Abstract
Background: Aging is associated with a decline in incremental LH pulse amplitude, which could be due to decreased GnRH secretion or impaired GnRH action.
Hypothesis: Inconsistent published studies of GnRH action in older men may be due to disparate sex-steroid milieus.
Facility: This study was conducted at a clinical translational-research unit.
Subjects: We studied 16 healthy men (8 young men and 8 older men).
Methods: An overnight transdermal testosterone (T) clamp was implemented before randomly ordered injections of 0, 2.5, 10, 25, 250, and 750 ng GnRH on separate days (96 study sessions).
Outcomes: LH responses were quantified by variable-waveform deconvolution analysis.
Results: The T clamp maintained age-invariant mean concentrations of total, bioavailable, and free T, SHBG, LH, FSH, and prolactin. By two-way analysis of covariance, GnRH dose (P < 0.001) but not age (0.15 ≤ P ≤ 0.83) determined mean, peak, incremental, and pulsatile LH responses. Statistical power (median) was 95, 98, 90, and 99% to detect a 30% or greater age contrast at P ≤ 0.05 in mean, peak, incremental, and pulsatile LH responses, and greater than 99% to detect a 30% or greater age contrast in bioavailable or total T concentrations. Higher GnRH doses (P < 0.001) abbreviated LH secretory bursts in both age groups.
Conclusion: In the face of eugonadal concentrations of total, bioavailable, and free T, young and older men exhibit remarkably similar LH responses to a 300-fold dose range of exogenous GnRH. Accordingly, previously reported disparate effects of age on GnRH action may reflect in part age-discrepant sex-steroid milieus.
When systemic testosterone levels are controlled experimentally, young and older men maintain similar LH responses to a 300-fold range of GnRH doses.
Aging in men is marked by gradual reductions in total, bioavailable, and free testosterone (T) as well as estradiol (E2) concentrations with a reciprocal rise in SHBG concentrations, as corroborated by longitudinal and cross-sectional studies in North America, Europe, and Australia (1). Although the mechanisms subserving progressive hypoandrogenemia in healthy older men are not established, aging is associated with diminished Leydig-cell number and decreased T responsiveness to injections of human chorionic gonadotropin and pulses of recombinant human LH (2). Analyses of intensively sampled 24-h LH profiles have further demonstrated a 30% increase in the frequency of ultradian LH pulses in the elderly male and a 50% decrement in incremental LH pulse amplitude, LH pulse area, and LH secretory-burst mass (3,4,5). This phenotype of LH pulsatility can be mimicked in young men by administering ketoconazole (KTCZ), an inhibitor of adrenal and testicular steroidogenesis, with glucocorticoid replacement (6). Intravenous T infusion or transdermal T delivery restores normal LH pulsatility, thereby verifying the key role of T in maintaining physiological LH pulse frequency and incremental LH pulse size (6,7).
Earlier assessments of the effects of GnRH on LH secretion in older men are confounded by several major factors. Limitations exist with respect to GnRH infusion dose (often a single pharmacological bolus), low sampling frequency and short sampling duration, type of LH assay, presence of obesity (body mass index ≥ 30 kg/m2), and irreproducibility across cohorts (5,8,9,10,11,12,13,14,15,16,17). In fact, LH responses to GnRH have been reported as greater, less, and similar in older compared with young men.
The incremental size of LH pulses can be viewed as a joint consequence of GnRH's dose-dependent stimulation of gonadotrope cells and sex-steroidal concentration-dependent feedback restraint of GnRH secretion and action (18,19). Therefore, valid comparison of gonadotrope responsiveness to exogenous GnRH would require either precise covariate adjustment for concomitant negative feedback or direct experimental control of T and E2 availability during GnRH testing. To implement the latter strategy, T and E2 concentrations were maintained at eugonadal levels in young and older men by combined administration of KTCZ/dexamethasone (DEX) and transdermal T. Under the overnight T clamp, each subject received double-blind, randomly ordered iv injections of saline (zero GnRH) and/or one of five doses of GnRH on separate mornings. The hypothesis was that age does not alter gonadotropin responses to dose-varying GnRH drive when systemic T availability is comparable.
Subjects and Methods
Subjects
A total of 16 healthy men participated. There were eight older (median age, 66 yr; range, 64–70 yr) and eight young (median age, 32 yr; range, 19–39 yr) volunteers. Median body mass index values in the young and older men were 23 and 24 kg/m2, respectively (P = not significant), with an absolute range of 21–26 kg/m2 for the combined cohorts. Each subject provided written informed consent approved by the local institutional review board. Medical inventory and physical examination (including testis size, libido, and potentia) were normal. There was no history of infertility, systemic disease, recent weight change, hormonal therapy, or psychoactive drug use. Fasting (0800 h) screening biochemical tests of metabolic, hematological, hepatic, and renal function were normal. Baseline endocrine evaluation was unremarkable for age, including serum T4 (normal range, 4–10 μg/dl), total T (≥300 ng/dl or ≥11 nmol/liter), E2 (<40 pg/ml or <140 pmol/liter), LH (2–15 IU/liter), FSH (2–20 IU/liter), and prolactin (2–15 μg/liter).
