Abstract
Objective:
To present recommendations for the prevention, education, and management of skin infections in athletes.
Background:
Trauma, environmental factors, and infectious agents act together to continually attack the integrity of the skin. Close quarters combined with general poor hygiene practices make athletes particularly vulnerable to contracting skin diseases. An understanding of basic prophylactic measures, clinical features, and swift management of common skin diseases is essential for certified athletic trainers to aid in preventing the spread of infectious agents.
Recommendations:
These guidelines are intended to provide relevant information on skin infections and to give specific recommendations for certified athletic trainers and others participating in athletic health care.
Keywords: tinea capitis, tinea corporis, herpes simplex, molluscum contagiosum, impetigo, folliculitis, furuncle, carbuncle, community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA)
The nature of athletics exposes the skin of its participants to a wide variety of stresses. Trauma, environmental factors, and infectious agents act together to continually attack the integrity of the skin. Combined with the close quarters shared by athletes and generally poor hygiene practices, it is not difficult to see why skin infections cause considerable disruption to individual and team activities.1 Skin infections in athletes are extremely common. Authors2 of a recent literature review investigating outbreaks of infectious diseases in competitive sports from 1922 through 2005 reported that more than half (56%) of all infectious diseases occurred cutaneously. Recognition of these diseases by certified athletic trainers (ATs), who represent the first line of defense against spread of these infections to other team members, is absolutely essential. Prophylactic measures and swift management of common skin infections are integral to preventing the spread of infectious agents. The following position statement and recommendations provide relevant information on skin infections and specific guidelines for ATs working with the athletes who contract them.
RECOMMENDATIONS
Based on the current research and literature, the National Athletic Trainers' Association (NATA) suggests the following guidelines for prevention, recognition, and management of athletes with skin infections. The recommendations are categorized using the Strength of Recommendation Taxonomy criterion scale proposed by the American Academy of Family Physicians3 on the basis of the level of scientific data found in the literature. Each recommendation is followed by a letter describing the level of evidence found in the literature supporting the recommendation: A means there are well-designed experimental, clinical, or epidemiologic studies to support the recommendation; B means there are experimental, clinical, or epidemiologic studies that provide a strong theoretical rationale for the recommendation; and C means the recommendation is based largely on anecdotal evidence at this time.
The recommendations have been organized into the following categories: prevention, education, and management of the skin infections. The clinical features of the most common skin lesions are presented in Table 1.
Table 1.
Clinical Features of Common Skin Infections
Prevention
-
Organizational support must be adequate to limit the spread of infectious agents.
The administration must provide the necessary fiscal and human resources to maintain infection control.30,31 Evidence Category: B
Custodial staffing must be increased to provide the enhanced vigilance required for a comprehensive infection-control plan. Evidence Category: C
Adequate hygiene materials must be provided to the athletes, including antimicrobial liquid (not bar) soap in the shower and by all sinks.7,32–35 Evidence Category: B
Infection-control policies should be included in an institution's policies and procedures manuals.22,31,36–38 Evidence Category: C
Institutional leadership must hold employees accountable for adherence to recommended infection-control practices.8,30,39–43 Evidence Category: B
Athletic departments should contract with a team dermatologist to assist with diagnosis, treatment, and implementation of infection control.44 Evidence Category: C
-
A clean environment must be maintained in the athletic training facility, locker rooms, and all athletic venues.
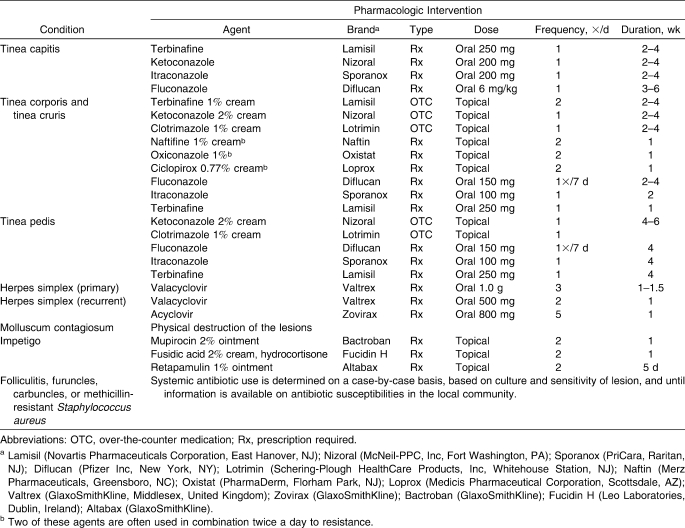
Cleaning and disinfection is primarily important for frequently touched surfaces such as wrestling mats, treatment tables, locker room benches, and floors.9,10,45,46 Evidence Category: A
A detailed, documented cleaning schedule must be implemented for all areas within the infection-control program, and procedures should be reviewed regularly. Evidence Category: C
The type of disinfectant or detergent selected for routine cleaning should be registered with the Environmental Protection Agency, and the manufacturer's recommendations for amount, dilution, and contact time should be followed.10,31,47 Evidence Category: B
-
Health care practitioners and athletes should follow good hand hygiene practices.31,48
-
When hands are visibly dirty, wash them with an acceptable antimicrobial cleanser from a liquid dispenser.48,49 Evidence Category: A
Correct hand-washing technique must be used, including wetting the hands first, applying the manufacturer's recommended amount of antimicrobial soap, rubbing the hands together vigorously for at least 15 seconds, rinsing the hands with water, and then drying them thoroughly with a disposable towel.48 Evidence Category: A
If hands are not visibly dirty, they can be decontaminated with an alcohol-based hand rub.17,18,41,50,51 Evidence Category: B
Hands should be decontaminated before and after touching the exposed skin of an athlete and after removing gloves.52–56 Evidence Category: B
-
-
Athletes must be encouraged to follow good overall hygiene practices.57–59
Athletes must shower after every practice and game with an antimicrobial soap and water over the entire body. It is preferable for the athletes to shower in the locker rooms provided by the athletic department.57 Evidence Category: B
Athletes should refrain from cosmetic body shaving.25 Evidence Category: B
Soiled clothing, including practice gear, undergarments, outerwear, and uniforms, must be laundered on a daily basis.10 Evidence Category: B
Equipment, including knee sleeves and braces, ankle braces, etc, should be disinfected in the manufacturer's recommended manner on a daily basis.58 Evidence Category: C
Athletes must be discouraged from sharing towels, athletic gear, water bottles, disposable razors, and hair clippers.57,59 Evidence Category: A
Athletes with open wounds, scrapes, or scratches must avoid whirlpools and common tubs. Evidence Category: C
Athletes are encouraged to report all abrasions, cuts, and skin lesions to and to seek attention from an AT for proper cleansing, treatment, and dressing. Evidence Category: CAll acute, uninfected wounds (eg, abrasions, blisters, lacerations) should be covered with a semiocclusive or occlusive dressing (eg, film, foam, hydrogel, or hydrocolloid) until healing is complete to prevent contamination from infected lesions, items, or surfaces. Evidence Category: C
Education
The sports medicine staff must educate everyone involved regarding infection-control policies and procedures.7,32–35,60
Administrators must be informed of the importance of institutional support to maintaining proper infection-control policies.7,32–35,60 Evidence Category: B
Coaches must be informed of the importance of being vigilant with their athletes about following infection-control policies to minimize the transmission of infectious agents.7,32–35,60 Evidence Category: B
-
Athletes need to be educated on their role in minimizing the spread of infectious diseases.
Follow good hygiene practices, including showering with antimicrobial soap and water after practices and games and frequent hand washing.57–59 Evidence Category: B
Have all practice and game gear laundered daily.10,17 Evidence Category: B
Avoid sharing of towels, athletic gear, water bottles, disposable razors, and hair clippers.57,59 Evidence Category: B
Perform daily surveillance and report all abrasions, cuts, and skin lesions to and seek attention from the athletic training staff for proper cleansing, treatment, and wound dressing. Evidence Category: C
The custodial staff must be included in the educational programs about infectious agents to be able to adequately help in daily disinfection of the facilities.10 Evidence Category: C
Management
Fungal Infections
-
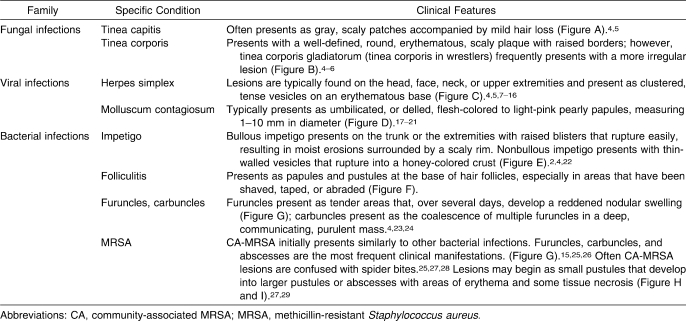
Tinea capitis (Figure A)
Diagnosis: A culture of lesion scrapings is the most definitive test, but a potassium hydroxide (KOH) preparation gives more immediate results.61 Evidence Category: B
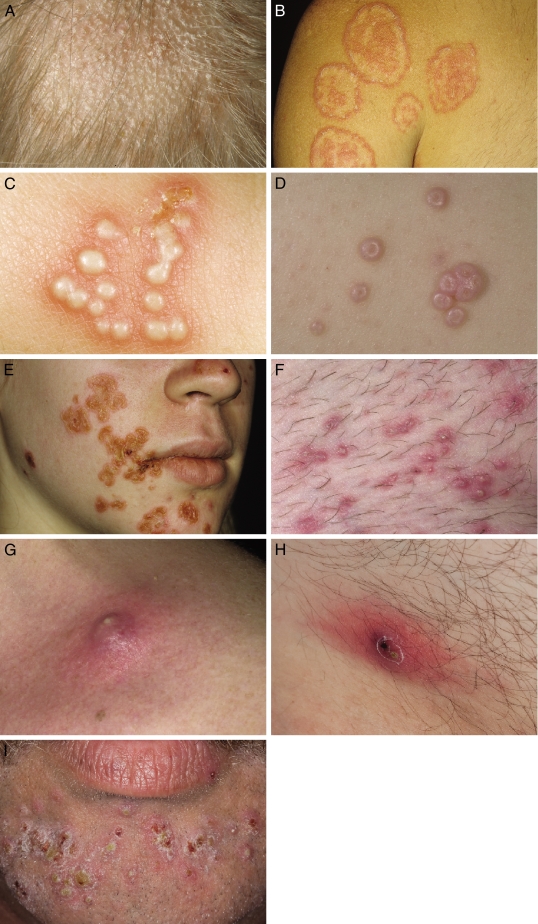
Treatment: Most patients have recalcitrant cases and should be treated with systemic antifungal agents: for example, a “cidal” antifungal drug, such as terbinafine, or alternative, such as fluconazole, itraconazole, or ketoconazole (Table 2). Adjunctive therapy with selenium sulfide shampoo is also recommended.4,57,61,62 Evidence Category: B
Criteria for return to competition: Athletes must have a minimum of 2 weeks of systemic antifungal therapy (Table 3).63,64 Evidence Category: B
-
Tinea corporis (Figure B)
Diagnosis: A culture of lesion scrapings is the most definitive test, but a KOH preparation gives more immediate results.61 Evidence Category: B
Treatment: Topical treatment with a cidal antifungal agent, such as terbinafine, naftifine, ciclopirox, or oxiconazole (or more than one of these), twice a day, is effective for localized lesions. More diffuse inflammatory conditions should be treated with systemic antifungal medication (Table 2).11,57,61,62,65 Evidence Category: B
Criteria for return to competition: Athletes must have used the topical fungicide for at least 72 hours, and lesions must be adequately covered with a gas-permeable membrane (Table 3).63,64 Evidence Category: B
Figure.
Skin diseases. A, Tinea capitis. B, Tinea corporis. C, Herpes simplex. D, Molluscum contagiosum. E, Impetigo. F, Folliculitis. G, Furuncle/carbuncle. H and I, Methicillin-resistant Staphylococcus aureus. All photos used with permission from www.dermnet.com.
Table 2.
Recommended Pharmacologic Treatment Regimens for Common Skin Infections
Table 3.
