Abstract
Context: Hypoparathyroidism is among the few hormonal insufficiency states not treated with replacement of the missing hormone. This is the first randomized controlled study in children comparing treatment with synthetic human PTH 1-34 and calcitriol.
Objective: The primary objective was to assess the efficacy and safety of long-term PTH 1-34 vs. calcitriol treatment in the maintenance of normal serum calcium values and renal calcium excretion in children with hypoparathyroidism.
Setting: The study was conducted at a clinical research center.
Subjects: Subjects included 12 children aged 5–14 yr with chronic hypoparathyroidism and without severe renal or hepatic insufficiency.
Study Design: The study was a 3-yr randomized parallel trial comparing twice-daily calcitriol (plus calcium and cholecalciferol in four daily doses) vs. sc PTH 1-34 treatment, with weekly or biweekly monitoring of serum and urine calcium.
Results: Mean predose serum calcium levels were maintained at, or just below, the normal range, and urine calcium levels remained in the normal range throughout the 3-yr study, with no significant differences between treatment groups. Creatinine clearance, corrected for body surface area, did not differ between groups and remained normal throughout the study. Markers of bone turnover were mildly elevated during PTH 1-34 therapy and remained within the normal range during calcitriol therapy. Mean bone mineral density Z-scores at the anterior-posterior lumbar spine, femoral neck, distal radius, and whole body remained within the normal range and did not differ between groups throughout the study. Similarly, height and weight percentiles did not differ between treatment groups and remained normal throughout the 3-yr follow-up.
Conclusion: We conclude that PTH 1-34 therapy is safe and effective in maintaining stable calcium homeostasis in children with hypoparathyroidism. Additionally, PTH 1-34 treatment allowed normal skeletal development because there were no differences in bone mineral accrual, linear growth, or weight gain between the two treatment arms over the 3-yr study period.
PTH 1-34 therapy is safe and effective in maintaining stable calcium homeostasis in children with hypoparathyroidism.
Hypoparathyroidism is a rare pediatric disorder, associated with low or undetectable PTH levels, which produces abnormalities in mineral metabolism that include hypocalcemia, hyperphosphatemia, and hypomagnesemia. The clinical presentation may include neuromuscular irritability causing tetany, muscle cramping, spasms, and seizures. In adults, the disorder is usually a complication of neck surgery or radiation. In children, the condition is most often idiopathic or due to inherited disorders such as autoimmune polyglandular failure syndrome type 1 or an activating mutation in the calcium-sensing receptor.
Children with hypoparathyroidism pose a particular therapeutic dilemma because recurrent episodes of hypocalcemia, if associated with seizures, may have adverse effects on brain development. However, overtreatment with calcitriol and calcium to avoid hypocalcemia may produce hypercalciuria because of the compromised physiological defenses against rising urine calcium in this disorder. Chronic episodic hypercalciuria, in turn, may produce nephrocalcinosis and permanent renal damage (1,2). Thus, vitamin D analog treatment of hypoparathyroidism in children involves the challenge, throughout the growth period, of adjusting treatment dosage to minimize both symptomatic hypocalcemia and asymptomatic, but potentially kidney-damaging, hypercalciuria.
As an alternative to vitamin D analogs, which are the sole Food and Drug Administration (FDA)-approved treatment, replacement therapy with the biologically active amino-terminal portion of the PTH molecule has been investigated during the past 2 decades. The initial pilot study of this treatment involved a 2-month course of PTH 1–38 in two adolescents with autoimmune hypoparathyroidism (3). Subsequently, studies with synthetic PTH 1-34 in adults with hypoparathyroidism showed, first, that once-daily PTH can maintain both serum and urine calcium within the normal range during a 12-wk period (4); second, that twice-daily PTH 1-34 provides effective short-term (12 wk) treatment with a reduced total daily dose, reduced bone turnover markers, and a decreased incidence of bone pain compared with a once-daily regimen (5); and, third, that twice-daily PTH 1-34 over a 3-yr period can maintain serum calcium at or just below normal with concurrent eucalciuria and with no change in bone mineral density (BMD), despite mild elevation of bone turnover markers (6).
Despite the evident rationale for PTH replacement in hypoparathyroidism, the first clinical studies of synthetic PTH 1-34 were for treatment and/or prevention of osteoporosis (7,8,9,10). The success of these studies led, in 2002, to FDA approval of recombinant human PTH 1-34 (rhPTH 1-34, teriparatide, Forteo; Eli Lilly, Indianapolis, IN) for the treatment of severe osteoporosis in adults. However, due to occurrence of osteosarcomas and death in rat toxicology studies of rhPTH 1-34 (11), the FDA-approved product label warned against pediatric use of rhPTH 1-34 and, despite the apparent absence of osteosarcomas during a decade of marketed adult rhPTH 1-34 use, safety concerns have prompted the manufacturer not to sponsor investigational use of rhPTH 1-34 in children.
