Abstract
Context: Mutations in TAC3 and TACR3 (encoding neurokinin B and its receptor) have been identified in Turkish patients with idiopathic hypogonadotropic hypogonadism (IHH), but broader populations have not yet been tested and genotype-phenotype correlations have not been established.
Objective: A broad cohort of normosmic IHH probands was screened for mutations in TAC3/TACR3 to evaluate the prevalence of such mutations and define the genotype/phenotype relationships.
Design and Setting: The study consisted of sequencing of TAC3/TACR3, in vitro functional assays, and neuroendocrine phenotyping conducted in tertiary care centers worldwide.
Patients or Other Participants: 345 probands, 18 family members, and 292 controls were studied.
Intervention: Reproductive phenotypes throughout reproductive life and before and after therapy were examined.
Main Outcome Measure: Rare sequence variants in TAC3/TACR3 were detected.
Results: In TACR3, 19 probands harbored 13 distinct coding sequence rare nucleotide variants [three nonsense mutations, six nonsynonymous, four synonymous (one predicted to affect splicing)]. In TAC3, one homozygous single base pair deletion was identified, resulting in complete loss of the neurokinin B decapeptide. Phenotypic information was available on 16 males and seven females with coding sequence variants in TACR3/TAC3. Of the 16 males, 15 had microphallus; none of the females had spontaneous thelarche. Seven of the 16 males and five of the seven females were assessed after discontinuation of therapy; six of the seven males and four of the five females demonstrated evidence for reversibility of their hypogonadotropism.
Conclusions: Mutations in the neurokinin B pathway are relatively common as causes of hypogonadism. Although the neurokinin B pathway appears essential during early sexual development, its importance in sustaining the integrity of the hypothalamic-pituitary-gonadal axis appears attenuated over time.
While the neurokinin B pathway appears essential during early sexual development, its importance in sustaining the integrity of the hypothalamic-pituitary-gonadal axis appears attenuated over time.
Humans with defects in GnRH secretion and/or action with resulting idiopathic hypogonadotropic hypogonadism (IHH) have been instrumental in defining the genetic control of this hormone. Human, animal, cellular, and bioinformatic models have been integrated to identify and understand the roles of genes affecting GnRH neuronal migration [KAL1 (1), FGFR1 (2), FGF8 (3), NELF (4), PROK2 (5,6,7,8), PROKR2 (6,8)], GnRH secretory activity [KISS1-R (9,10), GNRH1 (11,12,13)], pituitary GnRH responsiveness [GNRHR (14)], and, in some cases, functions yet to be clearly understood [CHD7 (15)]. The newest proteins on this list are neurokinin B (NKB, encoded by TAC3) and its cognate G protein-coupled receptor, NK3-R (encoded by TACR3) (16). NKB is a member of the tachykinin superfamily of neuropeptides that includes substance P and neurokinin A (17). Mutations in the genes encoding this ligand-receptor pair were recently identified in a Turkish population of normosmic IHH (nIHH) patients (16).
The mechanism(s) by which mutations in the NKB pathway cause GnRH deficiency and IHH are not yet clear. However, NKB is expressed in the same neurons that express kisspeptin, a member of the RF amide family of proteins (sharing the common C-terminal sequence Arg-Phe-NH2) (18,19). Although mutations in the gene encoding kisspeptin (KISS1) have yet to be reported in humans with GnRH deficiency, mutations in the gene encoding the kisspeptin receptor (KISS1-R or GPR54) have been identified in nIHH probands as well as an individuals with central precocious puberty (9,10,20,21,22,23). Kisspeptin is a powerful stimulus for GnRH-induced LH secretion, whereas paradoxically, NKB agonists appear to have an excitatory or inhibitory effect on GnRH-induced gonadotropin secretion in rodent models depending on gender and sex steroid milieu (24,25,26,27). This variability in response to NKB seems at odds with the discovery of loss of function mutations in the NKB signaling pathway in hypogonadotropic patients (16).
Because the original mutations in TAC3 and TACR3 were described only in Turkish patients, the goal of this study was to examine the prevalence of TAC3 and TACR3 mutations in a much larger, international cohort of patients. Once putative mutations were identified, their functional consequences were studied in vitro. The phenotypic consequences of mutation-carrying patients were investigated at several developmental windows including neonatal life, adolescence/early adulthood, and late adulthood (after sex steroid therapy) as evaluated by physical examination, gonadotropin pulse pattern, and fertility outcome.
The data from these studies demonstrate that functionally validated rare sequence variants within the tachykinin pathway profoundly impact the functioning of the hypothalamic-pituitary-gonadal (hpg) axis in late gestation. However, the effect of these same mutations appears to attenuate over time because a significant proportion of patients carrying mutations exhibited partial or complete reversal of their hypogonadotropism in adult life. These data thus suggest that patients with genetic mutations in this system may not require lifelong sex steroid replacement and may be capable of spontaneous fertility. Finally, these findings have importance for assembling the hierarchy of the various neuroendocrine and genetic determinants of GnRH secretion in the human during both adolescent puberty and the “mini-puberty” of neonatal life.
Patients and Methods
All study activities fell under research protocols approved by the Massachusetts General Hospital Institutional Review Board.
Patient cohorts
The TAC3 and TACR3 genes were screened in two groups: patients affected by nIHH, and prospectively recruited volunteers determined to have normal reproductive function by history and physical examination (192 Caucasians screened for all coding exons, and 100 Brazilians screened only for five nucleotide variants: G18D, I249V, Y256H, W275X, and R295S). Patients were referred directly to the Reproductive Endocrine Associates of Massachusetts General Hospital or the Developmental Endocrinology Unit of the Clinical Hospital in Sao Paulo, Brazil, for clinical evaluation, or they were referred by their physicians to participate in genetic studies. Whenever possible, patients were interviewed by both a physician and a genetics counselor using an Institutional Review Board-approved questionnaire to obtain a thorough medical and family history.
The diagnosis of nIHH was based on the failure to undergo normal sexual maturation by age 18 yr, low serum sex steroid levels in the setting of inappropriately low or normal gonadotropin levels, and no other abnormality detected on cranial imaging (28).
Olfactory testing employed the University of Pennsylvania identification test whenever possible (29). A score of at least the fifth centile based on sex and age was deemed normal. Of the 345 IHH patients who were screened, 107 were normosmic by olfactory testing, four were hyposmic, and the remaining 234 were normosmic by self-report.
