Abstract
Purpose: Vandetanib is a once-daily oral inhibitor of vascular endothelial growth factor receptor-2 and epidermal growth factor receptor tyrosine kinases that also inhibits rearranged during transfection kinase activity. Vandetanib (300 mg/d) has previously demonstrated antitumor activity in patients with advanced hereditary medullary thyroid cancer (MTC). This study investigated the efficacy and safety of 100 mg/d vandetanib in patients with advanced hereditary MTC.
Patients and Methods: Eligible patients with unresectable, measurable, locally advanced, or metastatic hereditary MTC received 100 mg/d vandetanib. Upon disease progression, eligible patients could enter postprogression treatment with 300 mg/d vandetanib until a withdrawal criterion was met. The primary objective was to assess the objective response rate by response evaluation criteria in solid tumors.
Results: The study comprised 19 patients (13 males, six females; mean age 45 yr). Confirmed objective partial responses were observed in three patients, yielding an objective response rate of 16% (95% confidence interval 3.4–39.6). Stable disease lasting 24 wk or longer was reported in a further 10 patients (53%); the disease control rate was therefore 68% (95% confidence interval 43.4–87.4). Serum levels of calcitonin and carcinoembryonic antigen showed a sustained 50% or greater decrease from baseline in 16% (three of 19) and 5% (one of 19) of patients, respectively. Adverse events were predominantly grade 1 or 2 and consistent with previous vandetanib monotherapy studies.
Conclusions: Vandetanib at a once-daily dose of 100 mg has clinically relevant antitumor activity in patients with locally advanced or metastatic hereditary MTC and an overall acceptable safety profile.
Vandetanib at 100 mg/day has clinically relevant antitumor activity and an acceptable safety profile in patients with advanced or metastatic hereditary medullary thyroid cancer.
Medullary thyroid cancer (MTC), a malignancy of the parafollicular C cells of the thyroid, accounts for up to 10% of all thyroid cancer cases (1) but accounts for a disproportionate number of thyroid cancer-related deaths (2). MTC occurs in both sporadic and hereditary settings, the latter accounting for approximately 25% of MTC cases. The hereditary form of MTC occurs as a component of the autosomal, dominantly inherited cancer syndromes multiple endocrine neoplasia (MEN) type 2A, MEN2B, and familial MTC (3) and is the most common cause of death in patients with these syndromes.
The only effective form of therapy is early thyroid resection when the disease is localized, with a 10-yr overall survival estimated at approximately 75–80% (4). However, there are limited treatment options for patients with disseminated disease (1). In this setting, neither systemic chemotherapy nor radiotherapy has been shown to prolong survival (5), underlining the importance of developing new approaches to treatment.
Vandetanib (AstraZeneca, Wilmington, DE) is a once-daily oral agent that selectively targets vascular endothelial growth factor receptor (VEGFR), epidermal growth factor receptor (EGFR) and RET (rearranged during transfection) signaling (6,7). Both RET and VEGFR are rational therapeutic targets in MTC. The protein product of the RET protooncogene is a cell membrane receptor tyrosine kinase that is known to play a key role in MTC pathogenesis (8). Mutations in RET are found in virtually all patients with hereditary MTC and in up to 50% of sporadic cases (9,10). These mutations result in the constitutive activation of the kinase function of RET and, in turn, the activation of a number of downstream kinase pathways that drive the neoplastic transformation of parafollicular C cells in the thyroid. Agents that inhibit RET kinase activity have been shown to decrease the proliferation of MTC cell lines (11). Increased expression of vascular endothelial growth factor, a potent proangiogenic factor, is characteristic of thyroid tumors (including MTC) and is associated with increased tumor growth and invasiveness (12). In addition, EGFR-dependent signaling may have a potential role in thyroid cancer (13,14) and as such represents an additional potential therapeutic target in MTC.
Phase I studies conducted in advanced solid tumors demonstrated that vandetanib monotherapy was well tolerated at doses up to 300 mg/d and that the pharmacokinetic profile supported once-daily dosing (15,16). A phase II open-label study (study code 6474IL0008) demonstrated that vandetanib 300 mg/d (the maximum tolerated dose) has clinical antitumor activity and a manageable adverse event profile in patients with advanced hereditary MTC (17). In the present study (study code D4200C00068), the clinical activity of vandetanib 100 mg/d was evaluated in patients with advanced hereditary MTC.
