Abstract
Why do complex-partial seizures in temporal lobe epilepsy (TLE) cause a loss of consciousness? Abnormal function of the medial temporal lobe is expected to cause memory loss, but it is unclear why profoundly impaired consciousness is so common in temporal lobe seizures. Recent exciting advances in behavioral, electrophysiological, and neuroimaging techniques spanning both human patients and animal models may allow new insights into this old question. While behavioral automatisms are often associated with diminished consciousness during temporal lobe seizures, impaired consciousness without ictal motor activity has also been described. Some have argued that electrographic lateralization of seizure activity to the left temporal lobe is most likely to cause impaired consciousness, but the evidence remains equivocal. Other data correlates ictal consciousness in TLE with bilateral temporal lobe involvement of seizure spiking. Nevertheless, it remains unclear why bilateral temporal seizures should impair responsiveness. Recent evidence has shown that impaired consciousness during temporal lobe seizures is correlated with large-amplitude slow EEG activity and neuroimaging signal decreases in the frontal and parietal association cortices. This abnormal decreased function in the neocortex contrasts with fast polyspike activity and elevated cerebral blood flow in limbic and other subcortical structures ictally. Our laboratory has thus proposed the “network inhibition hypothesis,” in which seizure activity propagates to subcortical regions necessary for cortical activation, allowing the cortex to descend into an inhibited state of unconsciousness during complex-partial temporal lobe seizures. Supporting this hypothesis, recent rat studies during partial limbic seizures have shown that behavioral arrest is associated with frontal cortical slow waves, decreased neuronal firing, and hypometabolism. Animal studies further demonstrate that cortical deactivation and behavioral changes depend on seizure spread to subcortical structures including the lateral septum. Understanding the contributions of network inhibition to impaired consciousness in TLE is an important goal, as recurrent limbic seizures often result in cortical dysfunction during and between epileptic events that adversely affects patients’ quality of life.
Keywords: cortex, EEG, fMRI, septal nuclei, slow waves, attention, temporal lobe epilepsy, thalamus
Introduction
Consciousness has been an exceeding difficult concept to define for researchers, clinicians, and philosophers alike. This is likely because consciousness is a complex phenomenon which encompasses various different processes. Plum and Posner (1980) have suggested that it is important to distinguish between the level of consciousness and the content of consciousness. The content of consciousness can be described as the substrate on which consciousness acts, and is composed of all other neural systems hierarchically organized into parallel sensory and motor systems that receive inputs, generate outputs, and perform internal processing on multiple levels (Blumenfeld, 2002). In turn, the level of consciousness also has multiple components, which can be summarized as the maintenance of three distinct but related processes: (i) the awake, alert state; (ii) attention; and (iii) awareness of self and environment (Blumenfeld, 2002, 2009). To study the neurobiological mechanisms of the awake, alert state necessary for consciousness, previous investigators have utilized various models such as sleep, coma, deep anesthesia, brain lesions, and epilepsy.
Generalized seizure disorders in humans, such as absence (petite mal) and tonic-clonic (grand mal) epilepsy, involve a pathological pattern of synchronous neuronal discharges in extensive networks throughout the brain, resulting in a loss of consciousness (Blumenfeld, 2005). Absence seizures are a form of generalized epilepsy in children characterized by rhythmic 3–4 Hz “spike-wave” discharges on electroencephalogram (EEG) produced by pathophysiological corticothalamic interactions (Avoli and Gloor, 1982; Blumenfeld and McCormick, 2000). These events are associated with brief 5–10 s episodes of unresponsiveness that have significant effects on a child's attentional abilities both during and between events (Levav et al., 2002; Mirsky and Van Buren, 1965). Conversely, generalized tonic-clonic seizures are characterized by fast excitatory discharges synchronously affecting neuronal networks in numerous brain regions, producing several minutes of unconsciousness and abnormal convulsive activity of widespread muscle groups (Blumenfeld et al., 2009; Morrell, 1993; Zifkin and Dravet, 2007).
It is perhaps not surprising that generalized seizures, involving extensive dysfunction of cortical and subcortical brain regions, cause significant impairments of consciousness. In temporal lobe epilepsy (TLE), however, seizures often originate from focal structures within the mesial temporal lobe, and frequently do not secondarily generalize or propagate to distal cortical areas. Yet, despite confinement of epileptic discharges to the temporal lobe and related limbic structures, seizures in TLE often cause a loss of consciousness. Understanding the mechanisms of impaired consciousness and cortical dysfunction during temporal lobe seizures has important clinical implications, as impaired consciousness causes motor vehicle accidents, drownings, poor work and school performance, and social stigmatization resulting in a major negative impact on patient quality of life (Drazkowski, 2007; Jacoby et al., 2005; Kobau et al., 2008; Sperling, 2004). In addition, previous investigations of TLE patients have found neocortical deficits including gray matter atrophy (Bonilha et al., 2006) and hypometabolism between seizures (Diehl et al., 2003; Nelissen et al., 2006), which may be related to neuropsychological sequelae and chronic cognitive impairments frequently suffered by these individuals (Helmstaedter and Kockelmann, 2006; Hermann et al., 1997; Laurent and Arzimanoglou, 2006). An appreciation of the long-range network effects of temporal lobe seizures on the neocortex may lead to a better grasp of epileptic mechanisms and a further understanding of the brain region interactions that underlie the conscious state. In this review, we will summarize previous investigations of TLE in humans and animal models, discuss what they suggest about the mechanisms of impaired consciousness during temporal lobe seizures, and advocate directions to further our understanding of this important problem.
The network inhibition hypothesis in TLE
Epilepsy is a debilitating neurological disorder that affects approximately 1% of the population in developed countries such as the United States (Devinsky, 2004). TLE is one of the most common epileptic disorders, characterized by seizures that frequently originate in limbic structures of the medial temporal lobe, such as the hippocampus and the amygdala (Engel, 1987; Williamson et al., 1993). As the temporal lobe is the most common site of origin of focal epileptic discharges, it has been suspected to be the most epileptogenic brain region (Engel et al., 2007). The etiology of TLE is frequently idiopathic, but it can also result from malignancy, trauma, infections, and vascular malformations (Engel and Williamson, 2007; Engel et al., 2007). Recurrent temporal lobe seizures often produce significant pathological changes, such as hippocampal sclerosis in two-thirds of patients, and can propagate from limbic structures to the temporal neocortex and other regions (Babb, 1987; de Lanerolle and Lee, 2005; Gloor, 1991; Williamson et al., 1993).
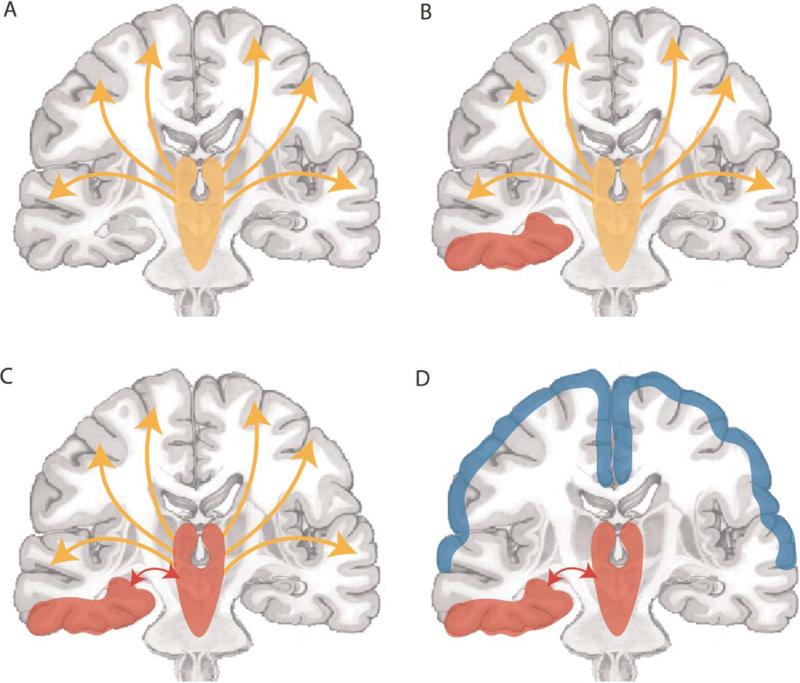
While discharges in TLE can propagate distally to produce a secondarily generalized tonic-clonic seizure, most temporal lobe seizures in patients taking anticonvulsant medications do not secondarily generalize. Thus, seizures in TLE are typically characterized as one of two major subtypes of partial seizures: complex-partial seizures, which result in a diminished level of consciousness during (ictal) and after (postictal) the event, and simple-partial seizures, which do not interfere with consciousness (ILAE, 1981). This clinical distinction is based primarily on symptomatology, and the neurobiological underpinnings of the divergent effects on consciousness seen in complex versus simple-partial seizures are not well understood. Various ideas have been proposed to explain how complex-partial seizures impair consciousness (Yu and Blumenfeld, 2008). Some evidence suggests that the laterality or bilaterality of temporal lobe involvement may be a primary determining factor of ictal alertness (Gloor et al., 1980; Hoffmann et al., 2008; Lux et al., 2002). Nevertheless, it has also been argued that one should not mistake postictal amnesia of the seizure — a probable result of bilateral temporal lobe dysfunction — with unconsciousness that also entails diminished responsiveness (Gloor, 1986). Therefore, another hypothesis is that while seizure lateralization may indeed be correlated with altered alertness in TLE, loss of consciousness more directly results from aberration of subcortical and brainstem structures that are necessary for maintaining the awake-alert state. As a sleep-like slow rhythm is frequently observed in frontal and parietal cortical regions during complex-partial seizures, our laboratory has hypothesized that “ictal neocortical slow activity” reflects remote effects of limbic seizures on other parts of the brain, resulting in a depressed cortical state responsible for the deficits in consciousness seen in TLE patients (Blumenfeld et al., 2004a, b). Figure 1 illustrates our “network inhibition hypothesis,” in which we postulate that consciousness is lost during complex-partial temporal lobe seizures because of seizure spread to midline subcortical and brainstem structures, leading in turn to bilateral cortical deactivation (Blumenfeld, 2009; Blumenfeld and Taylor, 2003; Norden and Blumenfeld, 2002). Evidence addressing this and other mechanistic theories of impaired consciousness in complex-partial TLE has been uncovered in previous behavioral, electrographic, and neuroimaging studies of temporal lobe seizures in both human patients and animal models of epilepsy.
Fig. 1.
Network inhibition hypothesis for loss of consciousness in complex-partial seizures. (A) Under normal conditions, the upper brainstem-diencephalic activating systems interact with the cerebral cortex to maintain normal consciousness. (B) A focal seizure involving the mesial temporal lobe unilaterally. (C) Propagation of seizure activity from the mesial temporal lobe to midline subcortical structures. (D) Disruption of the normal activating functions of the midline subcortical structures, together with the resulting depressed activity in bilateral regions of the frontoparietal association cortex, leads to loss of consciousness. Adapted with permission from Blumenfeld and Taylor (2003). Please see online version of this article for full color figure.
Behavioral semiology of temporal lobe seizures
While simple-partial temporal lobe seizures are frequently characterized by autonomic and/or psychic symptoms and epigastric sensations, complex-partial seizures of the temporal lobe often begin with an inhibition of purposeful motor activity and elicitation of automatic behavioral manifestations that are associated with unresponsiveness (ILAE, 1989). These automaton-like behaviors, termed “automatisms,” are typically manifested as repetitive orofacial movements such as lip-smacking, chewing, and swallowing that are not related to external stimuli in the individual's environment (Penfield, 1950). They can also manifest as “ambulatory automatisms” of the limbs or dystonic posturing ictally (Leung et al., 2000; Marks and Laxer, 1998).
Escueta et al. (1977) completed one of the earliest combined video-EEG analyses of complex-partial seizures and their behavioral correlates in TLE patients. The authors identified three clinical phases during most of the 76 seizures recorded, beginning with a motionless stare early in the event, followed by stereotypical movements, and with loss of consciousness later in the seizure (Escueta et al., 1977). Another study of 72 hippocampal or amygdalar seizures found that motionless staring or oroalimentary automatisms were the first behavioral manifestations during the majority of events (60%), with fewer seizures beginning with nonfocal discrete movements, perseverative stereotyped automatisms, or vocalizations (Maldonado et al., 1988). In general, whereas automatisms and other behavioral manifestations usually emerge early in a complex-partial seizure, while responsiveness may remain intact, consciousness in TLE is most profoundly affected late in the seizure and during the postictal period (Blumenfeld and Taylor, 2003). Rarely, automatisms have been described during simple-partial temporal lobe seizures with spared consciousness (Alarcon et al., 1998). For instance, one large investigation of 123 TLE patients reported that 7 individuals experienced prominent automatisms such as lip-smacking and swallowing without a loss of consciousness (Ebner et al., 1995).
Many patients report experiencing an “aura” at the beginning of a partial temporal lobe seizure, described as a warning symptom or vague signal of an impending event, likely representing early epileptiform activity below the threshold of scalp electrographic detection (Fried et al., 1995). One study of 96 seizures in 19 patients with stereotactic depth electrode implantation found that 67% of unilateral temporal lobe seizures began with an aura, while motionless stares, automatisms, and head-body turning were each manifested in approximately one-quarter of seizures (Quesney, 1986). In addition to auras preceding the epileptic event, psychic phenomena have been described in some patients during limbic seizures. In 1898, John Hughlings Jackson noted that medial temporal lobe seizures can result in a “dreamy state,” characterized by dramatic memory-like hallucinations such as déjà vu — the perception that one has previously experienced identical circumstances to the present situation (Jackson and Colman, 1898). After noting that certain patients with implanted intracranial electrodes experience these psychic phenomena during simple-partial seizures, Bancaud et al. (1994) reproduced these sensations of a dreamy state by electrically stimulating the anterior hippocampus, amygdala, or temporal neocortex. More severe psychotic symptoms or affective disturbances, such as hallucinations or intense emotional experiences, have also been described ictally in some individuals during temporal lobe seizures (Ardila, 1990; Boylan, 2002).
