease-associated channelopathies. Pore-loop mutations of Kir2.1 have been reported in one variant of periodic paralysis, the Andersen-Tawil syndrome (Plaster et al. 2001). These mutant subunits do not form functional Kir channels as homomultimers and exert a dominant-negative effect when co-expressed with wild-type Kir2.1 (Tristani-Firouzi et al. 2002).
For the NaV1.4 sodium channel, a cluster of nine missense mutations occurs in the IVS4 voltage sensor, and five lie in the outermost arginine at the R1 position, R1448C/H/L/P/S. All nine of these missense mutations produce the same clinical phenotype, paramyotonia congenita (PMC), which presents with myotonic stiffness that is aggravated by cooling and paradoxically worsens with repetitive muscle activity (paramyotonia). These PMC mutations all share a common biophysical profile as well, wherein the most pronounced defect observed in voltage-clamp studies of heterologously expressed channels is a 3- to 5-fold slower rate of inactivation (Chahine et al. 1994; Yang et al. 1994). These observations for disease-associated NaV1.4 mutations are consistent with biophysical studies using fluorophore-tagged S4 segments (Cha et al. 1999) or toxin-modified channels (Sheets & Hanck, 1995) to show that displacement of the voltage sensor in domain IV is tightly coupled to inactivation kinetics, whereas displacement of the voltage sensors in domains I–III is linked to channel activation.
Gating pore currents from S4 mutations in hypokalaemic periodic paralysis
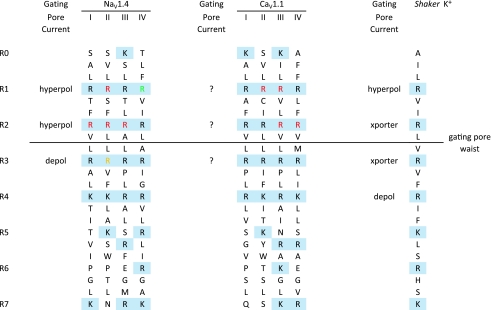
A new class of channel defect was recently discovered for HypoPP mutations in IIS4 of NaV1.4 (Sokolov et al. 2007; Struyk & Cannon, 2007). Historically, the first HypoPP mutations were identified in the α subunit of the skeletal muscle L-type Ca2+ channel CaV1.1. Curiously, all three mutations were in arginine residues of S4 segments in domains II and IV (Jurkat-Rott et al. 1994; Ptacek et al. 1994). This discovery generated a series of investigations on the voltage dependence of Ca2+ currents and on gating charge displacement (Lapie et al. 1996; Jurkat-Rott et al. 1998; Morrill & Cannon, 1999). Although some consistent changes were observed (slower activation and reduced current density), these defects could not readily account for the depolarization during an attack of HypoPP. Many years later, several HypoPP mutations were identified in NaV1.4 (Bulman et al. 1999; Davies et al. 2001; Sternberg et al. 2001; Matthews et al. 2009). This observation shattered the hypothesis that HypoPP is exclusively caused by mutations in CaV1.1 while mutations of NaV1.4 cause weakness in HyperPP or PMC. Moreover, the NaV1.4 defects in HypoPP were all missense mutations at arginines in S4 voltage sensors. In total, 15 missense mutations have been identified in families with HypoPP: 8 mutations in NaV1.4 are all at arginines in S4 of domains I, II, or III; 6 mutations in CaV1.1 are at arginines in S4 of domain II, III, or IV (see Fig. 1). The final mutation is a V876E in IIIS3 of CaV1.1, which segregated with HypoPP in a large multi-generation pedigree of a family from South Africa (Ke et al. 2009).
Figure 1. Alignment of S4 sequences for NaV1.4, CaV1.1 and Shaker K+ channels.
Positively charged residues are delineated with a shaded background (blue) for the R0 to R7 position. Mutations of these basic residues may produce a gating pore current activated by hyperpolarization (hyperpol), by depolarization (depol), or give rise to a proton transporter (xporter). The gating pore waist lies between R2 and R3, and voltage-dependent translocation of substituted R residues on S4 through this region regulates the flow of gating pore current. HypoPP mutations (red) are clustered at arginines in the R1 or R2 position of NaV1.4 and CaV1.1. Mutations of NaV1.4 at IV-R1 (green) are associated with PMC, not HypoPP, and do not produce gating pore current. A mixed variant of periodic paralysis is associated with mutations at NaV1.4 II-R3 (yellow) which causes a depolarization-activated gating pore.