Patients and Methods
Patients
Eligible patients had histologically confirmed, unresectable, measurable, locally advanced, or metastatic hereditary MTC with a confirmed diagnosis of MEN2A, MEN2B, or familial MTC by either germline RET mutation or family history. All patients had a World Health Organization performance status of 0–2; life expectancy 12 wk or longer; age 18 yr or older; and adequate cardiac, hematopoietic, hepatic, and renal function. Brain metastases were permitted if treated at least 4 wk before entry and clinically stable without steroid treatment for 1 wk. Patients who had received chemotherapy, radiotherapy, or major surgery less than 4 wk before the start of study therapy were excluded. The trial was approved by all relevant institutional ethical committees or review bodies and was conducted in accordance with the Declaration of Helsinki, good clinical practice, and the AstraZeneca policy on bioethics. Each patient provided written informed consent.
Study design and treatment
In this open-label, single-arm study, patients received once-daily oral doses of vandetanib 100 mg until disease progression, unacceptable toxicity, or withdrawal of consent. The primary objective of this study was to assess the objective response rate with vandetanib 100 mg/d monotherapy according to modified response evaluation criteria in solid tumors (RECIST) (18). Additional assessments included progression-free survival, disease control rate (percentage of subjects who have a best response of complete response, partial response or stable disease ≥24 wk), safety and tolerability, and biochemical response, as determined by the effect of vandetanib 100 mg/d on serum levels of calcitonin (CTN) and carcinoembryonic antigen (CEA).
Upon disease progression, all patients that the investigator believed may have been obtaining benefit from therapy could enter postprogression treatment with vandetanib 300 mg/d until objective disease progression occurred at this dose, or until another withdrawal criterion was met.
Assessments
Objective tumor assessments were conducted using modified RECIST and based on site-reviewed computed tomography or magnetic resonance imaging scans obtained at baseline and every 12 wk (±2 wk), while the patient was receiving vandetanib 100 mg/d treatment. Patients who entered postprogression treatment with vandetanib 300 mg/d had RECIST assessments performed every 24 wk (±2 wk), with the scan from the 100 mg discontinuation visit serving as a new baseline assessment for postprogression treatment. An objective complete or partial response was confirmed by repeat imaging at least 4 wk after the date of first response. If new hypointense or hypodense lesions appeared during one of the first two scheduled follow-up RECIST assessments, the baseline scans were reexamined. Isodense or isointense lesions identified in the same location during retrospective review of the baseline scans were recorded as nontarget lesions at baseline and followed up for progression. These lesions were recorded as new lesions if no isodense or isointense lesions were identified during retrospective review of the baseline scans.
Adverse events were assessed using National Cancer Institute's Common Terminology Criteria for Adverse Events (CTCAE; version 3). Dose adjustments were made as defined in the protocol for CTCAE grade 3 or 4 toxicity. Twelve-lead electrocardiograms were performed during screening, at wk 1, 2, 4, and 8 and then every 12 wk thereafter. Assessment of the corrected QT (QTc) interval using Bazett's correction was evaluated at each site and by central review, with prolongation defined as a single measurement of 550 msec or greater or an increase of 100 msec or greater from baseline; or two consecutive measurements (within 48 h of each other) that were 500 msec or greater but less than 550 msec or an increase of 60 msec or greater but less than 100 msec from baseline to a value 480 msec or greater.
Blood samples for CTN and CEA analysis were collected at baseline and throughout the study. Determination of serum CTN and CEA levels was performed at a central laboratory. Serum CTN levels were determined by an immunochemiluminescent method (Quest Diagnostics Nichols Institute, San Juan Capistrano, CA); serum CEA measurements were performed using a commercially available microparticle enzyme immunoassay (Abbott Diagnostics, Abbott Park, IL). For CTN and CEA analysis, a patient's best response was defined as follows: complete response, complete normalization of serum levels after treatment and confirmed with a repeat assessment; partial response, 50% or greater decrease from baseline levels maintained over a minimum of 4 wk; stable disease, between +50% and −50% change from baseline levels maintained for at least 4 wk; progressive disease, 50% or greater increase from baseline, maintained for at least 4 wk.
Statistical considerations
There was a target sample size of 15 evaluable patients in this study. This was selected on the basis that if no response was observed in 15 patients, the probability that the true objective response rate was 20% or greater would be less than 0.05. The primary assessment of objective response rate with associated 95% confidence intervals (CIs) was based on the 100-mg component of the study. All patients who received one dose or more of vandetanib were included in the safety and efficacy analyses. Estimates of progression-free survival (time from first dose of study drug to progression or death by any cause within 3 months of last tumor assessment) with associated 95% CIs were obtained using the Kaplan-Meier method. Median duration of response (interval between first confirmed objective response and evidence of progressive disease) and disease control rate (patients who had a best response of complete response or partial response or stable disease 24 wk or longer) were summarized with associated 95% CIs.