The 345 IHH probands represented a diverse racial mix: 213 were Caucasian (including 37 from Turkey), 25 were Asian, one was American Indian/Alaskan native, 12 were African American, one was mixed native American/Caucasian, and 93 were unknown [of these, 60 of 93 were Brazilian (30)] (Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org).
Of the nIHH probands, 248 were male, 95 were female, and two were unknown. These ratios are consistent with previously published reports demonstrating a male predominance in nIHH (31).
Of the nIHH pedigrees, 89 were familial and 80 were sporadic, with the remaining unknown as to their mode of inheritance. Of the familial cases, 43 could be classified as autosomal dominant, 27 as autosomal recessive, and two as X-linked.
Clinical studies
Of the 345 IHH patients screened, 97 were admitted to the General Clinical Research Center of Massachusetts General Hospital for blood sampling every 10 min for 12–24 h to assess endogenous GnRH-induced LH secretion as previously described (32).
Clinical assays
Blood-sampling studies were performed over a 29-yr period; therefore, two different immunoassay systems were used for the measurement of LH. Originally, LH measurements were made via RIA with a limit of detection of 0.8 IU/liter (33). Later, this assay was switched to an automated microparticle enzyme immunoassay (AxSYM System; Abbott Laboratories, Abbott Park, IL) with a limit of detection of 1.6 IU/liter. The microparticle enzyme immunoassay was calibrated using the same reference preparations as the RIA to make results comparable across data sets. The data regarding the presence or absence of LH pulses are based on the respective limits of detection of each system and integrated by virtue of a common standard in both assays that permitted interconversion.
Mutation analysis
Genomic DNA was extracted from peripheral blood leukocytes. Probands were screened by PCR amplification of exon segments and direct sequencing. The coding region of the TAC3 and TACR3 genes were amplified using PCR. The TAC3 gene (chromosome 12; GenBank accession no. NM 013251) consists of 10 exons. The TACR3 gene (chromosome 4; GenBank accession no. NM 001059) consists of five exons. Primers are outlined in Supplemental Data 1. All amplified products were sequenced using the AmpliTaq Dye Terminator Cycle Sequencing Kit and an ABI PRISM 377 DNA sequencer (Perkin-Elmer Corp., Foster City, CA). All sequence variations were found on both strands and confirmed in a separate PCR. All nucleotide changes were assessed for their presence in the National Center for Biotechnology Information database of single nucleotide polymorphisms, the expressed sequence tags database, and among control alleles.
Of the 345 probands in the cohort, 284 were also screened for mutations in genes known to be involved in IHH, including FGFR1 (n = 332), KISS1-R (n = 336), NELF (n = 274), GNRHR (n = 343), FGF8 (n = 332), GNRH1 (n = 200), KAL1 (n = 334), PROK2 (n = 330), and PROKR2 (n = 330).
Functional studies
African green monkey kidney fibroblast cells (COS-7 cells) were transiently transfected with 0.5 μg of either WT, G18D, I249V, Y256H, R295S, or Y315C NK3-R or empty vector (EV) (pcDNA3.1+) using GenePORTER Transfection Reagent (Gene Therapy Systems, San Diego, CA). Generation of NK3-R mutations and inositol phosphate (IP) assays are described in Supplemental Data 2.
Results
IHH and TACR3
Ethnic and gender distribution
Rare nucleotide variants in TACR3 were identified in 19 of 345 nIHH probands for a total prevalence of 5.5%. When the frequency of variants was examined in different racial subgroups, nucleotide changes were identified in 10 of 213 Caucasian probands (4.7%; four of the 10 probands were Turkish, for a 10.8% frequency in that ethnic subgroup), two of 25 were Asian probands (8%), and one of 12 was an African-American proband (8.3%) (Table S1). In probands where the racial designation was uncertain, one of 11 Hispanic probands (9%), four of 60 Brazilian probands (6.7%) (30), and one additional individual also harbored variants (Supplemental Table 1). Rare nucleotide variants were identified in 15 of 247 males (6.1%) and four of 95 females (4.2%) and thus occurred at approximately equal frequency in both genders.
Variants
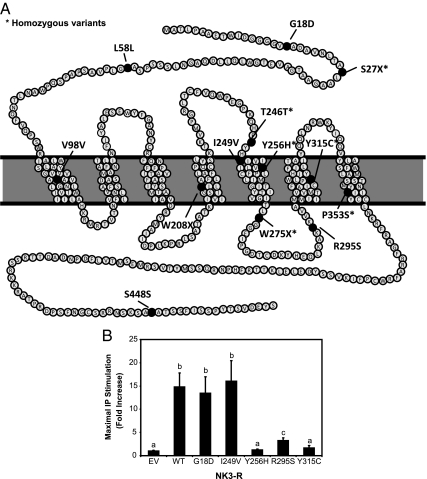
Of the 345 nIHH probands, 19 were found to harbor 13 distinct coding sequence nucleotide variants in TACR3 that were not observed in 292 controls (Table 1; also see Fig. 2). With the exception of P353S, which had previously been reported in the Turkish population (16), all other variants were novel. Three of the 13 variants were nonsense mutations (S27X, W280X, W275X); six of 13 variants were nonsynonymous (i.e. changing the amino acid: G18D, I249V, Y256H, R295S, Y315C, P353S); and the remaining four variants were synonymous (i.e. not changing the amino acid: L58L, V98V, T246T, S448S). However, the homozygous G>A transition underlying T246T occurred in the last base pair of exon 2 and was predicted to affect splicing (NNSPLICE 0.9). Of the nonsense, nonsynonymous, and putative splice site changes (n = 10), six were homozygous [S27X, W275X (present in three probands), Y256H, Y315C, P353S, T246T], and five variants were heterozygous [G18D, W208X, I249V, W275X (present in four probands), R295S].
Table 1.
