Abstract
Rapid eye movement (REM) sleep behavior disorder (RBD) is a parasomnia manifested by vivid, often frightening dreams associated with simple or complex motor behavior during REM sleep. Patients appear to “act out their dreams,” in which the exhibited behaviors mirror the content of the dreams, and the dream content often involves a chasing or attacking theme. The polysomnographic features of RBD include increased electromyographic tone +/- dream enactment behavior during REM sleep. Management with counseling and pharmacologic measures is usually straight-forward and effective.
In this review, the terminology, clinical and polysomnographic features, demographic and epidemiologic features, diagnostic criteria, differential diagnosis, and management strategies are discussed. Recent data on the suspected pathophysiologic mechanisms of RBD are also reviewed. The literature and our institutional experience on RBD are next discussed, with an emphasis on the RBD-neurodegenerative disease association and particularly the RBD-synucleinopathy association. Several issues relating to evolving concepts, controversies, and future directions are then reviewed, with an emphasis on idiopathic RBD representing an early feature of a neurodegenerative disease and particularly an evolving synucleinopathy. Planning for future therapies that impact patients with idiopathic RBD is reviewed in detail.
Keywords: REM sleep behavior disorder, parasomnia, synucleinopathy, neurodegenerative disease
Overview
Rapid eye movement sleep behavior disorder (RBD) is characterized by loss of normal skeletal muscle atonia during rapid eye movement (REM) sleep with prominent motor activity and dreaming. In this article, the clinical, epidemiologic, and polysomnographic features of RBD will be reviewed, followed by discussions on the criteria for diagnosis of RBD, and the management of this disorder. Recent data on the pathophysiologic underpinnings of RBD is next discussed. RBD associated with neurodegenerative disorders will then be discussed, with a particular emphasis on the RBD-synucleinopathy association. The concept of “idiopathic” RBD and the multitude of findings on ancillary studies in those labeled with idiopathic RBD will next be reviewed. Discussions on the evolving concepts and controversies will then follow. Finally, several issues pertaining to planning for future therapeutic trials in those with iRBD due to an underlying and evolving neurodegenerative disorder will be presented. Readers are directed to some key recent reviews on the fascinating parasomnia of RBD.1-3
The Core Aspects of RBD
Terminology
Some of the terms relating to REM sleep and RBD are confusing, and many reviews of RBD have not tended to elaborate on the ever-important issue of terminology.
Stage R
Stage R the new nomenclature proposed by the American Academy of Sleep Medicine, has changed the term “Stage REM” to “Stage R.”4 For historical and other reasons, the term “REM sleep” will be used throughout most of this article.
Dream enactment behavior (DEB)
Dream enactment behavior (DEB) this is a term that many investigators use to describe a history of recurrent nocturnal dream enactment behavior (abbreviated DEB). While almost all patients with RBD have a history of DEB (the few that don't are diagnosed purely on PSG – see below), not all patients with a history of DEB have RBD; similar behavior can also occur in untreated obstructive sleep apnea,5 as well as in sleepwalking and sleep terrors in adults, epilepsy, post-traumatic stress disorder, or as an effect of alcohol or drug administration or withdrawal.1, 6 Thus, most experts view that PSG confirmation of RBD is essential to make the diagnosis since DEB is not specific for RBD.
REM sleep without atonia (RSWA)
REM sleep without atonia (RSWA) this is the PSG-defined finding of increased electromyographic (EMG) tone during REM sleep (Stage R). Normally during REM sleep, there is active inhibition of EMG activity leading to complete or near complete atonia on the EMG derivations, but REM sleep without atonia (often abbreviated RSWA) represents the abnormal state of increased EMG tone. A major issue in PSG methodology currently is to establish what degree of EMG activity is considered within normal limits and what exceeds this and should be considered abnormal. This issue will be discussed further in the sections that follow.
REM sleep behavior disorder (RBD)
REM sleep behavior disorder (RBD) the term REM sleep behavior disorder (abbreviated RBD) is a distinct parasomnia characterized by both abnormal REM sleep electrophysiology – RSWA – and abnormal REM sleep behavior – a history of recurrent nocturnal DEB. Most experts view that the diagnosis of RBD cannot be applied unless both DEB and RSWA are present, and hence a polysomnogram is required to make the diagnosis. The shortened term “REM behavior disorder” is incomplete – it is the abnormal behaviors during REM sleep that the disorder of RBD represents (rapid eye movements or REMs occur during wakefulness – this is not relevant to the subject of RBD).
Subclinical RBD
Subclinical RBD some authors have equated the PSG finding of RSWA with subclinical RBD,7, 8 implying that such patients with increased EMG tone during REM sleep (Stage R) will subsequently develop clinical RBD. While we have certainly evaluated patients with RSWA who not only develop clinical RBD, but also a neurodegenerative disorder, this author would argue that there has not been a sufficient number of prospectively followed subjects with RSWA who subsequently develop clinical RBD to justify use of the term “subclinical RBD.” Hence, the PSG finding of RSWA should be applied, and longitudinal data will justify or refute equating RSWA with subclinical RBD.
Clinically probable RBD or probable RBD (pRBD)
Clinically probable RBD or probable RBD (pRBD) this term is synonymous with recurrent nocturnal DEB, and is being used more frequently at present in epidemiologic studies when PSG-confirmation of RBD is not feasible in all cases, and by investigators who are not able to perform sleep studies due to lack of availability of sleep centers and/or lack of reimbursement for PSGs.
Idiopathic RBD (iRBD)
Idiopathic RBD (iRBD) this term refers to RBD occurring in the absence of any other obvious associated neurologic disorder. There is a large amount of recent data suggesting that many patients with iRBD actually represent an evolving neurodegenerative disorder (discussed in detail later in this review), which has fostered many authors to qualify the term idiopathic RBD with surrounding quotation markers (ie, “idiopathic” RBD).
Secondary or symptomatic RBD
Secondary or symptomatic RBD these terms refer to the combination of RBD plus another neurologic disorder, such as narcolepsy or a neurodegenerative disease.
Considering some of these issues relating to terminology, one could propose some minor changes to some key terms as is shown in Table 1. These are merely suggestions based on this author's experience and opinions.
Table 1. Proposed Minor Changes to the Definitions and Diagnostic Criteria for REM Sleep Without Atonia and and REM Sleep Behavior Disorder.
| REM sleep without atonia (RSWA) |
| Abnormal EMG tone during REM sleep |
|
| Probable RBD |
| Abnormal behaviors during REM sleep |
|
| Definite RBD |
| Abnormal sleep behavior and abnormal EMG tone during REM sleep. Items A + B + C must be present for the diagnosis of definite RBD |
|
Illustrative Case
There are many illustrative examples in the literature. One patient evaluated at our institution fell asleep as a passenger on a transatlantic commercial flight and then exhibited punching and kicking behavior (who months later still recalled the dream of fighting animals in a cave). The behavior was interpreted as a seizure and the pilot urgently redirected the airliner back to the United States mainland for emergency medical care, resulting in tens of thousands of dollars being spent on the emergency landing, thousands more during the inpatient work-up of a suspected seizure, and requiring hundreds of passengers to change their travel plans. The initial inpatient evaluation was unrevealing, but the patient was prescribed phenytoin which he continued to use up to his initial evaluation at our institution several weeks after the plane incident. Periodic nocturnal dream enactment behavior had continued. The description of the prior spell and many other episodes of nocturnal dream enactment behavior over the preceding years, as described by the patient himself (he was a widower) were classic for RBD, and the diagnosis was confirmed on polysomnography. Phenytoin was discontinued, and low-dose clonazepam proved to provide complete control of his nightmares and behaviors. Upon re-evaluation two years later for a newly-developed rest tremor in one limb, shuffling gait, slowness of movement, etc., he had developed the cardinal features of Parkinson's disease.
Clinical Features
The demographic and clinical phenomenology of RBD are summarized in Table 2; examples of specific features and examples of behaviors can be found in numerous references.1-3, 9-27 One can characterize the three primary aspects of RBD as abnormal vocalizations, abnormal motor behavior, and altered dream mentation.
Table 2. Demographics and Clinical Phenomenology of REM Sleep Behavior Disorder.
| Demographics |
| Male gender predilection |
| Age of onset typically 40-70 years (range 15-80 years) |
| Clinical Phenomenology |
| Abnormal vocalizations – orating, yelling, swearing, screaming |
| Abnormal motor behavior – limb flailing, punching, kicking, lurching out of bed |
| Altered dream mentation – typically involve a chasing/attacking theme, with the insects, animals or other humans being the aggressors and the patient being the defender |
| Exhibited behaviors mirror dream content |
| Behaviors tend to occur in the latter half of the sleep period |
Abnormal vocalizations
While individuals may grunt, speak, laugh, or vocalize in a variety of ways during non-REM (NREM) and REM sleep, and such vocalizations are not necessarily “abnormal,” the vocalizations in RBD tend to be loud and suggest unpleasant dream mentation. Shouting, screaming, and swearing are common, and are often described as being very unlike the typical soft-spoken nature of the person's tendency to speak during wakefulness.
Abnormal motor behavior
Infrequent limb jerks are also common during sleep in individuals without RBD, but in those with RBD, the motor activity often begins with some repetitive jerking or movements, followed seconds later by more dramatic and seemingly purposeful activity such as punching, flailing as if to protect oneself, running, jumping out of bed, etc. It is during these behaviors that injuries to patients and their bedpartners can occur. Some bedpartners have attempted to awaken patients during an episode, and their comments and gestures become interwoven into the dream, sometimes resulting in injury. One spouse would keep a broom under her side of the bed and use it as her “wake-up poker” to abort her husband's RBD episode while she stood four feet away, yet on occasion this would be grabbed by her husband and used as a sword to fend his attackers. Bruises, pulled hair, limb fractures, and subdural hematomas have all been reported.
Altered dream mentation
Most patients view their dreams as nightmares, and the dream content often involves insects, animals, or people chasing or attacking them or their relatives or friends; the patient is almost always the defender and not the attacker. Many patients are able to recount the content of their dreams upon being awakened at the time of the behavior. Unlike most pleasant dreams and nightmares, when most individuals typically recall the details of dreams vividly upon awakening but seemingly forget almost all details by noon the following day, those with RBD can often recall vivid details of the nightmares for days, and sometimes for weeks or years. Those with significant dementia may not be able to recall and/or describe their dreams; in such cases, bedpartner observations of the abnormal behaviors are helpful.
The vocalizations and behaviors that are exhibited are strikingly consistent with the content of the dreams later reported by the patient – the behaviors mirror the dream content. Bedpartners are often very accurate at predicting what the patient had just dreamed about based on their observations of the dream enactment behavior. One vivid example involved a man who held his wife's head in a headlock while moving his legs as if running while both were attempting to sleep in bed, then exclaimed, “I'm gonna make that touchdown!” and then attempted to forcefully throw her head down toward the foot of the bed. When awakened, he recalled a dream in which he was running for a touchdown, and he spiked the “football” in the end zone. His wife knew precisely what he had been dreaming about.17
The timing of RBD episodes reflects when the patient is in REM sleep, and since the majority of REM sleep occurs during the latter half of the sleep period (particularly latter third of the sleep period, which for most individuals is after 3 am), RBD tends to be exhibited in the few hours prior to wake onset. There are exceptions to this, however, primarily in those who have narcolepsy (and thus enter REM sleep frequently within an hour of sleep onset) or who have an increased REM sleep drive due to sleep deprivation or untreated obstructive sleep apnea.
We have heard numerous descriptions from wives who experienced their first instance of being struck on their wedding night, thereby indicating that RBD can present in one's late teens or twenties. Many of these patients did not begin exhibiting features of a neurodegenerative disorder until 3-6 decades later. The frequency of DEB also varies widely – without treatment, some exhibit DEB every night, sometimes several times each night (presumably during most or all episodes of REM sleep), while others exhibit it no more than one night per month. Still others appear to exhibit clustering, with RBD occurring nightly for a week and then going months with little or no RBD, and then RBD occurring frequently some time later. Such patients are rarely able to identify an obvious trigger for the flurry of RBD. It is not known why the frequency varies so widely in patients with RBD.
Demographic and Epidemiologic Features
Most patients with RBD are male. Onset of symptoms varies widely, although most develop symptoms in the 40-70 age range. Those with RBD evolving before age 40 typically have narcolepsy (RBD and narcolepsy often coexist), although some whose RBD begins early in life can evolve into a neurodegenerative disorder decades later.28, 29 There is new data suggesting that the male predominance of RBD associated with PD (82%) and DLB (82%) is significantly less frequent than that associated with MSA (64%).30 This is a topic clearly worthy of further study if indeed there are sex differences on the specific type of neurodegenerative disease associated with RBD. Other published data on the frequency of RBD associated with neurodegenerative disease is largely based on convenience samples, and cannot be considered truly representative of the frequency of RBD associated with other neurodegenerative disorders.
The only published epidemiologic data on parasomnias in the population with relevance to RBD was based on telephone interviews in the United Kingdom. Ohayon et al. conducted interviews using the Sleep-EVAL system in 4972 individuals aged 15-100 years, and found 106 (2%) reported violent behaviors during sleep, most of whom were male.31 Twenty-five of these participants (0.5% of the sample) reported features highly suggestive of RBD. This single study has thus formed the basis for the estimated prevalence of RBD to be 0.5%.
An investigation of all patients residing in Olmsted County, Minnesota, who carried the diagnosis of PSG-confirmed RBD on April 1, 2008 was 0.02% (Table 3) (Boeve et al, unpublished data). This is by no means a sound epidemiologic study of the prevalence of RBD in a population, but the frequency of 20/100,000 could at least be considered a minimum estimate of the frequency of RBD in one county who have come to medical attention, undergone a PSG, and had the diagnosis confirmed. This is clearly a gross underestimate also when one considers that the sample from which these subjects were derived only includes participants in an aging and dementia research program, and while many residents of Olmsted County with PD also have probable RBD, they either do not have RBD confirmed by PSG, or do have PSG-confirmed RBD but are not participating in this particular research program.
Table 3. Clinical Features/Diagnoses in Residents of Olmsted County, Minnesota Who Are Participants in an Aging and Dementia Research Program and Had PSG-Proven RBD on January 1, 2008*.
| Primary clinical diagnoses | N |
| Normal neurologic functioning (idiopathic RBD) | 8 |
| Mild cognitive impairment (MCI) | 2 |
| MCI plus mild parkinsonian signs | 2 |
| Parkinson's disease with MCI | 1 |
| Dementia with Lewy bodies (DLB) | 7 |
| TOTAL | 20 |
Population of Olmsted County, Minnesota on 1/1/08 – approximately 100,000
20/100,000 = 0.02% = absolute minimum point prevalence of PSG-proven RBD
3 additional participants (2 with Parkinson's disease and 1 with DLB) have classic features of RBD but no REM sleep was attained on PSG; thus RBD is suspected (ie, probable RBD) but was not confirmed on PSG
Another dataset using the Mayo Sleep Questionnaire (discussed in more detail below) as a screening measure for probable RBD identified 79 (8.9%) of the 892 participants in the Mayo Clinic Study of Aging (MCSA) who screened positive for RBD;32 the MCSA is a population-based study assessing cognition, functional status, laboratory markers, neuroimaging markers, etc., in 70-89 year old community-dwelling residents of Olmsted County, Minnesota. While the RBD questions on the MSQ are not 100% specific for RBD (specificity is around 70% based on one validation study,33 our prior findings of increased frequency of parkinsonism,34 apathy and anxiety,35 and lower scores on measures of attention/executive functioning36 associated with positive screening for RBD on the MSQ in the MCSA, does suggest a large proportion of those who screen positive likely do have RBD.
Considering the growing data that “idiopathic” RBD may actually represent an evolving neurodegenerative disorder in a sizable proportion (to be discussed in more detail below), a well-designed epidemiologic study of the prevalence of RBD is clearly warranted.
Diagnostic Criteria
The 2nd edition of the International Classification of Sleep Disorders requires the following for the clinical diagnosis of RBD:37
Presence of RSWA on PSG
-
At least one of the following:
sleep-related, injurious, potentially injurious, or disruptive behaviors by history (i.e., dream enactment behavior), and/or
abnormal REM sleep behavior documented during polysomnographic monitoring,
Absence of EEG epileptiform activity during REM sleep unless RBD can be clearly distinguished from any concurrent REM sleep related seizure disorder
The sleep disorder is not better explained by another sleep disorder, medical or neurological disorder, mental disorder, medication use, or substance use disorder.
The American Academy of Sleep Medicine has critically evaluated the scoring methods of PSGs, and the formal diagnosis of RBD has changed slightly,4 and will likely change further as refinement occurs – this is discussed in more detail in the Polysomnographic Features section below.
Differential Diagnosis
The differential diagnosis of recurrent DEB includes the non-REM parasomnias (somnambulism, night terrors, confusional arousals), nocturnal panic attacks, nocturnal seizures, nightmares, noctural wandering associated with dementia, and obstructive sleep apnea (OSA). The history usually allows differentiation of these disorders from RBD. When diagnostic clarification is necessary, particularly when the risk for injury is high, the behaviors occur at any time of the night, other features suggesting an evolving neurodegenerative is present, or loud snoring and observed apnea suggestive of OSA is present, PSG with simultaneous video monitoring is warranted.
Polysomnographic Features
Those unfamiliar with the updated sleep stage characterization and scoring of PSGs should review the references which were authored by key authorities in the field of sleep medicine.38, 39 The new American Academy of Sleep Medicine Manual for the Scoring of Sleep and Related Events4 has maintained many of the criteria and definitions for REM sleep (stage R), primarily low amplitude mixed frequency EEG background, rapid eye movements, and low chin EMG tone. RSWA can be applied when there is 1) sustained muscle activity in REM sleep with 50% of the epoch having increased chin EMG amplitude, and/or 2) excessive transient muscle activity, defined by the presence of 5 or more mini-epochs (a 30 second epoch is divided into 10 3-second mini-epochs) in an epoch having transient muscle activity lasting at least 0.5 seconds. There was no minimum number of epochs showing abnormal muscle activity required for the RSWA designation – this was purposefully not stated as there is little good normative data.4
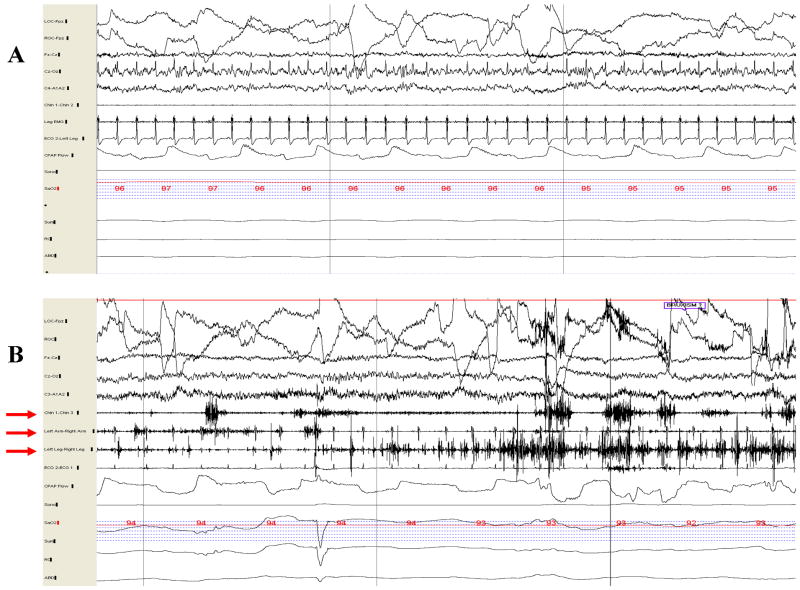
One can appreciate normal REM sleep/stage R as characterized by rapid eye movements, minimal to no EMG tone, and mixed alpha and theta activity on electroencephalography (EEG) as shown in Figure 1A. The characteristic electrophysiologic finding in patients with RBD is RSWA. This usually takes the form of a pathologic accentuation of transient muscle activity as shown in Figure 1B. Simultaneous video/PSG recording is essential for evaluating patients with suspected RBD, so that vocalizations and limb movements can be captured and viewed concurrently with PSG data. When vocalizations and/or limb movements emerge during REM sleep, without associated epileptiform activity on the EEG derivations (as in Figure 3B), the diagnosis of RBD is established. In our experience, violent and complex dream enactment behavior is relatively uncommon during single night PSG recordings; rather, increased EMG tone during REM sleep and sparse limb jerks are the norm.
Figure 1.
Thirty-second epoch polysomnograms showing normal REM sleep (A) and REM sleep without atonia—the electrophysiologic substrate for RBD (B). In A, note the absence of electromyographic (EMG) activity in the submental (Chin 1-Chin 2), and limb (Leg EMG) derivations, whereas increased EMG tone is present in the submental (Chin 1-Chin 3), upper limb (Left Arm-Right Arm), and lower limb (Left Leg-Right Leg) derivations in B (denoted by red arrows).