Return-to-Play Guidelines for Contact-Sport Athletes With Infectious Lesionsa
Viral Infections
-
Herpes simplex (Figure C)
Diagnosis: A culture of lesion scrapings is the most definitive test but may take days. A Tzanck smear that identifies herpes-infected giant cells may give more rapid, accurate results.1,57,61,66 Evidence Category: B
-
Treatment: New, active lesions may be treated with an oral antiviral medication, such as valacyclovir, to shorten the duration of the infection and lessen the chance of transmission.57,67–72 Evidence Category: B
Fully formed, ruptured, and crusted-over lesions are unaffected by antiviral medication. Evidence Category: B
-
Criteria for return to competition64
i. Athlete must be free of systemic symptoms, such as fever, malaise, etc. Evidence Category: B
ii. Athlete must have developed no new blisters for 72 hours. Evidence Category: B
iii. All lesions must be surmounted by a firm adherent crust. Evidence Category: B
iv. Athlete must have completed a minimum of 120 hours of systemic antiviral therapy. Evidence Category: B
v. Active lesions cannot be covered to allow participation. Evidence Category: B
-
Molluscum contagiosum (MC; Figure D)
Diagnosis: Clinical findings and microscopic inspection are the basis for diagnosis.73 Evidence Category: C
Treatment: Many anecdotal therapies have been suggested, but physical destruction of the lesions with a sharp curette is recommended.26,64,73–81 Evidence Category: B
Criteria for return to competition: Lesions should be curetted and covered with a gas-permeable membrane (Table 3).64 Evidence Category: B
Bacterial Infections
-
Impetigo (Figure E)
-
Diagnosis: The diagnosis of bacterial infections is primarily based on the history and characteristic appearance of the lesions.57 Evidence Category: B
Specimens for culture and antimicrobial susceptibility should be obtained from any questionable lesions.57 Evidence Category: B
-
Treatment: Culture and sensitivity of suspicious lesions will dictate treatment for all bacterial infections.
Topical mupirocin (Bactroban; GlaxoSmithKline, Middlesex, United Kingdom), fusidic acid (Fucidin H; Leo Pharma, Ballerup, Denmark), and retapamulin (Altabax; GlaxoSmithKline, Middlesex, United Kingdom) have been shown effective in treating impetigo.1,57,82,83 Evidence Category: B
-
Criteria for return to competition: Any suspicious lesions should be cultured and tested for antimicrobial sensitivity before the athlete returns to competition (Table 3).64Evidence Category: B
i. No new skin lesions for at least 48 hours. Evidence Category: B
ii. Completion of a 72-hour course of directed antibiotic therapy. Evidence Category: B
iii. No further drainage or exudate from the wound. Evidence Category: B
iv. Active infections may not be covered for competition.
-
-
Folliculitis/furuncles/carbuncles (Figure F and G)
-
Diagnosis: The diagnosis of bacterial infections is primarily based on the history and characteristic appearance of the lesions.57 Evidence Category: B
Specimens for culture and antimicrobial susceptibility should be obtained from any questionable lesions.57 Evidence Category: B
-
Treatment: Culture and sensitivity of suspicious lesions dictate treatment for all bacterial infections.57,84,85
i. Athlete must be referred to physician for incision, drainage, and culture. Evidence Category: B
ii. Antibiotic therapy must be initiated to control local cellulitis. Evidence Category: B
-
Criteria for return to competition: Any suspicious lesions should be cultured and tested for antimicrobial sensitivity before the athlete returns to competition (Table 3).64Evidence Category: B
i. No new skin lesions for at least 48 hours. Evidence Category: B
ii. Completion of a 72-hour course of directed antibiotic therapy. Evidence Category: B
iii. No further drainage or exudate from the wound. Evidence Category: B
iv. Active infections may not be covered for competition. Evidence Category: B
-
-
Methicillin-resistant Staphylococcus aureus (MRSA) (Figure H and I)
-
Diagnosis: The diagnosis of bacterial infections is primarily based on the history and characteristic appearance of the lesions. Evidence Category: B
i. The differential diagnosis for any potential Staphylococcus lesion must include MRSA.27,84,86,87 Evidence Category: B
ii. Reports of “spider bites” should be considered a possible sign for community-associated MRSA (CA-MRSA).84 Evidence Category: B
iii. Specimens for culture and antimicrobial susceptibility should be obtained from any questionable lesions.84,86 Evidence Category: B
-
Treatment: Recognition and referral of athletes with suspicious lesions are paramount. Evidence Category: B
-
Criteria for return to competition: Any suspicious lesions should be cultured and tested for antimicrobial sensitivity before the athlete returns to competition (Table 3).64Evidence Category: B
i. No new skin lesions for at least 48 hours. Evidence Category: B
ii.Completion of a 72-hour course of directed antibiotic therapy. Evidence Category: B
iii. No further drainage or exudate from the wound. Evidence Category: B
iv. Active infections may not be covered for competition. Evidence Category: B
-
Clinical Dermatology: A Color Guide to Diagnosis and Therapy by Habif94 and Skin Disease: Diagnosis and Treatment by Habif et al95 are excellent references for the recognition, diagnosis, and treatment of skin diseases, as is www.dermnet.com, a Web site that contains more than 23 000 images of skin diseases.
LITERATURE REVIEW
Transmission of the Infectious Agent
For the transmission of infectious agents, 3 basic elements are required: a source of the agent, an adequate susceptible host, and a mode of transmission for the agent to the host.31,96 Infectious agents in health care settings have been shown to come from many sources, including other patients,97–100 roommates, and visitors.99,101 These agents are also present in the athletic setting. The infected source may show active lesions or may be completely asymptomatic while in the incubation period of an infectious disease. It is, therefore, important to always assume that individuals are carriers of pathogenic microorganisms.
A very complex relationship exists between an infectious agent and a potential host patient.31 Many factors, including the immune state of the patient at the time of exposure, virulence of the infectious agent, quantity of the infectious innoculum, and medications taken by the patient (eg, corticosteroids) can affect the outcome after exposure to an infectious agent.31,102 Outcomes can range from no effect at all to asymptomatic colonization of the host to full symptomatic disease states.31 Athletes have unique characteristics that make them particularly susceptible hosts. They participate in high-risk activities103 and have constant assaults to the integrity of their skin,57 making transmission that much easier.
Transmission of infectious agents to the host can occur in a myriad of ways: through direct or indirect contact, droplets, airborne routes, or percutaneous or mucous membrane exposure.31 Direct transmission occurs when one infected person transfers the infectious agent to another through direct skin-to-skin contact.31 Indirect transmission refers to situations in which a susceptible person is infected by contact with a contaminated surface, such as a wrestling mat or contaminated clothing. Many cases of indirect transmission in the health care setting are found in the literature, including patient care devices,104–106 shared toys in pediatric wards,98 inadequately cleaned instruments,6,107–109 and poor hand hygiene,9,45 the latter of which is possibly the most common method of indirect transmission. Inadequate vigilance about hand washing is thought to be largely responsible for transferring infectious agents from one surface to another in health care settings, dramatically increasing disease transmission. Also, clothing has been shown to be contaminated with potential pathogens after coming in contact with infectious agents.110,111 Although supporting literature on indirect transmission in the athletic setting is lacking, it is not difficult to imagine the potential harm.
Droplet transmission occurs when infected droplets from sneezing, coughing, or talking make contact with the eyes, nose, or mouth of the host subject.112 Airborne transmission occurs when residue from evaporated droplets or dust particles stays suspended in the air for long periods of time and becomes inhaled by a susceptible host.113,114 In the athletic setting, the most common mode of transmission of skin diseases is direct or indirect contact from the source to the host. Other modes of transmission are beyond the scope of this review.
Prevention
First and foremost, for a prevention plan to be effective, the organization (university, high school, corporation, etc) should be committed to preventing disease transmission.31 This commitment should be manifested by including disease-transmission prevention in existing safety programs and policies and procedures manuals.22,36–38 These manuals should describe how the prevention principles will be applied, how infected persons will be identified, and how to communicate information about potentially infected persons to the proper personnel.31 Skin diseases, especially CA-MRSA, are reaching pandemic proportions, so organizations should be prepared to provide fiscal and human resources for controlling infection in an ever-changing environment.31
Furthermore, a culture of institutional safety shared by administrators, staff, and, in this case, athletes is essential to controlling infectious disease.30 Standard precautions and preventive measures must become the norm in athletic facilities for these programs to be implemented. Hospital-based studies have shown a direct correlation between high levels of “safety culture” and adherence to safe practices. Institutions that have seamlessly integrated these programs into their daily routine have had a high degree of success in keeping their stakeholders accountable for disease-prevention measures.39,40,43 This adherence to recommended practices can significantly minimize the transmission of infectious disease.8,41,42
Education about infectious-disease transmission and the recommended practices to minimize it should be an essential component to any infectious disease-prevention program. Understanding the science behind the recommended practices allows the health care team to more readily apply the standard precautions and modify them to their specific setting.7,32–35 Adherence to safety precautions is higher in groups that have received education in infectious-disease control.60
Hand hygiene is the single most important practice in reducing the transmission of infectious agents.31,48 Because of the significance of this issue, the Centers for Disease Control and Prevention assembled the Hand Hygiene Task Force, which wrote a 56-page document, “Guideline for Hand Hygiene in Health-Care Settings.”48 The guidelines48 include recommendations to wash hands with antimicrobial soap when the hands are visibly dirty49 or with an alcohol-based hand rub in the absence of visible soiling of the hands.17,18,41,50–52,115 Hands should always be decontaminated before54 and after contacting a patient's skin,52,53 after removing gloves,55,56 and after using the restroom.116–118 Trivial as it may seem, properly decontaminating the hands is of utmost importance. The correct technique for hand washing includes wetting the hands first, applying an appropriate amount of product, rubbing the hands together vigorously for at least 15 seconds, rinsing the hands with water, and then drying thoroughly with a disposable towel.48
The nature of athletic competition necessitates overall good personal hygiene practices. Close personal contact in both locker and dormitory rooms is a significant risk factor in disease transmission.57–59 Athletes are encouraged to shower with antimicrobial soap and water over the entire body immediately after each practice and game.57 Athletes should also be discouraged from cosmetic body shaving (ie, shaving a body area other than the face or legs), which has been shown to increase the risk of CA-MRSA more than 6-fold.25 Good personal hygiene decreases the colonization of bacteria58 and can be a first line of defense against transmission of infectious agents.
It is also important to maintain a clean environment in the athletic training room, locker rooms, and athletic venues. Cleaning and disinfection is primarily important for frequently touched surfaces, such as wrestling mats, treatment tables, and locker room benches and floors.9,10,45,46 An example of a cleaning schedule for a National Collegiate Athletic Association (NCAA) Division I wrestling program is provided in Table 4. Maintaining a properly cleaned and disinfected facility requires a team approach, including contributions from ATs, athletic administration, coaches, athletes, and custodial staff. Education of all involved parties is essential to minimizing transmission of infectious agents, and regular review of the cleaning procedures should be performed.48 The type of disinfectant or detergent selected for routine cleaning and disinfection is relatively unimportant, as long as it is registered by the Environmental Protection Agency and the manufacturer's recommendations for amount, dilution, and contact time are all followed.10,31,47 Some authors have suggested using a 1∶10 ratio of household bleach to tap water for routine environmental disinfection.119 Facility-based pathogen reservoirs most often result from a failure to follow the instructions rather than from the cleaning agent itself.24,120
Table 4.
A Sample Cleaning Schedule for a National Collegiate Athletic Association Division I Wrestling Programa
Soiled textiles, including towels, athletic clothing, elastic wraps, etc, can be reservoirs for infectious agents. Although these items can be significant contributors to infectious-disease transmission, if handled, transported, and laundered properly, the risk of transmission to a susceptible host is negligible.10 Another suggested potential risk factor for acquiring an infectious disease, sharing personal items such as bar soap, towels, water bottles, and protective equipment (eg, wrestling head gear), should be prohibited at all times.57,59 Athletic clothing and towels need to be laundered every day after practice, and equipment such as neoprene sleeves, knee braces, and other protective equipment should be disinfected with a 1∶10 bleach solution daily58 despite the fact that some authors121–123 have reported cases of contact dermatitis at this concentration.
The following sections provide literature support for fungal, viral, and bacterial infections. Background information, clinical features, diagnosis, treatment, prevention, and guidelines for return to competition will be presented for each of the infectious agents.
FUNGAL INFECTIONS
Dermatophytes (fungal organisms living in soil, on animals, or on humans124) include a group of fungi that infect and survive mostly on dead keratin cells in the stratum corneum of the epidermis. The infectious organisms responsible for fungal infections are typically from the Trichophyton genus.61 Specifically, Trichophyton tonsurans and Trichophyton rubrum are most often associated with tinea capitis and tinea corporis, respectively.61,125,126
Background
Chronic perspiration and the macerating affect of abrasive trauma contribute to the successful penetration of ubiquitous fungal elements, particularly in warm, moist areas such as the toe webs, inguinal creases, and axillary folds. In contact sports, the skin-on-skin contact of the participants and abrasions, both clinical and subclinical, also lend themselves to the passage of fungal infections from one athlete to the next. Dermatophyte infection can be manifested in many ways. Infections on the face and head are called tinea capitis, infections on the body are termed tinea corporis, infections in the groin are called tinea cruris, and infections of the feet are called tinea pedis.57,124 A number of authors107,125,127–130 have researched the epidemiologic considerations of this widespread cutaneous problem among athletes.
