Abstract
Context: Mobility limitation is associated with increased morbidity and mortality. The relationship between circulating testosterone and mobility limitation and physical performance is incompletely understood.
Objective: Our objective was to examine cross-sectional and prospective relations between baseline sex hormones and mobility limitations and physical performance in community-dwelling older men.
Design, Setting, and Participants: We conducted cross-sectional and longitudinal analyses of 1445 men (mean age 61.0 ± 9.5 yr) who attended Framingham Offspring Study examinations 7 and 8 (mean 6.6 yr apart). Total testosterone (TT) was measured by liquid chromatography tandem mass spectrometry at examination 7. Cross-sectional and longitudinal analyses of mobility limitation and physical performance were performed with continuous (per sd) and dichotomized [low TT and free testosterone (FT) and high SHBG vs. normal] hormone levels.
Main Outcome Measures: Self-reported mobility limitation, subjective health, usual walking speed, and grip strength were assessed at examinations 7 and 8. Short physical performance battery was performed at examination 7.
Results: Higher continuous FT was positively associated with short physical performance battery score (β = 0.13; P = 0.008), usual walking speed (β = 0.02; P = 0.048), and lower risk of poor subjective health [odds ratio (OR) = 0.72; P = 0.01]. In prospective analysis, 1 sd increase in baseline FT was associated with lower risk of developing mobility limitation (OR = 0.78; 95% confidence interval = 0.62–0.97) and progression of mobility limitation (OR = 0.75; 95% confidence interval = 0.60–0.93). Men with low baseline FT had 57% higher odds of reporting incident mobility limitation (P = 0.03) and 68% higher odds of worsening of mobility limitation (P = 0.007).
Conclusions: Lower levels of baseline FT are associated with a greater risk of incident or worsening mobility limitation in community-dwelling older men. Whether this risk can be reduced with testosterone therapy needs to be determined by randomized trials.
Low levels of free testosterone are associated with increased risk of incident or worsening mobility limitation in community-dwelling older men in the Framingham Heart Study.
The powerful demographic shift toward aging of human populations has focused attention on remediable factors that limit the ability of older individuals to live independently. Among individuals age 65 yr and older, 44% report some mobility limitation (1). Decline in mobility is associated with loss of independence (2) and increased risk of disability (3), institutionalization (4), decreased quality of life (5), and death (3,6). Mobility limitation in older individuals is undoubtedly multifactorial, but age-related declines in muscle mass, strength, and power are important contributors (7,8).
Total testosterone (TT) levels in men decline progressively with age (9,10,11,12,13,14). Because SHBG increases with age (9,10,15), the decline in free testosterone (FT) with aging is even greater than the decline in TT levels. Age-related decline in testosterone levels has been associated with reduced muscle mass and lower extremity strength in older men (16,17).
Only a few studies have addressed the relationships between circulating testosterone levels and mobility and physical function, and the data are conflicting. The Longitudinal Ageing Study Amsterdam reported a significant positive association between low TT and low grip strength in older men, but no statistically significant relationship with self-reported functional limitations was observed in that study (18). The Massachusetts Male Ageing Study found no relationship between TT and grip strength (19). An analysis of longitudinal data from two independent cohorts of older men showed no association between TT and FT and the decline in physical performance (20). The BACH/Bone Study concluded age-associated alterations in sex hormone levels play a minor role in age-related declines in muscle strength and physical performance (21). These studies measured testosterone levels by RIAs, whose accuracy has been questioned (22,23). Thus, the relationships among circulating levels of sex hormones and mobility limitations and physical performance in older men are inadequately understood.
Using data from the Framingham Offspring Study, we determined whether TT, FT, and SHBG are related cross-sectionally to mobility limitations, subjective health, and performance-based measures of physical function in community-dwelling older men. Additionally, in longitudinal analyses, we evaluated whether these hormones prospectively are associated with incident mobility limitation and worsening of mobility and subjective health in older men. We measured circulating TT levels by liquid chromatography tandem mass spectrometry (LC-MS/MS), widely considered the reference method for testosterone measurement (23).
Materials and Methods
The Boston University Institutional Review Board approved the study, and written informed consent was obtained from all participants.
Study sample
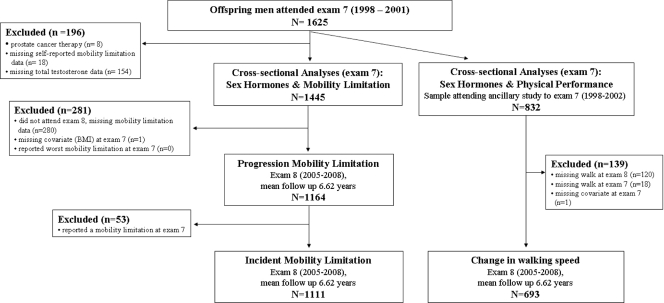
The Framingham Heart Study (FHS) began in 1948 as a prospective study to examine the risk factors for heart disease (24). In 1971, enrollment of offspring of the original cohort and spouses of the offspring constituted the Framingham Offspring Study (25). The offspring cohort has completed eight examinations at approximately 4- to 8-yr intervals. The men of the Offspring cohort who attended examination 7 (1998–2001) were eligible for the present study (n = 1625). Men with prostate cancer undergoing androgen deprivation therapy (n = 8) and those missing self-reported mobility data (n = 18) or TT (n = 154) at examination 7 were excluded, resulting in a sample size of 1445 for cross-sectional analyses (Fig. 1). To determine whether sex hormones are associated with incident and/or progression of mobility limitations, men who attended examination 8 (2005–2008) were examined on average 6.6 yr later. In the analysis of progression of mobility limitations, we excluded men who did not attend or had missing mobility data (n = 280) at examination 8 or missing covariate data (n = 1). We further excluded men reporting a mobility limitation at examination 7 (n = 53) when we examined incident mobility limitations. Hence, 1111 men were available to prospectively examine the association between circulating sex hormone levels and incident mobility limitations and 1164 men to determine the relationship between sex hormone levels and mobility limitation progression (Fig. 1).
Figure 1.
Study design.
