Abstract
Context:
Although originally manufactured for use in diagnostic imaging of internal structures, 2-cm-thick gel pads are also used as conducting media for therapeutic ultrasound over areas with bony prominences. Research on the ability of these pads to conduct enough energy to adequately heat tissues has provided mixed results. However, this research has mainly been performed on the triceps surae muscle, an area over which gel pads are not typically used. We wondered how much heating might be produced if a thinner pad was used over a tendon.
Objective:
To compare temperature rises in the human Achilles tendon during ultrasound treatments using ultrasound gel, a 2-cm-thick pad, and a 1-cm-thick pad.
Design:
Cross-sectional study.
Setting:
University therapeutic modality laboratory.
Patients or Other Participants:
Forty-eight healthy volunteers (24 women, 24 men).
Intervention(s):
We inserted a rigid thermocouple 1 cm deep into the Achilles tendon. Ultrasound was delivered at the following settings: 3 MHz, continuous, 1 W/cm2, 10 minutes.
Main Outcome Measure(s):
Temperature was recorded every 30 seconds for 10 minutes.
Results:
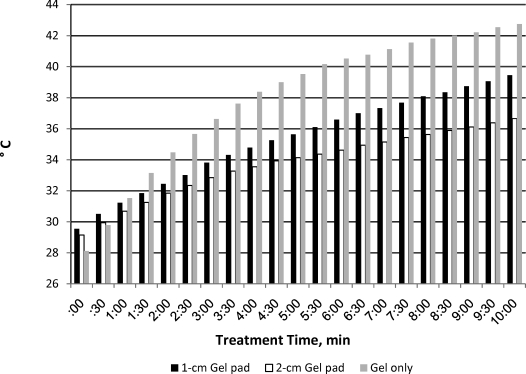
Temperature increased the most in the ultrasound gel group (increase = 13.3°C, peak = 42°C). The 1-cm-thick pad resulted in higher tendon temperature (increase = 9.3°C, peak = 37.8°C) than the 2-cm-thick pad (increase = 6.5°C, peak = 4.8°C). The 1-cm pad produced approximately 30% more heating than the 2-cm pad (SE = 0.72, P < .03).
Conclusions:
The thinner pad transmitted ultrasound more efficiently than the thicker pad. Thus, a gel pad of less than 1-cm thickness might be useful for superficial areas, such as the hands and ankles.
Keywords: therapeutic modalities, coupling agents, tissue temperatures
Key Points
Use of the 1-cm-thick gel pad resulted in greater tissue heating than use of the 2-cm-thick gel pad.
Participants' perceptions of heat did not differ for the 3 agents.
When properly applied, therapeutic ultrasound can increase tissue temperatures, which in turn increases blood flow and extensibility of tissues, relaxes muscle spasm, and provides analgesia to local tissues.1 A coupling medium is needed during ultrasound application to deliver sound energy to the tissues and prevent reflection of ultrasonic energy away from the treatment field.2 A coupling medium must be viscous, so that an adequate amount constantly remains between the sound head and the skin. The medium should be high in water content in order to transmit the sound waves while possessing low attenuation, so that the sound is not absorbed by the medium but transmitted into the tissues. Lastly, the medium should have a low susceptibility to bubble formation.2 Ultrasonic gel possesses all of these characteristics and is commonly regarded as the gold standard for ultrasound coupling.2–5
When ultrasound is applied over bony or irregular surfaces, an alternate coupling medium is required to ensure that the coupling agent maintains contact with the sound head. Methods include gel pads; gel-filled condoms; latex gloves full of gel, tap, or degassed water; and baths of tap or degassed water.3 The consensus3–6 is that the gel pad is an efficient transmitter of ultrasound energy, allowing the sound head to maintain contact with the coupling medium, which may provide fewer interruptions in ultrasound transmission.5
Parker Laboratories, Inc (Fairfield, NJ) produces an ultrasound gel pad that measures 9 cm in diameter and 2 cm in thickness, far thicker than the average 0.3- to 0.5-mm thickness of ultrasound gel used in the clinical setting. Some increased thickness is required to overcome the bony prominences and provide a smooth, level surface for the ultrasound sound head to glide over. This thick pad, however, may have detrimental effects on the treatment. It has been suggested7–9 that as the thickness of the coupling medium increases, transmissivity of the sound waves decreases, thus resulting in less heating. With this in mind, Parker Laboratories developed a 1-cm-thick round gel pad for use in our study. It is mainly composed of ultrasound gel in a solid, gelatinous form that is moldable to irregular surfaces. To date, however, no data have been provided as to the efficacy of this thinner gel pad when used as an ultrasound conducting medium. We conducted this study to compare temperature increase and patients' perceptions of heat during ultrasound treatment of the Achilles tendon using 3 media: commercially manufactured ultrasound gel, a 2-cm-thick gel pad, and a 1-cm-thick gel pad. If the 1-cm-thick pad performs well as an ultrasound medium, it might be useful in physical medicine and rehabilitation.