Clinical protocol
An indwelling iv catheter was placed in a forearm vein at 0645 h on the day of study, and blood samples (1.5 ml) were withdrawn every 10 min for 5 h beginning at 0800 h. The first 120 min of blood sampling served as the baseline (pre-GnRH injection). At 1000 h, a single dose of GnRH or saline was given by bolus iv injection double-blind, in randomly assigned order on separate mornings at least 3 d apart. Subjects each received GnRH doses of 0, 2.5, 10, 25, 250, and 750 ng/kg during the transdermal T clamp.
T clamp
To block adrenal and testicular steroidogenesis, KTCZ (1000 mg) was given orally with a nondairy snack at 2200 h. To maintain inhibition, a second dose of 400 mg KTCZ was administered orally at 0600 h in the same manner (6,20). DEX (0.75 mg, orally) was given concomitantly at 2200 h to prevent glucocorticoid deficiency. Administration of four times this glucocorticoid dose for 14 d does not alter gonadotropin responses to 100 ng/kg GnRH or 24-h LH pulsatility in men (21).
Assays
Blood samples were allowed to clot at room temperature, and sera were frozen at −20 C. LH concentrations were assayed by robotics-automated, LH β-subunit-directed, two-site monoclonal immunoradiometric assay (Nichols Institute, Inc., San Juan Capistrano, CA) (5). The sensitivity of the LH assay is 0.2 IU/liter (First International Reference Preparation), and the median interassay coefficient of variation is 8.5%. The median within-assay coefficient of variation was 5.8% (range, 3.9–6.5%). There is less than 0.03% cross-reactivity with free α- or LH β-subunit, FSH, or TSH. Concentrations of total T, E2, FSH, prolactin, and SHBG were measured in individual 2-h serum pools collected before GnRH injection (0800–1000 h, baseline) using RIA (sex steroids) and immunoradiometric assay (protein hormones; Diagnostic Products, Inc., Los Angeles, CA; and Diagnostic Laboratory Systems, Webster, TX) (21). Bioavailable and free T concentrations were assayed after ammonium-sulfate precipitation and equilibrium dialysis as described earlier (5).
Deconvolution analysis
LH concentration time series were analyzed using a recently developed automated deconvolution method, which was empirically validated using hypothalamo-pituitary sampling and simulated pulsatile time series (22,23,24). The Matlab-based algorithm first detrends the data and normalizes concentrations to the unit interval (0, 1). Second, the program creates multiple successively decremental potential pulse-time sets, each containing one fewer burst by a smoothing process (a nonlinear adaptation of the heat-diffusion equation). Third, a maximum-likelihood expectation estimation method calculates all secretion and elimination parameters simultaneously, conditional on each of the candidate pulse-time sets. Deconvolution parameters comprise basal secretion (β0), secretory-burst mass (η0, η1), random effects on burst mass (ςA), procedural/measurement error (ςε), and a three-parameter flexible γ secretory-burst waveform (β1, β2, β3). The fast half-life of LH was represented as 18 min constituting 63% of the decay amplitude and the slow half-life 90 min (25). Statistical model selection is performed to distinguish among the independently framed fits of the individual candidate pulse-time sets using the Akaike information criterion. The three parameters (and units) of interest in this study are basal and pulsatile secretion rates (concentration units per session), and waveform mode (time delay to maximal secretion after objectively estimated burst onset in minutes).
Statistical comparisons
The incremental rise in gonadotropin concentrations was defined as the algebraic difference between the GnRH-stimulated peak (maximal single concentration) and the mean (2-h) pre-GnRH LH or FSH concentration.
Two-way analysis of covariance (ANCOVA) was used to test the impact of GnRH dose on primary LH secretory measures and concentrations. The ANCOVA model entailed a two-factor (age) by five-factor (GnRH dose) design, in which the covariate was the subject's LH response on the saline-infusion (zero-dose GnRH) day (Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). LH-response measures were first transformed to the natural logarithmic scale to produce symmetric distributions and equalize measurement variability. The mixed-effects ANCOVA model (26) included two classification factors to estimate the main effect of age (young or old) and GnRH dose (five levels). Model parameters were estimated by way of residual maximum likelihood, and the variance-covariance matrix was modeled in the compound symmetry form. Tukey's honestly significantly different test was applied post hoc to allow protected comparisons among means (26). In the case of total T concentrations, the covariate was not significant, so a 2 × 5-factorial ANOVA model was employed (age × doses of GnRH) in a repeated-measures design applied to within-subject incremental T differences (value on day of GnRH dose minus value on the saline day).