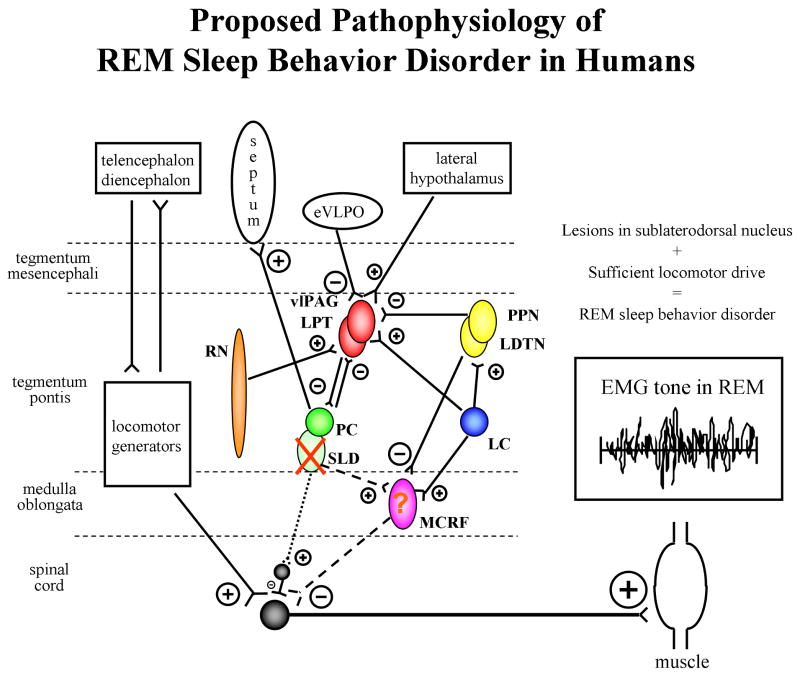
Figure 3. Proposed pathophysiology of REM sleep behavior disorder in humans.
Excitatory projections represented by ⊕, inhibitory projections represented by ⊖, with the size of these symbols representing the relative effect of each projection on the synapsing nuclei. Nuclei are represented by circles or ovals, with solid colored circles and ovals reflecting those with normal populations of neurons, and speckled circles and ovals reflecting those with significantly reduced populations of neurons. An X reflects ablation of a nucleus. The relative tonic influences of each projection are represented by line thickness, with thicker lines depicting stronger influences, thinner lines depicting weaker influences, and dashed and dotted lines depicting weak influences due to damage to neurons in the respective nuclei. The REM-off region is represented by the vlPAG and LPT in red, and the REM-on region is represented by the PC and SLD in green. The SLD (or analogous nucleus in humans) projects to spinal interneurons (“direct route,” denoted by the dotted line from SLD to spinal interneurons) and likely represents the final common pathway that causes active inhibition of skeletal muscle activity in REM sleep. The “indirect route,” denoted by the dashed line from SLD to the MCRF to the spinal interneurons, may also contribute to EMG atonia. However, in humans, it is not yet known whether lesions in structures which project to and from the MCRF, and lesioning the MCRF itself, are critical in affecting EMG atonia during REM sleep. EMG=electromyographic, eVLPO= extended part of the ventrolateral preoptic nucleus, LC=locus coeruleus, LDTN=laterodorsal tegmental nucleus, LPT=lateral pontine tegmentum, MCRF=magnocellular reticular formation, PC=pre-coeruleus, PPN=pedunculopontine nucleus, REM=rapid eye movement, RN=raphe nucleus, SLD=sublaterodorsal nucleus, vlPAG=ventrolateral part of the periaqueductal grey matter. See text for details. From Boeve et al, Brain 2007;130:2770-2788. Reprinted with permission from Oxford University Press.
Due to scheduling challenges and logistical issues, in which PSGs for evaluation of suspected RBD cannot be arranged in a timely manner at sleep disorder centers with procedures in place for assessing parasomnias, the sleep clinicians knowledgeable in the parasomnias attempt to work with local sleep clinicians to encourage them to perform PSGs with extra surface EMG leads on the limbs, and have all video/PSG data time-locked or even more simply unsynchronized to simultaneous video/PSG recording. The local sleep clinician is also requested to scrutinize the degree to which EMG tone is increased during REM sleep, whereas there should be essentially complete atonia. Frustratingly, this is very rarely accomplished, and thus the question of whether individual patients have REM sleep without atonia or overt RBD captured on PSG remains unanswered. This lack of consistency has many root causes, not the least of which being inadequate training of sleep fellows in the assessment and management of parasomnias, as well as the variable views of how to operationalize the assessment of what truly constitutes REM sleep without atonia vs normal EMG atonia during REM sleep. This issue will be discussed further in the section on Evolving Concepts, Controversies and Future Directions.
Management
The goals of therapy are to minimize the three cardinal features of the disorder – decrease the frequency and severity of the abnormal vocalizations (thereby reducing the embarrassing nature of screams and swearing with guests in the home, when traveling and sleeping in hotels, and when fishing/hunting/camping and sleeping in tents), decrease the frequency and severity of the abnormal behaviors (thereby reducing the risk of injury to the patient and bedpartner), and decrease the unpleasant dreams (thereby reducing the anticipatory concerns about nightmares which sometimes results in overt “sleep phobia”). All patients and their bedpartners should be counseled on simple steps to minimize injury, such as moving sharp and edged objects out of harms way, and placing a mattress or cushion of some type on the floor adjacent to the bed (many patients use foam rubber mattresses). A protective barrier placed on the side of the bed, such as any designed for infants to decrease the risk from falling off the bed, are variably effective.
Since most patients with RBD are male, it may be the “male pride” that keeps them from using barriers designed for infants, and other techniques have been used. Some have constructed plywood barriers placed along side the bed and on the bed in between the patient and spouse, with padding affixed to the sides of the plywood facing the patient. Others use a small mattress and place it on its side adjacent to the bed, with chairs leaning against the mattress to keep it in place. Some sleep in a sleeping bag in the bed in a cocoon-like fashion, with the open end of the sleeping bag toward the head tied as snuggly as possible. Some go to bed with oven mits on their hands, with shoestrings tied around the wrists to keep the mits in place. One man has used a rope with one end tied around him and the other end tied around the bedpost to alter his tendency to lurch and run out of bed. These and other colorful examples of safety ingenuity are described in other informative and entertaining sources1 – Carlos Schenck's text on parasomnias is a must-read for anyone interested in the RBD field.2
Clonazepam has been the drug of choice in those without significant cognitive impairment nor OSA, and is usually effective at 0.25-0.5 mg/night, but doses above 1 mg nightly are necessary in some patients.12, 22 Recent experience with melatonin shows that doses ranging from 3-12 mg/night can be effective either as sole therapy, or in conjunction with clonazepam when either melatonin or clonazepam alone is ineffective.40, 41 As noted in an excellent recent review on the subject of treatment,42 other drugs reported to improve RBD include pramipexole,43 donepezil,44 levodopa,45 carbamazepine,46 triazolam,22 and clozapine.22 We have also found quetiapine to be quite effective for managing RBD in many patients.
For unmarried couples who are dating, intimacy invitations to women by males with RBD are made only after considerable planning. Many married couples choose to sleep in separate bedrooms, with the resulting loss of intimacy. All of these accounts described above may sound somewhat comical to those unfamiliar with the disorder of RBD, but for those with frequent and severe episodes, preparing for sleep requires completing a nightly routine with potentially disastrous consequences when the drug and non-drug aspects of management are not followed.
As noted above, most patients experience a marked improvement in the frequency and severity of RBD features with medical therapy. Yet there are rare individuals whose RBD continues despite clonazepam being used in excess of 3 mg/night plus melatonin in excess of 12 mg/night. The addition of quetiapine, or levodopa, or a dopamine agonist sometimes provides benefit, other times not. We are aware of one patient whose RBD continued with nightly nightmares, screaming, and occasional injuries to himself despite clonazapem 4 mg/night, melatonin 12 mg/night, and quetiapine 100 mg/night, but with the addition of sodium oxybate at 3 grams at bedtime and 3 grams taken 3 hours later, his RBD was essentially completely controlled. He was later able to control RBD entirely with sodium oxybate monotherapy, yet if he forgot to take a dose, RBD would invariably occur. This author views sodium oxybate as a last resort, but for the very rare individuals with otherwise medically-refractory RBD, the benefit of markedly reduced risk of injury could be considered to outweigh the risks.
It is not clear why clonazepam, melatonin, and other agents improve RBD. Clonazepam reduces phasic activity in REM sleep, and while this agent clearly improves the three cardinal features in most patients, RSWA is still evident in those who undergo PSG while taking the drug. Melatonin has been shown to decrease the percentage of REM sleep epochs without muscle atonia and decrease the number of stage shifts in REM sleep, suggesting it has a more direct mode of action on REM sleep pathophysiology. One hypothesis is that it restores circadian modulation of REM sleep.40 Most sleep experts are frankly puzzled why melatonin has any effect on RBD.
There is recent data that some agents, particularly within the selective serotonin reuptake inhibitor (SSRI) and selective norepinephrine reuptake inhibitor (SNRI) classes, can precipitate or aggravate RBD in some individuals.47, 48 One interpretation is that this class of drugs sufficiently alter REM sleep physiology to seemingly induce RBD. Another interpretation is that in such patients, REM sleep control has already become dysregulated – perhaps due to an early evolving neurodegenerative disorder – and the SSRI or SNRI unmasks RBD which would have manifested months or years later (see section on Evolving Concepts and Controversies). In patients with both RBD and depression, which also often coexist, it may be best to favor an agent such as buproprion over the SSRI and SNRI agents due to its different pharmacologic properties.1
Pathophysiology
A comprehensive review of the known pathophysiology of RBD in the animal models and the hypothesized pathophysiology in humans can be found elsewhere;3 in this review, concepts and data pertaining to human RBD will be emphasized.
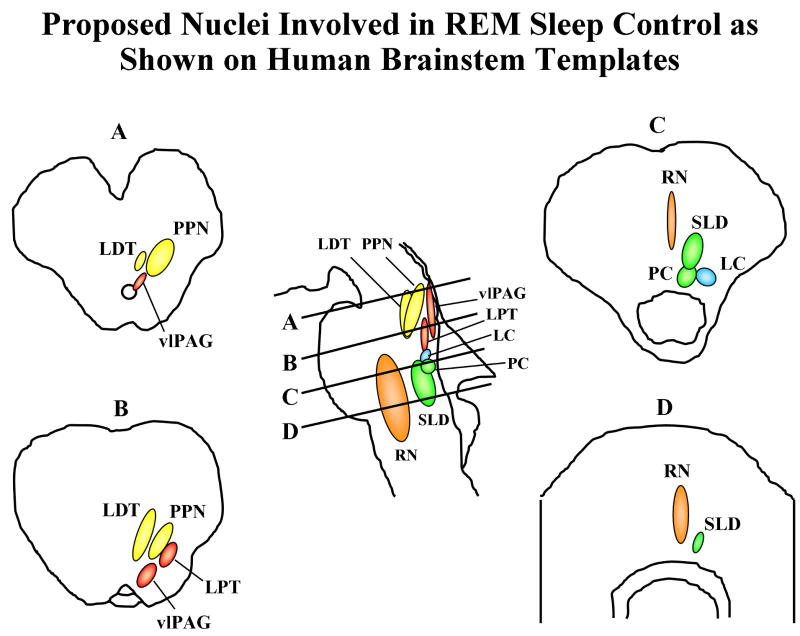
The proposed anatomic substrate for REM sleep control in humans is shown in Figure 2, and the proposed pathophysiology of human RBD is shown in Figure 3. The critical structures in the brainstem include the “REM-off” region consisting of the ventrolateral part of the periaqueductal gray matter (vlPAG) and lateral pontine tegmentum (LPT), the “REM-on” region consisting of the pre-coeruleus (PC) and sublaterodorsal nucleus (SLD), as well as the extended part of the ventrolateral preoptic nucleus (eVLPO), locus coeruleus (LC), laterodorsal tegmental nucleus (LDTN), pedunculopontine nucleus (PPN), and raphe nucleus (RN).
Figure 2. Proposed nuclei involved in REM sleep control as shown on human brainstem templates.
Letters represent cross-sectional views through the brainstem, with A corresponding to the pontomesencephalic junction, B to the upper/mid pons, C to lower/mid pons, and D just rostral to the pontomedullary junction. The REM-off region is represented by the vlPAG and LPT in red, and the REM-on region is represented by the PC and SLD in green. eVLPO= extended part of the ventrolateral preoptic nucleus, LC=locus coeruleus, LDTN=laterodorsal tegmental nucleus, LPT=lateral pontine tegmentum, PC=pre-coeruleus, PPN=pedunculopontine nucleus, REM=rapid eye movement, RN=raphe nucleus, SLD=sublaterodorsal nucleus, vlPAG=ventrolateral part of the periaqueductal grey matter. See text for details. From Boeve et al, Brain 2007;130:2770-2788. Reprinted with permission from Oxford University Press.
Studies in the cat and rat models suggested that there are two motor systems involved in normal REM sleep: one for generating muscle atonia and one for suppressing locomotor activity.49-57 The absence of motor activity in normal REM sleep occurs via active inhibition of spinal motoneurons plus reduced drive within locomotor generators. While phasic oculomotor and locomotor activity such as rapid eye movements and brief and low amplitude muscle twitches occur as normal phenomena in REM sleep, more elaborate motoric activity is directly or indirectly suppressed.18, 58 The final common pathway of spinal motor neuron inhibition was inferred to be via the medullary magnocellular reticular formation (MCRF); this inhibitory nucleus is known to suppress anterior horn cell activity via projections of the ventrolateral reticulospinal tract (VLST). The pontine nuclei described above are known to influence the REM and non-REM sleep circuits. In addition, midbrain and forebrain structures have been tied into these circuits: substantia nigra, hypothalamus, thalamus, basal forebrain, and frontal cortex.
The brainstem regions that have classically been considered in RBD pathophysiology based on lesion studies in cat include the MCRF, locus coeruleus/subcoeruleus complex, PPN, LDTN, and possibly substantia nigra (SN).49-57 Although these studies have identified components of REM sleep circuits, the primary sites and interactions have been debated. Lesions in the MCRF release the tonic inhibition on spinal motoneurons, leading to RSWA, but these lesions also destroyed fibers of passage. Lesions in the coeruleus/subcoeruleus complex cause RSWA, and the site and size of the lesion determines whether simple or complex behaviors are exhibited.49 There is also debate whether lesions in the PPN cause RSWA.45, 55, 56 The substantia nigra and the dopaminergic system have been proposed as a component of this REM sleep system, but there is minimal direct evidence to implicate the substantia nigra or dopaminergic dysfunction in RBD pathophysiology. Similarly, no convincing examples of RSWA nor RBD that have resulted from lesions in the diencephalon or telencephalon. Most evidence now suggests that populations of neurons that are considered “REM-on” cells in the subcoeruleus region are central to REM sleep and the associated EMG atonia.59, 60
The sublaterodorsal nucleus (SLD) identified by Boissard et al, which is equivalent to the subcoeruleus or peri-locus coeruleus in the cat, is the major structure responsible for REM sleep.61, 62 More recent work has led to the concept of a putative on/off switch for control of REM sleep.60
We have hypothesized that the structures and networks in humans are similar to the animal models,3 with the SLD or analogous nucleus with projections to spinal interneurons (“direct route”, denoted by the dotted line from SLD to spinal interneurons in Figure 3) being the final common pathway that causes active inhibition of skeletal muscle activity in REM sleep. The “indirect route” (denoted by the dashed line from SLD to spinal interneurons in Figure 3) can also contribute, with SLD lesioning causing reduced excitation of the MCRF, thereby causing a net reduced inhibition of spinal motoneurons (either directly or via spinal interneurons). It is not clear yet if lesioning or degeneration of the MCRF is sufficient to cause RBD in humans.
The locomotor generators, which are presumed to project to the spinal motoneurons either directly or indirectly via other brainstem nuclei, have yet to be identified and characterized. The neuronal circuitry for this process is poorly understood, and supratentorial influences on both the locomotor generators and the muscle atonia system are likely. One could predict that a variety of stimuli could alter locomotor drive and/or muscle atonia, such as other primary sleep disorders (e.g., obstructive sleep apnea), structural lesions in the brainstem, neurodegeneration, medications, illicit drugs, head trauma, etc.
This schema will require careful scrutiny based on meticulous histopathologic studies – this issue is discussed further in the concluding section.
The RBD – Neurodegenerative Disease Association
Analyses in Patients with RBD Plus Parkinsonism and/or Dementia
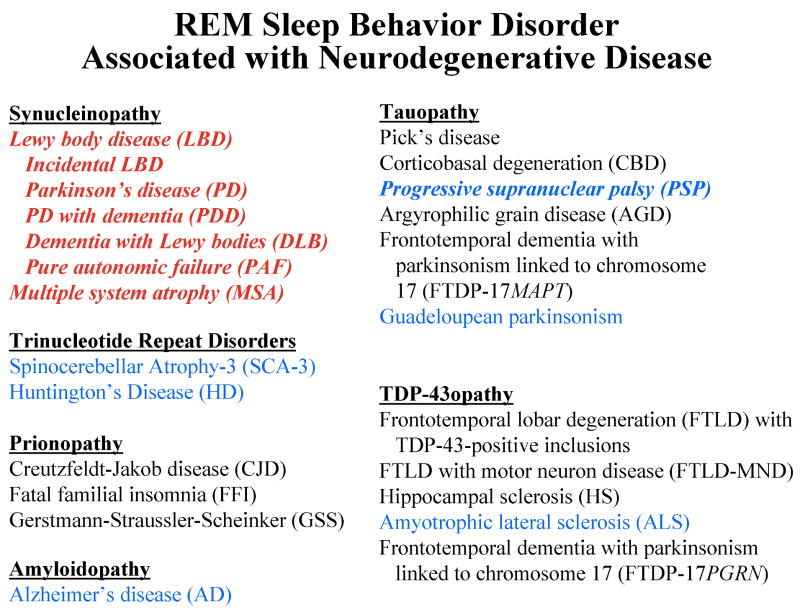
The major clinical syndromes and histopathologic disorders which cause dementia and/or parkinsonism are shown in Figure 4. As shown in this figure, numerous cases of RBD have been reported in association with certain neurodegenerative disorders, but not reported to date in association with most others. RBD is frequently associated with clinically-diagnosed Parkinson's disease,3, 9, 20-23, 25, 26, 40, 43, 47, 63-80 dementia with Lewy bodies,3, 17, 26, 27, 41, 80-87 and multiple system atrophy.9, 10, 20, 22, 25, 26, 88-98 Pure autonomic failure has also been reported.20, 99 RBD was identified in several members of a kindred with a parkin mutation,100 and Lewy body disease pathology has been reported in a different large kindred with parkin mutations.101 Many of the cases described in the reports noted above have had postmortem examination, and all such cases have had Lewy body disease or MSA confirmed at autopsy.
Figure 4.
The clinical syndromes and histopathologic disorders associated with each proteinopathy in the major neurodegenerative disorders which cause dementia and/or parkinsonism. Those syndromes and disorders which are commonly associated with REM sleep behavior disorder (RBD) are shown in red, and those which have been rarely associated with RBD are shown in blue. Those syndromes and disorders associated with RBD in which at least 1 pathologically-verified case has been identified are in italics. Those in black print have not been reported in association with RBD.
RBD has also been associated with other neurodegenerative disorders, albeit far les frequently. Spinocerebellar atrophy – type 3 (SCA-3) has also been reported associated with dream enactment behavior (although few have had PSG confirmation).102-104 Three (12%) of the 25 patients with Huntington's disease studied by Arnulf et al. had evidence of RBD.105 RSWA has been reported in a single case of sporadic CBD,7 but this patient did not have clinical RBD features. RBD has been reported in two cases of clinically-suspected sporadic PSP20, 22 and in 2/15 (13%) of a group of PSP subjects.106 We recently reported on a case who had the DLB phenotype including RBD, but had PSP pathology.3 One case of RBD with amyotrophic lateral sclerosis has also been identified.20 Guadeloupean parkinsonism (a tauopathy) has also been associated with RBD (7/9 patients studied), with 3 of these exhibiting RBD features many years prior to the onset of parkinsonism.107
One case of clinically-diagnosed Alzheimer's disease was associated with RBD,108 and neuropathologic analysis identified both Alzheimer's disease and Lewy body disease.109 We (Boeve et al, unpublished data) and others80, 110, 111 have observed sparse cases of RBD associated with clinically probable AD. In the study by Postuma et al,80 the neuropsychological profile of probable AD cases was indistinguishable to those with probable DLB, which suggests such cases have underlying LBD; these data are consistent with other data showing RBD associated with dementia but not visual hallucinations or parkinsonism likely reflects underlying LBD.83
Our updated clinicopathologic experience involving patients evaluated at Mayo Clinic Rochester and Mayo Clinic Jacksonville who had RBD associated with cognitive impairment/dementia and/or parkinsonism is shown in Table 4, which demonstrates the preponderance of LBD and MSA in this series (41/43=95%). The patient described above with PSP pathology, and a recently identified case with PSG-confirmed RBD who had atypical DLB clinical features yet AD pathology (Boeve et al, unpublished data) are the exceptions.
