Abstract
Context: Considerable evidence indicates that osteoblasts and adipocytes share a common progenitor cell in the bone marrow that is capable of mutually exclusive differentiation into the cell lineages responsible for bone and fat formation.
Objective: The purpose of this study was to examine the relation between bone acquisition and changes in marrow adiposity.
Design: This was a longitudinal study.
Outcome Measures and Subjects: Computed tomography measurements of femoral cortical bone area (CBA), cross-sectional area (CSA), and marrow density, and dual-energy x-ray absorptiometry (DXA) measurements of total body fat and lean mass (LM) were obtained in 39 healthy females (15–20 yr of age) at baseline and 18–24 months later.
Results: Marrow adiposity was inversely related to CBA at baseline and follow-up (r = 0.39 and 0.33; P = 0.015 and 0.039, respectively) but was not associated to CSA (r = 0.19 and 0.17; P = 0.24 and 0.32, respectively). The association between marrow fat and CBA persisted, even after controlling for body mass and DXA values of LM and femoral CSA. Gains in CBA during the course of the study were related to decreases in marrow fat (r = 0.41; P = 0.009), a relation that persisted, even after accounting for changes in bone size. Marrow fat was not associated to anthropometric measures or DXA values of body fat and LM (all r's between −0.15 and 0.19; P > 0.05).
Conclusions: Bone acquisition in the appendicular skeleton of healthy young females is inversely related to changes in marrow adiposity. These results provide support for the growing body of evidence indicating an inversely coupled relationship between osteogenesis and adipogenesis in the skeleton.
Bone acquisition in the appendicular skeleton is inversely related to changes in marrow adiposity.
Bone marrow houses a diverse population of cells belonging to several lineages, including stem cells of hematopoietic origin and those of mesenchymal origin, which hold the capacity to differentiate into osteoblasts, adipocytes, fibroblasts, chondrocytes, and myocytes. A large body of in vitro and animal literature indicates that, depending on the interplay of molecular, biochemical, and physical stimuli, mesenchymal stem cells (MSCs) differentiate into the cell lineages responsible for bone and fat formation through alternative activation of mutually exclusive transcriptional programs (1,2,3,4,5). Multiple basic studies have examined the roles of hormones and their receptors (6,7,8,9,10,11,12,13,14), cytokines and other serum factors (15,16,17,18,19), and mechanical stimuli (20,21) in the commitment of MSCs isolated from bone marrow stroma toward adipocytic or osteoblastic lineages.
It has been suggested that bone loss and osteoporosis in the elderly could be the consequence of a preferential age-related differentiation by MSCs into the adipocyte cell lineage at the expense of bone-forming osteoblasts (22,23,24,25,26,27,28,29,30,31,32). Support for this concept comes from histomorphometric and imaging studies showing an inverse association between bone mineral density and measures of marrow fat in older men and women (28,29,30,31,32). Some have even suggested that marrow adiposity could be an independent predictor of osteoporosis and fractures (25,26,27,28,29,31,32). Decreased mechanical loading on bone has been associated with increased marrow adiposity also in patients on prolonged bed rest (33). Moreover, we previously reported an inverse association between the amount of bone in the axial and appendicular skeletons and marrow adiposity in young men and women (34). Given the cross-sectional design of most of these studies, the strength of this evidence is limited. To definitively establish the link between osteogenesis and adipogenesis, we longitudinally studied whether bone acquisition in healthy young women was accompanied by a simultaneous decrease in marrow adiposity.
Subjects and Methods
Subjects
We enrolled 39 healthy white females 15–20 yr of age who had computed tomography (CT) determinations of bone and marrow density and dual-energy x-ray absorptiometry (DXA) bone measures obtained at baseline and 18–24 months later. At baseline, candidates for this study were excluded if they had a diagnosis of any underlying disease or chronic illness, they had been ill for longer than 2 wk during the previous 6 months, they had been admitted to the hospital at any time during the previous 3 yr, and they were taking any medications including oral contraceptives. Women with irregular menses, amenorrhea, or ovarian failure or who were pregnant were also excluded. All potential candidates underwent a general physical examination, including assessments of the degree of sexual development and a radiographic examination of the left hand and wrist. Only subjects who had reached sexual maturity, defined as Tanner V of sexual development (35), and skeletal maturity, defined as physeal closure in the phalanges and metacarpals using the radiographic atlas of Greulich and Pyle (36), were included in the study. Body mass index (BMI) percentiles were calculated based on the most current Centers for Disease Control and Prevention growth chart, which can be found at http://www.cdc.gov/growthcharts. The protocol for this study was approved by the institutional review board for clinical investigations at our institution, and all participants and/or their parents signed informed consent.