The striking homology of the HypoPP mutations in CaV1.1 and NaV1.4 suggested there may be a common functional defect, specific to substitutions at arginines in S4 segments, that may explain why mutations in two voltage-gated ion channels with very different physiological roles would produce a common clinical phenotype. The clue for where to look came from recent studies aimed at determining the conformational changes in S4 during gating. Histidine scanning mutagenesis of S4 in Shaker K+ channels was used as a tool to monitor gating-dependent accessibility of arginines to the intracellular or extracellular solutions, by measuring pH-dependent changes in gating charge displacement (Starace et al. 1997). Unexpectedly, these R→H mutants also supported ionic current carried by protons. Mutation of the outermost arginine at position R362 (R1H) created a proton-selective pore that was open at hyperpolarized potentials when the S4 was in the resting conformation ‘inward’ (Starace & Bezanilla, 2004), whereas the R4H replacement produced a gating pore that passed proton currents at depolarized potentials where S4 shifts to the activated conformation ‘outward’ (Starace & Bezanilla, 2001). A conceptual model has emerged where, in response to voltage changes, S4 translocates through a narrow crevasse or ‘gating pore’ of the channel complex. This pore has wide openings at either end, and a short waist, such that aqueous accessibility to S4 arginines shifts from one side of the membrane to the other in response to voltage (Yang et al. 1996). Conduction of ions through the gating pore occurs when residues of S4, or the crevasse-lining counterparts (Tombola et al. 2005), are mutated. The unitary conductance to protons was quite small, about 40 fS in a gradient of pHo 5/pHi 9.2 based on mean-variance analysis (Starace & Bezanilla, 2004). Conduction of protons along the S4 pathway, without a conventional pore domain, was subsequently identified as an intrinsic mechanism of permeation for the voltage-gated proton-selective Hv1 channel family (Ramsey et al. 2006; Sasaki et al. 2006). Replacement of R1 in the Shaker K+ channel by small hydrophobic residues (R1A/C/S/V) opened a conduction pathway at negative potentials that was permeable to alkali metal cations (Cs+ > K+ > Li+) and even guanadinium (Tombola et al. 2005). As with the proton currents for R1H channels, the unitary conductance was very small. For Shaker R1C the gating pore current at −200 mV was about 6% of the ‘α’ current through the conventional pore at +50 mV (Tombola et al. 2005). Assuming an open probability of 1 for each conduction pathway at these voltage extremes, and a linear open-channel I–V, then the unitary conductance of the gating pore is about 2% of that for the α pore. Considering that there are four R1C subunits in these homotetrameric channels, then the unitary conductance for each R1C is about 0.5% of the conductance for the α pore. The voltage-gated sodium channel is also capable of producing gating pore currents. Glutamine scanning mutagenesis of IIS4 in the rat brain sodium channel NaV1.2 showed that paired mutations at R1Q/R2Q resulted in gating pore currents activated by hyperpolarization, and the R2Q/R3Q pair was associated with gating pore currents activated by depolarization (Sokolov et al. 2005). Finally, a robust naturally occurring gating pore current has been identified in the flatworm KV3.2 channel, which contains the atypical S4 sequence with histidine at the R1 position and glycine at R3 (Klassen et al. 2008).
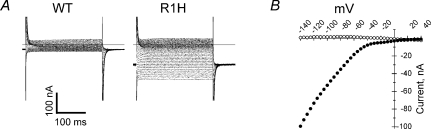
HypoPP mutations in S4 of NaV1.4 result in gating pore currents that are not detectable in wild-type channels (Fig. 2). All five HypoPP mutations in IIS4 have been screened by expression studies in oocytes and produced gating pore currents activated at hyperpolarized potentials (Sokolov et al. 2007; Struyk & Cannon, 2007; Struyk et al. 2008). The gating pore currents from IIS4-R1H and IIS4-R2H HypoPP mutations were carried exclusively by protons, whereas the gating pores produced by non-histidine mutations at IIS4-R2G/C/S were permeable to alkali metal cations (K+≈ Cs+ > Na+≈ Li+) and even guanadinium. The IIIS4-R2Q HypoPP mutation also supports a gating pore conductance active at hyperpolarized potentials and poorly selective among monovalent cations (Francis et al. 2010). Notably, the IVS4-R1C mutation associated with PMC does not give rise to detectable gating pore currents (ibid). These observations provide additional evidence that the four voltage-sensor domains in NaV channels are not equivalent and support the notion that an anomalous gating pore current is necessary and sufficient to cause HypoPP. Heterologous expression of CaV1.1 in non-muscle cells is poor, and this technical challenge has impeded the ability to test with sufficient sensitivity for gating pore currents from HypoPP mutations in the calcium channel. An unusual variant of periodic paralysis has been associated with missense mutations of NaV1.4 at IIS4-R3G/Q/W (Vicart et al. 2004). The clinical phenotype has features of HyperPP and normokalaemic periodic paralysis, but is clearly distinct from HypoPP. Heterologous expression studies show all three mutations produce gating pore currents, but the conductance activates with depolarization greater than −40 mV (Sokolov et al. 2008). Interestingly, mutagenesis studies with IIS4-R3Q in NaV1.2 did not produce a gating pore current (Sokolov et al. 2005). Only the double mutant NaV1.2 IIS4-R2Q/R3Q produced a gating pore current, which demonstrates isoform-specific differences in the ability to conduct gating pore currents in the NaV family.