Based on the perceived need for alternative treatment, however, we had already initiated, in the mid-1990s, studies of synthetic PTH 1-34 replacement therapy in children with hypoparathyroidism. Comparison of once-daily with twice-daily PTH 1-34 over a 14-wk period yielded results similar to those observed previously in adults (12). The current study examined the long-term results of synthetic PTH 1-34, in these same children, in a 3-yr randomized, parallel trial comparing twice-daily PTH 1-34 to twice-daily calcitriol with calcium and cholecalciferol supplementation. In addition to outcome measures described previously in adults, study of these growing children included observations of linear growth, weight gain, and bone mineral accrual.
Subjects and Methods
Subjects
The study was conducted at the National Institutes of Health (NIH) Clinical Center under a protocol approved by the Institutional Review Board of the National Institute of Child Health and Human Development and the FDA. All subjects and their parents gave written informed consent (or assent for younger children). Twelve children (eight males) aged 5–14 yr with chronic hypoparathyroidism were enrolled in the study. The baseline visit also served as the conclusion of the prior dose study (12). Accordingly, all subjects were receiving either once-daily or twice-daily PTH before the initiation of this study. The subjects in this study represented 70% of the hypoparathyroid children within the NIH intramural research program. The nonparticipating children included those who were ineligible or declined to participate.
The etiologies of hypoparathyroidism in this study were autoimmune polyglandular failure syndrome type 1 [brothers 1 and 8 and 6 and 11, from two different families (see Table 1)]; a sporadic activating mutation of the calcium receptor (patient 2) (13); and idiopathic (brothers 5 and 10 from a family with three other affected brothers and an affected father and uncle and siblings 7, 9, and 12 from a family with no affected parent or grandparent). Calcium-sensing receptor mutations were sought, and not found, in all patients with idiopathic familial hypoparathyroidism.
Table 1.
Baseline clinical and laboratory features of 12 children with hypoparathyroidism at study entry
| Patient no. | Age (yr) | Sex | Calcium (mmol/liter) (2.05–2.5) | Phosphorus (mmol/liter) (0.8–1.6) | Magnesium (mmol/liter) (0.65–1.05) | Alkaline phosphatase (U/liter) (100–320) | Urinary calcium (mmol per 24 h) (1.25–6.25) | Urinary phosphorus (13–42 mmol per 24 h) | Urinary magnesium (3–4.3 mmol per 24 h) | Corrected creatinine clearance (80–130 ml/min per 1.73 m2) |
|---|---|---|---|---|---|---|---|---|---|---|
| PTH | ||||||||||
| 1 | 10 | M | 1.84 | 1.96 | 0.71 | 461 | 5.25 | 26.0 | 5.22 | 148 |
| 2 | 7 | F | 1.86 | 2.33 | 0.53 | 207 | 4.61 | 15.8 | 4.78 | 71 |
| 3 | 8 | M | 1.93 | 2.51 | 0.70 | 300 | 1.86 | 13.7 | 2.09 | 80 |
| 4 | 12 | F | 2.16 | 2.09 | 0.77 | 178 | 7.11 | 28.3 | 5.61 | 141 |
| 5 | 12 | M | 2.04 | 2.50 | 0.66 | 550 | 4.08 | 27.8 | 6.00 | 94 |
| 6 | 14 | M | 1.68 | 2.41 | 0.65 | 287 | 6.07 | 20.0 | 5.34 | 119 |
| 7 | 10 | M | 1.70 | 2.09 | 0.62 | 248 | 8.87 | 20.3 | 6.96 | 86 |
| Mean ± sd | 10.2 ± 2.3 | 60% M | 1.89 ± 0.18 | 2.27 ± 0.22 | 0.66 ± 0.08 | 319 ± 137 | 5.41 ± 2.25 | 21.7 ± 5.8 | 5.14 ± 1.51 | 106 ± 31 |
| Calcitriol | ||||||||||
| 8 | 6 | M | 1.84 | 2.60 | 0.76 | 231 | 2.25 | 13.7 | 2.80 | 155 |
| 9 | 12 | F | 1.84 | 2.26 | 0.58 | 422 | 5.70 | 43.3 | 6.41 | 64 |
| 10 | 11 | M | 2.04 | 2.41 | 0.78 | 295 | 8.60 | 33.5 | 7.11 | 99 |
| 11 | 10 | M | 1.81 | 2.34 | 0.74 | 362 | 4.93 | 22.5 | 3.18 | 167 |
| 12 | 5 | F | 2.06 | 2.06 | 0.68 | 246 | 3.67 | 12.3 | 4.38 | 72 |
| Mean ± sd | 8.8 ± 2.9 | 71% M | 1.92 ± 0.12 | 2.33 ± 0.20 | 0.71 ± 0.08 | 311 ± 80 | 5.03 ± 2.39 | 25.1 ± 13.2 | 4.78 ± 1.92 | 111 ± 47 |
M, Male, F, female.