Measures of fat and bone
Subjects underwent CT measurements of bone and marrow using a General Electric Hilite Advantage scanner (General Electric Healthcare, Milwaukee, WI) and a standardized mineral reference phantom for simultaneous calibration (CT bone densitometry package; General Electric). All scans were obtained by the same CT technologist using the following technical factors: 120 kVp, 70 mAs, 2 sec, and 10-mm slice thickness. For this study, the cross-sectional area (CSA; square centimeters), cortical bone area (CBA; square centimeters), and cortical bone density (CBD; milligrams per cubic centimeter) at the midshafts of the femurs were obtained. Because of the thickness and the relative lack of porosity of the femurs in healthy young subjects, CBD values at this site reflect the material or true density of the bone (the amount of collagen and mineral in a given volume of bone) (37).
CT numbers express the measure of the linear attenuation of the x-ray beam through the medium in that space and are defined as Hounsfield units (HU), using the linear attenuation coefficient of water (HU = 0) and air (HU = −1000). Using these parameters, Hounsfield units for fat fall between a range of negative values (38). For the purpose of this study, CT values for marrow in Hounsfield units were converted into density values (grams per cubic centimeter) based on previously published studies that calculated CT attenuation values for human tissues (39,40,41). Because marrow is comprised of hematopoietic tissue (+Hounsfield units) with a density of 1.06 g/cm3, and fatty tissue (−Hounsfield units) with a density of 0.92 g/cm3, the higher the density of marrow tissue, the lower the fraction of marrow fat (41). This fraction changes during growth and throughout life in a predictable and orderly age-, bone-, and site-specific fashion (42,43,44). In the diaphyses of the long bones, the marrow reaches its adult pattern by 15 yr of age when it is mostly comprised of fat. Hence, at this site, CT values for the density of the marrow, even in young adulthood, mainly reflect the tissue density of fat because the influence of blood is minimized. The coefficients of variation for bone measurements in young adults are between 0.6 and 1.5% (37) and was calculated to be less than 1% for marrow density (34).
Measurements of total body fat (BF) and lean mass (LM) were obtained using a fan beam DXA densitometer (Delphi W; Hologic, Inc., Waltham, MA) in array mode and were analyzed with the manufacturer's software; the coefficients of variation for total BF and total LM measurements have been previously calculated to be 3.1 and 0.6%, respectively (45).
Statistical analyses
Student's t test for paired data were used to compare mean values between groups at baseline and at follow-up. Simple Pearson correlations were used to investigate the association between age, anthropometric and imaging parameters, and marrow density and to analyze the relations between changes in these variables. Multiple regression analyses were done using both the raw data and percent change of femoral CBA as the outcome measure and weight, height, BMI, DXA BF and LM, CSA, and marrow density changes as possible independent variables. StatView statistical software (SAS Institute Inc., Cary, NC) was used for these analyses. Values are expressed as mean ± sd, unless otherwise noted.
Results
Table 1 shows age, anthropometric measurements, and values for body composition and bone in all study subjects at baseline and follow-up. At follow up, subjects were significantly heavier, but not taller, and had greater body mass, DXA LM, and DXA BF than at baseline. Whereas both the CSA and CBA of the femur significantly increased (both P < 0.0001), femoral marrow adiposity significantly decreased (<0.001) at follow-up. In contrast, there was no significant difference in CBD (P = 0.396). All participants remained both healthy and medication free over the entire study.
Table 1.