Table 4. Updated clinicopathologic experience at Mayo Clinic from January 1990 to April 2009 of REM sleep behavior disorder associated with dementia and/or parkinsonism.
| Primary pathologic diagnoses | N |
| Lewy body disease | 36 |
| Mutliple system atrophy | 5 |
| Progressive supranuclear palsy | 1 |
| Alzheimer's disease | 1 |
| TOTAL | 43 |
Synucleinopathy pathology associated with RBD in this series: 41/43=95%
These data include several hundred autopsy-proven cases of Alzheimer's disease in the Mayo Alzheimer's Disease Research Center/Alzheimer's Disease Patient Registry, and also several hundreds autopsy-proven cases of PSP in the Society for PSP Brain Bank. Therefore, the vast majority of cases with RBD - with or without coexisting cognitive impairment and/or parkinsonism – represent an underlying synucleinopathy.
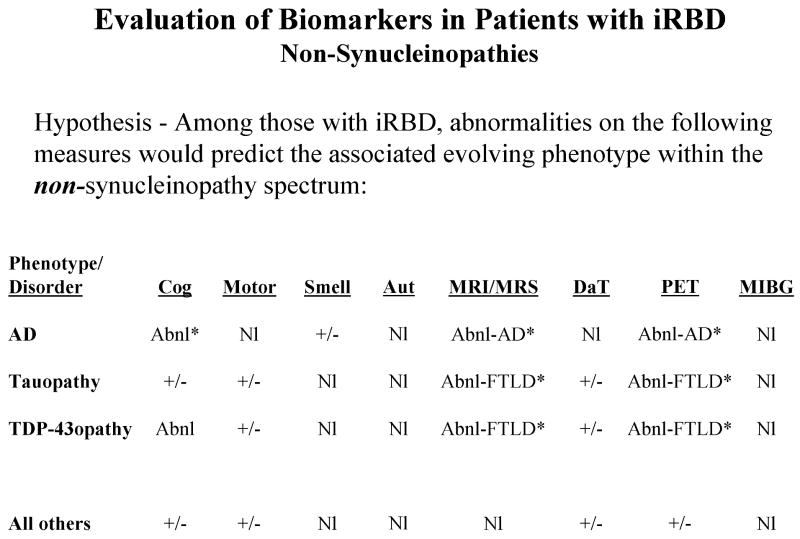
There are no published reports of RBD associated with Pick's disease, frontotemporal dementia, progressive nonfluent aphasia syndrome, semantic dementia, progressive subcortical gliosis, argyrophilic grain disease, frontotemporal lobar degeneration with ubiquitin- and TDP-43-positive inclusions, or dementia lacking distinctive histopathology.82 No evidence of RSWA or RBD was found in a recent study of 11 members of the pallido-ponto-nigral degeneration kindred who have the N279K mutation in the microtubule associated protein tau (MAPT), which represents a primary tauopathy.112 We have also not encountered RBD in any of our families with familial FTD +/- parkinsonism associated with other mutations in MAPT or with mutations in progranulin.113
Analyses in Patients with Idiopathic RBD
There are few studies published to date which involved patients with iRBD who have been followed prospectively. The seminal paper by Schenck et al. which launched the interest in the RBD-neurodegenerative disease association showed that among 29 iRBD patients who they followed longitudinally, 11 (38%) developed a parkinsonian disorder at a mean interval of 3.7 years after the diagnosis of RBD, and at a mean interval of 12.7 years after the onset of RBD.114 As of 2005, over 65% of their original group has developed parkinsonism and/or cognitive impairment.29 Four of these patients with iRBD plus cognitive impairment are currently being followed in the Mayo Alzheimer's Disease Research Center (kindly referred by Drs. Schenck and Mahowald), of whom 1 has nonamnestic mild cognitive impairment and the 3 others have classic DLB features.
Iranzo et al. reported on a series of 44 patients with iRBD with at least 2 years of clinical follow-up, of whom 20 (45%) patients developed a neurological disorder after a mean of 11.5 years from the reported onset of RBD.26 The newly-emerged disorders included PD (n=9), DLB (n=6), MSA (n=1), and mild cognitive impairment (n=4) in whom visuospatial dysfunction was prominent.26
Postuma et al. recently reported on a series of 93 patients with iRBD who they followed longitudinally, of whom 26/93 patients developed a neurodegenerative disorder: PD in 14, DLB in 7, probable AD in 4, and MSA in 1.80 The estimated 5-year risk of neurodegenerative disease was 17.7%, the 10-year risk was 40.6%, and the 12-year risk was 52.4%.80
Two cases with iRBD have had LBD pathology identified (termed “incidental LBD”). Uchiyama et al reported on a patient with a 20-yr history of RBD who had no cognitive or motor findings thoughout his clinical course. At autopsy, Lewy bodies were identified, particularly in the brainstem.115 We reported on a surgeon with PSG-proven RBD (onset age 57 years) and no other neurologic signs or symptoms who underwent neuropathologic examination upon his death at age 72.116 Histopathologic analysis showed Lewy body disease, with interestingly no significant neuronal loss or gliosis present in the substantia nigra or locus ceruleus.116
RBD and the Synucleinopathies
The disorders of LBD and MSA, which have prominent α-synuclein-positive pathology, are collectively termed “synucleinopathies.” Therefore, the clinical and pathologic data strongly supports the association of RBD with the synucleinopathies, and most experts in the field now regard patients with RBD plus cognitive impairment and/or parkinsonism likely have an underlying synucleinopathy.3, 26, 27, 76, 80, 82, 84, 110, 117-119 Furthermore, among the non-synucleinopathy disorders associated with RBD (e.g., PSP, SCA-3, and AD), patients have tended to have RBD evolve concurrently with or after the onset of parkinsonism, whereas RBD typically begins years or decades before the onset of cognitive and motor features of PD, DLB, MSA, and PAF. Hence, RBD preceding the motor and cognitive features of a neurodegenerative disorder may be particularly common in the synucleinopathies, and there is considerable interest to study patients with “idiopathic” RBD (discussed in much more detail in the sections that follow).
RBD, the Synucleinopathies, and Selective Vulnerability
The tendency of RBD to occur frequently in the synucleinopathies and rarely in the tauopathies and other neurodegenerative disorders supports the concept of selective vulnerability occurring in key brainstem neuronal networks in the synucleinopathies, and such neuronal networks are likely to be less dysfunctional or normal in the tauopathies and other neurodegenerative disorders.3, 26, 27, 80, 82, 84, 110, 117, 118 PD, DLB, MSA, the other few non-synucleinopathy disorders associated with RBD, and the rare RBD cases with structural brainstem lesions, may provide particular insights into RBD pathophysiology by demonstrating which neuronal networks are dysfunctional compared to the many disorders and cases that are not associated with RBD. Comparing the neuropathological findings (especially if neuronal quantification of key brainstem structures can be performed) in the rare cases of RBD associated with non-synucleinopathy disorders to the more common cases of non-synucleinopathy disorders not associated with RBD may be particularly enlightening.
RBD in the Context of the Braak Staging System for Parkinson's Disease
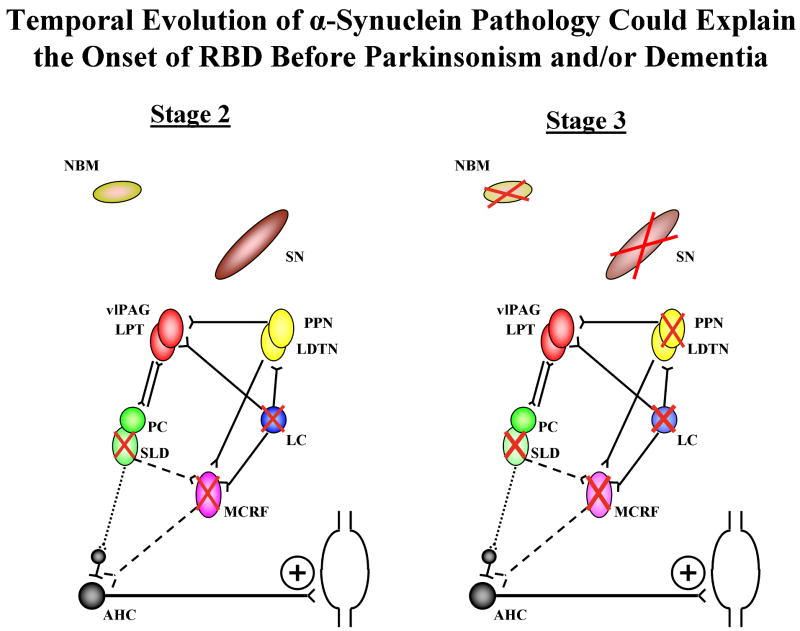
Braak et al. have proposed a staging system for the neuropathologic characterization of the phenotype of PD, and this system may be applicable to the timing of the evolution of RBD in the context of evolving LBD regardless if the clinical phenotype evolves as PD or DLB.3, 26, 76, 80, 84, 110, 117, 119-121 This staging system posits a temporal sequence of α-synuclein pathology in the brain beginning mainly in the medulla (and olfactory bulb) and gradually ascending to more rostral structures.120, 121 Dysfunction in the SLD +/- MCRF and peri-LC structures (Stage 2) could lead to RSWA and RBD, and more specifically, prominent degeneration in the SLD could be the critical nucleus involved. This temporal sequence of pathology could explain why RBD precedes parkinsonism and cognitive decline (Stages 3 and 4) and dementia (Stages 4-6) in many patients with Lewy body pathology. A schematic representation of this evolution through stages 2 and 3 are shown in Figure 5.
Figure 5.
Schematic of brainstem nuclei and connections pertinent to REM sleep, movement, and cognition. As per the Braak staging scheme, the temporal sequence of α-synuclein pathology begins mainly in the medulla and then ascends to the cortex (6 stages). In stage 1 (not shown), the dorsal IX/X motor nucleus, intermediate reticular zone, and olfactory bulb is affected, with presumably coexisting degenerative changes in these structures. In stage 2, there is progression in the structures involved in stage 1, plus the caudal raphe nuclei, MCRF, Peri-LC structures, and possibly SLD. RBD may evolve when sufficient degenerative changes have occurred in the SLD, peri-LC structures, and MCRF (denoted by red Xs within nuclei). In stage 3, there is progression in the structures involved in stage 2, plus the PPN, SN, and NBM (denoted by red Xs within nuclei). When sufficient degeneration occurs in the SN, then parkinsonism becomes manifest. When sufficient degeneration occurs in the NBM, then cognitive changes may become manifest. Additional α-synuclein pathology and neurodegeneration evolves in limbic and neocortical structures over stages 4-6 (not shown). This temporal sequence of pathology could explain why RBD precedes parkinsonism and dementia in many patients with Lewy body pathology.
Abbreviations: AHC=anterior horn cell, LC=locus coeruleus, LDTN=laterodorsal tegmental nucleus, LPT=lateral pontine tegmentum, MCRF=magnocellular reticular formation, NBM=nucleus basalis of Meynert, PC=pre-coeruleus, PPN=pedunculopontine nucleus, SLD=sublaterodorsal nucleus, SN=substantia nigra, vlPAG=ventrolateral part of the periaqueductal grey matter
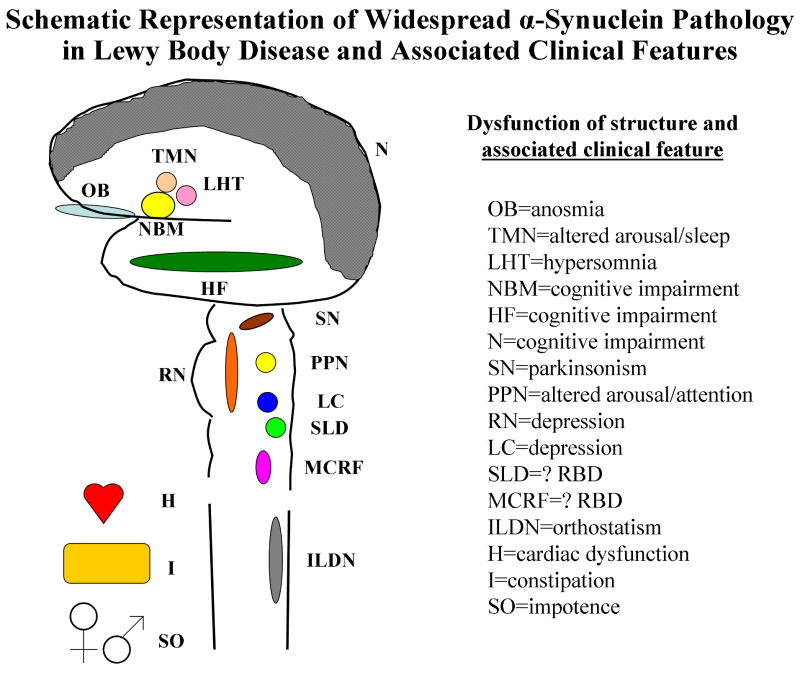
Over the few years since Braak et al. presented their staging scheme for PD, they and others have provided evidence that Lewy body disease is a more systemic process that clearly affects the peripheral autonomic nervous system, and in some cases actually may begin in the spinal cord prior to the brain, and the peripheral autonomic system even before the central nervous system.122-126 Lewy bodies and Lewy neurites have been found in the enteric nervous system, cardiac sympathetic system, and spinal cord (particularly intermediolateral cell column).122-126 A schematic of this representation and the likely structures and associated clinical features are shown in Figure 6. This schematic is admittedly overly-simplistic, and the actual contributions to the clinical features associated with LBD are surely more varied and complex than is depicted here. Yet the implications of this widespread pathology and possible evolution of LBD pathology from the periphery to the spinal cord and then ascending rostrally consistent with the Braak scheme provide a framework of testable hypotheses. The analyses addressing this framework are the focus of the next section.
Figure 6.
Schematic representation of the brain, spinal cord, and key peripheral and autonomic structures which can be affected by Lewy body disease pathology, and the clinical features associated with dysfunction of each structure. The structures (abbreviations) and likely associated clinical features are as follows: olfactory bulb (OB)=anosmia, tuberomamillary nucleus (TMN)=altered arousal/sleep, lateral hypothalamus (LHT)=hypersomnia, nucleus basalis of Meynert (NBM)=cognitive impairment, hippocampal formation (HF)=cognitive impairment, neocortex (N)=cognitive impairment, substantia nigra (SN)=parkinsonism, pedunculopontine nucleus (PPN)=altered arousal/attention, raphe nucleus (RN)=depression, locus ceruleus (LC)=depression, sublaterodorsal nucleus (SLD)=? RBD, magnocellular reticular formation (MCRF)=? RBD, intermediolateral cell column (ILDN)=orthostatism, sympathetic innervation of the heart (H)=cardiac dysfunction, enteric innervation of the intenstines (I)=constipation, and autonomic innervation of the sex organs (SO)=impotence
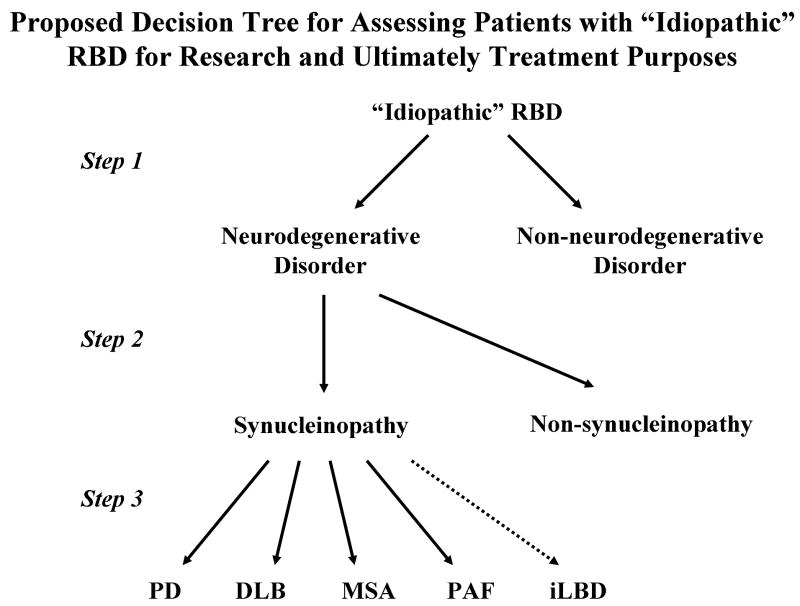
Characterization of Patients with “Idiopathic” RBD
“Idiopathic” RBD – A Potential Early Manifestation of a Neurodegenerative Disease
The retrospective and prospective data as reviewed above convincingly suggests that a significant proportion of those individuals with iRBD represent an early manifestation of an evolving neurodegenerative disease (which underscores why some authors qualify the term “idiopathic” with quotation marks). Many recent studies have used cross-sectional analyses with iRBD as the central feature and a variety of other clinical, neuropsychological, electrophysiological, and imaging modalities to test hypotheses, and almost all have supported the concept that most cases of iRBD likely reflect an evolving neurodegenerative disorder, which in most instances is LBD. The clinical and ancillary test findings associated with iRBD published to date are reviewed below.
