Abstract
Context: The CAG repeat polymorphism in the androgen receptor, denoted (CAG)n, is thought to (inversely) index androgen sensitivity. We hypothesized that (CAG)n would exhibit a modifying influence on the association between circulating total and calculated free testosterone (TT and FT) and physical frailty in aging men.
Objective: The objective of the study was to establish the influence of (CAG)n on the relation between circulating TT, FT, LH, SHBG, and frailty.
Design: This was a prospective cohort study of health and endocrine functioning in randomly selected men, with a baseline (T1: 1987–89) and two follow-up (T2: 1995–1997; T3: 2002–2004) visits.
Setting: This was an observational study of men residing in greater Boston, MA.
Participants: A total of 624 subjects aged 50–86 yr were retained.
Main Outcome Measures: The frailty phenotype was measured at T3. Components included weight loss, exhaustion, low physical activity, weakness, and slowness. Subjects exhibiting two of these five components were considered to be in an intermediate state, and those exhibiting three or more were considered frail.
Results: (CAG)n was positively associated with TT and FT. Multivariable regression analyses revealed no influence of CAG on longitudinal within-subject changes in hormone levels or cross-sectional (T3) associations between hormone concentrations and the prevalence of intermediate frailty or frailty. Models incorporating subjects' history of hormone decline produced similar negative results.
Conclusions: This population-based study does not support the hypothesis that interindividual differences in (CAG)n can account for a lack of association between circulating androgens and the frailty phenotype. Longitudinal analyses are needed to confirm these conclusions.
Variation in the length of the CAG repeat polymorphism does not appear to account for a prior observation of no association between serum testosterone levels and the frailty phenotype.
Frailty is a syndrome of aging characterized by generalized vulnerability to disability and loss of physiologic reserve. Although a consensus clinical definition has long proved elusive, the configuration advanced by Fried et al. (1) has gained widespread use as a measure of its physical manifestations (2,3,4) and, recently as a clinical outcome suitable for use in randomized trials (5). Validation studies show that this phenotype behaves as a syndrome (i.e. its components exhibit accumulative detrimental effects) and is associated with adverse downstream consequences including disability and death (1,6).
Declines in androgen concentrations are routinely cited as potential actors in the pathogenesis of frailty in men, most specifically through their role in age-related loss of muscle and bone mass (7,8,9,10,11). These assertions are supported by intervention studies in special populations. As a treatment for prostate cancer; for instance, androgen deprivation therapy induces rapid bone loss and increased risk of fracture (12,13,14). The evidence suggesting that androgen deprivation therapy accelerates the development of frailty is also strong (15).
On the other hand, the evidence concerning an association between endogenous androgen levels and frailty is mixed (16). A recent cross-sectional analysis (17) of data on healthy men enrolled in the Massachusetts Male Aging Study (MMAS) was suggestive of an association between serum SHBG levels and the frailty phenotype but demonstrated no comparable association for total or free testosterone (TT or FT). In cross-sectional and prospective analyses from the Osteoporotic Fractures in Men (MrOS) study, low bioavailable testosterone was associated with frailty measured concurrently and at 4-yr follow-up, although the latter appeared to be in part accounted for by adjustment for body composition and comorbidities (45).
Androgen effects are mediated by the androgen receptor (AR) and inversely associated with the length of the CAG repeat polymorphism, denoted (CAG)n, in exon 1 of the AR gene (18). An emerging hypothesis contends (19) that the tremendous interindividual variation observed in population-based studies of circulating androgen levels is in part explained by between-individual variation in androgen sensitivity, in part encoded by (CAG)n. Circulating androgen concentrations have been associated with (CAG)n in several samples (19,20,21,22,23), although not all (24,25,26), and some studies suggest an interactive relationship between androgens and (CAG)n in influencing body composition (27), bone mineral density (28), depressive symptoms (29), and age-related hormone declines (20). At least one study has demonstrated an apparent influence of (CAG)n on the rate of prostate growth after testosterone replacement in hypogonadism (30).
We hypothesized that confounding or effect modification by (CAG)n might mask an association between circulating androgens and the frailty phenotype, leading to an observation of no association (17). To test this hypothesis, we used data from the MMAS to construct longitudinal and cross-sectional statistical models investigating whether: 1) (CAG)n modifies the relation between age and within-subject changes in hormone concentrations, 2) (CAG)n influences cross-sectional associations between hormone concentrations and frailty, and 3) at a given point in time, (CAG)n has a moderating influence on the association between a subject's history of hormone decline and his current frailty status.
Subjects and Methods
The MMAS is a population-based prospective study of men living in and around Boston, MA. Its design and sampling scheme have been previously described (31). Briefly, male residents of greater Boston were drawn at random from the Massachusetts annual state census list, with sampling fractions adjusted to produce a uniform distribution between ages 40 and 70 yr. A total of 1709 respondents completed a baseline visit (T1) between 1987 and 1989. Follow-up visits were conducted after approximately 9 yr (T2: 1995–1997: n = 1134) and 15 yr (T3: 2002–2004: n = 853).
In early-morning visits to subjects' homes, trained interviewer/phlebotomists administered a standardized interview and obtained physical measures and blood samples. The New England Research Institutes Institutional Review Board approved all protocols, and written informed consent documentation was obtained from all subjects. Age, health history, employment, and other descriptive data were obtained using interview items. Race and ethnicity were measured by self-report according to self-report consistent with U.S. Office of Management and Budget guidelines.