Age, anthropometric parameters, total BF, total LM, and bone and marrow fat measurements at baseline and after 18–24 months in 39 females
| Baseline | Follow-up | Change, % | P value | |
|---|---|---|---|---|
| Age (yr) | 17.2 ± 1.4 (15.0–20.0) | 18.9 ± 1.3 (16.5–22.0) | <0.0001 | |
| Height (cm) | 162.9 ± 5.1 (154.8–176.8) | 163.1 ± 5.3 (154.8–177.8) | 0.1 ± 0.5 | 0.066 |
| Weight (kg) | 62.1 ± 8.5 (42.9–80.6) | 64.5 ± 10.0 (45.6–91.7) | 3.3 ± 6.6 | 0.004 |
| BMI (kg/m2) | 23.4 ± 3.1 (16.0–28.8) | 24.2 ± 3.6 (16.7–31.6) | 2.6 ± 6.4 | 0.016 |
| BMI percentile | 67.0 ± 25.1 (5.0–93.0) | 67.6 ± 24.4 (5.0–95.0) | 0.8 ± 18.7 | 0.660 |
| DXA LM (kg) | 33.97 ± 3.58 (27.59–41.74) | 34.82 ± 4.05 (27.59–42.68) | 2.0 ± 5.1 | 0.024 |
| DXA Fat mass (kg) | 20.17 ± 5.52 (7.15–30.46) | 21.54 ± 6.85 (10.54–40.33) | 6.3 ± 16.1 | 0.008 |
| CT femoral CSA (cm2) | 4.80 ± 0.47 (3.79–6.06) | 4.93 ± 0.45 (4.19–6.30) | 2.8 ± 3.4 | <0.0001 |
| CT femoral CBA (cm2) | 3.82 ± 0.33 (3.27–4.47) | 4.01 ± 0.34 (3.32–4.80) | 4.6 ± 4.2 | <0.0001 |
| CT femoral CBD (g/cm3) | 1152.4 ± 63.2 (1063.2–1280.5) | 1141.7 ± 58.7 (1060.9–1262.0) | 3.8 ± 3.3 | 0.396 |
| CT marrow density (g/cm3) | 0.96 ± 0.025 (0.93–1.045) | 0.98 ± 0.020 (0.95–1.042) | 1.5 ± 1.6 | <0.0001 |
Values are mean ± sd and range, in parentheses; P value for paired t test between baseline and follow-up groups.
The simple correlation between femoral CBA and age, anthropometric parameters, and imaging measures of bone, muscle, and fat at baseline and follow-up are shown in Table 2. Values for CBA at baseline and follow-up were directly related to height, weight, and measures of lean mass and femoral CSA but not associated with age or DXA measures of total body fat. In contrast, there was no significant association between marrow adiposity and age, height, weight, BMI, or DXA BF and LM at baseline and follow-up (all r's between −0.15 and 0.19; all P's > 0.05). CBD did not correlate with age, anthropometric measures, or values for marrow adiposity at baseline or follow-up (all P's > 0.05).
Table 2.
Simple Pearson correlations between femoral CBA and the variables age, anthropometric measures, body composition and bone parameters at baseline and after 18–24 months in 39 females
| Correlations with CT femoral CBA
|
||||
|---|---|---|---|---|
| Baseline
|
Follow-up
|
|||
| r | P | r | P | |
| Age (yr) | −0.09 | 0.608 | −0.26 | 0.111 |
| Height (cm) | 0.39 | 0.013 | 0.36 | 0.026 |
| Weight (kg) | 0.49 | 0.001 | 0.42 | 0.007 |
| BMI (kg/m2) | 0.32 | 0.046 | 0.30 | 0.066 |
| DXA LM (kg) | 0.49 | 0.003 | 0.53 | 0.001 |
| DXA fat mass (kg) | 0.26 | 0.134 | 0.24 | 0.156 |
| CT femoral CSA (cm2) | 0.83 | <0.0001 | 0.86 | <0.0001 |
| CT marrow density (g/cm3) | 0.39 | 0.015 | 0.33 | 0.039 |
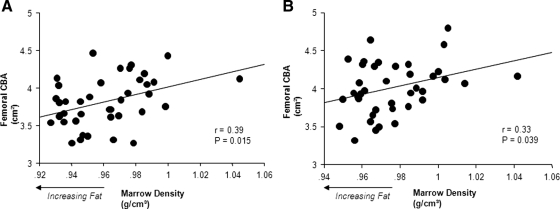
Marrow adiposity was inversely related to CBA at baseline and follow-up (Fig. 1) but was not associated with CSA (r = 0.19 and 0.17; P = 0.24 and 0.32, respectively). The reciprocal association between the amount of bone and marrow adiposity at baseline and follow-up persisted, even after controlling for body mass, DXA values for LM, and CT measures of femoral CSA (Table 3). Similar results were seen when weight and height replaced body mass as independent variables in the multiple regression model (data not shown).