Clinical Findings in “Idiopathic” REM Sleep Behavior Disorder
Anosmia/Dysosmia
Several studies have found impaired olfactory functioning in patients with iRBD. Stiasny-Kolster et al. studied 30 patients with clinical (idiopathic, n = 6; symptomatic, n = 13, mostly associated with narcolepsy) or subclinical (n = 11, associated with narcolepsy) RBD according to standard criteria and 30 age- and gender-matched healthy control subjects using ‘Sniffin’ Sticks'.119 RBD patients had a significantly higher olfactory threshold, lower discrimination score, and lower identification score. Compared with normative data, 97% of the iRBD patients had a pathologically increased olfactory threshold, 63% an impaired odor discrimination score, and 63% a decreased identification score. The authors concluded that iRBD patients with olfactory impairment might represent stage 2 preclinical α-synucleinopathy.119
Fantini et al. studied 54 consecutive PSG-confirmed iRBD patients and 54 age and gender-matched control subjects with the Brief University of Pennsylvania Smell Identification Test (B-UPSIT).127 They found 33 (61.1%) RBD patients versus 9 (16.6%) controls showing abnormal olfactory function (p < 0.0001). Difficulties in recognize paint thinner odorant showed the highest positive predictive value (0.95) for identifying iRBD. They interpreted these findings as showing that the olfactory deficit found in most iRBD patients are similar to that described in PD, and that dysnosmia may be a sign of a widespread neurodegenerative process. They also felt that its detection may help in identifying subjects at higher risk of developing an α-synucleinopathy disorder.127
Postuma et al. compared 25 patients with PSG-confirmed RBD without PD with age- and sex-matched controls.76 Olfaction was assessed using the B-UPSIT. When results were compared with age- and sex-adjusted normative values, 14 out of 25 patients with iRBD vs only 2 of 25 controls scored below the 25th percentile. The authors concluded that olfactory dysfunction, like many other potential early markers of Parkinson disease, are significantly abnormal in iRBD.76
Subtle Parkinsonism
In the same study noted above, the 25 subjects and 25 controls were assessed by the alternate tap test, Purdue Peg Board, and a timed up and go test measure of standing and walking. The results showed that many subjects exhibited subtle abnormalities on quantitative testing of motor and gait speed.76
Mihci et al. analyzed sleep and motor data among several hundred normal control participants (n=765) aged 70-89 in the Mayo Clinic Study of Aging – a population-based study of aging and cognition in Olmsted County, Minnesota.34 All participants who had a bedpartner had the Mayo Sleep Questionnaire (MSQ) completed, with an affirmative response to one question on RBD (and hence considered probable RBD) being 98% sensitive for definite RBD based on a PSG-validation analysis (Boeve et al, submitted). The motor subtest of the Unified Parkinson's Disease Rating Scale (UPDRS) was completed on all subjects, and parkinsonism was considered present if any subject had a score of 2 or greater in at least two of the core features: rigidity, bradykinesia, rest tremor, postural instability. Data were analyzed for subjects who underwent the key assessments and were considered cognitively normal. They found that 71 (9.3%) had parkinsonism alone, 46 (6.0%) had probable RBD alone, 14 (1.8%) had both parkinsonism and probable RBD, and 632 (82.6%) had neither. Parkinsonism was indeed significantly associated with probable RBD (p=0.002). The authors concluded that parkinsonism is associated with probable RBD among cognitively normal subjects in this population-based study, and longitudinal characterization and eventual neuropathologic examinations will be needed to determine which clinical phenotypes will evolve and whether LBD and MSA will be the primary underlying pathologies.34
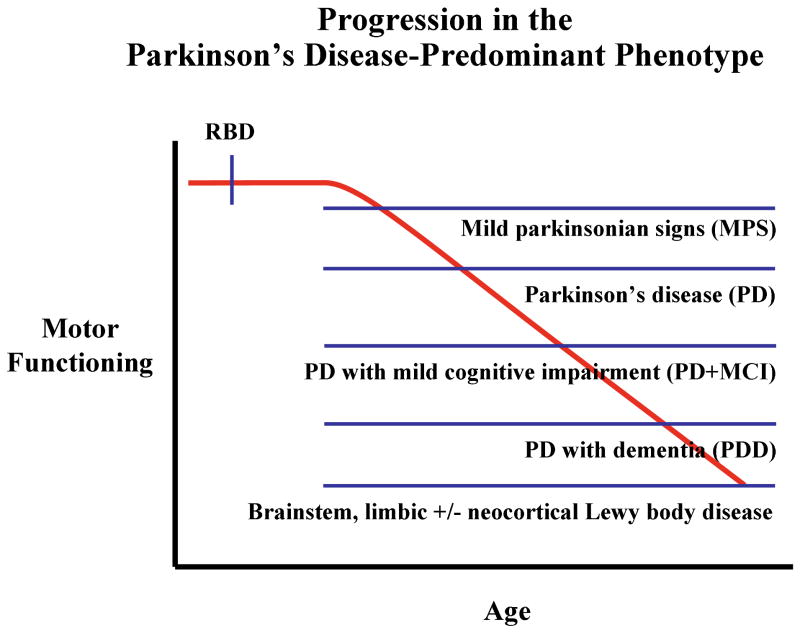
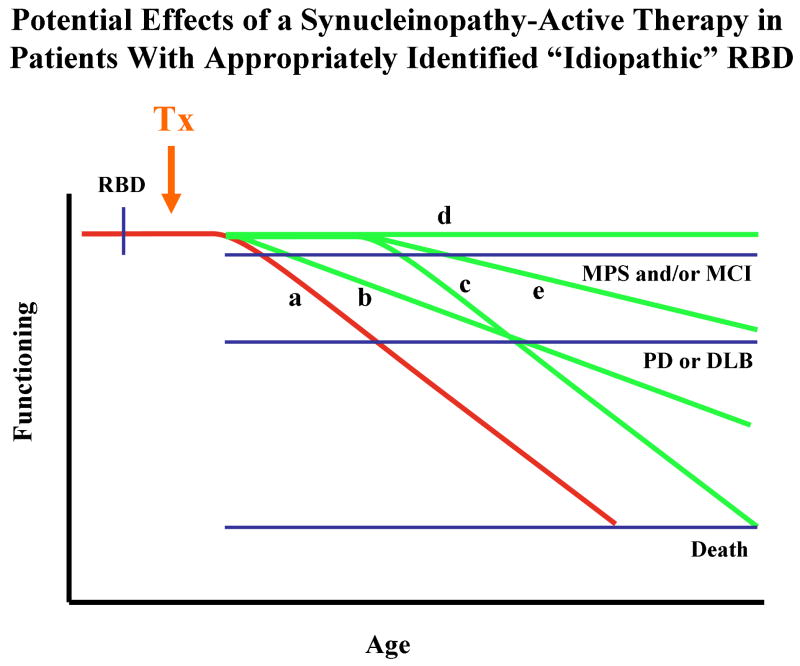
These findings and perspectives can be viewed conceptually as shown in Figure 7, in which the hypothesized progression in motor functioning with increasing age and disease severity in evolving Lewy body disease in the Parkinson's disease-predominant phenotype. The onset of RBD typically begins years or decades prior to the onset of subtle motor signs – these subtle signs are also termed “mild parkinsonian signs” and abbreviated as “MPS.”128 MPS may be asymptomatic and only detectable on clinical examination, or could be minimally symptomatic and not functionally disabling. Typical features of PD evolves months or years later. Over time, PD with mild cognitive impairment (PD+MCI) evolves, followed some time thereafter by parkinsonism which is progressively less levodopa-responsive despite aggressive dosing, and a full dementia syndrome (PD with dementia or PDD) becomes manifest.129, 130
Figure 7.
Schematic representation of the hypothesized progression in motor functioning with increasing age and disease severity in evolving Lewy body disease in the Parkinson's disease-predominant phenotype. The onset of RBD typically begins years or decades prior to the onset of subtle motor signs (mild parkinsonian signs or MPS); such motor signs may be asymptomatic and only detectable on clinical examination. Typical features of Parkinson's disease (PD) evolves months or years later. Over time, PD with mild cognitive impairment (PD+MCI) evolves, followed some time thereafter by parkinsonism which is less levodopa-responsive, and a full dementia syndrome (PD with dementia or PDD) becomes manifest.
Inherent in any schema such as this is the known individual variability, with some patients with PD living for more than 20 or 30 years with relatively preserved cognitive functioning, and some patients with PD never exhibiting RBD at all. However, most patients with “idiopathic” RBD who later develop typical features of PD evolve in the manner that is depicted in Figure 7.
Other
Additional findings on clinical measures in patients with iRBD include impairment on color discrimination,76 and higher reports of autonomic dysfunction (urinary dyscontrol, erectile function, and constipation).76 Depression and personality traits have also been studied, but no difference has been found between iRBD patients and controls.76. Apathy and anxiety has also been associated with probably RBD.35 Investigators with the Honolulu-Asia Aging Study found that subjective hypersomnolence was associated with increased incidence of subsequent Parkinson's disease.131 Although objective evidence of hypersomnolence was not found in patients with iRBD,1 this issue may be worthy of further study.
Cognitive and Neuropsychological Features/Findings in “Idiopathic” REM Sleep Behavior Disorder
Ferini-Strambi et al. evaluated 17 consecutive patients with iRBD and 17 matched controls on a battery of neuropsychological measures that assessed verbal and spatial memory, visual selective attention, cognitive set shifting, visuoconstructional abilities, visuospatial learning, verbal fluency, semantic fluency, and executive functions.132 The exclusion criteria included abnormalities on neurologic examination and a Mini-Mental State Exam score below 24. Results showed that the iRBD patients had lower scores on visuocontructional abilities and visuospatial learning. Because deficits in these domains have been demonstrated in patients with DLB,17, 81, 83, 133 the authors interpreted their results as possibly reflecting early DLB in these patients 132.
In a study by Massicotte-Marque et al., 14 patients with iRBD and 14 healthy control subjects underwent a neuropsychological evaluation.134 Compared to controls, patients with iRBD showed a lower performance on neuropsychological tests measuring attention, executive functions and learning, but not on tasks measuring delayed recall or visuospatial functioning. The authors concluded that the impaired cognitive profile in patients with iRBD is similar to that observed in early stages of some synucleinopathies.134
Terzaghi et al. compared the neuropsychological functions in 23 iRBD subjects to a group of healthy controls.135 Considering mean values, poorer performances were observed in the iRBD subjects on Word Span, Rey–Osterrieth's complex figure recall, Digit Span, and Logic Memory tests. On the basis of equivalent scores, the iRBD subjects performed significantly more poorly on tests of visuoconstructional learning abilities. They interpreted their data as showing the possible presence of cognitive deficits in iRBD sharing common features with Lewy body disease.135
Mild cognitive impairment (MCI) refers to the transitional state between normal aging and dementia.136 Most individuals with prominent memory impairment but preserved functioning in the other non-memory domains (often termed amnestic MCI and abbreviated aMCI) subsequently develop the typical clinical, neuropsychological, neurimaging, and neuropathological features of Alzheimer's disease.137 Other degenerative conditions such as dementia with Lewy bodies, frontotemporal dementia, primary progressive aphasia, corticobasal syndrome, and posterior cortical atrophy, as well as other etiologic categories of disease such as depression, cerebrovascular disease, metabolic disorders, etc., likely also evolve through an MCI transitional state, but little data has been published to support this view. The concept of MCI has therefore been further subtyped such that the clinical and neuropsychological profile of impairment can be extended beyond single domain MCI with amnesia (ie, amnestic MCI) to also include multiple domain MCI with amnesia, single domain nonamnestic MCI, and multiple domain nonamnestic MCI.137 This conceptual framework allows one to test hypotheses, and a few key questions pertaining to RBD and the synucleinopathies have recently been studied.
Based on the data accumulated thus far in RBD as well as DLB, one could hypothesize that 1) those individuals with RBD plus MCI likely reflect evolving LBD, and 2) the MCI subtypes with impairment maximal in the attention/executive and visuospatial functioning domains would be most likely to reflect evolving LBD. Molano et al. recently analyzed the clinical and neuropsychological data on all patients who were diagnosed with MCI, prospectively followed, and eventually underwent neuropathologic examination and had limbic +/- neocortical LBD.138 Eight subjects were identified, of whom 6 were male. Seven developed DLB prior to death; 1 died characterized as MCI. RBD preceded cognitive symptom onset in 6 cases by a median of 10 years (range 2-47 years). Each of the MCI subtypes were represented, with 7 of the 8 patients having impairment in the attention/executive functioning and/or visuospatial functioning domains. These authors concluded that LBD can pass through an MCI transitional state, with any MCI subtype can potentially evolve into DLB. Furthermore, since all cases with RBD and MCI eventually were shown to have autopsy-proven LBD, these data suggest that RBD plus MCI likely reflects brainstem and cerebral LBD.138
Molano et al. also compared the neuropsychological profiles among the same cohort described above in the analysis by Michi et al., in which normal control participants (n=765) aged 70-89 in the Mayo Clinic Study of Aging were the focus of analysis.36 All participants who had a bedpartner had the Mayo Sleep Questionnaire completed. The neuropsychological battery assessed performance in the attention/executive, memory, language, and visuospatial domains. A domain score was computed and transformed to a z-scale to allow comparisons. The frequency of probable RBD was 8.0%. Those with probable RBD had significantly lower median scores (25th-75th percentile scores) for the attention/executive domain. Scores were also lower in subjects with probable RBD vs. those without RBD across the other domains, although none reached statistical significance. The authors interpreted these findings consistent with very mild neuropsychological changes that may reflect evolving Lewy body disease in most subjects, although clearly longitudinal follow-up will be necessary to substantiate this hypothesis.36
While at first glance some of these studies appear to show inconsistent results, when one considers the domains that are most consistently impaired in PD and particularly DLB patients – attention/executive functions, learning, and visuospatial functioning – with relative preservation in delayed recall and confrontation naming measures – the data could still be interpreted as reflecting the pattern of impairment that is often seen in the phenotypes most commonly associated with synucleinopathy pathology. This pattern of impairment is not typical of Alzheimer's disease nor many of the tauopathies and TDP-43opathies. Furthermore, even though patients with PD and mild cognitive impairment or dementia, and those with DLB, tend to show abnormalities on measures of attention/executive functioning, learning, and visuospatial/visuoconstructive functioning, there is considerable variability across individuals. Hence one would expect to see variability across measures in individuals with iRBD. Some individuals with iRBD surely do not reflect an evolving synucleinopathy, or are so early in the disorder that only the brainstem and not the cerebrum is significantly affected by synucleinopathy pathology, neuronal loss, and neurochemical dysfunction. Investigators seeking to study neuropsychological functioning in iRBD patients should utilize measures which assess all cognitive domains, with several focused on attention/concentration, executive functioning, learning, and visuospatial/visuoconstructive functioning.
This author has also evaluated numerous patients with iRBD who have clear cognitive complaints, particularly problems with multitasking and anterograde memory processing that are impacting their ability to perform at their prior baseline at work and keep up with the daily tasks at home. To a seasoned clinician, these complaints sound neurologically-based, yet in many of these patients there is no obvious impairment on detailed neuropsychological testing. Many who have been followed longitudinally subsequently develop more obvious MCI or frank DLB. One must therefore conclude that many of the standard neuropsychological tests do not adequately capture the essence of what these patients are experiencing. We need measures that more adequately reflect their symptoms! This is an obvious area warranting further research.
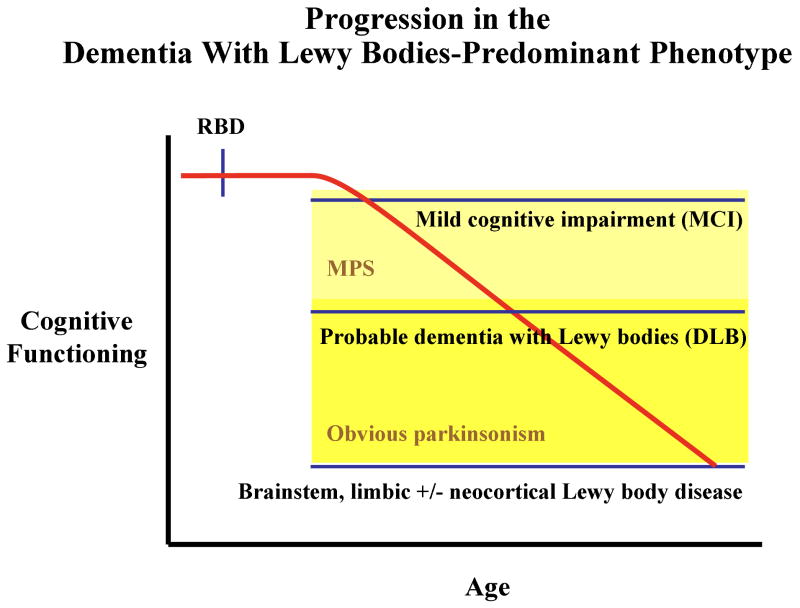
Similar to the concept of iRBD evolving into a PD-predominant phenotype as discussed above, one could hypothesis a similar progression in cognitive functioning with increasing age and disease severity in evolving LBD in the DLB-predominant phenotype (Figure 8). The onset of RBD typically begins years or decades prior to the onset of cognitive decline and a diagnosis of MCI, with subtle and often asymptomatic motor signs (ie, MPS – represented by the area shaded in light yellow) evolving concurrently or after the onset of cognitive decline. More obvious features of parkinsonism (represented by the area shaded in dark yellow) evolves no earlier than 1 year prior to the onset of dementia, thereby fulfilling the “one year rule” for the diagnosis of DLB.139 Over time, dementia, parkinsonism, and problematic neuropsychiatric features such as visual hallucinations and delusions tend to progress.
Figure 8.
Schematic representation of the hypothesized progression in cognitive functioning with increasing age and disease severity in evolving Lewy body disease in the dementia with Lewy bodies (DLB)-predominant phenotype. The onset of RBD typically begins years or decades prior to the onset of cognitive decline and a diagnosis of mild cognitive impairment (MCI), with subtle and often asymptomatic motor signs (mild parkinsonian signs or MPS – represented by the area shaded in light yellow) evolving concurrently or after the onset of cognitive decline. More obvious features of parkinsonism (represented by the area shaded in dark yellow) evolves no earlier than 1 year prior to the onset of dementia to warrant the DLB diagnosis. Over time, dementia, parkinsonism, and problematic neuropsychiatric features such as visual hallucinations and delusions tend to progress.
Like in the PD-predominant phenotype, inherent in any schema such as this is the known individual variability, with some patients with MCI not progressing to dementia even after 10 years, or some with autopsy proven LBD never exhibiting significant parkinsonism, and still others with DLB never exhibiting RBD at all. However, most patients with iRBD who later develop typical features of DLB evolve in the manner that is depicted here.
Electroencephalographic Findings in “Idiopathic” REM Sleep Behavior Disorder
Fantini et al. performed quantitative analyses of waking and REM sleep EEG in 15 patients with idiopathic RBD and in 15 matched controls.140 The exclusion criteria included abnormalities on neurologic examination and presence of affective disorders or dementia. EEG slowing was demonstrated in the RBD group, thus suggesting impaired cortical activity during both wakefulness and REM sleep. The authors interpreted these findings as possibly reflecting a very early sign of central nervous system dysfunction.140
In a study by Massicotte-Marque et al., 14 patients with iRBD and 14 healthy control subjects underwent waking EEG recordings.141 Compared to controls, patients with iRBD showed showed EEG slowing (higher delta and theta power) during wakefulness in all brain areas compared to controls. The authors concluded that waking EEG slowing in patients with iRBD is similar to that observed in early stages of some synucleinopathies.141
Neuroimaging Findings in “Idiopathic” REM Sleep Behavior Disorder
Structural Magnetic Resonance Imaging
Culebras and Moore reported on T2 signal changes on magnetic resonance imaging (MRI) in 6 patients with RBD and suggested vascular changes in the brainstem could disrupt REM sleep networks and result in RBD.142 However, the vast majority of patients with RBD in whom MRI has been performed have not corroborated this finding.12, 17, 22
Magnetic Resonance Spectroscopy
Miyamoto et al. detected an increase (compared to reference values from another institution) in the choline/creatine ratio on protein magnetic resonance spectroscopy (1H-MRS) in the pons of a 69 year old man with idiopathic RBD.143 Since other ratios were normal, the investigators interpreted these findings as demonstrating functional impairment at the cell membrane level.143
Iranzo et al. performed 1H-MRS in a larger sample of patients with idiopathic RBD (n=15) to determine if midbrain or pontine tegmentum abnormalities could be detected compared to matched controls (n=15).144 No significant differences in N-acetylaspartate/creatine, choline/creatine and myoinosito/creatine ratios were found between patients and controls, which they interpreted as suggesting that marked mesopontine neuronal loss or 1H-MRS detectable metabolic disturbances does not occur in idiopathic RBD.144
The discrepant findings between these two studies could be due in part to methodologic issues (location of regions of interest, reference values for determining abnormal results, etc.). No 1H-MRS studies with the medulla as the region of interest has been reported as yet in patients with idiopathic RBD; the Braak staging system of Parkinson's disease suggests that this region of interest may be worth investigating.120, 121
Single Photon Emission Computed Tomography
IPT-SPECT and IBZM-SPECT
Eisensehr et al. used (N)-(3-iodopropene-2-yl)-2beta-carbomethoxy-3beta-(4-chlorophenyl) tropane labeled with iodine 123 (IPT)-single photon emission computed tomography (SPECT), which reflects presynaptic dopaminergic transporter integrity, and (S)-2hydroxy-3iodo-6-methoxy-([1-ethyl-2-pyrrolidinyl]methyl) benzamide labeled with iodine 123 (IBZM-SPECT), which reflects postsynaptic dopaminergic D2 receptor integrity, to investigate dopaminergic parameters in patients with RBD, PD, and controls145 RBD cases had reduced striatal IPT uptake compared to controls, yet uptake was more similar (albeit symmetric) to PD cases. Furthermore, there was no significant difference in postsynaptic dopaminergic D2 receptors between RBD patients and controls. The reduction in dopaminergic transporters was thought to either be directly involved in RBD pathogenesis, or that RBD is the initial manifestation of PD.145 In a subsequent study, these investigators compared muscle activity in REM sleep on PSG and IPT- and IBZM-SPECT in normal controls (n=11), patients with idiopathic “subclinical” RBD (n=8), patients with idiopathic RBD (n=8), and patients with early Parkinson's disease (n=8).70 They found that the IPT uptake was highest in controls, lower for patients with “subclinical” RBD, still lower for patients with clinically manifest RBD, and lowest in patients with PD. Muscle activity during REM sleep was independently associated with reduction of striatal dopamine transporters. The IBZM uptake was not significantly different between the groups. They interpreted their findings as suggesting that there is a continuum of reduced striatal dopamine transporters involved in the pathophysiologic mechanisms causing increased muscle activity during REM sleep in patients with “subclinical” RBD.70
ECD-SPECT
Mazza et al. investigated the regional cerebral perfusion in patients with idiopathic REM behavior disorder (iRBD) by using (99m)Tc-Ethylene Cysteinate Dimer (ECD) SPECT on 8 patients with PSG-confirmed RBD and nine age-matched controls.146 They found increased perfusion in the pons and putamen bilaterally and in the right hippocampus, and decreased perfusion in frontal and temporoparietal cortices. The authors concluded that perfusional abnormalities in patients with iRBD were located in the brainstem, striatum, and cortex, and that such findings are consistent with the anatomic metabolic profile of Parkinson disease.146
I-123-FP-CIT-SPECT
In a study focused on olfactory function in patients with RBD, Stiasny-Kolner et al. performed [I-123] N-ω-fluoropropyl-2β-carbomethoxy-3β-(4-iodophenyl) nortropane (I-123-FP-CIT, also known as Ioflupane and marketed in Europe as DaTSCAN) SPECT in several of their patients with RBD, and found 3 patients with reduced nigrostriatal uptake: 1 with newly identified parkinsonism on clinical examination, and 2 with idiopathic RBD.119 The authors interpreted their findings as suggesting that iRBD patients with olfactory impairment might represent a preclinical α-synucleinopathy.119
Positron Emission Tomography
DTBZ-PET
Using dihydrotetrabenazine (DTBZ) positron emission tomography (PET), Albin et al. compared findings in elderly subjects with iRBD to those in similarly aged controls.147 Striatal binding of DTBZ was reduced in the iRBD subjects compared to controls, suggesting reduced dopaminergic substantia nigra neuron number. The authors concluded that this reduction is consistent with the hypothesis that RBD reflects an evolving degenerative parkinsonian disorder, and suggested that RBD either reflects dysfunction of the PPN secondary to basal ganglia dysfunction, or primary dysfunction of the PPN or other brainstem structures that is temporally associated with basal ganglia dysfunction.147
FDG-PET
Caselli et al. sought to determine if healthy adults reporting dream-enactment behavior (DEB+) have reduced cerebral metabolic rate for glucose (CMRgl) on fluorodeoxyglucose (FDG) PET in regions preferentially affected in patients with DLB.148 Among 17 cognitively normal patients with DEB+ and 17 control subjects (DEB-), the DEB+ group was associated with significantly lower CMRgl in several brain regions known to be preferentially affected in both DLB and Alzheimer disease (parietal, temporal, and posterior cingulate cortexes) and in several other regions, including the anterior cingulate cortex. The authors interpreted these findings as supporting further study of DEB as a possible risk factor for the development of DLB.148
PiB-PET
One of the most important advances in imaging in the aging and dementia field was the advent of Pittsburgh-compound B (PiB) PET, in which the PiB ligand binds to amyloid and allows visualization of cerebral amyloid in plaques and vessels in patients with Alzheimer's disease.149, 150 PiB-PET imaging findings in patients with RBD have not yet been published, but this imaging modality warrants a few words of caution for researchers in the RBD field. While some degree of controversy exists on the interpretation on PiB-positive PET scans, particularly in cognitive normal individuals, the use of PiB-PET imaging in DLB and PDD has recently begun151 and may have some applicability to imaging subjects with iRBD. On the one hand, it may seem reasonable to consider PiB-positive scans in those with iRBD as indicative of evolving Alzheimer's disease pathology, the facts that 1) some cognitively normal subjects have PiB-positive scans, and 2) most DLB patients have some degree of coexisting AD pathology and hence would likely have PiB-positive scans, make the use of PiB-PET imaging less than ideal as a “rule-out AD” biomarker for iRBD research purposes.