Probands bearing rare variants in TACR3 and TAC3
| Proband | Change (nucleotide) | Change (amino acid) | Functional consequences | Inheritance | Race | Coding mutation in other genes |
|---|---|---|---|---|---|---|
| TACR3 | ||||||
| Nonsense mutations | ||||||
| 1 | [c. 80 C>A]+[c. 80 C>A] | S27X/S27X | PTC | C | Nonea | |
| 2 | [c. 623 G>A]+[=] | W208X/N | PTC | F | C | Nonea |
| 3 | [c. 824 G>A]+[c. 824 G>A] | W275X/W275X | PTC | S | C | Noneb |
| 4 | [c. 824 G>A]+[c. 824 G>A] | W275X/W275X | PTC | F | C | Nonea |
| 5 | [c. 824 G>A]+[c. 824 G>A] | W275X/W275X | PTC | F | Noned | |
| 6 | [c. 824 G>A]+[=] | W275X/N | PTC | S | C | Nonea |
| 7 | [c. 824 G>A]+[=] | W275X/N | PTC | F | C | Nonea |
| 8 | [c. 824 G>A]+[=] | W275X/N | PTC | S | C | Noneb |
| 9 | [c. 172 C>T]+[=] and [c. 824 G>A]+[=] | L58 L/N and W275X/N | N/A and PTC | F | Noned | |
| Nonsynonymous changes | ||||||
| 10 | [c. 53 G>A]+[=] | G18D/N | = | S | Noned | |
| 11 | [c. 745 A>G]+[=] | I249V/N | = | F | H | Nonec |
| 12 | [c. 766 T>C]+[c. 766 T>C] | Y256H/Y256H | ↓↓↓IP | S | C | Nonea |
| 13 | [c. 885 A>C]+[=] | R295S/N | ↓↓↓IP | F | Noned | |
| 14 | [c. 944 A>G]+[c. 944 A>G] | Y315C/Y315C | ↓↓↓IP | F | Noneb | |
| 15 | [c. 1057 C>T]+[c. 1057 C>T] | P353S/P353S | ↓↓↓Calciume | C | Nonea | |
| Synonymous changes | ||||||
| 16 | [c. 294 G>C]+[=] | V98V/N | N/A | S | AA | Not screened |
| 17 | [c. 738 G>A]+[c.738 G>A] | T246T/T246T | Loss of splice site | S | A | GnRH1: het T58S 2 |
| 18 | [c. 1344 C>T]+[=] | S448S/N | N/A | (Adopted) | A | Noneb |
| 19 | [c. 1344 C>T]+[=] | S448S/N | N/A | C | Nonea | |
| TAC3 | ||||||
| 20 | [c. 60 Gdel]+[c. 60 Gdel] | G20fsX39/G20fsX39 | PTC | F | A | Noneb |
Screened for GnRHR, KISS1-R, FGFR1, FGF8, PROK2, PROKR2, NELF, GnRH1;
screened for GnRHR, KISS1-R, FGFR1, FGF8, PROK2, PROKR2, NELF;
screened for GnRHR, KISS1-R, FGF8;
screened for GnRHR, KISS1-R, FGFR1, FGF8, PROK2, PROKR2, GnRH1. N/A, Not applicable; N, normal allele; PTC, premature termination codon; F, familial; S, sporadic; C, Caucasian; A, Asian; AA, African-American; H, Hispanic; =, IP accumulation not different from WT; ↓↓↓, IP accumulation reduced compared to WT.
Previously published (16).
Figure 2.
A, Schematic of mutations in NK3-R. B, Effects of mutations in TACR3 on NKB-mediated activation of signal transduction. COS-7 cells transfected with wild-type (WT), G18D, I249V, Y256H, R295S, and Y315C NK3-R or EV were treated with NKB (10−7 m) for 1 h. A significant increase in IP accumulation occurred in cells transfected with WT, G18D, or I249V NK3-R. In contrast, there was a marked reduction in NKB-stimulated IP production in cells transfected with Y256H, R295S, or Y315C NK3-R, or with EV. a, b, and c denote significantly different fold increases in IP accumulation.
Of the eight probands bearing homozygous TACR3 variants, three were familial (only one of the three appeared to have an autosomal recessive mode of inheritance), three were sporadic, and two were unknown. Of the 11 probands bearing heterozygous (n = 10) or possible compound heterozygous (n = 1) variants, five were familial (one dominant, two recessive, and two unknown), four were sporadic, and two were unknown.
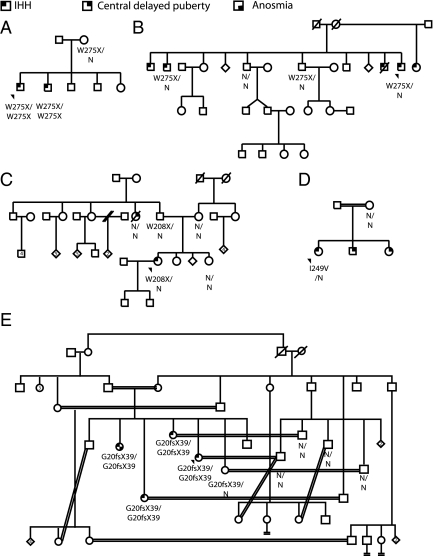
In familial cases, the identified nucleotide variants did not arise de novo but rather were present in at least one parent and all family members who were affected by IHH and whose DNA was available (Fig. 1). This observation suggests that TACR3 does not undergo frequent spontaneous mutagenesis.
Figure 1.
Pedigrees of familial cases of TACR3 and TAC3 mutations. With only one exception, all the affected family members had the same change as their respective probands. A, Proband 4; B, proband 7; C, proband 2; D, proband 11; E, proband 20.
Functional studies
In WT NK3-R transfected cells, NKB caused a 16.7 ± 3.9-fold induction of IP production; in contrast, cells transfected with EV failed to show any IP induction. Y256H, R295S, and Y315C NK-3R variants showed complete loss of function, with IP induction of 1.2 ± 0.2-fold, 3.2 ± 0.6-fold, and 1.7 ± 0.5-fold, respectively, over unstimulated levels. In contrast, cells transfected with the G18D or I249V NK3-R variant had a 13.5 ± 3.5-fold and 16.1 ± 4.4-fold IP induction, respectively, in response to 10−7 m NKB, not significantly different from cells transfected with WT NK3-R (Fig. 2).