Figure 1.
Correlations between CT values for the tissue density of the marrow and measures of CBA of the femur at baseline (A) and follow-up (B) in 39 females. Higher marrow density indicate a lesser amount of fat, hence the reciprocal relation between marrow adiposity and the amount of bone.
Table 3.
Multiple regression analyses for the prediction of femoral CBA at baseline and after 18–24 months in 39 females
| Femoral CBA (cm2)
|
||||
|---|---|---|---|---|
| Baseline
|
Follow-up
|
|||
| Standard β | P | Standard β | P | |
| BMI (kg/m2) | −0.012 | 0.917 | 0.004 | 0.974 |
| DXA LM (kg) | 0.094 | 0.478 | 0.019 | 0.882 |
| Femoral CSA (cm2) | 0.728 | <0.0001 | 0.832 | <0.0001 |
| Marrow density (g/cm3) | 0.248 | 0.015 | 0.179 | 0.038 |
| R2 adjusted | 0.7 | 0.77 | ||
There were strong correlations between changes in femoral CBA and changes in the CSA and marrow density of the femurs, regardless of whether the raw data or percentage changes were analyzed (Table 4). These relations were independent of each other and accounted for half of the variance in cortical bone changes (Table 5).
Table 4.
Simple Pearson correlations between the absolute and percent changes in femoral CBA and absolute and percent changes in anthropometric measures, body composition and bone parameters in 39 females
| Correlations with femoral CBA
|
||||
|---|---|---|---|---|
| Percent changes
|
Absolute changes
|
|||
| r | P | r | P | |
| Weight | 0.03 | 0.843 | 0.03 | 0.867 |
| Height | −0.09 | 0.586 | −0.10 | 0.567 |
| BMI | 0.04 | 0.812 | 0.04 | 0.815 |
| DXA LM | 0.25 | 0.164 | 0.26 | 0.140 |
| DXA fat mass | −0.03 | 0.881 | 0.04 | 0.831 |
| CT femoral CSA | 0.69 | <0.0001 | 0.68 | <0.0001 |
| CT marrow density | 0.41 | 0.009 | 0.41 | 0.009 |
Table 5.
Multiple regression analysis for the prediction of absolute and percent changes in femoral CBA in 39 females
| Femoral CBA
|
||||
|---|---|---|---|---|
| Percent changes
|
Absolute changes
|
|||
| Standard β | P | Standard β | P | |
| Femoral CSA | 0.631 | <0.0001 | 0.619 | <0.0001 |
| Marrow density | 0.268 | 0.026 | 0.262 | 0.032 |
| R2 adjusted | 0.52 | 0.50 | ||
Discussion
Previous cross-sectional studies, by us and others, have shown an inverse association between the amount of fat in the bone marrow and measures of bone mineral content (25,26,27). This longitudinal investigation provides further evidence for this association. Both at baseline and follow-up, 1.5–2 yr later, measures of cortical bone at the femoral shaft in healthy young women were inversely correlated to marrow adiposity. Moreover, increases in cortical bone were simultaneously related to significant decreases in marrow adiposity. The reciprocal relation between cortical bone acquisition and marrow fat persisted, even after controlling for other known determinants of bone mass, such as weight, height, and lean mass. Our ability to demonstrate this relationship in women soon after completion of sexual and skeletal maturity, when the skeletal remodeling and rates of bone accumulation are relatively low, underscores the strength of the interaction between bone and fat formation. Indeed, even a small gain of 3% in the cortical bone area at the midshaft of the femur was associated with significant decrease in marrow adiposity in the adjacent medullary canal.
