Abstract
Myxoma virus (MYXV) is a member of the Poxviridae family and prototype for the genus Leporipoxvirus. It is pathogenic only for European rabbits (Oryctolagus cuniculus), in which it causes a lethal disease called myxomatosis, and for two North American species, Sylvilagus audubonni and Sylvilagus nuttalli, in which it causes a less severe disease. MYXV replicates exclusively in the cytoplasm of the host cell, and its genome encodes 171 open reading frames. A number of these genes encode proteins that can interfere with or modulate host defense mechanisms, and several show promise in a clinical setting. Serp-1, for example, is in clinical trials for treatment of acute unstable coronary syndromes, and M-T7 has been shown to inhibit inflammatory responses in rabbit models of balloon angioplasty arterial injury. Additionally, although MYXV is not infectious in humans, it is able to productively infect a number of human cancer cell lines, but not normal human cells, and has also been shown to increase survival time in mouse models of human glioma. These characteristics suggest that MYXV could prove to be a viable therapeutic agent in a variety of clinical settings, including as an anti-inflammatory or anti-immune therapy, or as an oncolytic agent. MYXV is also an excellent model for poxvirus biology, pathogenesis, and host tropism studies. It is easily propagated in a number of cell lines, including adherent cells and suspension cultures, and minimal purification is required to provide a stock useful for in vivo and in vitro studies.
Keywords: Poxvirus, Myxoma virus, sucrose gradient purification, titration
Myxoma virus (MYXV) is a poxvirus that is the prototype of the genus Leporipoxvirus, and a member of the Poxviridae family. It is pathogenic only for European rabbits and two North American species of rabbits, and is harmless to humans. However, MYXV has been shown to productively infect a variety of human cancer cell lines originated from a diverse group of tissues (Sypula et al., 2004), and therefore has the potential for development as an oncolytic virus useful in treatment against a variety of cancers. Furthermore, MYXV is adept at evading and interfering with the host immune response and might serve as a source of immunomodulatory proteins that can be used as therapeutic agents in a variety of clinical settings (Lucas and McFadden, 2004). MYXV has also been established as a model to study poxvirus biology, pathogenesis, and host tropism.
Producing pure, high titer stocks of virus thus becomes critical for studying MYXV in the laboratory and developing it as a useful clinical agent. The virus is easily propagated on adherent cells or in suspension cultures, and grows in several cell lines, including RK13 (rabbit kidney epithelial), BHK-21 (baby hamster kidney), BGMK (Buffalo green monkey kidney), Vero (African green monkey kidney epithelial), BSC-40 (African green monkey kidney), and CV-1 (African green monkey kidney fibroblast) cells. Minimal purification is needed to provide a stock that is appropriate for both in vitro and in vivo work. The following protocols, which include propagation on both adherent and suspension cells, describe these methods for propagating, purifying, and quantifying stocks of MYXV that are untagged or that express fluorescent proteins such as green fluorescent protein (GFP), red fluorescent protein (RFP), tomato Red (tdRed), or other fluorescent proteins.
CAUTION: Although MYXV is not pathogenic for humans, it is considered a BSL-2 agent, and precautions for working with BSL-2 agents, including the proper use of personal protective equipment and handling in a Class II biosafety cabinet, are indicated. For more information regarding the safe handling of BSL-2 agents, refer to the U.S. Department of Health and Human Services’ “Biosafety in Microbiological and Biomedical Laboratories” (http://www.cdc.gov/od/OHS/biosfty/bmbl5/BMBL_5th_Edition.pdf).
Furthermore, additional precautions may be required by individual Institutional Biosafety Committees when working with other poxviruses in concert with MYXV because of concerns for the possibility of recombination between poxviruses. Potential institutional requirements may include, for instance, propagating two different poxviruses in separate incubators. Contact the local institutional biosafety office for policies and advice.
BASIC PROTOCOL 1: ROLLER BOTTLE PROPAGATION OF MYXV
This protocol describes propagation of MYXV on adherent CV-1, BSC-40, or BGMK cells.
Materials
Solutions and reagents
CV-1 cells (ATCC #CCL-70)
Complete MEM (Minimal Essential Medium - see recipe)
Phosphate-buffered saline (PBS; see recipe)
1X Trypsin-EDTA-PBS (see recipe)
1M HEPES
Concentrated MYXV stock
10mM Tris-HCl pH 8.0
Supplies and equipment
37°C water bath
Expanded surface roller bottles (Fisher cat. no. 06-419-9)
37°C roller bottle incubator or other incubator that will accommodate a small roller device (CO2 is not necessary)
Roller device that will fit in 37°C incubator
150cm2 tissue culture flasks
50 ml conical centrifuge tubes
Refrigerated low-speed table top centrifuge; e.g., Sorvall Legend RT, Beckman GS-6R, or equivalent
Inverted tissue culture microscope
Fluorescent microscope
CAUTION: All procedures should be performed in a Class II Biosafety cabinet, and liquid waste inactivated with BioClean, bleach, or other appropriate disinfectant to a final concentration of 10%. Autoclave all plasticware before disposing.
NOTE: Each expanded surface roller bottle will contain approximately 1 × 108 cells when grown to 80% confluency.
NOTE: Incubate all roller bottle cultures on a roller apparatus in a 37°C incubator without CO2. Adding HEPES to the bottles obviates the need for incubation with CO2 and reduces the potential for contamination.
NOTE: Perform all steps using aseptic technique, and assure that all reagents and supplies are sterile.
Prepare roller bottles
In a 37°C water bath, preheat the MEM medium, PBS, and 1X trypsin-EDTA-PBS.
-
1
Add 50 ml of complete MEM to each of 4 expanded surface roller bottles.
-
2
Roll bottles gently to wet entire surface.
-
3
Place bottles on roller at 37°C and roll for at least 30 min before seeding cells.
Prepare cells
-
4
Aspirate media from 4 confluent 150cm2 flasks of CV-1 cells into a vacuum flask, and rinse each flask by gently pipetting 10 ml of PBS onto the cell surface to remove any remaining FBS, which reduces the effectiveness of trypsin.
-
5
Aspirate PBS and add 5 ml of 1X trypsin-EDTA-PBS, recap the flasks, and rock back and forth to wet the cell monolayer. Place flasks at 37°C for 5–10 min.
-
6
When the cells become rounded (5–10 minutes), dislodge cells from flask surfaces by tapping the sides sharply, then add 8 ml complete MEM to each flask to inactivate trypsin. Pipette media up and down repeatedly to break up clumps of cells.
-
7
Divide cells into 2 × 50 ml conical centrifuge tubes, and spin for 5 min at 550 × g in a refrigerated low-speed table top centrifuge at 4°C.
-
8
Retrieve roller bottles from the roller at 37°C, and add 100 ml complete MEM to each, for a total of 150 ml media per bottle.
-
9
Aspirate media from conical centrifuge tubes and resuspend cells in a total of 20 ml of complete MEM.
-
10
Divide cell suspension equally among roller bottles (approximately 5 ml per bottle).
-
11
Add 150 µl of 1M HEPES to each bottle to maintain pH.
-
12
Tighten caps and place roller bottles on roller at 37°C.