The most common dermatophyte infection is tinea pedis, with prevalence rates ranging from 25% to 70% over the life span.127,129 A review of 10 recent reports has presented information on athletic teams infected with tinea corporis.125 As would be expected, the rates varied greatly from one group to another, depending in part upon the method of fungal identification and the fact that a certain level of penetration of infection into the team was necessary to identify the team for study. Certainly athletic teams at all levels of competition have no evidence of fungal infection. The rates of incidence of infection in the reported studies ranged from 20% to 77%. One overview128 performed in the mid-1980s indicated that 60% of college wrestlers and 52% of high school wrestlers demonstrated tinea infections at some time during the course of the season. Other investigators have reported that 84.7% of high school wrestling teams had at least 1 wrestler with tinea corporis131 and 95% of a Swedish club wrestling team exhibited cutaneous findings consistent with tinea infection, with 75% of those demonstrating positive cultures for T tonsurans.130
From information gathered in these studies, it is obvious that fungal infection rates among athletes vary widely. However, we can assume that any athletic team in which the problem has been identified can expect active infections in one-half to as many as three-fourths of its members, underlying the importance of aggressive treatment of isolated cases once they have been identified.
Clinical Features
The clinical presentation of cutaneous fungal infections is diverse. Tinea capitis often presents as gray scaly patches accompanied by mild hair loss.57,61 Tinea corporis, commonly known as ringworm, is characterized by a well-defined round, erythematous, scaly plaque with raised borders.57,61 Tinea corporis gladiatorum (tinea corporis among athletes)63,125 many times presents with a more irregular lesion, however.120 Tinea corporis is most commonly found on the head, neck, trunk, and upper extremities and only rarely affects the lower extremities.125,131 Tinea cruris presents with a well-defined erythematous plaque in the pubic and inguinal areas.66 Finally, tinea pedis presents in the toe webs, where macerated skin is usually accompanied by thick scaling or desquamation. Marginated erythema with advancing scales will often progress from the toe web to the entire sole of the foot and extend over the lateral margins in the “moccasin” distribution.62 Although early infection tends to be unilateral, bilateral involvement of the feet is common by the time the athlete seeks attention for the problem. Vesicle formation may appear near the advancing border, and the underside of the epithelium covering these vesicles is a rich source of fungal elements for diagnosis by both KOH preparation57 and fungal culture.57,61
Diagnosis
Although fungal cultures are more definitive than a KOH test, especially for specifically diagnosing the exact causative organism, 3 weeks may be required to determine that a culture is negative. Positive growth, of course, occurs more rapidly. The culture should be taken for ultimate confirmation, but a KOH preparation provides a more immediate determination of infection.61 In the hands of an experienced practitioner, these simple tests are invaluable for instituting immediate therapy, even though some KOH preparations lead to equivocal results. Very simply, scale obtained from a suspicious lesion is applied to a glass microscope slide, a 10% KOH solution is added, and a coverslip is applied. The slide is warmed, usually with a match, to degrade the keratin and expose the fungal elements.
Treatment
Athletes in noncontact sports or with localized cases may initially be treated with topical preparations as a conservative first-line approach.57,61 Topical treatments, including the cidal imidazoles, allylamines, and napthiomates, tend to be well tolerated by patients.57,61 More widespread, inflammatory, or otherwise difficult-to-treat cases may require the use of systemic antifungals, such as fluconazole or terbinafine,65 which can have substantial side effects.61
The topical cidal antifungals terbinafine, naftifine, ciclopirox, and oxiconazole are suggested11 with 2 to 4 times daily applications. Although this regimen may be effective in the off season, athletes in the midst of a competitive season should probably be treated immediately with oral terbinafine, itraconazole, or fluconazole. Topical treatment is typically required through the entire course of the season or at least 2 weeks in the off season. Typically, systemic treatment for common fungal infections should last 2 to 4 weeks. Scalp lesions can be particularly difficult to eradicate, so systemic therapy with medications such as terbinafine, ketoconazole, itraconazole, or fluconazole may be prescribed for up to 6 weeks; daily use of an antifungal shampoo such as ketoconazole or ciclopirox may be required in particularly virulent scalp infections in athletes.4,62 A summary of common treatment regimens for dermatophyte infections is presented in Table 2.
Prevention
In athletes who are prone to tinea pedis, careful attention to drying the feet is a necessity, including careful towel drying, particularly of the toe webs. The regular application of foot powder or 20% aluminum chloride (Drysol; Person & Covey, Inc, Glendale, CA) is also valuable. Wearing shower shoes in the locker room may be beneficial. Daily changing of athletic socks and even blow drying of the feet and athletic shoes have also been recommended.62 Immediately showering after each training session and thoroughly drying all areas, especially intertriginous areas, is recommended, as well as the use of absorbent sports briefs and the application of bacteriostatic powder, such as Zeasorb-AF (Stiefel Laboratories, Inc, Research Triangle Park, NC), to the axillae and groin.
Wrestlers represent a particularly difficult and crucial subset in terms of preventing fungal infections. Wrestlers with extensive active lesions, which can be identified on visual inspection by ATs and coaches, must be withheld from all contact. Wrestlers who have demonstrated a particular susceptibility for tinea corporis in the course of the competitive season have been successfully treated prophylactically throughout the entire season with a low dosage of fluconazole (150 mg every other week or 200 mg per month).1,62,132 Wrestlers with particularly recalcitrant (persistent or recurrent) infections should have their family members and animals (eg, dogs, cats, and farm animals) examined as well, because these may be reservoirs for reinfecting the athletes.133
Careful attention is also required to disinfecting wrestling mats. Although several investigators134 were not able to isolate fungal organisms from mats, T tonsurans has been cultured from a mat immediately after use.135 The cleaning of mats is probably not as significant as the concern about skin-to-skin contact, but careful disinfecting greatly reduces and may even eliminate tinea infections in some wrestling teams.130 Daily cleaning of the mats with chlorine-containing disinfectant sprays, at least during the course of the competitive season, is recommended (Table 4).
Return to Competition
Athletes with tinea may return to sport only after they are cleared by the examining physician or AT.64 Clearance to compete can only be given if the lesions have adequately responded to treatment, which generally requires 3 days of topical treatment in minor cases or 2 weeks of systemic treatment in more severe cases.63 Athletes with solitary or closely clustered, localized lesions will not be disqualified if the lesions are in a body location that can be covered securely. The barrier preparation should be a dressing, such as Opsite (Smith & Nephew, London, United Kingdom) or Bioclusive (Johnson & Johnson, Langhorne, PA) followed by Pro Wrap (Fabrifoam, Exton, PA) and stretch tape. Dressings should be changed after each match so that the lesion can air dry64 (Table 3).
VIRAL INFECTIONS
Two primary viral infections are prevalent in athletic populations: herpes simplex and MC. Herpes simplex infection is common among athletes, especially those engaged in activities with full skin-on-skin contact, such as wrestling57,136,137 and rugby.1,57,137 Molluscum contagiosum is a highly infectious pox virus skin infection caused by the MC virus, which is classified within the family of poxviruses (Poxviridae).75
Herpes Simplex
Background
Herpes infection is caused by the herpes simplex virus (HSV), and outbreaks in athletes that spread throughout the entire team have been widely reported.128,136 In a study of high school wrestlers at one summer wrestling camp, 60 of 175 wrestlers at the camp developed herpes lesions.136 In the general population, up to 60% of college students possessed antibodies for HSV.138 Herpes infections specifically contracted by athletes were first studied in 1964,139 but then there was a 24-year hiatus in the literature between the earlier clinical publications and the flurry of clinical reports between 1988 and 1992.128,136,140,141
Clinical Features
Clinical features of HSV have been well described in the medical literature since 1964.139 After an incubation period of 3 to 10 days, patients develop a variety of systemic signs or symptoms depending on their preexisting immunity to HSV. Symptoms can range in severity from a mild viral prodromal illness to an almost influenza-like illness with symptoms of fever, severe malaise, prostration, polyarthralgias, polymyalgias, pharyngitis, and conjunctivitis. Physical signs of the infection in athletes can include disseminated skin lesions and complications of conjunctivitis, keratitis, stomatitis, meningitis, arthritis, and hepatitis as well as marked lymphadenopathy and hepatosplenomegaly. Secondary infection with S aureus is common and often simulates bacterial folliculitis.
It is important for ATs to recognize the unique clinical features of HSV infection because an innocuous-appearing HSV lesion on the lip of an athlete can infect many other athletes who lack immunity against the virus. Recurrent HSV infections typically appear as a localized cluster of tense vesicles on the lip; however, it is important to note that particles from the virus reside latently in the dorsal root ganglia of the host's sensory nerves. Thus, recurrent HSV infections can appear in areas other than the lip and oftentimes in areas of previous outbreaks.57,142 Typically, HSV lesions are located on the head, face, neck, or upper extremities.19,46,68,128 The outbreak is usually preceded by symptoms that can include irritability, headache, tingling, and burning or itching of the skin at the site of recurrence.61 Whether athletes are contagious during the prodromal period is unclear. However, we know that individuals with recurrent HSV labialis (fever blisters or cold sores) can shed the virus intermittently between episodes and in the absence of lesions,143 and these individuals may represent a reservoir of virus for infecting previously uninfected athletes. The presence of HSV in the secretions of uninfected athletes is a significant one that needs to be investigated. If proven, a strong case could be made for season-long daily prophylaxis of all individuals on a team.
After the prodrome described above, a primary HSV outbreak often includes widespread clustered vesicles on an erythematous base in areas of contact of the head and neck, trunk, and arms in infected athletes.61,144 Many times, numerous clustered perifollicular vesicles crust rapidly, giving the false impression of folliculitis. The vesicles may continue to erupt for a period of 7 to 10 days and eventually evolve into dry, crusted lesions.
Diagnosis
The diagnosis of HSV is often delayed for days and misdiagnosed as occlusion, bacterial folliculitis, or other pyoderma because HSV clinically can simulate these conditions very closely. A high index of suspicion and clinical expertise is critical in evaluating athletes and diagnosing HSV. Viral culture of vesicle scrapings is the most definitive diagnostic tool, but results can take days.1,57,61,66 A Tzanck smear that identifies herpes-infected giant cells is invaluable in making the correct diagnosis while awaiting the culture results.1,57,61
The AT plays a very important and proactive role in the epidemiologic control of skin infections in athletes. This role begins with daily skin examinations before practices and games or matches. Any athletes with suspicious lesions should be immediately triaged to the team physician for disposition the same day. Whenever possible, ATs should establish relationships with local dermatologists to handle all their skin evaluation needs. An individual suspected of having a contagious skin disease should be immediately isolated from other team members until he or she is examined by the team dermatologist and the skin infection is properly managed. Implementation of these stringent epidemiologic-based concepts can result in a significant reduction in the incidence of skin infections among members of athletic teams.
Treatment
Treatment of primary HSV is most effective with antiviral drugs such as acyclovir or valacyclovir.69 Acyclovir represented the original therapy for HSV,70,71 but the unwieldy dosing pattern of 5 times a day made compliance an issue.67 The typical dosing regimen for valacyclovir, however, is 500 mg twice daily for 7 days.67,68 Once the lesions are fully formed, ruptured, and crusted over, antiviral medications are no longer effective.57 Topical antiviral creams have proven to be ineffective.72
Retrospectively, Anderson44 evaluated HSV outbreaks in Minnesota high school wrestlers during the 1999 season. Statistical analysis of these data confirmed the importance of properly screening and triaging all athletes with suspicious skin lesions for diagnosis and treatment before allowing further contact with other wrestlers. The average time from exposure to outbreak was 6.8 ± 1.70 days, with a 32.7% probability of transmission to sparring partners in a group.
Prevention
In an evidence-based study, Anderson68 reported on the prophylactic use of valacyclovir and concluded that wrestlers with a history of HSV for more than 2 years were adequately treated with valacyclovir 500 mg/d, and those with a history of lesions for less than 2 years showed reductions in HSV infections with 1 g/d of valacyclovir.145
Return to Competition
According to NCAA guidelines,64 the athlete may not return to participation until he or she has received 5 days of oral antiviral therapy and all lesions have a dried, adherent crust (Table 3).