In contrast, there was no significant association between marrow density and values for femoral CSA. The presence of a reciprocal relation between the changes in marrow adiposity and bone acquisition in the femur, but no association between marrow fat and the overall femoral cross-sectional dimensions, are consistent with the notion that marrow stem cells regulate osteogenesis primarily at the endocortical surface. These findings in a relatively small group of young women are similar to those of a recent longitudinal study from Australia in older men undergoing an 18-month targeted bone-loading exercise program that resulted in reduced femoral marrow adiposity. Furthermore, the decrease in marrow adiposity was related to increased femoral cortical bone (due to reduced endocortical bone loss) but no change in the overall periosteal apposition or femoral cross-sectional size (46). That such strikingly comparable findings were obtained in two cohorts of different gender, ages, and from two continents with different environmental conditions underscore the generalizability of the relation between bone and fat formation.
Marrow adiposity in the femur was not related to weight, BMI, or DXA values for total BF mass at baseline or follow-up. These findings corroborate previous studies suggesting the independence of these two fat depots and a distinct metabolic function of marrow fat (31,47). Indeed, we recently found that measures of marrow adiposity were not related to any anthropometric parameters or surrogates of adiposity, such as the waist to hip ratio, or to the amount of visceral or sc fat in healthy young adults. Moreover, unlike other fat depots, marrow adiposity was not associated with BP, measures of carotid wall thickness, fasting glucose, fasting insulin, insulin resistance, or lipid profile (48). It should be noted, however, that in extreme cases of malnutrition such as in anorexia nervosa, an inverse correlation between bone marrow fat content and BMI or sc adipose tissue has been reported (49) and that weight loss in patients with anorexia is associated with an increased amount of marrow fat (50).
A technical characteristic regarding CT must be considered for the appropriate interpretation of the current results. Beam hardening and the preferential loss of lower-energy photons from a polychromatic x-ray beam would cause a decrease of CT attenuation values in the center of the marrow cavity (51). The greater amount of cortical bone at follow-up would likely have caused a greater beam-hardening effect, resulting in an overestimation of the amount of marrow fat. It is possible that these errors minimize the strength of the relation we found between changes in bone and fat in the femurs. There are several additional limitations. We chose subjects who had achieved skeletal maturity to adjust for the confounding effect of skeletal growth on marrow conversion. Whereas it is impossible to completely control for the influence of other developmental factors because we found significantly less marrow fat at follow-up, our results cannot be attributed to differences in the timing or completeness of marrow conversion. Although we examined only the appendicular skeleton, previous cross-sectional studies have shown bone acquisition in the axial skeleton to also be inversely related to marrow adiposity. Moreover, because MSCs are closely bound to endosteal and trabecular surfaces and because trabeculae have substantially greater surface areas adjacent to the marrow cavity (52), this relation is likely to be even more discernible in the axial skeleton.
In conclusion, bone acquisition in the appendicular skeleton of healthy young women is inversely related to marrow adiposity. The results of this study represent the clinical correlate of basic research information showing that marrow adipocytes and osteoblasts share a common progenitor (1,2,3,4,5). Deciphering the mechanisms that influence the inseparable reciprocal transformation of MSCs into osteoblasts or adipocytes could lead to the development of strategies to maximize bone mass and prevent osteoporosis.
Footnotes
This work was supported by Grant DAMD17-01-1-0817 from the Department of the Army and Grant ROI-AR052744-02 from the National Institutes of Health.
Disclosure Summary: N.D.I., A.O.M., K.G., T.A.L.W., F.D., and V.G. have nothing to declare.
First Published Online April 14, 2010
Abbreviations: BF, Body fat; BMI, body mass index; CBA, cortical bone area; CBD, cortical bone density; CSA, cross-sectional area; CT, computed tomography; DXA, dual-energy x-ray absorptiometry; HU, Hounsfield unit; LM, lean mass; MSC, mesenchymal cell.