-
13
After 1 day check for even cell distribution using an inverted microscope. On day 2 or 3, cells should be 80% confluent; verify this under the microscope. The time it takes for cells to become confluent may vary and is dependent on the growth rate of a particular cell line.
Infect cell cultures
-
14
On the second or third day, when cells appear to be about 80% confluent, remove 100 ml of media from roller bottles, leaving 50 ml.
-
15
Add virus to each roller bottle for a multiplicity of infection (MOI) of 0.02–0.05 (add 2.0–5.0 × 106 virus per bottle when cells are grown to 80% confluency [1 × 108 cells per bottle]).
-
16
Roll bottles at 37°C for 1 hr.
-
17
Retrieve bottles and add 100 ml fresh complete MEM to each bottle.
-
18
Add 100 µl of 1M HEPES to each bottle to maintain pH.
-
19
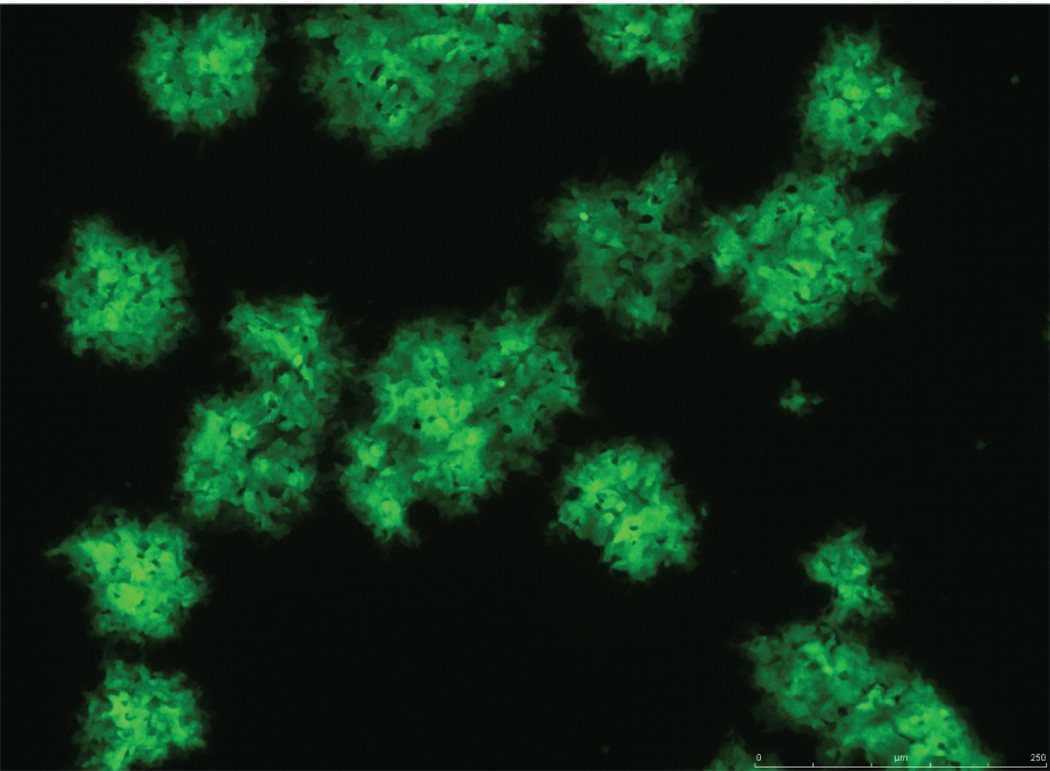
Return bottles to 37°C and roll for 48–72 hr. (This time period depends on the cell line and MYXV variant; e.g., a mutant virus). After 48 hr, if the MYXV is tagged with a fluorescent protein, examine the bottles using a fluorescence microscope, if the microscope is set up to allow this. Look for fluorescent foci, clusters of cells that appear sick, as shown in figure 1. If the virus is not tagged with a fluorescent protein, look for foci using the inverted tissue culture microscope. Cytopathic effect (CPE) and foci are easily visualized in a highly infected culture. Cells should be harvested before they begin to lift from the surface of the bottle, which they will do as the infection progresses.
Figure 1.
vMYX-GFP grown on adherent CV-1 cells. 48 hr post-infection. Image provided by Eric Bartee.
Harvest cells and virus
-
20
Remove media from the roller bottles.
-
21
Gently rinse cells with 40 ml PBS per bottle without disturbing the cell surface.
-
22
Add 50 ml 1X trypsin-EDTA-PBS to each bottle.
-
23
Roll bottles at 37°C for approx. 15–20 minutes.
-
24
Remove bottles and swirl liquid around the wall of the bottles to remove cells.
-
25
Collect the cells into 50 ml conical centrifuge tubes.
-
26
Rinse bottles with 20–25 ml PBS per bottle and add to tubes.
-
27
Pellet cells by centrifuging at 1930 × g for 5 min in a low-speed centrifuge at 4°C.
-
28
Discard the supernatant and resuspend pellets containing virus in 5 ml of 10mM Tris-HCl, pH 8.0 for each roller bottle, or a total of 20 ml.
-
29
Freeze resuspended cells containing virus in a 50 ml conical centrifuge tube at −80°C until ready to purify.
ALTERNATE PROTOCOL 1: PROPAGATION OF FLUORESCENT PROTEIN-TAGGED MYXV IN SPINNER FLASKS
MYXV can also be grown in suspension cultures of BHK-21 cells. Adherent BHK-21 cells used to seed spinner flasks are grown in dishes in DMEM; when transferred to the spinner flasks, the cells are cultured in IMDM medium, an enriched medium that is ideal for culturing cells at high density. Bellco makes a spinner flask that has an impeller, a built-in suspended rotating magnet, and side arms with removable caps that allow for easy transfer of media and virus in and out of the flask. These caps are left loose but secure to allow gas exchange in a CO2 incubator. This alternate protocol is adapted for following the growth of MYXV that is tagged with a fluorescent protein and is not intended for growing untagged virus.
Additional Materials (Also see Basic Protocol 1)
Solutions and reagents
BHK-21 cells (ATCC # CCL-10)
Complete DMEM (Dulbecco’s Modified Eagle Medium - see recipe)
Complete IMDM (Iscove’s Modified Dulbecco’s Medium - see recipe)
Supplies and equipment
10cm tissue culture plates
CO2 incubator
Sterile disposable pipettes
125 ml and 500 ml spinner flasks (Bellco µ-Carrier Spinner Flask)
Magnetic stir plate
60 cm tissue culture dish or 6-well culture dish
500 ml centrifuge bottles
Sorvall SLA-3000 rotor or equivalent
High speed centrifuge, such as Sorvall RC-6 or equivalent
CAUTION: All procedures should be performed in a Class II Biosafety cabinet, and liquid waste inactivated with BioClean, bleach, or other appropriate disinfectant to a final concentration of 10%. Autoclave all plasticware before disposing.
NOTE: Do not use detergent to clean spinner flasks. Instead, use tap water at high pressure to first wash the flask, impeller, and lid. Follow with a rinse with high pressure tap distilled water. Finally, half-fill the flask with ultra-pure pyrogen-free water, reassemble with impeller and lid, and shake well. Repeat this 3 to 4 times, then drain well and autoclave.