We also determined the cross-sectional relationships between sex hormone levels and Short physical performance battery (SPPB), timed usual walk, and grip strength using a subset of men from an ancillary study to examination 7 that included the physical performance battery (n = 832, 1998–2002). Furthermore, we examined the association between sex hormone levels and the change in timed usual walk and hand grip strength among men who attended examination 8 (n = 693).
Measurement of circulating sex hormones
Serum TT, FT, and SHBG levels were measured at examination 7. TT level was measured by LC-MS/MS, as described (26,27,28). The sensitivity of the assay was 2 ng/dl, and interassay coefficients of variation (CV) were 7.8, 5.9, and 3.5% in samples with testosterone concentrations of 250, 500, and 1000 ng/dl, respectively. SHBG levels were measured using an immunofluorometric assay (DELFIA-Wallac, Inc., Turku, Finland). The interassay CV were 8.3, 7.9, and 10.9%, and intraassay CV were 7.3, 7.1, and 8.7%, respectively, in the low, medium, and high pools (29,30). FT was calculated by using the law of mass action equation (31,32). Calculated FT concentrations differ systematically from those measured by equilibrium dialysis and vary with the algorithm used for calculating FT (33).
Healthy men aged 19–40 yr enrolled in the FHS Generation 3 (children of the Offspring participants) cohort free of cardiovascular disease, cancer, diabetes, hypertension, smoking, hypercholesterolemia, and obesity (n = 456) served as the referent population to determine normative sex hormone values. For the purpose of this study, low TT and FT levels were defined as less than the 2.5th percentile for TT and FT of the referent population (TT <348.3 ng/dl; FT <70.0 pg/ml) and high SHBG levels as more than the 97.5th percentile of the referent sample (SHBG >81.6 nmol/liter).
Self-reported measurement of mobility limitation
At examinations 7 and 8, trained technicians queried participants about mobility limitations using a modified Rosow-Breslau questionnaire (34), which has been shown to have high test-retest reliability in other large population-based studies (35,36). Participants were asked if they were able to 1) do heavy work around the house, like shovel snow or wash windows, walls, or floors without help; 2) walk half a mile without help (about four to six blocks); and 3) walk up and down one flight of stairs (37). At examination 7, the last item was asked as part of the Katz Activities of Daily Living scale with the following directive: during the course of a normal day, can you walk up and down one flight of stairs independently or do you need human assistance or the use of a device? Response choices included 1) no help needed, independent; 2) uses device, independent; 3) human assistance needed, minimally dependent; 4) dependent; and 5) do not do during a normal day. If the participant reported independence, he was considered able to perform the mobility task. A participant was considered to have a mobility limitation if he reported an inability to do one or more of the three items on the scale.
Subjective health
A standard single-item subjective health measure was used, “In general, how is your health now?” (examination 7) or “In general, how would you say your health is?” (examination 8). Response options at examination 7 included poor, fair, good, or excellent, and at examination 8, there was an additional response option of very good. The responses were reduced to a binary variable for analyses; 0 for good health (responses of good, very good, and excellent) and 1 for poor health (responses of poor or fair health).
Observed physical performance measures
Hand grip strength and performance-based measures of physical function were measured by trained technicians at an ancillary study to Offspring examination 7 (1998–2002). Measurements of hand grip strength and walking speed were repeated at Offspring examination 8.
SPPB
The SPPB is a validated battery that evaluates lower extremity function by measuring standing balance, gait speed, and time to rise from a chair five times (38). The standing balance measure was assigned a score ranging from 0–4, and gait speed and chair stands were assigned a score ranging from 1–4, with 4 indicating the highest level of performance. A summary performance score from 2 (worst) to 12 (best) was calculated by summing the individual scores.
Standing balance test.
Participants were asked to maintain balance in three positions: feet in side by side position, feet in semi-tandem position, and feet in tandem position. For each of the three positions, participants were timed to a maximum of 10 sec. Participants were assigned a score of 0 if they were unable to hold the side-by-side standing position for 10 sec, a score of 1 if they could hold the side-by-side standing position for 10 sec but were unable to hold a semi-tandem position for 10 sec, a score of 2 if they could hold a semi-tandem position for 10 sec but were unable to hold a full-tandem position for 3 sec, a score of 3 if they could stand in a full-tandem position for 3–9 sec, or a score of 4 if they could stand in a full-tandem position for 10 sec.
Measured walk.
Usual walking speed was assessed by asking the participants to walk at their usual pace over a 4-m course. Participants were allowed to use walking aids if necessary but not the assistance of another person. The test was repeated twice, and the faster of the two trials was used. Walking speed was scored as follows: less than 0.47 m/sec = 1; 0.47–0.64 m/sec = 2; 0.65–0.82 m/sec = 3; and 0.83 m/sec or faster = 4. For individuals who did not attempt or complete the walk, the value was set to the maximum value obtained by any individual.
Chair stand test.
Participants were asked to stand from a sitting position in a straight-backed chair without using their arms. If they were able to perform the task, they were asked to stand up and sit down five times, as quickly as possible. The time required to perform five chair stands was scored as follows: more than 16.6 sec = 1; 13.7–16.6 sec = 2; 11.2–13.6 sec = 3; and 11.1 sec or less = 4. If participants were unable to perform this task, then a score of 60 sec was assigned.
Hand grip strength
Grip strength was measured in both hands using an adjustable Jamar hydraulic dynamometer (Sammons Preston, Inc., Bolingbrook, IL). Participants were seated in a chair with elbow flexed at a 90° angle. Each trial consisted of a maximum squeeze for 3 sec. Three trials were performed with each hand, and the best performance in the six trials was used as the hand grip strength value.
Statistical analyses
Cross-sectional analyses
Baseline descriptive statistics (means ± sd) for continuous variables and percent for dichotomous variables were generated. Cross-sectional associations among sex hormones and binary self-reported mobility limitation and subjective health were assessed using multiple logistic regression, and multiple linear regression was used for continuous outcomes (usual walking speed, handgrip strength, and SPPB score).