Protocol
This was a randomized, parallel, open-label, 3-yr study comparing calcitriol (plus calcium and cholecalciferol supplementation) vs. PTH 1-34 replacement therapy. After completion of the prior short-term study comparing once-daily vs. twice-daily PTH 1-34 (12), subjects were assigned randomly, in blocks of four, to receive, at 0900 and 2100 h, either twice-daily sc PTH 1-34 (0.4 μg/kg mean initial dose) or twice-daily calcitriol (initially 0.25 μg/dose) plus combined calcium (1200 mg/d) and cholecalciferol (800 IU/d) supplementation divided into four daily doses. Synthetic human parathyroid hormone 1-34 (purchased from Bachem Inc., Torrance, CA) was prepared at the NIH Clinical Center for human administration as previously described (4).
Calcitriol and PTH 1-34 dose adjustments were permitted as indicated clinically throughout the study, with the dose of PTH 1-34 being adjusted in increments or decrements of approximately 15% to maintain urine and serum calcium within the normal range. Both serum calcium and 24-h urine calcium were measured daily during an initial 1-wk inpatient admission, once weekly during the next 2 months, and biweekly thereafter. For several subjects, weekly collections continued if they experienced recurrent symptoms of hypocalcemia or intermittent episodes of malabsorption. Blood and urine samples were sent to the NIH by overnight express mail.
Dietary intake of calcium ranged from 700 to 1600 mg of elemental calcium based on dietary history and results of a food frequency questionnaire obtained at each clinic visit. Except for magnesium supplementation in all but two patients, no study participant received phosphate binders, diuretics, or other medications that affected serum calcium, magnesium, or phosphorus levels.
Clinical monitoring
Subjects were seen in the NIH Clinical Center outpatient clinic every 6 months. During this 3-d visit, patients provided two 24-h urine and two blood samples. Blood samples were obtained approximately 10–11 h after their medication doses, usually before the morning dose. Food frequency and symptom questionnaires were completed by patients with the assistance of their parents at each clinic visit.
Study end points
The primary outcome measures were serum and urine calcium levels. Secondary outcome measures were serum phosphorus, magnesium, 1,25-dihydroxyvitamin D3, 25-hydroxyvitamin D3, and serum and urine markers of bone turnover. Additional secondary outcome measures included kidney function (creatinine clearance corrected for body surface area), linear growth (calculated from heights which were the average of three Harpenden stadiometer measurements), body weight, and BMD. BMD measurements of the spine, forearm, hip, and whole body were performed on a QDR 2000 scanner using dual-energy x-ray absorptiometry (Hologic, Inc., Bedford MA). Height and weight percentiles were determined according to Centers for Disease Control and Prevention reference data (14). BMD and content Z-scores were based on standards generated by the Bone Mineral Density in Childhood Study (15).
The serum calcium, phosphorus, and magnesium levels determined for each patient at different time points throughout the study were the average of six values obtained within a 2-month period, with two of the values being obtained on consecutive days at a scheduled 6-month NIH clinic visit. Most of the levels represent predose morning values, with a few representing predose evening values to accommodate patient schedules. The corresponding urine values are also the mean of six determinations obtained on the same days. All blood and urine samples were measured at the NIH Clinical Center except for serum 25-hydroxy- and 1,25-dihydroxyvitamin D3, serum osteocalcin, and urine pyridinoline and deoxypyridinoline, which were measured at Quest Diagnostics (San Juan Capistrano, CA).
Statistical methods
Data were assessed first for treatment effect across time using mixed models for repeated measures, and second for trend over time as determined by slopes with the use of one-sample t tests. Continuous data were compared between groups by two-sample t test, and categorical data were analyzed by χ2 or Fisher's exact tests, as appropriate. P ≤ 0.05 were considered statistically significant. The Bonferroni adjustment for multiple comparisons was applied to comparisons at specific time points, with only the adjusted P values being reported. Data are mean ± sd except in figures, where mean ± se is presented. All data were analyzed using SAS version 9.1 (SAS Institute Inc., Cary, NC).