(CAG)n was obtained at T2 via a method previously described (20). In brief, DNA was extracted from whole-blood buffy coats, and the CAG repeat region of the AR was amplified using PCR with fluorescent-labeled sense primers.
Frailty phenotype
Components of the frailty construct were obtained (17) at T3. The measurement of frailty in MMAS was intended to mirror as closely as possible the definitions used by Fried et al. (1) in the Cardiovascular Health Study.
Measures taken as components of the frailty phenotype included weight loss, exhaustion, low physical activity levels, slowness (measured by walking speed), and weakness (measured by grip strength). In MMAS, men were classified as either nonfrail (displaying zero of the five traits described above), intermediate (one to two traits), or frail (three or more traits).
Excess weight loss was indicated by a subjects' loss of weight being equal to at least the 20th percentile weight loss between T2 and T3 among men at least 60 yr of age, i.e. in excess of 7 kg. Exhaustion was measured as in Cardiovascular Health Study according to individual items obtained from the Center for Epidemiologic Studies-Depression scale (32). Low physical activity was taken to be indicated by a subject's activity being below the 20th percentile among MMAS subjects of at least 60 yr (17).
Subjects' height and weight were measured using methods validated for use in large population-based field studies (33), and grip strength was measured using a Jamar handheld dynomometer (Sammons Preston, Bolingbrook, IL). Slowness was defined as being at or below the 20th percentile of the overall sample in walking speed as measure by the Physical Performance Test (34), after stratification according to median sample height. Weakness was indicated by a subjects' being below the 20th percentile of the overall sample in grip strength (maximum of two measurements), after stratification according to quartiles of body mass index. Extensive additional details have been published previously (17).
Serum hormones
Serum hormones may be influenced by elements of experimental design (35). Accordingly, the MMAS took steps to minimize bias and imprecision. Blood samples were drawn within 4 h of subjects' waking to reduce the impact of diurnal variation in hormone concentrations. To counteract episodic hormone secretion, two samples were obtained at each visit, 30 min apart, and were pooled in equal aliquots at the time of assay. Blood was kept in an ice-cooled container and centrifuged within 6 h of study visit. Serum was stored in 5 ml scintillation vials at −20 C, shipped to the laboratory within 1 wk by same-day courier, and stored at −70 C until the time of assay. All assays were performed at the Endocrine Laboratory, University of Massachusetts Medical Center, under the direction of Christopher Longcope, MD.
TT was measured with RIA kits from Diagnostic Products Corp. (Los Angeles, CA). TT interassay coefficients of variation (CVs) were 8.0, 9.0, and 8.3% at T1, T2, and T3, respectively. Age-specific TT concentrations were consistent with those obtained in other major epidemiologic studies of serum T. The proportion of serum FT was calculated using the mass action equations described by Södergård et al. (36), with association constants taken from Vermeulen et al. (37). Serum SHBG was measured using RIA kits at T1 and T2 and at T3 by chemiluminescent enzyme immunometric assay using the Diagnostic Products Immulite technology; interassay CVs were 10.9, 7.9, and 3.0% at T1, T2, and T3, respectively. LH concentrations were measured by RIA kits from Ciba Corning (Medfield, MA) at T1 (CV 9.5%), the Abbott Diagnostics (Chicago, IL) IMx system at T2 (CV = 6.9%), and Diagnostic Products Immulite at T3 (CV 3.2%).
Analytic sample and statistical methods
Of the 1709 men in the original MMAS cohort, 795 participated at both T2 [at which time (CAG)n was measured] and T3 (when frailty components were obtained). From this group, men who reported stroke (n = 56) or Parkinson's disease (n = 1) or were taking levodopa-carbidopa (a treatment for Parkinson's disease, n = 3) or donepezil (a treatment for Alzheimer's disease, n = 7) at either time point were excluded. Of the remaining subjects, those who did not have enough data to determine overall frailty status (n = 67), were missing hormone data at both time points (n = 15), or were missing (CAG)n at T2 (n = 22) were excluded, yielding an analytic sample of 624 men.
Statistical analyses were performed using SAS software version 9.1 (SAS Institute, Cary, NC) and the R system version 2.8.1 (38). Exploratory analyses incorporating graphical and tabular displays were employed to assess crude associations between hormones, age, and frailty. Generalized additive models were used to obtain graphical depictions including estimation of mean trends. For cross-sectional models with frailty as the outcome, a logit link function was employed.
Given the substantial skew in the data, LH values were log transformed (base e) before formal analyses. Bivariate linear associations between hormone concentrations, SHBG concentrations, and (CAG)n were assessed using Pearson correlation statistics evaluated at T2 [when (CAG)n was assessed].
To assess the influence of (CAG)n on longitudinal changes in hormone values, we used mixed-effects regression (39) with subject treated as a random effect. These models separated subjects' age into two parts: baseline age and time in study. The latter is taken to quantify subjects' aging from baseline (T1) to study follow-ups (T2, T3) in a model that controls for age at study entry. The influence of (CAG)n on trends in hormones was evaluated using an interaction term constructed by multiplying (CAG)n by time in study. This effect can be interpreted as the mean difference in per-year change in the relevant hormone outcome observed between two subjects whose (CAG)n differ by one. The statistical significance of these and other model effects were evaluated using Wald tests.
To formally assess the influence of (CAG)n on the association between hormones and frailty, we used logistic regression analyses of the frailty outcome (obtained at T3 only). First, we constructed models of frailty as a function of (CAG)n, age at T3, of each hormone concentration, and the interaction between that hormone and (CAG)n, which may be interpreted as the difference in the strength of the hormone/frailty association observed for subjects whose (CAG)n differ by one. In primary analyses, the intermediate and frail populations were combined to produce a dichotomous outcome comparing intermediate and frail populations together to other subjects. Secondary analyses compared frail subjects with all others.