Cardiac MIBG Imaging
Recent evidence supports the notion that cardiac sympathetic involvement is a very early event in the evolution of Lewy body disease in humans, occurring prior to the deposition of α-synuclein pathology in the central nervous system in many patients.126 Cardiac (123)I-metaiodobenzylguanidine (MIBG) reflects cardiac sympathetic integrity, and reduced uptake on MIBG imaging is consistent with the loss of sympathetic terminals in the heart. Ample data now exists on reduced cardiac MIBG uptake being present in patients with Lewy body disease regardless if the disorder is exhibited as PD, DLB, PD with dementia, or pure autonomic failure (PAF).152-154 Several investigators have therefore suggested that MIBG could be used to identify who has underlying LBD regardless of the other clinical features being exhibited.
Further support of this hypothesis has come from cardiac MIBG imaging in patients with idiopathic RBD, in which uptake is reduced.155-157 In contrast, cardiac MIBG uptake in MSA – another synucleinopathy often associated with RBD – is typically within normal limits.156, 158 Since the topography of degeneration in MSA is almost exclusively the central nervous system,159-161 while LBD involves the peripheral autonomic and sensorimotor systems and multiple networks in the central nervous system, the evidence accumulated thus far suggests that reduced cardiac MIBG uptake may be one of the most specific biomarkers for underlying LBD. The sensitivity of cardiac MIBG imaging for LBD is not yet known, and this technique does have several limitations such as questionable validity in those with other medical disorders (e.g., diabetes mellitus) that affect cardiac sympathetic integrity, high cost, and limited availability. Yet additional work with cardiac MIBG imaging in idiopathic RBD, particularly in countries outside of Japan (all MIBG publications on RBD have emanated from the excellent work from several centers in Japan), is clearly warranted.
Evolving Concepts, Controversies, and Future Directions in REM Sleep Behavior Disorder
Does the RBD Diagnosis Require Polysomnography?
The simple answer to this question is “yes.” If a patient is exhibiting recurrent dream enactment behavior, and the dreams are viewed as unpleasant, and the potential for injury to the patient or bedpartner is high, PSG with synchronous video monitoring is essential. Given the implications of a formal PSG-confirmed diagnosis of RBD, and the known tendency for some patients with moderate to severe obstructive sleep apnea (OSA)5 to have a history of recurrent dream enactment behavior associated with dreams involving a chasing or attacking theme (which is essentially identical to that of RBD), a PSG is imperative. This author has evaluated numerous middle-aged males with concerns about RBD and the future implications thereof, but rather minimal historical and examination findings to suggest clinically significant OSA, yet on PSG moderate to severe OSA is found, EMG atonia during REM sleep is normal, and nasal CPAP subsequently eliminates the unpleasant dreams and recurrent dream enactment behavior completely. A PSG should be strongly considered in each and every patient with suspected RBD, and should be performed whenever feasible particularly if the potential for injury is high.
However, there are many circumstances when PSG confirmation of RBD is not feasible or possible (see below). Yet there is growing appreciation of the importance of identifying patients with RBD for clinical and particularly research purposes. PSG confirmation of RBD is mandatory in the current diagnostic criteria schemes.
When Is a Diagnosis of “Probable RBD” Justified?
In the instances when 1) PSG cannot be performed for whatever reason, 2) is performed and little or no apparent REM sleep is present, or 3) the EMG tone during REM sleep is equivocally elevated, this author would suggest the use of the term “probable RBD” for those who otherwise have a classic history of recurrent dream enactment behavior associated with dreams involving a chasing or attacking them; the confidence in this diagnosis is heightened further if there are no historical features (loud disruptive snoring, observed choking/gasping/apnea during sleep) or anatomic findings (narrow oropharynx, large neck circumference, obesity) to suggest OSA, nor any features to strongly suspect nocturnal seizures or sleepwalking. The diagnosis of “probable RBD” could also be justified if questionnaires are shown to be adequately sensitive and specific for RBD based on PSG validation (see below).
How Accurate Are Screening Measures for RBD?
PSG is essential to establish the diagnosis of RBD, but the procedure does require appropriate monitoring equipment, including time synchronized video recordings, specially trained technologists, bed availability in a sleep laboratory, and clinicians who can interpret the data. The procedure is costly (>$1000 per study at most centers for a clinical PSG), especially for patients with limited insurance coverage. Subjects must be willing and able to sleep in a sleep laboratory and undergo monitoring. Some patients with coexisting neurologic disorders are too cognitively or physically impaired to tolerate and undergo an adequate study, are too uncooperative to permit all monitoring equipment to remain in place, have a risk of falls during the night, or are institutionalized. Since the background EEG is often so abnormal in those with moderate to severe dementia, determining which epochs represent REM sleep on PSG can be difficult if not impossible. Some even with only mild cognitive impairment have features of status dissociatus, in which an experienced sleep clinician cannot determine a sleeping versus a waking EEG with certainty even when viewing the synchronous video. Some patients have no REM sleep on a PSG. Due to the limited number of sleep disorders center in many countries, PSG is not possible even when clearly medically warranted. In some patients, the dream enactment behavior is so infrequent and mild that a clinical PSG is difficult to justify. Reimbursement for PSG is not always covered by insurance and federal health care plans. Especially for research purposes, it could be useful to use a validated measure to screen for RBD by querying the bedpartners of patients who are cognitively impaired, severely disabled or deceased. It is also impractical to perform PSGs in large numbers of subjects in epidemiologic studies of sleep disorders. Therefore, questionnaires that adequately screen for RBD could be useful for clinical and research purposes.
Mayo Sleep Questionnaire
Our group developed the Mayo Sleep Questionnaire (MSQ), which is a 16-item measure that poses questions about RBD, periodic limb movements during sleep, restless legs syndrome, sleepwalking, obstructive sleep apnea, and sleep-related leg cramps. There were two versions initially developed – one completed by the patient and one completed by his/her bedpartner/informant. Our early pilot data using the MSQ through 2002,162, 163 in which responses on the MSQ were compared with the findings on PSG, indicated that the sensitivity and specificity were higher for the bedpartner/informant version compared to the patient version, regardless of whether the patient was cognitively impaired or not. Since 2002, we have therefore only used the bedpartner/informant version of the MSQ. The measure is available for anyone to use. Our updated validation data in two groups of subjects who have had the MSQ completed by their bedpartner and undergone PSG – an aging and dementia cohort (n=159) and a population-based cohort of community-dwelling elderly subjects (n=70) has identified an affirmative response to one question was ≥98% sensitive and ≥69% specific for PSG-confirmed RBD; specificity increased depending on the responses to additional questions on RBD particularly if there was no history to suggest OSA (Boeve et al, submitted).
Semistructured Questionnaire Based on ICSD-R Diagnostic Criteria for RBD
The Bologna, Genova, Parma and Pisa Universities group for the study of REM Sleep Behavior Disorder (RBD) in Parkinson's Disease used a questionnaire-based method to study RBD in PD patients.73 Six trained neurologists used a semistructured questionnaire based on ICSD-R diagnostic criteria for RBD to evaluate 200 PD patients and their caregivers. RBD was defined according to clinical ICSD-R minimal diagnostic criteria [1]: Limb or body movements associated with dream mentation (criterion B) in the presence of at least one of the following: harmful or potentially harmful sleep behaviors (criterion C1); dreams appear to be “acted out” (criterion C2); sleep behaviors disrupt sleep continuity (criterion C3). They found that 34% of their PD patients had a history suggestive of RBD.73
REM Sleep Behavior Disorder Screening Questionnaire
Stiasny-Kolster et al. developed the REM Sleep Behavior Disorder Screening Questionnaire (RBDSQ), which is a 10-item patient self-rating questionnaire (maximum total score of 13 points) covering the clinical features of RBD.164 They studied 54 patients with PSG-confirmed RBD, 160 control subjects in whom RBD was excluded by history and PSG, and 133 unselected healthy subjects who did not undergo PSG. The found that the mean RBDSQ score in the RBD group was 9.5 points compared with 4.6 points in control group, and using an RBDSQ score of five points as a positive test result, the results yielded a sensitivity of 96% and a specificity of 56%.164
The high sensitivity and ease of use for the MSQ and RBDSQ questionnaires make either measure appealing as a screening tool for RBD, with the MSQ likely more appropriate for use in those with cognitive impairment/dementia since the responses are provided by bedpartners. In those who screen positive using either measure but are unable or unwilling to undergo PSG, or who have little or no apparent REM sleep during a PSG, then a diagnosis of “probable RBD” would be justified.
Does REM Sleep Without Atonia Represent Subclinical RBD?
There is debate as to the terminology and clinical significance of increased EMG tone during REM sleep (i.e., REM sleep without atonia or RSWA) in patients who have never exhibited dream enactment behavior. Such patients have been classified as having “preclinical” or “subclinical” RBD. While (at least in our experience) some patients with RSWA subsequently develop clinical RBD, and a few of these patients have subsequently developed PD or DLB, not all patients with RSWA have developed clinical RBD. There is no prospective systematic study of patients with RSWA that has been carried out to clarify this issue. Thus, RSWA appears to be the more appropriate terminology rather than preclinical or subclinical RBD based on the available data, and future research can involve patients with RSWA and follow them longitudinally to determine outcome and predictors of outcome.
What Are the Optimum Criteria for Assessing EMG Tone During REM Sleep on PSG?
Most individuals who undergo PSG have unequivocal findings in REM sleep, with either essentially complete EMG atonia during REM sleep (as shown in Figure 3A) or clearly increased EMG tone during REM sleep (as shown in Figure 3B) +/- obvious vocalizations and motor activity such as limb flailing or punching. When sleep clinicians view the PSG, it is rather straight-forward and comments such as “clearly normal EMG atonia” or “clearly increased EMG tone” (also with comments on the observed abnormal behaviors) are made in the results or interpretation of the PSG. Yet some have equivocal findings, with occasional periods of increased EMG tone during tonic and (more often) phasic REM sleep while normal EMG atonia exists over most of the REM sleep epochs. These findings pose challenges for the clinician interpreting the PSG, particularly if one of the indications for PSG was “rule in” or “rule out” RBD; the “rule out” determination is made even more difficult when no apparent dream enactment behavior occurred on the synchronous video during the periods of increased EMG tone.
Frauscher et al framed the current state of affairs in assessing EMG tone during REM sleep very well:165
“The International Classification of Sleep Disorders-2 defined REM sleep without atonia as the ‘electromyographic (EMG) finding of excessive amounts of sustained or intermittent elevation of submental EMG tone or excessive phasic submental or (upper or lower) limb EMG twitching.’ This definition has several limitations. First, a precise definition of ‘excessive amounts of tonic and phasic EMG activity’ was not provided, since normal values of these measures are unknown. Second, it is not stated how the tonic and phasic EMG activity have to be measured. Third, it is unclear which muscle or combination of muscles of the body (either axial or extremity muscles, lower or upper extremity muscles, or proximal or distal extremity muscles) provides the highest rates of abnormal REM sleep EMG activity in RBD.”
These concerns have led several groups to study this issue further, and some have developed scoring systems to not only qualify the EMG tone as normal or abnormal, but also to quantify the degree to which EMG tone is abnormal.165-169 There are pros and cons with each of the proposed systems, with the main drawback of manual systems being the time and effort required to assess the mini-epochs of REM sleep. The automated systems are more appealing for obvious reasons; whether any become standard practice – even among academic centers interested in this issue – remains to be seen.
Do All Patients With iRBD Represent an Early Neurodegenerative Disease?
The answer to this question is certainly “no.” There are many other medical conditions which have been associated with RBD and some of these may be etiologically related. Certain medications, particularly some of the selective serotonin reuptake inhibitors (SSRIs) and selective noradrenergic reuptake inhibitors (SNRIs) appear to increase EMG tone during REM sleep +/- precipitate or aggravate RBD.47, 48 While it may seem reasonable to presume that anyone with RBD who has no other known medical condition associated with RBD and does not take SSRIs or SNRIs are most likely to represent those who have an underlying evolving neurodegenerative disorder, this is purely speculative at this point. It is also important to again note that a 10-20 year history of RBD has been associated with otherwise asymptomatic LBD,115, 116 so even in the setting of RBD representing a manifestation of an early neurodegenerative disorder, not all such patients will experience changes in motor or cognitive functioning during their lifetime.
The RBD-SSRI/SNRI association raises other questions – why do some individuals using a medication in either of these classes appear to develop RBD features, while millions of individuals take one of these medications and do not develop RBD features? Are some of the SSRI and SNRI medications actually “unmasking” RBD that would have evolved months or years later had they not used these medications? This issue is worthy of further study.
How Should Clinicians Discuss the RBD-Neurodegenerative Disease Association with iRBD Patients?
Some experts believe there is insufficient evidence to conclude that most individuals with iRBD represent an evolving neurodegenerative disorder, and even among those that are exhibiting RBD due to underlying Lewy body disease, not all such patients subsequently exhibit parkinsonism or dementia despite 10-20 years of RBD features.115, 116 Thus, the feeling is that discussing iRBD as a possible risk factor for a neurodegenerative disorder, particularly in an era when no therapy exists, is not appropriate and no discussion is warranted as only undue anxiety would result.
Other experts feel that there is sufficient evidence to warrant discussion with iRBD patients (+/- their spouses) about the RBD-neurodegenerative disease association, with the timing of such a discussion dependent on the circumstances of the situation – if the patient resides nearby the center where the diagnosis was made and longitudinal follow-up is planned, then this discussion can occur at some point along the course of follow-up. This author views the RBD-neurodegenerative disease association similar to an autosomal dominant mutation with incomplete penetrance, with the big unknowns being if and when any other signs of a neurodegenerative disorder could become manifest.
Clinicians should also be mindful of the internet savvy nature of most of the public, whereby newly-diagnosed patients with iRBD very likely search on “RBD” or “REM behavior disorder” or “REM sleep behavior disorder” and may be acutely anxious about the information on various websites concerning increased future risk of PD or dementia, and may also be angry with their physician for not sharing this information with them at the time of diagnosis. Plus, many highly educated middle-aged males read about this association and attempt to contact experts in the RBD field, which is certainly understandable as they are seeking medication and non-medication approaches to stave off parkinsonism and dementia.
This author tends to discuss this issue briefly at the time of diagnosis, and in more detail over subsequent visits, again depending on the circumstances with each individual patient. It may be prudent for sleep medicine clinicians, neurologists, and psychiatrists to consult with their medical genetics colleagues for further advice on discussing this important issue with patients.
What is the Specificity of RBD for the Synucleinopathies?
Whenever a hypothesis is proposed, it warrants scientific scrutiny from several groups of investigators to test the hypothesis and ultimately support it, refute it, or refine/qualify it. This has appropriately occurred in the RBD field, particularly testing the hypothesis that RBD associated with neurodegenerative disease usually reflects an underlying synucleinopathy.82 The roots of this hypothesis were based on the wealth of clinical and clinicopathologic studies available as of 2001, and continued observation of how frequently RBD has been associated with PD, DLB, and MSA particularly and the more rare association of RBD with AD, PSP, SCA-3, Guadalupian parkinsonism, and Huntington's disease. RBD has not been reported in association with several other neurodegenerative disorders. Most of the studies published since the original hypothesis have involved clinically- +/- genetically-diagnosed patients, and the gold standard for almost any study in neurodegenerative disease research requires gross and microscopic analyses of tissue, with the latter analyses being done using appropriate routine and immunocytochemical staining techniques. This latter point has been emphasized in the setting of parkinsonism associated mutations in the leucine-rich repeat kinase 2 (LRRK2) gene, in which pleomorphic pathology has been found: Lewy body Parkinson's disease, diffuse Lewy body disease, nigral degeneration without distinctive histopathology, and PSP-like pathology.170 In other words, analyses involving patients with RBD during life are clearly important, but neuropathologic examination is crucial to assessing the RBD-proteinopathy and RBD-pathophysiology mechanisms.
While our own ongoing clinicopathologic experience as reviewed above has shown an overwhelming tendency for RBD to be associated with the disorders collectively termed the synucleinopathies, our own data has also revealed one case of PSP and one case of AD with RBD. Thus, RBD is not 100% specific for the synucleinopathies, nor did we ever expect it to be, given the known variability in humans. Yet the tendency for RBD to occur frequently in the synucleinopathies and infrequently in the non-synucleinopathies suggests that the selective vulnerability of key brainstem networks involved in RBD pathophysiology in the synucleinopathies is relatively consistent, and these same brainstem networks tend not to be similarly affected in the non-synucleinopathies. Furthermore, when RBD precedes the onset of parkinsonism and/or cognitive impairment by several years, the literature would suggest that such cases are much more likely to represent an evolving synucleinopathy, whereas RBD evolving concurrently with or after the onset of parkinsonism and/or cognitive impairment has occurred more frequently in the non-synucleinopathy disorders. Plus, some of these non-synucleinopathy cases appeared to have been diagnosed more on the basis of the PSG, with a rather minimal history of dream enactment behavior. Hence, not only the presence of RBD may be important to the specificity of RBD to which neurodegenerative disease process is at play, but the timing of RBD onset as well as frequency and severity of dream enactment behavior appear to have relevance. These issues should be studied with more rigor.
What are the Implications of the Evolution of RBD and Other Clinical Features in the PD- and DLB-Predominant Phenotypes on the Topography of Degeneration in LBD?
A clinical feature or sign in neurodegenerative disease reflects sufficient neuronal/glial/neurotransmitter dysfunction in a critical neuronal network. While some of these features or signs have known or suspected networks of dysfunction, the underlying substrate for other features or signs is less clear. Parkinsonism most likely is associated with dopaminergic depletion due to sufficient degeneration in the nigrostriatal system, and cognitive impairment is likely associated, at least in part, with cholinergic depletion due to basal forebrain/limbic system/neocortex degeneration. It is highly likely that RBD reflects sufficient degeneration in brainstem networks as described above. The underlying pathobiology of visual hallucinations in DLB and PDD, and fluctuations in cognition/arousal in DLB, is not well understood, but these tend to be features that evolve well after the onset of cognitive impairment and parkinsonism in DLB and PDD.80, 171
Along this same line of reasoning, the evolution of clinical features must reflect the topography of degeneration over time. Many patients with DLB tend to experience RBD prior to cognitive impairment, with subsequent development of parkinsonism. This evolution suggests that dysfunction in the pontomedullary circuitry precedes dysfunction in the basal forebrain, limbic system and neocortical circuitry as well dysfunction in the nigrostratial circuitry. One could argue, as others recently have,172 that this evolution of features and presumed pathophysiologic basis does not support the Braak staging scheme of PD, yet it may provide some clues into LBD pathoanatomy of the DLB phenotype.