Longitudinal analyses
The primary analyses employed multiple logistic regression to examine the relation between circulating sex hormone levels and 1) incident mobility limitation in men free of limitations at examination 7 and 2) decrease in subjective health between examination 7 and 8 from good or excellent to poor or fair. In secondary analyses, we examined progression of mobility limitations and decline in subjective health from examination 7 to 8, defined as a change of one or more response levels on the Rosow-Breslau scale or subjective health question (moving on Rosow-Breslau scale from 0 to 1, from 1 to 2, etc.). In additional analyses, we used multiple linear regression models to examine whether sex hormones measured at examination 7 were associated with change in gait speed and grip strength at examination 8 while adjusting for gait speed and grip strength at baseline (examination 7).
To account for potential confounders (variables related to outcomes that might affect the strength of association), all models were adjusted for age, body mass index (BMI), smoking, and comorbidities (cardiovascular disease and cancer) at examination 7. However, the univariate association of TT and FT levels with the Framingham physical activity index, a measure of physical activity, was either very weak (Pearson's correlation coefficient for TT = 0.07; P = 0.008) or not significant (Pearson correlation coefficient for FT = 0.01; P = 0.6867). Therefore, the analyses were not adjusted for physical activity index.
Furthermore, to examine the potential threshold effect, where hormone concentrations below a certain level relate to risk of poor outcomes, both cross-sectional and longitudinal analyses were repeated, defining low levels of TT and FT and high levels of SHBG based on the 2.5th percentile cutoff obtained from the Generation 3 healthy reference sample. Statistical significance level was set at two-sided P < 0.05.
Results
Demographic data
The baseline characteristics of men in our study sample with sex hormone and mobility data are shown in Table 1. The men in our sample were on average 61.0 yr at baseline with mean TT, FT, and SHBG levels of 583 ± 227 ng/dl, 86 ± 32 pg/ml, and 58 ± 27 nmol/liter, respectively, and 15.4% had low TT, 31.6% had low FT, and 15.5% had high SHBG levels. The proportion of men with self-reported mobility limitation at baseline was 6.4 and 7.1% of men reported poor subjective health. In the sample of men with physical performance data, the mean SPPB score was 10.9, usual walking speed 1.25 m/sec, and hand grip strength 42.4 kg. Thirteen percent (n = 144) of the sample reported an occurrence of mobility limitation at examination 8, and 14% (n = 163) reported a progression of their mobility limitation from examination 7 to 8.
Table 1.
Baseline characteristics at examination 7
| Characteristic | Cross-sectional analyses
|
Longitudinal analyses: men with incident mobility limitation data (n = 1111) | |
|---|---|---|---|
| Men with mobility limitation data (n = 1445) | Men with physical performance data (n = 832) | ||
| Age (yr) | 61.0 (9.5) | 61.6 (9.3) | 59.6 (9.0) |
| Smoking (%) | 12.7 | 11.5 | 11.8 |
| Alcohol consumption, drinks/wk (%) | |||
| None | 27.3 | 28.9 | 25.8 |
| 1–14 | 69.1 | 67.4 | 71.1 |
| >14 | 3.6 | 3.7 | 3.1 |
| BMI (kg/m2) | 28.8 (4.5) | 28.8 (4.5) | 28.7 (4.5) |
| Prevalent cardiovascular disease (%) | 17.6 | 17.3 | 13.1 |
| Cancer (%) | 9.7 | 10.8 | 7.7 |
| TT (ng/dl) | 583.5 (226.5) | 584.4 (229.4) | 589.9 (228.7) |
| FT (pg/ml) | 86.1 (31.8) | 86.2 (32.0) | 88.6 (31.4) |
| SHBG (nmol/liter) | 58.2 (26.7) | 58.2 (26.3) | 56.4 (25.5) |
| Men with low TT (%)a | 15.4 | 15.1 | 15.0 |
| Men with low FT (%) | 31.6 | 31.3 | 27.7 |
| Men with high SHBG (%) | 15.5 | 15.5 | 14.0 |
| Self-reported mobility limitation (%) | 6.4 | 5.8 | 0.0 |
| Poor subjective health (%) | 7.1 | 6.4 | 4.9 |
| SPPB summary score | 10.9 (1.30) | 11.0 (1.10) | |
| Usual walking speed (m/sec) | 1.25 (0.30) | 1.28 (0.29) | |
| Grip strength (kg) | 42.4 (12.5) | 43.5 (12.0) | |
Values are mean (sd) for continuous variables and percentages for dichotomous characteristics. To convert TT to SI units (nanomoles per liter), multiply TT concentrations in nanograms per deciliter by 0.0347. To convert FT to SI units (picomoles per liter), multiply FT concentration in picograms per milliliter by 3.47.
Sex hormones were defined as low or high vs. normal using healthy reference sample of FHS Generation 3 men. TT and FT levels below the 2.5th percentile of the referent sample (TT <348.3 ng/dl; FT <70.0 pg/ml) were deemed low, and SHBG levels above the 97.5th percentile of the referent sample (SHBG, 81.6 nmol/liter) were deemed high levels.
Cross-sectional relation between sex hormones and mobility and physical performance
The cross-sectional associations between circulating levels of sex hormones and self-reported mobility limitations and subjective health at examination 7 are presented in Table 2. TT and SHBG were not significantly associated with mobility limitation or subjective health. FT levels were not significantly associated with mobility but were associated with subjective health. As FT increased, the chances of reporting poor subjective health decreased; 1 sd increase in FT was associated with a 28% decrease in the odds of reporting poor subjective health [multivariable-adjusted odds ratio (OR) = 0.72; 95% confidence interval (CI) = 0.56–0.94]. Compared with men with normal FT levels, men with low FT levels had an increased risk of reporting poor subjective health (OR = 1.61; 95% CI = 1.02–2.55). The cross-sectional associations between circulating levels of sex hormones and physical performance measures at baseline examination 7 are presented in Table 3. TT and SHBG were not significantly associated with any of the physical performance measures. FT levels were significantly associated with SPPB score and usual walking speed. As FT increased, SPPB score and usual walking speed increased as well; each sd increase in FT was associated with a 0.13-U increase in SPPB score (P = 0.008) and 0.02 m/sec increase in usual walking speed (P = 0.048). Men with low FT were also more likely to have lower grip strength (adjusted mean difference between men with low and high FT = −2.01; 95% CI = −3.95 to −0.07) than those with normal FT. Low TT and high SHBG were not significantly associated with any mobility or physical performance measure.
Table 2.