NOTE: For spinner cultures, a magnetic stir plate is placed in a CO2 incubator to accommodate the spinner flask(s).
NOTE: Addition or removal of media or virus to or from the spinner flask should always be done through one of the two capped side arm ports using a sterile disposable pipette. Prior to incubation, both caps are placed loosely but securely on the port to allow CO2 exchange in the incubator.
NOTE: Perform all steps using aseptic technique, and assure that all reagents and supplies are sterile.
Prepare Seed Cell Culture
-
1
Seed 2×106 BHK-21 cells in each of 3 10cm tissue culture dishes in 10 ml complete DMEM.
-
2
Grow in a CO2 incubator at 37°C.
-
3
After 2–3 days check for confluency using an inverted microscope. When the cells are approximately 90% confluent, the dishes are ready to split.
Prepare Spinner Flask
-
4
Pre-warm complete IMDM at 37°C.
-
5
Carefully remove the cap from one of the side ports of the flask.
-
6
Add 30 ml of complete IMDM to the flask and recap.
Seed Starter Spinner Flask
-
7
Pre-warm PBS and trypsin-EDTA-PBS at 37°C.
-
8
Remove media from the 10 cm dishes of BHK-21 cells and rinse cells by gently adding 10 ml PBS to the inner edge of the dishes, using care not to disturb the cell monolayer.
-
9
Remove the PBS and add 2 ml of 1X trypsin-EDTA-PBS to plates.
-
10
Rock plates back and forth to wet cell monolayer, then place plates at 37°C for 5–10 min.
-
11
Remove plates from incubator, and dislodge cells from the dish surface by gently tapping the sides of the dishes. The cells should dislodge easily.
-
12
Add 8 ml complete IMDM to each dish to inactivate the trypsin, and pipette the media up and down repeatedly to break up clumps of cells.
-
13
Transfer cells to the spinner flask containing 30 ml IMDM through one of the side arm ports. The total volume will now be approximately 60 ml. Replace the cap loosely but securely, and loosen the second side arm cap to allow CO2 exchange.
-
14
Place spinner flask on a stir plate in the CO2 incubator at 37°C, and stir gently (100–250 rpm).
-
15
Culture will need to grow for 3–4 days until media is slightly yellow and cells, when viewed under the microscope, are clumped. Check for clumps after 1 or 2 days by carefully removing from the side arm port approximately 2 ml of culture and place into a 60 mm cell culture dish or other small dish appropriate for viewing under the inverted microscope. Follow the culture in this way for another day or two until ready.
Infect cell culture
-
16
When cells have grown for 3–4 days and appear as described in step 15 above, carefully transfer the entire 60 ml culture from the 125 ml spinner flask into a 500 ml spinner flask prepared as before and containing 400 ml of pre-warmed complete IMDM.
-
17
Inoculate the culture immediately with 5 × 108 virus and replace the cap on the side arm. Both side arm caps should be loose, but secure, to allow gas exchange.
-
18
Place the spinner flask on a stir plate in the CO2 incubator at 37°C, and stir gently at 100–250 rpm.
-
19
At 3–4 days post-infection, remove 2–3 ml of the culture into a 60 mm cell culture dish as before and check cells under the fluorescent microscope. When over 90–95% of the cells fluoresce, the virus is ready to be harvested.
Harvest cells and virus
-
20
Collect cell suspension in 2 × 500 ml centrifuge bottles.
-
21
Pellet cells and virus at 4,225 × g (5,000 rpm in a SLA-3000 rotor) for 15 min at 4°C in an RC-6 or equivalent high-speed centrifuge.
-
22
Discard the supernatant and resuspend pellets containing virus in a total of 20 ml of 10mM Tris-HCl, pH 8.0.
-
23
Freeze resuspended cells containing virus in a 50 ml conical centrifuge tube at −80°C until purification.
ALTERNATE PROTOCOL 2: SMALL-SCALE MYXV AMPLIFICATION AND CRUDE VIRUS STOCK PURIFICATION
MYXV stock can also be amplified on BGMK cells using 4–6 confluent 150cm2 flasks. This method is useful for preparing a smaller crudely purified stock of virus after making, for example, a new recombinant virus. The virus preparation will contain cellular debris that cannot be removed by centrifugation and may inhibit certain applications.
Additional Materials (also see Basic Protocol 1 and Alternate Protocol 1)
BGMK cells
Solutions and Reagents
Swelling buffer (see recipe)
Supplies and Equipment
Optional: Platform rocker
Sterile cell scrapers
40 ml Dounce homogenizer with a loose pestle (autoclaved)
Oak Ridge tubes (Fisher cat. no. NC9472311)
Sorvall SA600 rotor or equivalent
Optional: Cup sonicator
Cryotubes (1.5 or 2 ml)
CAUTION: All procedures should be performed in a Class II Biosafety cabinet, and liquid waste inactivated with BioClean, bleach, or other appropriate disinfectant to a final concentration of 10%. Autoclave all plasticware before disposing.
NOTE: Perform all steps using aseptic technique, and assure that all reagents and supplies are sterile.
Prepare cells
-
1
Seed 4–6 150cm2 flasks with BGMK cells and grow to confluency.
Infect cells
-
2
Aspirate media from the flasks.
-
3
Infect the cells at a MOI of 0.05 with virus diluted in 5 ml complete DMEM media per flask. The media should just cover the cell surface to prevent cells from drying. A confluent 150cm2 flask will contain approximately 1×107 cells; infect with 5×105 virus per flask.
-
4
Infect at 37°C for 1 hr with shaking on a platform rocker placed in a CO2 incubator; alternatively, rock flasks gently every 15 minutes to prevent drying of cells.
-
5
1 hr post-infection add 25 ml of complete DMEM media to each flask.
-
6
Incubate flasks at 37°C under CO2 for 48–72 hr, depending on progression of the infection. The infected cells should remain attached to the flask. If amplifying a recombinant virus expressing a fluorescent protein, the virus will be ready to harvest when 90–100% of the cells appear to express the fluorescent protein when viewed under a fluorescent microscope.
-
7
Harvest the infected cells by scraping directly into the growth medium in the flasks.
-
8
Transfer the cell suspension into 50 ml conical centrifuge tubes and spin down the cells at 1930 × g in a low-speed refrigerated centrifuge at 4°C for 10–15 minutes.
-
9
Discard the supernatant and resuspend the cell pellets in a total of 10–15 ml of swelling buffer. Swell cells by incubating on ice for 20 minutes. Cells can be stored at −80°C at this point.
Homogenize cells containing virus
-
10
Transfer infected cell suspension to an autoclaved 40 ml Dounce homogenizer that has been pre-chilled on ice and homogenize with a loose pestle for 80 strokes.
-
11
Transfer the homogenate to a 50 ml conical tube and spin down cell debris at 500 × g for 5 minutes at 4°C.
-
12
Transfer the supernatant to a sterile Oak Ridge tube for high-speed pelleting. The supernatant contains most of the virus.
-
13
Resuspend the pellet from step 11 in 10 ml swelling buffer and dounce again with 60–70 strokes to collect residual virus.