References
- Hong JH, Hwang ES, McManus MT, Amsterdam A, Tian Y, Kalmukova R, Mueller E, Benjamin T, Spiegelman BM, Sharp PA, Hopkins N, Yaffe MB 2005 TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science 309:1074–1078 [DOI] [PubMed] [Google Scholar]
- Otto F, Thornell AP, Crompton T, Denzel A, Gilmour KC, Rosewell IR, Stamp GW, Beddington RS, Mundlos S, Olsen BR, Selby PB, Owen MJ 1997 Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell 89:765–771 [DOI] [PubMed] [Google Scholar]
- Nakashima K, de Crombrugghe B 2003 Transcriptional mechanisms in osteoblast differentiation and bone formation. Trends Genet 19:458–466 [DOI] [PubMed] [Google Scholar]
- Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, Spiegelman BM, Mortensen RM 1999 PPARγ is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell 4:611–617 [DOI] [PubMed] [Google Scholar]
- Rodríguez JP, Montecinos L, Ríos S, Reyes P, Martínez J 2000 Mesenchymal stem cells from osteoporotic patients produce a type I collagen-deficient extracellular matrix favoring adipogenic differentiation. J Cell Biochem 79:557–565 [DOI] [PubMed] [Google Scholar]
- Nuttall ME, Patton AJ, Olivera DL, Nadeau DP, Gowen M 1998 Human trabecular bone cells are able to express both osteoblastic and adipocytic phenotype: implications for osteopenic disorders. J Bone Miner Res 13:371–382 [DOI] [PubMed] [Google Scholar]
- Dang ZC, van Bezooijen RL, Karperien M, Papapoulos SE, Löwik CW 2002 Exposure of KS483 cells to estrogen enhances osteogenesis and inhibits adipogenesis. J Bone Miner Res 17:394–405 [DOI] [PubMed] [Google Scholar]
- Doglio A, Dani C, Fredrikson G, Grimaldi P, Ailhaud G 1987 Acute regulation of insulin-like growth factor-I gene expression by growth hormone during adipose cell differentiation. EMBO J 6:4011–4016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque G, Macoritto M, Kremer R 2004 Vitamin D treatment of senescence accelerated mice (SAM-P/6) induces several regulators of stromal cell plasticity. Biogerontology 5:421–429 [DOI] [PubMed] [Google Scholar]
- Green H, Kehinde O 1975 An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell 5:19–27 [DOI] [PubMed] [Google Scholar]
- Kindblom JM, Gevers EF, Skrtic SM, Lindberg MK, Göthe S, Törnell J, Vennström B, Ohlsson C 2005 Increased adipogenesis in bone marrow but decreased bone mineral density in mice devoid of thyroid hormone receptors. Bone 36:607–616 [DOI] [PubMed] [Google Scholar]
- Bonnelye E, Aubin JE 2005 Estrogen receptor-related receptor α: a mediator of estrogen response in bone. J Clin Endocrinol Metab 90:3115–3121 [DOI] [PubMed] [Google Scholar]
- Wronski TJ, Walsh CC, Ignaszewski LA 1986 Histologic evidence for osteopenia and increased bone turnover in ovariectomized rats. Bone 7:119–123 [DOI] [PubMed] [Google Scholar]
- Martin RB, Chow BD, Lucas PA 1990 Bone marrow fat content in relation to bone remodeling and serum chemistry in intact and ovariectomized dogs. Calcif Tissue Int 46:189–194 [DOI] [PubMed] [Google Scholar]
- Doerrler W, Feingold KR, Grunfeld C 1994 Cytokines induce catabolic effects in cultured adipocytes by multiple mechanisms. Cytokine 6:478–484 [DOI] [PubMed] [Google Scholar]
- Ignotz RA, Massagué J 1985 Type β transforming growth factor controls the adipogenic differentiation of 3T3 fibroblasts. Proc Natl Acad Sci USA 82:8530–8534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama Y, Takeuchi Y, Suzawa M, Fukumoto S, Murayama H, Yamato H, Fujita T, Kurokawa T, Matsumoto T 1998 Reduced expression of interleukin-11 in bone marrow stromal cells of senescence-accelerated mice (SAMP6): relationship to osteopenia with enhanced adipogenesis. J Bone Miner Res 13:1370–1377 [DOI] [PubMed] [Google Scholar]