Adverse events
Subjects completed a symptom questionnaire at each NIH follow-up visit. Tracked symptoms included numbness, spasms, muscle weakness, joint pain, fatigue, bone pain, and depression. Subjects were asked to rate the severity and duration of each symptom.
Results
Baseline characteristics of the PTH 1-34 treatment group (n = 7, ages 7–14 yr, five males) did not differ significantly from the calcitriol treatment group (n = 5, ages 5–12 yr, three males, Table 1). Of the 14 subjects in the prior short-term study of once-daily vs. twice-daily PTH 1-34 (12), two were not included in analyses of this study: one had recently turned 18 yr old and entered the similarly designed long-term adult study (6), and one was randomized to the calcitriol arm of this study but discontinued before any data were collected because of the anticipated inconvenience of study procedures. Additionally, three subjects from one family [subject 7 (PTH group) and subjects 9 and 12 (calcitriol group)] discontinued midway through the study (after 1.5 yr) due to the inconvenience of travel to NIH. At baseline, all but two subjects (subjects 3 and 8) required magnesium supplementation. Furthermore, before randomization, five subjects (subjects 4, 5, 8, 9, 11) were receiving short-term twice-daily PTH; two (subjects 4, 5) were randomized to continue this PTH treatment regimen during the 3-yr study. Seven subjects were receiving once-daily PTH (subjects 1, 2, 3, 6, 7, 10, 12); five were randomized to the twice-daily PTH treatment arm during the long-term study (subjects 1, 2, 3, 6, 7).
Dose
Mean ± sd PTH 1-34 dose over the 3-yr study was 0.6 ± 0.5 μg/kg (approximately 27 μg/dose) and mean ± sd calcitriol dose was 0.009 ± 0.004 μg/kg (approximately 0.3 μg/dose), each administered twice daily. Mean ± sd magnesium supplement was 272 ± 175 mg/d for PTH 1-34 subjects and 296 ± 197 mg/d for calcitriol subjects. Calcium carbonate (300 mg) combined with cholecalciferol (200 IU) was given as a pill four times daily.
Primary end points
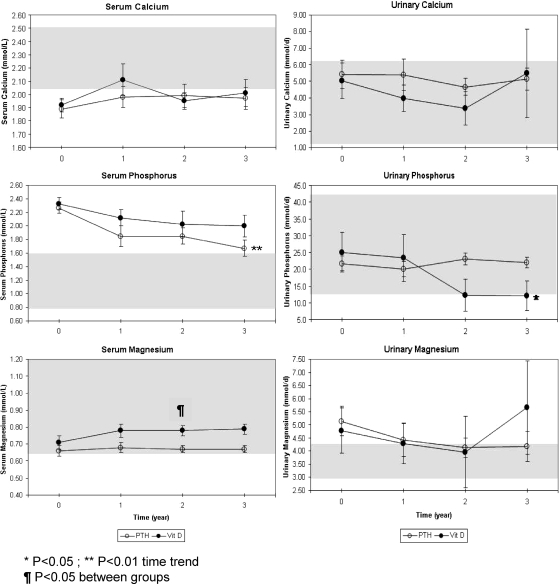
Mean ± sd serum calcium levels showed no differences across time or between treatments (1.96 ± 0.04 vs. 1.99 ± 0.05 mmol/liter for the PTH 1-34 and calcitriol groups, respectively, Fig. 1) and remained at or just below the normal range throughout the study. Mean ± sd urine calcium excretion also showed no difference across time or between treatments, whether expressed in millimoles per day (Fig. 1) or in milligrams per kilogram body weight per day (4.9 ± 0.8 vs. 4.4 ± 1.1 mg/kg · d for the PTH 1-34 and calcitriol groups, respectively, P = 0.73) and were within the upper half of the normal range throughout the study.
Figure 1.
Mean ± sem mineral values in blood and 24-h urine collections measured in children receiving PTH 1-34 (PTH) vs. calcitriol, calcium, and cholecalciferol (vitamin D3) for treatment of hypoparathyroidism.