METHODS
Study Design
A randomized single factorial design guided this study, with 3 levels of the independent variable (ultrasound gel only, 2-cm-thick gel pad, 1-cm-thick gel pad). Four dependent variables were measured: total temperature change, time to peak temperature, and patient rating of heat at the middle (5 minutes) and end (10 minutes) of the treatment.
Participants
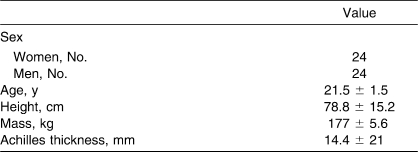
Forty-eight people, 24 women and 24 men, volunteered for the study. Participants were free of lower leg injury for at least 6 months before the study. Demographic data are presented in the Table. The study was approved by the university institutional review board, and volunteers gave written informed consent.
Table.
Participants' Demographic Characteristics
Instruments
Therapeutic ultrasound was produced via the Omnisound 3000 (Accelerated Care Plus Corp, Reno, NV) delivered at a frequency of 3 MHz. The ultrasound lead zirconate titanate crystal was 5 cm2 with an effective radiating area of 4.2 cm2 and a beam nonuniformity ratio of 3.9.
A 32-mm, 23-gauge rigid thermocouple (Phystek MT23/3; Physitemp Instruments, Inc, Clifton, NJ) was inserted into a portal made by one 20-gauge needle (length = 31.75 mm) into the target tissue. The rigid thermocouple was connected to an Iso-Thermex machine (Columbus Instruments, Columbus, OH), which was interfaced with a personal computer. According to the manufacturer, the Iso-Thermex is accurate to ± 0.1°C. Ultrasound gel (Parker Laboratories) and Aquaflex gel pads, either 2 or 1 cm thick (Parker Laboratories), served as the coupling media for this study (Figures 1 and 2).
Figure 1.
Treating the Achilles tendon using the ultrasound gel.
Figure 2.
Treating the Achilles tendon using the 1-cm-thick gel pad.
Procedures
Upon entry into the room, each participant drew a number corresponding to 1 of the 3 groups. The participant then assumed a prone position on a padded treatment table with the ankles hanging off the table. To keep the volunteer in the open-packed position with plantar flexion of 0° to 10°, he or she was told to relax the ankles.4 Our target tissue was the largest aspect of the Achilles tendon as determined by a caliper measurement. We chose this technique instead of a specific distance from the heel because of the natural variances in Achilles tendon size. The area was marked with a pen and cleansed with a Betadine swab (Purdue Products LP, Stamford, CT). A sterile needle was inserted in the posterior aspect of the Achilles tendon to help make a small portal for the rigid thermocouple. The needle was then removed and the thermocouple inserted through the portal made by the needle to a depth of 1 cm into the posterior aspect of the Achilles tendon. The thermocouple was secured with a 30-mm strip of medical tape (0.5-cm Transpore; 3M, St. Paul, MN); the tape was torn in half vertically, with 1 strip each placed above and below the thermocouple. The tape remained out of the treatment area and was used to ensure that the thermocouple remained in the desired location and at the desired depth. For the ultrasound treatment site, an area twice the size of the ultrasound head was marked on the top of the Achilles tendon at a right angle to the thermocouple. Baseline temperature was determined by a variance of less than 0.5°C over a 3-minute period, measured every 30 seconds. In this study, we discovered that the mean time to baseline temperature was 8.68 minutes (±2.21 minutes). Treatment started immediately after baseline temperature was reached and was performed at a right angle to the thermocouple. The sound head was moved at a rate of approximately 4 cm/s. We did not use a metronome because heating rates in an area twice the size of the sound head are similar for velocities of 2 cm/s to 8 cm/s when templates or tracings are used.10
Temperature was recorded every 30 seconds for the 10-minute treatment duration. At 5 minutes into the treatment and at the end of the treatment, participants were asked to rate the amount of heat they felt from the treatment on a verbal analog scale from 1 to 10, 1 being no heat and 10 being the same as if the patient was being burned.