Figure 2. Hyperpolarization-activated gating pore current in the R1H HypoPP mutation of NaV1.4.
A, currents elicited by 300 ms step depolarizations between −140 mV and +30 mV from a holding potential of −100 mV in oocytes expressing wild-type (WT) or R1H channels. Saturating concentration of TTX has been added to suppress INa. B, steady-state I–V relation, after subtraction of linear background leak: WT, open circles; R1H, filled circles. Modified from Struyk & Cannon (2007) with permission.
Implications of a gating pore current for muscle fibre excitability
The identification of gating pore currents in association with HypoPP is a major advancement, but the impact of this discovery hinges upon establishing a mechanistic link between this unusual current and susceptibility to depolarization-induced attacks of weakness in the setting of low extracellular K+. One obvious consideration is the relatively small amplitude of gating pore currents. To address this question, it is crucial to estimate the gating pore current density in muscle fibres, and consensus is lacking on this point. Sokolov et al. (2007) estimate that the gating pore current, Igp, is about 1% of the central pore Na+ current, INa, based on favourable recordings where the expression level was well-matched to measure both INa and Igp after TTX application in the same egg. We estimated a 50-fold smaller Igp, about 0.02% of INa, based on the ratio of Igp to total gating charge displacement and the assumption of 12 charges per channel (Struyk et al. 2008). The peak Na+ conductance in mammalian skeletal muscle is ∼50 mS cm−2, and so in the heterozygous state, the predicted gating pore conductance at the resting potential is 5 μS cm−2 or ∼1% of the total membrane resting conductance. Under physiological conditions at rest, a steady inward current of ∼0.5 μA cm−2 would flow, carried by protons for R→H mutant channels and by Na+ for all other missense R/X mutations. Curiously, the HypoPP clinical phenotype is nearly indistinguishable for R→H mutations compared to other substitutions (Sternberg et al. 2001). One hypothesis for the basis of this similarity, despite very different charge carriers, is that the Na+/H+ exchanger effectively converts a proton leak to a Na+ leak (Jurkat-Rott & Lehmann-Horn, 2007).
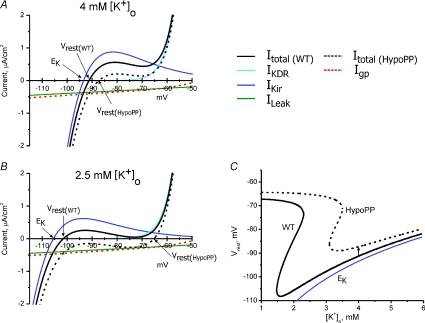
The mechanism by which the relatively small-amplitude Igp produces susceptibility to large depolarized shifts of Vrest in hypokalaemia with a loss of fibre excitability has been linked to the balance between the inward Igp and the small outward K+ current passed by the inward rectifier IKir under resting conditions (Struyk & Cannon, 2008). The phenomenon depends critically on the non-linear I–V characteristics of IKir. Indeed, an approximation based on ohmic behaviour predicts only a small depolarization of Vrest by Igp of magnitude: ΔVrest= (0.5 μA cm−2)/(200 μS cm−2) = 2.5 mV. (A conductance of 200 μS cm−2, or about 20% of the total resting conductance of a muscle fibre, is used for this calculation because Cl− is passively distributed in the steady state, and therefore the large Cl− conductance does not contribute to setting Vrest.) The non-linear behaviour of the steady-state I–V relation of mammalian skeletal muscle is shown by the simulated response in Fig. 3, which consists of three components, Imem=IKir+IKDR+ILeak, where IKDR is the delayed rectifier and ILeak is a small ohmic conductance with a reversal potential of 0 mV. Although the Cl− conductance accounts for ∼80% of the total membrane conductance of muscle at rest, and on a rapid time scale of milliseconds is very important for repolarization and prevention of myotonic after-discharges, it has almost no influence on the steady-state I–V relation because of passive redistribution of Cl−. As a result, Vrest is determined by a balance of two relatively small currents, the outward component of the IKir and the inward ILeak. A reduction in extracellular K+ will not only cause a hyperpolarized shift of EK, but also produces a leftward shift of the outward ‘hump’ of IKir. Under extreme conditions, Such as [K+]o of ∼1 mm, the outward hump of IKir is overwhelmed at all voltages by the inward ILeak. Vrest will now be set by a balance between ILeak and IKDR. Moreover, the outward current from IKDR becomes substantial only for V > −55 mV and therefore the balance point between ILeak and IKDR is determined primarily by the voltage at which the KDR channel activates (i.e. by gating behaviour, rather than by reversal potential). The end result is an apparent decoupling of Vrest from EK to produce the paradoxical depolarization of Vrest in low external K+ that has been observed for cells in which IKir is the predominant K+ conductance for normally setting Vrest, as in skeletal muscle (Carmeliet, 1982) and heart (Siegenbeek van Heukelom, 1991). The gating pore produced by HypoPP mutations in arginines of S4 segments has a conductance that is of comparable amplitude to the endogenous ILeak (Fig. 3B). The impact on Vrest is that the catastrophic depolarization as external K+ is lowered now occurs at a higher [K+]o, around 2.5–3.0 mm instead of ∼1 mm (Struyk & Cannon, 2008). The phase plot of Vrest as a function of [K+]o (Fig. 3C, arrow) shows that while Igp causes only a small 4 mV depolarization of Vrest in 4.0 mm[K+]o, Igp induces a critical rightward shift of 1.5 mm in the catastrophic break point such that susceptibility to depolarization now occurs in the physiological range for [K+]o fluctuations.