Molluscum Contagiosum
Background
In the United States between 1990 and 1999, 280 000 physician visits per year for MC were estimated.146 The prevalence of MC in children has been reported to be as high as 7.4%147 and considerably higher148 in more confined communities. Several authors149,150 have found no sex differences in the incidence of MC, whereas others147 showed boys to be affected more often. The infection is commonly seen in younger children; however, because of skin-to-skin transmission, it is not uncommon for athletes, including swimmers,147,151 cross-country runners,5 and wrestlers,152 to demonstrate MC infection in areas of direct contact with bodily secretions from other athletes. In addition to contact exposure, certain predisposing factors, such as atopic dermatitis, increase the likelihood of developing MC.153 Many times in these individuals, a small, particularly itchy patch of eczema can develop around the lesions a month or more after their onset.154 In addition, immunocompromised individuals and those on systemic steroids are at increased risk of developing extensive MC infections.28,155–157 Paradoxical immunosuppression in young, conditioned athletes has been described as a predisposing factor to explain the prevalence of infection in this population.96
Clinical Features
The clinical features of MC are fairly characteristic and usually do not present a diagnostic dilemma. The lesions typically are umbilicated, or delled, flesh-colored to light-pink pearly papules, measuring 1 to 10 mm in diameter.73 Although usually a benign, self-resolving infection in nonimmunosuppressed people,29 MC left untreated can persist for 2 to 4 years before clearing spontaneously.158 Untreated MC can present a number of problems in athletes, including the development of secondary pyodermas with S aureus and an eczematous eruption surrounding individual lesions.75 Rupture of molluscum papules can result in furuncle-like lesions that can heal with depressed varicelliform-type scars.75,120 In fact, scarring after long-standing, untreated MC is not uncommon.75
Diagnosis
Because of the characteristic nature of MC lesions, the diagnosis of clinically suspicious lesions is routinely made on clinical examination. If the diagnosis is still uncertain, a Tzanck smear can be done on the crushed core contents of an individual molluscum papule to look for molluscum bodies, which appear on electron microscopic analysis as large, brick-shaped virus particles in positive samples. The MC lesions can occur as solitary lesions or be clustered (usually no more than 20) on body surfaces and, at times, be inoculated extensively into hair-growing areas, such as the beard or pubic area.73
Treatment
Numerous anecdotal therapies have been used for the treatment of MC, including agents such as cantharidin81 and salicylic acid79 and modalities such as cryotherapy74 and pulsed-dye laser.77 More recently, topical immunomodulators such as imiquimod have been used with varying degrees of success.26,76 Evidence-based reviews20 of reported anecdotal treatment modalities for molluscum show no definite statistical evidence of benefits to these therapies.
Physical destruction of scattered MC lesions with a sharp curette is recommended as the preferred method of treatment by many authors,64,75,78,80 but little evidence-based research has been conducted using randomized controlled trials to evaluate its success. Physical destruction of the lesions is useful for rapidly clearing an athlete's skin and, thus, allowing participation in events and preventing both autoinoculation and spread to other athletes.80,159 Curettage can be done easily with or without the use of topical anesthetic creams. When extensive, MC can be a reason for an athlete's disqualification from participation; however, solitary lesions can be appropriately covered or curetted before competition, according to the NCAA Wrestling Championships Handbook.64 Although a recent evidence-based medicine review failed to determine any standard effective therapies,160 the most efficient way to clear this infection rapidly and return the athlete to participation is simple curettage of lesions.
Prevention
Prevention of the spread of this highly contagious infection is best accomplished by meticulous hygiene after exposure to another athlete's skin secretions or inanimate objects that have come in contact with secretions from other athletes, such as swimming pool benches, towels, gym equipment, and wrestling mats.
Return to Competition
In most cases, the athlete must undergo some type of treatment before returning to competition. At this time, the NCAA requires athletes to have the lesions curetted or removed before return to play, although localized or solitary lesions may be covered with a gas-permeable dressing followed by stretch tape64 (Table 3).
BACTERIAL INFECTIONS
Bacterial infections are most commonly caused by various gram-positive strains of Streptococcus and Staphylococcus bacteria.1,161 As much as 30% of the healthy population is colonized with Staphylococcus bacteria in the anterior nares.162 Outbreaks of S aureus infections have been reported in football, basketball, and rugby players.12,59,163
Impetigo
Background
Impetigo is a contagious superficial bacterial infection, or pyoderma, of the skin caused by S aureus and group A β-hemolytic Streptococcus.57,137,161 Impetigo is classified as bullous or nonbullous forms.57
Clinical Features
Bullous impetigo presents with superficial blisters (bullae) that rupture easily. The eruptions are typically moist and surrounded by a scaly rim.164 Nonbullous impetigo, the most common form, initially presents with a thin-walled vesicle followed rapidly by rupture and desquamation to expose a raw, denuded surface covered with a yellowish-brown or honey-colored serous crusting in the perinasal and periorofacial areas.1,57,164
Diagnosis
Diagnosis of bacterial infections is primarily based on the history and characteristic appearance of the lesions, but with the increasing vigilance regarding antibiotic- resistant strains of Staphylococcus infections, scrapings or drainage samples of the lesions should be cultured.57
Treatment
Although impetigo has no standard therapy, the management guidelines include culture and sensitivity of suspicious lesions and treatment with appropriate topical or oral (or both) antibiotics. Good evidence shows that topical mupirocin, fusidic acid, or retapamulin is as effective or more effective and has fewer side effects than oral antibiotics.83 Other authors1,82,83 have recommended antibiotics such as dicloxacillin and cephalexin; or, if the athlete is allergic to penicillins, erythromycin may be used effectively.1
Return to Competition
Any suspicious lesions should be cultured and tested for antimicrobial sensitivity before return to competition. In general, return to competition after bacterial infections should not be allowed until the athlete has completed a 72-hour course of directed antibiotic therapy, has no further drainage or exudate from the wounds, and has developed no new lesions for at least 48 hours.64 Also, because of the communicable nature of bacterial infections, active lesions should not be covered to allow for participation.64
Folliculitis, Furuncles, and Carbuncles
Background
Folliculitis, furuncles, and carbuncles are caused by follicular-based S aureus infections that arise in areas of high friction and perspiration.57
Clinical Features
Folliculitis presents as a myriad of perifollicular papules and pustules on hair-bearing areas, especially in areas that have been shaved, taped, or abraded.94 Furuncles, or boils, are also follicular-based S aureus infections presenting as tender areas that, over a period of a few days, develop a reddened nodular swelling.57,84,85 The lesions are essentially a perifollicular abscess that often progresses to spontaneous rupture and drainage.57 Multiple furuncles that coalesce into a common, purulent mass, called a carbuncle, can be associated with surrounding cellulitis.57,94
Diagnosis
Diagnosis of folliculitis, furuncles, or carbuncles should follow the same progression as the diagnosis of impetigo. All diagnostic decisions should be based on the history and characteristic appearance of the lesions, with scraping or drainage samples of the lesions cultured to rule out antibiotic-resistant strains of Staphylococcus infections.57
Treatment
Athletes with folliculitis should be referred for culture of purulent perifollicular lesions and appropriate antibiotics.57 Simple furuncles may be treated with warm compresses to promote drainage, but more fluctuant furuncles and carbuncles require incision and drainage.57,84,165 After drainage, the athlete needs systemic antimicrobial therapy and close follow-up.57,84,85 These lesions must be managed properly with incision, drainage, and antibiotics to control surrounding cellulitis.84 As mentioned previously, furuncles and carbuncles may be caused by antibiotic-resistant strains of the Staphylococcus bacteria, so it is essential that this diagnosis be considered. Although ATs are not expected to manage these Staphylococcus abscesses, the athlete must be referred to a knowledgeable physician who will perform incision and drainage when necessary and treat with oral antibiotics.
Return to Competition
Guidelines for return to competition after folliculitis, furuncles, or carbuncles are the same as for impetigo. Suspicious lesions should be cultured and tested for antimicrobial sensitivity, and return to participation should not be allowed until the athlete has completed at least 72 hours of directed antibiotic therapy, no drainage or exudate is visible from the wound, and no new lesions have developed in the previous 48 hours. Bacterial infections cannot be covered to allow for participation.64
Methicillin-Resistant S aureus
Background
In the early 1960s, an antibiotic-resistant strain of S aureus known as MRSA was described.87,166 Methicillin-resistant S aureus has acquired the mecA gene167,168 and is resistant to β-lactam antibiotics, including penicillins and cephalosporins,13,86,88 although resistance to other classes of antibiotics, such as fluoroquinolones and tetracyclines, is increasing.14,84,88 Until recently, MRSA was thought to be exclusively a hospital-acquired infection.2,86,88 In the mid- to late 1990s, however, MRSA infections started to be detected in the community outside the typical health care settings,2,86,168,169 being diagnosed in athletes participating in football,25,170,171 wrestling,21 and fencing,171 where as many as 70% of team members required hospitalization and intravenous antibiotic therapy.170,171 In one study,172 the mortality attributable to MRSA infections was estimated to be as high as 22%. This new manifestation of MRSA, called CA-MRSA, is reported to be the most frequent cause of skin infections seen in emergency rooms across the country.169,173 In one hospital in Texas, the number of CA-MRSA cases increased from 9 in 1999 to 459 in 2003.174 Although the spectrum of MRSA appears to be similar in both types (furuncles, carbuncles, and abscesses are most commonly reported27,84,86,88), CA-MRSA contains isolates that are distinct from those of MRSA acquired in the health care setting.86,169,175 Risk factors associated with MRSA include recent hospitalization, outpatient visits, or close contact with a person with risk factors.176 The risk factors for CA-MRSA are not as well defined, and it is not uncommon for patients with no identifiable risk factors to become infected.177
An alarming increase in the prevalence of MRSA nasal colonization has been noted in both healthy children15 and adults.178 Nasal colonization of MRSA isolates in healthy children increased from 2.2% to 9.2%15 and in healthy adults from 0.8% to 7.3% between 2001 and 2004.86,179 Additionally, transmission of CA-MRSA is quite easy in close-contact settings, such as locker rooms and athletic fields,175 so prevention, recognition, and proper management of MRSA are important responsibilities for the AT.
Clinical Features
Prompt recognition of bacterial infections in athletes is vital to preventing both the spread of this highly contagious infection to other team members and contamination of athletic facilities where athletes congregate. Health care professionals should always consider CA-MRSA in the differential diagnosis of all patients presenting with symptoms associated with Staphylococcus disease.
Initially, CA-MRSA infections present similarly to other bacterial infections.27,84,86,87 Furuncles, carbuncles, and abscesses are the most frequent clinical manifestations.88,180 The lesions may begin as small pustules and develop into larger pustules or abscesses with areas of erythema and some tissue necrosis.84,170 In several documented cases,16,84,87 patients and their caregivers have confused CA-MRSA lesions with spider bites.
Diagnosis
With any presentation of a skin and soft tissue infection compatible with that caused by S aureus or history of a “spider bite,” MRSA must be included in the differential diagnosis.84 Any abscess or purulent skin lesion, particularly with signs of severe local or systemic infection, should be cultured for MRSA isolates and antimicrobial susceptibility.84,86
Treatment
It is critical for ATs to understand the proper recognition, dispensation, and management of MRSA infections. An athlete with a suspected MRSA infection must be immediately isolated from other team members and referred to a knowledgeable physician. The physician, who must maintain close contact with the AT in such cases, should abide by the evolving guidelines for the management of these infections. Individual treatment should be guided by local susceptibility data, because prevalence of resistance to antimicrobial agents varies geographically and is likely to change over time.84 Although evidence from controlled clinical trials is presently insufficient to establish optimal treatment regimens for MRSA, several antimicrobial therapies have been proposed.84 Mild to moderate cases in patients with no significant comorbidities still respond well to β-lactam agents.84 Some experts84,91 suggest that a prevalence of 10% to 15% of S aureus isolates, however, means that a change to alternative antimicrobial therapies might be needed. Alternative agents (both oral and parenteral) include vancomycin,84 clindamycin,92 daptomycin,23 tigecycline,93 minocycline, trimethoprim-sulfamethoxazole,89 rifampin,84,89 and linezolid.84,86
Given their potential for rapid development of resistance, some antimicrobial agents are discouraged for the treatment of MRSA. Specifically, these agents include fluoroquinolones (ciprofloxacin and levofloxacin)88,90 and macrolides/azalides (erythromycin, clarithromycin, and azithromycin).88
Prevention
Currently, no oral antibiotic prophylaxis is recommended for bacterial infections. Some authors137,138,180 have discussed using agents such as mupirocin and antiseptic body washes to eliminate S aureus nasal colonization in healthy patients, although very limited data have examined the association between MRSA colonization and subsequent infection.181 Prophylaxis is best accomplished by following standard infection-control precautions, good hand hygiene, and overall hygiene practices as recommended earlier.
Return to Competition
Because of the prevalence and virulent nature of CA-MRSA, any suspicious lesions should be cultured and tested for antimicrobial sensitivity before the athlete returns to participation. In general, after a bacterial infection, return to play should not be allowed until the athlete has completed a 72-hour course of directed antibiotic therapy, has no further drainage or exudate from the wounds, and has developed no new lesions for at least 48 hours.64 Also, because of the communicable nature of bacterial infections, active lesions cannot be covered to allow participation.64
CONCLUSIONS
Certified ATs and other athletic team health care providers must be able to identify the signs and symptoms of common skin diseases in athletes. This position statement outlines the current recommendations to educate the stakeholders in their athletic programs about minimizing disease transmission, preventing the spread of infectious agents, and improving the recognition and management of common skin diseases in athletes.