For the ultrasound gel group, approximately 7 mL of the gel was applied directly to the skin over the treatment field. For the gel pad groups, the treatments were performed with the gel pad between the transducer and the skin and a thin layer of ultrasound gel on top of the pad to help reduce friction between the sound head and the pad. If, at any point during the treatment, the participant felt discomfort, the treatment was terminated. The treatment was terminated early for only 1 participant, who was in the gel group.
After the treatment, the thermocouple was removed and the insertion site cleansed with 70% isopropyl alcohol and covered with a plastic bandage. The thermocouple was disinfected by soaking overnight in Cidex (Johnson & Johnson, Arlington, TX).4
Statistical Analysis
The mean temperature increases for each of the 3 treatment groups and the participants' ratings of heat were evaluated with four 1 × 3 analyses of variance. A Tukey-Kramer post hoc test was used to determine where any differences were located in the data. All analyses were conducted using SAS (version 9.1; SAS Institute Inc, Cary, NC).
RESULTS
Intratendon temperature was greatest in the ultrasound gel group (overall increase = 13.3°C ± 0.73°C, peak temperature = 42°C, F2,43 = 22.06, P < .0001).
Temperature increases and patient ratings of heat were different by treatment (F6,82 = 6.04, P < .0001). However, ratings of heat were not different among the 3 groups with 5 minutes remaining (2.4 ± 1.43, F2,25 = 1.50, P = .23) or at the end of the treatment (3.21 ± 1.94, F2,26 = 2.35, P = .11) One participant in the gel-only group, however, stopped the treatment at the 9-minute mark because of discomfort. This was the only occurrence of a treatment being terminated before the fully prescribed time.
DISCUSSION
Three-megahertz ultrasound delivered through a 100% ultrasound gel medium produced the highest temperature increase of the 3 coupling media groups.11–13 Although the gel was superior in our study, the gel pads still produced a heating effect. Thus, the gel pad, whether 2 cm or 1 cm thick (with a thin coating of ultrasound gel on top to reduce friction), provides an adequate coupling medium for therapeutic ultrasound when indirect ultrasound is indicated.
Based on our findings, we believe that the thinner the medium, the greater the tissue heating. In fact, even though the 2-cm gel pad resulted in an increase of 6.5°C, the peak temperature was only 34.8°C. Because the treatment area was an extremity, we did not expect temperatures to reach 39°C–40°C; however, the thinner gel medium did reach 42°C.
In our study, the thickness of the gel pads was important. Casarotto et al7 investigated the thickness of coupling media with 4 commonly accepted coupling agents. White petroleum was compared with ultrasound gel, mineral oil, and degassed water at thicknesses of 0.3 mm and 0.5 mm. Their patients felt the transducer head became hotter with use of white petroleum. In general, when the thickness of the coupling agent increased, the transducer temperature increased, and the transmissivity (measured by the heat produced in the tissue) decreased.7 However, they used different coupling agents, so their results need to be taken with caution.
Other researchers have studied the thickness of ultrasound media. Poltawski and Watson9 reported that when the layer of couplant was increased from 0.2 mm to 0.6 mm, the mean transmitted power output from the transducer was reduced from 6.2 W to 5.85 W. Although this reduction in transmissivity was statistically significant, the authors suggested it might also become clinically significant with different treatment frequencies and settings.9 Our results also indicate that the transmissivity of the ultrasound was reduced as the thickness of the coupling media increased, but this finding may be due to the difference in our measurement techniques. Poltawski and Watson9 measured the wattage that passed through the coupling media; we chose to measure transmissivity by the temperature increase in the target tissue.
Our results differed from those of Bishop et al4 and Merrick et al6 in that we found a difference between using gel alone and using the gel pad. This may have been due to methodologic differences among the studies. We treated the Achilles tendon, whereas Bishop et al4 treated muscle. We applied ultrasound gel only to the top of the gel pad, whereas Bishop et al4 applied it to both the top and bottom of the pad. Merrick et al6 did not apply any gel to the gel pad and still found that gel alone and ultrasound gel pads were equivalent. The different results may have been due to the depth of the measurement (3 cm below the skin surface) and the tissue treated (muscle of the medial calf) used by Merrick et al.6
The rate of temperature increase per minute in tendon using 3 MHz ultrasound at 1 W/cm2 has been reported as 3.45 times as high as that of the heating rate of muscle tissue.14 Chan et al14 demonstrated that when treating the patellar tendon in an area 2 times the effective radiating area of the sound head at 3 MHz and 1 W/cm2, temperature increased at a rate of 2.1°C per minute, compared with 1.2°C per minute for 4 times the effective radiating area.