Data are given as the mean ± sem. Experiment-wise P < 0.05 was construed as statistically significant. Statistical power was estimated as summarized in Supplemental Table 2.
Results
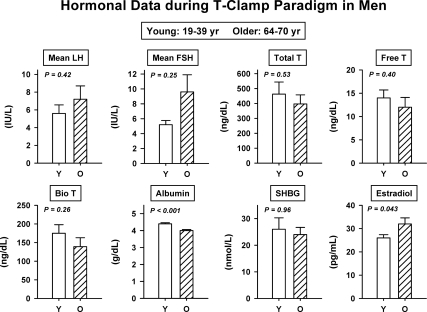
The T-clamp paradigm yielded comparable mean pooled 2-h (0800–1000 h) serum concentrations of LH, FSH, prolactin, SHBG, total T, bioavailable and free T (all P > 0.10) in young and older men (Fig. 1). Two-way ANOVA of incremental (GnRH-infused minus saline-infused value of) total T concentrations on the five separate GnRH-infusion days in the eight young and eight older men disclosed no effect of age (P = 0.38) or GnRH dose (P = 0.94) and no interaction (P = 0.56). The same was true for both bioavailable and free T (P > 0.20). There was a weak effect of age (P = 0.043) on mean serum E2 concentrations measured under the T clamp, viz., 26 ± 1.4 (young) and 32 ± 2.6 (older) pg/ml (multiply by 3.68 for pmol/liter). Serum albumin and IGF-I were lower in older subjects than in young subjects.
Figure 1.
Comparability of mean serum LH, FSH, total T, free T, bioavailable T, SHBG, and prolactin but not albumin concentrations in eight young and eight older healthy men. Subjects were each studied after six overnight KTCZ/DEX transdermal T-addback clamps. P values reflect two-tailed, pooled-variance unpaired t testing. Data are expressed as mean ± sem.
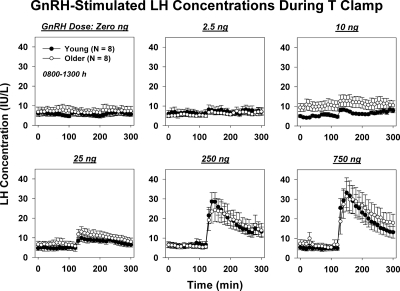
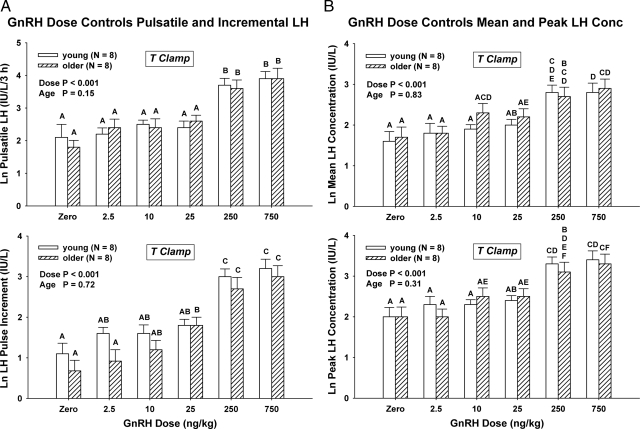
Mean 5-h LH concentration vs. time profiles are given for saline and each of five GnRH doses in young and older men in Fig. 2. Two-way ANCOVA revealed a strong effect of GnRH dose (P < 0.001) on pulsatile LH secretion (viz. summed LH secretory-burst mass after GnRH injection) but no effect of age (P = 0.15) (Fig. 3A, top). The influence of GnRH dose on pulsatile LH secretion was selective because basal (nonpulsatile) LH secretion was independent of both age (P = 0.64) and GnRH dose (P = 0.16). In addition, the incremental rise in LH concentrations induced by GnRH was unrelated to age (P = 0.72) but was positively affected by GnRH dose (P < 0.001) (Fig. 3A, bottom). The post-GnRH mean (3-h) LH concentration was also unrelated to age (P = 0.83) but was strongly influenced by GnRH dose (P < 0.001) (Fig. 3B, top). The absence of an age dependence of LH responses was further affirmed by two-way ANCOVA of peak LH concentrations attained after saline and the five active GnRH doses (age, P = 0.31; GnRH dose, P < 0.001) (Fig. 3B, bottom). Normalizing LH responses as the ratio of LH to E2 or T concentrations preserved the GnRH dose effect (P < 0.001) but did not unveil any effect of age (0.20 ≤ P ≤ 0.71). The ANCOVA results of all four LH-response models are summarized in Supplemental Table 1.