Acknowledgments
We gratefully acknowledge the efforts of B. J. Anderson, MD; Wilma F. Bergfeld, MD, FAAD; Daniel Monthley, MS, ATC; Jeffrey Stoudt, MA, ATC; James Thornton, MA, ATC, PES; James Leyden, MD; and the Pronouncements Committee in the preparation of this document.
The NATA publishes its position statements as a service to promote the awareness of certain issues to its members. The information contained in the position statement is neither exhaustive nor exclusive to all circumstances or individuals. Variables such as institutional human resource guidelines, state or federal statutes, rules, or regulations, as well as regional environmental conditions, may impact the relevance and implementation of these recommendations. The NATA advises its members and others to carefully and independently consider each of the recommendations (including the applicability of same to any particular circumstance or individual). The position statement should not be relied upon as an independent basis for care, but rather as a resource available to NATA members or others. Moreover, no opinion is expressed herein regarding the quality of care that adheres to or differs from NATA's position statements. The NATA reserves the right to rescind or modify its position statements at any time.
REFERENCES
- 1.Adams B. B. Dermatologic disorders of the athlete. Sports Med. 2002;32(5):309–321. doi: 10.2165/00007256-200232050-00003. [DOI] [PubMed] [Google Scholar]
- 2.Turbeville S. D., Cowan L. D., Greenfield R. A. Infectious disease outbreaks in competitive sports: a review of the literature. Am J Sports Med. 2006;34(11):1860–1865. doi: 10.1177/0363546505285385. [DOI] [PubMed] [Google Scholar]
- 3.Ebell M. H., Siwek J., Weiss B. D., et al. Strength of recommendation taxonomy (SORT): a patient-centered approach to grading evidence in the medical literature. Am Fam Physician. 2004;69(3):548–556. [PubMed] [Google Scholar]
- 4.Allen H. B., Honig P. J., Leyden J. J., McGinley K. J. Selenium sulfide: adjunctive therapy for tinea capitis. Pediatrics. 1982;69(1):81–83. [PubMed] [Google Scholar]
- 5.Commens C. A. Cutaneous transmission of molluscum contagiosum during orienteering competition. Med J Aust. 1987;146(2):117. doi: 10.5694/j.1326-5377.1987.tb136293.x. [DOI] [PubMed] [Google Scholar]
- 6.Srinivasan A., Wolfenden L. L., Song X., et al. An outbreak of Pseudomonas aeruginosa infections associated with flexible bronchoscopes. N Engl J Med. 2003;348(3):221–227. doi: 10.1056/NEJMoa021808. [DOI] [PubMed] [Google Scholar]
- 7.Beekmann S. E., Vaughn T. E., McCoy K. D., et al. Hospital bloodborne pathogens programs: program characteristics and blood and body fluid exposure rates. Infect Control Hosp Epidemiol. 2001;22(2):73–82. doi: 10.1086/501867. [DOI] [PubMed] [Google Scholar]
- 8.Tokars J. I., McKinley G. F., Otten J., et al. Use and efficacy of tuberculosis infection control practices at hospitals with previous outbreaks of multidrug-resistant tuberculosis. Infect Control Hosp Epidemiol. 2001;22(7):449–455. doi: 10.1086/501933. [DOI] [PubMed] [Google Scholar]
- 9.Bhalla A., Pultz N. J., Gries D. M., et al. Acquisition of nosocomial pathogens on hands after contact with environmental surfaces near hospitalized patients. Infect Control Hosp Epidemiol. 2004;25(2):164–167. doi: 10.1086/502369. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Guidelines for environmental infection control in health-care facilities. Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC) MMWR Morb Mortal Wkly Rep. 2003;52(RR10):1–42. [PubMed] [Google Scholar]
- 11.Vasily D. B., Foley J. J. More on tinea corporis gladiatorum. J Am Acad Dermatol. 2002;46:1–2. doi: 10.1067/mjd.2002.120603. [DOI] [PubMed] [Google Scholar]
- 12.Decker M. D., Lybarger J. A., Vaughn W. K., Hutcheson R. H., Jr, Schaffner W. An outbreak of staphylococcal skin infections among river rafting guides. Am J Epidemiol. 1986;124(6):969–976. doi: 10.1093/oxfordjournals.aje.a114486. [DOI] [PubMed] [Google Scholar]
- 13.Crawford S. E., Boyle-Vavra S., Daum R. S. Community associated methicillin-resistant Staphylococcus aureus. In: Hooper D. C., Scheld M., editors. Emerging Infections. Vol 7. Washington, DC: ASM Press; 2007. pp. 153–179. [Google Scholar]
- 14.Frazee B. W., Lynn J., Charlebois E. D., Lambert L., Lowery D., Perdreau-Remington F. High prevalence of methicillin-resistant Staphylococcus aureus in emergency department skin and soft tissue infections. Ann Emerg Med. 2005;45(3):311–320. doi: 10.1016/j.annemergmed.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 15.Creech C. B., 2nd, Kernodle D. S., Alsentzer A., Wilson C., Edwards K. M. Increasing rates of nasal carriage of methicillin-resistant Staphylococcus aureus in healthy children. Pediatr Infect Dis J. 2005;24(7):617–621. doi: 10.1097/01.inf.0000168746.62226.a4. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. Outbreaks of community-associated methicillin-resistant Staphylococcus aureus skin infections—Los Angeles County, California, 2002–2003. MMWR Morb Mortal Wkly Rep. 2003;52(5):88. [PubMed] [Google Scholar]
- 17.Bischoff W. E., Reynolds T. M., Sessler C. N., Edmond M. B., Wenzel R. P. Handwashing compliance by health care workers: the impact of introducing an accessible, alcohol-based hand antiseptic. Arch Intern Med. 2000;160(7):1017–1021. doi: 10.1001/archinte.160.7.1017. [DOI] [PubMed] [Google Scholar]
- 18.Larson E. L., Aiello A. E., Bastyr J., et al. Assessment of two hand hygiene regimens for intensive care unit personnel. Crit Care Med. 2001;29(5):944–951. doi: 10.1097/00003246-200105000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Stacey A., Atkins B. Infectious diseases in rugby players: incidence, treatment and prevention. Sports Med. 2000;29(3):211–220. doi: 10.2165/00007256-200029030-00005. [DOI] [PubMed] [Google Scholar]
- 20.Brandrup F., Asschenfeldt P. Molluscum contagiosum-induced comedo and secondary abscess formation. Pediatr Dermatol. 1989;6(2):118–121. doi: 10.1111/j.1525-1470.1989.tb01008.x. [DOI] [PubMed] [Google Scholar]
- 21.Lindenmayer J. M., Schoenfeld S., O'Grady R., Carney J. K. Methicillin-resistant Staphylococcus aureus in a high school wrestling team and the surrounding community. Arch Intern Med. 1998;158(8):895–899. doi: 10.1001/archinte.158.8.895. [DOI] [PubMed] [Google Scholar]
- 22.Kohn L. T., Corrigan J., Donaldson M. S. To Err Is Human: Building a Safer Health System. Washington, DC: National Academy Press; 2000. [PubMed] [Google Scholar]
- 23.Carpenter C. F., Chambers H. F. Daptomycin: another novel agent for treating infections due to drug-resistant gram-positive pathogens. Clin Infect Dis. 2004;38(7):994–1000. doi: 10.1086/383472. [DOI] [PubMed] [Google Scholar]
- 24.Malik R. E., Cooper R. A., Griffith C. J. Use of audit tools to evaluate the efficacy of cleaning systems in hospitals. Am J Infect Control. 2003;31(3):181–187. doi: 10.1067/mic.2003.34. [DOI] [PubMed] [Google Scholar]
- 25.Begier E. M., Frenette K., Barrett N. L., et al. A high-morbidity outbreak of methicillin-resistant Staphylococcus aureus among players on a college football team, facilitated by cosmetic body shaving and turf burns. Clin Infect Dis. 2004;39(10):1446–1453. doi: 10.1086/425313. [DOI] [PubMed] [Google Scholar]
- 26.Theos A. U., Cummins R., Silverberg N. B., Paller A. S. Effectiveness of imiquimod cream 5% for treating childhood molluscum contagiosum in a double-blind, randomized pilot trial. Cutis. 2004;74(2) [PubMed] [Google Scholar]
- 27.Miller L. G., Perdreau-Remington F., Bayer A. S., et al. Clinical and epidemiologic characteristics cannot distinguish community-associated methicillin-resistant Staphylococcus aureus infection from methicillin-susceptible S aureus infection: a prospective investigation. Clin Infect Dis. 2007;44(4):471–482. doi: 10.1086/511033. [DOI] [PubMed] [Google Scholar]
- 28.Matis W. L., Triana A., Shapiro R., Eldred L., Polk B. F., Hood A. F. Dermatologic findings associated with human immunodeficiency virus infection. J Am Acad Dermatol. 1987;17(5, pt 1):746–751. doi: 10.1016/s0190-9622(87)70257-6. [DOI] [PubMed] [Google Scholar]
- 29.Ordoukhanian E., Lane A. T. Warts and molluscum contagiosum: beware of treatments worse than the disease. Postgrad Med. 1997;101(2) doi: 10.3810/pgm.1997.02.167. [DOI] [PubMed] [Google Scholar]
- 30.Pronovost P. J., Nolan T., Zeger S., Miller M., Rubin H. How can clinicians measure safety and quality in acute care? Lancet. 2004;363(9414):1061–1067. doi: 10.1016/S0140-6736(04)15843-1. [DOI] [PubMed] [Google Scholar]
- 31.Siegel J. D., Rhinehart E., Jackson M., Chiarello L. Health Care Infection Control Practices Advisory Committee: 2007 guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control. 2007;35(10)(suppl 2):S65–S164. doi: 10.1016/j.ajic.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonten M. J., Kollef M. H., Hall J. B. Risk factors for ventilator-associated pneumonia: from epidemiology to patient management. Clin Infect Dis. 2004;38(8):1141–1149. doi: 10.1086/383039. [DOI] [PubMed] [Google Scholar]
- 33.Tablan O. C., Anderson L. J., Besser R., Bridges C., Hajjeh R. CDC; Healthcare Infection Control Practices Advisory Committee. Guidelines for preventing health-care–associated pneumonia, 2003: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. MMWR Morb Mortal Wkly Rep. 2003;53(RR-3):1–36. [PubMed] [Google Scholar]
- 34.Macartney K. K., Gorelick M. H., Manning M. L., Hodinka R. L., Bell L. M. Nosocomial respiratory syncytial virus infections: the cost-effectiveness and cost-benefit of infection control. Pediatrics. 2000;106(3):520–526. doi: 10.1542/peds.106.3.520. [DOI] [PubMed] [Google Scholar]
- 35.Ostrowsky B. E., Trick W. E., Sohn A. H., et al. Control of vancomycin-resistant Enterococcus in health care facilities in a region. N Engl J Med. 2001;344(19):1427–1433. doi: 10.1056/NEJM200105103441903. [DOI] [PubMed] [Google Scholar]
- 36.Burke J. P. Infection control: a problem for patient safety. N Engl J Med. 2003;348(7):651–656. doi: 10.1056/NEJMhpr020557. [DOI] [PubMed] [Google Scholar]
- 37.Gerberding J. L. Hospital-onset infections: a patient safety issue. Ann Intern Med. 2002;137(8):665–670. doi: 10.7326/0003-4819-137-8-200210150-00011. [DOI] [PubMed] [Google Scholar]