There are several aspects to consider. The Braak staging system was indeed proposed for LBD as it relates to the phenotype and evolution of signs in PD and not DLB 120, 121. Many investigators have viewed this staging system to be quite consistent with the usual evolution of features in PD, where RBD and anosmia occur prior to parkinsonism with subsequent development of dementia, visual hallucinations and delusions. In other words, a “bottom-to-top” or ascending progression of Lewy neurites, Lewy bodies, and neuronal degeneration as proposed in Stages 1-6 of the Braak scheme explains the evolution of features in typical PD quite well 120, 121. A similar evolution of degenerative changes may occur in both PD and DLB, and the timing of clinical features may reflect when the critical thresholds of neuronal network degeneration are reached. Neurons with Lewy bodies and/or Lewy neurites may reflect diseased/dysfunctional neurons, but clinical manifestations do not evolve until a sufficient threshold of degeneration has occurred. For example, if an 80% depletion of dopaminergic neurons is needed to manifest parkinsonism, and a 50% depletion of cholinergic neurons is needed to manifest cognitive impairment, it would be reasonable to suggest a “bottom-to-top” progression of LBD pathology could still explain the onset of cognitive symptoms prior to parkinsonism if both systems are affected gradually over years but the thresholds for the expression of clinical deficits are different such that cognitive impairment becomes evident prior to parkinsonism. In other words, the evolution of features in the DLB phenotype does not necessarily refute the Braak staging system for LBD progression.
Another explanation is that, at least in some cases, a “top-to-bottom” or descending progression of degenerative changes from the neocortex/limbic system to the nigrostriatal system may better explain the onset of cognitive impairment prior to parkinsonism in many DLB cases. Or a more patchy and discontinuous evolution of progression could evolve. Most cases of evolving AD develop amnestic MCI with subsequent develop of language, attention/executive, and visuospatial dysfunction tend to follow the Braak stages of neurofibrillary tangle deposition, but there are certainly cases of atypical AD (which tend to have “hippocampal sparing AD” pathology) which have presented as focal cortical syndromes such as progressive aphasia,173 corticobasal syndrome,174 or posterior cortical atrophy175 with relative preservation of memory who clearly did not have neurofibrillary tangle deposition evolve in the typical manner of AD. Therefore, there will always be exceptions to any model of neurodegenerative disease progression, and surely LBD will evolve in more than one manner.
Yet if the majority of cases in any disease evolves in a relatively consistent fashion, this suggests common dysfunctional mechanisms of system biology. RBD may present years or decades prior to the onset of cognitive impairment in patients diagnosed with DLB or PD. This finding supports the bottom-to-top progression with variations in the relative degree of degeneration in critical structures. One therefore could predict that in DLB and PDD, visual hallucinations and fluctuations would be caused by sufficient degeneration and/or neurochemical dysfunction in the diencephalon and telencephalon, or at least a diffuse topography of degeneration/dysfunction. More work in understanding the hallucinatory and fluctuating phenomenology as well as sleep-wake mechanisms is necessary before the underlying substrates of visual hallucinations and fluctuations are known.
How Should Clinicopathologic Studies Be Performed in Patients With RBD?
It is imperative to plan for autopsy in any willing individual with iRBD as well as those with RBD associated with any neurodegenerative disease. Ideally, performing detailed histologic examinations in those who had PSG-proven RBD and those who had no history of RBD-like behavior and had PSG confirming normal EMG atonia during REM sleep, will be most informative.
There are many challenges to studying tissue to determine which are the key populations of neurons +/- glia that are critical to RBD pathophysiology. Whether the brainstem networks involve the nuclei proposed in the RBD pathophysiology scheme as shown in Figures 2 and 3, or others in combination with these nuclei, or other nuclei completely different than those proposed, needs to be examined. Stereologic studies which involve neuronal count quantification, while certainly very laborious, are likely to be informative. Yet some of the brainstem nuclei have unclear borders, or they span a convoluted area of tissue which complicates the technical aspects of quantifying multiple nuclei.
Implications of “Idiopathic” RBD for Future Drug Trials in the Synucleinopathies
As noted above, RBD tends to precede the onset of parkinsonism or dementia in patients with MSA, PD, and DLB by years or decades.1, 17, 22, 63, 65, 81, 82, 84, 91, 92, 109, 114, 176, 177 Almost 40% of patients with iRBD in one series were subsequently found to have developed a parkinsonian disorder,114 and continued follow-up of this cohort has shown approximately 65% have now developed parkinsonism and/or cognitive impairment.29 RBD preceded dementia and/or parkinsonism in 67% of another series,84 and preceded the onset of cognitive impairment in autopsy-proven LBD by a median of 10 years.138 The prospectively-followed cohorts by Iranzo et al. and Postuma et al. are particularly important.26, 80 Therefore, iRBD may represent the harbinger of an evolving neurodegenerative disorder, which in most cases may be a synucleinopathy.1, 3, 24, 26, 76, 80, 84, 110, 117-119
Thus, if iRBD represents the earliest clinical manifestation of an evolving neurodegenerative disorder, the presence of RBD may be particularly relevant early in the course of a neurodegenerative disease when intervention may be most critical. Agents that may positively affect synucleinopathy pathophysiology could be instituted in patients with appropriately-identified iRBD and potentially delay or prevent the development of cognitive impairment or parkinsonism (see Figure 9 and accompanying legend). This is an exciting avenue for future drug therapy, but considerable work is still necessary to identify patients with iRBD, develop biomarkers and predictors of those with an underlying synucleinopathy, and study the natural history of patients with iRBD so that eventual clinical trials can be powered to the estimated effect sizes of therapies.
Figure 9.
Schematic representation of the hypothesized progression in functioning with increasing age and disease severity in evolving Lewy body disease (LBD) in the Parkinson's disease (PD)- and dementia with Lewy bodies (DLB)-predominant phenotypes, and the potential effect of therapy. The onset of REM sleep behavior disorder (RBD) typically begins many years prior to the onset of cognitive decline and a diagnosis of mild cognitive impairment (MCI), and/or many years prior to the onset of motor decline and detectable mild parkinsonian signs (MPS). Progression typically continues over subsequent years to either PD or DLB, and ultimately on to death. This evolution of progression in the current era of primarily symptomatic treatments, with no agent convincingly showing altered progression in the underlying disease process of LBD, is depicted by the red line marked “a”.
One could envision at least four potential effects of a synucleinopathy-active therapy (labeled “Tx” and instituted along the time course as shown by the orange arrow) in patients with appropriately identified “idiopathic” RBD. The agent could slow down the rate of progression (as shown by the green line marked “b”), or delay the onset of cognitive and/or motor decline (as shown by the green line marked “c”), or prevent progression to cognitive and motor decline altogether (as shown by the green line marked “d”). Or, perhaps a synucleinopathy-active therapy could delay the onset of symptoms and slow down the rate of progression (as shown by the green line marked “e”).
A Proposed Decision Tree
A proposed decision tree for assessing patients with iRBD could be formulated somewhat like what is presented in Figure 10. One would first seek to determine in individual patients who is manifesting features of an early neurodegenerative disorder and who is not (step 1). Perhaps many of the clinical features and ancillary test findings discussed in this review, and hopefully many others yet to be identified or developed, will function as accurate biomarkers. Next, if the biomarkers are sufficiently specific to pinpoint the underlying proteinopathy (ie, synucleinopathy vs non-synucleinopathy), then such biomarkers will function in steps 1 and 2; if not, new biomarkers with adequate sensitivity and specificity for proteinopathies and particularly synucleinopathies will need to be developed. Finally, it remains to be seen if agents that ultimately affect synucleinopathy pathophysiology will be safe and efficacious in all of the synucleinopathies regardless of whether the pathologic process is LBD or MSA, or the phenotype is PD-predominant, DLB-predominant, MSA-predominant, or PAF-predominant.
Figure 10.
Proposed decision tree for assessing patients with “idiopathic” RBD for research and ultimately treatment purposes. Presuming that one of the ultimate goals of identifying patients with apparently “idiopathic” RBD is to commence one or more therapies which could delay the onset of cognitive impairment and/or parkinsonism, slow down the course, or halt progression of an underlying neurodegenerative disorder altogether, one would first seek to determine in individual patients who is manifesting features of an early neurodegenerative disorder and who is not (step 1). Perhaps many of the clinical features and ancillary test findings covered in this review, and hopefully many others yet to be developed, will function as accurate biomarkers. Next, if the biomarkers are sufficiently specific to pinpoint the underlying proteinopathy (ie, synucleinopathy vs non-synucleinopathy), then such biomarkers will function in steps 1 and 2; if not, new biomarkers with adequate sensitivity and specificity for proteinopathies and particularly synucleinopathies will need to be developed. Finally, it remains to be seen if agents that ultimately affect synucleinopathy pathophysiology will be safe and efficacious in all of the synucleinopathies regardless of whether the pathologic process is LBD or MSA, or the phenotype is PD-predominant, DLB-predominant, MSA-predominant, or PAF-predominant. Incidental LBD (iLBD) will be impossible to predict without pathology. The challenge for investigators at present is to study adequate numbers of patients with “idiopathic” RBD with a spectrum of clinical tests and potential biomarkers longitudinally to assess the natural history and prepare for future clinical trials.
Abbreviations: DLB=dementia with Lewy bodies, MSA=multiple system atrophy PAF=pure autonomic failure, PD=Parkinson's disease, RBD=REM sleep behavior disorder
Biomarkers
The term “biomarker” is used very loosely in this context, and is meant to represent any clinical feature or finding, and any finding on a diagnostic test, which may indicate a particular disorder or disease state. A list of potential biomarkers which have been or could be studied in patients with iRBD is shown in Figure 11 [note that this author emphasizes the work by the many esteemed members of the International RBD Study Group (Chair: Prof. Dr. med. Wolfgang Oertel) who have used/studied many of these biomarkers, or are suggesting to use/study these biomarkers, deserve the credit for moving the RBD field forward on biomarker research]. This is clearly a partial list of potential biomarkers which will surely be expanded upon over the years to come.
Figure 11.
List of potential biomarkers to study in patients with idiopathic RBD.
Based on the published data for certain biomarkers in specific phenotypes/disorders, as well as the suspected findings despite no data published as yet, one could propose a profile of abnormalities on various biomarkers in patients with iRBD resulting from an underlying synucleinopathy (Figure 12). The phenotypes/diseases include incidental Lewy body disease (iLBD), which is the term applied to those individuals who have no obvious neurologic impairment but have LBD found at autopsy, PD, DLB, MSA, and PAF. One would predict a different pattern of normal (Nl) and abnormal (Abnl) findings based on the biomarkers being performed. Some results are more difficult to predict, such as whether any abnormalities on smell testing, autonomic testing, and DaTSCAN would occur in iLBD. Also, some abnormal findings may be particularly suggestive of specific phenotypes/disorders, such as the neuropsychological profile of impairment in DLB.
Figure 12.
Hypothesized profile of abnormalities on various biomarkers in patients with iRBD resulting from an underlying synucleinopathy. The phenotypes/diseases include incidental Lewy body disease (iLBD), Parkinson's disease (PD), dementia with Lewy bodies (DLB), multiple system atrophy (MSA), and pure autonomic failure (PAF). One would predict a different pattern of normal (Nl) and abnormal (Abnl) findings based on the biomarkers being performed, with some results more difficult to predict (represented by “+/-”). Some abnormal findings may be particularly suggestive of specific phenotypes/disorders (represented by an asterisk).
Abbreviations: Cog=cognitive/neuropsychological testing, Aut=autonomic studies, MRI=magnetic resonance imaging, MRS=magnetic resonance spectroscopy, DaT=DaTSCAN, PET=positron emission tomography of the brain using various ligands, MIBG=Cardiac (123)I-metaiodobenzylguanidine imaging.
A different profile of abnormalities on various biomarkers in patients with iRBD resulting from an underlying non-synucleinopathy degenerative disorder could also be predicted, as shown in Figure 13). The biomarkers that would be more normal than not include smell testing (despite the literature on olfaction being impaired early in AD, it now appears many of these patients have co-existing LBD,178, 179 and the findings on neuropsychological testing, MRI, MRS, and FDG-PET are distinctly different – at least from DLB and PDD. As described above, cardiac MIBG is typically normal in disorders outside of the PD-PDD-DLB-PAF spectrum. Findings on DaTSCAN tend to be normal in AD, but it remains to be seen if findings are typically abnormal in PSP and CBD as few such cases with DaTSCAN performed antemortem have undergone autopsy.
Figure 13.
Hypothesized profile of abnormalities on various biomarkers in patients with iRBD resulting from an underlying non-synucleinopathy neurodegenerative disorder. The phenotypes/diseases include Alzheimer's disease (AD), one of the tauopathies (Pick's disease, corticobasal degeneration, progressive supranuclear palsy, multisystem tauopathy, or frontotemporal dementa with parkinsonism associated with a mutation in the microtubule associated protein tau), or one of the TDP-43-associated pathologies (frontotemporal lobar degeneration [FTLD] with TDP-43 positive inclusions, FTLD with motor neuron disease, or FTLD with a mutation in progranulin). One would predict a different pattern of normal (Nl) and abnormal (Abnl) findings based on the biomarkers being performed, with some results more difficult to predict (represented by “+/-”). Some abnormal findings may be particularly suggestive of specific phenotypes/disorders (represented by an asterisk).
Abbreviations: Cog=cognitive/neuropsychological testing, Aut=autonomic studies, MRI=magnetic resonance imaging, MRS=magnetic resonance spectroscopy, DaT=DaTSCAN, PET=positron emission tomography of the brain using various ligands, MIBG=Cardiac (123)I-metaiodobenzylguanidine imaging
The challenges
The challenge for basic scientists is to develop therapies that impact the cascade of events involved in synucleinopathy and other neurodegenerative disorder pathophysiologies. The challenge for clinical investigators at present is to study adequate numbers of patients with “idiopathic” RBD longitudinally with a spectrum of clinical tests and potential biomarkers to assess the natural history of the current state of no truly disease-altering therapies, and prepare for future clinical trials. The hurdles are even more challenging when one considers that the funding cycles for most federal and private funding institutions run over 5 year periods, and the unique work of Postuma et al. suggests that 50% of those with iRBD develop parkinsonism and/or dementia in 12 years. This is a core “nature of the beast” in this line of research – LBD appears to evolve slowly over several decades in many individuals, but this cannot thwart our attempts to plan for disease-altering therapies. Yet it is extremely important to realize for therapeutic advances – a minor effect on pathophysiology early in the disease course in a process that evolves over decades could potentially delay or alter the clinical manifestations by many years such that morbidity or mortality could occur outside of the neurodegenerative realm. Securing funding to plan for future therapeutic trials will require ingenuity by investigators, a large investment in infrastructure and biomarker technologies, and frank optimism on the part of funding agencies, but surely most would agree that the literature amply suggests that the scientific basis for this line of future research is sound.
The optimism
It is difficult to envision the heightened optimism even a decade ago in the potential of impacting a neurodegenerative process early in its course. Idiopathic RBD is one of the more intriguing clinical curiosities in medicine, and certainly in sleep medicine and neurology. Idiopathic RBD likely represents a window of opportunity for impacting neurodegenerative disease like few other disorders, particularly among the apparent sporadic cases. The scientific community should educate the public on the importance of early diagnosis and treatment of RBD (being ever mindful of the anxiety over what the disorder may represent), and follow iRBD longitudinally with a battery of clinical assessments and potential biomarkers, while we await agents that impact neurodegenerative disease pathophysiology.