We also constructed models incorporating the longitudinal history of subjects into assessments of frailty at T3. These included baseline age, baseline hormone concentrations, per-year change in hormone concentrations, (CAG)n, and an interaction term constructed from the latter two effects. These interaction terms were used to test the hypothesis that (CAG)n might influence the association between the history of change in hormones from baseline and the likelihood of frailty at T3. Again, Wald tests were used to determine statistical significance.
Results
Table 1 describes the analytic sample at all three study waves. By the third study wave (T3), more than 40% of subjects were at least 70 yr old. The prevalence of diagnosed age-related disease increased rapidly with time; for instance, subject reports of diagnosed hypertension were much more common at T3 (49%) than baseline (22%). At T3, a majority of subjects remained employed (55%), and 74% were currently married. At T3, some 49% of subjects were classified as intermediate; a much smaller number (8%) were frail. The mean TT and FT declined sharply with time.
Table 1.
Selected characteristics of the analytic sample (n = 624)
| T1 (1987–1989) | T2 (1995–1997) | T3 (2002–2004) | |
|---|---|---|---|
| Demographic and health characteristics, n (%)a | |||
| Age (yr) | |||
| 40–49 | 267 (42.8%) | 22 (3.5%) | 0 (0.0%) |
| 50–59 | 228 (36.5%) | 268 (42.9%) | 133 (21.3%) |
| 60–69 | 128 (20.5%) | 225 (36.1%) | 233 (37.3%) |
| 70–79 | 1 (0.2%) | 109 (17.5%) | 210 (33.7%) |
| 80–86 | 0 (0.0%) | 0 (0.0%) | 48 (7.7%) |
| Race | |||
| White | 612 (98.2%) | 596 (96.1%) | 604 (97.3%) |
| Black | 5 (0.8%) | 8 (1.3%) | 4 (0.6%) |
| Other | 6 (1.0%) | 16 (2.6%) | 13 (2.1%) |
| Marital status | |||
| Never married | 57 (9.1%) | 45 (7.2%) | 54 (8.7%) |
| Currently married | 480 (76.9%) | 487 (78.2%) | 461 (73.9%) |
| Divorced | 77 (12.3%) | 68 (10.9%) | 67 (10.7%) |
| Widowed | 10 (1.6%) | 23 (3.7%) | 42 (6.7%) |
| Employed | 555 (88.9%) | 441 (70.8%) | 341 (54.6%) |
| Education | |||
| Less than high school | 41 (6.6%) | 33 (5.3%) | 35 (5.6%) |
| At least some college | 78 (12.5%) | 77 (12.5%) | 99 (15.9%) |
| College or postgraduate study | 505 (80.9%) | 508 (82.2%) | 490 (78.5%) |
| Annual household income | |||
| Less than $40,000 | 163 (26.6%) | 143 (23.8%) | 135 (22.5%) |
| $40,000–79,000 | 281 (45.9%) | 203 (33.8%) | 203 (33.8%) |
| $80,000 or more | 168 (27.5%) | 254 (42.3%) | 262 (43.7%) |
| Health status | |||
| Diabetes mellitus | 18 (2.9%) | 36 (5.8%) | 62 (9.9%) |
| Hypertension | 139 (22.3%) | 215 (34.5%) | 303 (48.6%) |
| Heart disease | 42 (6.7%) | 84 (13.5%) | 142 (22.8%) |
| Current smoking | 114 (18.3%) | 58 (9.3%) | 43 (6.9%) |
| Hormone concentrations, mean ± sd | |||
| TT, ng/dl | 526.1 ± 169.5 | 454.8 ± 156.0 | 418.6 ± 164.8 |
| FT, ng/dl | 13.6 ± 5.2 | 10.6 ± 3.7 | 7.2 ± 2.8 |
| SHBG, nm | 31.8 ± 16.3 | 34.4 ± 16.4 | 51.3 ± 20.0 |
| LH, IU/liter | 4.7 ± 2.8 | 5.7 ± 4.5 | 4.6 ± 4.4 |
| Frailty (T3 only), n (%)a | |||
| Degree of frailty | |||
| None | 251 (43.1%) | ||
| Intermediate | 286 (49.1%) | ||
| Frail | 46 (7.9%) | ||
| Frailty component | |||
| Weight loss | 53 (8.6%) | ||
| Exhaustion | 77 (12.4%) | ||
| Low physical activity | 105 (17.6%) | ||
| Slowness | 135 (21.9%) | ||
| Weakness | 134 (22.9%) |
Some subjects missing selected data; proportions calculated w/respect to nonmissing records.
In this sample, (CAG)n ranged from eight to 37, thus spanning the typical range, with a mean ± sd of 22.1 ± 3.0, and a median of 22. Simple associations between frailty status, (CAG)n, and hormone concentrations are described in Table 2. A significant cross-sectional association between FT and frailty observable at T2 and T3; this has previously been shown (17) to be accounted for by control for other covariates.
Table 2.