- Stringer B, Waddington R, Houghton A, Stone M, Russell G, Foster G 2007 Serum from postmenopausal women directs differentiation of human clonal osteoprogenitor cells from an osteoblastic toward an adipocytic phenotype. Calcif Tissue Int 80:233–243 [DOI] [PubMed] [Google Scholar]
- Diascro Jr DD, Vogel RL, Johnson TE, Witherup KM, Pitzenberger SM, Rutledge SJ, Prescott DJ, Rodan GA, Schmidt A 1998 High fatty acid content in rabbit serum is responsible for the differentiation of osteoblasts into adipocyte-like cells. J Bone Miner Res 13:96–106 [DOI] [PubMed] [Google Scholar]
- Li YJ, Batra NN, You L, Meier SC, Coe IA, Yellowley CE, Jacobs CR 2004 Oscillatory fluid flow affects human marrow stromal cell proliferation and differentiation. J Orthop Res 22:1283–1289 [DOI] [PubMed] [Google Scholar]
- David V, Martin A, Lafage-Proust MH, Malaval L, Peyroche S, Jones DB, Vico L, Guignandon A 2007 Mechanical loading down-regulates peroxisome proliferator-activated receptor γ in bone marrow stromal cells and favors osteoblastogenesis at the expense of adipogenesis. Endocrinology 148:2553–2562 [DOI] [PubMed] [Google Scholar]
- Quarto R, Thomas D, Liang CT 1995 Bone progenitor cell deficits and the age-associated decline in bone repair capacity. Calcif Tissue Int 56:123–129 [DOI] [PubMed] [Google Scholar]
- Mullender MG, van der Meer DD, Huiskes R, Lips P 1996 Osteocyte density changes in aging and osteoporosis. Bone 18:109–113 [DOI] [PubMed] [Google Scholar]
- Chan GK, Duque G 2002 Age-related bone loss: old bone, new facts. Gerontology 48:62–71 [DOI] [PubMed] [Google Scholar]
- Burkhardt R, Kettner G, Böhm W, Schmidmeier M, Schlag R, Frisch B, Mallmann B, Eisenmenger W, Gilg T 1987 Changes in trabecular bone, hematopoiesis and bone marrow vessels in aplastic anemia, primary osteoporosis, and old age: a comparative histomorphometric study. Bone 8:157–164 [DOI] [PubMed] [Google Scholar]
- Meunier P, Aaron J, Edouard C, Vignon G 1971 Osteoporosis and the replacement of cell populations of the marrow by adipose tissue. A quantitative study of 84 iliac bone biopsies. Clin Orthop Relat Res 80:147–154 [DOI] [PubMed] [Google Scholar]
- Verma S, Rajaratnam JH, Denton J, Hoyland JA, Byers RJ 2002 Adipocytic proportion of bone marrow is inversely related to bone formation in osteoporosis. J Clin Pathol 55:693–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung DK, Griffith JF, Antonio GE, Lee FK, Woo J, Leung PC 2005 Osteoporosis is associated with increased marrow fat content and decreased marrow fat unsaturation: a proton MR spectroscopy study. J Magn Reson Imaging 22:279–285 [DOI] [PubMed] [Google Scholar]
- Griffith JF, Yeung DK, Antonio GE, Lee FK, Hong AW, Wong SY, Lau EM, Leung PC 2005 Vertebral bone mineral density, marrow perfusion, and fat content in healthy men and men with osteoporosis: dynamic contrast-enhanced MR imaging and MR spectroscopy. Radiology 236:945–951 [DOI] [PubMed] [Google Scholar]
- Griffith JF, Yeung DK, Antonio GE, Wong SY, Kwok TC, Woo J, Leung PC 2006 Vertebral marrow fat content and diffusion and perfusion indexes in women with varying bone density: MR evaluation. Radiology 241:831–838 [DOI] [PubMed] [Google Scholar]
- Shen W, Chen J, Punyanitya M, Shapses S, Heshka S, Heymsfield SB 2007 MRI-measured bone marrow adipose tissue is inversely related to DXA-measured bone mineral in Caucasian women. Osteoporos Int 18:641–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrli FW, Hopkins JA, Hwang SN, Song HK, Snyder PJ, Haddad JG 2000 Cross-sectional study of osteopenia with quantitative MR imaging and bone densitometry. Radiology 217:527–538 [DOI] [PubMed] [Google Scholar]
- Trudel G, Payne M, Mädler B, Ramachandran N, Lecompte M, Wade C, Biolo G, Blanc S, Hughson R, Bear L, Uhthoff HK 2009 Bone marrow fat accumulation after 60 days of bed rest persisted 1 year after activities were resumed along with hemopoietic stimulation: the Women International Space Simulation for Exploration study. J Appl Physiol 107:540–548 [DOI] [PubMed] [Google Scholar]