Cross-sectional associations between baseline circulating sex hormone levels and self-reported mobility limitation at examination 7 (n = 1445)
| Multivariable logistic regression
|
||||
|---|---|---|---|---|
| Mobility limitation
|
Subjective health
|
|||
| OR (95% CI) | P | OR (95% CI) | P | |
| Continuous hormone levels | ||||
| TT | 0.89 (0.70–1.13) | 0.34 | 0.80 (0.63–1.01) | 0.06 |
| FT | 0.80 (0.61–1.03) | 0.09 | 0.72 (0.56–0.94) | 0.01 |
| SHBG | 1.13 (0.91–1.40) | 0.28 | 1.05 (0.85–1.31) | 0.64 |
| Dichotomized sex hormone levels | ||||
| Low TT | 1.12 (0.62–2.02) | 0.70 | 1.09 (0.62–1.89) | 0.77 |
| Low FT | 1.29 (0.80–2.08) | 0.30 | 1.61 (1.02–2.55) | 0.04 |
| High SHBG | 1.31 (0.75–2.28) | 0.34 | 1.20 (0.69–2.09) | 0.51 |
Continuous hormone levels and OR values are for 1 sd change in sex hormone levels. All models were adjusted for age, BMI, smoking, and comorbidities (cancer and cardiovascular disease) at examination 7. Sex hormones were defined as low or high vs. normal using healthy reference sample of FHS Generation 3 men. Low TT and FT levels were those below the 2.5th percentile of the referent sample (TT <348.3 ng/dl; FT <70.0 pg/ml), and SHBG levels above the 97.5th percentile of the referent sample (SHBG, 81.6 nmol/liter) represented high SHBG levels. Low FT levels (<70 pg/ml) were associated with increased risk (OR = 1.61) of poor subjective health; each sd increase in FT level was associated with a 28% decease (OR = 0.72) in risk of reporting poor subjective health.
Table 3.
Cross-sectional associations between baseline circulating sex hormone levels and physical performance at examination 7 (n = 832)
| Multivariable linear regression
|
||||||
|---|---|---|---|---|---|---|
| SPPB score
|
Usual walking speed (m/sec)
|
Grip strength (kg)
|
||||
| β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | |
| Continuous hormone levels | ||||||
| TT | 0.06 (−0.03–0.15) | 0.18 | 0.02 (−0.006–0.04) | 0.15 | 0.35 (−0.55–1.24) | 0.45 |
| FT | 0.13 (0.03–0.22) | 0.008 | 0.02 (0.0001–0.04) | 0.048 | 0.57 (−0.33–1.48) | 0.21 |
| SHBG | −0.08 (−0.17–0.02) | 0.13 | −0.01 (−0.04–0.009) | 0.23 | −0.41 (−1.38–0.56) | 0.41 |
| Dichotomized sex hormone levels | ||||||
| Low TT | −0.14 (−0.40–0.11) | 0.27 | −0.01 (−0.07–0.05) | 0.65 | −1.73 (−4.16–0.70) | 0.16 |
| Low FT | −0.13 (−0.34–0.07) | 0.20 | −0.03 (−0.08–0.01) | 0.16 | −2.01 (−3.95–0.07) | 0.04 |
| High SHBG | −0.08 (−0.33–0.18) | 0.55 | −0.05 (−0.11–0.007) | 0.09 | 0.38 (−2.13–2.88) | 0.77 |
Continuous hormone levels and β−values are for 1 sd change in sex hormone levels. All models were adjusted for age, BMI, smoking, and comorbidities (cancer and cardiovascular disease) at examination 7. Sex hormones were defined as low or high vs. normal using healthy reference sample of FHS Generation 3 men. Low TT and FT levels were those below the 2.5th percentile of the referent sample (TT <348.3 ng/dl; FT <70.0 pg/ml), and SHBG levels above the 97.5th percentile of the referent sample (SHBG, 81.6 nmol/liter) represented high SHBG levels. Low FT levels (<70 pg/ml) were associated with decreased grip strength. Each sd increase in FT level was associated with 0.13 U increase in SPPB score and 0.02 m/sec increase in usual walking speed.
Longitudinal relation between sex hormones and mobility and physical performance
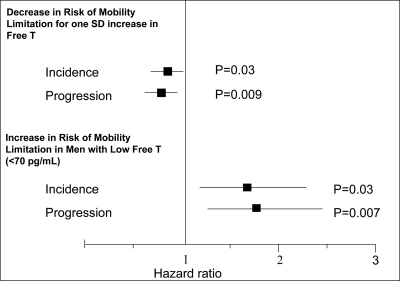
The results of our primary analyses of the impact of hormones on development of mobility limitations and decline in subjective health are presented in Table 4. Baseline low FT was a significant predictor of incident mobility limitation (Fig. 2). As FT increased by 1 sd, the risk of developing mobility limitation decreased by 22% (OR = 0.78; 95% CI = 0.62–0.97). FT levels were also significantly associated with progression of mobility limitation (OR = 0.75; 95% CI = 0.60–0.93). Thus, men were 25% less likely to report worsening mobility limitation for each sd increase in circulating FT. No significant relationships were observed between FT and subjective health in the longitudinal analysis. TT, FT, and SHBG levels were not significantly associated with change in usual walking speed or handgrip strength from examination 7 to 8 (data not shown).
Table 4.
Longitudinal relations between baseline circulating sex hormone levels and incident mobility limitation: multivariable logistic regression (n = 1111)
| Mobility limitation
|
Subjective health
|
|||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Continuous hormone levels | ||||
| TT | 0.90 (0.74–1.09) | 0.28 | 1.09 (0.80–1.47) | 0.59 |
| FT | 0.78 (0.62−0.97) | 0.03 | 0.87 (0.62–1.22) | 0.42 |
| SHBG | 1.11 (0.91–1.34) | 0.31 | 1.29 (0.97–1.72) | 0.08 |
| Dichotomized sex hormone levels | ||||
| Low TT | 1.46 (0.91–2.34) | 0.12 | 0.48 (0.19–1.24) | 0.13 |
| Low FT | 1.57 (1.06–2.32) | 0.03 | 1.23 (0.64–2.38) | 0.53 |
| High SHBG | 1.29 (0.80–2.10) | 0.30 | 1.59 (0.74–3.43) | 0.24 |
OR values are for 1 sd change in hormone levels. For incidence, the sample excludes subjects at exam 7 with mobility limitation and poor subjective health. For progression, the sample excludes subjects at exam 7 with worst response choice for mobility limitation and subjective health. All models were adjusted for age, BMI, smoking, and comorbidities (cancer and cardiovascular disease). Sex hormones were defined as low or high vs. normal using healthy reference sample of FHS Generation 3 men. Low TT and FT levels were those below the 2.5th percentile of the referent sample (TT <348.3 ng/dl; FT <70.0 pg/ml), and SHBG levels above the 97.5th percentile of the referent sample (SHBG, 81.6 nmol/liter) represented high SHBG levels. Low FT levels (<70 pg/ml) at examination 7 were associated with increased risk (OR = 1.57) of developing mobility limitation at examination 8. Each sd increase in FT level at examination 7 was associated with a 22% decrease (OR = 0.78) in risk of reporting mobility limitation at examination 8.