- 38.Shulman L., Ost D. Managing infection in the critical care unit: how can infection control make the ICU safe? Crit Care Clin. 2005;21(1) doi: 10.1016/j.ccc.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 39.Clarke S. P., Rockett J. L., Sloane D. M., Aiken L. H. Organizational climate, staffing, and safety equipment as predictors of needlestick injuries and near-misses in hospital nurses. Am J Infect Control. 2002;30(4):207–216. doi: 10.1067/mic.2002.123392. [DOI] [PubMed] [Google Scholar]
- 40.Gershon R. R., Karkashian C. D., Grosch J. W., et al. Hospital safety climate and its relationship with safe work practices and workplace exposure incidents. Am J Infect Control. 2000;28(3):211–221. doi: 10.1067/mic.2000.105288. [DOI] [PubMed] [Google Scholar]
- 41.Pittet D., Hugonnet S., Harbarth S., et al. Effectiveness of a hospital-wide programme to improve compliance with hand hygiene: Infection Control Programme. Lancet. 2000;356(9238):1307–1312. doi: 10.1016/s0140-6736(00)02814-2. [DOI] [PubMed] [Google Scholar]
- 42.Sherertz R. J., Ely E. W., Westbrook D. M., et al. Education of physicians-in-training can decrease the risk for vascular catheter infection. Ann Intern Med. 2000;132(8):641–648. doi: 10.7326/0003-4819-132-8-200004180-00007. [DOI] [PubMed] [Google Scholar]
- 43.Vaughn T. E., McCoy K. D., Beekmann S. E., Woolson R. E., Torner J. C., Doebbeling B. N. Factors promoting consistent adherence to safe needle precautions among hospital workers. Infect Control Hosp Epidemiol. 2004;25(7):548–555. doi: 10.1086/502438. [DOI] [PubMed] [Google Scholar]
- 44.Anderson B. J. Skin infections in Minnesota high school state tournament wrestlers: 1997–2006. Clin J Sport Med. 2007;17(6):478–480. doi: 10.1097/JSM.0b013e31815ac43d. [DOI] [PubMed] [Google Scholar]
- 45.Duckro A. N., Blom D. W., Lyle E. A., Weinstein R. A., Hayden M. K. Transfer of vancomycin-resistant enterococci via health care worker hands. Arch Intern Med. 2005;165(3):302–307. doi: 10.1001/archinte.165.3.302. [DOI] [PubMed] [Google Scholar]
- 46.Hota B. Contamination, disinfection, and cross-colonization: are hospital surfaces reservoirs for nosocomial infection? Clin Infect Dis. 2004;39(8):1182–1189. doi: 10.1086/424667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rutala W. A., Weber D. J. Disinfection and sterilization in health care facilities: what clinicians need to know. Clin Infect Dis. 2004;39(5):702–709. doi: 10.1086/423182. [DOI] [PubMed] [Google Scholar]
- 48.Centers for Disease Control and Prevention. Guideline for hand hygiene in health-care settings: recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. MMWR Morb Mortal Wkly Rep. 2002;51(RR-16):1–45. [PubMed] [Google Scholar]
- 49.Larson E. A causal link between handwashing and risk of infection? Examination of the evidence. Infect Control Hosp Epidemiol. 1988;9(1):28–36. doi: 10.1086/645729. [DOI] [PubMed] [Google Scholar]
- 50.Boyce J. M. Scientific Basis for Handwashing With Alcohol And Other Waterless Antiseptic Principles and Practices in Healthcare Facilities. Washington, DC: Association for Professionals in Infection Control and Epidemiology; 2001. [Google Scholar]
- 51.Maury E., Alzieu M., Baudel J. L., et al. Availability of an alcohol solution can improve hand disinfection compliance in an intensive care unit. Am J Respir Crit Care Med. 2000;162(1):324–327. doi: 10.1164/ajrccm.162.1.9908118. [DOI] [PubMed] [Google Scholar]
- 52.Ehrenkranz N. J., Alfonso B. C. Failure of bland soap handwash to prevent hand transfer of patient bacteria to urethral catheters. Infect Control Hosp Epidemiol. 1991;12(11):654–662. doi: 10.1086/646261. [DOI] [PubMed] [Google Scholar]
- 53.McFarland L. V., Mulligan M. E., Kwok R. Y., Stamm W. E. Nosocomial acquisition of Clostridium difficile infection. N Engl J Med. 1989;320(4):204–210. doi: 10.1056/NEJM198901263200402. [DOI] [PubMed] [Google Scholar]
- 54.Mortimer E. A., Jr, Lipsitz P. J., Wolinsky E., Gonzaga A. J., Rammelkamp C. H., Jr Transmission of staphylococci between newborns: importance of the hands to personnel. Am J Dis Child. 1962;104(3):289–295. doi: 10.1001/archpedi.1962.02080030291012. [DOI] [PubMed] [Google Scholar]
- 55.Olsen R. J., Lynch P., Coyle M. B., Cummings J., Bokete T., Stamm W. E. Examination gloves as barriers to hand contamination in clinical practice. JAMA. 1993;270(3):350–353. [PubMed] [Google Scholar]
- 56.Tenorio A. R., Badri S. M., Sahgal N. B., et al. Effectiveness of gloves in the prevention of hand carriage of vancomycin-resistant Enterococcus species by health care workers after patient care. Clin Infect Dis. 2001;32(5):826–829. doi: 10.1086/319214. [DOI] [PubMed] [Google Scholar]
- 57.Cordoro K. M., Ganz J. E. Training room management of medical conditions: sports dermatology. Clin Sports Med. 2005;24(3) doi: 10.1016/j.csm.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 58.Luke A., d'Hemecourt P. Prevention of infectious diseases in athletes. Clin Sports Med. 2007;26(3):321–344. doi: 10.1016/j.csm.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nguyen D. M., Mascola L., Brancoft E. Recurring methicillin-resistant Staphylococcus aureus infections in a football team. Emerg Infect Dis. 2005;11(4):526–532. doi: 10.3201/eid1104.041094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Michalsen A., Delclos G. L., Felknor S. A., et al. Compliance with universal precautions among physicians. J Occup Environ Med. 1997;39(2):130–137. doi: 10.1097/00043764-199702000-00010. [DOI] [PubMed] [Google Scholar]
- 61.Pleacher M. D., Dexter W. W. Cutaneous fungal and viral infections in athletes. Clin Sports Med. 2007;26(3):397–411. doi: 10.1016/j.csm.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 62.Basler R. S. Skin problems in athletics. In: Mellion M. B., Walsh W. M., Madden C., editors. Team Physician's Handbook. Philadelphia, PA: Hanley & Belfus; 2002. pp. 311–325. [Google Scholar]
- 63.Beller M., Gessner B. D. An outbreak of tinea corporis gladiatorum on a high school wrestling team. J Am Acad Dermatol. 1994;31(2, pt 1):197–201. doi: 10.1016/s0190-9622(94)70145-8. [DOI] [PubMed] [Google Scholar]
- 64.Vasily D. B., Foley J. J. Guidelines for disposition of skin infections. In: Halpin T., editor. NCAA 2004 Division I Wrestling Championships Handbook. Indianapolis, IN: National Collegiate Athletic Association; 2004. pp. 21–23. [Google Scholar]
- 65.Kohl T. D., Martin D. C., Berger M. S. Comparison of topical and oral treatments for tinea gladiatorum. Clin J Sport Med. 1999;9(3):161–166. doi: 10.1097/00042752-199907000-00008. [DOI] [PubMed] [Google Scholar]
- 66.Fitzpatrick T. B., Johnson R. A., Wolff K., editors. Cutaneous Fungal Infections. 3rd ed. New York, NY: McGraw-Hill; 1997. [Google Scholar]
- 67.Adams B. B. New strategies for the diagnosis, treatment, and prevention of herpes simplex in contact sports. Curr Sports Med Rep. 2004;3(5):277–283. doi: 10.1249/00149619-200410000-00008. [DOI] [PubMed] [Google Scholar]
- 68.Anderson B. J. The effectiveness of valacyclovir in preventing reactivation of herpes gladiatorum in wrestlers. Clin J Sport Med. 1999;9(2):86–90. doi: 10.1097/00042752-199904000-00008. [DOI] [PubMed] [Google Scholar]
- 69.Balfour H. H., Jr Antiviral drugs. N Engl J Med. 1999;340(16):1255–1268. doi: 10.1056/NEJM199904223401608. [DOI] [PubMed] [Google Scholar]
- 70.Becker T. M. Herpes gladiatorum: a growing problem in sports medicine. Cutis. 1992;50(2):150–152. [PubMed] [Google Scholar]
- 71.Halstead M. E., Bernhardt D. T. Common infections in the young athlete. Pediatr Ann. 2002;31(1):42–48. doi: 10.3928/0090-4481-20020101-10. [DOI] [PubMed] [Google Scholar]
- 72.Marques A. R. Herpes Simplex. Vol 2. 6th ed. New York, NY: McGraw Hill; 2003. [Google Scholar]
- 73.Rogers M., Barnetson R. S. C. Diseases of the skin. In: Campbell A. G. M., McIntosh N., editors. Forfar and Arneil's Textbook of Pediatrics. 5th ed. New York, NY: Churchill Livingstone; 1998. pp. 1633–1635. [Google Scholar]
- 74.Barton S. E., Chard S. Facial molluscum: treatment with cryotherapy and podophyllotoxin. Int J STD AIDS. 2002;13(4):277–278. doi: 10.1258/0956462021924974. [DOI] [PubMed] [Google Scholar]
- 75.Brown J., Janniger C. K., Schwartz R. A., Silverberg N. B. Childhood molluscum contagiosum. Int J Dermatol. 2006;45(2):93–99. doi: 10.1111/j.1365-4632.2006.02737.x. [DOI] [PubMed] [Google Scholar]
- 76.Campanelli A., Krischer J., Saurat J. H. Topical application of imiquimod and associated fever in children. J Am Acad Dermatol. 2005;52(1):E1. doi: 10.1016/j.jaad.2004.10.858. [DOI] [PubMed] [Google Scholar]
- 77.Hancox J. G., Jackson J., McCagh S. Treatment of molluscum contagiosum with the pulsed dye laser over a 28-month period. Cutis. 2003;71(5):414–416. [PubMed] [Google Scholar]
- 78.Kakourou T., Zachariades A., Anastasiou T., Architectonidou E., Georgala S., Theodoridou M. Molluscum contagiosum in Greek children: a case series. Int J Dermatol. 2005;44(3):221–223. doi: 10.1111/j.1365-4632.2004.02074.x. [DOI] [PubMed] [Google Scholar]
- 79.Leslie K. S., Dootson G., Sterling J. C. Topical salicylic acid gel as a treatment for molluscum contagiosum in children. J Dermatolog Treat. 2005;16(5–6):336–340. doi: 10.1080/09546630500430521. [DOI] [PubMed] [Google Scholar]
- 80.Lowy D. R. Molluscum contagiosum. In: Fitzpatrick T. B., Freedberg I. M., editors. Fitzpatrick's Dermatology in General Medicine. Vol 2. 5th ed. New York, NY: McGraw-Hill; 1999. pp. 2478–2481. [Google Scholar]
- 81.Ross G. L., Orchard D. C. Combination topical treatment of molluscum contagiosum with cantharidin and imiquimod 5% in children: a case series of 16 patients. Australas J Dermatol. 2004;45(2):100–102. doi: 10.1111/j.1440-0960.2004.00066.x. [DOI] [PubMed] [Google Scholar]
- 82.Adams B. B. Sports dermatology. Adolesc Med. 2001;12(2) [PubMed] [Google Scholar]
- 83.Koning S., Verhagen A. P., van Suijlekom-Smit L. W. A., Morris A., Butler C. C., van der Wouden J. C. Interventions for impetigo. Cochrane Database Syst Rev. 2004. p. CD003261. doi: 10./002/14651858.CD003261.pub2. [DOI] [PubMed]
- 84.Gorwitz R. J., Jernigan D. B., Powers J. H., Jernigan J. A. Strategies for clinical management of MRSA in the community: summary of an experts' meeting convened by the Centers for Disease Control and Prevention, 2006. http://www.cdc.gov/ncidod/dhqp/ar_mrsa_ca.html. Accessed March 24, 2010.