(CAG)n and hormone concentrations by third wave (T3) frailty status
| Not frail (n = 251)
|
Intermediate (n = 286)
|
Frail (n = 46)
|
P value | ||||
|---|---|---|---|---|---|---|---|
| Mean | sd | Mean | sd | Mean | sd | ||
| (CAG)n | 22.1 | (2.97) | 22.1 | (3.09) | 22.3 | (2.58) | 0.897 |
| T1 | |||||||
| Testosterone (ng/dL) | 534.7 | (167.80) | 512.2 | (168.12) | 523.1 | (187.57) | 0.312 |
| FT (ng/dl) | 13.6 | (5.04) | 13.3 | (4.97) | 12.7 | (5.79) | 0.437 |
| SHBG (nm) | 32.6 | (16.79) | 31.5 | (16.79) | 34.3 | (15.11) | 0.489 |
| LH | 4.7 | (2.90) | 4.7 | (2.81) | 4.9 | (2.97) | 0.951 |
| T2 | |||||||
| Testosterone (ng/dl) | 472.1 | (154.96) | 442.5 | (156.35) | 442.1 | (165.66) | 0.078 |
| FT (ng/dl) | 11.2 | (3.75) | 10.4 | (3.63) | 9.0 | (3.37) | <0.001 |
| SHBG (nm) | 34.2 | (16.10) | 33.7 | (15.44) | 42.6 | (22.67) | 0.002 |
| LH | 5.5 | (3.60) | 5.8 | (5.10) | 6.2 | (4.95) | 0.511 |
| T3 | |||||||
| Testosterone (ng/dl) | 438.6 | (163.92) | 406.1 | (157.52) | 410.7 | (186.19) | 0.081 |
| FT (ng/dl) | 7.6 | (2.76) | 7.0 | (2.78) | 5.8 | (2.44) | <0.001 |
| SHBG (nm) | 50.8 | (19.86) | 50.7 | (19.76) | 63.5 | (22.70) | <0.001 |
| LH | 4.2 | (3.96) | 4.7 | (4.72) | 5.6 | (3.86) | 0.151 |
| Rate of change, T1 to T3 | |||||||
| Testosterone (ng/dl · yr) | −6.4 | (11.58) | −7.2 | (12.42) | −6.7 | (12.76) | 0.738 |
| FT (ng/dl/yr) | −0.4 | (0.31) | −0.4 | (0.37) | −0.4 | (0.33) | 0.643 |
| SHBG (nm/yr) | 1.2 | (1.25) | 1.3 | (1.38) | 2.0 | (1.46) | 0.003 |
| LH | −0.0 | (0.24) | 0.0 | (0.25) | 0.0 | (0.20) | 0.063 |
Table 3 displays crude associations between serum, hormone concentrations, and (CAG)n at T3. Calculated FT was negatively associated with LH, but TT was not. (CAG)n displayed significant but modest associations with both.
Table 3.
Pearson correlation between hormones and (CAG)n (P value), MMAS T2
| SHBG | LH | (CAG)n | |
|---|---|---|---|
| TT | 0.49 (<0.001) | −0.01 (0.98) | 0.08 (0.05) |
| FT | −0.14 (<0.001) | 0.13 (0.002) | |
| SHBG | 0.20 (<0.001) | −0.06 (>0.11) | |
| LH | −0.03 (0.52) |
Influence of (CAG)n on age/hormone associations
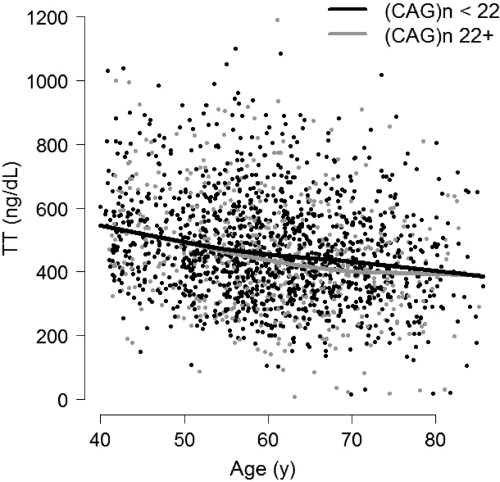
We first used exploratory graphical analyses to assess evidence in favor of an interactive longitudinal relation between age and (CAG)n in predicting hormone levels. An example is provided in Fig. 1. For the purposes of illustration, (CAG)n has been dichotomized at 22. There is no evidence that (CAG)n modifies the relation between age and TT, as is indicated by the fact that the slopes of the fitted lines displayed here are nearly identical. Exploratory results for other hormones (not shown) were similar.
Figure 1.
TT vs. age, stratified according to median (CAG)n, over the three waves of the MMAS. Fitted lines are generated by locally weighted scatterplot smoothing. (CAG)n does not modify the relation between TT and age, as indicated by the similarity in the slope of the two fitted lines.
Formal tests of the hypothesis of an interaction between age and (CAG)n in predicting hormone levels are provided in Table 4. Consideration of estimated interaction effects provides little evidence that (CAG)n has an influence on the longitudinal relation between age and hormone levels. For instance, the apparent change in per-year decline in TT per intersubject difference unit in (CAG)n was small (−0.15 ng/dl) and statistically nonsignificant (P = 0.34), which is consistent with exploratory longitudinal analyses as depicted in Fig. 1.
Table 4.