Figure 3. The gating pore current increases the susceptibility to paradoxical depolarization in low extracellular [K+].
The steady-state I–V relation for mammalian skeletal muscle was simulated by the combination of an inward rectifier K+ current, IKir, a delayed rectifier K+ current, IKDR, and a leakage current with a reversal potential of 0 mV, ILeak, as in Struyk & Cannon (2008). A, in 4 mm[K+]o the resting potential of −91.3 mV is determined primarily from the balance of an inward ILeak and outward IKir. Addition of a gating pore current, Igp, to simulate HypoPP (dashed red line), shifts the I–V relation downward (dashed black line) but results in only a small depolarization of Vrest to −87.3 mV. B, reduction of [K+]o to 2.5 mm shifts EK and IKir to more negative potentials, with a predicted hyperpolarization of Vrest to −101.6 mV in WT fibres. For HypoPP fibres, however, Vrest depolarizes to −65.3 mV (arrow) because the combination of inward currents (ILeak+Igp) exceeds the outward current from IKir. Under these conditions, which would result in paralysis from inactivation of sodium channels, Vrest is now set by the balance of IKDR and the inward currents. Because KDR channels are active only for >−55 mV, Vrest is strongly dependent on KDR gating, which results in an apparent decoupling of Vrest from EK. C, phase plot of Vrest as a function of [K+]o for simulated WT and HypoPP fibres. The inward gating pore current in HypoPP fibres causes only a small depolarization of Vrest from −91.3 mV to −87.3 mV in 4 mm[K+]o (arrow). More importantly, the catastrophic depolarization of Vrest shifts leftward from 1.5 mm[K+]o for WT to 3 mm for HypoPP. The Nernst potential for K+, EK, is shown for comparison (blue line).
Pharmacological studies support the theoretical notion that a balance between IKir and ILeak±Igp is the critical determinant for the paradoxical depolarization in low K+ that triggers an attack of HypoPP. Barium toxicity produces a secondary form of hypokalaemic periodic paralysis (Gallant, 1983), and the Kir channel is very sensitive to Ba2+ block. Studies in mouse muscle that were designed to detect the paradoxical depolarization as a function of [K+]o showed that in response to a 2 mm K+ challenge normal fibres hyperpolarized by 8 mV whereas fibres in 10 μm Ba2+ (50% block of IKir) depolarized by 17 mV (Struyk & Cannon, 2008). Reduction of KATP channel activity has been reported in excised patches from human HypoPP fibres with the CaV1.1-R528H mutation (Tricarico et al. 1999). Moreover, administration of insulin reduced the outward limb of IKir in human HypoPP fibres, making them more susceptible to aberrant depolarization (Ruff, 1999). Taken together, these observations suggest that attacks of HypoPP triggered by carbohydrate ingestion may be linked to insulin secretion with the attendant reduction in IKir specifically from an increased intracellular ratio of [ATP]/[ADP] which would inhibit KATP channels. A secondary insulin effect would be a shift of extracellular K+ into the myoplasm from increased activity of the Na+/K+-ATPase. Both effects, decreased KATP activity and reduced extracellular [K+], would contribute to the development of paradoxical depolarization and weakness. The reciprocal experiment, to increase the inward leak rather than decreasing Kir, also showed a shift of the catastrophic break point of Vrest to higher [K+]o. In control conditions, the paradoxical depolarization occurred in 1.5 mm[K+]o, whereas a leak produced by the ionophore amphotericin caused the depolarization shift to occur at 2.5 mm (Jurkat-Rott et al. 2009).