Our results indicate that the temperature rise in the tendon was not as drastic as that noted by Chan et al.14 When using gel only and treating the Achilles tendon at the same settings, we found the temperature rise to be 1.33°C per minute. This value is 2.2 times the established 0.6°C per minute temperature rise found in human muscle tissue,15 compared with the 3.45 times greater value seen by Chan et al.14
We believe that the difference noted between these results may stem from the presence of bone in the treatment field in the Chan et al14 study, causing ultrasound energy to reflect back into the tissue and increasing the heating rate. In our study, no bone was present in the treatment field, preventing the potential reflection of ultrasound energy into the treatment tissue.
Merrick et al6 compared the same 2-cm-thick gel pad that we used with 100% gel and direct ultrasound. They reported no difference between the coupling media but noted that the thickness of the gel pad may have been a factor.6 They hypothesized that a thinner gel pad might have increased the temperature further. These different results than in our study may have been due to the treatment frequencies (1 MHz, 1.5 W/cm2), duration (7 minutes), and tissue targeted (triceps surae) by Merrick et al.6
We found the mean temperature rises were 6.5°C ± 0.72°C for the 2-cm pad and 9.3°C ± 0.75°C for the 1-cm-thick pad. Thus, pad thickness may affect the length of the ultrasound treatment necessary to achieve a vigorous heating. We also found differences between the use of gel alone and either of the gel pads.
Our primary focus was to determine if peak temperatures differed during ultrasound treatments using 2 thicknesses of gel pads. If we look at the peak temperatures reached for therapeutic effects, only the ultrasound gel produced heating well above 40°C. The average temperature of this group was 42°C, high enough to be therapeutic but below the threshold for tissue destruction (45°C). This group started at a low baseline (30°C), which is not uncommon in a limb.
Our results contrasted with those of Bishop et al4 with regard to patient comfort. Of 18 participants, 8 reported that the treatment was uncomfortable when using the gel pad alone but not when the gel pad was coated on both sides with gel or the gel was used alone for a 10-minute treatment. Only 1 of our volunteers reported feeling uncomfortable and asked for the treatment to be stopped during the 10-minute treatment; this was in the gel-only group. Coating the gel pad only on the top with gel might have diminished discomfort during this procedure.
By far, the ultrasound gel produced the highest temperatures in our study. We did not objectively identify any differences in how the gel pads conformed to uneven areas. When the areas being treated are less contoured, we believe gel is the best option. However, in very contoured areas, where the sound head does not lie flat on the skin's surface, we think the 1-cm gel pad could be advantageous. The 1-cm gel pad may also be beneficial when treating open wounds, providing a sterile, bacteriostatic, conforming coupling medium to place over the wound. The gel pad may allow for reduced friction between the coupling media and the wound.
The cleanup required to remove gel from the wound may be eliminated by using the 1-cm gel pad. Presence of the pad may also prevent the sound head from coming too close to, or in direct contact with, the open wound and contaminating the gel, which may reduce the risk of cross-contamination among patients. Our results demonstrate that when indirect ultrasound is indicated, such as over a bony prominence or an open wound, the 1-cm-thick ultrasound gel pad provides sufficient coupling to achieve a heating effect.
The difference between the heating rates of the 2-cm and 1-cm pads may also be clinically significant: 6.5°C ± 3.12°C for the former and 9.2°C ± 2.97°C for the latter (Figure 3). All temperatures, except for the single participant who ended the treatment early, were highest when the treatment ended at 10 minutes.
Figure 3.
Mean temperature rise per minute for the 1-cm-thick and 2-cm-thick gel pads and gel only.
The results of this study are limited by the following factors: participants' age range (18 to 30 years), location of the treatment (posterior aspect of the human Achilles tendon), ultrasound machine (Omnisound 3000), depth of the treatment tissue (1 cm), length and settings for the treatment (3 MHz, continuous, 1 W/cm2, 10 minutes), size of the treatment area (twice the size of the sound head), speed of the movement of the sound head (approximately 4 cm/s), and the coupling agents (gel only, 2-cm-thick gel pad, 1-cm-thick gel pad).