Acknowledgments
Supported by grants AG 15866, AG 16574, AG 06786, P50 NS40256, and the Robert H. and Clarice Smith and Abigail Van Buren Alzheimer's Disease Research Program of the Mayo Foundation
References
- 1.Schenck C, Mahowald M. REM sleep behavior disorder: Clinical, developmental, and neuroscience perspectives 16 years after its formal identification in SLEEP. Sleep. 2002;25:120–138. doi: 10.1093/sleep/25.2.120. [DOI] [PubMed] [Google Scholar]
- 2.Schenck C. Paradox Lost - Midnight in the Battleground of Sleep and Dreams - Violent Moving Nightmares, REM Sleep Behavior Disorder. Extreme-Nights, LLC; 2005. [Google Scholar]
- 3.Boeve B, Silber M, Saper C, et al. Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease. Brain. 2007;130:2770–2788. doi: 10.1093/brain/awm056. [DOI] [PubMed] [Google Scholar]
- 4.Iber C, Ancoli-Israel S, Chesson A, Quan S, the American Academy of Sleep Medicine The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications 2007 [Google Scholar]
- 5.Iranzo A, Santamaria J. Severe obstructive sleep apnea/hypopnea mimicking REM sleep behavior disorder. Sleep. 2005;28:203–206. doi: 10.1093/sleep/28.2.203. [DOI] [PubMed] [Google Scholar]
- 6.Schenck C, Milner D, Hurwitz T, Bundlie S, Mahowald M. A polysomnographic and clinical report on sleep-related injury in 100 adult patients. Amer J Psychiatr. 1989;146:1166–1173. doi: 10.1176/ajp.146.9.1166. [DOI] [PubMed] [Google Scholar]
- 7.Kimura K, Tachibana N, Toshihiko A, Kimura J, Shibasaki H. Subclinical REM sleep behavior disorder in a patient with corticobasal degeneration. Sleep. 1997;20:891–894. doi: 10.1093/sleep/20.10.891. [DOI] [PubMed] [Google Scholar]
- 8.Pareja J, Caminero A, Masa J, Dobato J. A first case of progressive supranuclear pasy and pre-clinical REM sleep behavior disorder presenting as inhibition of speech during wakefulness and somniloquy with phasic muscle twitching during REM sleep. Neurologia. 1996;11:304–306. [PubMed] [Google Scholar]
- 9.Schenck CH, Bundlie SR, Ettinger MG, Mahowald MW. Chronic behavioral disorders of human REM sleep: a new category of parasomnia. Sleep. 1986;9:293–308. doi: 10.1093/sleep/9.2.293. [DOI] [PubMed] [Google Scholar]
- 10.Schenck CH, Bundlie SR, Patterson AL, Mahowald MW. Rapid eye movement sleep behavior disorder. A treatable parasomnia affecting older adults. JAMA. 1987;257:1786–1789. [PubMed] [Google Scholar]
- 11.Schenck CH, Milner DM, Hurwitz TD, Bundlie SR, Mahowald MW. A polysomnographic and clinical report on sleep-related injury in 100 adult patients. Amer J Psychiatry. 1989;146:1166–73. doi: 10.1176/ajp.146.9.1166. [DOI] [PubMed] [Google Scholar]
- 12.Schenck C, Mahowald M. A polysomnographic, neurologic, psychiatric and clinical outcome report on 70 consecutive cases with REM sleep behavior disorder (RBD): sustained clonzepam efficacy in 89.5% of 57 treated patients. Clev Clin J Med. 1990;57(suppl):10–24. [Google Scholar]
- 13.Schenck C, Hurwitz T, Mahowald M. REM sleep behaviour disorder: an update on a series of 96 patients and a review of the world literature. J Sleep Res. 1993;2:224–231. doi: 10.1111/j.1365-2869.1993.tb00093.x. [DOI] [PubMed] [Google Scholar]
- 14.Schenck CH, Mahowald MW. Long-term, nightly benzodiazepine treatment of injurious parasomnias and other disorders of disrupted nocturnal sleep in 170 adults. Amer J Med. 1996;100:333–7. doi: 10.1016/S0002-9343(97)89493-4. [DOI] [PubMed] [Google Scholar]
- 15.Schenck CH, Mahowald MW. REM sleep parasomnias. Neurologic Clinics. 1996;14:697–720. doi: 10.1016/s0733-8619(05)70281-4. [DOI] [PubMed] [Google Scholar]
- 16.Gross P. REM sleep behavior disorder causing bilateral subdural hematomas. Sleep Res. 1992;21:204. [Google Scholar]
- 17.Boeve BF, Silber MH, Ferman TJ, et al. REM sleep behavior disorder and degenerative dementia: an association likely reflecting Lewy body disease. Neurology. 1998;51:363–370. doi: 10.1212/wnl.51.2.363. [DOI] [PubMed] [Google Scholar]
- 18.Mahowald M, Schenck C. REM sleep behavior disorder. In: Kryger M, Roth T, Dement W, editors. Principles and practice of sleep medicine. WB Saunders; Philadelphia: 2000. pp. 724–741. [Google Scholar]
- 19.Dyken M, Lin-Dyken D, Seaba P, Yamada T. Violent sleep-related behavior leading to subdural hemorrhage. Arch Neurol. 1995;52:318–321. doi: 10.1001/archneur.1995.00540270114028. [DOI] [PubMed] [Google Scholar]
- 20.Sforza E, Krieger J, Petiau C. REM sleep behavior disorder: clinical and physiopathological findings. Sleep Med Rev. 1997;1:57–69. doi: 10.1016/s1087-0792(97)90006-x. [DOI] [PubMed] [Google Scholar]
- 21.Comella C, Nardine T, Diederich N, Stebbins G. Sleep-related violence, injury, and REM sleep behavior disorder in Parkinson's disease. Neurology. 1998;51:526–529. doi: 10.1212/wnl.51.2.526. [DOI] [PubMed] [Google Scholar]
- 22.Olson E, Boeve B, Silber M. Rapid eye movement sleep behavior disorder: demographic, clinical, and laboratory findings in 93 cases. Brain. 2000;123:331–339. doi: 10.1093/brain/123.2.331. [DOI] [PubMed] [Google Scholar]
- 23.Gagnon JF, Medard MA, Fantini M, et al. REM sleep behavior disorder and REM sleep without atonia in Parkinson's disease. Neurology. 2002;59:585–589. doi: 10.1212/wnl.59.4.585. [DOI] [PubMed] [Google Scholar]
- 24.Turner RS. Idiopathic rapid eye movement sleep behavior disorder is a harbinger of dementia with Lewy bodies. J Geriatr Psychiatr Neurol. 2002;15:195–9. doi: 10.1177/089198870201500404. [DOI] [PubMed] [Google Scholar]
- 25.Iranzo A, Santamaria J, Rye DB, et al. Characteristics of idiopathic REM sleep behavior disorder and that associated with MSA and PD. Neurology. 2005;65:247–252. doi: 10.1212/01.wnl.0000168864.97813.e0. [DOI] [PubMed] [Google Scholar]
- 26.Iranzo A, Molinuevo J, Santamaría J, et al. Rapid-eye-movement sleep behaviour disorder as an early marker for a neurodegenerative disorder: a descriptive study. Lancet Neurol. 2006;5:572–577. doi: 10.1016/S1474-4422(06)70476-8. [DOI] [PubMed] [Google Scholar]
- 27.Boeve B, Silber M, Ferman T, et al. REM Sleep Behavior Disorder in Parkinson's Disease, Dementia with Lewy Bodies, and Multiple System Atrophy. In: Bedard M, et al., editors. Mental and Behavioral Dysfunction in Movement Disorders. Humana Press; Totowa: 2003. pp. 383–397. [Google Scholar]
- 28.Claassen D, Josephs K, Ahlskog J, et al. REM sleep behavior disorder may precede PD, DLB, or MSA by up to half a century. Neurology. 2009;72(Suppl 3):A324. doi: 10.1212/WNL.0b013e3181ec7fac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schenck C, Bundlie S, Mahowald M. REM behavior disorder (RBD): Delayed emergence of parkinsonism and/or dementia in 65% of older men initially diagnosed with idiopathic RBD, and an analysis of the minimum & maximum tonic and/or phasic electromyographic abnormalities found during REM sleep. Sleep. 2003;26:A316. [Google Scholar]
- 30.Tang J, Boeve B, Tippmann-Peikert M, Silber M. Gender effect in patients with REM sleep behavior disorder associated with multiple system atrophy compared with Parkinson's disease and dementia with Lewy bodies. Neurology. 2009;72(Suppl 3):A325. [Google Scholar]
- 31.Ohayon M, Caulet M, Priest R. Violent behavior during sleep. J Clin Psychiatr. 1997;58:369–376. [PubMed] [Google Scholar]
- 32.Molano J, Boeve B, Roberts R, et al. Frequency of sleep disorders in the community-dwelling elderly: The Mayo Clinic Study of Aging. Neurology. 2009;72(Suppl 3):A107. [Google Scholar]
- 33.Boeve B, Molano J, Ferman T, et al. Screening for REM sleep behavior disorder in patients with cognitive impairment and/or parkinsonism: Updated validation data on the Mayo Sleep Questionnaire. Neurology. 2009;72:A248. [Google Scholar]
- 34.Mihci E, Molano J, Boeve B, et al. Parkinsonism is associated with probable REM sleep behavior disorder in cognitively normal elderly subjects: The Mayo Clinic Study of Aging. Neurology. 2008;70(Suppl 1):A150. [Google Scholar]
- 35.Molano J, Mihci E, Boeve B, et al. Anxiety and apathy are associated with probable REM sleep behavior disorder among cognitively normal elderly subjects: The Mayo Clinic Study of Aging. Neurology. 2008;70(Suppl 1):A146. [Google Scholar]
- 36.Molano J, Mihci E, Boeve B, et al. Lower scores in attention/executive functioning is associated with probable REM sleep behavior disorder among cognitively normal elderly subjects: The Mayo Clinic Study of Aging. Neurology. 2008;70(Suppl 1):A66. [Google Scholar]
- 37.International Classification of Sleep Disorders: Diagnostic and Coding Manual. 2nd. American Academy of Sleep Medicine; Westchester, IL: 2005. [Google Scholar]
- 38.Silber M, Ancoli-Israel S, Bonnet M, et al. The visual scoring of sleep in adults. J Clin Sleep Med. 2007;15:121–131. [PubMed] [Google Scholar]
- 39.Redline S, Budhiraja R, Kapur V, et al. The scoring of respiratory events in sleep: reliability and validity. J Clin Sleep Med. 2007;15:169–200. [PubMed] [Google Scholar]
- 40.Kunz D, Bes F. Melatonin as a therapy in REM sleep behavior disorder patients: An open-labeled pilot study on the possible influence of melatonin on REM-sleep regulation. Mov Disord. 1999;14:507–511. doi: 10.1002/1531-8257(199905)14:3<507::aid-mds1021>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 41.Boeve B, Silber M, Ferman T. Melatonin for treatment of REM sleep behavior disorder in neurologic disorders: results in 14 patients. Sleep Med. 2003;4:281–284. doi: 10.1016/s1389-9457(03)00072-8. [DOI] [PubMed] [Google Scholar]
- 42.Gagnon J, Postuma R, Montplaisir J. Update on the pharmacology of REM sleep behavior disorder. Neurology. 2006;67:742–747. doi: 10.1212/01.wnl.0000233926.47469.73. [DOI] [PubMed] [Google Scholar]
- 43.Fantini M, Gagnon JF, Filipini D, Montplaisir J. The effects of pramipexole in REM sleep behavior disorder. Neurology. 2003;61:1418–1420. doi: 10.1212/wnl.61.10.1418. [DOI] [PubMed] [Google Scholar]
- 44.Ringman J, Simmons J. Treatment of REM sleep behavior disorder with donepezil: a report of three cases. Neurology. 2000;55:870–871. doi: 10.1212/wnl.55.6.870. [DOI] [PubMed] [Google Scholar]
- 45.Rye D. Contributions of the pedunculopontine region to normal and altered REM sleep. Sleep. 1997;20:757–788. doi: 10.1093/sleep/20.9.757. [DOI] [PubMed] [Google Scholar]
- 46.Bamford C. Carbamazepine in REM sleep behavior disorder. Sleep. 1993;16:33–34. [PubMed] [Google Scholar]
- 47.Onofrj M, Luciano AL, Thomas A, Iacono D, D'Andreamatteo G. Mirtazapine induces REM sleep behavior disorder (RBD) in parkinsonism. Neurology. 2003;60:113–115. doi: 10.1212/01.wnl.0000042084.03066.c0. [DOI] [PubMed] [Google Scholar]
- 48.Winkelman J, James L. Serotonergic antidepressants are associated with REM sleep without atonia. Sleep. 2004;15:317–321. doi: 10.1093/sleep/27.2.317. [DOI] [PubMed] [Google Scholar]
- 49.Hendricks J, Morrison A, Mann G. Different behaviors during paradoxical sleep without atonia depend on pontine lesion site. Brain Res. 1982;239:81–105. doi: 10.1016/0006-8993(82)90835-6. [DOI] [PubMed] [Google Scholar]
- 50.Jouvet M, Delorme F. Locus coeruleus et sommeil paradoxal. C R Soc Biol. 1965;159:895–899. [Google Scholar]
- 51.Lai Y, Siegel J. Medullary regions mediating atonia. J Neurosci. 1988;8:4790–4796. doi: 10.1523/JNEUROSCI.08-12-04790.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lai Y, Siegel J. Muscle tone suppression and stepping produced by stimulation of midbrain and rostral pontine reticular formation. J Neurosci. 1990;10:2727–2734. doi: 10.1523/JNEUROSCI.10-08-02727.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lai Y, Siegel J. Brainstem-mediated locomotion and myoclonic jerks. I. Neural substrates. Brain Res. 1997;745:257–264. doi: 10.1016/s0006-8993(96)01177-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lai Y, Siegel J. Brainstem-mediated locomtion and myoclonic jerks. II. Pharmacological effects. Brain Res. 1997;745:265–270. doi: 10.1016/s0006-8993(96)01180-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morrison A. The pathophysiology of REM-sleep behavior disorder. Sleep. 1998;21:446. [PubMed] [Google Scholar]
- 56.Rye D. The pathophysiology of REM-sleep behavior disorder. Sleep. 1998;21:446–449. [PubMed] [Google Scholar]
- 57.Shouse M, Siegel J. Pontine regulation of REM sleep components in cats: integrity of the pedunculopontine tegmentum (PPT) is important for phasic events but unnecessary for atonia during REM sleep. Brain Res. 1992;571:50–63. doi: 10.1016/0006-8993(92)90508-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mahowald MW, Schenck CH. Rem sleep without atonia--from cats to humans. Arch Ital Biol. 2004;142:469–78. [PubMed] [Google Scholar]
- 59.Siegel J. The stuff dreams are made of: anatomical substrates of REM sleep. Nature Neurosci. 2006;9:721–722. doi: 10.1038/nn0606-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lu J, Sherman D, Devor M, Saper C. A putative flip-flop switch for control of REM sleep. Nature. 2006;441:589–594. doi: 10.1038/nature04767. [DOI] [PubMed] [Google Scholar]
- 61.Boissard R, Gervasoni D, Schmidt M, et al. The rat ponto-medullary network responsible for paradoxical sleep onset and maintenance: a combined microinjection and functional neuroanatomical study. Eur J Neurosci. 2002;16:1959–1973. doi: 10.1046/j.1460-9568.2002.02257.x. [DOI] [PubMed] [Google Scholar]
- 62.Boissard E, Fort P, Gervasoni D, Barbagli B, Luppi P. Localization of the GABAergic and non-GABAergic neurons projecting to the sublaterodorsal nucleus and potentially gating paradoxical sleep onset. Eur J Neurosci. 2003;18:1627–1639. doi: 10.1046/j.1460-9568.2003.02861.x. [DOI] [PubMed] [Google Scholar]
- 63.Silber M, Ahlskog J. REM sleep behavior disorder in parkinsonian syndromes. Sleep Res. 1992;21:313. [Google Scholar]
- 64.Silber M, Dexter D, Ahlskog J, Hauri P, Shepard J. Abnormal REM sleep motor activity in untreated Parkinson's disease. Sleep Res. 1993;22:274. [Google Scholar]
- 65.Tan A, Salgado M, Fahn S. Rapid eye movement sleep behavior disorder preceding Parkinson's disease with therapeutic response to levodopa. Mov Disord. 1996;11:214–216. doi: 10.1002/mds.870110216. [DOI] [PubMed] [Google Scholar]
- 66.Rye D, Johnston L, Watts R, Bliwise D. Juvenile Parkinson's disease with REM sleep behavior disorder, sleepiness, and daytime REM onset. Neurology. 1999;53:1868–1872. doi: 10.1212/wnl.53.8.1868. [DOI] [PubMed] [Google Scholar]
- 67.Arnulf I, Bonnet AM, Damier P, et al. Hallucinations, REM sleep, and Parkinson's disease: a medical hypothesis. Neurology. 2000;55:281–288. doi: 10.1212/wnl.55.2.281. [DOI] [PubMed] [Google Scholar]
- 68.Onofrj M, Thomas A, D'Andreamatteo G, et al. Incidence of RBD and hallucination in patients affected by Parkinson's disease: 8-year follow-up. Neurol Sci. 2002;23:S91–S94. doi: 10.1007/s100720200085. [DOI] [PubMed] [Google Scholar]
- 69.Onofrj M, Luciano AL, Iacono D, et al. HLA typing does not predict REM sleep behaviour disorder and hallucinations in Parkinson's disease. Mov Disord. 2003;18:337–340. doi: 10.1002/mds.10409. [DOI] [PubMed] [Google Scholar]
- 70.Eisensehr I, Linke R, Tatsch K, et al. Increased muscle activity during rapid eye movement sleep correlates with decrease of striatal presynaptic dopamine transporters. IPT and IBZM SPECT imaging in subclinical and clinically manifest idiopathic REM sleep behavior disorder, Parkinson's disease, and controls. Sleep. 2003;26:507–512. doi: 10.1093/sleep/26.5.507. [DOI] [PubMed] [Google Scholar]
- 71.Ozekmekci S, Apaydin H, Kilic E. Clinical features of 35 patients with Parkinson's disease displaying REM behavior disorder. Clin Neurol Neurosurg. 2005;107:306–309. doi: 10.1016/j.clineuro.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 72.Pacchetti C, Manni R, Zangaglia R, et al. Relationship between hallucinations, delusions, and rapid eye movement sleep behavior disorder in Parkinson's disease. Mov Disord. 2005;20:1439–1448. doi: 10.1002/mds.20582. [DOI] [PubMed] [Google Scholar]
- 73.Scaglione C, Vignatelli L, Plazzi G, et al. REM sleep behaviour disorder in Parkinson's disease: a questionnaire-based study. Neurol Sci. 2005;25:316–321. doi: 10.1007/s10072-004-0364-7. [DOI] [PubMed] [Google Scholar]
- 74.Hanoglu L, Ozer F, Meral H, Dincer A. Brainstem 1H-MR spectroscopy in patients with Parkinson's disease with REM sleep behavior disorder and IPD patients without dream enactment behavior. Clin Neurol Neurosurg. 2006;108:129–134. doi: 10.1016/j.clineuro.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 75.Sinforiani E, Zangaglia R, Manni R, et al. REM sleep behavior disorder, hallucinations, and cognitive impairment in Parkinson's disease. Mov Disord. 2006;21:462–466. doi: 10.1002/mds.20719. [DOI] [PubMed] [Google Scholar]
- 76.Postuma R, Lang A, Massicotte-Marquez J, Montplaisir J. Potential early markers of Parkinson disease in idiopathic REM sleep behavior disorder. Neurology. 2006;66:845–851. doi: 10.1212/01.wnl.0000203648.80727.5b. [DOI] [PubMed] [Google Scholar]
- 77.Gjerstad M, Boeve B, Wentzel-Larsen T, Aarsland D, Larsen J. Occurrence and clinical correlates of REM sleep behavior disorder in patients with Parkinson's disease over time. J Neurol Neurosurg Psychiatry. 2007 Jun 8; doi: 10.1136/jnnp.2007.116830. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 78.Postuma R, Gagnon J, Vendette M, Charland K, Montplaisir J. Manifestations of Parkinson disease differ in association with REM sleep behavior disorder. Mov Disord. 2008;23:1665–1672. doi: 10.1002/mds.22099. [DOI] [PubMed] [Google Scholar]
- 79.Postuma R, Gagnon J, Vendette M, Charland K, Montplaisir J. REM sleep behaviour disorder in Parkinson's disease is associated with specific motor features. J Neurol Neurosurg Psychiatry. 2008;79:1117–1121. doi: 10.1136/jnnp.2008.149195. [DOI] [PubMed] [Google Scholar]
- 80.Postuma R, Gagnon J, Vendette M, et al. Quantifying the risk of neurodegenerative disease in idiopathic REM sleep behavior disorder. Neurology. 2009 Jan 7; doi: 10.1212/01.wnl.0000340980.19702.6e. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ferman TJ, Boeve BF, Smith GE, et al. REM sleep behavior disorder and dementia: cognitive differences when compared with AD. Neurology. 1999;52:951–957. doi: 10.1212/wnl.52.5.951. [DOI] [PubMed] [Google Scholar]
- 82.Boeve B, Silber M, Ferman T, Lucas J, Parisi J. Association of REM sleep behavior disorder and neurodegenerative disease may reflect an underlying synucleinopathy. Mov Disord. 2001;16:622–630. doi: 10.1002/mds.1120. [DOI] [PubMed] [Google Scholar]
- 83.Ferman T, Boeve B, Smith G, et al. Dementia with Lewy bodies may present as dementia with REM sleep behavior disorder without parkinsonism or hallucinations. J Internat Neuropsychol Soc. 2002;8:907–914. doi: 10.1017/s1355617702870047. [DOI] [PubMed] [Google Scholar]
- 84.Boeve B, Silber M, Parisi J, et al. Synucleinopathy pathology and REM sleep behavior disorder plus dementia or parkinsonism. Neurology. 2003;61:40–45. doi: 10.1212/01.wnl.0000073619.94467.b0. [DOI] [PubMed] [Google Scholar]
- 85.Massironi G, Galluzzi S, Frisoni G. Drug treatment of REM sleep behavior disorders in dementia with Lewy bodies. Int Psychogeriatr. 2003;15:377–383. doi: 10.1017/s1041610203009621. [DOI] [PubMed] [Google Scholar]
- 86.Ferman T, Smith G, Boeve B, et al. DLB fluctuations: Specific features that reliably differentiate DLB from AD and normal aging. Neurology. 2004;62:181–187. doi: 10.1212/wnl.62.2.181. [DOI] [PubMed] [Google Scholar]
- 87.Ferman T, Smith G, Boeve B, et al. Neuropsychological differentiation of dementia with Lewy bodies from normal aging and Alzheimer's disease. Clin Neuropsychol. 2006;20 doi: 10.1080/13854040500376831. [DOI] [PubMed] [Google Scholar]
- 88.Plazzi G, Corsini R, Provini F, et al. REM sleep behavior disorder in multiple system atrophy. Neurology. 1997;48:1094–1097. doi: 10.1212/wnl.48.4.1094. [DOI] [PubMed] [Google Scholar]
- 89.Tachibana N, Kimura K, Kitajima K, et al. REM sleep motor dysfunction in multiple system atrophy: with special emphasis on sleep talk as its early clinical manifestation. J Neurol Neurosurg Psychiatry. 1997;63:678–681. doi: 10.1136/jnnp.63.5.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Quera Salva M, Guilleminault C. Olivopontocerebellar degeneration, abnormal sleep, and REM sleep without atonia. Neurology. 1986;36:576–577. doi: 10.1212/wnl.36.4.576. [DOI] [PubMed] [Google Scholar]