-
14
Spin again at 500 × g for 5 minutes. Add this supernatant to the supernatant collected in step 12.
Recover virus by centrifugation
-
15
Centrifuge the pooled supernatants at 20,850 × g (12,000 rpm in a Sorvall SA600 or equivalent rotor) for 60 minutes at 4°C.
-
16
Resuspend the virus pellet in 2 ml of DMEM containing 10% FBS by pipetting or vortexing until all clumps are dispersed. Optional: Sonicate virus suspension for 1 minute to break up clumps. Sonicator cup should contain ½ ice and ½ water to keep virus cool.
-
17
Titrate virus according to Basic Protocol 3, and store at −80°C in 0.2–1.0 ml aliquots in cryotubes.
BASIC PROTOCOL 2: PURIFICATION OF MYXV OVER SUCROSE
Virus is first homogenized and then pelleted through a 36% w/v sucrose cushion. This level of purification is sufficient for many protocols, including in vivo work. However, for some procedures, a more purified preparation is necessary. An assay that measures cytokine induction, for example, would require the use of highly purified virus that is free from any cellular components; this assures that any activity measured is solely a result of viral factors. To obtain a level of purification sufficient for such assays, virus that is first pelleted through 36% sucrose is then banded over a 24–40% sucrose step gradient, collected, and concentrated.
Materials
Solutions and Reagents
10mM Tris-HCl, pH 8.0 at 4°C
24, 28, 32, 36, and 40% w/v Sucrose in 10 mM Tris-HCl pH 8.0 (see recipe) at 4°C
Supplies and Equipment
37°C water bath
Cup sonicator
40 ml Dounce homogenizer with loose pestle (autoclaved)
50 ml conical centrifuge tubes
Sterile individually wrapped pipettes
40 ml Ultraclear ultracentrifuge tubes for SW28 rotor (Beckman Coulter cat. no. 344058)
12 ml Ultraclear ultracentrifuge tubes for SW41 rotor (Beckman Coulter cat. no. 344059)
Ultracentrifuge with Beckman SW28 and SW41 rotors or equivalents
Sterile Pasteur pipettes
2 ml cryotubes
CAUTION: All procedures should be performed in a Class II Biosafety cabinet, and liquid waste inactivated with BioClean, bleach, or other appropriate disinfectant to a final concentration of 10%. Autoclave all plasticware before disposing.
NOTE: Sucrose step gradients should be prepared the day before they are needed and placed at 4°C overnight prior to use.
NOTE: All steps are performed at 4°C.
NOTE: Perform all steps using aseptic technique, and assure that all reagents and supplies are sterile.
Homogenize Harvested Virus
-
1
Quickly thaw suspension from harvested cells and virus (see Basic Protocol 1 and Alternate Protocol 1) at 37°C until just thawed. Do not allow to warm up.
-
2
In the same 50 ml conical tube in which the virus suspension was frozen, sonicate in a cup sonicator 2 times for 1 min each with a 30 sec to 1 min rest between sonication steps to prevent virus from warming up. Sonicator cup should contain ½ ice and ½ water to keep virus cooled.
-
3
Transfer suspension (10 ml) into an autoclaved 40 ml Dounce homogenizer prechilled on ice and homogenize with a loose pestle for 20 strokes.
-
4
Transfer the homogenate to a 50 ml conical centrifuge tube and spin at 1040 × g for 15 min in a low-speed table top centrifuge at 4°C to remove nuclei and other cell debris.
-
5
Save the supernatant and resuspend the pellet in 5 ml of 10mM Tris-HCl, pH 8.0.
-
6
Repeat step #3 and #4, except centrifuge at 1930 × g for 15 min at 4°C.
-
7
Remove the supernatant and discard the pellet.
-
8
Combine supernatants.
Pellet Virus through a Sucrose Cushion
-
9
Pipette 16 ml of cold 36% sucrose in 10mM Tris-HCl, pH 8.0 into a 40 ml Ultraclear tube. Set up a second balance tube with 16 ml of 36% sucrose overlaid with 20 ml of water or 10mM Tris-HCl, pH 8.0.
-
10
Gently pipette 20 ml of the homogenate containing virus on top of the sucrose layer, as shown in figure 2, being careful to avoid mixing of the two solutions. The tube should be filled to within 0.5–1.0 cm from the top to prevent tube collapse during centrifugation. Gently pipette cold 10mM Tris-HCl, pH 8.0 over the virus homogenate if more volume is required in the tube and to balance weight for both the tubes.
-
11
Load the sample and balance tubes into buckets and centrifuge for 80 min at 58,267 × g (18,000 rpm in SW28 rotor) at 4°C.
-
12
Discard the supernatant and resuspend the virus pellet in 1 ml of cold 10mM Tris-HCl, pH 8.0 if no further purification is desired, and determine the titer by foci forming assay. Store in 0.2 ml aliquots at −80°C in 2 ml cryotubes. If further purification by sucrose step gradient is desired, resuspend the pellet in 1.4 ml of cold 10mM Tris-HCl, pH 8.0 and proceed to step 13.
Figure 2.
Schematic of SW28 Ultraclear tube with 36% sucrose pad overlaid with virus suspension. Virus is pelleted through sucrose for removal of cell debris.
Further purify virus through a sucrose step gradient
-
13
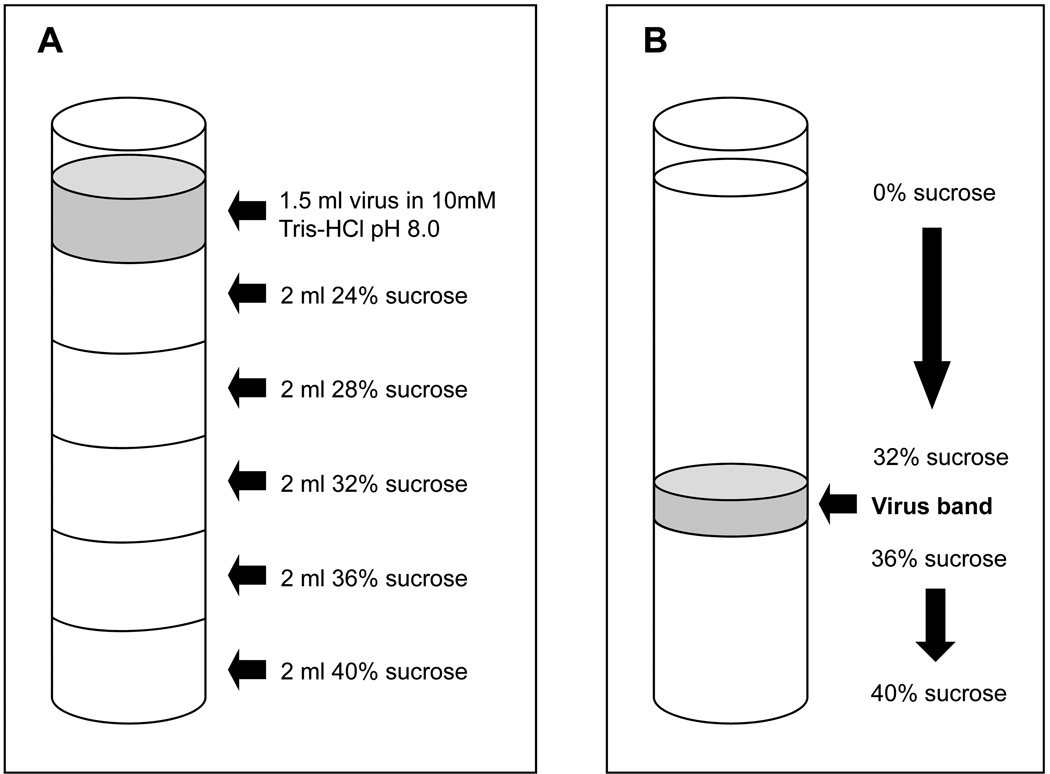
The day before use, prepare two 24–40 % sucrose step gradients in 12 ml Ultraclear ultracentrifuge tubes made with 2 ml each of cold 24, 28, 32, 36, and 40 % sucrose in 10 mM Tris-HCl pH8.0, as illustrated in figure 3A. Start by pipetting 2 ml of the 40% sucrose into the bottom of the tube, and gently layer each successively less concentrated sucrose solution on top of the other, being careful to avoid mixing of solutions. The top sucrose layer will contain 24% sucrose, and one gradient will serve as a balance tube. Store the gradients at 4°C overnight.