Clinical phenotypes
Probands carrying rare variants in TACR3 exhibited a broad range of phenotypes. Of males carrying coding sequence variants in TACR3 and in whom information was available (n = 15 probands and one family member), 15 of 16 or 94% had microphallus [stretched penile length of less than 10.5 cm (34)] as assessed by questionnaire or physical examination at first presentation (in most cases) (Table 2). Surprisingly, despite this high prevalence of microphallus, only two probands (no. 7 and no. 13) had cryptorchidism. Testes size was generally small but did range from 1–12 ml, suggesting that some probands had undergone partial sexual maturation (35) (Table 2). Of females carrying sequence variants in TACR3 and in whom information was available (n = 3), none had spontaneous thelarche or menarche (Table 3).
Table 2.
Phenotypic characterization of male probands and family members harboring rare variants in TACR3 before and after treatment
| Proband | Before treatment
|
Treatment | After treatment
|
Fertility | Evidence for neuroendocrine recovery | ||||
|---|---|---|---|---|---|---|---|---|---|
| Testis size; phallus size | T levels (ng/dl) | No. of pulses/mean LH (q 10 min sampling × 12 h for LH) | Testis size; phallus size | T levels (ng/dl) | No. of pulses/mean LH (q 10 min sampling × 12 h for LH) | ||||
| 1 | − | − | − | T | 0.5 ml; 6 cm | − | − | No | − |
| 3 | ″Small phallus″a | 14 | − | T | 4–5 ml; 9.5 cm | − | − | No | − |
| 4 | 1 ml; 7 cm | − | − | T | − | − | − | No | − |
| 5 | 2 ml; 7 cm | 35 | − | T | 3 ml; 10 cm | − | − | No | − |
| 6 | 2 ml; 5 cm | 16 | 0/1.2 | T | 8 ml; ″increased phallus″ | 38 | 2/3.2 | S | Spontaneous fertility |
| 7 | 2 ml; ″small phallus″ | 57 | 0/0.8 | T, GnRH pump, hCG | 10 ml; ″NL phallus″ | 68 | 8/7.9 | No | ↑LH pulses |
| 8 | 6 ml; 4 cm | <28 | − | T, hCG+FSH | 25 ml | 285 | − | S | Spontaneous fertility |
| 9 | 6 ml; 12.5 cm | 34 | − | T | 8 ml; 12.5 cm | − | − | No | − |
| 10 | 2 ml | <14 | − | − | − | − | − | − | − |
| 12 | 5 ml; ″small phallus″ | 65 | − | T | 12 ml; ″NL phallus″ | 222 | 4/7.5 | Not tried | ↑T |
| 13 | 2 ml; 6 cm | 30 | T | 9 cm | − | − | No | − | |
| 15 | − | − | − | T, hCG | 6 ml; 8 cm | ″Low″ | − | No | No |
| 16 | 12 ml; ″small phallus″a | 16 | − | T | 13 ml; 10 cm | 246 | 5/7.4 | Not tried | ↑T |
| 17 | ″Small phallus″a | − | − | T | 3 ml | − | − | No | − |
| 19 | 1–2 ml; ″small phallus″ | − | − | T | 1–2 ml; 7 cm | − | − | Yes | − |
| Family members | |||||||||
| 4b | − | − | − | − | − | − | − | − | − |
| 7b | 4 ml; ″small phallus″ | 35 | 0/1.7 | T, GnRH | 11 ml; ″NL phallus″ | 505 | 6/9.1 | No | ↑LH pulses |
NL, Normal; −, unknown; S, spontaneous; hCG, human chorionic gonadotropin; ↑, increased.
These assessments were made at birth; all others were made at the first presentation for abnormal pubertal development.
Table 3.
Phenotypic characterization of female probands and family members harboring rare variants in TACR3 and TAC3 before and after treatment
| Proband | Before treatment
|
Treatment | After treatment
|
Fertility | Evidence for neuroendocrine recovery | ||||
|---|---|---|---|---|---|---|---|---|---|
| Breast | Menarche | No. of pulses/mean LH (q 10 min sampling × 12 h for LH) | Breast | Menarche | No. of pulses/mean LH (q 10 min sampling × 12 h for LH) | ||||
| TACR3 | |||||||||
| 2 | No | No | 0/0.8 | E/P | III | Yes | 0/0.9 | No | No |
| 11 | No | No | 0/1.6 | E/P | IV | Yes | − | − | − |
| 14 | − | − | − | E/P | − | Yes | − | − | − |
| 18 | No | No | − | E/Pa | − | Yes | − | − | − |
| TAC3 | |||||||||
| 20 | No | No | − | E/P | Yes | Yes | − | S | Spontaneous fertility, spontaneous vaginal bleeding |
| Family members | |||||||||
| 20b | No | No | − | E/P | Yes | Yes | − | S | Spontaneous fertility |
| 20c | No | No | − | E/P | Yes | Yes | − | No | Spontaneous regular vaginal bleeding |
| 20d | No | No | − | E/P | Yes | Yes | − | No | Vaginal bleeding after progesterone monotherapy |
E/P, Estrogens/progesterone; S, spontaneous; −, unknown.
Also clomiphene, pulsatile GnRH, and gonadotropins.
Probands were also evaluated after treatment was discontinued (most commonly sex steroids). In striking contrast to the signs of severe hypogonadotropism that accompanied their initial presentation (pre treatment), several probands with TACR3 mutations showed evidence for spontaneous activity of their hpg axis post treatment in adulthood. Three males [no. 6 with W275X/N, no. 8 with W275X/N, no. 12 with Y256H/Y256H (functionally validated)] exhibited significant increases in testicular volume while on androgens, suggestive of endogenous gonadotropin stimulation of Sertoli cell complement (Table 2). Notably, no. 6 and no. 8 also achieved fertility in the absence of gonadotropin/GnRH therapy.