Results
Patients
Nineteen patients recruited between August 2006 and May 2007 received initial treatment with vandetanib 100 mg/d. Patient demographics and characteristics are summarized in Table 1. All patients had undergone prior thyroidectomy. Eighteen patients had metastatic disease and one patient had locally advanced disease to the lymph nodes. At the time of data cutoff (January 31, 2008), 11 patients were continuing to receive vandetanib 100 mg/d. The remaining patients had discontinued initial treatment because of withdrawal of consent (n = 1), adverse events (n = 3), or disease progression (n = 4). One patient received only one dose of vandetanib 100 mg owing to voluntary discontinuation from the study and was not evaluable for tumor response. All four patients with disease progression entered postprogression treatment with vandetanib 300 mg/d, and three of these patients were receiving vandetanib 300 mg/d at data cutoff. As of January 2010, 12 patients were still receiving vandetanib, including five at the increased dose of 300 mg/d.
Table 1.
Demographic and baseline characteristics of patients
| Characteristics | n |
|---|---|
| Number of patients | 19 |
| Male | 13 |
| Female | 6 |
| Mean age (yr) (range) | 45 (22–79) |
| Mean time since diagnosis (yr) (range) | 13 (5–33) |
| Disease stage | |
| IVa (%) | 1 (5) |
| IVc (%) | 18 (95) |
| Metastatic sites | |
| Adrenal | 1 |
| Bone | 8 |
| Gastrointestinal | 1 |
| Hepatic | 16 |
| Lymph nodes | 8 |
| Neck | 4 |
| Respiratory | 8 |
| Other | 1 |
| World Health Organization performance status | |
| 0 (normal activity) | 16 |
| 1 (restricted activity) | 1 |
| 2 (in bed ≤50% of the time) | 2 |
| Associated endocrinopathies | 17 (90) |
| MEN2A (%) | 1 (5) |
| MEN2B (%) | 1 (5) |
| Familial MTC (%) | |
| Codon location of RET germline mutation (%) | |
| 380 | 1 (5) |
| 608 | 1 (5) |
| 618 | 2 (11) |
| 620 | 2 (11) |
| 634 | 9 (47) |
| 768 | 1 (5) |
| 918 | 1 (5) |
| Unknown | 2 (11) |
Efficacy
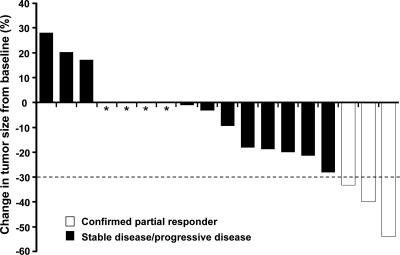
Assessment of RECIST best response is summarized in Table 2. According to investigator assessments, three patients (16%) met the criteria for having an objective partial response, defined as a 30% or greater decrease in the sum of longest diameter measurements by RECIST. The median duration of response (from onset of response) at the time of data cutoff was 168 d. Stable disease 24 wk or longer was reported in 10 patients (53%); the disease control rate (complete response + partial response + stable disease 24 wk or greater) was therefore 68% (13 of 19 patients). Three patients had progressive disease as a best response. Figure 1 shows the maximum reduction (or smallest increase) from baseline in the sum of the longest diameters of target lesions for each evaluable patient. Eleven patients (58%) experienced some tumor shrinkage during vandetanib 100 mg/d treatment.
Table 2.
Tumor assessment (RECIST best response)
| Best RECIST response | All patients (n = 19) |
|---|---|
| Assessment of best response | |
| Complete response (%) | 0 |
| Confirmed partial response (%) | 3 (16) |
| Stable disease 24 wk or longer (%) | 10 (53) |
| Stable disease 8 wk or longer, less than 24 wk (%) | 2 (11) |
| Progressive disease (%) | 3 (16) |
| Not evaluable (%) | 1 (5) |
| Objective response rate (CR + PR) (%) (95% CI) | 16 (3.4–39.6) |
| Disease control rate (CR + PR + SD ≥ 24 wk) (%) (95% CI) | 68 (43.4–87.4) |
CI, Confidence interval; CR, complete response; PR, partial response; SD, stable disease.