-
14
Sonicate the virus in a cup sonicator 2 times for 1 min each with a 30 sec to 1 min rest between sonication steps to prevent virus from warming up. The sonicator cup should contain ½ ice and ½ water to keep virus cool.
-
15
Gently pipette 1.4 ml of the sonicated virus mixture on the top of one of the gradients without mixing with the top sucrose solution; use water or 10mM Tris HCl, pH 8.0 in the balance tube gradient.
-
16
Secure the gradients into two buckets of the SW41 rotor and spin for 40 min at 33,518 × g (14,000 rpm in SW41 rotor) at 4°C.
-
17
Remove the gradients from the rotor buckets, and with a sterile Pasteur pipette, carefully remove liquid from above the virus band shown in figure 3B, and discard. Remove as much liquid as possible without disturbing the virus band.
-
18
Using a sterile Pasteur pipette, carefully collect the virus band and place on ice in a fresh 12 ml Ultraclear tube. Discard the remaining liquid in the gradient tube.
-
19
Dilute the collected virus band up to 12 ml with cold 10mM Tris-HCl, pH 8.0, and prepare a balance tube.
-
20
Spin in SW41 rotor for 60 min at 43,780 × g (16,000 rpm in SW41 rotor) at 4°C.
-
21
Discard the supernatant and resuspend the virus pellet in 1 ml of cold 10mM Tris-HCl, pH 8.0, and determine amount of virus harvested by OD measurement.
Figure 3.
Schematic of SW41 Ultraclear centrifuge tube with: (A) 24–40% sucrose step gradient prepared with virus overlay prior to centrifugation; and (B) virus band after centrifugation. MYXV bands between the 32% and 36% sucrose layers.
OD260nm MEASUREMENT
-
22
Make a 1:250 dilution of the virus in water.
-
23
Using water as a blank, measure the OD260nm. Calculate virus yield as indicated in Table 1.
-
24
Store virus in 0.2 ml aliquots at −80°C in cryotubes.
Table 1.
Calculation of virus yield by OD260
| VIRUS PARTICLES/ML VIRUS SUSPENSION: 1 OD260nm = 1.2×1010 particles/ml | ||
| VIRUS PARTICLES/CELL (1:250 dilution): | ||
|
|
||
BASIC PROTOCOL 3: QUANTIFICATION OF PURIFIED MYXV BY FOCI FORMING ASSAY
MYXV forms foci, areas of clumped infected cells, rather than plaques, and titer is expressed in foci forming units/ml of culture (FFU/ml). Determining the titer can be done in a variety of ways, depending on whether the virus is untagged, has a fluorescent tag, or other reporter gene. For untagged virus, the infected cell monolayer and foci are stained with crystal violet (Kotwal and Abrahams, 2004). For a fluorescent protein-tagged MYXV, titer is determined by counting foci under a fluorescent microscope, and for titration of MYXV having the reporter gene lac Z (vMyxlac), X-gal staining can be used.
Materials
Solutions and Reagents
CV-1 cells
PBS (for splitting cells to seed 6-well dish)
Trypsin-EDTA-PBS (for splitting cells to seed 6-well dish)
Complete MEM medium
Crystal Violet solution (see recipe)
Neutral buffered formalin (see recipe)
X-gal staining solution (see recipe)
Supplies and Equipment
Inverted tissue culture microscope
6-well tissue culture dishes
CO2 incubator
Sterile 1.5 ml microcentrifuge tubes
Optional: Platform rocker
Fluorescent microscope
Optional: cup sonicator
CAUTION: All procedures should be performed in a Class II Biosafety cabinet, and liquid waste inactivated with BioClean, bleach, or other appropriate disinfectant to a final concentration of 10%. Autoclave all plasticware before disposing.
CAUTION: Dispose of all chemicals in the appropriate manner.
NOTE: Perform all steps using aseptic technique, and assure that all reagents and supplies are sterile.
Seed Cells
-
1
Seed a 6-well tissue culture dish with 1/5 of a nearly confluent 75cm2 tissue culture flask of CV-1 cells. One flask, when nearly confluent, should contain approximately 5–6 × 106 cells, and each well of the 6-well dish should be seeded with 2–4 × 105 cells.
-
2
Mix the appropriate volume of cells with complete MEM to a final volume of 12 ml for seeding each of the 6 wells.
-
3
Pipette 2 ml of the mixture into each well of a 6-well tissue culture dish.
-
4
Incubate at 37°C under CO2. When cells appear to be 80–90% confluent (usually the next day), they are ready to infect.
Infect Cells
-
5
Thaw virus to be titrated at room temperature until just thawed.
-
6
Optional: If clumps are present in the virus prep, it may be sonicated for 10–15 sec in a cup sonicator containing ½ ice and ½ water. Do not sonicate for longer than 10–15 sec.
-
7
Make serial dilutions of virus in complete MEM as described in Table 2.
-
8
Aspirate media from the 6-well dish. Add 500µl of each dilution to a single well in the dish.
-
9
Adsorb the virus on the cells by incubating 1 hr at 37°C under CO2. Every 10 min, remove the dish from the incubator and rock gently to redistribute liquid. A platform rocker can also be used to ensure uniform distribution of inoculation medium on cell surface.
-
10
After 1 hr, add 1.5 ml complete MEM to each well.
-
11
Incubate 2–5 days at 37°C under CO2.
-
12
Count the foci using the appropriate method for the virus being titrated.
Table 2.