Secondary end points
Phosphorus, magnesium, and vitamin D
Mean ± sd serum phosphorus levels showed no difference between treatments (1.79 ± 0.09 vs. 2.02 ± 0.11 for the PTH 1-34 and calcitriol groups, respectively, P = 0.13, Fig. 1) and remained above the normal range throughout the study. In the PTH 1-34 group, however, there was a significant downward trend over time (P < 0.01), with mean serum phosphorus level at 3 yr only slightly above normal. Mean ± sd urine phosphorus levels did not differ across time or by treatment group whether expressed as millimoles per day (Fig. 1) or as milligrams per kilogram body weight per day (15.9 ± 1.7 vs. 10.9 ± 2.2 mg/kg · d for the PTH 1-34 and calcitriol groups, respectively, P = 0.10). In the calcitriol group, however, there was a significant downward trend over time in urine phosphorus level (P < 0.05), with values at the lower limit of normal at the 2- and 3-yr time points.
Mean ± sd serum magnesium levels were similar across time and between treatments (0.68 ± 0.02 vs. 0.75 ± 0.03 mmol/liter for the PTH 1-34 and calcitriol groups, respectively, P = 0.06, Fig. 1) but were lower in the PTH group at 2 and 2.5 yr (0.67 ± 0.02 vs. 0.78 ± 0.03 mmol/liter, P < 0.05, and 0.67 ± 0.02 vs. 0.82 ± 0.01 mmol/liter, P < 0.05, respectively). Mean ± sd urine magnesium levels were at or above the upper limit of the normal range throughout the study (Fig. 1), with no difference across time or between treatments (2.6 ± 0.4 vs. 2.3 ± 0.5 mg/kg · d for the PTH 1-34 and calcitriol groups, respectively).
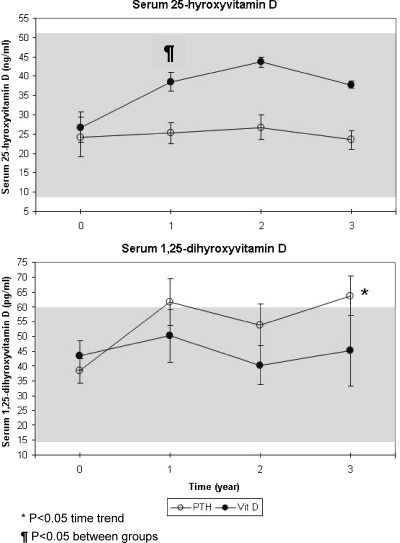
Mean ± sd serum 1,25-dihydroxyvitamin D3 levels did not differ between treatments (60 ± 6 vs. 47 ± 7 pg/ml for the PTH 1-34 and calcitriol groups, respectively, P = 0.20, Fig. 2). However, a significant upward trend over time was observed in the PTH-treated but not the calcitriol-treated patients (P < 0.05). Mean ± sd 25-hydroxyvitamin D3 levels were consistently lower in the PTH-treated patients (23 ± 1 vs. 34 ± 2 ng/ml for the PTH 1-34 and calcitriol groups, respectively, P < 0.01, Fig. 2), which most likely reflects the supplemental cholecalciferol administered (in combination with calcium) to the calcitriol-treated patients.
Figure 2.
Mean ± sem serum vitamin D levels measured in children receiving PTH 1-34 (PTH) vs. calcitriol, calcium, and cholecalciferol for treatment of hypoparathyroidism.
Kidney function
Mean ± sd creatinine clearance, corrected for body surface area, did not differ across time or between treatments (105 ± 8 vs. 108 ± 10 ml/min per 1.7 m2 for the PTH 1-34 and calcitriol groups, respectively).
Markers of bone turnover
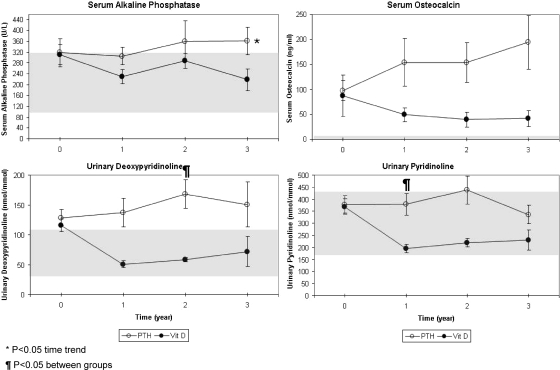
Except for serum alkaline phosphatase, in which a weak upward trend (P < 0.05, Fig. 3) in PTH-treated subjects but no overall difference compared with calcitriol-treated subjects was observed, mean ± sd markers of bone remodeling were significantly higher in the PTH-treated group (155 ± 30 vs. 36 ± 36 ng/ml for serum osteocalcin, P < 0.05; 152 ± 14 vs. 52 ± 18 nmol/mmol creatinine for urine deoxypyridinoline, P < 0.01; 397 ± 28 vs. 193 ± 35 nmol/mmol creatinine for urine pyridinoline, P < 0.01, Fig. 4). Additionally, statistically significant differences between treatment groups were seen at 1.5, 2, and 2.5 yr for urine deoxypyridinoline and at 1 and 1.5 yr for urine pyridinoline.