Figure 1.
Maximum reduction from baseline (or smallest increase from baseline for patients with no reductions) in target lesion size. Baseline radiographic measurements of target lesions were compared with measurements over the course of the study to determine the best change in target lesion size for each patient with data; one nonevaluable patient is not shown. Each bar represents one patient. The dashed line represents the threshold for partial response. *, Four patients with a best response of 0% change from baseline (all stable disease ≥24 wk).
At the time of data cutoff, five patients (26%) had progressed (RECIST defined progression, n = 4; death within 3 months of last RECIST assessment, n = 1) and 14 patients had no progression and were alive at the time of analysis; the estimated median progression-free survival could not be determined due to the insufficient number of progression events during follow-up.
Of the four patients who received vandetanib 300 mg/d during the postprogression period, one patient had a documented RECIST progression and completed the study. The remaining three patients had not experienced RECIST progression in the postprogression period at data cutoff and continued to receive vandetanib 300 mg/d. None of the four patients had experienced an objective response during the postprogression period.
There were no symptomatic diarrhea responses among the patients in the study who were evaluable for symptomatic response (abnormal stool at baseline).
Analysis of serum tumor markers and RET germline mutation status
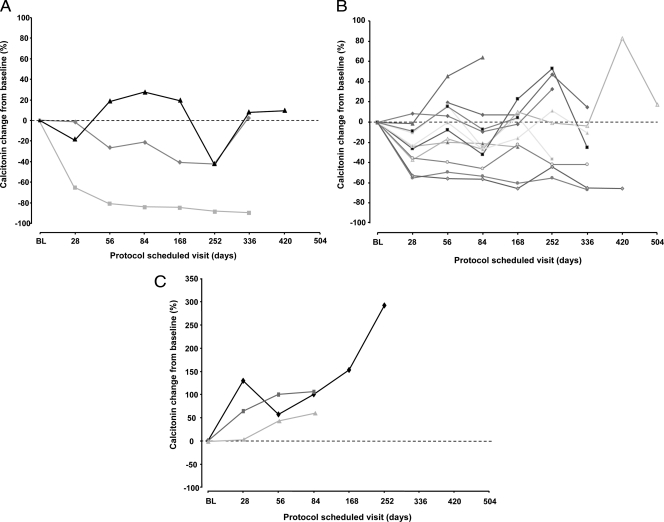
Decreases from baseline in serum levels of CTN and CEA met the criteria for a biochemical partial response in three patients (16%) and one patient (5%), respectively. A comparison of RECIST best response with best CTN and CEA responses is shown in Table 3. Although only one of the patients with a CTN partial response also had a confirmed objective partial response by RECIST (the other two patients had stable disease), all three patients with a confirmed partial response experienced a greater than 42% reduction from baseline in serum CTN. The patient with a CEA partial response also met the criteria for CTN partial response but not objective response (best RECIST response of stable disease ≥8 wk, <24 wk). A marked upward trend in both serum CTN and CEA levels was noted in the three patients with a best objective response of progressive disease. Changes in serum CTN and CEA levels over time for individual patients, grouped according to best RECIST response, are shown in Fig. 2 and Supplemental Fig. S1, published as supplemental data on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org, respectively. There was no apparent association between RET germline mutation and clinical outcome in this study (Table 3).
Table 3.