Preparing Serial Dilutions for MYXV Titration
| Tube no. | Vol. virus | Vol. Medium | Resulting Dilution |
|---|---|---|---|
| 1 | 10 µl virus stock | 990 µl | 10−2 dilution |
| 2 | 10 µl from tube 1 | 990 µl | 10−4 dilution |
| 3 | 100 µl from tube 2 | 900 µl | 10−5 dilution |
| 4 | 100 µl from tube 3 | 900 µl | 10−6 dilution |
| 5 | 100 µl from tube 4 | 900 µl | 10−7 dilution |
| 6 | 100 µl from tube 5 | 900 µl | 10−8 dilution |
Score Foci
Scoring fluorescent protein-tagged virus
Titrating MYXV that is tagged with a fluorescent protein such as GFP, RFP, or tdRed is readily accomplished by counting foci under a fluorescent microscope. View each well of the plate under the microscope and count the foci in wells that have <100 foci. It should be possible to get counts for at least two wells. Calculate the titer (FFU/ml), which = number of foci × dilution × 2. Average the FFU/ml for each countable well.
Scoring untagged virus
If the virus is not tagged with a fluorescent protein, the cells are stained with crystal violet and foci counted under an inverted microscope.
Aspirate media from wells.
Add 200–500 µl of crystal violet solution to each well, applying gently along the side of the wells to avoid disrupting cells.
Swirl gently and incubate for up to 1 hr at room temperature.
Aspirate the stain and invert plates to dry the wells.
Count foci and calculate FFU/ml as above.
Scoring MYXV with a lac Z reporter gene: X-Gal staining
For titration of MYXV having the reporter gene lac Z (vMyxlac), X-gal staining can be used for β-galactosidase activity. After staining, blue foci can be counted with the naked eye (Stanford et al., 2007)
Aspirate media from the infected cells
Wash the cells once with cold PBS (2ml/well).
Fix the infected cells using 2 ml neutral buffered formalin for 5 min at room temperature.
Remove the formalin solution and rinse the cells 3× with 2 ml PBS/well for 4 min per wash.
Add 2 ml of the X-gal staining solution to the cells for 4 to 8 hours at room temperature.
Remove the stain, count the blue foci as seen in figure 4, and calculate FFU/ml as above.
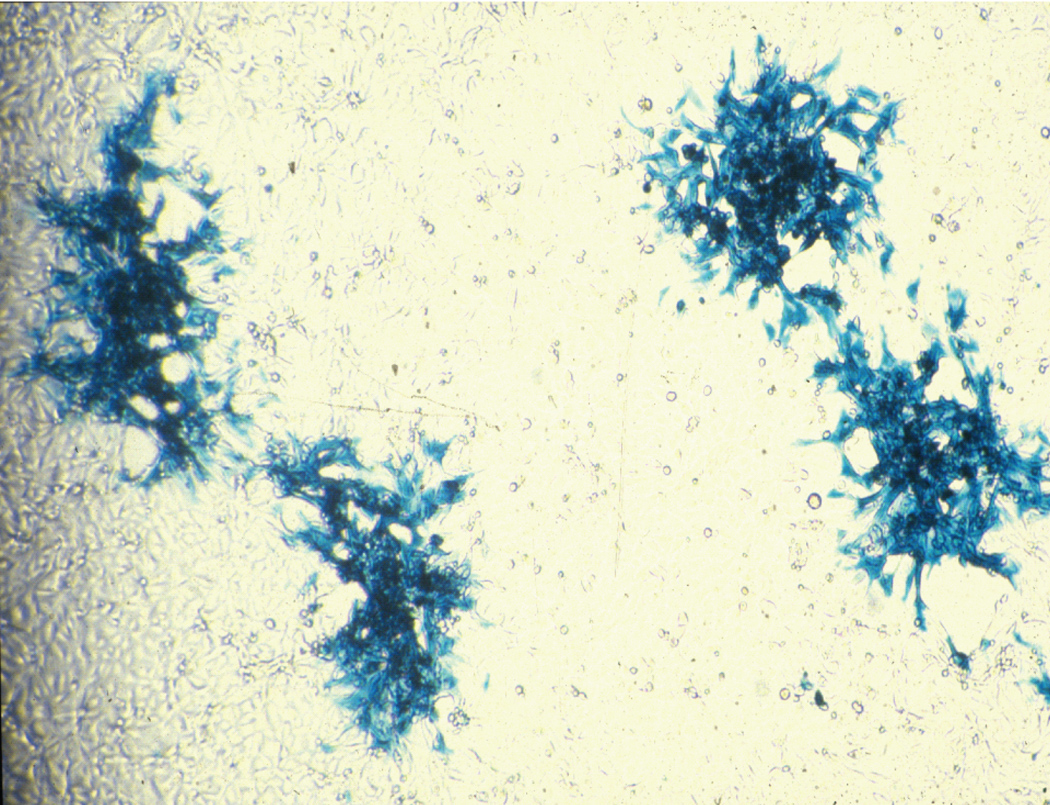
Figure 4.
vMYXlac grown on adherent BGMK cells and stained with X-gal.
REAGENTS AND SOLUTIONS
Use ultra-pure pyrogen free water in all recipes and protocols steps. Information on common stock solutions can be found in APPENDIX 2A, and for suppliers, refer to SUPPLIERS APPENDIX.
Complete Dulbecco’s Modified Eagle Medium (DMEM - Invitrogen cat. no. 11965-126)
1 liter of DMEM
100 ml Heat-inactivated Fetal Bovine Serum (FBS) (Invitrogen cat. no 26140-079) (see Heat-inactivated FBS below for heat-inactivation instructions)
10 ml Penicillin/Streptomycin solution (Invitrogen cat. no. 15140-163)
10 ml L-Glutamine 100X (Invitrogen cat. no. 25030-164)
Complete Iscove’s Modified Dulbecco’s Medium (IMDM - Invitrogen cat. no. 12440-079)
1 liter of IMDM
20 ml Heat-inactivated FBS
10 ml Penicillin/Streptomycin solution
10 ml L-Glutamine 100X
Complete Minimum Essential Medium (MEM - Invitrogen cat. no. 11095-114)
1 liter of MEM
50 ml Heat-inactivated FBS
10 ml Penicillin/Streptomycin solution
10 ml L-Glutamine 100X
10 ml Sodium Pyruvate (Invitrogen cat. no. 11360-070)
10 ml Non-essential Amino Acids (Invitrogen cat. no. 11140-050)
Crystal Violet Solution
0.1% w/v crystal violet dissolved in 20% ethanol
Heat-inactivated FBS
Thaw a 500 ml bottle of FBS overnight at 4°C. If it is still frozen the next day, finish thawing at 37°C. Heat inactivate by heating at 56°C for 1 hr. Store at −20°C in smaller convenient aliquots.
Neutral Buffered Formalin (10% v/v 37% formaldehyde solution in PBS)
Measure out 10 ml 37% formaldehyde solution and dilute to 100 ml with PBS
PBS
Per liter:
8 g NaCl (137mM)
1.44 g Na2HPO4 (10mM)
0.2 g KCl (2.7mM)
0.24 g KH2PO4 (2mM)
Dissolve in 900 ml ultra-pure pyrogen free water.
Check pH and adjust to pH 7.4 with dilute HCl if necessary.
Bring volume to 1 L with ultra-pure pyrogen free water and autoclave in 500 ml bottles for 20 min.
Store indefinitely at room temperature or at 4°C after opening.