Figure 3.
Mean ± sem bone turnover markers in blood and 24-h urine collections in children with hypoparathyroidism treated with PTH 1-34 (PTH) vs. calcitriol, calcium, and cholecalciferol.
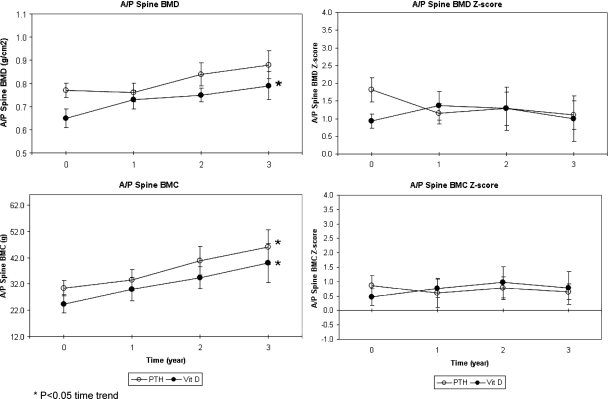
Figure 4.
Mean ± sem spine BMD and BMC in children receiving PTH 1-34 (PTH) vs. calcitriol, calcium, and cholecalciferol for treatment of hypoparathyroidism.
Bone density
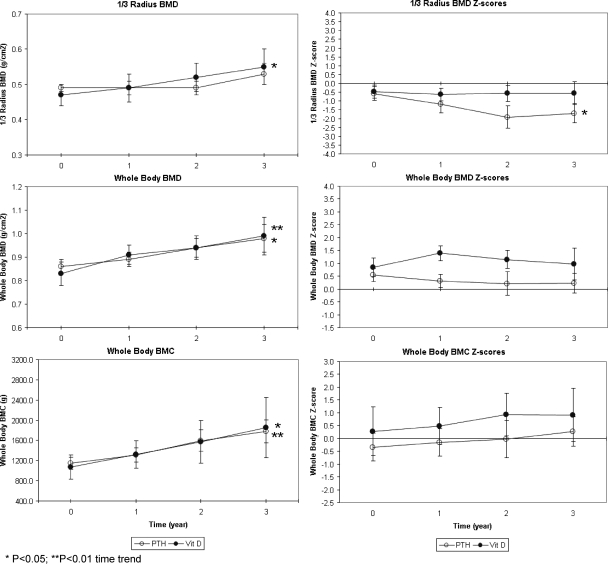
BMD Z-score did not differ across time or between treatment groups at the anterior-posterior (A/P) lumbar spine, femoral neck, total femur (data not shown), distal third of the radius, or whole body (Figs. 4 and 5), with the exception of a significant downward trend over time at the distal radius of the PTH 1-34 group (P < 0.05, Fig. 5). As expected for growing children, BMD increased at all sites for both treatment groups, and these upward trends over time were statistically significant at all sites for the calcitriol group (P < 0.05, except for P < 0.01 for the whole body BMD, Fig. 5) and at the total femur (data not shown) and whole body for the PTH 1-34 group (P < 0.05, Fig. 5).
Figure 5.
Mean ± sem whole-body and radius BMD and BMC in children receiving PTH 1-34 (PTH) vs. calcitriol, calcium, and cholecalciferol for treatment of hypoparathyroidism.
Bone mineral content (BMC) Z-score did not differ across time or between treatment groups at the A/P lumbar spine or whole body (Fig. 5). As expected for growing children, BMC showed consistent upward trends for both treatment groups, which were statistically significant for the PTH 1-34 group at the A/P lumbar spine (P < 0.05, Fig. 4), the whole body (P < 0.01, Fig. 5), and the calcitriol group at the A/P lumbar spine and whole body (P < 0.05, Figs. 4 and 5).
Growth
Mean ± sd height percentile did not differ across time or between treatment groups (47 ± 13 vs. 53 ± 15 for the PTH 1-34 and calcitriol groups, respectively, P = 0.76), indicating normal linear growth of both groups throughout the study. Likewise, mean ± sd weight percentile showed no significant differences over time or between treatment groups (54 ± 13 vs. 63 ± 15 for the PTH 1-34 and calcitriol groups, respectively, P = 0.68).