Following complex-partial temporal lobe seizures, patients often experience a postictal state of impaired consciousness, confusion, and amnesia of the event (Gloor, 1986). Blum et al. (1996) studied 23 epileptic patients to assess the individuals’ insight into seizure occurrence and frequency. Looking at both complex-partial and secondarily generalized events, the authors observed that only one-quarter of patients always endorsed having had a seizure immediately after they regained consciousness, and 30% of individuals never acknowledged experiencing a documented seizure. Although not all seizures in this report originated from temporal lobe foci, those that did were most likely to be associated with postictal amnesia (Blum et al., 1996). Another investigation found a positive correlation between amnesia for previously documented auras and seizure severity, further suggesting that increased levels of temporal dysfunction may be more likely to produce postictal amnesia (Schulz et al., 1995).
Behavioral manifestations during limbic epileptic events in TLE patients raise interesting questions about whether loss of consciousness in temporal lobe seizures results from: (i) a progressive spread of excitatory epileptic discharges to other brain regions, such as the neocortex, or (ii) long-range depressive effects, dissimilar from seizure propagation, that suppress distant cortical and subcortical brain structures critical for vigilance. Electrophysiological and neuroimaging explorations of diminished ictal responsiveness in TLE have focused on correlating deficits in responsiveness with temporal lobe lateralization of seizure activity, and more recently have characterized the effects of limbic seizures on bilateral cortical and subcortical networks necessary for maintaining consciousness.
EEG correlates of impaired consciousness in human TLE
Limbic seizures originate from mesial temporal structures on either the left or right side of the brain. Lateralization of behavioral signs such as automatisms can provide insight into the side of seizure onset, albeit with some inconsistency (Saint-Hilaire and Lee, 2000). It has also been proposed that lateralization or bilaterality of temporal lobe seizures may be the predominant feature predicting either loss or preservation of consciousness ictally. Some have hypothesized that the left hemisphere, which is dominant for language in most humans, is primarily responsible for the conscious state (Albert et al., 1976; Ebner et al., 1995; Schwartz, 1967). One study utilizing the Wada test found that while temporary inactivation of the right hemisphere does not interfere with consciousness, impaired left hemispheric activity causes unresponsiveness to both verbal and somatic stimuli (Franczek et al., 1997). Correlating seizure lateralization with awareness of the events, Inoue and Mihara (1998) found that TLE patients who were unaware of their seizures typically had onset of epileptic activity in the mesial temporal lobe of language-dominant (typically left) side. A more recent investigation similarly described that ictal behavioral arrest was more common during left-sided temporal seizures than those beginning on the right side (Hoffmann et al., 2008). Furthermore, Lux et al. (2002) looked at vocalizations during complex-partial seizures and reported that while patients with left temporal seizure activity had ictal memory loss and impairment of both expressive and receptive speech, those with seizure activity limited to the right temporal lobe rarely exhibited these impairments during the event. Another study examined ictal and postictal speech deficits in TLE and found that unintelligible vocalizations were more commonly associated with seizures originating in the language-dominant temporal lobe, while coherent speech was generally noted during discharges originating from the nondominant side (Gabr et al., 1989). Since involvement of the dominant temporal lobe more often causes language impairment, it is possible that apparent impaired consciousness could be an artifact of the testing procedures, which usually depend on responses to verbal questions and commands. This could lead to a bias in studies attempting to lateralize impaired consciousness in temporal lobe seizures, since nonverbal aspects of consciousness are more difficult to evaluate.
Another hypothesis is that unilateral temporal lobe seizure activity alone might not be sufficient to cause a loss of consciousness, and that bilateral limbic discharges are required to elicit unresponsiveness. Herbert Jasper (1964) was one of the first to propose that ictal automatisms and amnesia in TLE depend on seizure activity involving both temporal lobes. Gloor et al. (1980) described that 74% of temporal lobe seizures that resulted in a loss of consciousness showed bitemporal ictal involvement on EEG. Subsequently, several investigators have offered further evidence that seizures involving bilateral temporal lobe cortices are more likely to cause impairments in vigilance and responsiveness than those with unilateral localization (Bancaud et al., 1994; Inoue and Mihara, 1998; Pedley, 1992). These studies imply that bilaterality of temporal lobe involvement in TLE may be an important predictive factor of ictal impairment of consciousness. Nonetheless, while it may appear intuitive that bilateral temporal lobe dysfunction can result in amnesia due to aberrant limbic activity (Milner, 1972), temporal lobe structures are not often considered necessary for consciousness. This can be illustrated by the classic case of patient H.M. who was unable to form new memories after bilateral mesial temporal lobectomy, yet remained alert, responsive, and interactive — thus, “conscious” — postoperatively (Scoville and Milner, 1957). Might bilaterality of temporal lobe involvement represent a noncausal variable, correlated with impaired alertness during limbic seizures, but not the direct source of loss of consciousness? For instance, in one study by Munari et al. (1980), while complex-partial seizures did more commonly engage the temporal lobes bilaterally compared to simple-partial seizures, it was found that seizures with impaired consciousness were also approximately twice the duration of simple-partial events and were more likely to recruit structures outside of the temporal lobe. This implies that seizure severity and extratemporal involvement may also serve as predictors of ictal loss of consciousness in addition to temporal lobe laterality.
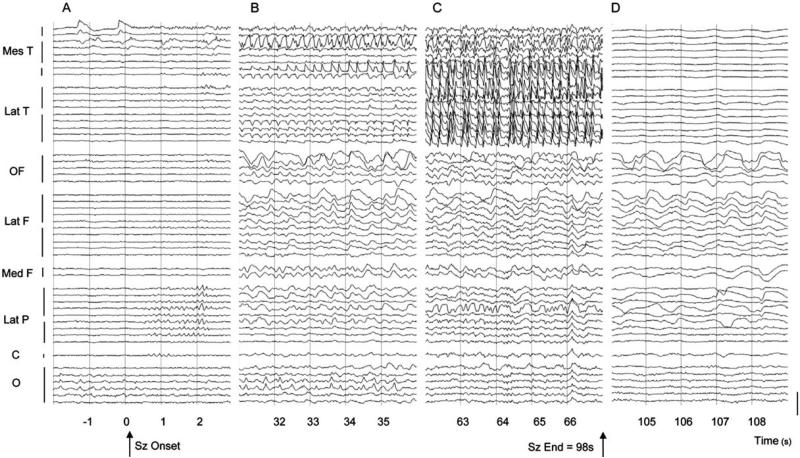
While the majority of electrographic studies of TLE have focused on temporal lobe localization of seizure activity, examining the effects of limbic discharges on other brain regions that are important for consciousness is an essential endeavor to further understand these problems. In patients with intracranial electrodes implanted in the thalamus, Bertashius (1991) found that thalamic nuclei were often affected by epileptic activity during temporal lobe seizures. More recently, Bartolomei and colleagues have observed that in patients with mesial TLE seizures, thalamocortical synchrony was specifically correlated with loss of consciousness ictally (Guye et al., 2006). Moreover, although epileptic discharges during temporal lobe seizures are typically characterized by fast, polyspike electro-graphic signals in both the seizure focus and loci of propagation, slow EEG rhythms have also been uncovered in neocortical regions ictally in TLE. Specifically, several previous intracranial EEG studies have revealed large-amplitude slow oscillatory activity in frontal and parietal neocortical regions during complex-partial temporal lobe seizures (Eisenschenk et al., 2001; Franaszczuk et al., 1994; Lieb et al., 1991; Mayanagi et al., 1996). Despite the dissimilarity of these slow waves to fast seizure spiking, they have traditionally been interpreted as the spread of seizure activity to distal brain structures. In a recent intracranial EEG study of complex-partial temporal lobe seizures, our laboratory also found large-amplitude 1–2 Hz slow waves in the frontoparietal neocortices ictally, most prominent in the orbitofrontal cortex (Fig. 2) (Blumenfeld et al., 2004b). This slow activity in the association cortices during seizures was starkly contrasted with polyspike activity in the seizing temporal lobe, as it did not contain fast or sharp components characteristic of epileptic discharges (Fig. 2A–C). Furthermore, the neocortical slow activity persisted into the postictal period of impaired consciousness, and closely resembled the large-amplitude cortical oscillations typically seen during slow-wave sleep (Fig. 2D). These findings suggest that ictal neocortical slow activity, perhaps resulting from seizure spread to subcortical structures like the thalamus that are involved in cortical activation, may contribute to loss of consciousness during complex-partial seizures. Nevertheless, limited spatial sampling during human intracranial EEG studies of epilepsy restricts our understanding of distal brain effects during temporal lobe seizures. Further insight into the hypotheses addressing impaired consciousness in TLE has been achieved using neuroimaging techniques, which have served as critical tools to correlate ictal consciousness with involvement of anatomical regions throughout the brain.
Fig. 2.
Example of intracranial EEG recording during a mesial temporal seizure. (A) Seizure onset with low-voltage fast activity emerging from periodic spiking in the mesial temporal contacts. Later in this interval (0–30 s after seizure onset) spike-and-slow activity later appeared in the mesial as well as the lateral temporal contacts (not shown). (B) Sample from 30 to 60 s after seizure onset. Rhythmic spike and sharp wave activity continues in the temporal lobe, while the frontal and parietal contacts show large amplitude irregular slow-wave activity. (C) Sample from 60 to 90 s after seizure onset. Spike and polyspike-and-wave activity is present in the temporal lobe, with ongoing slow waves in the neocortex. (D) Postictal suppression is seen in temporal lobe contacts, with continued irregular slowing in the frontoparietal neocortex. Ipsilateral contacts only are shown. Bars along left margin indicate electrode contacts from different strips, rows, or depth electrodes in the indicated brain regions. Calibration bar on right is 1000 μV. Montage is referential to mastoid. Mes T, mesial temporal; Lat T, lateral temporal; OF, orbital frontal; Lat F, lateral frontal; Med F, medial frontal; Lat P, lateral parietal; C, perirolandic (pre- and post-central gyri); O, occipital. Adapted with permission from Blumenfeld et al. (2004b).
Neuroimaging insights into impaired consciousness in human TLE
Why do complex-partial temporal lobe seizures cause unconsciousness even though EEG recordings typically show seizure activity confined to temporal cortex? As discussed previously, partial seizures in TLE often cause other functional deficits beyond those expected from local limbic impairment, such as repetitive automaton-like movements (Loddenkemper and Kotagal, 2005), dystonic posturing of the limbs (Marks and Laxer, 1998), and neuroendocrine changes (Bauer, 2001; Quigg et al., 2002). It has therefore been proposed that even when temporal lobe seizures do not propagate, they may cause remote dysfunction in other brain regions, leading to these disturbances and altered consciousness (Blumenfeld et al., 2004a, b; Van Paesschen et al., 2003). Several neuroimaging studies have investigated brain regions affected by limbic seizure activity. Of the functional imaging techniques available to study TLE, single photon emission computed tomography (SPECT) has advantages over positron emission tomography (PET) or functional magnetic resonance imaging (fMRI), as the SPECT radiotracer can be injected during the seizure, with subsequent imaging performed after the event. This alleviates challenges related to movement artifact during seizures with other imaging methods (Englot and Blumenfeld, 2009; Kim et al., 2009).
Using SPECT, investigators have found increased cerebral blood flow (CBF) associated with epileptic activity in the temporal lobe on the side of seizure onset (Andersen et al., 1990; Bonte et al., 1983; Duncan et al., 1990). Other SPECT studies have shown bilateral involvement of the thalamus and upper brainstem during temporal lobe seizures (Hogan et al., 2006; Tae et al., 2005), with some providing evidence that impaired consciousness during these events is correlated with increased perfusion in these regions (Blumenfeld et al., 2004a; Lee et al., 2002; Mayanagi et al., 1996). Imaging results of thalamic involvement in TLE complement evidence of elevated activity in the thalamus ictally detected using intracranial EEG (Arthuis et al., 2009; Guye et al., 2006; Rosenberg et al., 2006), and of interictal thalamic atrophy or dysfunction often associated with mesial TLE (Chang et al., 2008; Gong et al., 2008; Hetherington et al., 2007; Labate et al., 2008; Natsume et al., 2003; Riederer et al., 2008). Given these findings, it is possible that medial diencephalic and brainstem connections known to be important for arousal may contribute to behavioral arrest during complex-partial seizures of the temporal lobe.
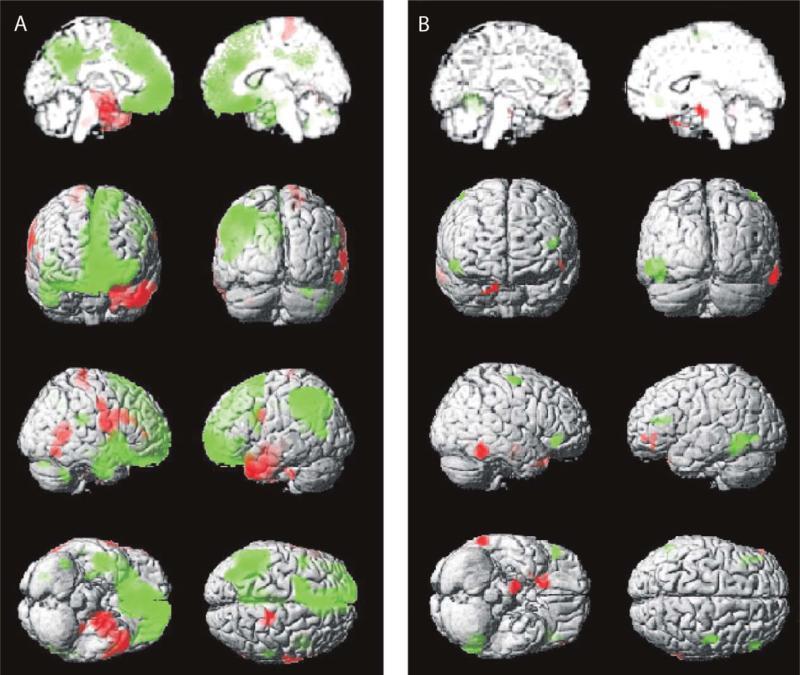
In addition to revealing areas of increased perfusion ictally in TLE, some neuroimaging studies have also shown reduced CBF in frontal and parietal cortices during complex-partial seizures, simultaneous to increases in the seizing temporal lobe (Chang et al., 2002; Menzel et al., 1998; Rabinowicz et al., 1997). For instance, Van Paesschen et al. (2003) performed ictal SPECT during complex-partial seizure in 24 patients with intractable mesial TLE. The authors observed temporal lobe hyperperfusion ipsilateral to seizures onset that was inversely associated with hypoperfusion in the frontal lobes during scans in all patients. In another recent SPECT investigation, our laboratory reported that neocortical decreases in CBF during complex-partial temporal lobe seizures were associated with deficits in consciousness, as no reductions were seen on average during simple-partial temporal lobe seizures (Fig. 3) (Blumenfeld et al., 2004a). Specifically, statistical parametric maps of complex-partial seizures indicated CBF elevations in the temporal lobe on the side of seizure onset, as well as midline subcortical-diencephalic structures, while CBF was diminished in widespread frontal and parietal association cortices ictally (Fig. 3A). However, widespread bilateral CBF decreases in the higher-order association cortices were not present during simple-partial seizures (Fig. 3B). SPECT signal decreases during complex-partial seizures were seen in the same frontoparietal cortical regions as ictal neocortical slow activity (Fig. 2). In addition, like neocortical slow oscillations, cortical hypoperfusion was observed during both the ictal and postictal periods of temporal lobe seizures, when consciousness remains impaired (Blumenfeld et al., 2004a, b).