Mixed-effects regression models assessing the influence of (CAG)n influence on longitudinal associations between age and hormone outcomes
| Hormone outcome and predictors | Change in hormone outcome per unit change in predictor | P values |
|---|---|---|
| TT (ng/dl) | ||
| Age at baseline (yr) | −1.82 | 0.006 |
| Time in study | −4.06 | 0.24 |
| (CAG)n | 3.89 | 0.06 |
| Interaction: (CAG)n × time in study | −0.15 | 0.34 |
| FT (ng/dl) | ||
| Age at baseline (yr) | −0.14 | <0.001 |
| Time in study | −0.30 | 0.002 |
| (CAG)n | 0.14 | 0.004 |
| Interaction: (CAG)n × time in study | −0.01 | 0.24 |
| SHBG (nm) | ||
| Age at baseline (yr) | 0.54 | <0.001 |
| Time in study | 0.94 | 0.009 |
| (CAG)n | −0.31 | 0.17 |
| Interaction: (CAG)n × time in study | 0.01 | 0.45 |
| Log LH (IU/liter) | ||
| Age at baseline (yr) | 0.013 | <0.01 |
| Time in study | −0.009 | 0.44 |
| (CAG)n | −0.004 | 0.56 |
| Interaction: (CAG)n × time in study | < 0.001 | 0.83 |
Interaction terms (bolded) estimate the influence of (CAG)n on the longitudinal relation between aging and hormone concentrations. They are statistically nonsignificant in each model, as is consistent with exploratory analyses (Fig. 1).
Influence of (CAG)n on hormone/frailty associations
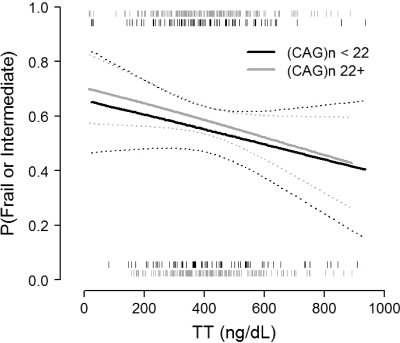
We used exploratory graphical analyses to assess whether (CAG)n could modify associations between hormones and the prevalence of frailty or intermediate frailty in cross-sectional models focusing on data at T3. An example is provided in Fig. 2. We observed little evidence that (CAG)n modified the association between TT and frailty; the relation between TT and frailty/intermediate frailty among subjects with (CAG)n < 22 (the median value) was similar to that among subjects with (CAG)n 22 or greater. Again, results for other hormone measures (not shown) were similar.
Figure 2.
Frailty and intermediate frailty vs. TT at the third wave of the MMAS, stratified by (CAG)n. Solid lines depict fitted values from regression of frailty on FT, using a generalized additive model and logit link function; 95% confidence intervals are presented with dotted lines. Rug plots at the top and bottom of the figure depict raw TT values for frail and intermediate (top) and nonfrail (bottom) subjects. There is little evidence that (CAG)n modifies the relation between TT and frailty, as indicated by the similarity in the fitted lines and the substantial overlap in confidence regions.
Cross-sectional logistic regression models using data from T3 produced results only in line with these exploratory findings. Although previous results indicated associations between SHBG and the frailty construct (17), models controlling for age at T3 and incorporating hormone effects displayed no significant modification of that association by (CAG)n (results not shown). Likewise there was little evidence that (CAG)n modified the generally nonsignificant association between TT or FT and frailty.
Models incorporating history of hormone decline produced similar results. Table 5 provides example presentations of potential statistical interactions between (CAG)n and recent hormone changes, controlling for baseline age and hormone concentrations. Here there was little evidence to indicate that (CAG)n modifies the (nonsignificant) associations between changes in serum hormone or SHBG concentrations and the likelihood of frailty/intermediate frailty, as is indicated by the statistical nonsignificance of potential interaction effects. For instance, these models indicate that the essentially nonexistent association between TT and frailty/intermediate frailty differs by a very small and nonsignificant amount (odds ratio 0.98, P = 0.54) between subjects whose (CAG)n differs by one. Simpler models that did not include the hypothesize interaction but simply assessed the relation between hormones and frailty controlling for (CAG)n as a confounder likewise revealed no association between hormone changes and frailty at T3 (models not shown).
Table 5.
Multiple logistic regression analysis of the frailty construct, incorporating within-subject history of changes in hormone concentrations
| Hormone predictors (model outcome is frailty) | Odds ratios | P values |
|---|---|---|
| TT (ng/dl) | ||
| Interaction: (CAG)n × per-year change in testosterone | 0.98 | 0.54 |
| FT (ng/dl) | ||
| Interaction: (CAG)n × per-year change in FT | 0.99 | >0.99 |
| SHBG (nm) | ||
| Interaction: (CAG)n × per-year change in SHBG | 0.91 | 0.72 |
| LH (IU/liter) | ||
| Interaction: (CAG)n × change in LH | 0.93 | 0.62 |
Odds ratios depicted are for a dichotmous outcome combining intermediate and frailty vs. other (see Subjects and Methods). The effects depicted describe the apparent multiplicative influence of a 1-unit intersubject difference in CAG on the mean relation between per-year changes in hormones and the odds of frailty or incident frailty at T3, in which the models control for baseline age and baseline hormone concentration as well as the per-year change in hormone concentration and (CAG)n main effects. Interactions are nonsignificant in all cases.
Models controlling for a wider class of confounders (Table 1) generated no change in results. In addition, models replacing the frailty outcome with its individual components such as grip strength produced no evidence that (CAG)n modified the previously reported (17) relations between hormones and those components (models not shown). Finally, secondary analyses contrasting frail subjects with all others (see Subjects and Methods) did not differ in their conclusions from analyses described above.
Discussion
A previous analysis (20) considered (CAG)n in the context of T concentrations from the first two waves (T1, T2) of the MMAS. That analysis showed that (CAG)n was negatively associated with total T concentrations at T2 in models controlling for baseline (T1) T concentrations as a covariate. This finding suggested the hypothesis that (CAG)n regulates within-subject changes in T levels to a degree observable even in otherwise healthy populations. With the collection of a third wave (T3) of data in the MMAS, we have here been able to formally test this hypothesis using longitudinal regression and statistical interaction terms, with supporting exploratory analyses provided by stratification.