Conclusions
Channel gating is commonly affected by channelopathies, and the causative mutations often reside in the S1–S4 voltage sensor domain. An additional anomaly, the gating pore current, has been identified for missense mutations at arginine residues in S4 voltage sensors of the skeletal muscle sodium channel NaV1.4. Several lines of evidence support the hypothesis that the gating pore current plays a causative role in the pathogenesis of HypoPP. All eight HypoPP mutations identified thus far in NaV1.4 are at arginines in the R1 or R2 position. Six of seven HypoPP mutations in CaV1.1 are at homologous S4 arginines, and the final mutation is in S3, which potentially could also give rise to a gating pore current. Six of the NaV1.4 HypoPP mutations have been tested by functional expression, and all produce gating pore currents activated at hyperpolarized potentials. Moreover, the gating pore hypothesis provides a common mechanism by which mutations of either NaV1.4 or CaV1.1 cause a common clinical phenotype. Finally, model simulations demonstrate that gating pore currents, although small in amplitude, are sufficient to cause paradoxical depolarization in the setting of mild hypokalaemia. Over 45 missense mutations at arginines in S4 voltage sensors of NaV, CaV and KV channels have been identified in various channelopathies, and it is likely that gating pore currents may also play a role in epilepsy, migraine and cardiac arrhythmia. The gating pore current also represents an opportunity for new therapeutic strategies. Agents that block the gating pore current, without affecting channel gating, would be ideal drugs to treat these disorders.
Acknowledgments
Work in the author's laboratory has been supported by NIAMS (AR42703) of the NIH, and the Muscular Dystrophy Association.
References
- Adrian RH, Bryant SH. On the repetitive discharge in myotonic muscle fibres. J Physiol. 1974;240:505–515. doi: 10.1113/jphysiol.1974.sp010620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft FM. Ion Channels and Disease. Oxford: Elsevier; 2000. [Google Scholar]
- Bulman DE, Scoggan KA, van Oene MD, Nicolle MW, Hahn AF, Tollar LL, Ebers GC. A novel sodium channel mutation in a family with hypokalemic periodic paralysis. Neurology. 1999;53:1932–1936. doi: 10.1212/wnl.53.9.1932. [DOI] [PubMed] [Google Scholar]
- Cannon SC. Pathomechanisms in channelopathies of skeletal muscle and brain. Annu Rev Neurosci. 2006;29:387–415. doi: 10.1146/annurev.neuro.29.051605.112815. [DOI] [PubMed] [Google Scholar]
- Cannon SC, Brown RH, Jr, Corey DP. A sodium channel defect in hyperkalemic periodic paralysis: potassium-induced failure of inactivation. Neuron. 1991;6:619–626. doi: 10.1016/0896-6273(91)90064-7. [DOI] [PubMed] [Google Scholar]
- Cannon SC, Brown RH, Jr, Corey DP. Theoretical reconstruction of myotonia and paralysis caused by incomplete inactivation of sodium channels. Biophys J. 1993;65:270–288. doi: 10.1016/S0006-3495(93)81045-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon SC, Strittmatter SM. Functional expression of sodium channel mutations identified in families with periodic paralysis. Neuron. 1993;10:317–326. doi: 10.1016/0896-6273(93)90321-h. [DOI] [PubMed] [Google Scholar]
- Carmeliet E. Induction and removal of inward-going rectification in sheep cardiac Purkinje fibres. J Physiol. 1982;327:285–308. doi: 10.1113/jphysiol.1982.sp014232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha A, Ruben PC, George ALJ, Fujimoto E, Bezanilla F. Voltage sensors in domains III and IV, but not I and II, are immobilized by Na+ channel fast inactivation. Neuron. 1999;22:73–87. doi: 10.1016/s0896-6273(00)80680-7. [DOI] [PubMed] [Google Scholar]
- Chahine M, George AL, Jr, Zhou M, Ji S, Sun W, Barchi RL, Horn R. Sodium channel mutations in paramyotonia congenita uncouple inactivation from activation. Neuron. 1994;12:281–294. doi: 10.1016/0896-6273(94)90271-2. [DOI] [PubMed] [Google Scholar]