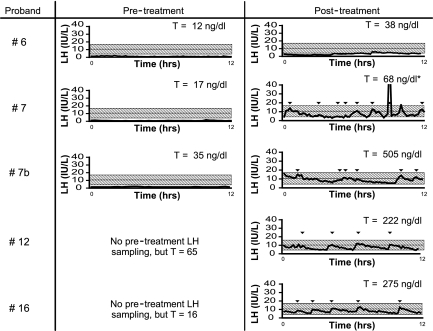
Four male probands underwent blood sampling every 10 min to assess endogenous GnRH-induced LH pulsatility (Table 2 and Fig. 3). Within this group, no. 12 [Y256H/Y256H (functionally validated)] had 5-ml testes and a testosterone (T) level of 65 ng/dl pre treatment. After androgen treatment, he had 12-ml testes, T level of 222 ng/dl, and four LH pulses during 12 h of blood sampling every 10 min. Similarly, patient 16 (V98V/N) had a T level of 16 ng/dl before treatment but 246 ng/dl after treatment, accompanied by five LH pulses in 12 h. Proband 6 (W275X/N) had a complete absence of GnRH-induced LH pulses before starting sex steroid therapy; after treatment, two LH pulses were detectable. Despite this modest change in his neuroendocrine profile, years later, this patient conceived a child in the absence of GnRH or gonadotropin therapy (genetic paternity not confirmed). Proband 7 (W275X/N) also had no LH pulses before androgen therapy but after treatment demonstrated robust LH pulses (although his T levels remained subnormal) (Table 2 and Fig. 3). Notably, one of the brothers of proband 7 (7b) who carried the same W275X mutation demonstrated an active LH pattern on his post treatment sampling study but with normal T levels (Figs. 1B and 3 and Table 2). Another brother with IHH also showed a “normalized” hpg axis after treatment based on hormone levels, but no DNA is available to confirm his TACR3 genotype (Fig. 1B).
Figure 3.
Blood sampling every 10 min regarding reversal probands. Left, Studies before treatment. Right, Studies after discontinuation of treatment. *, T value represents a single blood sample taken within 5 months of the sampling study.
IHH and TAC3
For TAC3, one female proband from a consanguineous family (no. 20) was found to harbor a homozygous frameshift mutation (c.G60del) leading to a premature termination codon in the precursor upstream of the NKB decapeptide (Supplemental Fig. 1). After treatment with sex steroids, this individual conceived a child without fertility medications, but she subsequently suffered an early pregnancy loss. One of her sisters carrying the same homozygous mutation (no. 20b) also conceived spontaneously, and she carried the pregnancy to term (Table 3 and Fig. 1). Sister 20c has not conceived but has regular menstrual cycles (Table 3 and Fig. 1). Sister 20d has had a positive withdrawal bleed to a progesterone challenge but does not cycle spontaneously (Table 3 and Fig. 1).
Discussion
This report focuses on reproductive phenotypes before and after sex steroid therapy in hypogonadotropic patients with rare nucleotide variants in the NKB pathway. Although information regarding the probands and family members comes from several different sources with variable depth of phenotyping, therapies, and intensity of follow-up, two striking biological features emerge. Most males carrying TACR3 variants for whom physical examination evidence was available (94%) had microphallus identified either neonatally or later in life. This frequency stands in sharp contrast to an 8% prevalence of microphallus in an nIHH cohort (n = 42) selected without consideration of genetic etiology (35). Because phallic growth is strongly influenced by the integrity of the in utero activity of the hpg axis in late gestation (36), this consistent presence of microphallus suggests that NKB-stimulated signaling plays an important role as a driver of GnRH secretory activity during this critical window of development. Second, the majority of patients who were assessed longitudinally after discontinuation of sex steroid therapy exhibited evidence of spontaneous partial or complete recovery of their reproductive axis (10 of 12 or 83%), as determined by increases in LH pulsatility, T, testicular volume, the presence of withdrawal bleeding, spontaneous menstrual cycles, and/or spontaneous pregnancy (10 individuals with rare sequence variants: 9 of 10 with termination, frameshift, or functionally validated mutations; and one of 10 with heterozygous synonymous change). Although determining the true incidence of amelioration of IHH is limited by the difficulty of assessing patients off therapy, the high prevalence of reversal reported here stands in sharp distinction to the much lower reported incidence of recovery from GnRH deficiency in a patient cohort bearing a variety of genetic mutations (∼10%) (28). Thus, TAC3/TACR3 genetic defects may portend future prognosis in IHH and foreshadow the possibility of discontinuing sex steroid or gonadotropin therapy later in life. More importantly, perhaps, this striking phenotype strongly suggests that the role of the NKB system in GnRH secretion may be less critical in adult life than during late gestation and the early neonatal period. This finding is in contrast to patients with DAX1 mutations causing adrenal hypoplasia congenita (IHH and adrenal insufficiency), who can have active hpg axes in neonatal life followed by adolescent hypogonadotropism (37). Moreover, the TAC3/TACR3 phenotype appears to be distinct from the phenotypes associated with another secretory product of the same neurons, kisspeptin, and its cognate receptor, KISS1-R, where reversal of IHH has not yet been described. Of course, this latter point must be interpreted with caution because the number of well-studied mutations in the KISS1/KISS1-R system is much smaller at this time (9,10,20,21).
Nonsense mutations in the ligand (TAC3) and receptor (TACR3) provide an important opportunity to examine the most severe dosing of gene impairment, thereby allowing a contrast of phenotypic severity as well as a contrast between the two sexes. For example, two females with a homozygous frameshift mutation in TAC3 conceived spontaneously. Another continued having spontaneous regular menstrual cycles after discontinuation of sex steroid treatment. Because the homozygous TAC3 mutation led to a complete absence of peptide, these patients demonstrate that the ligand, NKB, is dispensable for GnRH synthesis and secretion during the adult period. Because the tachykinin pathway is already well known to be promiscuous, other tachykinins, or even completely different ligands might compensate for their absence of NKB (17).
In parallel to this ligand mutation, four men had homozygous nonsense mutations in TACR3. Although patient 3 (homozygous W275X) was born with a microphallus, no follow-up is available to determine whether his hypogonadal state persisted or showed evidence for recovery later in life. Similarly, little follow-up information is available on the other male homozygous patients: no. 1 (S27X/S27X), no. 4 and no. 5 (W275X/W275X), as well as no. 17 (with the presumed homozygous splice site mutation T246T/T246T). However, proband 12 (Y256H/Y256H; validated loss-of-function in vitro) presented at age 21 with a history of microphallus and a hypogonadal T level (65 ng/dl) but subsequently demonstrated robust LH pulses off therapy with T levels of 222 ng/dl later in adulthood. This observation suggests that patients with markedly abnormal signaling through the NKB receptor can still achieve robust GnRH synthesis and secretion during adulthood.