Comparison of RECIST best response with best CTN/CEA response and RET germline mutation status
| Patienta | Best tumor response (largest percent change in tumor size) | Best CTN response (percent change from baseline)b | Best CEA response (percent change from baseline)b | RET germline mutationc |
|---|---|---|---|---|
| 1 | Confirmed PR (−54.0) | SD (−42.3) | SD (−16.4) | C380R |
| 2 | Confirmed PR (−40.0) | PR (−89.4) | SD (−83.7) | E768D |
| 3 | Confirmed PR (−33.3) | SD (−43.0) | SD (+4.4) | C634R |
| 4 | SD ≥ 24 wk (−28.1) | PR (−66.8) | SD (−28.0) | C634R |
| 5 | SD ≥ 24 wk (−21.4) | SD (−36.8) | SD (−24.6) | C620R |
| 6 | SD ≥ 24 wk (−20.0) | SD (−24.6) | SD (−32.4) | C634L |
| 7 | SD ≥ 24 wk (−18.8) | SD (+7.1) | PD (+72.0) | C618S |
| 8 | SD ≥ 24 wk (−9.5) | SD (−9.7) | SD (−9.63) | NE |
| 9 | SD ≥ 24 wk (−1) | SD (−37.0) | SD (+4.7) | C620R |
| 10 | SD ≥ 24 wk (0) | SD (−31.8) | SD (−6.9) | M918T |
| 11 | SD ≥ 24 wk (0) | SD (−45.8) | SD (−25.1) | C634R |
| 12 | SD ≥ 24 wk (0) | SD (−27.8) | SD (−10.4) | C634Y |
| 13 | SD ≥ 24 wk (0) | PR (−65.4) | PR (−62.1) | C634R |
| 14 | SD ≥ 8 wk, <24 wk (−18.2) | SD (−15.7) | SD (−37.8) | C608S |
| 15 | SD ≥ 8 wk, <24 wk (−3.2) | SD (−1.4) | SD (−32.9) | NE |
| 16 | PD (+17.2) | SD (+3.2) | SD (+26.2) | C618S |
| 17 | PD (+20.3) | PD (+57.0) | SD (+44.2) | C634R |
| 18 | PD (+28.1) | PD (+64.0) | SD (+37.6) | C634R |
NE, Not evaluable; PR, partial response; SD, stable disease.
One patient not evaluable for objective response (RECIST).
CTN and CEA response criteria: PR, 50% or greater decrease from baseline levels maintained over a minimum of 4 wk; SD, between +50% and −50% change from baseline levels maintained over a minimum of 4 wk.
Codon and amino acid substitution.
Figure 2.
Serum CTN levels [percent change from baseline (BL)] in patients with a best objective response (RECIST) of partial response (n = 3) (A), stable disease (n = 12) (B), and progressive disease (n = 3) (C).
Safety and tolerability
The mean duration of vandetanib 100 mg treatment was 262 d (range 1–501); the duration of therapy for the 300 mg (postprogression) treatment was 80 d (range 14–177). Vandetanib 100 mg/d dosing was interrupted and then reduced in two patients because of adverse events. One patient had a dose interruption for asymptomatic QTc prolongation (>500 and <500 msec on two consecutive occasions), which occurred on d 173 of vandetanib 100 mg/d treatment. QTc prolongation resolved 4 d after dose interruption, and the patient was restarted at a lower dose of vandetanib 100 mg every other day. The remaining patient had a dose interruption and subsequent reduction (vandetanib 100 mg every other day) due to the development of diabetes insipidus. This patient, who had a history of hypertension, subsequently discontinued vandetanib treatment due to renal insufficiency, which was considered by the investigator to be vandetanib related. Two other patients discontinued vandetanib 100 mg/d treatment due to an adverse event. One patient experienced muscle weakness that led to the discontinuation of vandetanib and that was considered by the investigator to be vandetanib related. The remaining patient died due to aspiration pneumonia, which was considered to be unrelated to vandetanib.
The majority of adverse events for patients receiving vandetanib 100 mg were reported as CTCAE grade 1 or 2. Table 4 summarizes the most common adverse events, which included diarrhea (nine patients, 47%), fatigue (eight patients, 42%), rash (five patients, 26%), and constipation (four patients, 21%). Six patients (26%) experienced seven CTCAE grade 3 adverse events [muscular weakness, hypertension, myalgia, phaeochromocytoma, asymptomatic QTc prolongation, diplopia, visual disturbance each (n = 1)], and there was one CTCAE grade 4 adverse event (diabetes insipidus). These events were generally manageable with symptomatic treatment, dose interruption, or reduction.
Table 4.
Adverse events, irrespective of causality, reported in three or more patientsa
| Adverse event | Total (n = 19 patients) |
|---|---|
| Diarrhea, n (%) | 9 (47) |
| Fatigue, n (%) | 8 (42) |
| Rash, n (%) | 5 (26) |
| Constipation, n (%) | 4 (21) |
| Anorexia, n (%) | 3 (16) |
| Back pain, n (%) | 3 (16) |
| Nausea, n (%) | 3 (16) |
| Photosensitivity reaction, n (%) | 3 (16) |
All of these events were CTCAE grade 1 or 2.
Elevations in TSH and hypothyroidism have been previously noted in patients receiving tyrosine kinase inhibitors, and these patients require thyroid hormone replacement (19,20). All 19 patients receiving vandetanib in this study had undergone prior thyroidectomy and were receiving thyroid hormone replacement before entering study. Baseline TSH data were available for 17 patients, and in these patients an increase in TSH levels was observed, reaching a maximum by d 84 after the start of vandetanib treatment [5.1-fold (mean) and 7.3-fold (median) increases over baseline]. No patients were reported to have symptomatic hypothyroidism, but thyroid hormone replacement therapy was increased in two patients.