- Cummins TR, Zhou J, Sigworth FJ, Ukomadu C, Stephan M, Ptacek LJ, Agnew WS. Functional consequences of a Na+ channel mutation causing hyperkalemic periodic paralysis. Neuron. 1993;10:667–678. doi: 10.1016/0896-6273(93)90168-q. [DOI] [PubMed] [Google Scholar]
- Davies NP, Eunson LH, Samuel M, Hanna MG. Sodium channel gene mutations in hypokalemic periodic paralysis: an uncommon cause in the UK. Neurology. 2001;57:1323–1325. doi: 10.1212/wnl.57.7.1323. [DOI] [PubMed] [Google Scholar]
- Davies NP, Imbrici P, Fialho D, Herd C, Bilsland LG, Weber A, Mueller R, Hilton-Jones D, Ealing J, Boothman BR, Giunti P, Parsons LM, Thomas M, Manzur AY, Jurkat-Rott K, Lehmann-Horn F, Chinnery PF, Rose M, Kullmann DM, Hanna MG. Andersen-Tawil syndrome: new potassium channel mutations and possible phenotypic variation. Neurology. 2005;65:1083–1089. doi: 10.1212/01.wnl.0000178888.03767.74. [DOI] [PubMed] [Google Scholar]
- Francis D, Struyk A, Cannon SC. Arginine mutations in the S4 VSD of NaV1.4 associated with hypokalemic periodic paralysis, but not paramyotonia, create a gating pore conductance. Biophys J. 2010;98:111a. [Google Scholar]
- Gallant EM. Barium-treated mammalian skeletal muscle: similarities to hypokalaemic periodic paralysis. J Physiol. 1983;335:577–590. doi: 10.1113/jphysiol.1983.sp014552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward LJ, Kim JS, Lee MY, Zhou H, Kim JW, Misra K, Salajegheh M, Wu FF, Matsuda C, Reid V, Cros D, Hoffman EP, Renaud JM, Cannon SC, Brown RH. Targeted mutation of mouse skeletal muscle sodium channel produces myotonia and potassium-sensitive weakness. J Clin Invest. 2008;118:1437–1449. doi: 10.1172/JCI32638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurkat-Rott K, Lehmann-Horn F. Do hyperpolarization-induced proton currents contribute to the pathogenesis of hypokalemic periodic paralysis, a voltage sensor channelopathy? J Gen Physiol. 2007;130:1–5. doi: 10.1085/jgp.200709834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurkat-Rott K, Lehmann-Horn F, Elbaz A, Heine R, Gregg RG, Hogan K, Powers PA, Lapie P, Vale-Santos JE, Weissenback J, Fontaine B. A calcium channel mutation causing hypokalemic periodic paralysis. Hum Mol Genet. 1994;3:1415–1419. doi: 10.1093/hmg/3.8.1415. [DOI] [PubMed] [Google Scholar]
- Jurkat-Rott K, Uetz U, Pika-Hartlaub U, Powell J, Fontaine B, Melzer W, Lehmann-Horn F. Calcium currents and transients of native and heterologously expressed mutant skeletal muscle DHP receptor α1 subunits (R528H) FEBS Lett. 1998;423:198–204. doi: 10.1016/s0014-5793(98)00090-8. [DOI] [PubMed] [Google Scholar]
- Jurkat-Rott K, Weber MA, Fauler M, Guo XH, Holzherr BD, Paczulla A, Nordsborg N, Joechle W, Lehmann-Horn F. K+-dependent paradoxical membrane depolarization and Na+ overload, major and reversible contributors to weakness by ion channel leaks. Proc Natl Acad Sci U S A. 2009;106:4036–4041. doi: 10.1073/pnas.0811277106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke T, Gomez CR, Mateus HE, Castano JA, Wang QK. Novel CACNA1S mutation causes autosomal dominant hypokalemic periodic paralysis in a South American family. J Hum Genet. 2009;54:660–664. doi: 10.1038/jhg.2009.92. [DOI] [PubMed] [Google Scholar]
- Klassen TL, Spencer AN, Gallin WJ. A naturally occurring omega current in a Kv3 family potassium channel from a platyhelminth. BMC Neurosci. 2008;9:52. doi: 10.1186/1471-2202-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch MC, Steinmeyer K, Lorenz C, Ricker K, Wolf F, Otto M, Zoll B, Lehmann-Horn F, Grzeschik KH, Jentsch TJ. The skeletal muscle chloride channel in dominant and recessive human myotonia. Science. 1992;257:797–800. doi: 10.1126/science.1379744. [DOI] [PubMed] [Google Scholar]
- Kubisch C, Schmidt-Rose T, Fontaine B, Bretag AH, Jentsch TJ. ClC-1 chloride channel mutations in myotonia congenita: variable penetrance of mutations shifting the voltage dependence. Hum Mol Genet. 1998;7:1753–1760. doi: 10.1093/hmg/7.11.1753. [DOI] [PubMed] [Google Scholar]