Fig. 3.
Complex-partial, but not simple-partial, temporal lobe seizures are associated with significant CBF decreases in frontoparietal neocortical regions. Statistical parametric maps depict CBF increases in red and decreases in green. Changes ipsilateral to seizure onset are shown on the left side of the brain, and contralateral changes on the right side of the brain (combining patients with left and right onset seizures). (A) Complex-partial seizures arising from the temporal lobe are associated with significant CBF increases and decreases in widespread brain regions. Sixty to ninety seconds after seizure onset, increases occur mainly in the ipsilateral temporal lobe, while decreases occur in the ipsilateral > contralateral frontal and parietal association cortex (n =  ). (B) Simple-partial seizures arising from the temporal lobe are not associated with widespread CBF changes (n =
). (B) Simple-partial seizures arising from the temporal lobe are not associated with widespread CBF changes (n =  ). For (A) and (B), extent threshold, k =
). For (A) and (B), extent threshold, k = 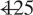 voxels (voxel size =
voxels (voxel size = 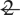 × 2 × 2 mm). Height threshold, P =
× 2 × 2 mm). Height threshold, P = 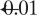 . Equivalently, only voxel clusters greater than 1 cm3 in volume and with Z scores greater than 2.33 are displayed. Adapted with permission from Blumenfeld et al. (2004a). Please see online version of this article for full color figure.
. Equivalently, only voxel clusters greater than 1 cm3 in volume and with Z scores greater than 2.33 are displayed. Adapted with permission from Blumenfeld et al. (2004a). Please see online version of this article for full color figure.
The above neuroimaging and electrophysiological findings raise the question: is ictal unconsciousness in TLE more directly related to deactivation of the frontoparietal neocortex following ictal recruitment of subcortical regions, rather than being caused by the spread of excitatory seizure activity to bilateral temporal or association cortices? In some respects, this possibility echoes ideas of 19th-century neurologist John Hughlings Jackson, who after observing several epileptic seizures, proposed that consciousness is impaired when higher cortical function becomes disorganized and lacks integrative ability (Jackson et al., 1931). Jackson considered the “highest nervous centers” to be the substrata of consciousness, representing complex cortical regions responsible for the coordination of various inputs and outputs throughout the body, and he hypothesized that impaired consciousness resulted when discharges engaged these regions. Jackson proposed that if a seizure begins in a subordinate structure or series of structures, consciousness will be lost if this discharge spread to alter higher-order processes, or if a sufficiently large number of subordinate structures become recruited (Jackson et al., 1931; Yamauchi, 1998).
Neuroimaging and EEG characterizations of cortical activity during complex-partial temporal seizures raise many interesting questions regarding loss of consciousness during these events. For instance, involvement of which subcortical structures is important in the production of ictal neocortical slow rhythms? Does the phenomenon result from an active inhibitory process directly affecting the cortex, or from disruption of normal neocortical activation, allowing the cortex to temporarily resort to a depressed state resembling coma, deep anesthesia, or sleep (Cowan and Wilson, 1994; Haider et al., 2006; Steriade et al., 1993)? Or, in contrast, do ictal neocortical slow rhythms simply represent the propagation of excitatory seizure activity to distal cortical regions? To further address these issues, it is useful to discuss invasive recordings of neuronal activity recorded in animal models of TLE.
Network effects of temporal lobe seizures in animal models
While human studies of neurological disease possess the greatest validity, animal models of TLE activity allow controlled mechanistic studies which can be valuable in understanding both network effects and behavioral manifestations associated with complex-partial seizures. Behavioral correlates of electrographic temporal lobe seizures have been extensively studied in rodent models of TLE. For instance, the classic scale created by Ronald Racine (1972) allows investigators to rate behavioral limbic seizures from those including only mild manifestations such as staring and behavioral arrest (class 0) or facial automatisms (class 1) to those with more prominent convulsions characterized by head nodding (class 2) or progressively worsening clonic activity (classes 3–5). Thus, animal behavior during Racine class 0 or 1 seizures resembles the semiology associated with human complex-partial temporal lobe seizures.
Similar to human investigations, several rat studies of TLE have shown involvement of the thalamus during partial limbic seizures (Bertram et al., 2001, 2008; Blumenfeld et al., 2007; Englot et al., 2008). Significant neuronal loss has been described in the medial, dorsal, and rhomboid thalamic nuclei associated with limbic seizures, with lidocaine-mediated inhibition of the midline thalamus reducing the duration of epileptic discharges (Bertram et al., 2001). Also, as discussed below, our laboratory has described bilateral involvement of the thalamus on fMRI during electrically stimulated limbic seizures (Englot et al., 2008).
How might aberrant activity in the thalamus and other subcortical structures that are important for alertness lead to a loss of consciousness? One possibility is that these regions may help propagate excitatory activity directly to the neocortex. Alternatively, our network inhibition hypothesis proposes that seizure activity in one part of the brain, particularly the mesial temporal lobe, may cause inhibition of normal subcortical activating systems, and thereby indirectly deactivate frontoparietal cortical regions necessary for consciousness. Given the inherent network properties of the central nervous system, intense activation of one region might result in functional changes in adjacent or remote areas, even without spread of excitation to these other areas. Common examples in which increased activity in one cortical region can cause an inhibitory surround include normal visual or somatosensory processing (Angelucci et al., 2002; Brumberg et al., 1996; Derdikman et al., 2003; McCasland et al., 1991; Sengpiel et al., 1997). Some animal studies have provided evidence that a similar process may occur in epilepsy focally, such as in abnormal surround inhibition adjacent to cortical seizure foci (Collins, 1978; Prince and Wilder, 1967; Schwartz and Bonhoeffer, 2001). However, in TLE the affected frontoparietal neocortex does not lie immediately adjacent to the mesial temporal lobe, suggesting that more complex long-range network mechanisms may play a role.
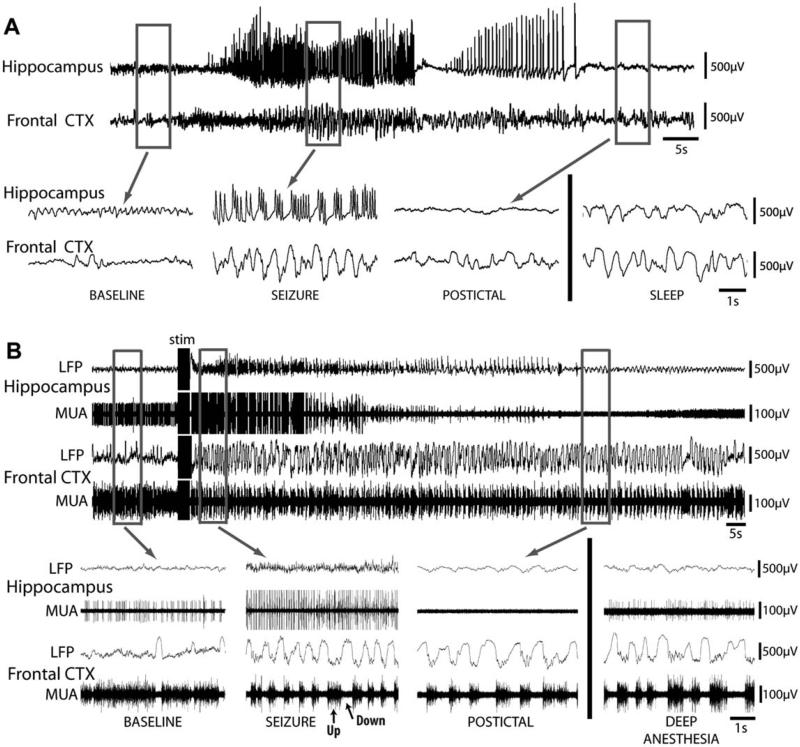
Our laboratory recently performed a multi-modal study of ictal neocortical slow oscillations during partial limbic seizures in both lightly anesthetized and awake-behaving rats (Englot et al., 2008). We observed that spontaneous partial limbic seizures in awake-behaving animals were associated with fast 9–12 Hz fast polyspike seizure activity in hippocampal EEG, as expected. However, in the frontal cortex, we observed large amplitude 1–3 Hz slow waves (Fig. 4A) that more closely resembled slow-wave sleep oscillations (Fig. 4A, bottom right) than seizure spiking. These partial seizures were associated with mild behavioral manifestations such as behavioral arrest and facial automatism (Racine class 0–1), differing dramatically from convulsive activity seen during secondarily generalized seizures in the same animals — the latter of which were associated with fast polyspike activity in the frontal cortex (Englot et al., 2008).
Fig. 4.
Partial limbic seizures in rats produce ictal neocortical slow waves in the orbitofrontal cortex (CTX). (A) Local field potentials (LFP) in the hippocampus and orbitofrontal cortex during a spontaneous partial seizure associated with behavioral arrest in an awake-behaving rat. Hippocampal recordings reveal large-amplitude, fast polyspike activity during the seizure, while frontal cortical recordings show large-amplitude 1–3 Hz slow waves during and after the seizure without considerable propagation of fast spike activity. Ictal neocortical slow activity resembles large-amplitude slow rhythms seen in the frontal cortex during an episode of natural sleep, recorded in the same animal at a different time (bottom, right). LFP recordings are filtered 0.3–100 Hz. (B) Example of LFP and multiunit activity (MUA) recordings during an electrically stimulated partial seizure in a lightly anesthetized rat. During baseline, recordings show a stable theta rhythm in hippocampal LFP and low-voltage beta activity with occasional slow waves in the orbitofrontal cortex (see also inset). MUA recordings reveal relatively stable neuronal firing in both areas. During the seizure, hippocampal LFP recordings show 9–10 Hz fast polyspike activity ictally associated with population spikes in MUA recordings. Population spikes are often up to 10 times larger in amplitude than individual baseline units and are thus shown truncated here. In the orbitofrontal cortex, 1–2 Hz large-amplitude slow waves are seen in LFP recordings, associated with Up and Down states (arrows) of neuronal firing in MUA recordings. No fast polyspike activity is present in the frontal cortex LFPs. After the seizure, hippocampal activity is depressed whereas frontal slow oscillations persist postictally. Recordings from the same rat under deep anesthesia at a different time are also shown (bottom right), during which slow activity is present in the frontal cortex. LFP recordings are filtered 0.1–100 Hz and MUA recordings are filtered 400 Hz–20 kHz. Adapted with permission from Englot et al. (2008).
Electrically stimulated partial hippocampal seizures in lightly anesthetized animals also revealed similar patterns of local field potential (LFP) fast activity and large population spikes in multiunit activity (MUA) seen in the hippocampus ictally (Fig. 4B). This activity differed from decreased firing with Up and Down states of neuronal firing in the frontal cortex ictally, which resembled Up and Down states seen during deep anesthesia (Fig. 4B, bottom right). These Up and Down states appeared similar to neocortical firing patterns commonly seen in other studies of cortical depression, such as during deep sleep and anesthesia (Cowan and Wilson, 1994; Haider et al., 2006; Steriade et al., 1993).
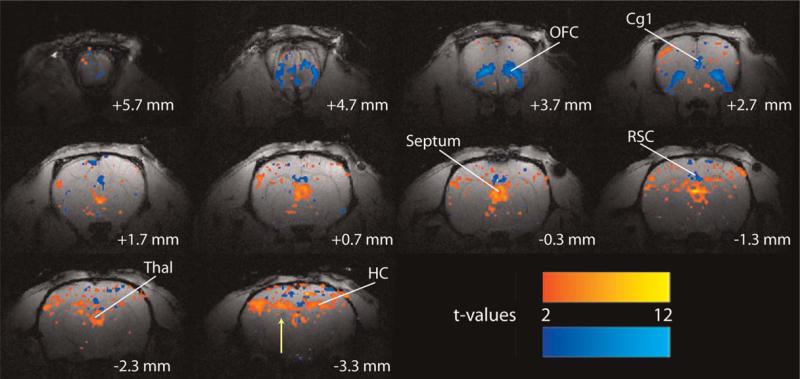
CBF measurements using laser Doppler flow-metry (LDF) showed that while cortical seizure propagation (i.e., secondary generalization) was associated with elevated CBF, cortical perfusion decreased during partial seizures with ictal slow activity (Englot et al., 2008). Finally, fMRI recordings of electrically stimulated partial seizures revealed decreased blood oxygen level dependent (BOLD) signal in the orbital frontal cortex ictally, contrasted by elevations in the hippocampus, as well as the septal nuclei and thalamus (Fig. 5). These studies complement electrophysiology and neuroimaging investigations of human TLE to further suggest that ictal neocortical slow activity is associated with diminished neocortical activity, not excitatory seizure propagation to the cortex.
Fig. 5.