Discussion
There is a lack of effective therapy for patients with metastatic MTC and new treatment options are urgently needed. Targeted therapy may represent a new therapeutic approach for advanced MTC, and several early-phase clinical studies have investigated the utility of targeted agents in thyroid cancer (21,22,23,24). The current study demonstrates that vandetanib 100 mg/d has antitumor activity in patients with locally advanced or metastatic hereditary MTC. Durable confirmed objective partial responses were observed in three of 19 patients (16%), and a further 10 patients experienced long-term stable disease. In an open-label phase II study of vandetanib 300 mg/d in advanced MTC patients, 20% of patients (six of 30) experienced a confirmed partial response and a further 53% (16 of 30) experienced long-term stable disease (17). Taken together, these two studies demonstrate that both the 100 and 300 mg/d dose levels of vandetanib have antitumor activity in this setting. These studies were open-label, single-arm trials, and no direct comparison of the efficacy at each dose level has been conducted. Therefore, no conclusions can be made with regard to the relative efficacy of the 100 and 300 mg/d doses in MTC. The 300 mg/d dose has been considered appropriate for evaluation in a further phase III study, and the results from the current study demonstrate that antitumor activity may still be observed in patients requiring dose reductions to 100 mg/d.
Preliminary results suggest that multikinase inhibitors that share the ability to inhibit RET and VEGFR kinases have efficacy in MTC (21,25,26). However, the relative contribution of RET and/or VEGFR inhibition (as well as activity vs. other targets) is currently unknown and merits further investigation. Specifically, vandetanib is a selective inhibitor of RET, VEGFR, and EGFR and activity vs. each of these targets could contribute to the antitumor effects of this agent observed in patients with advanced MTC (11,12,13,14).
Serum CTN and CEA levels are routinely used as an indicator of tumor progression in MTC because they are believed to be a useful indicator of tumor burden (2,27). A clear association between serum CTN and CEA marker response and objective response based on RECIST criteria was difficult to define in this study.
Vandetanib 100 mg/d was well tolerated in the majority of patients in this study. Two patients discontinued the study due to a vandetanib-related adverse event, and vandetanib 100 mg dosing was interrupted and then reduced in two patients because of adverse events. The duration of treatment for the 11 patients remaining on vandetanib 100 mg at data cutoff ranged from 212 to 501 d, with three patients receiving more than a year of treatment. Most adverse events were CTCAE grade 1 or 2 and were manageable. Diarrhea, fatigue, and rash were the most common adverse events reported in this study, and these are consistent with previous vandetanib monotherapy studies (28,29).
In conclusion, vandetanib 100 mg/d has clinical antitumor activity in patients with advanced or metastatic hereditary MTC. An international, randomized, placebo-controlled phase III trial (ZETA; 6474IL0058) of vandetanib 300 mg/d in patients with locally advanced or metastatic MTC (hereditary or sporadic) has completed recruitment and is being analyzed.
Supplementary Material
Acknowledgments
This study, including editorial assistance provided by Chris Watson (Mudskipper Bioscience), was supported financially by AstraZeneca.
Footnotes
Disclosure Summary: B.G.R. has consulted for, has served on an advisory board for, and has received honoraria from AstraZeneca; L.P.-A. has consulted for Roche, Pfizer, and Eli Lilly; A.K. and J.V. are employees of and have stock options in AstraZeneca; R.H. has nothing to declare.
First Published Online April 6, 2010
For editorial see page 2621
Abbreviations: CEA, Carcinoembryonic antigen; CI, confidence interval; CTCAE, Common Terminology Criteria for Adverse Events; CTN, calcitonin; EGFR, epidermal growth factor receptor; MEN, multiple endocrine neoplasia; MTC, medullary thyroid cancer; QTc, corrected QT; RECIST, response evaluation criteria in solid tumors; RET, rearranged during transfection; VEGFR, vascular endothelial growth factor receptor.