Figure 2.
Longitudinal analyses of incident mobility limitation. Continuous FT level hazard ratios are for 1 sd increase in hormone levels, adjusting for age, BMI, smoking, and comorbidities (cardiovascular disease and cancer). As shown in the upper panel, each sd increase in FT level was associated with 22% (OR = 0.78; 95% CI = 0.62–0.97) decrease in the risk of developing mobility limitation and 25% decrease in the risk of worsening mobility limitation (progression). The lower panel shows the association of low FT (<2.5th percentile (<70.0 pg/ml)) at baseline examination 7 with the risk of developing (incident) mobility limitation at examination 8 or of reporting worsening mobility limitation (progression) at examination 8. The FT hazard ratios were adjusted for age, BMI, smoking, and comorbidities. The squares indicate point estimates for hormones, and the lines indicate 95% CI.
Men with low FT were 57% more likely to develop mobility limitation on follow-up (OR = 1.57; 95% CI = 1.06–2.32) and 68% more likely to experience worsening of mobility limitation (OR = 1.68; 95% CI = 1.16–2.45) compared with men with normal FT (Table 4 and Figure 2). Low TT and high SHBG were not associated with either incident mobility limitation or progression of mobility limitation.
Discussion
In our community-based sample of men, higher baseline FT levels (both continuous and threshold values) were significantly associated with lower odds of an incident mobility limitation. Baseline FT was also a significant correlate of progression of mobility limitation, consistent with the incidence findings. Furthermore, FT was positively associated with faster baseline usual walking speed and SPPB score, a valid measure of lower extremity function and an important determinant of mobility. Thus, baseline FT is a significant correlate of both self-reported and performance-based measures of mobility. TT and SHBG were not associated with any of the mobility or physical performance measures.
According to the free hormone hypothesis, FT, representing the unbound hormone, is considered the biologically active fraction of testosterone. Although bioavailable testosterone has been reported to be associated with self-reported mobility limitation, muscle strength, and physical performance measures (39), we did not analyze bioavailable testosterone because it is a calculated multiple of FT. However, recent data suggest that SHBG-bound testosterone may be internalized through endocytic pits after binding to the megalin receptor and may also be biologically relevant (40). Indeed, in the Massachusetts Male Ageing Study, SHBG, rather than TT or FT, was associated with frailty (41). Our data support the free hormone hypothesis and suggest that FT may mediate most of the effects of testosterone on physical function measures because we did not find any relationship between SHBG and TT with mobility or physical performance measures.
FT levels were associated with subjective health in cross-sectional but not longitudinal analyses. Although testosterone may not be causally related to subjective health, it is possible that factors that contribute to poor subjective health such as comorbid conditions may also lower testosterone levels. It is possible that individuals whose health deteriorated between examinations 7 and 8 did not return for follow-up. Of the men that did not return for examination 8, or had missing incident mobility limitation at examination 8, 11.8% had reported poor subjective health at examination 7 compared with 7.1% of men in the total sample. This may also explain why fewer men reported poor subjective health at examination 8. Therefore, the positive longitudinal associations between FT and mobility limitations and their progression are all the more remarkable.
The observed association between FT and mobility measures has biological plausibility. Testosterone is an important determinant of skeletal muscle mass (17) and increases muscle mass by promoting myogenic differentiation of multipotent mesenchymal stem cells (42,43) and by stimulating muscle protein synthesis (44,45). Testosterone administration also increases maximal voluntary strength and power in men (29,30,46). However, the association of testosterone with physical function measures in epidemiological studies has been inconsistent. Although some studies have found testosterone levels to be related to self-reported (19) as well as performance-based measures of physical function (18,21,47), frailty (48), and falls (39), a recent prospective analysis of two cohorts did not find any significant association of either TT or FT with decline in physical function or muscle strength (20). The effects of testosterone therapy on physical function measures in randomized testosterone trials have been heterogeneous. Some trials have reported improvements in gait speed, stair climbing power, and composite measures of physical function (49,50), whereas others failed to find significant effects (51,52,53). However, older men included in the first-generation testosterone trials were not uniformly hypogonadal (51,52,53). Also, most of the studies included healthy older men without functional limitations and used tests of physical function that had low ceiling (54). Finally, testosterone doses in some trials were small and did not significantly raise serum testosterone (51,52). We have shown that testosterone administration in young and older men is associated with dose-dependent increments in skeletal muscle mass and maximal voluntary strength (30,55,56).
We observed that circulating FT levels were significantly associated with both SPPB scores and walking speed. Each sd increase in FT was associated with a 0.13-U increase in SPPB score and 0.02-m/sec in usual walking speed. Perera et al. (57) have deemed a 0.5-U change in SPPB score and a 0.05-m/sec change in gait speed to be clinically important changes. Thus, testosterone levels have a small but significant effect on these measures of physical performance. Indeed, testosterone is only one of many physiological processes that regulate complex functions such as walking, although it is an important remediable factor and, therefore, the subject of current investigation.
Our study has significant strengths. First, the FHS cohort included community-dwelling men over a wider age range than has been included in some other studies that were focused mostly on older men. The longitudinal design of our analyses lends strength to our inferences. We adjusted our analyses for potential confounders, including age, BMI, smoking, and comorbidities. This is the first population study to evaluate the relationship between mobility and physical function with TT levels measured by LC-MS/MS, widely considered the gold standard for the measurement of testosterone levels (23). We defined reference ranges of testosterone levels by using healthy young men, age 19–40 yr, and evaluated the relationship between androgens and mobility based on these population-based thresholds.