Using traditional Mendelian principles, patients bearing heterozygous mutations in autosomal recessively inherited genes, such as those encoding NKB and its receptor, would not be predicted to manifest a disease phenotype. However, heterozygous mutations in the gene encoding another G protein-coupled receptor [prokineticin 2 receptor (PROKR2)] have now been reported as contributors to the IHH disease phenotype, either alone or in the presence of heterozygous mutations in other as-yet-to-be-identified genes/pathways (8). Thus, patients with homozygous nonsense mutations in TACR3 might be hypothesized to have more severe phenotypes than those bearing heterozygous mutations. Three probands with homozygous W275X presented with microphallus; however, three of four men bearing heterozygous nonsense mutations also had microphallus, suggesting that despite a smaller number of intact TACR3 alleles, the severity of early androgen deficiency was comparable. Importantly, whereas the vast majority of the patients with mutations in this series were screened for mutations in the other genes currently known to cause IHH, strikingly only one rare heterozygous variant (13) was identified in another gene (proband 17, bearing heterozygous T58S in GnRH1) (Table 1).
Despite the number of mutations identified in the NKB pathway, our knowledge of how this ligand/receptor pair influences GnRH secretion remains incomplete. In rodents and sheep, NKB and its receptor are coexpressed in neurons that express kisspeptin and dynorphin (18,19), raising the possibility of neurokinins acting indirectly to modulate GnRH secretion. Like many other genes involved in the pathogenesis of GnRH deficiency, NKB and its receptor are also expressed in various other organs (uterus and ovary) (38,39), opening the possibility that mutations in this pathway can act at multiple levels of the hpg axis to cause dysfunction. In contrast to patients with nIHH, mice with targeted deletions of Tacr3, at least in the strains tested to date, do not have gross reproductive defects (40), and mice in which the TAC3-equivalent gene Tac2 has been selectively deleted have yet to be reported.
Mutations in the NKB system are relatively common as causes of hypogonadism, occurring in more than 5% of a nIHH population that spans several ethnic groups. Although additional studies will help to illuminate the biology and interactions of the neurokinin pathway, the severe hypogonadotropism observed during early neonatal life in patients with mutations in the ligand/receptor pair juxtaposed against later markers of recovery suggests that NKB and its receptor play disparate roles in different windows of reproductive development.
Supplementary Material
Acknowledgments
We are grateful to the Brazilian contributors, Heraldo Mendes Garmes, Maria Tereza M. Baptista, Gil Guerra, Jr., and Maria Adelaide Albergaria Pereira, who referred patients for molecular analysis.
Footnotes
S.B.S. and J.E.H. received grant support from the National Center for Research Resources (M01-RR-01066, Harvard Clinical and Translational Science Center). The Brazilian work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP Grant 05/04726-0) (to A.C.L.) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq Grant 300209/2008-8) (to A.C.L.). W.A. is supported by the Medical Research Council UK (MRC Program Grant G0900567) and the European Commission (FP7 Collaborative Project EuroDSD). S.B.S., U.B.K., and W.F.C. received grant support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health through a cooperative agreement (U54 5U54HD028138) as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research. J.E.H. received grant support from National Institutes of Health R01-HD-42708.
Disclosure Summary: E.G., C.T., S.D.N., M.G.A., A.A.D., V.A.H., A.P.A., J.C., E.T., L.F.G.S., E.M.F.C., B.B.d.M., M.d.C., A.L., E.B., M.O., R.Q., J.K.A., S.E.S., and T.R.C. have nothing to declare. Grant support received by other authors is acknowledged above.
First Published Online March 23, 2010
For editorial see page 2625
Abbreviations: EV, Empty vector; hpg, hypothalamic-pituitary-gonadal (axis); IHH, idiopathic hypogonadotropic hypogonadism; IP, inositol phosphate; nIHH, normosmic IHH; NKB, neurokinin B; NK3-R, tachykinin receptor 3; T, testosterone.
Harvard Center for Reproductive Sciences and Reproductive Endocrine Unit (E.G., M.G.A., A.A.D., V.A.H., J.E.H., W.F.C., S.B.S.), Department of Medicine, Massachusetts General Hospital, Boston, Massachusetts 02114; Laboratório de Hormônios e Genética Molecular (C.T., E.T., L.F.G.S., E.M.F.C., B.B.d.M., A.C.L.), Serviço de Endocrinologia - Divisão de Clínica Médica I, Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo 05403-900 São Paulo, Brazil; Harvard Center for Reproductive Sciences and Division of Endocrinology, Diabetes, and Hypertension (S.D.N., A.P.A., J.C., U.B.K.), Brigham and Women's Hospital and Harvard Medical School, Boston, Massachusetts 02115; Departamento de Clínica Médica (M.d.C.), Universidade de São Paulo, Faculdade de Medicina de Ribeirão Preto, Monte Alegre 14049900 Ribeirao Preto, Sao Paulo, Brazil; Section of Endocrinology (A.L.), University Hospital of Brasilia and Molecular Pharmacology Laboratory, Faculty of Health Sciences, University of Brasilia, DF 70910-900 Brasilia, Brazil; Department of Endocrinology (E.B., M.O.), Gulhane School of Medicine, Ankara 06018, Turkey; Endocrinology Research Group (R.Q.), Institute for Human Genetics, University of Newcastle-upon-Tyne, Newcastle-upon-Tyne NE1 3BZ, United Kingdom; Division of General Internal Medicine (J.K.A.), University of Washington Medical Center, Seattle, Washington 98195; Clinical Genetics Unit (S.E.S., T.R.C.), Birmingham Women's Hospital National Health Service Foundation Trust, Edgbaston, Birmingham B15 2TG, United Kingdom; and Centre for Endocrinology, Diabetes, and Metabolism (W.A.), School of Clinical and Experimental Medicine, University of Birmingham, Birmingham B15 2TT, United Kingdom