Adverse events
Analysis of symptom occurrence at any time during the follow-up showed no significant differences between groups. Serious adverse events included hypocalcemia on two occasions in patient 2. This subject had chronically low serum calcium levels due to calcium-sensing receptor activating mutation, and both episodes were characterized by worsened, symptomatic hypocalcemia associated with flu-like symptoms and fever. Both episodes resolved with an outpatient iv calcium infusion. Two other patients were hospitalized for complications of autoimmune polyglandular failure syndrome type 1 (patients 4 and 8) not associated with abnormal calcium levels. During study year three, there was a single report of bone pain (subject 7, Table 1) from one child receiving PTH. There were no seizures or intensive care unit admissions in any subject.
Discussion
Within the context of a closely monitored research study, with 3-yr follow-up of a small number of hypoparathyroid children, the current results establish that PTH 1-34 and calcitriol treatment are similar in maintaining normal serum and urine calcium, kidney function, bone mineral accrual, and linear growth. These observations are novel because there are no prior long-term studies comparing PTH and vitamin D treatment in such children.
Neither of the two treatments restored normal mineral homeostasis because serum calcium was slightly below the normal range during both treatments, and serum phosphorus was consistently above normal in both treatment groups. Additionally, most patients required magnesium supplementation to maintain serum magnesium within the low normal range, with high normal or slightly elevated urine magnesium levels.
As in the previous study in adults (6), bone turnover markers were at the upper limit of normal or elevated in the PTH-treated patients, but this difference was not associated with a difference in bone mineral accrual or linear growth, which appeared to proceed normally in both treatment groups. This observation is especially important, given the FDA warning against the use in children of rhPTH 1-34 (Forteo; Eli Lilly) because of 2-yr carcinogenicity studies yielding osteosarcoma in rats (11). These studies, performed at supraphysiological dosage throughout the lifetime of rats with normal parathyroid function, have raised safety concerns regarding long-term PTH 1-34 use that resulted in the product label warning against its noninvestigational use in children. Additionally, the observation of chronically elevated bone biomarkers, in the current 3-yr study of twice-daily administration, highlights the importance of continued careful investigation of this regimen and of the desirability of developing a more physiological regimen of PTH replacement.
Limitations of this study include the small sample size and relatively short duration of treatment in a lifelong disorder. The recruitment of hypoparathyroid children was likely biased, to some degree, toward a population who are more difficult to manage or less satisfied with their conventional therapy. Because few serious adverse events were observed, which were largely unrelated to treatment, we cannot compare the likelihood of events such as tetany or seizures due to undertreatment or hypercalcemic crisis due to overtreatment. Likewise, this study provides no insight into whether the theoretical advantage of PTH 1-34 in reducing urine calcium will or will not translate over a longer period into a reduced frequency of nephrocalcinosis or decline in kidney function. Lastly, the efficacy findings in this closely monitored research study in highly motivated families may not translate into real-world effectiveness in less closely monitored patients or less motivated families.
Looking forward at this early stage in the development of replacement therapy for hypoparathyroidism, we are left with many unanswered questions, not dissimilar from those involving insulin replacement for diabetes many decades ago. Can the efficacy of the current rapid-acting PTH 1-34 formulation be improved by more frequent daily injections or continuous sc infusion as with the insulin pump in diabetes? Can stable, long-acting formulations of PTH be developed that, like newer basal insulin formulations, would provide 24-h control of calcium metabolism with a once-daily dose? Would vitamin D3 supplementation in PTH-treated patients, by raising 25-hydroxyvitamin D3 levels, improve the consistency of PTH 1-34 efficacy or allow similar efficacy with a lower dose? Although the current results are encouraging, these or other refinements of this therapy will be required before the abnormal mineral homeostasis of these patients is normalized and the quality of their lives fully restored.
Acknowledgments
The authors acknowledge the contributions of the National Institutes of Health Clinical Center Fellows and the One North West nursing staff. We greatly appreciate the contributions of nutritionist Nancy Sebring.
Footnotes
This work was supported by the Intramural Research Program of the National Institute of Child Health and Human Development.
Present address for N.S.: Biostatistics and Clinical Epidemiology Service, Clinical Center, National Institutes of Health, Bethesda, Maryland.
Disclosure Summary: G.B.C. is a retired employee, share owner, and consultant for Eli Lilly (although Eli Lilly had no involvement with this study). The other authors have nothing to disclose.