Our study also has some limitations. First, epidemiological studies including longitudinal studies can define associations but not causality. Second, our study population was white, and therefore, our findings may not be generalizable to other race/ethnicities. Also, of the 1445 men who were evaluated for mobility limitation at examination 7, 280 did not return for examination 8 or had missing mobility limitation data at examination 8. Importantly, men who did not return for examination 8 had a higher frequency of mobility limitation, lower SPPB score, and slower walking speed at baseline than those who did return for examination 8. Thus, it is possible that some of the subjects with poor health whose health deteriorated did not return for follow-up, thus diluting the observed effects. Therefore, our longitudinal analyses likely represent a conservative estimate of the association between FT levels and mobility limitation and walking speed. We did not have sex hormones measured at examination 8 to evaluate the correlation between the change in testosterone levels and incident mobility. We did not measure estradiol levels and were unable to dissect out the possible role of aromatization on these outcomes. Serum testosterone levels are affected by pulsatile, diurnal, and circannual rhythms, and single samples ignore rhythmic hormone secretion. Our analyses show that single early morning testosterone levels, obtained in a manner similar to that by physicians in real practice, were associated with mobility limitation and some other measures of physical function. Therefore, even though our models did not factor in the complexities of biological rhythms, they are in concordance with the need of practitioners to depend on conveniently obtained single samples. Finally, the Framingham cohort was younger and healthier than some other epidemiological studies, resulting in fewer events and lower rates of worsening of physical function; this may have reduced the statistical power. We had 0.685 power to detect an association of similar magnitude for TT to that obtained from FT at an α of 0.05. The follow-up of these men over a still longer period of time resulting in potentially more events could increase the power.
These data have clinical implications. Mobility is one of the most important physical functions, essential for independent living. Our data show that men with low FT had a 57% greater risk of developing a mobility limitation and a 68% higher risk of deterioration in their mobility. Whether this risk can be reduced with exogenous testosterone therapy in older men with mobility limitation and low free testosterone levels would need to be determined by a randomized clinical trial.
Footnotes
This work was supported by National Institute on Aging, 1RO1AG31206; Boston Claude D. Pepper Older Americans Independence Center, 5P30 AG31679; and NIH grants AG029451-01A2 and 2R01 AG16495. The Framingham Heart Study of the National Heart, Lung, and Blood Institute of the National Institutes of Health and Boston University School of Medicine was supported by the National Heart, Lung, and Blood Institute's Framingham Heart Study contract No. N01-HC-25195.
Disclosure Summary: S.B. received research support from the Centers for Disease Control Foundation.
First Published Online April 9, 2010
For editorial see page 2634
Abbreviations: BMI, Body mass index; CI, confidence interval; FHS, Framingham Heart Study; FT, free testosterone; LC-MS/MS, liquid chromatography tandem mass spectrometry; OR, odds ratio; SPPB, short physical performance battery; TT, total testosterone.
References
- Shumway-Cook A, Ciol MA, Yorkston KM, Hoffman JM, Chan L 2005 Mobility limitations in the Medicare population: prevalence and sociodemographic and clinical correlates. J Am Geriatr Soc 53:1217–1221 [DOI] [PubMed] [Google Scholar]
- Rubenstein LZ, Powers CM, MacLean CH 2001 Quality indicators for the management and prevention of falls and mobility problems in vulnerable elders. Ann Intern Med 135:686–693 [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB 1995 Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med 332:556–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bonsdorff M, Rantanen T, Laukkanen P, Suutama T, Heikkinen E 2006 Mobility limitations and cognitive deficits as predictors of institutionalization among community-dwelling older people. Gerontology 52:359–365 [DOI] [PubMed] [Google Scholar]
- Groessl EJ, Kaplan RM, Rejeski WJ, Katula JA, King AC, Frierson G, Glynn NW, Hsu FC, Walkup M, Pahor M 2007 Health-related quality of life in older adults at risk for disability. Am J Prev Med 33:214–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metter EJ, Schrager M, Ferrucci L, Talbot LA 2005 Evaluation of movement speed and reaction time as predictors of all-cause mortality in men. J Gerontol A Biol Sci Med Sci 60:840–846 [DOI] [PubMed] [Google Scholar]
- Hurley BF 1995 Age, gender, and muscular strength. J Gerontol A Biol Sci Med Sci 50(Spec No):41–44 [DOI] [PubMed] [Google Scholar]
- Lauretani F, Russo CR, Bandinelli S, Bartali B, Cavazzini C, Di Iorio A, Corsi AM, Rantanen T, Guralnik JM, Ferrucci L 2003 Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol 95:1851–1860 [DOI] [PubMed] [Google Scholar]
- Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, Bremner WJ, McKinlay JB 2002 Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab 87:589–598 [DOI] [PubMed] [Google Scholar]
- Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR 2001 Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab 86:724–731 [DOI] [PubMed] [Google Scholar]
- Morley JE, Kaiser FE, Perry 3rd HM, Patrick P, Morley PM, Stauber PM, Vellas B, Baumgartner RN, Garry PJ 1997 Longitudinal changes in testosterone, luteinizing hormone, and follicle-stimulating hormone in healthy older men. Metabolism 46:410–413 [DOI] [PubMed] [Google Scholar]