- Di Iorgi N, Rosol M, Mittelman SD, Gilsanz V 2008 Reciprocal relation between marrow adiposity and the amount of bone in the axial and appendicular skeleton of young adults. J Clin Endocrinol Metab 93:2281–2286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner JM 1978 Physical growth and development. In: Forfar JO, Arnell CC, eds. Textbook of pediatrics. 2nd ed. Edinburgh, Scotland: Churchill Livingstone; 249–303 [Google Scholar]
- Greulich WW, Pyle SI 1959 Radiographic atlas of skeletal development of the hand and wrist. 2nd ed. Stanford, CA: Stanford University Press [Google Scholar]
- Hangartner TN, Gilsanz V 1996 Evaluation of cortical bone by computed tomography. J Bone Miner Res 11:1518–1525 [DOI] [PubMed] [Google Scholar]
- Hounsfield GN1980 Computed medical imaging. Science 210:22–28 [DOI] [PubMed] [Google Scholar]
- White DR, Woodard HQ, Hammond SM1987 Average soft-tissue and bone models for use in radiation dosimetry. Br J Radiol 60:907–913 [DOI] [PubMed] [Google Scholar]
- Woodard HQ, White DR1982 Bone models for use in radiotherapy dosimetry. Br J Radiol 55:277–282 [DOI] [PubMed] [Google Scholar]
- Schneider W, Bortfeld T, Schlegel W2000 Correlation between CT numbers and tissue parameters needed for Monte Carlo simulations of clinical dose distributions. Phys Med Biol 45:459–478 [DOI] [PubMed] [Google Scholar]
- Moore SG, Dawson KL1990 Red and yellow marrow in the femur: age-related changes in appearance at MR imaging. Radiology 175:219–223 [DOI] [PubMed] [Google Scholar]
- Moore SG, Bisset GS, 3rd, Siegel MJ, Donaldson JS1991 Pediatric musculoskeletal MR imaging. Radiology 179:345–360 [DOI] [PubMed] [Google Scholar]
- Vande Berg BC, Lecouvet FE, Moysan P, Maldague B, Jamart J, Malghem J1997 MR assessment of red marrow distribution and composition in the proximal femur: correlation with clinical and laboratory parameters. Skeletal Radiol 26:589–596 [DOI] [PubMed] [Google Scholar]
- Gilsanz V, Wren TA, Sanchez M, Dorey F, Judex S, Rubin C2006 Low-level, high-frequency mechanical signals enhance musculoskeletal development of young women with low BMD. J Bone Miner Res 21:1464–1474 [DOI] [PubMed] [Google Scholar]
- Daly R, Ebeling P, Kukuljan S, Harvey S, Nowson C2009 Effects of Exercise on Appendicular Bone Marrow Fat and Its Relation to Changes in Bone Density, Structure and Strength in Older Men. J Bone Miner Res 24 (Suppl 1) Available at http://wwwasbmrorg/Meetings/AnnualMeeting [Google Scholar]
- Justesen J, Stenderup K, Ebbesen EN, Mosekilde L, Steiniche T, Kassem M 2001 Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology 2:165–171 [DOI] [PubMed] [Google Scholar]
- Di Iorgi N, Mittelman SD, Gilsanz V 2008 Differential effect of marrow adiposity and visceral and subcutaneous fat on cardiovascular risk in young, healthy adults. Int J Obes (Lond) 32:1854–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredella MA, Fazeli PK, Miller KK, Misra M, Torriani M, Thomas BJ, Ghomi RH, Rosen CJ, Klibanski A 2009 Increased bone marrow fat in anorexia nervosa. J Clin Endocrinol Metab 94:2129–2136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abella E, Feliu E, Granada I, Millá F, Oriol A, Ribera JM, Sánchez-Planell L, Berga LI, Reverter JC, Rozman C 2002 Bone marrow changes in anorexia nervosa are correlated with the amount of weight loss and not with other clinical findings. Am J Clin Pathol 118:582–588 [DOI] [PubMed] [Google Scholar]
- Curry TS, Dowdey JE, Murry RC 1990 Christensen's physics of diagnostic radiology. 4th ed. Malvern, PA: Lea, Febiger [Google Scholar]
- Gurevitch O, Slavin S, Feldman AG 2007 Conversion of red bone marrow into yellow—cause and mechanisms. Med Hypotheses 69:531–536 [DOI] [PubMed] [Google Scholar]