Example of BOLD increases and decreases during an electrically stimulated partial limbic seizure. During partial limbic seizures, BOLD fMRI signal increases are observed in the hippocampus, thalamus, and septal nuclei. Prominent BOLD decreases are seen in the orbitofrontal, anterior cingulate, and retrosplenial/posterior cingulate cortices. The arrow signifies the hippocampal electrode artifact. t-maps are shown for the first 30 s of seizure activity (10 consecutive fMRI images acquired every 3 s) versus 30 s baseline and are superimposed on high-resolution anatomical images. Slices are shown from anterior to posterior, with approximate coordinates relative to bregma (Englot et al., 2008). Color bars indicate t-values for increases (warm colors) and decreases (cold colors). The display threshold is t = 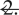 Cg1, anterior cingulate cortex; HC, hippocampus; OFC, orbitofrontal cortex; RSC, retrosplenial/posterior cingulate cortex; Thal, thalamus. Adapted with permission from Englot et al. (2008). Please see online version of this article for full color figure.
Cg1, anterior cingulate cortex; HC, hippocampus; OFC, orbitofrontal cortex; RSC, retrosplenial/posterior cingulate cortex; Thal, thalamus. Adapted with permission from Englot et al. (2008). Please see online version of this article for full color figure.
The mechanistic differences between cortical polyspike propagation during secondarily generalized seizures versus ictal neocortical slow activity during complex-partial seizures are not fully understood. The pathways responsible for secondary generalization of temporal lobe seizures have been previously studied (Bertashius, 1991; Gloor et al., 1993; Spencer et al., 1987; Wilson et al., 1990). Some have suggested that the orbitofrontal cortex plays an important role in cortical seizure propagation (Lieb et al., 1991; Wilson and Engel, 1993), possessing a high degree of functional connectivity with the hippocampus in TLE (Catenoix et al., 2005; Wilson and Engel, 1993). It is thus possible that exploitation of normal circuits connecting the hippocampus and neocortex, which are important for memory storage (Lavenex and Amaral, 2000; Thierry et al., 2000), can contribute to abnormal cortical activity in limbic epilepsy. Perhaps the orbitofrontal cortex acts as gateway for neocortical propagation of epileptic discharges, maintaining a diminished state of function during smaller complex-partial seizures that is overwhelmed by exceedingly intense excitation during secondarily generalized limbic seizures.
Neuroimaging studies of limbic seizures in rats provide preliminary insight into brain regions which may play a role in ictal neocortical slow activity. In addition to hippocampal excitation, we observed fMRI BOLD increases in the medial thalamus during partial limbic seizures (Fig. 5). Given the similarities between ictal slow activity and cortical slow waves that occur during slow-wave sleep, perhaps similar reductions in subcortical arousal systems contribute to this phenomenon (Gervasoni et al., 2004; Jones, 2002; Steriade et al., 1991). We also saw considerable activations in the lateral septum during partial limbic seizures (Fig. 5) which were larger and more consistent than changes in any other region, and peaked relatively early compared to signal fluctuations in other areas (Englot et al., 2008). The septum is an anterior and medial region in the basal forebrain which can be divided into several subregions. The lateral septal nuclei consist mainly of GABAergic inhibitory neurons, while the medial septal nuclei consist mainly of cholinergic neurons (Colom, 2006). The lateral septal nuclei receive their major inputs from the hippocampal formation, and then project heavily to the medial septum–diagonal band of Broca complex, as well as to various hypothalamic areas, the mammillary complex, the ventral tegmental area, and other regions in the basal forebrain (Colom et al., 2006; Irle and Markowitsch, 1986; Mesulam and Mufson, 1984; Risold and Swanson, 1997). GABAergic neurons in the lateral septum are, therefore, poised to produce widespread inhibition in a variety of subcortical structures. The septum is considered a nodal point where ascending non-rhythmic inputs from the brainstem and hypothalamus are converted into rhythmical signals, which are then transmitted to the hippocampus and neocortex (Bland and Colom, 1993; Bland et al., 1994; Vertes and Kocsis, 1997). Thus, the septal nuclei play an important role in regulating normal hippocampal rhythms (Cavazos et al., 1997; Colom, 2006), and may also contribute anti-epileptogenic effects by preventing hyperexcitable limbic states (Colom et al., 2006; Colom and Garrido-Sanabria, 2007). This introduces a few novel questions: does the septum also contribute to the production of rhythmic ictal neocortical slow activity, or does it play an important inhibitory role in limiting limbic seizure propagation?
During preliminary mechanistic studies in the rat, we applied focal electrical stimuli to three regions showing fMRI activation during partial limbic seizures — the dorsal hippocampus, mediodorsal thalamus, and lateral septum (Englot et al., 2009). Our goal was to mimic “seizure” activity in each region individually, without actually producing a seizure that would propagate to the other regions. Interestingly, while stimulations of the hippocampus and medial thalamus did not result in notable behavioral alterations or cortical electrographic effects, stimulating the lateral septum did produce large-amplitude 1–3 Hz slow oscillations in the frontal cortex and behavioral arrest resembling changes during ictal neocortical slow activity (Englot et al., 2009). Furthermore, by preventing lateral septal recruitment in hippocampal seizures via surgical transection of the fornix — the primary white matter tract that permits hippocampal-septal communication — both neocortical slow oscillations and behavioral arrest were abolished during limbic seizures (Englot et al., 2009). These findings allow novel insights into a potential role of septal activity in the production of ictal neocortical slow waves and diminished responsiveness during complex-partial temporal lobe seizures.
The network inhibition hypothesis revisited
As proposed in our network inhibition hypothesis (Fig. 1), it is possible that ictal aberration of normal activity in subcortical arousal systems may contribute to unconsciousness during complex-partial temporal lobe seizures. Some attention has been directed toward the importance of thalamocortical interactions in this phenomenon, but the intense lateral septal involvement during partial limbic seizures in rats has led us to also consider a possible role for the septum in eliciting ictal neocortical slow activity. While the majority of neurons in the lateral septal nuclei are inhibitory cells that release gamma-aminobutyric acid (GABA), no direct projections to orbitofrontal cortex have been found, to our knowledge (Colom et al., 2006; Risold and Swanson, 1997). However, previous animal studies have suggested that the lateral septum does project to other regions involved in normal cortical activation, such as the hypothalamus and basal forebrain (Cirino and Renaud, 1985; Irle and Markowitsch, 1986; Mesulam and Mufson, 1984; Varoqueaux and Poulain, 1999), in addition to its most prominent projections to the medial septum (Colom et al., 2006; Risold and Swanson, 1997). It is thus possible that lateral septal activation, such as during limbic seizures or electrical stimulation, may result in increased inhibition of the basal forebrain or hypothalamic regions, leading secondarily to diminished cortical excitation. First we consider a relatively simple model. For instance, many projections in the basal forebrain release acetylcholine onto the neocortex — a major source of cortical activation in the awake state (Duque et al., 2000). Might lateral septal neurons inhibit these nearby acetylcholinergic projections, leading secondarily to a loss of cortical activation?
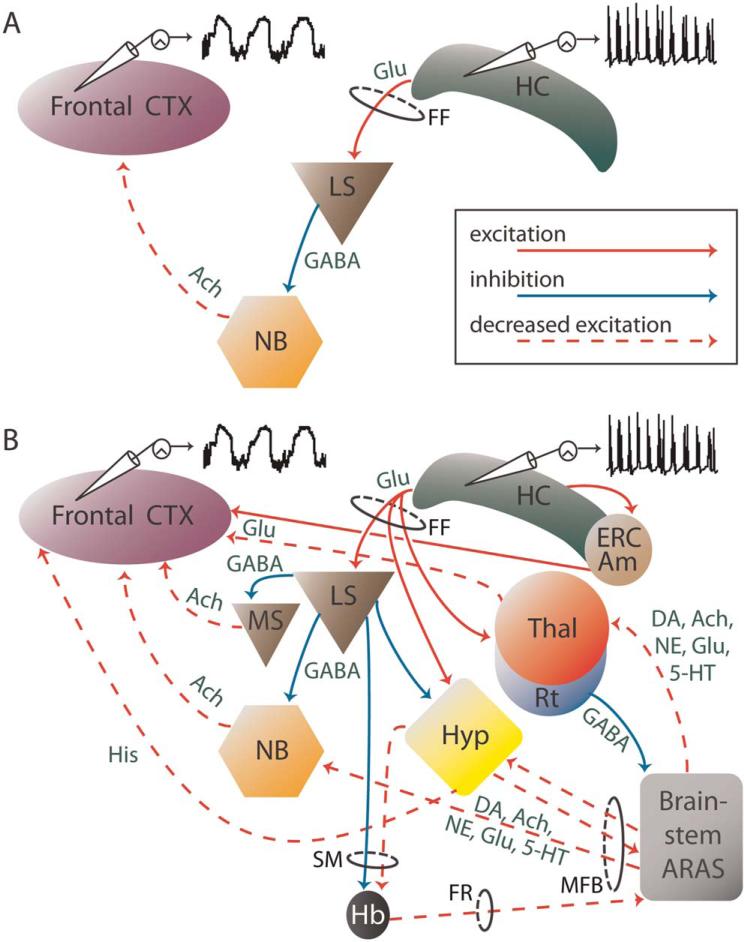
As depicted schematically in Figure 6, human and animal results to date have led us to hypothesize about numerous potential mechanistic contributions to ictal neocortical slow activity during complex-partial temporal lobe seizures. For instance, it is possible that during a partial limbic seizure, abnormal excitation of the hippocampus produces lateral septal activation via glutamatergic projections traveling in the fornix (Fig. 6A). This may in turn lead to inhibition of the nucleus basalis by GABAergic projections from the lateral septum, which then results in diminished acetylcholinergic activation of the frontal cortex by the nucleus basalis. Receiving less excitatory input, the neocortex defaults to an inhibited state, allowing slow oscillatory activity to emerge, and behavior to become diminished. Interestingly, past studies have shown that lesions of the nucleus basalis in the rat produce large-amplitude slow oscillations in the neocortex (Bringmann, 1996; Buzsaki et al., 1988), resembling cortical slow waves seen during sleep and partial limbic seizures. However, aberration of normal thalamocortical interactions are also likely to affect cortical states, as are changes in other brain regions known to be important in arousal which we have not yet explored. Therefore, it is important to broaden our mechanistic hypothesis to encompass other possible network alterations.
Fig. 6.
Schematic diagram illustrating possible network mechanisms of ictal neocortical slow activity. (A) A simplified schematic diagram, showing that excitation of the hippocampus during a seizure may activate the lateral septum via glutamatergic projections in the fornix. This leads in turn to GABAergic inhibition of the nucleus basalis, which then results in diminished acetylcholinergic activation of the frontal cortex by the nucleus basalis, and thus allows the cortex to enter a depressed state associated with ictal neocortical slow activity. (B) A more complex diagram based on (A), adding other network changes that may contribute to ictal neocortical slow oscillations during hippocampal seizure activity. These mechanisms include the inhibitory influence of the lateral septum and the thalamic reticular nucleus onto subcortical structures and the ascending reticular activation system. This in turn leads to decreased excitatory input to the frontal neocortex by various activating structures, such as the thalamus, hypothalamus, and the nucleus basalis — ultimately resulting in cortical depression. Anatomical structures are labeled with black lettering, while neurotransmitters are listed in green. Recordings in the hippocampus and frontal cortex show example LFPs in each region. 5-HT, serotonin; Ach, acetylcholine; AM, amygdala; ARAS, ascending reticular activation system; CTX, cortex; DA, dopamine; ERC, entorhinal cortex; FF, fimbria-fornix; FR, fasciculus retroflexus; GABA, gamma-aminobutyric acid; Glu, glutamate; Hb, habenula; HC, hippocampus; His, histamine; MFB, medial forebrain bundle; LS, lateral septum; MS, medial septum; NB, nucleus basalis; NE, norepinephrine; Rt, thalamic reticular nucleus; SM, stria medullaris, Thal, thalamus. Please see online version of this article for full color figure.
Figure 6B illustrates several additional structures and pathways that may contribute to ictal neocortical slow activity. These potential mechanisms include GABAergic inhibitory influence from regions that may be activated by limbic seizure activity — including the lateral septum and the thalamic reticular nucleus — onto subcortical and brainstem structures which play a role in arousal. For instance, the ascending reticular activating system of the rostral brainstem is comprised of several cell populations important for wakefulness. These include serotonergic neurons in the dorsal raphe (Richerson, 2004), norepinephrine-containing projections from the locus coeruleus (Berridge and Foote, 1996), dopamine release from the pedunculopontine tegmental region and the substantia nigra pars compacta (Dunbar et al., 1992; Dzirasa et al., 2006), acetylcholinergic fibers from the pontomesencephalic tegmentum (Jones, 2008; Woolf and Butcher, 1986), as well as several glutamatergic projections (Jones, 2003). Alterations in these regions during temporal lobe seizures could result from inhibitory signals from the reticular thalamic nucleus (Parent and Steriade, 1984; Steriade et al., 1984), or from diminished excitatory impulses originating from the hypothalamus (Baev et al., 1985; Purves et al., 1992) or habenula (Cuello et al., 1978; Irle et al., 1984) — areas which receive projections from the septal region (Kawaja et al., 1990; Risold and Swanson, 1997).
It is known that projections from the ascending reticular formation convey arousal signals to the cortex primarily through the basal forebrain, thalamus, and hypothalamus (Siegel, 2004). In turn, the hypothalamus provides excitatory input to the neocortex via histaminergic projections in the pre-optic region of the anterior hypothalamus (Lin et al., 1994), and hypocretin/orexin neurons in the posterior hypothalamus (Sakurai, 2005; Saper et al., 2001). The hypothalamus also sends input to brainstem activating regions through the habenula via the stria medullaris and subsequent fasciculus retroflexus (Blander and Wise, 1989; Goto et al., 2005; Semba and Fibiger, 1992). Hence, it is possible that the hypothalamus contributes to the behavioral and neocortical effects of partial limbic seizures, perhaps by providing less cortical or brainstem excitation after becoming inhibited by lateral septal projections (Staiger and Wouterlood, 1990; Varoqueaux and Poulain, 1999), or alternatively through aberration of normal function by direct seizure propagation (Bastlund et al., 2005; Quigg et al., 1999; Silveira et al., 2000).