References
- Franco B, Guioli S, Pragliola A, Incerti B, Bardoni B, Tonlorenzi R, Carrozzo R, Maestrini E, Pieretti M, Taillon-Miller P, Brown CJ, Willard HF, Lawrence C, Persico MG, Camerino G, Ballabio A 1991 A gene deleted in Kallmann's syndrome shares homology with neural cell adhesion and axonal path-finding molecules. Nature 353:529–536 [DOI] [PubMed] [Google Scholar]
- Dodé C, Levilliers J, Dupont JM, De Paepe A, Le Dû N, Soussi-Yanicostas N, Coimbra RS, Delmaghani S, Compain-Nouaille S, Baverel F, Pêcheux C, Le Tessier D, Cruaud C, Delpech M, Speleman F, Vermeulen S, Amalfitano A, Bachelot Y, Bouchard P, Cabrol S, Carel JC, Delemarre-van de Waal H, Goulet-Salmon B, Kottler ML, Richard O, Sanchez-Franco F, Saura R, Young J, Petit C, Hardelin JP 2003 Loss-of-function mutations in FGFR1 cause autosomal dominant Kallmann syndrome. Nat Genet 33:463–465 [DOI] [PubMed] [Google Scholar]
- Falardeau J, Chung WC, Beenken A, Raivio T, Plummer L, Sidis Y, Jacobson-Dickman EE, Eliseenkova AV, Ma J, Dwyer A, Quinton R, Na S, Hall JE, Huot C, Alois N, Pearce SH, Cole LW, Hughes V, Mohammadi M, Tsai P, Pitteloud N 2008 Decreased FGF8 signaling causes deficiency of gonadotropin-releasing hormone in humans and mice. J Clin Invest 118:2822–2831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K, Acierno Jr JS, Seminara SB 2004 Characterization of the human nasal embryonic LHRH factor gene, NELF, and a mutation screening among 65 patients with idiopathic hypogonadotropic hypogonadism (IHH). J Hum Genet 49:265–268 [DOI] [PubMed] [Google Scholar]
- Matsumoto S, Yamazaki C, Masumoto KH, Nagano M, Naito M, Soga T, Hiyama H, Matsumoto M, Takasaki J, Kamohara M, Matsuo A, Ishii H, Kobori M, Katoh M, Matsushime H, Furuichi K, Shigeyoshi Y 2006 Abnormal development of the olfactory bulb and reproductive system in mice lacking prokineticin receptor PKR2. Proc Natl Acad Sci USA 103:4140–4145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodé C, Teixeira L, Levilliers J, Fouveaut C, Bouchard P, Kottler ML, Lespinasse J, Lienhardt-Roussie A, Mathieu M, Moerman A, Morgan G, Murat A, Toublanc JE, Wolczynski S, Delpech M, Petit C, Young J, Hardelin JP 2006 Kallmann syndrome: mutations in the genes encoding prokineticin-2 and prokineticin receptor-2. PLoS Genet 2:e175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitteloud N, Zhang C, Pignatelli D, Li JD, Raivio T, Cole LW, Plummer L, Jacobson-Dickman EE, Mellon PL, Zhou QY, Crowley Jr WF 2007 Loss-of-function mutation in the prokineticin 2 gene causes Kallmann syndrome and normosmic idiopathic hypogonadotropic hypogonadism. Proc Natl Acad Sci USA 104:17447–17452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole LW, Sidis Y, Zhang C, Quinton R, Plummer L, Pignatelli D, Hughes VA, Dwyer AA, Raivio T, Hayes FJ, Seminara SB, Huot C, Alos N, Speiser P, Takeshita A, Van Vliet G, Pearce S, Crowley Jr WF, Zhou QY, Pitteloud N 2008 Mutations in prokineticin 2 and prokineticin receptor 2 genes in human gonadotrophin-releasing hormone deficiency: molecular genetics and clinical spectrum. J Clin Endocrinol Metab 93:3551–3559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E 2003 Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA 100:10972–10976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno Jr JS, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley Jr WF, Aparicio SA, Colledge WH 2003 The GPR54 gene as a regulator of puberty. N Engl J Med 349:1614–1627 [DOI] [PubMed] [Google Scholar]
- Cattanach BM, Iddon CA, Charlton HM, Chiappa SA, Fink G 1977 Gonadotrophin-releasing hormone deficiency in a mutant mouse with hypogonadism. Nature 269:338–340 [DOI] [PubMed] [Google Scholar]
- Bouligand J, Ghervan C, Tello JA, Brailly-Tabard S, Salenave S, Chanson P, Lombès M, Millar RP, Guiochon-Mantel A, Young J 2009 Isolated familial hypogonadotropic hypogonadism and a GNRH1 mutation. N Engl J Med 360:2742–2748 [DOI] [PubMed] [Google Scholar]
- Chan YM, de Guillebon A, Lang-Muritano M, Plummer L, Cerrato F, Tsiaras S, Gaspert A, Lavoie HB, Wu CH, Crowley Jr WF, Amory JK, Pitteloud N, Seminara SB 2009 GNRH1 mutations in patients with idiopathic hypogonadotropic hypogonadism. Proc Natl Acad Sci USA 106:11703–11708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Roux N, Young J, Misrahi M, Genet R, Chanson P, Schaison G, Milgrom E 1997 A family with hypogonadotropic hypogonadism and mutations in the gonadotropin-releasing hormone receptor. N Engl J Med 337:1597–1602 [DOI] [PubMed] [Google Scholar]
- Kim HG, Kurth I, Lan F, Meliciani I, Wenzel W, Eom SH, Kang GB, Rosenberger G, Tekin M, Ozata M, Bick DP, Sherins RJ, Walker SL, Shi Y, Gusella JF, Layman LC 2008 Mutations in CHD7, encoding a chromatin-remodeling protein, cause idiopathic hypogonadotropic hypogonadism and Kallmann syndrome. Am J Hum Genet 83:511–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Ozbek MN, Imamoglu S, Akalin NS, Yuksel B, O'Rahilly S, Semple RK 2009 TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat Genet 41:354–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida TA, Rojo J, Nieto PM, Pinto FM, Hernandez M, Martín JD, Candenas ML 2004 Tachykinins and tachykinin receptors: structure and activity relationships. Curr Med Chem 11:2045–2081 [DOI] [PubMed] [Google Scholar]
- Burke MC, Letts PA, Krajewski SJ, Rance NE 2006 Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: morphologic evidence of interrelated function within the arcuate nucleus. J Comp Neurol 498:712–726 [DOI] [PubMed] [Google Scholar]