These tests do not support the hypothesis that (CAG)n modifies the association between within-subject changes in age and serum hormone (or SHBG) concentrations. Cross-sectional analyses of frailty data at T3 in turn provide no evidence that (CAG)n modifies the association between hormone concentrations and the frailty construct as described by Fried and colleagues (1,6). These findings did not change when history of hormone decline was explicitly incorporated into these cross-sectional analyses. They also did not vary according to whether the dichotomous outcome variable contrasted frail and intermediate subjects to all others or rather contrasted frail subjects to all subjects either intermediate or robust. From the consistency of these results, we conclude that confounding by (CAG)n does not mask an association between testosterone (TT and FT) and frailty in the MMAS sample and that our prior finding (17) of no association between testosterone and the overall frailty construct in that population cannot be attributed to a lack of consideration of (CAG)n in that analysis.
Although large epidemiological studies of the association between circulating androgens and frailty in men are rare, androgen decline and general hormonal dysregulation are routinely described as contributing to the pathogenesis of frailty and particular in the development of sarcopenia. Consistent with this reasoning, we previously observed (17), despite no overall association with the composite frailty context, a negative association between circulating testosterone and the prevalence of specific frailty components (weakness and low physical activity). As with the overall frailty construct, however, further examination of these associations here revealed no modifying influence of (CAG)n.
An apparent lack of influence of (CAG)n in frailty might nevertheless be attributed to the diversity and nonspecificity of the various components of the frailty construct. Similar difficulties attend discussions of the role of (CAG)n and androgen sensitivity in the context of outcomes assumed to be influenced by androgen concentrations, such as bone mineral density or hormone levels themselves (24). It may simply be that the modulating influence of (CAG)n on androgen sensitivity is too subtle to be reliably observed in population-based studies of circulating concentrations.
Alternatively, it may simply be that testosterone contributes little to frailty as defined here. Rockwood and colleagues (40,41) have consistently shown that the development of frailty is closely tied to the number of illnesses affecting an individual. Thus, the effect of illness would overwhelm any small effect of anabolic hormones. In addition, many of the components of frailty are not necessarily affected by testosterone. Weight loss may be due to sarcopenia but is also seen with cachexia, anorexia, and malabsorption, none of which are due to low testosterone (42). Exhaustion and low physical activity are more likely to be due to alterations in oxygen availability to tissues, dysphoria, or inherent lack of enthusiasm to exercise. Slowness is often associated with arthritic pain. Finally, the common presence of low 25-hydroxyvitamin D levels may further confound any association with testosterone (43). More explicit consideration of multihormone interactions (11) and models positing direct causal relations between (CAG)n, hormones, and specific changes in body composition and function (44) may be required to elicit a clearer picture. Recent data (21) from the European Male Aging Study further indicate that the association between (CAG)n and increased circulating estrogen concentrations bears closer scrutiny.
The analyses described above have some limitations. Chief among these is the lack of longitudinal data on the frailty construct (collected at T3 only). Although we attempted to incorporate history of hormone decline in our consideration of frailty as an outcome, it is possible that longitudinal data on frailty itself would reveal a longitudinal association between frailty and circulating hormone levels and possibly a role for (CAG)n in determining the strength of that association. An additional limitation is that due to the nature of the MMAS population, many of the subjects described here are relatively young, and comparatively few are frail. Whereas the evidence described here best supports the hypothesis of no influence of (CAG)n on the hormones/frailty relationship, it is possible that an analysis in and older population with greater morbidity would achieve greater power to detect such influences, particularly in the longitudinal setting.
The results reported here demonstrate a lack of evidence for a role of the CAG repeat polymorphism in the AR to mediate the association between circulating androgen levels and the frailty construct. Further and more complex study is necessary to elicit a more complete understanding of the male hormonal path to frailty.
Footnotes
Study design and data collection of this work were supported by the National Institutes of Health (National Institute of Diabetes and Digestive and Kidney Diseases Grants DK44995 and DK51345 and National Institute on Aging Grant AG04673). Analyses described here were supported through an unrestricted educational grant to New England Research Institutes, Inc. from GlaxoSmithKline.
Disclosure Summary: R.E.W. and R.V.C. are employees of and have equity interests in GlaxoSmithKline. J.E.M. has equity interests in Mattem Vet Pharmaceuticals, has consulted and lectured for Solvay Pharmaceuticals, and has received grant support from PAR Pharmaceuticals. All other authors have nothing to disclose.
First Published Online April 21, 2010
For editorial see page 2634
Abbreviations: AR, Androgen receptor; (CAG)n, CAG repeat polymorphism; CV, coefficient of variation; FT, free testosterone; MMAS, Massachusetts Male Aging Study; TT, total testosterone.