- Simon D, Preziosi P, Barrett-Connor E, Roger M, Saint-Paul M, Nahoul K, Papoz L 1992 The influence of aging on plasma sex hormones in men: the Telecom Study. Am J Epidemiol 135:783–791 [DOI] [PubMed] [Google Scholar]
- Wu FC, Tajar A, Pye SR, Silman AJ, Finn JD, O'Neill TW, Bartfai G, Casanueva F, Forti G, Giwercman A, Huhtaniemi IT, Kula K, Punab M, Boonen S, Vanderschueren D 2008 Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab 93:2737–2745 [DOI] [PubMed] [Google Scholar]
- Zmuda JM, Cauley JA, Kriska A, Glynn NW, Gutai JP, Kuller LH 1997 Longitudinal relation between endogenous testosterone and cardiovascular disease risk factors in middle-aged men. A 13-year follow-up of former Multiple Risk Factor Intervention Trial participants. Am J Epidemiol 146:609–617 [DOI] [PubMed] [Google Scholar]
- Muller M, den Tonkelaar I, Thijssen JH, Grobbee DE, van der Schouw YT 2003 Endogenous sex hormones in men aged 40–80 years. Eur J Endocrinol 149:583–589 [DOI] [PubMed] [Google Scholar]
- Roy TA, Blackman MR, Harman SM, Tobin JD, Schrager M, Metter EJ 2002 Interrelationships of serum testosterone and free testosterone index with FFM and strength in aging men. Am J Physiol Endocrinol Metab 283:E284–E294 [DOI] [PubMed] [Google Scholar]
- Baumgartner RN, Waters DL, Gallagher D, Morley JE, Garry PJ 1999 Predictors of skeletal muscle mass in elderly men and women. Mech Ageing Dev 107:123–136 [DOI] [PubMed] [Google Scholar]
- Schaap LA, Pluijm SM, Smit JH, van Schoor NM, Visser M, Gooren LJ, Lips P 2005 The association of sex hormone levels with poor mobility, low muscle strength and incidence of falls among older men and women. Clin Endocrinol (Oxf) 63:152–160 [DOI] [PubMed] [Google Scholar]
- O'Donnell AB, Travison TG, Harris SS, Tenover JL, McKinlay JB 2006 Testosterone, dehydroepiandrosterone, and physical performance in older men: results from the Massachusetts Male Aging Study. J Clin Endocrinol Metab 91:425–431 [DOI] [PubMed] [Google Scholar]
- Schaap LA, Pluijm SM, Deeg DJ, Penninx BW, Nicklas BJ, Lips P, Harris TB, Newman AB, Kritchevsky SB, Cauley JA, Goodpaster BH, Tylavsky FA, Yaffe K, Visser M 2008 Low testosterone levels and decline in physical performance and muscle strength in older men: findings from two prospective cohort studies. Clin Endocrinol (Oxf) 68:42–50 [DOI] [PubMed] [Google Scholar]
- Araujo AB, Travison TG, Bhasin S, Esche GR, Williams RE, Clark RV, McKinlay JB 2008 Association between testosterone and estradiol and age-related decline in physical function in a diverse sample of men. J Am Geriatr Soc 56:2000–2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhasin S, Zhang A, Coviello A, Jasuja R, Ulloor J, Singh R, Vesper H, Vasan RS 2008 The impact of assay quality and reference ranges on clinical decision making in the diagnosis of androgen disorders. Steroids 73:1311–1317 [DOI] [PubMed] [Google Scholar]
- Rosner W, Auchus RJ, Azziz R, Sluss PM, Raff H 2007 Position statement: Utility, limitations, and pitfalls in measuring testosterone: an Endocrine Society position statement. J Clin Endocrinol Metab 92:405–413 [DOI] [PubMed] [Google Scholar]
- Dawber TR, Meadors GF, Moore Jr FE 1951 Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health 41:279–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP 1979 An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol 110:281–290 [DOI] [PubMed] [Google Scholar]
- Sattler FR, Castaneda-Sceppa C, Binder EF, Schroeder ET, Wang Y, Bhasin S, Kawakubo M, Stewart Y, Yarasheski KE, Ulloor J, Colletti P, Roubenoff R, Azen SP 2009 Testosterone and growth hormone improve body composition and muscle performance in older men. J Clin Endocrinol Metab 94:1991–2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sir-Petermann T, Codner E, Pérez V, Echiburú B, Maliqueo M, Ladrón de Guevara A, Preisler J, Crisosto N, Sánchez F, Cassorla F, Bhasin S 2009 Metabolic and reproductive features before and during puberty in daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab 94:1923–1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesper HW, Botelho JC, Shacklady C, Smith A, Myers GL 2008 CDC project on standardizing steroid hormone measurements. Steroids 73:1286–1292 [DOI] [PubMed] [Google Scholar]
- Bhasin S, Storer TW, Javanbakht M, Berman N, Yarasheski KE, Phillips J, Dike M, Sinha-Hikim I, Shen R, Hays RD, Beall G 2000 Testosterone replacement and resistance exercise in HIV-infected men with weight loss and low testosterone levels. JAMA 283:763–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhasin S, Woodhouse L, Casaburi R, Singh AB, Mac RP, Lee M, Yarasheski KE, Sinha-Hikim I, Dzekov C, Dzekov J, Magliano L, Storer TW 2005 Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab 90:678–688 [DOI] [PubMed] [Google Scholar]
- Mazer NA 2009 A novel spreadsheet method for calculating the free serum concentrations of testosterone, dihydrotestosterone, estradiol, estrone and cortisol: with illustrative examples from male and female populations. Steroids 74:512–519 [DOI] [PubMed] [Google Scholar]
- Vermeulen A, Verdonck L, Kaufman JM 1999 A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 84:3666–3672 [DOI] [PubMed] [Google Scholar]
- Sartorius G, Ly LP, Sikaris K, McLachlan R, Handelsman DJ 2009 Predictive accuracy and sources of variability in calculated free testosterone estimates. Ann Clin Biochem 46:137–143 [DOI] [PubMed] [Google Scholar]
- Rosow I, Breslau N 1966 A Guttman health scale for the aged. J Gerontol 21:556–559 [DOI] [PubMed] [Google Scholar]
- Beckett LA, Brock DB, Lemke JH, Mendes de Leon CF, Guralnik JM, Fillenbaum GG, Branch LG, Wetle TT, Evans DA 1996 Analysis of change in self-reported physical function among older persons in four population studies. Am J Epidemiol 143:766–778 [DOI] [PubMed] [Google Scholar]