CONCLUSIONS
For the greatest temperature rise in the target tissue, ultrasound gel was the best coupling medium of the 3 agents we studied. However, when a situation arises warranting the use of indirect ultrasound, the 1-cm-thick ultrasound gel pad coated with gel on the top provides sufficient coupling to achieve a heating effect.
With regard to the participants' ratings of heat, the results were not significant. Thus, the clinician may apply the treatment with any of the 3 coupling media without reservation.
REFERENCES
- 1.Dyson M. The Use of Ultrasound in Sports Physiotherapy. Edinburgh, Scotland: Churchill Livingstone; 1989. pp. 120–128. [Google Scholar]
- 2.Docker M. F., Foulkes D. J., Patrick M. K. Ultrasound couplants for physiotherapy. Physiotherapy. 1982;68(4):124–125. [PubMed] [Google Scholar]
- 3.Klucinec B., Scheidler M., Denegar C., Domholdt E., Burgess S. Transmissivity of coupling agents used to deliver ultrasound through indirect methods. J Orthop Sports Phys Ther. 2000;30(5):263–269. doi: 10.2519/jospt.2000.30.5.263. [DOI] [PubMed] [Google Scholar]
- 4.Bishop S., Draper D. O., Knight K. L., Feland B. J., Eggett D. Human tissue-temperature rise during ultrasound treatments with the Aquaflex gel pad. J Athl Train. 2004;39(2):126–131. [PMC free article] [PubMed] [Google Scholar]
- 5.Klucinec B. The effectiveness of the Aquaflex gel pad in the transmission of acoustic energy. J Athl Train. 1996;31(4):313–317. [PMC free article] [PubMed] [Google Scholar]
- 6.Merrick M. A., Mihalyov M. R., Roethemeier J. L., Cordova M. L., Ingersoll C. D. A comparison of intramuscular temperatures during ultrasound treatments with coupling gel or gel pads. J Orthop Sports Phys Ther. 2002;32(5):216–220. doi: 10.2519/jospt.2002.32.5.216. [DOI] [PubMed] [Google Scholar]
- 7.Casarotto R. A., Adamowski J. C., Fallopa F., Bacanelli F. Coupling agents in therapeutic ultrasound: acoustic and thermal behavior. Arch Phys Med Rehabil. 2004;85(1):162–165. doi: 10.1016/s0003-9993(03)00293-4. [DOI] [PubMed] [Google Scholar]
- 8.Enwemeka C., Prange K., Shah S., Smejkal M., Zalman M. Relative transmissivity of ultrasound coupling media of various thicknesses. Paper presented at: 10th International Congress of World Confederation for Physical Therapy; June 25–30, 1995; Washington, DC.
- 9.Poltawski L., Watson T. Relative transmissivity of ultrasound coupling agents commonly used by therapists in the UK. Ultrasound Med Biol. 2007;33(1):120–128. doi: 10.1016/j.ultrasmedbio.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 10.Weaver S. L., Demchak T. J., Stone M. B., Brucker J. B., Burr P. O. Effect of transducer velocity on intramuscular temperature during a 1-MHz ultrasound treatment. J Orthop Sports Phys Ther. 2006;36(5):320–325. doi: 10.2519/jospt.2006.2157. [DOI] [PubMed] [Google Scholar]
- 11.Ashton D. F., Draper D. O., Myrer J. W. Temperature rise in human muscle during ultrasound treatments using Flex-all as a coupling agent. J Athl Train. 1998;33(2):136–140. [PMC free article] [PubMed] [Google Scholar]
- 12.Gulick D. T., Ingram N., Krammes T., Wilds C. Comparison of tissue heating using 3MHz ultrasound with T-Prep versus Aquasonic gel. Phys Ther Sport. 2005;6(3):131–136. [Google Scholar]
- 13.Rubley M. D., Peluga M. N., Mendoza J., et al. Ultrasound heating of the Achilles tendon: a comparison of direct and indirect application techniques [abstract] J Athl Train. 2008;43(3 suppl):S-58–S-59. [Google Scholar]
- 14.Chan A. K., Myrer J. W., Measom G. J., Draper D. O. Temperature changes in human patellar tendon in response to therapeutic ultrasound. J Athl Train. 1998;33(2):130–135. [PMC free article] [PubMed] [Google Scholar]
- 15.Draper D. O., Castel J. C., Castel D. Rate of temperature increase in human muscle during 1 MHz and 3 MHz continuous ultrasound. J Orthop Sports Phys Ther. 1995;22(4):142–150. doi: 10.2519/jospt.1995.22.4.142. [DOI] [PubMed] [Google Scholar]