- Lapie P, Goudet C, Nargeot J, Fontaine B, Lory P. Electrophysiological properties of the hypokalaemic periodic paralysis mutation (R528H) of the skeletal muscle α1s subunit as expressed in mouse L cells. FEBS Lett. 1996;382:244–248. doi: 10.1016/0014-5793(96)00173-1. [DOI] [PubMed] [Google Scholar]
- Lehmann-Horn F, Jurkat-Rott K. Voltage-gated ion channels and hereditary disease. Physiol Rev. 1999;79:1317–1372. doi: 10.1152/physrev.1999.79.4.1317. [DOI] [PubMed] [Google Scholar]
- Lehmann-Horn F, Rüdel R, Dengler R, Lorkovic H, Haass A, Ricker K. Membrane defects in paramyotonia congenita with and without myotonia in a warm environment. Muscle Nerve. 1981;4:396–406. doi: 10.1002/mus.880040508. [DOI] [PubMed] [Google Scholar]
- Lehmann-Horn F, Rüdel R, Jurkat-Rott K. Nondystrophic myotonias and periodic paralyses. In: Engel AG, Franzini-Armstrong C, editors. Myology. 3rd edn. New York: McGraw-Hill; 2004. pp. 1257–1300. [Google Scholar]
- Lossin C. A catalog of SCN1A variants. Brain Dev. 2009;31:114–130. doi: 10.1016/j.braindev.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Matthews E, Labrum R, Sweeney MG, Sud R, Haworth A, Chinnery PF, Meola G, Schorge S, Kullmann DM, Davis MB, Hanna MG. Voltage sensor charge loss accounts for most cases of hypokalemic periodic paralysis. Neurology. 2009;72:1544–1547. doi: 10.1212/01.wnl.0000342387.65477.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrill JA, Cannon SC. Effects of mutations causing hypokalaemic periodic paralysis on the skeletal muscle L-type Ca2+ channel expressed in Xenopus laevis oocytes. J Physiol. 1999;520:321–336. doi: 10.1111/j.1469-7793.1999.00321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaster NM, Tawil R, Tristani-Firouzi M, Canun S, Bendahhou S, Tsunoda A, Donaldson MR, Iannaccone ST, Brunt E, Barohn R, Clark J, Deymeer F, George AL, Jr, Fish FA, Hahn A, Nitu A, Ozdemir C, Serdaroglu P, Subramony SH, Wolfe G, Fu YH, Ptacek LJ. Mutations in Kir2.1 cause the developmental and episodic electrical phenotypes of Andersen's syndrome. Cell. 2001;105:511–519. doi: 10.1016/s0092-8674(01)00342-7. [DOI] [PubMed] [Google Scholar]
- Ptacek LJ, Tawil R, Griggs RC, Engel AG, Layzer RB, Kwiecinski H, McManis PG, Santiago L, Moore M, Fouad G, Bradley P, Leppert MF. Dihydropyridine receptor mutations cause hypokalemic periodic paralysis. Cell. 1994;77:863–868. doi: 10.1016/0092-8674(94)90135-x. [DOI] [PubMed] [Google Scholar]
- Pusch M. Myotonia caused by mutations in the muscle chloride channel gene CLCN1. Hum Mutat. 2002;19:423–434. doi: 10.1002/humu.10063. [DOI] [PubMed] [Google Scholar]
- Ramsey IS, Moran MM, Chong JA, Clapham DE. A voltage-gated proton-selective channel lacking the pore domain. Nature. 2006;440:1213–1216. doi: 10.1038/nature04700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüdel R, Lehmann-Horn F, Ricker K, Kuther G. Hypokalemic periodic paralysis: in vitro investigation of muscle fibre membrane parameters. Muscle Nerve. 1984;7:110–120. doi: 10.1002/mus.880070205. [DOI] [PubMed] [Google Scholar]
- Ruff RL. Insulin acts in hypokalemic periodic paralysis by reducing inward rectifier K+ current. Neurology. 1999;53:1556–1563. doi: 10.1212/wnl.53.7.1556. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Takagi M, Okamura Y. A voltage sensor-domain protein is a voltage-gated proton channel. Science. 2006;312:589–592. doi: 10.1126/science.1122352. [DOI] [PubMed] [Google Scholar]
- Sheets MF, Hanck DA. Voltage-dependent open-state inactivation of cardiac sodium channels: gating current studies with Anthopleurin-A toxin. J Gen Physiol. 1995;106:617–640. doi: 10.1085/jgp.106.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegenbeek van Heukelom J. Role of the anomalous rectifier in determining membrane potentials of mouse muscle fibres at low extracellular K+ J Physiol. 1991;434:549–560. doi: 10.1113/jphysiol.1991.sp018485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolov S, Scheuer T, Catterall WA. Ion permeation through a voltage-sensitive gating pore in brain sodium channels having voltage sensor mutations. Neuron. 2005;47:183–189. doi: 10.1016/j.neuron.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Sokolov S, Scheuer T, Catterall WA. Gating pore current in an inherited ion channelopathy. Nature. 2007;446:76–78. doi: 10.1038/nature05598. [DOI] [PubMed] [Google Scholar]