24, 28, 32, 36, and 40% w/v Sucrose in 10 mM Tris-HCl pH 8.0
Per 500 ml:
120, 140, 160, 180, or 200 g sucrose, respectively
Dissolve in 400 ml 10 mM Tris-HCl pH 8.0, then adjust volume to 500 ml. Filter sterilize using a 0.2µm bottle-top filter, and store at 4°C indefinitely in 250 ml aliquots.
Swelling Buffer
Per 500 ml:
5 ml 1M Tris-HCl pH 8.0
1 ml 1M MgCl2
Adjust volume to 500 ml with water and autoclave for 20 minutes in convenient aliquots.
Store at 4°C indefinitely
1X Trypsin-EDTA-PBS
Per liter:
20 ml 2.5% (10X) Trypsin (Invitrogen cat. no. 15090-046)
10 ml 0.5M EDTA, pH 8.0
10 ml penicillin/streptomycin solution
Bring volume to 1 L with PBS.
Filter sterilize through a 0.2µm bottle-top filter
Store in convenient aliquots at −20°C indefinitely.
X-Gal Staining Solution
Make the following stock solutions
20 mg/ml X- Gal [5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside] solution (dissolved in N,N-dimethyl formamide - make fresh each time)
500mM K3Fe(CN)6 (Potassium ferricyanide) – 82.3 g/500 ml (autoclave and store at 4°C)
500mM K4Fe(CN)6·3H2O (Potassium ferrocyanide) – 105.6 g/500 ml (autoclave and store at 4°C)
1M MgCl2
For 20 ml X-Gal staining solution
1 ml of 20 mg/ml X-Gal solution (1 mg/ml final)
200 µl 500mM potassium ferricyanide (5 mM final)
200 µl 500mM potassium ferrocyanide (5 mM final)
20 µl 1M MgCl2 (1mM final)
18.58 ml PBS
Make this solution just prior to use and cover with foil. Make a fresh stock each time it is used.
COMMENTARY
Background Information
MYXV is a rabbit specific poxvirus that causes myxomatosis, a devastating and lethal disease in European rabbits (Oryctolagus cuniculus). It has also been shown to cause disease in two North American species, Sylvilagus audubonni and Sylvilagus nuttalli, but in these rabbits pathogenesis differs from that seen with classical myxomatosis (Regnery, 1971; Silvers et al., 2009). Myxomatosis is characterized by severe systemic cellular immunosuppression which leads to respiratory complications and death. However, in its evolutionary hosts, which belong to the genus Sylvilagus, and include the South American tapeti (Sylvilagus brasilensis) and the North American brush rabbit (Sylvilagus bachmani), MYXV is slowly cleared and causes only mild disease and a benign tumor at the infection site (Stanford, Werden, and McFadden, 2007; McFadden, 2005). To date, neither MYXV infection nor seroconversion in humans has been observed, even where the virus is widely distributed in rabbits and in carrier mosquitoes (Stanford and McFadden, 2007). Because of its high species specificity, MYXV was used in Australia in the 1950’s as a biological control for rabbits that were introduced in 1859, and which quickly multiplied and caused devastating damage to agriculture and the environment (Fenner and Ratcliffe, 1965).
Like other members of the poxvirus family, MYXV replicates exclusively in the cytoplasm of infected cells, and its genome consists of a single double stranded DNA (dsDNA) which encodes 171 open reading frames (Cameron et al., 1999). The central part of the genome encodes approximately 100 essential genes that are conserved among the members of poxvirus genera. The rest of the genes, including two copies each of the 12 genes that map within the terminal inverted repeats, encode proteins that interfere with and modulate host defense mechanisms. A number of these proteins share a sequence similarity with host cellular genes, suggesting a coevolutionary path (Johnston and McFadden, 2003). Some, called viroceptors, are secreted and able to bind specific ligands such as TNF, for example. Others, known as virokines, are also secreted, and imitate host immune inhibitors, while viromitigators function as host range factors that inhibit apoptosis (Johnston and McFadden, 2003; Kerr and McFadden, 2002). These characteristics give MYXV possible utility in a number of therapeutic settings. One of the MYXV-encoded immunomodulatory proteins, Serp-1, is now in clinical trials (conducted by VIRON Therapeutics, Inc.) for acute unstable coronary syndromes (unstable angina and small heart attacks). The M-T7 protein of MYXV, a secreted glycoprotein that inhibits rabbit gamma interferon, has also been shown to inhibit inflammatory responses in rabbit models of balloon angioplasty injury to arteries (Liu et al., 2000), and it is likely that a variety of other immunomodulatory proteins can be developed as anti-inflammatory or anti-immune therapeutics.
MYXV is also able to productively infect a variety of human cancer cell lines, but not normal human cells, and in in vivo studies, MYXV has also been shown to provide long-term survival in mouse models of human glioma (Lun et al., 2005). Although it was previously unknown whether MYXV could reliably target human primary cancer cells while sparing normal cells, in recent studies, normal and leukemic hematopoietic stem and progenitor cells (HSPCs) were treated with MYXV, resulting in infection of a significant number of the leukemic cells, and in only a small number of the normal cells. Additionally, normal HSPCs from healthy human bone marrow treated ex vivo with MYXV and xenotransplanted into sublethally irradiated NOG mice readily engrafted, and no evidence for MYXV replication was found in tissues at necropsy (Kim et al., 2009). These results open the door for MYXV to potentially join vaccinia virus, another poxvirus, as a new oncolytic virotherapy candidate.
Troubleshooting
Tissue Culture Problems
Although contamination with bacteria, fungi, and yeast is common in cell culture, there are preventive measures that can be taken. Reduce contamination by using careful aseptic technique and performing all cell culture activities in a biosafety cabinet; regularly wipe down cabinet and equipment surfaces with 70% ethanol. Change gloves often, keep the time flasks and media bottles are open to a minimum, and if possible, use individually wrapped sterile disposable pipettes. Antibiotics such as penicillin and streptomycin may be used routinely in media; alternatively, an antibiotic-antimycotic agent (Invitrogen cat. no. 15240-062) containing a 100X concentration of penicillin and streptomycin, as well as 25 µg amphotericin B/ml, may be used. Commercially prepared media is recommended, but if media is prepared in the lab from a powdered mix, it is important to use very clean glassware and ultra-pure, pyrogen-free water when preparing it.
Mycoplasma is a more difficult contaminant to detect and treat, because unlike other types of contamination, it is not visible under the microscope, nor is it sensitive to antibiotics commonly used in cell culture. It can affect both cell growth and the propagation of virus in those cells, so it is important to test cultures regularly and treat if necessary.
A PCR-based test kit for mycoplasma detection is available from Sigma (cat. no. MP0025-1KT), and should cultures test positive, Plasmocin (InvivoGen), an antibiotic effective against mycoplasma, is available in concentrations for both prophylaxis and treatment. For treatment of contaminated cultures, cells should be split into media containing Plasmocin (cat. no. ant-mpt) at a concentration of 25µg/ml, maintained at that concentration for two weeks, and retested. If mycoplasma is still present, an additional one week treatment and/or increase of Plasmocin concentration to 37.5µg/ml may be necessary. For prophylactic maintenance of cell cultures, Plasmocin (cat. no. ant-mpp) is used in media at a concentration of 5µg/ml, but is not typically used unless mycoplasma is a recurring problem.