- Crawford SL, Jette AM, Tennstedt SL 1997 Test-retest reliability of self-reported disability measures in older adults. J Am Geriatr Soc 45:338–341 [DOI] [PubMed] [Google Scholar]
- Brorsson B, Asberg KH 1984 Katz index of independence in ADL. Reliability and validity in short-term care. Scand J Rehabil Med 16:125–132 [PubMed] [Google Scholar]
- Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB 1994 A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 49:M85–M94 [DOI] [PubMed] [Google Scholar]
- Orwoll E, Lambert LC, Marshall LM, Blank J, Barrett-Connor E, Cauley J, Ensrud K, Cummings SR 2006 Endogenous testosterone levels, physical performance, and fall risk in older men. Arch Intern Med 166:2124–2131 [DOI] [PubMed] [Google Scholar]
- Hammes A, Andreassen TK, Spoelgen R, Raila J, Hubner N, Schulz H, Metzger J, Schweigert FJ, Luppa PB, Nykjaer A, Willnow TE 2005 Role of endocytosis in cellular uptake of sex steroids. Cell 122:751–762 [DOI] [PubMed] [Google Scholar]
- Mohr BA, Bhasin S, Kupelian V, Araujo AB, O'Donnell AB, McKinlay JB 2007 Testosterone, sex hormone-binding globulin, and frailty in older men. J Am Geriatr Soc 55:548–555 [DOI] [PubMed] [Google Scholar]
- Singh R, Artaza JN, Taylor WE, Gonzalez-Cadavid NF, Bhasin S 2003 Androgens stimulate myogenic differentiation and inhibit adipogenesis in C3H 10T1/2 pluripotent cells through an androgen receptor-mediated pathway. Endocrinology 144:5081–5088 [DOI] [PubMed] [Google Scholar]
- Singh R, Bhasin S, Braga M, Artaza JN, Pervin S, Taylor WE, Krishnan V, Sinha SK, Rajavashisth TB, Jasuja R 2009 Regulation of myogenic differentiation by androgens: cross talk between androgen receptor/β-catenin and follistatin/transforming growth factor-β signaling pathways. Endocrinology 150:1259–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky IG, Balagopal P, Nair KS 1996 Effects of testosterone replacement on muscle mass and muscle protein synthesis in hypogonadal men: a clinical research center study. J Clin Endocrinol Metab 81:3469–3475 [DOI] [PubMed] [Google Scholar]
- Ferrando AA, Sheffield-Moore M, Paddon-Jones D, Wolfe RR, Urban RJ 2003 Differential anabolic effects of testosterone and amino acid feeding in older men. J Clin Endocrinol Metab 88:358–362 [DOI] [PubMed] [Google Scholar]
- Bhasin S, Storer TW, Berman N, Callegari C, Clevenger B, Phillips J, Bunnell TJ, Tricker R, Shirazi A, Casaburi R 1996 The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med 335:1–7 [DOI] [PubMed] [Google Scholar]
- Szulc P, Claustrat B, Marchand F, Delmas PD 2003 Increased risk of falls and increased bone resorption in elderly men with partial androgen deficiency: the MINOS study. J Clin Endocrinol Metab 88:5240–5247 [DOI] [PubMed] [Google Scholar]
- Cawthon PM, Ensrud KE, Laughlin GA, Cauley JA, Dam TT, Barrett-Connor E, Fink HA, Hoffman AR, Lau E, Lane NE, Stefanick ML, Cummings SR, Orwoll ES 2009 Sex hormones and frailty in older men: the osteoporotic fractures in men (MrOS) study. J Clin Endocrinol Metab 94:3806–3815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill KT, Weltman AL, Gentili A, Patrie JT, Fryburg DA, Hanks JB, Urban RJ, Veldhuis JD 2002 Single and combined effects of growth hormone and testosterone administration on measures of body composition, physical performance, mood, sexual function, bone turnover, and muscle gene expression in healthy older men. J Clin Endocrinol Metab 87:5649–5657 [DOI] [PubMed] [Google Scholar]
- Page ST, Amory JK, Bowman FD, Anawalt BD, Matsumoto AM, Bremner WJ, Tenover JL 2005 Exogenous testosterone (T) alone or with finasteride increases physical performance, grip strength, and lean body mass in older men with low serum T. J Clin Endocrinol Metab 90:1502–1510 [DOI] [PubMed] [Google Scholar]
- Emmelot-Vonk MH, Verhaar HJ, Nakhai Pour HR, Aleman A, Lock TM, Bosch JL, Grobbee DE, van der Schouw YT 2008 Effect of testosterone supplementation on functional mobility, cognition, and other parameters in older men: a randomized controlled trial. JAMA 299:39–52 [DOI] [PubMed] [Google Scholar]
- Nair KS, Rizza RA, O'Brien P, Dhatariya K, Short KR, Nehra A, Vittone JL, Klee GG, Basu A, Basu R, Cobelli C, Toffolo G, Dalla Man C, Tindall DJ, Melton 3rd LJ, Smith GE, Khosla S, Jensen MD 2006 DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med 355:1647–1659 [DOI] [PubMed] [Google Scholar]
- Snyder PJ, Peachey H, Hannoush P, Berlin JA, Loh L, Lenrow DA, Holmes JH, Dlewati A, Santanna J, Rosen CJ, Strom BL 1999 Effect of testosterone treatment on body composition and muscle strength in men over 65 years of age. J Clin Endocrinol Metab 84:2647–2653 [DOI] [PubMed] [Google Scholar]
- LeBrasseur NK, Bhasin S, Miciek R, Storer TW 2008 Tests of muscle strength and physical function: reliability and discrimination of performance in younger and older men and older men with mobility limitations. J Am Geriatr Soc 56:2118–2123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhasin S, Woodhouse L, Casaburi R, Singh AB, Bhasin D, Berman N, Chen X, Yarasheski KE, Magliano L, Dzekov C, Dzekov J, Bross R, Phillips J, Sinha-Hikim I, Shen R, Storer TW 2001 Testosterone dose-response relationships in healthy young men. Am J Physiol Endocrinol Metab 281:E1172–E1181 [DOI] [PubMed] [Google Scholar]
- Storer TW, Magliano L, Woodhouse L, Lee ML, Dzekov C, Dzekov J, Casaburi R, Bhasin S 2003 Testosterone dose-dependently increases maximal voluntary strength and leg power, but does not affect fatigability or specific tension. J Clin Endocrinol Metab 88:1478–1485 [DOI] [PubMed] [Google Scholar]
- Perera S, Mody SH, Woodman RC, Studenski SA 2006 Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc 54:743–749 [DOI] [PubMed] [Google Scholar]