- Sokolov S, Scheuer T, Catterall WA. Depolarization-activated gating pore current conducted by mutant sodium channels in potassium-sensitive normokalemic periodic paralysis. Proc Natl Acad Sci U S A. 2008;105:19980–19985. doi: 10.1073/pnas.0810562105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starace DM, Bezanilla F. Histidine scanning mutagenesis of basic residues of the S4 segment of the Shaker K+ channel. J Gen Physiol. 2001;117:469–490. doi: 10.1085/jgp.117.5.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starace DM, Bezanilla F. A proton pore in a potassium channel voltage sensor reveals a focused electric field. Nature. 2004;427:548–553. doi: 10.1038/nature02270. [DOI] [PubMed] [Google Scholar]
- Starace DM, Stefani E, Bezanilla F. Voltage-dependent proton transport by the voltage sensor of the Shaker K+ channel. Neuron. 1997;19:1319–1327. doi: 10.1016/s0896-6273(00)80422-5. [DOI] [PubMed] [Google Scholar]
- Sternberg D, Maisonobe T, Jurkat-Rott K, Nicole S, Launay E, Chauveau D, Tabti N, Lehmann-Horn F, Hainque B, Fontaine B. Hypokalaemic periodic paralysis type 2 caused by mutations at codon 672 in the muscle sodium channel gene SCN4A. Brain. 2001;124:1091–1099. doi: 10.1093/brain/124.6.1091. [DOI] [PubMed] [Google Scholar]
- Struyk AF, Cannon SC. A Na+ channel mutation linked to hypokalemic periodic paralysis exposes a proton-selective gating pore. J Gen Physiol. 2007;130:11–20. doi: 10.1085/jgp.200709755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struyk AF, Cannon SC. Paradoxical depolarization of Ba2+-treated muscle exposed to low extracellular K+: insights into resting potential abnormalities in hypokalemic paralysis. Muscle Nerve. 2008;37:326–337. doi: 10.1002/mus.20928. [DOI] [PubMed] [Google Scholar]
- Struyk AF, Markin VS, Francis D, Cannon SC. Gating pore currents in DIIS4 mutations of NaV1.4 associated with periodic paralysis: saturation of ion flux and implications for disease pathogenesis. J Gen Physiol. 2008;132:447–464. doi: 10.1085/jgp.200809967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombola F, Pathak MM, Isacoff EY. Voltage-sensing arginines in a potassium channel permeate and occlude cation-selective pores. Neuron. 2005;45:379–388. doi: 10.1016/j.neuron.2004.12.047. [DOI] [PubMed] [Google Scholar]
- Tricarico D, Servidei S, Tonali P, Jurkat-Rott K, Camerino DC. Impairment of skeletal muscle adenosine triphosphate-sensitive K+ channels in patients with hypokalemic periodic paralysis. J Clin Invest. 1999;103:675–682. doi: 10.1172/JCI4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tristani-Firouzi M, Jensen JL, Donaldson MR, Sansone V, Meola G, Hahn A, Bendahhou S, Kwiecinski H, Fidzianska A, Plaster N, Fu YH, Ptacek LJ, Tawil R. Functional and clinical characterization of KCNJ2 mutations associated with LQT7 (Andersen syndrome) J Clin Invest. 2002;110:381–388. doi: 10.1172/JCI15183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicart S, Sternberg D, Fournier E, Ochsner F, Laforet P, Kuntzer T, Eymard B, Hainque B, Fontaine B. New mutations of SCN4A cause a potassium-sensitive normokalemic periodic paralysis. Neurology. 2004;63:2120–2127. doi: 10.1212/01.wnl.0000145768.09934.ec. [DOI] [PubMed] [Google Scholar]
- Yang N, George AL, Jr, Horn R. Molecular basis of charge movement in voltage-gated sodium channels. Neuron. 1996;16:113–122. doi: 10.1016/s0896-6273(00)80028-8. [DOI] [PubMed] [Google Scholar]
- Yang N, Ji S, Zhou M, Ptacek LJ, Barchi RL, Horn R, George AL., Jr Sodium channel mutations in paramyotonia congenita exhibit similar biophysical phenotypes in vitro. Proc Nat Acad Sci U S A. 1994;91:12785–12789. doi: 10.1073/pnas.91.26.12785. [DOI] [PMC free article] [PubMed] [Google Scholar]