Low Virus Yield
Mycoplasma is a leading cause of low virus yield when propagating MYXV; cells should be routinely tested and, if necessary, treated. It is also important to determine an optimum multiplicity of infection (MOI) when infecting cells, which for MYXV is MOI = 0.02 – 0.05. A CO2 level of 5% and temperature of 37°C should be carefully maintained in incubators, as well.
Anticipated Results
Virus yields of 1 × 1010 ffu/ml are typical with Basic Protocol 1 and Alternate Protocol 1 after purification. Yields from Alternate Protocol 2 range from 1 × 108 to1 × 109 ffu/ml.
Time Considerations
For Basic Protocol 1 and Alternate Protocols 1 and 2, most time will be spent waiting for cells to reach confluency after seeding. It may be convenient to seed cells in the middle of the week to be ready to inoculate with virus on a Friday; the infection can be incubated over the weekend and virus harvested on a Monday in most instances. Harvesting virus involves only a 5 min spin to collect cells from Basic Protocol 1, and a 15 min spin for Alternate Protocol 1. If following Alternate Protocol 2, virus is collected by scraping cells from flasks, followed by a 10–15 minute centrifugation step, and 20 minute cell-swelling step. Harvested virus from all three protocols can then be frozen and purified at a later time. For Basic Protocol 1 and Alternate Protocol 1 this involves homogenizing virus, pelleting through a sucrose cushion, and purifying on sucrose step gradients, all of which can be easily accomplished in a single day. However, the sucrose step gradients must be prepared the day before needed and stored at 4°C overnight prior to use. For Alternate Protocol 2, the crude purification can be completed in a morning and includes Dounce homogenizing, two 5 minute spins, and a 1 hr centrifugation step to pellet virus.
Titrating virus is done over several days. On day one, cells are seeded into a 6-well dish, and on day two, virus is diluted and applied to the plate for a 1 hr incubation, followed by removal of the inoculum and addition of media. This process takes about 2 hrs, and is followed by a 2–5 day incubation. Staining and/or counting foci can be easily accomplished in about 2 hrs unless the virus requires X-gal staining. The wash and staining process takes less than an hour, but it can take from 4–8 hr for color to develop so that foci can be counted.
Storage
MYXV is stable for a number of years when stored at −80°C, but a reduction in titer by one log is likely after 10 years or more. It should be stored in 0.2–1.0 ml aliquots in cryotubes, as repeated freezing/thawing will reduce the titer by one or two logs. Aliquot in an appropriate volume according to specific needs.
LITERATURE CITED
- 1.Cameron C, Hota-Mitchell S, Chen L, Barrett J, Cao JX, Macaulay C, Willer D, McFadden G. The complete DNA sequence of myxoma virus. Virology. 1999;264:298–318. doi: 10.1006/viro.1999.0001. [DOI] [PubMed] [Google Scholar]
- 2.Fenner F, Ratcliffe FN. Myxomatosis. Cambridge, England: Cambridge Univ. Press; 1965. [Google Scholar]
- 3.Johnston J, McFadden G. Poxvirus immunomodulation strategies: current perspectives. J. Virology. 2003;77:6093–6100. doi: 10.1128/JVI.77.11.6093-6100.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kerr P, McFadden G. Immune responses to myxoma virus. Viral Immunology. 2002;15:229–246. doi: 10.1089/08828240260066198. [DOI] [PubMed] [Google Scholar]
- 5.Kim M, Madlambayan GJ, Rahman MM, Smallwood SE, Meacham AM, Hosaka K, Scott EW, Cogle CR, McFadden G. Myxoma virus targets primary human leukemic stem and progenitor cells while sparing normal hematopoietic stem and progenitor cells. Leukemia. 2009;23:2313–2317. doi: 10.1038/leu.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kotwal GJ, Abrahams M-R. Growing poxviruses and determining virus titer. In: Isaacs SN, editor. Methods in Molecular Biology, Vol. 269: Vaccinia Virus and Poxvirology Methods and Protocols. New Jersey: Humana Press; 2004. pp. 101–112. [DOI] [PubMed] [Google Scholar]
- 7.Liu L, Lalani A, Dai E, Seet B, Macauley C, Singh R, Fan L, McFadden G, Lucas A. The viral anti-inflammatory chemokine-binding protein M-T7 reduces intimal hyperplasia after vascular injury. J. Clin. Investig. 2000;105:1613–1621. doi: 10.1172/JCI8934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lucas A, McFadden G. Secreted immunomodulatory viral proteins as novel biotherapeutics. J. Immunol. 2004;173:4765–4774. doi: 10.4049/jimmunol.173.8.4765. [DOI] [PubMed] [Google Scholar]
- 9.Lun X, Yang W, Alain T, Shi Z-Q, Muzik H, Barrett JW, McFadden G, Bell J, Hamilton MG, Senger DL, Forsyth PA. Myxoma virus is a novel oncolytic virus with significant antitumor activity against experimental human gliomas. Cancer Res. 2005;65:9982–9990. doi: 10.1158/0008-5472.CAN-05-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McFadden G. Poxvirus tropism. Nat Rev Microbiol. 2005;3:201–213. doi: 10.1038/nrmicro1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Regnery DC. The epidemic potential of Brazilian myxoma virus (Lausanne strain) for three species of North American cottontails. Am. J. Epidemiol. 1971;94:514–519. doi: 10.1093/oxfordjournals.aje.a121349. [DOI] [PubMed] [Google Scholar]
- 12.Silvers L, Barnard D, Knowlton F, Inglis B, Labudovic A, Holland MK, Janssens PA, van Leeuwen BH, Kerr PJ. Host-specificity of myxoma virus: Pathogenesis of South American and North American strains of myxoma virus in two North American lagommorph species. Vet. Microbiol. 2009 doi: 10.1016/j.vetmic.2009.09.031. doi:10.1016/j.vetmic.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 13.Stanford MM, Barrett JW, Nazarian SH, Werden S, McFadden G. Oncolytic virotherapy synergism with signaling inhibitors: rapamycin increases myxoma virus tropism for human tumor cells. J. Virol. 2007;81:1251–1260. doi: 10.1128/JVI.01408-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stanford MM, McFadden G. Myxoma virus and oncolytic virotherapy: a new biological weapon in the war against cancer. Expert Opin Biol Ther. 2007;7:1415–1425. doi: 10.1517/14712598.7.9.1415. [DOI] [PubMed] [Google Scholar]
- 15.Stanford MM, Werden SJ, McFadden G. Myxoma virus in the European rabbit: interactions between the virus and its susceptible host. Vet Res. 2007;38:299–318. doi: 10.1051/vetres:2006054. [DOI] [PubMed] [Google Scholar]
- 16.Sypula J, Wang F, Ma Y, Bell J, McFadden G. Myxoma virus tropism in human tumor cells. Gene Ther Mol Biol. 2004;8:103–114. [Google Scholar]
INTERNET RESOURCES
- 1.Website for U.S. Department of Health and Human Services’ Biosafety in Microbiological and Biomedical Laboratories (BMBL) 5th Edition. http://www.cdc.gov/od/OHS/biosfty/bmbl5/BMBL_5th_Edition.pdf.