Another important structure to consider in the mechanism of ictal neocortical slow oscillations is the amygdaloid complex. The amygdala has been shown to play a significant role in epileptiform activity of the temporal lobe (Aroniadou-Anderjaska et al., 2008; Klueva et al., 2003; McIntyre and Gilby, 2008) as well as modulation of arousal signals in both local (Pare and Gaudreau, 1996; Velasco et al., 1989) and distant cortical regions (Dringenberg and Vanderwolf, 1996; Stock et al., 1981). There is some evidence that the amygdala may play a notable role in acetylcholinergic neocortical activation (Dringenberg and Vanderwolf, 1996). Furthermore, given shared connections between the amygdaloid complex and septum via fibers in the stria terminalis and amygdalofugal pathways (Leonard and Scott, 1971), it is important to consider possible effects of convergent signals from septal and hippocampal regions onto the amygdala in modulating neocortical rhythms during limbic seizures. The entorhinal cortex is also a major afferent and efferent pathway to the hippocampus (Chrobak et al., 2000; McIntyre and Gilby, 2008), and could therefore contribute in important ways to spread of ictal activity to both cortical and subcortical structures, and to modulation of neocortical activity.
Finally, the possible role of the medial septum in ictal neocortical slow activity requires further investigation. In addition to the well-known hippocampal connections, the medial septum–diagonal band of Broca complex does have some cholinergic cortical projections, albeit mostly to posterior cortical regions (Gaykema et al., 1990), and previous rat studies have suggested that medial septal lesions may impair performance in attention-related tasks (Brandner and Schenk, 1998).
In summary, a large number of subcortical structures could potentially contribute to behavioral changes during limbic seizures, and further investigations will be crucial as the network underpinnings of ictal neocortical slow activity continue to be unraveled.
Future directions
The human and animal studies of TLE summarized here provide characterization and preliminary insight into the mechanistic underpinnings of impaired consciousness and ictal neocortical slow rhythms during complex-partial temporal lobe seizures. However, much remains unknown about how focal seizure activity in the temporal lobe leads to a loss of consciousness ictally, and additional investigations — including studies in animal models within which mechanistic interventions are feasible — are needed to further elucidate this problem.
It will be beneficial in future studies to perform invasive measurements of neuronal activity in awake-behaving animals, during which behavior can be studied simultaneously. One mechanistic possibility we have discussed is that lateral septal involvement in partial seizures may result in inhibition of the acetylcholinergic activating system in the nucleus basalis via GABAergic septal projections, leading secondarily to neocortical depression. Further experiments addressing this hypothesis should include electrophysiological recordings from the nucleus basalis during limbic seizures to determine if the firing of acetylcholinergic neurons is indeed suppressed. We should also determine whether stimulation of the nucleus basalis prevents ictal neocortical slow rhythms during seizures by diminishing inhibitory influence from the lateral septum onto the basal forebrain. Direct measurements of changes in neurotransmitter levels in the neocortex during partial seizures represent another key direction, as they may provide further insight into the neurophysiological underpinnings of ictal cortical changes. Utilizing microdialysis or recent voltammetric biosensor techniques (Parikh et al., 2007; Rutherford et al., 2007) may be useful in detecting cortical neurotransmitter fluctuations during ictal neocortical slow activity. Moreover, surgical and pharmacological interventions geared toward preventing distal effects of complex-partial seizures in animals are likely to be fruitful. While in preliminary rat experiments, preventing lateral septal recruitment during electrically stimulated limbic seizures via fornix transection diminished both the electrographic and behavioral correlates of ictal neocortical slow oscillations, the utilization of more localized, reversible methods to prevent subcortical discharges during seizures should be considered. Also, in vivo intracellular recordings of pyramidal neurons in neocortical regions affected by ictal slow waves might permit supplementary insight into whether ictal neocortical slow activity is associated with diminished excitatory postsynaptic potentials, which would suggest decreased levels of excitatory neurotransmitters, or increased inhibitory postsynaptic potentials, implying elevated inhibitory neurotransmission in the cortex.
Animal fMRI studies can produce high spatial resolution images of structures affected by temporal lobe seizures, but potentially important regions have not yet been visualized ictally. Future scans should examine in particular areas important for neocortical activation that have also been shown to be involved in limbic seizures, such as the hypothalamus, amygdala, and upper brainstem, which are difficult to image with surface coils and will therefore require more innovative technical advances. Additional exploration should include electrophysiological recordings, stimulations, and inactivations of these structures during partial limbic seizures.
Although modeling consciousness in animal studies bears obvious limitations, complex-partial seizures in humans interfere with the most basic level of consciousness — the awake, alert state — which we believe can be reasonably modeled in basic research. For example, the behavioral correlates of ictal neocortical slow activity in rats include decreased locomotion and behavioral arrest. While quantitative measurements of performance using controlled behavioral tasks during limbic seizures have not yet been pursued, these will be important to include in upcoming investigations. For instance, simple response-time and Pavlovian conditioning tasks can be utilized to test both attention (responding to a stimulus) and learning (conditioning of a stimulus) during ictal neocortical slow activity in rats.
To translate this work into the human arena, additional human studies will be necessary as well. Further intracranial studies prospectively evaluating human behavior with both widespread cortical (Blumenfeld et al., 2004b) and subcortical (Arthuis et al., 2009; Guye et al., 2006) electro-physiological measurements will be necessary to more fully identify the specific anatomical regions and activity patterns associated with behavioral impairment. Prospective, standardized, and quantitative methods for patient assessment, including computerized and manually administered testing batteries are needed, which should be continuously available to enhance the quality and quantity of behavioral data obtained in conjunction with electrophysiological studies. Treatment of impaired consciousness in epilepsy will continue to be aimed first and foremost at preventing seizures. However, in some patients, stopping all seizures is not feasible, so treatments that can at least prevent impaired consciousness during seizures will be beneficial. Thus, ultimately, therapeutic interventions such as disconnection procedures, neurostimulation, or medication trials may soon become possible, with the goal of preventing impaired consciousness during partial seizures.
Conclusions: the consciousness system and TLE
In the mid-20th century, Wilder Penfield and Herbert Jasper hypothesized that the brainstem and diencephalon play critical roles in integrating brain activity across both cerebral hemispheres (Jasper, 1991; Penfield, 1958). It was observed that most epileptic patients suffered little to no impairment of consciousness after wide resection of cerebral cortical structures or the corpus callosum, although applying pressure to the brainstem resulted in immediate and reversible loss of consciousness (Penfield, 1958). Penfield and Jasper deduced that the neuroanatomical basis of consciousness involved more than the cerebral cortex, already known to be the seat of various cognitive processes (Jasper, 1991). They argued that the “indisputable substratum” of consciousness was rooted in diencephalic and upper brainstem regions. Jasper (1964) also posited that activation of only the amygdala and hippocampus was insufficient to induce automatisms, and the impairment of consciousness required the involvement of widespread subcortical structures. Furthermore, neurochemical studies suggested the presence of diffuse ascending modulatory systems that project from the diencephalon and brainstem that interact with, but are anatomically separate from, the sensory and motor systems (Jasper, 1991). Work summarized in this review, examining both human TLE patients and animal models, suggests that functional aberration of these same networks may also underlie ictal neocortical slow activity and loss of consciousness during complex-partial temporal lobe seizures. As we have discussed, factors which may increase the likelihood of network aberration during partial temporal lobe seizures include: (i) lateralization of discharges to the dominant hemisphere, (ii) bilateral temporal lobe involvement, (iii) increased seizure length and severity, and (iv) dysfunction of subcortical structures important for arousal.
Although the full mechanisms of ictal neocortical slow activity in TLE remain unknown, progress to date allows us to expand upon the ideas of Jasper and Penfield and define a “consciousness system” within which dysfunction during epileptic seizures results in temporary loss of the awake, alert state. We propose that the consciousness system includes (i) the upper brainstem, (ii) subcortical structures such as the medial thalamus and basal forebrain region (possibly including the septal nuclei), (iii) anterior and posterior interhemispheric regions (cingulate, medial frontal cortex, and precuneus/retrosplenial cortex), and (iv) lateral frontal and parietal association cortices (Blumenfeld, 2009). It is likely that when seizure activity significantly disrupts normal activity in these areas, consciousness is adversely affected secondary to either cortical seizure propagation, as in primary-generalized, or secondarily generalized seizures, or neocortical deactivation, as in complex-partial temporal lobe seizures. In contrast, epileptic events that do not involve regions in the consciousness system, such as simple-partial temporal lobe seizures, do not cause a loss of consciousness. While significant questions remain regarding the mechanisms of impaired consciousness during partial temporal lobe seizures, recent advances in our understanding of ictal neocortical slow activity and related network effects will help guide future studies geared toward preventing the adverse neocortical effects of complex-partial seizures.
Acknowledgments
This work was supported by NIH R01 NS049307 (HB) and F30 NS59074 (DJE), a Donaghue Foundation Investigator Award (HB), and by the Betsy and Jonathan Blattmachr family.
References
- Alarcon G, Elwes RDC, Polkey CE, Binnie CD. Ictal oroalimentary automatisms with preserved consciousness: Implications for the pathophysiology of automatisms and relevance to the international classification of seizures. Epilepsia. 1998;39:1119–1122. doi: 10.1111/j.1528-1157.1998.tb01300.x. [DOI] [PubMed] [Google Scholar]
- Albert ML, Silverberg R, Reches A, Berman M. Cerebral dominance for consciousness. Archives of Neurology. 1976;33:453–454. doi: 10.1001/archneur.1976.00500060059013. [DOI] [PubMed] [Google Scholar]
- Andersen AR, Waldemar G, Dam M, Fuglsang-Frederiksen A, Herning M, Kruse-Larsen C. SPECT in the presurgical evaluation of patients with temporal lobe epilepsy — A preliminary report. Acta Neurochirurgica Supplementum. 1990;50:80–83. doi: 10.1007/978-3-7091-9104-0_16. [DOI] [PubMed] [Google Scholar]
- Angelucci A, Levitt JB, Lund JS. Anatomical origins of the classical receptive field and modulatory surround field of single neurons in macaque visual cortical area V1. Progress in Brain Research. 2002;136:373–388. doi: 10.1016/s0079-6123(02)36031-x. [DOI] [PubMed] [Google Scholar]
- Ardila A. Partial cognitive seizures. Neuropsychiatry, Neuropsychology, and Behavioral Neurology. 1990;2:175–182. [Google Scholar]
- Aroniadou-Anderjaska V, Fritsch B, Qashu F, Braga MF. Pathology and pathophysiology of the amygdala in epileptogenesis and epilepsy. Epilepsy Research. 2008;78:102–116. doi: 10.1016/j.eplepsyres.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthuis M, Valton L, Regis J, Chauvel P, Wendling F, Naccache L, et al. Impaired consciousness during temporal lobe seizures is related to increased long-distance cortical-subcortical synchronization. Brain. 2009;132:2091–2101. doi: 10.1093/brain/awp086. [DOI] [PubMed] [Google Scholar]
- Avoli M, Gloor P. Interaction of cortex and thalamus in spike and wave discharges of feline generalized penicillin epilepsy. Experimental Neurology. 1982;76:196–217. doi: 10.1016/0014-4886(82)90112-1. [DOI] [PubMed] [Google Scholar]