First Published Online April 14, 2010
Abbreviations: A/P, anterior-posterior; BMC, bone mineral content; BMD, bone mineral density; rhPTH 1-34, recombinant human PTH 1-34.
References
- Weber G, Cazzuffi MA, Frisone F, de Angelis M, Pasolini D, Tomaselli V, Chiumello G 1988–1989 Nephrocalcinosis in children and adolescents: sonographic evaluation during long-term treatment with 1,25-dihydrocholecalciferol. Child Nephrol Urol 9:273–276 [PubMed] [Google Scholar]
- Santos F, Smith MJ, Chan JC 1986 Hypercalciuria associated with long-term administration of calcitriol (1,25-dihydroxyvitamin D). Am J Dis Child 140:139–142 [DOI] [PubMed] [Google Scholar]
- Strögmann W, Bohrn E, Woloszezuk W 1990 First experiences in the substitution treatment of hypoparathyroidism with synthetic human parathyroid hormone. Monatsschr Kinderheilkd 138:141–146 [PubMed] [Google Scholar]
- Winer K, Yanovski JA, Cutler Jr GB 1996 Synthetic human parathyroid hormone 1-34 vs calcitriol and calcium in the treatment of hypoparathyroidism: results of a randomized crossover trial. JAMA 276:631–636 [PubMed] [Google Scholar]
- Winer KK, Yanovski JA, Sarani B, Cutler Jr GB 1998 A randomized, crossover trial of once-daily vs twice-daily human parathyroid hormone 1-34 in the treatment of hypoparathyroidism. J Clin Endocrinol Metab 83:3480–3486 [DOI] [PubMed] [Google Scholar]
- Winer KK, Ko CW, Reynolds JC, Dowdy K, Keil M, Peterson D, Gerber LH, McGarvey C, Cutler Jr GB 2003 Long-term treatment of hypoparathyroidism: a randomized controlled study comparing parathyroid hormone-(1-34) versus calcitriol and calcium. J Clin Endocrinol Metab 88:4214–4220 [DOI] [PubMed] [Google Scholar]
- Slovik DM, Neer RM, Potts Jr JT 1981 Short-term effects of synthetic human parathyroid hormone 1-34 administration on bone mineral metabolism in osteoporotic patients. J Clin Invest 68:1261–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slovik DM, Rosenthal DI, Doppelt SH, Potts Jr JT, Daly MA, Campbell JA, Neer RM 1986 Restoration of spinal bone in osteoporotic men by treatment with parathyroid hormone 1-34 and 1,25 dihydroxyvitamin D. J Bone Miner Res 1:377–381 [DOI] [PubMed] [Google Scholar]
- Finkelstein JS, Klibanski A, Schaefer EH, Hornstein MD, Schiff I, Neer RM 1994 Parathyroid hormone for the prevention of bone loss induced by estrogen deficiency. N Engl J Med 331:1618–1623 [DOI] [PubMed] [Google Scholar]
- Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH 2001 Effect of parathyroid hormone 1-34 on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344:1434–1441 [DOI] [PubMed] [Google Scholar]
- Vahle JL, Sato M, Long GG, Young JK, Francis PC, Engelhardt JA, Westmore MS, Linda Y, Nold JB 2002 Skeletal changes in rats given daily subcutaneous injections of recombinant human parathyroid hormone (1-34) for 2 years and relevance to human safety. Toxicol Pathol 30:312–321 [DOI] [PubMed] [Google Scholar]
- Winer KK, Sinaii N, Peterson D, Sainz Jr B, Cutler Jr GB 2008 Effects of once versus twice-daily parathyroid hormone1-34 therapy in children with hypoparathyroidism. J Clin Endocrinol Metab 93:3389–3395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron J, Winer KK, Yanovski JA, Cunningham AW, Laue L, Zimmerman D, Cutler Jr GB 1996 Mutations in the Ca-sensing receptor gene cause autosomal dominant and sporadic hypoparathyroidism. Hum Mol Genet 5:601–606 [DOI] [PubMed] [Google Scholar]
- Kuczmarski R, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL 2002 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 246:1–190 [PubMed] [Google Scholar]
- Kalkwarf HJ, Zemel BS, Gilsanz V, Lappe JM, Horlick M, Oberfield S, Mahboubi S, Fan B, Frederick MM, Winer K, Shepherd JA 2007 The bone mineral density in childhood study (BMDCS): bone mineral content and density according to age, sex, and race. J Clin Endocrinol Metab 92:2087–2099 [DOI] [PubMed] [Google Scholar]