- 85.Lowy F. D. Staphylococcus aureus infections. N Engl J Med. 1998;339(8):520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 86.Daum R. S. Clinical practice. Skin and soft-tissue infections caused by methicillin-resistant Staphylococcus aureus. N Engl J Med. 2007;357(4):380–390. doi: 10.1056/NEJMcp070747. [DOI] [PubMed] [Google Scholar]
- 87.Rihn J. A., Michaels M. G., Harner C. D. Community-acquired methicillin-resistant Staphylococcus aureus: an emerging problem in the athletic population. Am J Sports Med. 2005;33(12):1924–1929. doi: 10.1177/0363546505283273. [DOI] [PubMed] [Google Scholar]
- 88.Fridkin S. K., Hageman J. C., Morrison M., et al. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352(14):1436–1444. doi: 10.1056/NEJMoa043252. [DOI] [PubMed] [Google Scholar]
- 89.Iyer S., Jones D. H. Community-acquired methicillin-resistant Staphylococcus aureus skin infection: a retrospective analysis of clinical presentation and treatment of a local outbreak. J Am Acad Dermatol. 2004;50(6):854–858. doi: 10.1016/j.jaad.2003.12.043. [DOI] [PubMed] [Google Scholar]
- 90.Jones M. E., Critchley I. A., Karlowsky J. A., et al. In vitro activities of novel nonfluorinated quinolones PGE 9262932 and PGE 9509924 against clinical isolates of Staphylococcus aureus and Streptococcus pneumoniae with defined mutations in DNA gyrase and topoisomerase IV. Antimicrob Agents Chemother. 2002;46(6):1651–1657. doi: 10.1128/AAC.46.6.1651-1657.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kaplan S. L. Treatment of community-associated methicillin-resistant Staphylococcus aureus infections. Pediatr Infect Dis J. 2005;24(5):457–458. doi: 10.1097/01.inf.0000164162.00163.9d. [DOI] [PubMed] [Google Scholar]
- 92.Martinez-Aguilar G., Hammerman W. A., Mason E. O., Jr, Kaplan S. L. Clindamycin treatment of invasive infections caused by community-acquired, methicillin-resistant and methicillin-susceptible Staphylococcus aureus in children. Pediatr Infect Dis J. 2003;22(7):593–598. doi: 10.1097/01.inf.0000073163.37519.ee. [DOI] [PubMed] [Google Scholar]
- 93.Postier R. G., Green S. L., Klein S. R., Ellis-Grosse E. J., Loh E. Results of a multicenter, randomized, open-label efficacy and safety study of two doses of tigecycline for complicated skin and skin-structure infections in hospitalized patients. Clin Ther. 2004;26(5):704–714. doi: 10.1016/s0149-2918(04)90070-7. [DOI] [PubMed] [Google Scholar]
- 94.Habif T. P. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 4th ed. Philadelphia, PA: Mosby; 2004. [Google Scholar]
- 95.Habif T. P., Campbell J. L. J., Chapman M. S., Dinulos J. G. H., Zug K. A. Skin Disease: Diagnosis and Treatment. Philadelphia, PA: Elsevier; 2005. [Google Scholar]
- 96.Hughes W. T. The athlete: an immunocompromised host. Adv Pediatr Infect Dis. 1997;13:79–99. [PubMed] [Google Scholar]
- 97.Bridges C. B., Kuehnert M. J., Hall C. B. Transmission of influenza: implications for control in health care settings. Clin Infect Dis. 2003;37(8):1094–1101. doi: 10.1086/378292. [DOI] [PubMed] [Google Scholar]
- 98.Hall C. B. Nosocomial respiratory syncytial virus infections: the “Cold War” has not ended. Clin Infect Dis. 2000;31(2):590–596. doi: 10.1086/313960. [DOI] [PubMed] [Google Scholar]
- 99.Saiman L., Siegel J. Cystic Fibrosis Foundation. Infection control recommendations for patients with cystic fibrosis: microbiology, important pathogens, and infection control practices to prevent patient-to-patient transmission. Infect Control Hosp Epidemiol. 2003;24(5)(suppl):S6–S52. doi: 10.1086/503485. [DOI] [PubMed] [Google Scholar]
- 100.Varia M., Wilson S., Sarwal S., et al. Investigation of a nosocomial outbreak of severe acute respiratory syndrome (SARS) in Toronto, Canada. CMAJ. 2003;169(4):285–292. [PMC free article] [PubMed] [Google Scholar]
- 101.Munoz F. M., Ong L. T., Seavy D., Medina D., Correa A., Starke J. R. Tuberculosis among adult visitors of children with suspected tuberculosis and employees at a children's hospital. Infect Control Hosp Epidemiol. 2002;23(10):568–572. doi: 10.1086/501972. [DOI] [PubMed] [Google Scholar]
- 102.Osterholm M. T., Hedberg C. W., Moore K. A. The epidemiology of infectious diseases. In: Mandell G. L., Bennett J. E., Dolin R., editors. Principles and Practice of Infectious Diseases, 5th ed. Philadelphia, PA: Churchill Livingstone; 2000. pp. 161–163. [Google Scholar]
- 103.Pate R. R., Trost S. G., Levin S., Dowda M. Sports participation and health-related behaviors among US youth. Arch Pediatr Adolesc Med. 2000;154(9):904–911. doi: 10.1001/archpedi.154.9.904. [DOI] [PubMed] [Google Scholar]
- 104.Centers for Disease Control and Prevention (CDC) Nosocomial hepatitis B virus infection associated with reusable fingerstick blood sampling devices—Ohio and New York City, 1996. MMWR Morb Mortal Wkly Rep. 1997;46(10):217–221. [PubMed] [Google Scholar]
- 105.Centers for Disease Control and Prevention (CDC) Transmission of hepatitis B virus among persons undergoing blood glucose monitoring in long-term-care facilities—Mississippi, North Carolina, and Los Angeles County, California, 2003–2004. MMWR Morb Mortal Wkly Rep. 2005;54(9):220–223. [PubMed] [Google Scholar]
- 106.Desenclos J. C., Bourdiol-Razes M., Rolin B., et al. Hepatitis C in a ward for cystic fibrosis and diabetic patients: possible transmission by spring-loaded finger-stick devices for self-monitoring of capillary blood glucose. Infect Control Hosp Epidemiol. 2001;22(11):701–707. doi: 10.1086/501849. [DOI] [PubMed] [Google Scholar]
- 107.Kirschke D. L., Jones T. F., Craig A. S., et al. Pseudomonas aeruginosa and Serratia marcescens contamination associated with a manufacturing defect in bronchoscopes. N Engl J Med. 2003;348(3):214–220. doi: 10.1056/NEJMoa021791. [DOI] [PubMed] [Google Scholar]
- 108.Schelenz S., French G. An outbreak of multidrug-resistant Pseudomonas aeruginosa infection associated with contamination of bronchoscopes and an endoscope washer-disinfector. J Hosp Infect. 2000;46(1):23–30. doi: 10.1053/jhin.2000.0800. [DOI] [PubMed] [Google Scholar]
- 109.Weber D. J., Rutala W. A. Lessons from outbreaks associated with bronchoscopy. Infect Control Hosp Epidemiol. 2001;22(7):403–408. doi: 10.1086/501924. [DOI] [PubMed] [Google Scholar]
- 110.Perry C., Marshall R., Jones E. Bacterial contamination of uniforms. J Hosp Infect. 2001;48(3):238–241. doi: 10.1053/jhin.2001.0962. [DOI] [PubMed] [Google Scholar]
- 111.Zachary K. C., Bayne P. S., Morrison V. J., Ford D. S., Silver L. C., Hooper D. C. Contamination of gowns, gloves, and stethoscopes with vancomycin-resistant enterococci. Infect Control Hosp Epidemiol. 2001;22(9):560–564. doi: 10.1086/501952. [DOI] [PubMed] [Google Scholar]
- 112.Papineni R. S., Rosenthal F. S. The size distribution of droplets in the exhaled breath of healthy human subjects. J Aerosol Med. 1997;10(2):105–116. doi: 10.1089/jam.1997.10.105. [DOI] [PubMed] [Google Scholar]
- 113.Bloch A. B., Orenstein W. A., Ewing W. M., et al. Measles outbreak in a pediatric practice: airborne transmission in an office setting. Pediatrics. 1985;75(4):676–683. [PubMed] [Google Scholar]
- 114.Coronado V. G., Beck-Sague C. M., Hutton M. D., et al. Transmission of multidrug-resistant Mycobacterium tuberculosis among persons with human immunodeficiency virus infection in an urban hospital: epidemiologic and restriction fragment length polymorphism analysis. J Infect Dis. 1993;168(4):1052–1055. doi: 10.1093/infdis/168.4.1052. [DOI] [PubMed] [Google Scholar]
- 115.Widmer A. F. Replace hand washing with use of a waterless alcohol hand rub? Clin Infect Dis. 2000;31(1):136–143. doi: 10.1086/313888. [DOI] [PubMed] [Google Scholar]
- 116.Doebbeling B. N., Li N., Wenzel R. P. An outbreak of hepatitis A among health care workers: risk factors for transmission. Am J Public Health. 1993;83(12):1679–1684. doi: 10.2105/ajph.83.12.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rodriguez E. M., Parrott C., Rolka H., Monroe S. S., Dwyer D. M. An outbreak of viral gastroenteritis in a nursing home: importance of excluding ill employees. Infect Control Hosp Epidemiol. 1996;17(9):587–592. doi: 10.1086/647390. [DOI] [PubMed] [Google Scholar]
- 118.Standaert S. M., Hutcheson R. H., Schaffner W. Nosocomial transmission of Salmonella gastroenteritis to laundry workers in a nursing home. Infect Control Hosp Epidemiol. 1994;15(1):22–26. doi: 10.1086/646813. [DOI] [PubMed] [Google Scholar]
- 119.Mayfield J. L., Leet T., Miller J., Mundy L. M. Environmental control to reduce transmission of Clostridium difficile. Clin Infect Dis. 2000;31(4):995–1000. doi: 10.1086/318149. [DOI] [PubMed] [Google Scholar]
- 120.Denton M., Wilcox M. H., Parnell P., et al. Role of environmental cleaning in controlling an outbreak of Acinetobacter baumannii on a neurosurgical intensive care unit. J Hosp Infect. 2004;56(2):106–110. doi: 10.1016/j.jhin.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 121.Eun H. C., Lee A. Y., Lee Y. S. Sodium hypochlorite dermatitis. Contact Dermatitis. 1984;11(1):45. doi: 10.1111/j.1600-0536.1984.tb00172.x. [DOI] [PubMed] [Google Scholar]
- 122.Hostynek J. J., Wilhelm K. P., Cua A. B., Maibach H. I. Irritation factors of sodium hypochlorite solutions in human skin. Contact Dermatitis. 1990;23(5):316–324. doi: 10.1111/j.1600-0536.1990.tb05165.x. [DOI] [PubMed] [Google Scholar]
- 123.Osmundsen P. E. Contact dermatitis due to sodium hypochlorite. Contact Dermatitis. 1978;4(3):177–178. doi: 10.1111/j.1600-0536.1978.tb03779.x. [DOI] [PubMed] [Google Scholar]
- 124.Murphy G. F. The skin. In: Cotran R. S., Kumar V., Collins T., editors. Robbins Pathologic Basis of Disease. 6th ed. Philadelphia, PA: WB Saunders; 1999. pp. 1170–1213. [Google Scholar]
- 125.Adams B. B. Tinea corporis gladiatorum. J Am Acad Dermatol. 2002;47(2):286–290. doi: 10.1067/mjd.2002.120603. [DOI] [PubMed] [Google Scholar]
- 126.Foster K. W., Ghannoum M. A., Elewski B. E. Epidemiologic surveillance of cutaneous fungal infection in the United States from 1999 to 2002. J Am Acad Dermatol. 2004;50(5):748–752. doi: 10.1016/s0190-9622(03)02117-0. [DOI] [PubMed] [Google Scholar]
- 127.Auger P., Marquis G., Joly J., Attye A. Epidemiology of tinea pedis in marathon runners: prevalence of occult athlete's foot. Mycoses. 1993;36(1–2):35–41. doi: 10.1111/j.1439-0507.1993.tb00685.x. [DOI] [PubMed] [Google Scholar]
- 128.Becker T. M., Kodsi R., Bailey P., Lee F., Levandowski R., Nahmias A. J. Grappling with herpes: herpes gladiatorum. Am J Sports Med. 1988;16(6):665–669. doi: 10.1177/036354658801600620. [DOI] [PubMed] [Google Scholar]
- 129.Drake L. A., Dinehart S. M., Farmer E. R., et al. Guidelines of care for superficial mycotic infections of the skin: tinea corporis, tinea cruris, tinea faciei, tinea manuum, and tinea pedis. Guidelines/Outcomes Committee. American Academy of Dermatology. J Am Acad Dermatol. 1996;34(2, pt 1):282–286. doi: 10.1016/s0190-9622(96)80135-6. [DOI] [PubMed] [Google Scholar]
- 130.Hradil E., Hersle K., Nordin P., Faergemann J. An epidemic of tinea corporis caused by Trichophyton tonsurans among wrestlers in Sweden. Acta Derm Venereol. 1995;75(4):305–306. doi: 10.2340/0001555575305306. [DOI] [PubMed] [Google Scholar]