- Babb TL, Brown WJ. Pathological findings in epilepsy. In: Engel J Jr., editor. Surgical treatments of the epilepsies. Raven Press; New York: 1987. pp. 511–540. [Google Scholar]
- Baev KV, Berezovskii VK, Kebkalo TG, Savos'kina LA. Projections of neurons of the hypothalamic locomotor region to brain stem and spinal cord structures in the cat. Neirofiziologiia. 1985;17:817–823. [PubMed] [Google Scholar]
- Bancaud J, Brunet-Bourgin F, Chauvel P, Halgren E. Anatomical origin of deja vu and vivid ‘memories’ in human temporal lobe epilepsy. Brain. 1994;117:71–90. doi: 10.1093/brain/117.1.71. [DOI] [PubMed] [Google Scholar]
- Bastlund JF, Jennum P, Mohapel P, Penschuck S, Watson WP. Spontaneous epileptic rats show changes in sleep architecture and hypothalamic pathology. Epilepsia. 2005;46:934–938. doi: 10.1111/j.1528-1167.2005.63204.x. [DOI] [PubMed] [Google Scholar]
- Bauer J. Interactions between hormones and epilepsy in female patients. Epilepsia. 2001;42(Suppl. 3):20–22. doi: 10.1046/j.1528-1157.2001.042suppl.3020.x. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Foote SL. Enhancement of behavioral and electroencephalographic indices of waking following stimulation of noradrenergic beta-receptors within the medial septal region of the basal forebrain. Journal of Neuroscience. 1996;16:6999–7009. doi: 10.1523/JNEUROSCI.16-21-06999.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertashius KM. Propagation of human complex-partial seizures: A correlation analysis. Electroencephalography and Clinical Neurophysiology. 1991;78:333–340. doi: 10.1016/0013-4694(91)90095-l. [DOI] [PubMed] [Google Scholar]
- Bertram EH, Mangan PS, Zhang D, Scott CA, Williamson JM. The midline thalamus: Alterations and a potential role in limbic epilepsy. Epilepsia. 2001;42:967–978. doi: 10.1046/j.1528-1157.2001.042008967.x. [DOI] [PubMed] [Google Scholar]
- Bertram EH, Zhang D, Williamson JM. Multiple roles of midline dorsal thalamic nuclei in induction and spread of limbic seizures. Epilepsia. 2008;49:256–268. doi: 10.1111/j.1528-1167.2007.01408.x. [DOI] [PubMed] [Google Scholar]
- Bland BH, Colom LV. Extrinsic and intrinsic properties underlying oscillation and synchrony in limbic cortex. Progress in Neurobiology. 1993;41:157–208. doi: 10.1016/0301-0082(93)90007-f. [DOI] [PubMed] [Google Scholar]
- Bland BH, Oddie SD, Colom LV, Vertes RP. Extrinsic modulation of medial septal cell discharges by the ascending brainstem hippocampal synchronizing pathway. Hippocampus. 1994;4:649–660. doi: 10.1002/hipo.450040604. [DOI] [PubMed] [Google Scholar]
- Blander A, Wise RA. Anatomical mapping of brain stimulation reward sites in the anterior hypothalamic area: Special attention to the stria medullaris. Brain Research. 1989;483:12–16. doi: 10.1016/0006-8993(89)90029-2. [DOI] [PubMed] [Google Scholar]
- Blum DE, Eskola J, Bortz JJ, Fisher RS. Patient awareness of seizures. Neurology. 1996;47:260–264. doi: 10.1212/wnl.47.1.260. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H. Neuroanatomy through clinical cases. Sinauer Associates Publishers, Inc.; Sunderland, MA: 2002. [Google Scholar]
- Blumenfeld H. Consciousness and epilepsy: Why are patients with absence seizures absent? Progress in Brain Research. 2005;150:271–286. doi: 10.1016/S0079-6123(05)50020-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld H. Epilepsy and consciousness. In: Laureys S, Tononi G, editors. The neurology of consciousness: Cognitive neuroscience and neuropathology. Elsevier; New York, NY: 2009. [Google Scholar]
- Blumenfeld H, McCormick DA. Corticothalamic inputs control the pattern of activity generated in thalamocortical networks. The Journal of Neuroscience. 2000;20:5153–5162. doi: 10.1523/JNEUROSCI.20-13-05153.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld H, McNally KA, Vanderhill SD, Paige AL, Chung R, Davis K, et al. Positive and negative network correlations in temporal lobe epilepsy. Cerebral Cortex. 2004a;14:892–902. doi: 10.1093/cercor/bhh048. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H, Rivera M, McNally KA, Davis K, Spencer DD, Spencer SS. Ictal neocortical slowing in temporal lobe epilepsy. Neurology. 2004b;63:1015–1021. doi: 10.1212/01.wnl.0000141086.91077.cd. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H, Rivera M, Vasquez JG, Shah A, Ismail D, Enev M, et al. Neocortical and thalamic spread of amygdala kindled seizures. Epilepsia. 2007;48:254–262. doi: 10.1111/j.1528-1167.2006.00934.x. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H, Taylor J. Why do seizures cause loss of consciousness? The Neuroscientist. 2003;9:301–310. doi: 10.1177/1073858403255624. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H, Varghese GI, Purcaro MJ, Motelow JE, Enev M, McNally KA, et al. Cortical and subcortical networks in human secondarily generalized tonic-clonic seizures. Brain. 2009;132:999–1012. doi: 10.1093/brain/awp028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilha L, Rorden C, Appenzeller S, Coan AC, Cendes F, Li LM. Gray matter atrophy associated with duration of temporal lobe epilepsy. NeuroImage. 2006;32:1070–1079. doi: 10.1016/j.neuroimage.2006.05.038. [DOI] [PubMed] [Google Scholar]
- Bonte FJ, Devous MD, Sr., Stokely EM, Homan RW. Single-photon tomographic determination of regional cerebral blood flow in epilepsy. American Journal of Neuroradiology. 1983;4:544–546. [PMC free article] [PubMed] [Google Scholar]
- Boylan LS. Peri-ictal behavioral and cognitive changes. Epilepsy and Behavior. 2002;3:16–26. doi: 10.1006/ebeh.2001.0305. [DOI] [PubMed] [Google Scholar]
- Brandner C, Schenk F. Septal lesions impair the acquisition of a cued place navigation task: Attentional or memory deficit? Neurobiology of Learning and Memory. 1998;69:106–125. doi: 10.1006/nlme.1997.3814. [DOI] [PubMed] [Google Scholar]
- Bringmann A. Behaviour-related effects of nicotine on slow EEG waves in basal nucleus-lesioned rats. Naunyn-Schmiedeberg's Archives of Pharmacology. 1996;353:168–174. doi: 10.1007/BF00168754. [DOI] [PubMed] [Google Scholar]
- Brumberg JC, Pinto DJ, Simons DJ. Spatial gradients and inhibitory summation in the rat whisker barrel system. Journal of Neurophysiology. 1996;76:130–140. doi: 10.1152/jn.1996.76.1.130. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Bickford RG, Ponomareff G, Thal LJ, Mandel R, Gage FH. Nucleus basalis and thalamic control of neocortical activity in the freely moving rat. The Journal of Neuroscience. 1988;8:4007–4026. doi: 10.1523/JNEUROSCI.08-11-04007.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catenoix H, Magnin M, Guenot M, Isnard J, Mauguiere F, Ryvlin P. Hippocampal-orbitofrontal connectivity in human: An electrical stimulation study. Clinical Neurophysiology. 2005;116:1779–1784. doi: 10.1016/j.clinph.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Cavazos JE, Wang CJ, Sitoh YY, Ng SE, Tien RD. Anatomy and pathology of the septal region. Neuroimaging Clinics of North America. 1997;7:67–78. [PubMed] [Google Scholar]
- Chang CP, Yen DJ, Yu SM, Liu RS, Chang HF, Hsieh HJ, et al. Unilateral thalamic hypometabolism in patients with temporal lobe epilepsy. Journal of the Formosan Medical Association. 2008;107:567–571. doi: 10.1016/S0929-6646(08)60170-9. [DOI] [PubMed] [Google Scholar]
- Chang DJ, Zubal IG, Gottschalk C, Necochea A, Stokking R, Studholme C, et al. Comparison of statistical parametric mapping and SPECT difference imaging in patients with temporal lobe epilepsy. Epilepsia. 2002;43:68–74. doi: 10.1046/j.1528-1157.2002.21601.x. [DOI] [PubMed] [Google Scholar]
- Chrobak JJ, Lorincz A, Buzsaki G. Physiological patterns in the hippocampo-entorhinal cortex system. Hippocampus. 2000;10:457–465. doi: 10.1002/1098-1063(2000)10:4<457::AID-HIPO12>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Cirino M, Renaud LP. Influence of lateral septum and amygdala stimulation on the excitability of hypothalamic supraoptic neurons. An electrophysiological study in the rat. Brain Research. 1985;326:357–361. doi: 10.1016/0006-8993(85)90045-9. [DOI] [PubMed] [Google Scholar]
- Collins RC. Use of cortical circuits during focal penicillin seizures: An autoradiographic study with [14C]deoxyglucose. Brain Research. 1978;150:487–501. doi: 10.1016/0006-8993(78)90815-6. [DOI] [PubMed] [Google Scholar]
- Colom LV. Septal networks: Relevance to theta rhythm, epilepsy and Alzheimer's disease. Journal of Neurochemistry. 2006;96:609–623. doi: 10.1111/j.1471-4159.2005.03630.x. [DOI] [PubMed] [Google Scholar]
- Colom LV, Garcia-Hernandez A, Castaneda MT, Perez-Cordova MG, Garrido-Sanabria ER. Septo-hippocampal networks in chronically epileptic rats: Potential antiepileptic effects of theta rhythm generation. Journal of Neurophysiology. 2006;95:3645–3653. doi: 10.1152/jn.00040.2006. [DOI] [PubMed] [Google Scholar]
- Colom LV, Garrido-Sanabria E. Modulation of normal and altered hippocampal excitability states by septal networks. Journal of Neuroscience Research. 2007;85:2839–2843. doi: 10.1002/jnr.21276. [DOI] [PubMed] [Google Scholar]
- Cuello AC, Emson PC, Paxinos G, Jessell T. Substance P containing and cholinergic projections from the habenula. Brain Research. 1978;149:413–429. doi: 10.1016/0006-8993(78)90484-5. [DOI] [PubMed] [Google Scholar]
- Cowan RL, Wilson CJ. Spontaneous firing patterns and axonal projections of single corticostriatal neurons in the rat medial agranular cortex. Journal of Neurophysiology. 1994;71:17–32. doi: 10.1152/jn.1994.71.1.17. [DOI] [PubMed] [Google Scholar]
- de Lanerolle NC, Lee TS. New facets of the neuropathology and molecular profile of human temporal lobe epilepsy. Epilepsy and Behavior. 2005;7:190–203. doi: 10.1016/j.yebeh.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Derdikman D, Hildesheim R, Ahissar E, Arieli A, Grinvald A. Imaging spatiotemporal dynamics of surround inhibition in the barrels somatosensory cortex. Journal of Neuroscience. 2003;23:3100–3105. doi: 10.1523/JNEUROSCI.23-08-03100.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O. Diagnosis and treatment of temporal lobe epilepsy. Reviews in Neurological Diseases. 2004;1:2–9. [PubMed] [Google Scholar]
- Diehl B, LaPresto E, Najm I, Raja S, Rona S, Babb T, et al. Neocortical temporal FDG-PET hypometabolism correlates with temporal lobe atrophy in hippocampal sclerosis associated with microscopic cortical dysplasia. Epilepsia. 2003;44:559–564. doi: 10.1046/j.1528-1157.2003.36202.x. [DOI] [PubMed] [Google Scholar]
- Drazkowski J. An overview of epilepsy and driving. Epilepsia. 2007;48(Suppl. 9):10–12. doi: 10.1111/j.1528-1167.2007.01392.x. [DOI] [PubMed] [Google Scholar]
- Dringenberg HC, Vanderwolf CH. Cholinergic activation of the electrocorticogram: An amygdaloid activating system. Experimental Brain Research. 1996;108:285–296. doi: 10.1007/BF00228101. [DOI] [PubMed] [Google Scholar]
- Dunbar JS, Hitchcock K, Latimer M, Rugg EL, Ward N, Winn P. Excitotoxic lesions of the pedunculopontine tegmental nucleus of the rat. II. Examination of eating and drinking, rotation, and reaching and grasping following unilateral ibotenate or quinolinate lesions. Brain Research. 1992;589:194–206. doi: 10.1016/0006-8993(92)91278-m. [DOI] [PubMed] [Google Scholar]
- Duncan R, Patterson J, Hadley DM, Wyper DJ, McGeorge AP, Bone I. Tc99m HMPAO single photon emission computed tomography in temporal lobe epilepsy. Acta Neurologica Scandinavica. 1990;81:287–293. doi: 10.1111/j.1600-0404.1990.tb01557.x. [DOI] [PubMed] [Google Scholar]
- Duque A, Balatoni B, Detari L, Zaborszky L. EEG correlation of the discharge properties of identified neurons in the basal forebrain. Journal of Neurophysiology. 2000;84:1627–1635. doi: 10.1152/jn.2000.84.3.1627. [DOI] [PubMed] [Google Scholar]
- Dzirasa K, Ribeiro S, Costa R, Santos LM, Lin SC, Grosmark A, et al. Dopaminergic control of sleep-wake states. The Journal of Neuroscience. 2006;26:10577–10589. doi: 10.1523/JNEUROSCI.1767-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner A, Dinner DS, Noachtar S, Luders H. Automatisms with preserved responsiveness: A lateralizing sign in psychomotor seizures. Neurology. 1995;45:61–64. doi: 10.1212/wnl.45.1.61. [DOI] [PubMed] [Google Scholar]
- Eisenschenk S, Gilmore RL, Cibula JE, Roper SN. Lateralization of temporal lobe foci: Depth versus subdural electrodes. Clinical Neurophysiology. 2001;112:836–844. doi: 10.1016/s1388-2457(01)00517-x. [DOI] [PubMed] [Google Scholar]
- Engel J. Outcome with respect to epileptic seizures. In: Engel J, editor. Surgical treatment of the epilepsies. Raven Press; New York, NY: 1987. pp. 553–571. [Google Scholar]
- Engel J, Williamson PD. Limbic seizures. In: Engel J, Pedley TA, editors. Epilepsy: A comprehensive textbook. Lippincott Williams & Wilkins; Philadelphia, PA: 2007. pp. 541–552. [Google Scholar]
- Engel J, Williamson PD, Wieser HG. Mesial temporal lobe epilepsy with hippocampal sclerosis. In: Engel J, Pedley TA, editors. Epilepsy: A comprehensive textbook. Lippincott Williams & Wilkins; Philadelphia, PA: 2007. pp. 2479–2486. [Google Scholar]