- Amstalden M, Coolen LM, Hemmerle AM, Billings HJ, Connors JM, Goodman RL, Lehman MN 2010 Neurokinin 3 Receptor Immunoreactivity in the Septal Region, Preoptic Area and Hypothalamus of the Female Sheep: Colocalization in Neurokinin B Cells of the Arcuate Nucleus but not in Gonadotrophin-Releasing Hormone Neurones. J Neuroendocrinol 22:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple RK, Achermann JC, Ellery J, Farooqi IS, Karet FE, Stanhope RG, O'Rahilly S, Aparicio SA 2005 Two novel missense mutations in G protein-coupled receptor 54 in a patient with hypogonadotropic hypogonadism. J Clin Endocrinol Metab 90:1849–1855 [DOI] [PubMed] [Google Scholar]
- Lanfranco F, Gromoll J, von Eckardstein S, Herding EM, Nieschlag E, Simoni M 2005 Role of sequence variations of the GnRH receptor and G protein-coupled receptor 54 gene in male idiopathic hypogonadotropic hypogonadism. Eur J Endocrinol 153:845–852 [DOI] [PubMed] [Google Scholar]
- Tenenbaum-Rakover Y, Commenges-Ducos M, Iovane A, Aumas C, Admoni O, de Roux N 2007 Neuroendocrine phenotype analysis in five patients with isolated hypogonadotropic hypogonadism due to a L102P inactivating mutation of GPR54. J Clin Endocrinol Metab 92:1137–1144 [DOI] [PubMed] [Google Scholar]
- Teles MG, Bianco SD, Brito VN, Trarbach EB, Kuohung W, Xu S, Seminara SB, Mendonca BB, Kaiser UB, Latronico AC 2008 A GPR54-activating mutation in a patient with central precocious puberty. N Engl J Med 358:709–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval-Guzmán T, Rance NE 2004 Central injection of senktide, an NK3 receptor agonist, or neuropeptide Y inhibits LH secretion and induces different patterns of Fos expression in the rat hypothalamus. Brain Res 1026:307–312 [DOI] [PubMed] [Google Scholar]
- Corander MP, Challis BG, Thompson EL, Jovanovic Z, Loraine Tung YC, Rimmington D, Huhtaniemi IT, Murphy KG, Kemal Topaloglu A, Yeo GS, O'Rahilly S, Dhillo WS, Semple RK, Coll AP 2010 The effects of neurokinin B (NKB) upon gonadotrophin release in male rodents. J Neuroendocrinol 22:181–187 [DOI] [PubMed] [Google Scholar]
- Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA 2009 Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci 29:11859–11866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman AS, Navarro VM, Kim J, Clifton D, Steiner RA 2009 Sex differences in the regulation of Kiss1/NKB neurons in juvenile mice: implications for the timing of puberty. Am J Physiol Endocrinol Metab 297: E1212–E1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raivio T, Falardeau J, Dwyer A, Quinton R, Hayes FJ, Hughes VA, Cole LW, Pearce SH, Lee H, Boepple P, Crowley Jr WF, Pitteloud N 2007 Reversal of idiopathic hypogonadotropic hypogonadism. N Engl J Med 357:863–873 [DOI] [PubMed] [Google Scholar]
- Doty RL, Shaman P, Kimmelman CP, Dann MS 1984 University of Pennsylvania Smell Identification Test: a rapid quantitative olfactory function test for the clinic. Laryngoscope 94:176–178 [DOI] [PubMed] [Google Scholar]
- Pimenta JR, Zuccherato LW, Debes AA, Maselli L, Soares RP, Moura-Neto RS, Rocha J, Bydlowski SP, Pena SD 2006 Color and genomic ancestry in Brazilians: a study with forensic microsatellites. Hum Hered 62:190–195 [DOI] [PubMed] [Google Scholar]
- Waldstreicher J, Seminara SB, Jameson JL, Geyer A, Nachtigall LB, Boepple PA, Holmes LB, Crowley Jr WF 1996 The genetic and clinical heterogeneity of gonadotropin-releasing hormone deficiency in the human. J Clin Endocrinol Metab 81:4388–4395 [DOI] [PubMed] [Google Scholar]
- Hayes FJ, McNicholl DJ, Schoenfeld D, Marsh EE, Hall JE 1999 Free α-subunit is superior to luteinizing hormone as a marker of gonadotropin-releasing hormone despite desensitization at fast pulse frequencies. J Clin Endocrinol Metab 84:1028–1036 [DOI] [PubMed] [Google Scholar]
- Filicori M, Butler JP, Crowley Jr WF 1984 Neuroendocrine regulation of the corpus luteum in the human. Evidence for pulsatile progesterone secretion. J Clin Invest 73:1638–1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrich PN, Vasconcelos JS, Damião R, Silva EA 2007 Penile anthropometry in Brazilian children and adolescents. J Pediatr (Rio J) 83:441–446 [DOI] [PubMed] [Google Scholar]
- Pitteloud N, Hayes FJ, Boepple PA, DeCruz S, Seminara SB, MacLaughlin DT, Crowley Jr WF 2002 The role of prior pubertal development, biochemical markers of testicular maturation, and genetics in elucidating the phenotypic heterogeneity of idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab 87:152–160 [DOI] [PubMed] [Google Scholar]
- Herman RA, Jones B, Mann DR, Wallen K 2000 Timing of prenatal androgen exposure: anatomical and endocrine effects on juvenile male and female rhesus monkeys. Horm Behav 38:52–66 [DOI] [PubMed] [Google Scholar]
- Takahashi T, Shoji Y, Shoji Y, Haraguchi N, Takahashi I, Takada G 1997 Active hypothalamic-pituitary-gonadal axis in an infant with X-linked adrenal hypoplasia congenita. J Pediatr 130:485–488 [DOI] [PubMed] [Google Scholar]
- Patak E, Candenas ML, Pennefather JN, Ziccone S, Lilley A, Martín JD, Flores C, Mantecón AG, Story ME, Pinto FM 2003 Tachykinins and tachykinin receptors in human uterus. Br J Pharmacol 139:523–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löffler S, Schulz A, Brylla E, Nieber K, Spanel-Borowski K 2004 Transcripts of neurokinin B and neurokinin 3 receptor in superovulated rat ovaries and increased number of corpora lutea as a non-specific effect of intraperitoneal agonist application. Regul Pept 122:131–137 [DOI] [PubMed] [Google Scholar]
- Siuciak JA, McCarthy SA, Martin AN, Chapin DS, Stock J, Nadeau DM, Kantesaria S, Bryce-Pritt D, McLean S 2007 Disruption of the neurokinin-3 receptor (NK3) in mice leads to cognitive deficits. Psychopharmacology (Berl) 194:185–195 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.