References
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA 2001 Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56:M146–M156 [DOI] [PubMed] [Google Scholar]
- Morley JE, Haren MT, Rolland Y, Kim MJ 2006 Frailty. Med Clin North Am 90:837–847 [DOI] [PubMed] [Google Scholar]
- Topinková E 2008 Aging, disability and frailty. Ann Nutr Metab 52(Suppl 1):S6–S11 [DOI] [PubMed] [Google Scholar]
- Walston J, Hadley EC, Ferrucci L, Guralnik JM, Newman AB, Studenski SA, Ershler WB, Harris T, Fried LP 2006 Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc 54:991–1001 [DOI] [PubMed] [Google Scholar]
- Fairhall N, Aggar C, Kurrle SE, Sherrington C, Lord S, Lockwood K, Monaghan N, Cameron ID 2008 Frailty Intervention Trial (FIT). BMC Geriatr 8:27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandeen-Roche K, Xue QL, Ferrucci L, Walston J, Guralnik JM, Chaves P, Zeger SL, Fried LP 2006 Phenotype of frailty: characterization in the women's health and aging studies. J Gerontol A Biol Sci Med Sci 61:262–266 [DOI] [PubMed] [Google Scholar]
- Morley JE, Kaiser FE, Sih R, Hajjar R, Perry 3rd HM 1997 Testosterone and frailty. Clin Geriatr Med 13:685–695 [PubMed] [Google Scholar]
- Morley JE, Kim MJ, Haren MT 2005 Frailty and hormones. Rev Endocr Metab Disord 6:101–108 [DOI] [PubMed] [Google Scholar]
- Roubenoff R 2000 Sarcopenia: a major modifiable cause of frailty in the elderly. J Nutr Health Aging 4:140–142 [PubMed] [Google Scholar]
- Srinivas-Shankar U, Wu FCW 2009 Frailty and muscle function: role for testosterone? Front Horm Res 37:133–149 [DOI] [PubMed] [Google Scholar]
- Maggio M, Cappola AR, Ceda GP, Basaria S, Chia CW, Valenti G, Ferrucci L 2005 The hormonal pathway to frailty in older men. J Endocrinol Invest 28:15–19 [PubMed] [Google Scholar]
- Daniell HW 2001 Osteoporosis due to androgen deprivation therapy in men with prostate cancer. Urology 58:101–107 [DOI] [PubMed] [Google Scholar]
- Allain TJ 2006 Prostate cancer, osteoporosis and fracture risk. Gerontology 52:107–110 [DOI] [PubMed] [Google Scholar]
- Higano CS 2008 Androgen-deprivation-therapy-induced fractures in men with nonmetastatic prostate cancer: what do we really know? Nat Clin Pract Urol 5:24–34 [DOI] [PubMed] [Google Scholar]
- Bylow K, Mohile SG, Stadler WM, Dale W 2007 Does androgen-deprivation therapy accelerate the development of frailty in older men with prostate cancer?: a conceptual review. Cancer 110:2604–2613 [DOI] [PubMed] [Google Scholar]
- Muller M, Grobbee DE, Thijssen JH, van den Beld AW, van der Schouw YT 2003 Sex hormones and male health: effects on components of the frailty syndrome. Trends Endocrinol Metab 14:289–296 [DOI] [PubMed] [Google Scholar]
- Mohr BA, Bhasin S, Kupelian V, Araujo AB, O'Donnell AB, McKinlay JB 2007 Testosterone, sex hormone-binding globulin, and frailty in older men. J Am Geriatr Soc 55:548–555 [DOI] [PubMed] [Google Scholar]
- Zitzmann M, Nieschlag E 2003 The CAG repeat polymorphism within the androgen receptor gene and maleness. Int J Androl 26:76–83 [DOI] [PubMed] [Google Scholar]
- Crabbe P, Bogaert V, De Bacquer D, Goemaere S, Zmierczak H, Kaufman JM 2007 Part of the interindividual variation in serum testosterone levels in healthy men reflects differences in androgen sensitivity and feedback set point: contribution of the androgen receptor polyglutamine tract polymorphism. J Clin Endocrinol Metab 92:3604–3610 [DOI] [PubMed] [Google Scholar]
- Krithivas K, Yurgalevitch SM, Mohr BA, Wilcox CJ, Batter SJ, Brown M, Longcope C, McKinlay JB, Kantoff PW 1999 Evidence that the CAG repeat in the androgen receptor gene is associated with the age-related decline in serum androgen levels in men. J Endocrinol 162:137–142 [DOI] [PubMed] [Google Scholar]
- Huhtaniemi IT, Pye SR, Limer KL, Thomson W, O'Neill TW, Platt H, Payne D, John SL, Jiang M, Boonen S, Borghs H, Vanderschueren D, Adams JE, Ward KA, Bartfai G, Casanueva F, Finn JD, Forti G, Giwercman A, Han TS, Kula K, Lean ME, Pendleton N, Punab M, Silman AJ, Wu FC 2009 Increased estrogen rather than decreased androgen action is associated with longer androgen receptor CAG repeats. J Clin Endocrinol Metab 94:277–284 [DOI] [PubMed] [Google Scholar]
- Skjærpe PA, Giwercman YL, Giwercman A, Svartberg J 16 December 2008 Androgen receptor gene polymorphism and the metabolic syndrome in 60–80 years old Norwegian men (Internet). Int J Androl 10.1111/j.1365-2605.2008.00942.x [DOI] [PubMed] [Google Scholar]
- Stanworth RD, Kapoor D, Channer KS, Jones TH 2008 Androgen receptor CAG repeat polymorphism is associated with serum testosterone levels, obesity and serum leptin in men with type 2 diabetes. Eur J Endocrinol 159:739–746 [DOI] [PubMed] [Google Scholar]
- Van Pottelbergh I, Lumbroso S, Goemaere S, Sultan C, Kaufman JM 2001 Lack of influence of the androgen receptor gene CAG-repeat polymorphism on sex steroid status and bone metabolism in elderly men. Clin Endocrinol (Oxf) 55:659–666 [DOI] [PubMed] [Google Scholar]