- Englot DJ, Blumenfeld H. Functional MRI in basic epilepsy research. In: Schwartzkroin P, editor. Encyclopedia of basic epilepsy research. Elsevier; London, UK: 2009. [Google Scholar]
- Englot DJ, Mishra AM, Mansuripur PK, Herman P, Hyder F, Blumenfeld H. Remote effects of focal hippocampal seizures on the rat neocortex. The Journal of Neuroscience. 2008;28:9066–9081. doi: 10.1523/JNEUROSCI.2014-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englot DJ, Modi B, Mishra AM, DeSalvo M, Hyder F, Blumenfeld H. Cortical deactivation induced by subcortical network dysfunction in limbic seizures. 2009. In review. [DOI] [PMC free article] [PubMed]
- Escueta AV, Kunze U, Waddell G, Boxley J, Nadel A. Lapse of consciousness and automatisms in temporal lobe epilepsy: A videotape analysis. Neurology. 1977;27:144–155. doi: 10.1212/wnl.27.2.144. [DOI] [PubMed] [Google Scholar]
- Franaszczuk PJ, Bergey GK, Kaminski MJ. Analysis of mesial temporal seizure onset and propagation using the directed transfer function method. Electroencephalography and Clinical Neurophysiology. 1994;91:413–427. doi: 10.1016/0013-4694(94)90163-5. [DOI] [PubMed] [Google Scholar]
- Franczek S, Demakis GJ, Pennell EB, DeBose C, Gilmore RL. Revisited: Cerebral dominance for consciousness. Electroencephalography and Clinical Neurophysiology. 1997;102:24P–25P. [Google Scholar]
- Fried I, Spencer DD, Spencer SS. The anatomy of epileptic auras: Focal pathology and surgical outcome. Journal of Neurosurgery. 1995;83:60–66. doi: 10.3171/jns.1995.83.1.0060. [DOI] [PubMed] [Google Scholar]
- Gabr M, Luders H, Dinner D, Morris H, Wyllie E. Speech manifestations in lateralization of temporal lobe seizures. Annals of Neurology. 1989;25:82–87. doi: 10.1002/ana.410250113. [DOI] [PubMed] [Google Scholar]
- Gaykema RP, Luiten PG, Nyakas C, Traber J. Cortical projection patterns of the medial septum-diagonal band complex. The Journal of Comparative Neurology. 1990;293:103–124. doi: 10.1002/cne.902930109. [DOI] [PubMed] [Google Scholar]
- Gervasoni D, Lin SC, Ribeiro S, Soares ES, Pantoja J, Nicolelis MA. Global forebrain dynamics predict rat behavioral states and their transitions. The Journal of Neuroscience. 2004;24:11137–11147. doi: 10.1523/JNEUROSCI.3524-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloor P. Consciousness as a neurological concept in epileptology: A critical review. Epilepsia. 1986;27(Suppl. 2):S14–S26. doi: 10.1111/j.1528-1157.1986.tb05737.x. [DOI] [PubMed] [Google Scholar]
- Gloor P. Mesial temporal sclerosis: Historical background and overview from a modern perspective. In: Luders H, editor. Epilepsy surgery. Vol. 77. Raven Press; New York, NY: 1991. pp. 689–702. [Google Scholar]
- Gloor P, Olivier A, Ives J. Loss of consciousness in temporal lobe epilepsy: Observations obtained with stereotaxic depth electrode recordings and stimulations. In: Canger R, Angeleri F, Penry JK, editors. Advances in epileptology: The XIth Epilepsy International Symposium. Raven Press; New York, NY: 1980. pp. 349–353. [Google Scholar]
- Gloor P, Salanova V, Olivier A, Quesney LF. The human dorsal hippocampal commissure. An anatomically identifiable and functional pathway. Brain. 1993;116:1249–1273. doi: 10.1093/brain/116.5.1249. [DOI] [PubMed] [Google Scholar]
- Gong G, Concha L, Beaulieu C, Gross DW. Thalamic diffusion and volumetry in temporal lobe epilepsy with and without mesial temporal sclerosis. Epilepsy Research. 2008;80:184–193. doi: 10.1016/j.eplepsyres.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Goto M, Canteras NS, Burns G, Swanson LW. Projections from the subfornical region of the lateral hypothalamic area. The Journal of Comparative Neurology. 2005;493:412–438. doi: 10.1002/cne.20764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guye M, Regis J, Tamura M, Wendling F, McGonigal A, Chauvel P, et al. The role of corticothalamic coupling in human temporal lobe epilepsy. Brain. 2006;129:1917–1928. doi: 10.1093/brain/awl151. [DOI] [PubMed] [Google Scholar]
- Haider B, Duque A, Hasenstaub AR, McCormick DA. Neocortical network activity in vivo is generated through a dynamic balance of excitation and inhibition. Journal of Neuroscience. 2006;26:4535–4545. doi: 10.1523/JNEUROSCI.5297-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstaedter C, Kockelmann E. Cognitive outcomes in patients with chronic temporal lobe epilepsy. Epilepsia. 2006;47(Suppl. 2):96–98. doi: 10.1111/j.1528-1167.2006.00702.x. [DOI] [PubMed] [Google Scholar]
- Hermann BP, Seidenberg M, Schoenfeld J, Davies K. Neuropsychological characteristics of the syndrome of mesial temporal lobe epilepsy. Archives of Neurology. 1997;54:369–376. doi: 10.1001/archneur.1997.00550160019010. [DOI] [PubMed] [Google Scholar]
- Hetherington HP, Kuzniecky RI, Vives K, Devinsky O, Pacia S, Luciano D, et al. A subcortical network of dysfunction in TLE measured by magnetic resonance spectroscopy. Neurology. 2007;69:2256–2265. doi: 10.1212/01.wnl.0000286945.21270.6d. [DOI] [PubMed] [Google Scholar]
- Hoffmann JM, Elger CE, Kleefuss-Lie AA. Lateralizing value of behavioral arrest in patients with temporal lobe epilepsy. Epilepsy and Behavior. 2008;13:634–636. doi: 10.1016/j.yebeh.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Hogan RE, Kaiboriboon K, Bertrand ME, Rao V, Acharya J. Composite SISCOM perfusion patterns in right and left temporal seizures. Archives of Neurology. 2006;63:1419–1426. doi: 10.1001/archneur.63.10.1419. [DOI] [PubMed] [Google Scholar]
- ILAE Proposal for revised clinical and electroencephalographic classification of epileptic seizures. From the Commission on Classification and Terminology of the International League Against Epilepsy. Epilepsia. 1981;22:489–501. doi: 10.1111/j.1528-1157.1981.tb06159.x. [DOI] [PubMed] [Google Scholar]
- ILAE Proposal for revised classification of epilepsies and epileptic syndromes. Commission on Classification and Terminology of the International League Against Epilepsy. Epilepsia. 1989;30:389–399. doi: 10.1111/j.1528-1157.1989.tb05316.x. [DOI] [PubMed] [Google Scholar]
- Inoue Y, Mihara T. Awareness and responsiveness during partial seizures. Epilepsia. 1998;39:7–10. doi: 10.1111/j.1528-1157.1998.tb05142.x. [DOI] [PubMed] [Google Scholar]
- Irle E, Markowitsch HJ. Afferent connections of the substantia innominata/basal nucleus of Meynert in carnivores and primates. Journal für Hirnforschung. 1986;27:343–367. [PubMed] [Google Scholar]
- Irle E, Sarter M, Guldin WO, Markowitsch HJ. Afferents to the ventral tegmental nucleus of Gudden in the mouse, rat, and cat. The Journal of Comparative Neurology. 1984;228:509–541. doi: 10.1002/cne.902280406. [DOI] [PubMed] [Google Scholar]
- Jackson J, Colman W. Case of epilepsy with tasting movements and ‘dreamy state’ — Very small patch of softening in the left uncinate gyrus. Brain. 1898;21:580–590. [Google Scholar]
- Jackson JH, Taylor J, Holmes G, Walshe F. Selected writings of John Hughlings Jackson. Hodder and Stoughton; London: 1931. [Google Scholar]
- Jacoby A, Snape D, Baker GA. Epilepsy and social identity: The stigma of a chronic neurological disorder. Lancet Neurology. 2005;4:171–178. doi: 10.1016/S1474-4422(05)01014-8. [DOI] [PubMed] [Google Scholar]
- Jasper HH. Some physiological mechanisms involved in epileptic automatisms. Epilepsia. 1964;5:1–20. doi: 10.1111/j.1528-1157.1964.tb04341.x. [DOI] [PubMed] [Google Scholar]
- Jasper HH. Current evaluation of the concepts of centrencephalic and cortico-reticular seizures. Electroencephalography and Clinical Neurophysiology. 1991;78:2–11. doi: 10.1016/0013-4694(91)90012-s. [DOI] [PubMed] [Google Scholar]
- Jones BE. Arousal systems. Frontiers in Bioscience. 2003;8:s438–s451. doi: 10.2741/1074. [DOI] [PubMed] [Google Scholar]
- Jones BE. Modulation of cortical activation and behavioral arousal by cholinergic and orexinergic systems. Annals of the New York Academy of Sciences. 2008;1129:26–34. doi: 10.1196/annals.1417.026. [DOI] [PubMed] [Google Scholar]
- Jones EG. Thalamic circuitry and thalamocortical synchrony. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2002;357:1659–1673. doi: 10.1098/rstb.2002.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaja MD, Flumerfelt BA, Hrycyshyn AW. Synaptic organization of septal projections in the rat medial habenula: A wheat germ agglutinin-horseradish peroxidase and immunohistochemical study. Synapse. 1990;6:45–54. doi: 10.1002/syn.890060106. [DOI] [PubMed] [Google Scholar]
- Kim SH, Zubal IG, Blumenfeld H. Epilepsy localization by ictal and interictal SPECT. In: Van Heertum R, Ichise M, Tikofsky RS, editors. Functional cerebral SPECT and PET imaging. Lippincott Williams & Wilkins; Philadelphia, PA: 2009. [Google Scholar]
- Klueva J, Munsch T, Albrecht D, Pape HC. Synaptic and non-synaptic mechanisms of amygdala recruitment into temporolimbic epileptiform activities. European Journal of Neuroscience. 2003;18:2779–2791. doi: 10.1111/j.1460-9568.2003.02984.x. [DOI] [PubMed] [Google Scholar]
- Kobau R, Zahran H, Thurman DJ, Zack MM, Henry TR, Schachter SC, et al. Epilepsy surveillance among adults — 19 states, Behavioral Risk Factor Surveillance System, 2005. MMWR Surveillance Summaries. 2008;57:1–20. [PubMed] [Google Scholar]
- Labate A, Cerasa A, Gambardella A, Aguglia U, Quattrone A. Hippocampal and thalamic atrophy in mild temporal lobe epilepsy: A VBM study. Neurology. 2008;71:1094–1101. doi: 10.1212/01.wnl.0000326898.05099.04. [DOI] [PubMed] [Google Scholar]
- Laurent A, Arzimanoglou A. Cognitive impairments in children with nonidiopathic temporal lobe epilepsy. Epilepsia. 2006;47(Suppl. 2):99–102. doi: 10.1111/j.1528-1167.2006.00703.x. [DOI] [PubMed] [Google Scholar]
- Lavenex P, Amaral DG. Hippocampal-neocortical interaction: A hierarchy of associativity. Hippocampus. 2000;10:420–430. doi: 10.1002/1098-1063(2000)10:4<420::AID-HIPO8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Lee KH, Meador KJ, Park YD, King DW, Murro AM, Pillai JJ, et al. Pathophysiology of altered consciousness during seizures: Subtraction SPECT study. Neurology. 2002;59:841–846. doi: 10.1212/wnl.59.6.841. [DOI] [PubMed] [Google Scholar]
- Leonard CM, Scott JW. Origin and distribution of the amygdalofugal pathways in the rat: An experimental neuroanatomical study. Journal of Comparative Neurology. 1971;141:313–329. doi: 10.1002/cne.901410304. [DOI] [PubMed] [Google Scholar]
- Leung LS, Ma J, McLachlan RS. Behaviors induced or disrupted by complex partial seizures. Neuroscience and Biobehavioral Reviews. 2000;24:763–775. doi: 10.1016/s0149-7634(00)00035-x. [DOI] [PubMed] [Google Scholar]
- Levav M, Mirsky AF, Herault J, Xiong L, Amir N, Andermann E. Familial association of neuropsychological traits in patients with generalized and partial seizure disorders. Journal of Clinical and Experimental Neuropsychology. 2002;24:311–326. doi: 10.1076/jcen.24.3.311.985. [DOI] [PubMed] [Google Scholar]
- Lieb JP, Dasheiff RB, Engel J., Jr. Role of the frontal lobes in the propagation of mesial temporal lobe seizures. Epilepsia. 1991;32:822–837. doi: 10.1111/j.1528-1157.1991.tb05539.x. [DOI] [PubMed] [Google Scholar]
- Lin JS, Sakai K, Jouvet M. Hypothalamopreoptic histaminergic projections in sleep-wake control in the cat. European Journal of Neuroscience. 1994;6:618–625. doi: 10.1111/j.1460-9568.1994.tb00306.x. [DOI] [PubMed] [Google Scholar]
- Loddenkemper T, Kotagal P. Lateralizing signs during seizures in focal epilepsy. Epilepsy and Behavior. 2005;7:1–17. doi: 10.1016/j.yebeh.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Lux S, Kurthen M, Helmstaedter C, Hartje W, Reuber M, Elger CE. The localizing value of ictal consciousness and its constituent functions: A video-EEG study in patients with focal epilepsy. Brain. 2002;125:2691–2698. doi: 10.1093/brain/awf276. [DOI] [PubMed] [Google Scholar]
- Maldonado HM, Delgado-Escueta AV, Walsh GO, Swartz BE, Rand RW. Complex partial seizures of hippocampal and amygdalar origin. Epilepsia. 1988;29:420–433. doi: 10.1111/j.1528-1157.1988.tb03741.x. [DOI] [PubMed] [Google Scholar]
- Marks WJ, Jr., Laxer KD. Semiology of temporal lobe seizures: Value in lateralizing the seizure focus. Epilepsia. 1998;39:721–726. doi: 10.1111/j.1528-1157.1998.tb01157.x. [DOI] [PubMed] [Google Scholar]
- Mayanagi Y, Watanabe E, Kaneko Y. Mesial temporal lobe epilepsy: Clinical features and seizure mechanism. Epilepsia. 1996;37(Suppl. 3):57–60. doi: 10.1111/j.1528-1157.1996.tb01823.x. [DOI] [PubMed] [Google Scholar]
- McCasland JS, Carvell GE, Simons DJ, Woolsey TA. Functional asymmetries in the rodent barrel cortex. Somatosensory and Motor Research. 1991;8:111–116. doi: 10.3109/08990229109144735. [DOI] [PubMed] [Google Scholar]
- McIntyre DC, Gilby KL. Mapping seizure pathways in the temporal lobe. Epilepsia. 2008;49(Suppl. 3):23–30. doi: 10.1111/j.1528-1167.2008.01507.x. [DOI] [PubMed] [Google Scholar]
- Menzel C, Grunwald F, Klemm E, Ruhlmann J, Elger CE, Biersack HJ. Inhibitory effects of mesial temporal partial seizures onto frontal neocortical structures. Acta Neurologica Belgica. 1998;98:327–331. [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. Neural inputs into the nucleus basalis of the substantia innominata (Ch4) in the rhesus monkey. Brain. 1984;107(1):253–274. doi: 10.1093/brain/107.1.253. [DOI] [PubMed] [Google Scholar]