- 91.Tison F, Wenning G, Quinn N, Smith S. REM sleep behavior disorder as the presenting symptom of multiple system atrophy. J Neurol Neurosurg Psychiatry. 1995;58:379–380. doi: 10.1136/jnnp.58.3.379-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wright B, Rosen J, Buysse D, Reynolds C, Zubenko G. Shy-Drager syndrome presenting as a REM behavioral disorder. J Geriatr Psychiatry Neurol. 1990;3:110–113. doi: 10.1177/089198879000300210. [DOI] [PubMed] [Google Scholar]
- 93.Manni R, Morini R, Martignoni E, et al. Nocturnal sleep in multisystem atrophy with autonomic failure: polygraphic findings in ten patients. J Neurol. 1993;240:247–250. doi: 10.1007/BF00818713. [DOI] [PubMed] [Google Scholar]
- 94.Coccagna G, Martinelli P, Zucconi M, Cirignotta F, Ambrosetto G. Sleep-related respiratory and haemodynamic changes in Shy-Drager syndrome: a case report. J Neurol. 1985;232:310–313. doi: 10.1007/BF00313872. [DOI] [PubMed] [Google Scholar]
- 95.Tachibana N, Oka Y. Longitudinal change in REM sleep components in a patient with multiple system atrophy associated with REM sleep behavior disorder: paradoxical improvement of nocturnal behaviors in a progressive neurodegenerative disease. Sleep Med. 2004;5:155–158. doi: 10.1016/j.sleep.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 96.Vetrugno R, Provini F, Cortelli P, et al. Sleep disorders in multiple system atrophy: a correlative video-polysomnographic study. Sleep Med. 2004;5:21–30. doi: 10.1016/j.sleep.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 97.Schmeichel A, Buchhalter L, Low P, et al. Mesopontine cholinergic neuron involvement in Lewy body dementia and multiple system atrophy. Neurology. 2008;70:368–373. doi: 10.1212/01.wnl.0000298691.71637.96. [DOI] [PubMed] [Google Scholar]
- 98.Gaig C, Iranzo A, Tolosa E, et al. Pathological description of a non-motor variant of multiple system atrophy. J Neurol Neurosurg Psychiatry. 2008;79:1399–1400. doi: 10.1136/jnnp.2008.145276. [DOI] [PubMed] [Google Scholar]
- 99.Weyer A, Minnerop M, Abele M, Klockgether T. REM sleep behavioral disorder in pure autonomic failure (PAF) Neurology. 2006;66:608–609. doi: 10.1212/01.WNL.0000198251.94422.F3. [DOI] [PubMed] [Google Scholar]
- 100.Kumru H, Santamaria J, Tolosa E, et al. Rapid eye movement sleep behavior disorder in parkinsonism with parkin mutations. Ann Neurol. 2004;56:599–603. doi: 10.1002/ana.20272. [DOI] [PubMed] [Google Scholar]
- 101.Pramstaller P, Schlossmacher M, Jacques T, et al. Lewy body Parkinson's disease in a large pedigree with 77 Parkin mutation carriers. Ann Neurol. 2005;58:411–422. doi: 10.1002/ana.20587. [DOI] [PubMed] [Google Scholar]
- 102.Fukutake T, Shinotoh H, Nishino H, et al. Homozygous Machado-Joseph disease presenting as REM sleep behavior disorder and prominent psychiatric symptoms. Eur J Neurol. 2002;9:97–100. doi: 10.1046/j.1468-1331.2002.00335.x. [DOI] [PubMed] [Google Scholar]
- 103.Friedman J. Presumed rapid eye movement behavior disorder in Machado-Joseph disease (spinocerebellar ataxia type 3. Mov Disord. 2002;17:1350–1353. doi: 10.1002/mds.10269. [DOI] [PubMed] [Google Scholar]
- 104.Iranzo A, Muñoz E, Santamaria J, et al. REM sleep behavior disorder and vocal cord paralysis in Machado-Joseph disease. Mov Disord. 2003;18:1179–1183. doi: 10.1002/mds.10509. [DOI] [PubMed] [Google Scholar]
- 105.Arnulf I, Nielsen J, Lohmann E, et al. Rapid eye movement sleep disturbances in Huntington disease. Arch Neurol. 2008;65:482–488. doi: 10.1001/archneur.65.4.482. [DOI] [PubMed] [Google Scholar]
- 106.Arnulf I, Merino-Andreu M, Bloch F, et al. REM sleep behavior disorder and REM sleep without atonia in patients with progressive supranuclear palsy. Sleep. 2005;28:349–354. [PubMed] [Google Scholar]
- 107.De Cock V, Lannuzel A, Verhaeghe S, et al. REM sleep behavior disorder in patients with guadeloupean parkinsonism, a tauopathy. Sleep. 2007;30:1026–1032. doi: 10.1093/sleep/30.8.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schenck CH, Garcia-Rill E, Skinner RD, Anderson ML, Mahowald MW. A case of REM sleep behavior disorder with autopsy-confirmed Alzheimer's disease: postmortem brain stem histochemical analyses. Biol Psychiatry. 1996;40:422–425. doi: 10.1016/0006-3223(96)00070-4. [DOI] [PubMed] [Google Scholar]
- 109.Schenck CH, Mahowald MW, Anderson ML, et al. Lewy body variant of Alzheimer's disease (AD) identified by postmortem ubiquitin staining in a previously reported case of AD associated with REM sleep behavior disorder. Biol Psychiatry. 1997;42:527–528. doi: 10.1016/S0006-3223(97)00228-X. letter. [DOI] [PubMed] [Google Scholar]
- 110.Gagnon JF, Postuma R, Mazza S, Doyon J, Montplaisir J. Rapid-eye-movement sleep behaviour disorder and neurodegenerative diseases. Lancet Neurol. 2006;5:424–432. doi: 10.1016/S1474-4422(06)70441-0. [DOI] [PubMed] [Google Scholar]
- 111.Gagnon J, Petit D, Fantini M, et al. REM sleep behavior disorder and REM sleep without atonia in probable Alzheimer disease. Sleep. 2006;29:1321–1325. doi: 10.1093/sleep/29.10.1321. [DOI] [PubMed] [Google Scholar]
- 112.Boeve B, Lin SC, Strongosky A, Dickson D, Wszolek Z. Absence of REM sleep behavior disorder in eleven members of the PPND kindred. Arch Neurol. 2006;63:268–272. doi: 10.1001/archneur.63.2.268. [DOI] [PubMed] [Google Scholar]
- 113.Kelley B, Haidar W, Boeve B, et al. Prominent phenotypic variability associated with mutations in Progranulin. Neurobiol Aging. 2007 Oct 18; doi: 10.1016/j.neurobiolaging.2007.08.022. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schenck CH, Bundlie SR, Mahowald MW. Delayed emergence of a parkinsonian disorder in 38% of 29 older men initially diagnosed with idiopathic rapid eye movement sleep behaviour disorder. Neurology. 1996;46:388–393. doi: 10.1212/wnl.46.2.388. [DOI] [PubMed] [Google Scholar]
- 115.Uchiyama M, Isse K, Tanaka K, et al. Incidental Lewy body disease in a patient with REM sleep behavior disorder. Neurology. 1995;45:709–712. doi: 10.1212/wnl.45.4.709. [DOI] [PubMed] [Google Scholar]
- 116.Boeve B, Dickson D, Olson E, et al. Insights into REM sleep behavior disorder pathophysiology in brainstem-predominant Lewy body disease. Sleep Med. 2007 doi: 10.1016/j.sleep.2006.08.017. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Boeve B, Silber M, Ferman T. REM sleep behavior disorder in Parkinson's disease and dementia with Lewy bodies. J Ger Psychiatry Neurol. 2004;17:146–157. doi: 10.1177/0891988704267465. [DOI] [PubMed] [Google Scholar]
- 118.Boeve B, Saper C. REM sleep behavior disorder: A possible early marker for synucleinopathies. Neurology. 2006;66:796–797. doi: 10.1212/01.wnl.0000209264.61035.bb. [DOI] [PubMed] [Google Scholar]
- 119.Stiasny-Kolster K, Doerr Y, Möller J, et al. Combination of ‘idiopathic’ REM sleep behaviour disorder and olfactory dysfunction as possible indicator for -synucleinopathy demonstrated by dopamine transporter FP-CIT-SPECT. Brain. 2005;128:126–137. doi: 10.1093/brain/awh322. [DOI] [PubMed] [Google Scholar]
- 120.Braak H, Del Tredici K, Rub U, et al. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 121.Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res. 2004;318:121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- 122.Wakabayashi K, Takahashi H. Neuropathology of autonomic nervous system in Parkinson's disease. Eur Neurol. 1997;38 2:2–7. doi: 10.1159/000113469. [DOI] [PubMed] [Google Scholar]
- 123.Klos K, Ahlskog J, Josephs K, et al. Alpha-synuclein pathology in the spinal cords of neurologically asymptomatic aged individuals. Neurology. 2006;66:1100–1102. doi: 10.1212/01.wnl.0000204179.88955.fa. [DOI] [PubMed] [Google Scholar]
- 124.Fujishiro H, Frigerio R, Burnett M, et al. Cardiac sympathetic denervation correlates with clinical and pathologic stages of Parkinson's disease. Mov Disord. 2008;23:1085–1092. doi: 10.1002/mds.21989. [DOI] [PubMed] [Google Scholar]
- 125.Dickson D, Fujishiro H, DelleDonne A, et al. Evidence that incidental Lewy body disease is pre-symptomatic Parkinson's disease. Acta Neuropathol. 2008;115:437–444. doi: 10.1007/s00401-008-0345-7. [DOI] [PubMed] [Google Scholar]
- 126.Braak H, Del Tredici K. Neuroanatomy and pathology of sporadic Parkinson's disease. Adv Anat Embryol Cell Biol. 2009;201:1–119. [PubMed] [Google Scholar]
- 127.Fantini M, Postuma R, Montplaisir J, Ferini-Strambi L. Olfactory deficit in idiopathic rapid eye movements sleep behavior disorder. Brain Research Bulletin. 2006;70:386–390. doi: 10.1016/j.brainresbull.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 128.Louis E, Bennett D. Mild Parkinsonian signs: An overview of an emerging concept. Mov Disord. 2007;22:1681–1688. doi: 10.1002/mds.21433. [DOI] [PubMed] [Google Scholar]
- 129.Aarsland D, Andersen K, Larsen J, Lolk A, Kragh-Sorensen P. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch Neurol. 2003;60:387–392. doi: 10.1001/archneur.60.3.387. [DOI] [PubMed] [Google Scholar]
- 130.Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord. 2007;22:1689–1707. doi: 10.1002/mds.21507. [DOI] [PubMed] [Google Scholar]
- 131.Abbott R, Ross G, White L, et al. Excessive daytime sleepiness and subsequent development of Parkinson disease. Neurology. 2005;65:1442–1446. doi: 10.1212/01.wnl.0000183056.89590.0d. [DOI] [PubMed] [Google Scholar]
- 132.Ferini-Strambi L, Di Gioia M, Castronovo V, et al. Neuropsychological assessment in idiopathic REM sleep behavior disorder (RBD): Does the idiopathic form of RBD really exist? Neurology. 2004;62:41–45. doi: 10.1212/01.wnl.0000101726.69701.fa. [DOI] [PubMed] [Google Scholar]
- 133.Mori E, Shimomura T, Fujimori M, et al. Visuoperceptual impairment in dementia with Lewy bodies. Arch Neurol. 2000;57:489–493. doi: 10.1001/archneur.57.4.489. [DOI] [PubMed] [Google Scholar]
- 134.Massicotte-Marquez J, Décary A, Gagnon J, et al. Executive dysfunction and memory impairment in idiopathic REM sleep behavior disorder. Neurology. 2008;70:1250–1257. doi: 10.1212/01.wnl.0000286943.79593.a6. [DOI] [PubMed] [Google Scholar]
- 135.Terzaghi M, Sinforiani E, Zucchella C, et al. Cognitive performance in REM sleep behaviour disorder: a possible early marker of neurodegenerative disease? Sleep Med. 2008;9:343–351. doi: 10.1016/j.sleep.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 136.Petersen R, Smith G, Waring S, et al. Mild cognitive impairment: Clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 137.Petersen R. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 138.Molano J, Boeve B, Ferman T, et al. Mild cognitive impairment +/- subsequent dementia associated with underlying Lewy body disease. Neurology. 2009;72:A248. [Google Scholar]
- 139.McKeith I, Dickson D, Lowe J, et al. Dementia with Lewy bodies: Diagnosis and management: Third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 140.Fantini ML, Gagnon JF, Petit D, et al. Slowing of electroencephalogram in rapid eye movement sleep behavior disorder. Ann Neurol. 2003;53:774–780. doi: 10.1002/ana.10547. [DOI] [PubMed] [Google Scholar]
- 141.Massicotte-Marquez J, Carrier J, Decary A, et al. Slow-wave sleep and delta power in rapid eye movement sleep behavior disorder. Ann Neurol. 2005;57:277–282. doi: 10.1002/ana.20373. [DOI] [PubMed] [Google Scholar]
- 142.Culebras A, Moore J. Magnetic resonance findings in REM sleep behavior disorder. Neurology. 1989;39:1519–1523. doi: 10.1212/wnl.39.11.1519. [DOI] [PubMed] [Google Scholar]
- 143.Miyamoto M, Miyamoto T, Kubo J, et al. Brainstem function in rapid eye movement sleep behavior disorder: the evaluation of brainstem function by proton MR spectroscopy (1H-MRS) Psychiatr Clin Neurosci. 2000;54:350–351. doi: 10.1046/j.1440-1819.2000.00710.x. [DOI] [PubMed] [Google Scholar]
- 144.Iranzo A, Santamaria J, Pujol J, et al. Brainstem proton magnetic resonance spectroscopy in idopathic REM sleep behavior disorder. Sleep. 2002;25:867–870. [PubMed] [Google Scholar]
- 145.Eisensehr I, Linke R, Noachtar S, et al. Reduced striatal dopamine transporters in idiopathic rapid eye movement sleep behaviour disorder: Comparison with Parkinson's disease and controls. Brain. 2000;123:1155–1160. doi: 10.1093/brain/123.6.1155. [DOI] [PubMed] [Google Scholar]
- 146.Mazza S, Soucy J, Gravel P, et al. Assessing whole brain perfusion changes in REM sleep behavior disorder. Neurology. 2006;67:1618–1622. doi: 10.1212/01.wnl.0000242879.39415.49. [DOI] [PubMed] [Google Scholar]
- 147.Albin R, Koeppe R, Chervin R, et al. Decreased striatal dopaminergic innervation in REM sleep behavior disorder. Neurology. 2000;55:1410–1412. doi: 10.1212/wnl.55.9.1410. [DOI] [PubMed] [Google Scholar]
- 148.Caselli R, Chen K, Bandy D, et al. A preliminary fluorodeoxyglucose positron emission tomography study in healthy adults reporting dream-enactment behavior. Sleep. 2006;29:927–933. doi: 10.1093/sleep/29.7.927. [DOI] [PubMed] [Google Scholar]
- 149.Klunk W, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 150.Jack C, Jr, Lowe V, Weigand S, et al. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer's disease: implications for sequence of pathological events in Alzheimer's disease. Brain. 2009 Mar 31; doi: 10.1093/brain/awp062. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gomperts S, Rentz D, Moran E, et al. Imaging amyloid deposition in Lewy body diseases. Neurology. 2008;71:903–910. doi: 10.1212/01.wnl.0000326146.60732.d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Taki J, Yoshita M, Yamada M, Tonami N. Significance of 123I-MIBG scintigraphy as a pathophysiological indicator in the assessment of Parkinson's disease and related disorders: it can be a specific marker for Lewy body disease. Ann Nucl Med. 2004;18:453–461. doi: 10.1007/BF02984560. [DOI] [PubMed] [Google Scholar]
- 153.Orimo S, Amino T, Itoh Y, et al. Cardiac sympathetic denervation precedes neuronal loss in the sympathetic ganglia in Lewy body disease. Acta Neuropathol. 2005;109:583–588. doi: 10.1007/s00401-005-0995-7. [DOI] [PubMed] [Google Scholar]
- 154.Oka H, Yoshioka M, Morita M, et al. Reduced cardiac 123I-MIBG uptake reflects cardiac sympathetic dysfunction in Lewy body disease. Neurology. 2007;69:1460–1465. doi: 10.1212/01.wnl.0000277450.49788.82. [DOI] [PubMed] [Google Scholar]
- 155.Miyamoto T, Miyamoto M, Inoue Y, et al. Reduced cardiac 123I-MIBG scintigraphy in idiopathic REM sleep behavior disorder. Neurology. 2006;67:2236–2238. doi: 10.1212/01.wnl.0000249313.25627.2e. [DOI] [PubMed] [Google Scholar]
- 156.Miyamoto T, Miyamoto M, Suzuki K, et al. 123I-MIBG cardiac scintigraphy provides clues to the underlying neurodegenerative disorder in idiopathic REM sleep behavior disorder. Sleep. 2008;31:717–723. doi: 10.1093/sleep/31.5.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Oguri T, Tachibana N, Mitake S, Kawanishi T, Fukuyama H. Decrease in myocardial 123I-MIBG radioactivity in REM sleep behavior disorder: two patients with different clinical progression. Sleep Med. 2008;9:583–585. doi: 10.1016/j.sleep.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 158.Braune S, Reinhardt M, Schnitzer R, Riedel A, Lücking C. Cardiac uptake of [123I]MIBG separates Parkinson's disease from multiple system atrophy. Neurology. 1999;53:1020–1025. doi: 10.1212/wnl.53.5.1020. [DOI] [PubMed] [Google Scholar]
- 159.Benarroch EE, Schmeichel AM. Depletion of cholinergic mesopontine neurons in multiple system atrophy: A substrate for REM behavior disorder? Neurology. 2002;58(suppl 3):A345. doi: 10.1212/wnl.59.6.944. [DOI] [PubMed] [Google Scholar]
- 160.Benarroch E, Schmeichel A, Low P, et al. Involvement of medullary regions controlling sympathetic output in Lewy body disease. Brain. 2005;128:338–344. doi: 10.1093/brain/awh376. [DOI] [PubMed] [Google Scholar]
- 161.Benarroch EE, Schmeichel AM, Sandroni P, Low PA, Parisi JE. Brainstem respiratory control: Substrates of respiratory failure of multiple system atrophy. Acta Neuropathol (Berl) 2007;113:75–80. doi: 10.1007/s00401-006-0150-0. [DOI] [PubMed] [Google Scholar]
- 162.Boeve B, Silber M, Ferman T, Smith G. Validation of a questionnaire for the diagnosis of REM sleep behavior disorder. Sleep. 2002;25(abstr suppl):A486. [Google Scholar]
- 163.Boeve B, Silber M, Ferman T, Smith G, Petersen R. Validation of a questionnaire for the diagnosis of REM sleep behavior disorder. Neurology. 2002;58(suppl 3):A509. [Google Scholar]
- 164.Stiasny-Kolster K, Mayer G, Schäfer S, et al. The REM sleep behavior disorder screening questionnaire--a new diagnostic instrument. Mov Disord. 2007;22:2386–2393. doi: 10.1002/mds.21740. [DOI] [PubMed] [Google Scholar]
- 165.Frauscher B, Iranzo A, Högl B, et al. Quantification of electromyographic activity during REM sleep in multiple muscles in REM sleep behavior disorder. Sleep. 2008;31:724–731. doi: 10.1093/sleep/31.5.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lapierre O, Montplaisir J. Polysomnographic features of REM sleep behavior disorder: development of a scoring method. Neurology. 1992;42:1371–1374. doi: 10.1212/wnl.42.7.1371. [DOI] [PubMed] [Google Scholar]
- 167.Ferri R, Franceschini C, Zucconi M, et al. Searching for a marker of REM sleep behavior disorder: submentalis muscle EMG amplitude analysis during sleep in patients with narcolepsy/cataplexy. Sleep. 2008;31:1409–1417. [PMC free article] [PubMed] [Google Scholar]
- 168.Mayer G, Kesper K, Ploch T, et al. Quantification of tonic and phasic muscle activity in REM sleep behavior disorder. J Clin Neurophysiol. 2008;25:48–55. doi: 10.1097/WNP.0b013e318162acd7. [DOI] [PubMed] [Google Scholar]
- 169.Bliwise D, Rye D. Elevated PEM (phasic electromyographic metric) rates identify rapid eye movement behavior disorder patients on nights without behavioral abnormalities. Sleep. 2008;31:853–857. doi: 10.1093/sleep/31.6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zimprich A, Biskup S, Leitner P, et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 171.Smith G, Boeve B, Pankratz V, et al. Time course of diagnostic features of Lewy body disease. Neurology. 2009;72(Suppl 3):A246. [Google Scholar]
- 172.Burke R, Dauer W, Vonsattel J. A critical evaluation of the Braak staging scheme for Parkinson's disease. Ann Neurol. 2008;64:485–491. doi: 10.1002/ana.21541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Josephs K, Duffy J, Strand E, et al. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain. 2006;129:1385–1398. doi: 10.1093/brain/awl078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Boeve BF, Maraganore DM, Parisi JE, et al. Pathologic heterogeneity in clinically diagnosed corticobasal degeneration. Neurology. 1999;53:795–800. doi: 10.1212/wnl.53.4.795. [DOI] [PubMed] [Google Scholar]
- 175.Tang-Wai D, Graff-Radford N, Boeve B, et al. Clinical, genetic, and neuropathologic characteristics of posterior cortical atrophy. Neurology. 2004;63:1168–1174. doi: 10.1212/01.wnl.0000140289.18472.15. [DOI] [PubMed] [Google Scholar]
- 176.Turner RS, Chervin RD, Frey KA, Minoshima S, Kuhl DE. Probable diffuse Lewy body disease presenting as REM sleep behavior disorder. Neurology. 1997;49:523–527. doi: 10.1212/wnl.49.2.523. [DOI] [PubMed] [Google Scholar]
- 177.Turner R, D'Amato C, Chervin R, Blaivas M. The pathology of REM sleep behavior disorder with comorbid Lewy body dementia. Neurology. 2000;55:1730–1732. doi: 10.1212/wnl.55.11.1730. [DOI] [PubMed] [Google Scholar]
- 178.McShane R, Nagy Z, Esiri M, et al. Anosmia in dementia is associated with Lewy bodies rather than Alzheimer's pathology. J Neurol Neurosurg Psychiatry. 2001;70:739–743. doi: 10.1136/jnnp.70.6.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Olichney J, Murphy C, Hofstetter C, et al. Anosmia is very common in the Lewy body variant of Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2005;76:1342–1347. doi: 10.1136/jnnp.2003.032003. [DOI] [PMC free article] [PubMed] [Google Scholar]