- Walsh S, Zmuda JM, Cauley JA, Shea PR, Metter EJ, Hurley BF, Ferrell RE, Roth SM 2005 Androgen receptor CAG repeat polymorphism is associated with fat-free mass in men. J Appl Physiol 98:132–137 [DOI] [PubMed] [Google Scholar]
- Zitzmann M, Brune M, Kornmann B, Gromoll J, von Eckardstein S, von Eckardstein A, Nieschlag E 2001 The CAG repeat polymorphism in the AR gene affects high density lipoprotein cholesterol and arterial vasoreactivity. J Clin Endocrinol Metab 86:4867–4873 [DOI] [PubMed] [Google Scholar]
- Lapauw B, Goemaere S, Crabbe P, Kaufman JM, Ruige JB 2007 Is the effect of testosterone on body composition modulated by the androgen receptor gene CAG repeat polymorphism in elderly men? Eur J Endocrinol 156:395–401 [DOI] [PubMed] [Google Scholar]
- Stiger F, Brändström H, Gillberg P, Melhus H, Wolk A, Michaelsson K, Kindmark A 2008 Association between repeat length of exon 1 CAG microsatellite in the androgen receptor and bone density in men is modulated by sex hormone levels. Calcif Tissue Int 82:427–435 [DOI] [PubMed] [Google Scholar]
- Seidman SN, Araujo AB, Roose SP, McKinlay JB 2001 Testosterone level, androgen receptor polymorphism, and depressive symptoms in middle-aged men. Biol Psychiatry 50:371–376 [DOI] [PubMed] [Google Scholar]
- Zitzmann M, Depenbusch M, Gromoll J, Nieschlag E 2003 Prostate volume and growth in testosterone-substituted hypogonadal men are dependent on the CAG repeat polymorphism of the androgen receptor gene: a longitudinal pharmacogenetic study. J Clin Endocrinol Metab 88:2049–2054 [DOI] [PubMed] [Google Scholar]
- O'Donnell AB, Araujo AB, McKinlay JB 2004 The health of normally aging men: the Massachusetts Male Aging Study (1987–2004). Exp Gerontol 39:975–984 [DOI] [PubMed] [Google Scholar]
- Radloff L 1977 The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1:385–401 [Google Scholar]
- McKinlay S, Kipp D, Johnson P, Downey K, Carleton R, A field approach for obtaining physiological measures in surveys of general populations: response rates, reliability and costs. In: Proc Fourth Conference on Health Survey Research Methods, Washington, DC, 1984. U.S. Department of Health and Human Services-Public Health Service Publication No. 84-3346. Washington, DC: U.S. Government Printing Office [Google Scholar]
- Reuben DB, Siu AL 1990 An objective measure of physical function of elderly outpatients. The Physical Performance Test. J Am Geriatr Soc 38:1105–1112 [DOI] [PubMed] [Google Scholar]
- Gray A, Berlin JA, McKinlay JB, Longcope C 1991 An examination of research design effects on the association of testosterone and male aging: results of a meta-analysis. J Clin Epidemiol 44:671–684 [DOI] [PubMed] [Google Scholar]
- Södergård R, Bäckström T, Shanbhag V, Carstensen H 1982 Calculation of free and bound fractions of testosterone and estradiol-17β to human plasma proteins at body temperature. J Steroid Biochem 16:801–810 [DOI] [PubMed] [Google Scholar]
- Vermeulen A, Verdonck L, Kaufman JM 1999 A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 84:3666–3672 [DOI] [PubMed] [Google Scholar]
- R Development Core Team 2008 R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN, 3-900051-07-0; URL, http://www.r-project.org [Google Scholar]
- Laird NM, Ware JH 1982 Random-effects models for longitudinal data. Biometrics 38:963–974 [PubMed] [Google Scholar]
- Rockwood K, Abeysundera MJ, Mitnitski A 2007 How should we grade frailty in nursing home patients? J Am Med Dir Assoc 8:595–603 [DOI] [PubMed] [Google Scholar]
- Song X, MacKnight C, Latta R, Mitnitski AB, Rockwood K 2007 Frailty and survival of rural and urban seniors: results from the Canadian Study of Health and Aging. Aging Clin Exp Res 19:145–153 [DOI] [PubMed] [Google Scholar]
- Morley JE, Thomas DR 2008 Cachexia: new advances in the management of wasting diseases. J Am Med Dir Assoc 9: 205–210 [DOI] [PubMed] [Google Scholar]
- Shardell M, Hicks GE, Miller RR, Kritchevsky S, Andersen D, Bandinelli S, Cherubini A, Ferrucci L 2009 Association of low vitamin D levels with the frailty syndrome in men and women. J Gerontol A Biol Sci Med Sci 64:69–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo AB, Travison TG, Bhasin S, Esche GR, Williams RE, Clark RV, McKinlay JB 2008 Association between testosterone and estradiol and age-related decline in physical function in a diverse sample of men. J Am Geriatr Soc 56:2000–2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon PM, Ensrud KE, Laughlin GA, Cauley JA, Dam TT, Barrett-Connor E, Fink HA, Hoffman AR, Lau E, Lane NE, Stefanick ML, Cummings SR, Orwoll ES; Osteoporotic Fractures in Men (MrOS) Research Group 2009 Sex hormones and frailty in older men: the Osteoporotic Fractures in Men (MrOS) Study. J Clin Endocrinol Metab 94:3806–3815 [DOI] [PMC free article] [PubMed] [Google Scholar]