- Milner B. Disorders of learning and memory after temporal lobe lesions in man. Clinical Neurosurgery. 1972;19:421–446. doi: 10.1093/neurosurgery/19.cn_suppl_1.421. [DOI] [PubMed] [Google Scholar]
- Mirsky AF, Van Buren JM. On the nature of the “absence” in centrencephalic epilepsy: A study of some behavioral, electroencephalographic, and autonomic factors. Electroencephalography and Clinical Neurophysiology. 1965;18:334–348. doi: 10.1016/0013-4694(65)90053-2. [DOI] [PubMed] [Google Scholar]
- Morrell MJ. Differential diagnosis of seizures. Neurologic Clinics. 1993;11:737–754. [PubMed] [Google Scholar]
- Munari C, Bancaud J, Bonis A, Stoffels C, Szikla G, Talairach J. Impairment of consciousness in temporal lobe seizures: A stereoelectroencephalographic study. In: Canger R, Angeleri F, Penry JK, editors. Advances in epileptology: The XIth Epilepsy International Symposium. Raven Press; New York: 1980. pp. 111–114. [Google Scholar]
- Natsume J, Bernasconi N, Andermann F, Bernasconi A. MRI volumetry of the thalamus in temporal, extratemporal, and idiopathic generalized epilepsy. Neurology. 2003;60:1296–1300. doi: 10.1212/01.wnl.0000058764.34968.c2. [DOI] [PubMed] [Google Scholar]
- Nelissen N, Van Paesschen W, Baete K, Van Laere K, Palmini, Van Billoen H, et al. Correlations of interictal FDG-PET metabolism and ictal SPECT perfusion changes in human temporal lobe epilepsy with hippocampal sclerosis. NeuroImage. 2006;32:684–695. doi: 10.1016/j.neuroimage.2006.04.185. [DOI] [PubMed] [Google Scholar]
- Norden AD, Blumenfeld H. The role of subcortical structures in human epilepsy. Epilepsy and Behavior. 2002;3:219–231. doi: 10.1016/s1525-5050(02)00029-x. [DOI] [PubMed] [Google Scholar]
- Pare D, Gaudreau H. Projection cells and interneurons of the lateral and basolateral amygdala: Distinct firing patterns and differential relation to theta and delta rhythms in conscious cats. The Journal of Neuroscience. 1996;16:3334–3350. doi: 10.1523/JNEUROSCI.16-10-03334.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent A, Steriade M. Midbrain tegmental projections of nucleus reticularis thalami of cat and monkey: A retrograde transport and antidromic invasion study. The Journal of Comparative Neurology. 1984;229:548–558. doi: 10.1002/cne.902290408. [DOI] [PubMed] [Google Scholar]
- Parikh V, Kozak R, Martinez V, Sarter M. Prefrontal acetylcholine release controls cue detection on multiple timescales. Neuron. 2007;56:141–154. doi: 10.1016/j.neuron.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedley T. Classifications of seizures and epilepsy. In: Resor S, Kutt H, editors. The medical treatment of epilepsy. Informa Health Care; New York, NY: 1992. p. 8. [Google Scholar]
- Penfield W. Epileptic automatism and the centrencephalic integrating system. Research Publications: Association for Research in Nervous And Mental Disease. 1950;30:513–528. [PubMed] [Google Scholar]
- Penfield W. Centrencephalic integrating system. Brain. 1958;81:231–234. doi: 10.1093/brain/81.2.231. [DOI] [PubMed] [Google Scholar]
- Plum F, Posner JB. The diagnosis of stupor and coma. Davis; Philadelphia, PA: 1980. [Google Scholar]
- Prince DA, Wilder BJ. Control mechanisms in cortical epileptogenic foci. “Surround” inhibition. Archives of Neurology. 1967;16:194–202. doi: 10.1001/archneur.1967.00470200082007. [DOI] [PubMed] [Google Scholar]
- Purves D, Augustine G, Fitzpatrick D, Katz L, Williams S, McNamara J, et al. Emotions. Neuroscience. Sinauer Associates Publishers, Inc.; Sunderland, MA: 1992. [Google Scholar]
- Quesney F. Clinical and EEG features of complex partial seizures of temporal lobe origin. Epilepsia. 1986;27:S27–S45. doi: 10.1111/j.1528-1157.1986.tb05738.x. [DOI] [PubMed] [Google Scholar]
- Quigg M, Clayburn H, Straume M, Menaker M, Bertram EH., III Hypothalamic neuronal loss and altered circadian rhythm of temperature in a rat model of mesial temporal lobe epilepsy. Epilepsia. 1999;40:1688–1696. doi: 10.1111/j.1528-1157.1999.tb01585.x. [DOI] [PubMed] [Google Scholar]
- Quigg M, Kiely JM, Shneker B, Veldhuis JD, Bertram EH., III Interictal and postictal alterations of pulsatile secretions of luteinizing hormone in temporal lobe epilepsy in men. Annals of Neurology. 2002;51:559–566. doi: 10.1002/ana.10188. [DOI] [PubMed] [Google Scholar]
- Rabinowicz AL, Salas E, Beserra F, Leiguarda RC, Vazquez SE. Changes in regional cerebral blood flow beyond the temporal lobe in unilateral temporal lobe epilepsy. Epilepsia. 1997;38:1011–1014. doi: 10.1111/j.1528-1157.1997.tb01484.x. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalography and Clinical Neurophysiology. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nature Reviews. Neuroscience. 2004;5:449–461. doi: 10.1038/nrn1409. [DOI] [PubMed] [Google Scholar]
- Riederer F, Lanzenberger R, Kaya M, Prayer D, Serles W, Baumgartner C. Network atrophy in temporal lobe epilepsy: A voxel-based morphometry study. Neurology. 2008;71:419–425. doi: 10.1212/01.wnl.0000324264.96100.e0. [DOI] [PubMed] [Google Scholar]
- Risold PY, Swanson LW. Connections of the rat lateral septal complex. Brain Research. Brain Research Reviews. 1997;24:115–195. doi: 10.1016/s0165-0173(97)00009-x. [DOI] [PubMed] [Google Scholar]
- Rosenberg DS, Mauguiere F, Demarquay G, Ryvlin P, Isnard J, Fischer C, et al. Involvement of medial pulvinar thalamic nucleus in human temporal lobe seizures. Epilepsia. 2006;47:98–107. doi: 10.1111/j.1528-1167.2006.00375.x. [DOI] [PubMed] [Google Scholar]
- Rutherford EC, Pomerleau F, Huettl P, Stromberg I, Gerhardt GA. Chronic second-by-second measures of l-glutamate in the central nervous system of freely moving rats. Journal of Neurochemistry. 2007;102:712–722. doi: 10.1111/j.1471-4159.2007.04596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Hilaire JM, Lee MA. Localizing and lateralizing value of epileptic symptoms in temporal lobe epilepsy. Canadian Journal of Neurological Sciences. 2000;27(Suppl. 1):S1–S5. doi: 10.1017/s031716710000055x. discussion S20-1. [DOI] [PubMed] [Google Scholar]
- Sakurai T. Roles of orexin/hypocretin in regulation of sleep/wakefulness and energy homeostasis. Sleep Medicine Reviews. 2005;9:231–241. doi: 10.1016/j.smrv.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Saper CB, Chou TC, Scammell TE. The sleep switch: Hypothalamic control of sleep and wakefulness. Trends in Neuroscience. 2001;24:726–731. doi: 10.1016/s0166-2236(00)02002-6. [DOI] [PubMed] [Google Scholar]
- Schulz R, Luders HO, Noachtar S, May T, Sakamoto A, Hothausen H, et al. Amnesia of the epileptic aura. Neurology. 1995;45:231–235. doi: 10.1212/wnl.45.2.231. [DOI] [PubMed] [Google Scholar]
- Schwartz B. Hemispheric dominance and consciousness. Acta Neurologica Scandinavica. 1967;43:513–525. doi: 10.1111/j.1600-0404.1967.tb05757.x. [DOI] [PubMed] [Google Scholar]
- Schwartz TH, Bonhoeffer T. In vivo optical mapping of epileptic foci and surround inhibition in ferret cerebral cortex. Nature Medicine. 2001;7:1063–1067. doi: 10.1038/nm0901-1063. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. Journal of Neurology, Neurosurgery, and Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba K, Fibiger HC. Afferent connections of the laterodorsal and the pedunculopontine tegmental nuclei in the rat: A retro- and antero-grade transport and immunohistochemical study. The Journal of Comparative Neurology. 1992;323:387–410. doi: 10.1002/cne.903230307. [DOI] [PubMed] [Google Scholar]
- Sengpiel F, Sen A, Blakemore C. Characteristics of surround inhibition in cat area 17. Experimental Brain Research. 1997;116:216–228. doi: 10.1007/pl00005751. [DOI] [PubMed] [Google Scholar]
- Siegel J. Brain mechanisms that control sleep and waking. Naturwissenschaften. 2004;91:355–365. doi: 10.1007/s00114-004-0541-9. [DOI] [PubMed] [Google Scholar]
- Silveira DC, Klein P, Ransil BJ, Liu Z, Hori A, Holmes GL, et al. Lateral asymmetry in activation of hypothalamic neurons with unilateral amygdaloid seizures. Epilepsia. 2000;41:34–41. doi: 10.1111/j.1528-1157.2000.tb01502.x. [DOI] [PubMed] [Google Scholar]
- Spencer SS, Williamson PD, Spencer DD, Mattson RH. Human hippocampal seizure spread studied by depth and subdural recording: The hippocampal commissure. Epilepsia. 1987;28:479–489. doi: 10.1111/j.1528-1157.1987.tb03676.x. [DOI] [PubMed] [Google Scholar]
- Sperling MR. The consequences of uncontrolled epilepsy. CNS Spectrums. 2004;9:98–101. 106–9. doi: 10.1017/s1092852900008464. [DOI] [PubMed] [Google Scholar]
- Staiger JF, Wouterlood FG. Efferent projections from the lateral septal nucleus to the anterior hypothalamus in the rat: A study combining phaseolus vulgaris-leucoagglutinin tracing with vasopressin immunocytochemistry. Cell Tissue Research. 1990;261:17–23. doi: 10.1007/BF00329434. [DOI] [PubMed] [Google Scholar]
- Steriade M, Dossi RC, Pare D, Oakson G. Fast oscillations (20–40 Hz) in thalamocortical systems and their potentiation by mesopontine cholinergic nuclei in the cat. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:4396–4400. doi: 10.1073/pnas.88.10.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Nunez A, Amzica F. A novel slow (<1 Hz) oscillation of neocortical neurons in vivo: Depolarizing and hyperpolarizing components. Journal of Neuroscience. 1993;13:3252–3265. doi: 10.1523/JNEUROSCI.13-08-03252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Parent A, Hada J. Thalamic projections of nucleus reticularis thalami of cat: A study using retrograde transport of horseradish peroxidase and fluorescent tracers. The Journal of. Comparative Neurology. 1984;229:531–547. doi: 10.1002/cne.902290407. [DOI] [PubMed] [Google Scholar]
- Stock G, Rupprecht U, Stumpf H, Schlor KH. Cardiovascular changes during arousal elicited by stimulation of amygdala, hypothalamus and locus coeruleus. Journal of the Autonomic Nervous System. 1981;3:503–510. doi: 10.1016/0165-1838(81)90083-7. [DOI] [PubMed] [Google Scholar]
- Tae WS, Joo EY, Kim JH, Han SJ, Suh YL, Kim BT, et al. Cerebral perfusion changes in mesial temporal lobe epilepsy: SPM analysis of ictal and interictal SPECT. Neuroimage. 2005;24:101–110. doi: 10.1016/j.neuroimage.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Thierry AM, Gioanni Y, Degenetais E, Glowinski J. Hippocampo-prefrontal cortex pathway: Anatomical and electrophysiological characteristics. Hippocampus. 2000;10:411–419. doi: 10.1002/1098-1063(2000)10:4<411::AID-HIPO7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Van Paesschen W, Dupont P, Van Driel G, Van Billoen H, Maes A. SPECT perfusion changes during complex partial seizures in patients with hippocampal sclerosis. Brain. 2003;126:1103–1111. doi: 10.1093/brain/awg108. [DOI] [PubMed] [Google Scholar]
- Varoqueaux F, Poulain P. Projections of the mediolateral part of the lateral septum to the hypothalamus, revealed by Fos expression and axonal tracing in rats. Anatomia Embryologia (Berlin) 1999;199:249–263. doi: 10.1007/s004290050226. [DOI] [PubMed] [Google Scholar]
- Velasco JM, Fernandez de Molina A, Perez D. Suprarhinal cortex response to electrical stimulation of the lateral amygdala nucleus in the rat. Experimental Brain Research. 1989;74:168–172. doi: 10.1007/BF00248290. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Kocsis B. Brainstem-diencephaloseptohippocampal systems controlling the theta rhythm of the hippocampus. Neuroscience. 1997;81:893–926. doi: 10.1016/s0306-4522(97)00239-x. [DOI] [PubMed] [Google Scholar]
- Williamson PD, French JA, Thadani VM, Kim JH, Novelly RA, Spencer SS, et al. Characteristics of medial temporal lobe epilepsy: II. Interictal and ictal scalp electroencephalography, neuropsychological testing, neuroimaging, surgical results, and pathology. Annals of Neurology. 1993;34:781–787. doi: 10.1002/ana.410340605. [DOI] [PubMed] [Google Scholar]
- Wilson CL, Engel J., Jr. Electrical stimulation of the human epileptic limbic cortex. Advances in Neurology. 1993;63:103–113. [PubMed] [Google Scholar]
- Wilson CL, Isokawa M, Babb TL, Crandall PH. Functional connections in the human temporal lobe. I. Analysis of limbic system pathways using neuronal responses evoked by electrical stimulation. Experimental Brain Research. 1990;82:279–292. doi: 10.1007/BF00231248. [DOI] [PubMed] [Google Scholar]
- Woolf NJ, Butcher LL. Cholinergic systems in the rat brain: III. Projections from the pontomesencephalic tegmentum to the thalamus, tectum, basal ganglia, and basal forebrain. Brain Research Bulletin. 1986;16:603–637. doi: 10.1016/0361-9230(86)90134-6. [DOI] [PubMed] [Google Scholar]
- Yamauchi T. Impairment of consciousness during epileptic seizures with special reference to neuronal mechanisms. Epilepsia. 1998;39:16–20. doi: 10.1111/j.1528-1157.1998.tb05144.x. [DOI] [PubMed] [Google Scholar]
- Yu L, Blumenfeld H. Theories of impaired consciousness in epilepsy. Annals of the New York Academy of Sciences. 2009;1157:48–60. doi: 10.1111/j.1749-6632.2009.04472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zifkin BG, Dravet C. Generalized tonic-clonic seizures. In: Engel J, Pedley TA, editors. Epilepsy: A comprehensive textbook. Lippincott Williams & Wilkins; Philadelphia, PA: 2007. pp. 353–362. [Google Scholar]