- 131.Kohl T. D., Giesen D. P., Moyer J., Jr, Lisney M. Tinea gladiatorum: Pennsylvania's experience. Clin J Sport Med. 2002;12(3):165–171. doi: 10.1097/00042752-200205000-00004. [DOI] [PubMed] [Google Scholar]
- 132.Kohl T. D., Martin D. C., Nemeth R., Hill T., Evans D. Fluconazole for the prevention and treatment of tinea gladiatorum. Pediatr Infect Dis J. 2000;19(8):717–722. doi: 10.1097/00006454-200008000-00009. [DOI] [PubMed] [Google Scholar]
- 133.Frieden I. J., Howard R. Tinea capitis: epidemiology, diagnosis, treatment, and control. J Am Acad Dermatol. 1994;31(3, pt 2):S42–S46. doi: 10.1016/s0190-9622(08)81266-2. [DOI] [PubMed] [Google Scholar]
- 134.Kohl T. D., Lisney M. Tinea gladiatorum: wrestling's emerging foe. Sports Med. 2000;29(6):439–447. doi: 10.2165/00007256-200029060-00006. [DOI] [PubMed] [Google Scholar]
- 135.el Fari M., Graser Y., Presber W., Tietz H. J. An epidemic of tinea corporis caused by Trichophyton tonsurans among children (wrestlers) in Germany. Mycoses. 2000;43(5):191–196. doi: 10.1046/j.1439-0507.2000.00558.x. [DOI] [PubMed] [Google Scholar]
- 136.Belongia E. A., Goodman J. L., Holland E. J., et al. An outbreak of herpes gladiatorum at a high-school wrestling camp. N Engl J Med. 1991;325(13):906–910. doi: 10.1056/NEJM199109263251302. [DOI] [PubMed] [Google Scholar]
- 137.Reinberg J., Ailor S. K., Dyer J. A. Common sports-related dermatologic infections. Mo Med. 2007;104(2):119–123. [PubMed] [Google Scholar]
- 138.Oxman M. Herpes simplex virus. In: Gorbach S. L., Bartlett J. G., Blacklow N. R., editors. Infectious Diseases. 2nd ed. Philadelphia, PA: WB Saunders; 1998. pp. 2022–2062. [Google Scholar]
- 139.Selling B., Kibrick S. An outbreak of herpes simplex among wrestlers (herpes gladiatorum) N Engl J Med. 1964;270:979–982. doi: 10.1056/NEJM196405072701903. [DOI] [PubMed] [Google Scholar]
- 140.Centers for Disease Control and Prevention. Herpes gladiatorum at a high school wrestling camp: Minnesota. MMWR Morb Mortal Wkly Rep. 1990;39(5):69–71. [PubMed] [Google Scholar]
- 141.Holland E. J., Mahanti R. L., Belongia E. A., et al. Ocular involvement in an outbreak of herpes gladiatorum. Am J Ophthalmol. 1992;114(6):680–684. doi: 10.1016/s0002-9394(14)74044-9. [DOI] [PubMed] [Google Scholar]
- 142.Whitley R. J., Kimberlin D. W., Roizman B. Herpes simplex viruses. Clin Infect Dis. 1998;26(3):541–553. doi: 10.1086/514600. [DOI] [PubMed] [Google Scholar]
- 143.da Silva L. M., Guimaraes A. L., Victoria J. M., Gomes C. C., Gomez R. S. Herpes simplex virus type 1 shedding in the oral cavity of seropositive patients. Oral Dis. 2005;11(1):13–16. doi: 10.1111/j.1601-0825.2004.01058.x. [DOI] [PubMed] [Google Scholar]
- 144.Stalkup J., Yeung-Yue K., Brentjens M. Human herpes viruses. In: Bolognia J., Jorizzo J., Rapini R., editors. Dermatology. New York, NY: Mosby; 2003. pp. 1235–1253. [Google Scholar]
- 145.Anderson B. J. Valacyclovir to expedite the clearance of recurrent herpes gladiatorum. Clin J Sport Med. 2005;15(5):364–366. doi: 10.1097/01.jsm.0000181468.44397.ce. [DOI] [PubMed] [Google Scholar]
- 146.Molino A. C., Fleischer A. B., Jr, Feldman S. R. Patient demographics and utilization of health care services for molluscum contagiosum. Pediatr Dermatol. 2004;21(6):628–632. doi: 10.1111/j.0736-8046.2004.21602.x. [DOI] [PubMed] [Google Scholar]
- 147.Niizeki K., Kano O., Kondo Y. An epidemic study of molluscum contagiosum: relationship to swimming. Dermatologica. 1984;169(4):197–198. doi: 10.1159/000249604. [DOI] [PubMed] [Google Scholar]
- 148.Overfield T. M., Brody J. A. An epidemiologic study of molluscum contagiosum in Anchorage, Alaska. J Pediatr. 1966;69(4):640–642. doi: 10.1016/s0022-3476(66)80053-7. [DOI] [PubMed] [Google Scholar]
- 149.Koning S., Bruijnzeels M. A., van Suijlekom-Smit L. W., van der Wouden J. C. Molluscum contagiosum in Dutch general practice. Br J Gen Pract. 1994;44(386):417–419. [PMC free article] [PubMed] [Google Scholar]
- 150.Relyveld J., Bergink A. H., Nijhuis H. G. J. Epidemiologica notes: leg ulcers, warts and dying circumstances. Huisarts en Wetenschap. 1988;31:266–267. [Google Scholar]
- 151.Fujisawa S., Ishizaka N., Morioka S., Usami Y., Ariga T. Aftercare of medical check-up. Pediatr Dermatol. 1982;5:129. [Google Scholar]
- 152.Nardi G. Molluschi contagiosi e lottatori. Dermosifilograf. 1934;9:110–116. [Google Scholar]
- 153.Keipert J. A. The association of molluscum contagiosum and infantile eczema. Med J Aust. 1971;1(5):267–270. [PubMed] [Google Scholar]
- 154.Beaulieu P., Pepin E., Aboucaya P., et al. Molluscum contagiosum: epidemiological study of 452 cases in private practice [Molluscum contagiosum etude épidémiologique de 452 observations en pratique libérale] Nouv Dermatol. 2000;19(3):231. [Google Scholar]
- 155.Euvrard S., Kanitakis J., Cochat P., Cambazard F., Claudy A. Skin diseases in children with organ transplants. J Am Acad Dermatol. 2001;44(6):932–939. doi: 10.1067/mjd.2001.113465. [DOI] [PubMed] [Google Scholar]
- 156.Gottlieb S. L., Myskowski P. L. Molluscum contagiosum. Int J Dermatol. 1994;33(7):453–461. doi: 10.1111/j.1365-4362.1994.tb02853.x. [DOI] [PubMed] [Google Scholar]
- 157.Husak R., Garbe C., Orfanos C. E. Mollusca contagiosa in HIV infection. Clinical manifestation, relation to immune status and prognostic value in 39 patients [Mollusca contagiosa bei HIV-Infektion Klinische Manifestation, Beziehung zum Immunstatus und prognostische Wertigkeit bei 39 Patienten] Hautarzt. 1997;48(2):103–109. doi: 10.1007/s001050050554. [DOI] [PubMed] [Google Scholar]
- 158.Sturt R. J., Muller H. K., Francis G. D. Molluscum contagiosum in villages of the West Sepik District of New Guinea. Med J Aust. 1971;2(15):751–754. doi: 10.5694/j.1326-5377.1971.tb92526.x. [DOI] [PubMed] [Google Scholar]
- 159.Sterling J. C., Kurtz J. B. Viral infections. In: Champion R. H., Burton J. L., Ebling F. J. G., editors. Rook/Wilkinson/Ebling Textbook of Dermatology. Vol 6. Oxford, United Kingdom: Blackwell; 1998. pp. 1005–1008. [Google Scholar]
- 160.Kress D. W. Nonmelanoma Skin Cancer and Cutaneous Viral Diseases: An Update. Rockville, MD: Elsevier/International Medical News Group and Skin Disease Education Foundation; 2007. Molluscum contagiosum: an evidence-based review; pp. 8–10. [Google Scholar]
- 161.Powell F. C. Sports dermatology. J Eur Acad Dermatol Venerol. 1994;3(1):1–15. [Google Scholar]
- 162.Kluytmans J., van Belkum A., Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997;10(3):505–520. doi: 10.1128/cmr.10.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Centers for Disease Control. Staphylococcal infections in wrestlers: Iowa. MMWR Morb Mortal Wkly Rep. 1962;11:152. [Google Scholar]
- 164.Barton L. L., Friedman A. D. Impetigo: a reassessment of etiology and therapy. Pediatr Dermatol. 1987;4(3):185–188. doi: 10.1111/j.1525-1470.1987.tb00776.x. [DOI] [PubMed] [Google Scholar]
- 165.Stevens D. L., Bisno A. L., Chambers H. F., et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41(10):1373–1406. doi: 10.1086/497143. [DOI] [PubMed] [Google Scholar]
- 166.Barrett F. F., McGehee R. F., Jr, Finland M. Methicillin-resistant Staphylococcus aureus at Boston City Hospital: bacteriologic and epidemiologic observations. N Engl J Med. 1968;279(9):441–448. doi: 10.1056/NEJM196808292790901. [DOI] [PubMed] [Google Scholar]
- 167.Ma X. X., Ito T., Tiensasitorn C., et al. Novel type of staphylococcal cassette chromosome mec identified in community-acquired methicillin-resistant Staphylococcus aureus strains. Antimicrob Agents Chemother. 2002;46(4):1147–1152. doi: 10.1128/AAC.46.4.1147-1152.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Naimi T. S., LeDell K. H., Como-Sabetti K., et al. Comparison of community- and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA. 2003;290(22):2976–2984. doi: 10.1001/jama.290.22.2976. [DOI] [PubMed] [Google Scholar]
- 169.Klevens R. M., Morrison M. A., Nadle J., et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298(15):1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 170.Rihn J. A., Posfay-Barbe K., Harner C. D., et al. Community-acquired methicillin-resistant Staphylococcus aureus outbreak in a local high school football team unsuccessful interventions. Pediatr Infect Dis J. 2005;24(9):841–843. doi: 10.1097/01.inf.0000177287.11971.d4. [DOI] [PubMed] [Google Scholar]
- 171.Centers for Disease Control and Prevention (CDC) Methicillin-resistant Staphylococcus aureus infections among competitive sports participants: Colorado, Indiana, Pennsylvania, and Los Angeles County, 2000–2003. MMWR Morb Mortal Wkly Rep. 2003;52(33):793–795. [PubMed] [Google Scholar]
- 172.Selvey L. A., Whitby M., Johnson B. Nosocomial methicillin-resistant Staphylococcus aureus bacteremia: is it any worse than nosocomial methicillin-sensitive Staphylococcus aureus bacteremia? Infect Control Hosp Epidemiol. 2000;21(10):645–648. doi: 10.1086/501707. [DOI] [PubMed] [Google Scholar]
- 173.Moran G. J., Krishnadasan A., Gorwitz R. J., et al. Methicillin-resistant S aureus infections among patients in the emergency department. N Engl J Med. 2006;355(7):666–674. doi: 10.1056/NEJMoa055356. [DOI] [PubMed] [Google Scholar]
- 174.Purcell K., Fergie J. Epidemic of community-acquired methicillin-resistant Staphylococcus aureus infections: a 14-year study at Driscoll Children's Hospital. Arch Pediatr Adolesc Med. 2005;159(10):980–985. doi: 10.1001/archpedi.159.10.980. [DOI] [PubMed] [Google Scholar]
- 175.Kazakova S. V., Hageman J. C., Matava M., et al. A clone of methicillin-resistant Staphylococcus aureus among professional football players. N Engl J Med. 2005;352(5):468–475. doi: 10.1056/NEJMoa042859. [DOI] [PubMed] [Google Scholar]
- 176.Beam J. W., Buckley B. Community-acquired methicillin-resistant Staphylococcus aureus: prevalence and risk factors. J Athl Train. 2006;41(3):337–340. [PMC free article] [PubMed] [Google Scholar]
- 177.Said-Salim B., Mathema B., Kreiswirth B. N. Community-acquired methicillin-resistant Staphylococcus aureus: an emerging pathogen. Infect Control Hosp Epidemiol. 2003;24(6):451–455. doi: 10.1086/502231. [DOI] [PubMed] [Google Scholar]
- 178.Hidron A. I., Kourbatova E. V., Halvosa J. S., et al. Risk factors for colonization with methicillin-resistant Staphylococcus aureus (MRSA) in patients admitted to an urban hospital: emergence of community-associated MRSA nasal carriage. Clin Infect Dis. 2005;41(2):159–166. doi: 10.1086/430910. [DOI] [PubMed] [Google Scholar]
- 179.Baggett H. C., Hennessy T. W., Leman R., et al. An outbreak of community-onset methicillin-resistant Staphylococcus aureus skin infections in southwestern Alaska. Infect Control Hosp Epidemiol. 2003;24(6):397–402. doi: 10.1086/502221. [DOI] [PubMed] [Google Scholar]
- 180.Laupland K. B., Conly J. M. Treatment of Staphylococcus aureus colonization and prophylaxis for infection with topical intranasal mupirocin: an evidence-based review. Clin Infect Dis. 2003;37(7):933–938. doi: 10.1086/377735. [DOI] [PubMed] [Google Scholar]
- 181.Ellis M. W., Hospenthal D. R., Dooley D. P., Gray P. J., Murray C. K. Natural history of community-acquired methicillin-resistant Staphylococcus aureus colonization and infection in soldiers. Clin Infect Dis. 2004;39(7):971–979. doi: 10.1086/423965. [DOI] [PubMed] [Google Scholar]