References
- Schlumberger M, Carlomagno F, Baudin E, Bidart JM, Santoro M 2008 New therapeutic approaches to treat medullary thyroid carcinoma. Nat Clin Pract Endocrinol Metab 4:22–32 [DOI] [PubMed] [Google Scholar]
- Ball DW 2007 Medullary thyroid cancer: therapeutic targets and molecular markers. Curr Opin Oncol 19:18–23 [DOI] [PubMed] [Google Scholar]
- Marx SJ 2005 Molecular genetics of multiple endocrine neoplasia types 1 and 2. Nat Rev Cancer 5:367–375 [DOI] [PubMed] [Google Scholar]
- Roman S, Lin R, Sosa JA 2006 Prognosis of medullary thyroid carcinoma: demographic, clinical, and pathologic predictors of survival in 1252 cases. Cancer 107:2134–2142 [DOI] [PubMed] [Google Scholar]
- Orlandi F, Caraci P, Mussa A, Saggiorato E, Pancani G, Angeli A 2001 Treatment of medullary thyroid carcinoma: an update. Endocr Relat Cancer 8:135–147 [DOI] [PubMed] [Google Scholar]
- Carlomagno F, Vitagliano D, Guida T, Ciardiello F, Tortora G, Vecchio G, Ryan AJ, Fontanini G, Fusco A, Santoro M 2002 ZD6474, an orally available inhibitor of KDR tyrosine kinase activity, efficiently blocks oncogenic RET kinases. Cancer Res 62:7284–7290 [PubMed] [Google Scholar]
- Wedge SR, Ogilvie DJ, Dukes M, Kendrew J, Chester R, Jackson JA, Boffey SJ, Valentine PJ, Curwen JO, Musgrove HL, Graham GA, Hughes GD, Thomas AP, Stokes ES, Curry B, Richmond GH, Wadsworth PF, Bigley AL, Hennequin LF 2002 ZD6474 inhibits vascular endothelial growth factor signaling, angiogenesis, and tumor growth following oral administration. Cancer Res 62: 4645–4655 [PubMed] [Google Scholar]
- Santoro M, Carlomagno F 2006 Drug insight: small molecule inhibitors of protein kinases in the treatment of thyroid cancer. Nat Clin Pract Endocrinol Metab 2:42–52 [DOI] [PubMed] [Google Scholar]
- Marsh DJ, Mulligan LM, Eng C 1997 RET proto-oncogene mutations in multiple endocrine neoplasia type 2 and medullary thyroid carcinoma. Horm Res 47:168–178 [DOI] [PubMed] [Google Scholar]
- Santoro M, Melillo RM, Carlomagno F, Fusco A, Vecchio G 2002 Molecular mechanisms of RET activation in human cancer. Ann NY Acad Sci 963:116–121 [DOI] [PubMed] [Google Scholar]
- Cohen MS, Hussain HB, Moley JF 2002 Inhibition of medullary thyroid carcinoma cell proliferation and RET phosphorylation by tyrosine kinase inhibitors. Surgery 132:960–966; discussion 966–967 [DOI] [PubMed] [Google Scholar]
- Bunone G, Vigneri P, Mariani L, Butó S, Collini P, Pilotti S, Pierotti MA, Bongarzone I 1999 Expression of angiogenesis stimulators and inhibitors in human thyroid tumors and correlation with clinical pathological features. Am J Pathol 155:1967–1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Johansson HE, Bergholm UI, Westermark KM, Grimelius LE 1997 Expression of c-Myc, TGF-α and EGF-receptor in sporadic medullary thyroid carcinoma. Acta Oncol 36:407–411 [DOI] [PubMed] [Google Scholar]
- Mitsiades CS, Kotoula V, Poulaki V, Sozopoulos E, Negri J, Charalambous E, Fanourakis G, Voutsinas G, Tseleni-Balafouta S, Mitsiades N 2006 Epidermal growth factor receptor as a therapeutic target in human thyroid carcinoma: mutational and functional analysis. J Clin Endocrinol Metab 91:3662–3666 [DOI] [PubMed] [Google Scholar]
- Tamura T, Minami H, Yamada Y, Yamamoto N, Shimoyama T, Murakami H, Horiike A, Fujisaka Y, Shinkai S, Tahara M, Kawada K, Ebi H, Sasaki Y, Jiang H, Saijo N 2006 A Phase I dose-escalation study of ZD6474 in Japanese patients with solid, malignant tumors. J Thorac Oncol 1:1002–1009 [PubMed] [Google Scholar]
- Holden SN, Eckhardt SG, Basser R, de Boer R, Rischin D, Green M, Rosenthal MA, Wheeler C, Barge A, Hurwitz HI 2005 Clinical evaluation of ZD6474, an orally active inhibitor of VEGF and EGF receptor signaling, in patients with solid, malignant tumors. Ann Oncol 16:1391–1397 [DOI] [PubMed] [Google Scholar]
- Wells Jr SA, Gosnell JE, Gagel RF, Moley J, Pfister D, Sosa JA, Skinner M, Krebs A, Vasselli J, Schlumberger M 2010 Vandetanib for the treatment of patients with locally advanced or metastatic hereditary medullary thyroid cancer. J Clin Oncol 28:767–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG 2000 New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216 [DOI] [PubMed] [Google Scholar]
- de Groot JW, Zonnenberg BA, Plukker JT, van Der Graaf WT, Links TP 2005 Imatinib induces hypothyroidism in patients receiving levothyroxine. Clin Pharmacol Ther 78:433–438 [DOI] [PubMed] [Google Scholar]
- Desai J, Yassa L, Marqusee E, George S, Frates MC, Chen MH, Morgan JA, Dychter SS, Larsen PR, Demetri GD, Alexander EK 2006 Hypothyroidism after sunitinib treatment for patients with gastrointestinal stromal tumors. Ann Intern Med 145:660–664 [DOI] [PubMed] [Google Scholar]
- Schlumberger MJ, Elisei R, Bastholt L, Wirth LJ, Martins RG, Locati LD, Jarzab B, Pacini F, Daumerie C, Droz JP, Eschenberg MJ, Sun YN, Juan T, Stepan DE, Sherman SI 2009 Phase II study of safety and efficacy of motesanib in patients with progressive or symptomatic, advanced or metastatic medullary thyroid cancer. J Clin Oncol 27:3794–3801 [DOI] [PubMed] [Google Scholar]
- Sherman SI, Wirth LJ, Droz JP, Hofmann M, Bastholt L, Martins RG, Licitra L, Eschenberg MJ, Sun YN, Juan T, Stepan DE, Schlumberger MJ 2008 Motesanib diphosphate in progressive differentiated thyroid cancer. N Engl J Med 359:31–42 [DOI] [PubMed] [Google Scholar]
- Cohen EE, Rosen LS, Vokes EE, Kies MS, Forastiere AA, Worden FP, Kane MA, Sherman E, Kim S, Bycott P, Tortorici M, Shalinsky DR, Liau KF, Cohen RB 2008 Axitinib is an active treatment for all histologic subtypes of advanced thyroid cancer: results from a Phase II study. J Clin Oncol 26:4708–4713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta-Abramson V, Troxel AB, Nellore A, Puttaswamy K, Redlinger M, Ransone K, Mandel SJ, Flaherty KT, Loevner LA, O'Dwyer PJ, Brose MS 2008 Phase II trial of sorafenib in advanced thyroid cancer. J Clin Oncol 26:4714–4719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober F, Hermann M, Handler A, Krotia G 2007 Effect of sorafenib in symptomatic metastatic medullary thyroid cancer. J Clin Oncol 25:617S [Google Scholar]
- Salgia R, Sherman S, Hong D, Ng C, Frye J, Janisch L, Ratain M, Kurzrock R 2008 A phase I study of XL184, a RET, VEGFR2 and MET kinase inhibitor in patients with advanced malignancies, including patients with medullary thyroid cancer. J Clin Oncol 26:158S [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laure Giraudet A, Al Ghulzan A, Aupérin A, Leboulleux S, Chehboun A, Troalen F, Dromain C, Lumbroso J, Baudin E, Schlumberger M 2008 Progression of medullary thyroid carcinoma: assessment with calcitonin and carcinoembryonic antigen doubling times. Eur J Endocrinol 158:239–246 [DOI] [PubMed] [Google Scholar]
- Kiura K, Nakagawa K, Shinkai T, Eguchi K, Ohe Y, Yamamoto N, Tsuboi M, Yokota S, Seto T, Jiang H, Nishio K, Saijo N, Fukuoka M 2008 A randomized, double-blind, phase IIa dose-finding study of Vandetanib (ZD6474) in Japanese patients with non-small cell lung cancer. J Thorac Oncol 3:386–393 [DOI] [PubMed] [Google Scholar]
- Natale RB, Bodkin D, Govindan R, Sleckman BG, Rizvi NA, Capó A, Germonpré P, Eberhardt WE, Stockman PK, Kennedy SJ, Ranson M 2009 Vandetanib versus gefitinib in patients with advanced non-small-cell lung cancer: results from a two-part, double-blind, randomized phase II study. J Clin Oncol 27:2523–2529 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.