Figure 2.
Saline or GnRH-stimulated LH concentrations measured every 10 min for 5 h during T clamp in young and older men. Data are expressed as mean ± sem.
Figure 3.
A, Pulsatile LH secretion [summed mass (IU) of LH secreted per unit distribution volume (liter)] (top) and incremental (peak minus mean baseline) LH concentration responses (bottom) after bolus iv injection of saline or 2.5, 10, 25, 250, and 750 ng/kg GnRH in eight young and eight older men during a T clamp. Data are expressed as mean ± sem on the natural logarithmic scale. By two-way mixed-effects ANCOVA, overall P was < 0.001 with the indicated individual effects of age and GnRH dose. Means with unshared (unique) alphabetic superscripts differ significantly by post hoc Tukey's honestly significantly different test. Thus, A and B differ, whereas A and AB or AB and B do not. B, Comparable bar-graph plots summarizing mean (top) and peak (bottom) LH responses to a 300-fold dose range of GnRH. Statistical summaries and power analysis are given in Supplemental Tables 1 and 2.
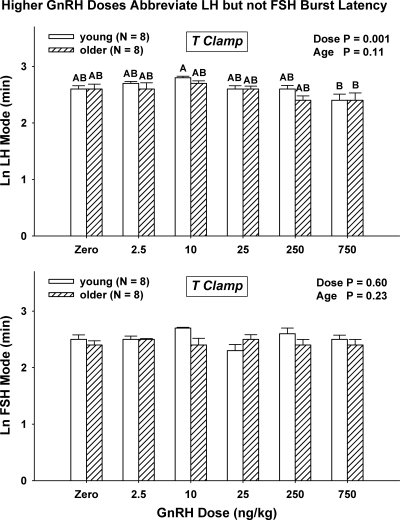
The waveform of secretory bursts can be characterized by the algebraic mode of the burst, viz., the time latency between burst onset and maximal secretion. Two-way ANCOVA disclosed that GnRH dose (P < 0.001), but not age (P = 0.11), regulates LH secretory-burst mode (overall, P < 0.001) (Fig. 4, top). In particular, the highest dose of GnRH reduced the mode in young and older men significantly and comparably from 14 ± 0.76 and 14 ± 1.1 min (saline) to 11 ± 1.2 and 12 ± 1.5 min (GnRH, 750 ng/kg), respectively. There were no effects of age or GnRH dose on the mode for FSH secretory bursts (GnRH dose effect, P = 0.60; age, P = 0.23) (Fig. 4, bottom). The grand mean mode for FSH secretory bursts was 12 ± 2.0 min.
Figure 4.
Increasing doses of GnRH, but not age, decrease the mode of LH (top) but not FSH (bottom) secretory bursts (time delay from stimulus to maximal LH secretion rate) in healthy men. See Fig. 3 for format.
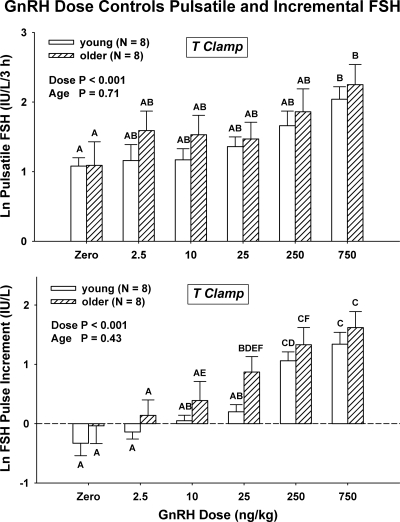
As shown in Supplemental Fig. 1 (bottom), GnRH dose controlled pulsatile and incremental FSH release (both P < 0.001 by ANCOVA) (Fig. 5). GnRH also augmented peak (P = 0.018) (but not mean, P = 0.066) FSH concentrations dose-dependently. In contrast, age did not influence these measures (0.43 ≤ P ≤ 1.0). Age was associated with elevated basal (nonpulsatile) FSH secretion [4.6 ± 1.8 vs. 1.8 ± 0.6 IU/liter/5 h (P = 0.016 via sign test)].
Figure 5.
GnRH dose controls pulsatile FSH secretion (summed mass of FSH secreted in bursts, top) and incremental FSH concentrations (peak minus mean value, bottom) independently of age. See Fig. 3 for representation of data.
Supplemental Table 2 gives statistical power estimates for detecting a 30% contrast due to age at each of the five doses of GnRH for each of the four models of LH responses. In each model, power rose with GnRH dose. Median (range) estimates of power were 99 (85–99)% for pulsatile LH secretion, 90 (50–94)% for incremental LH response, 95 (65–97)% for mean LH concentration, and 98 (90–99)% for peak LH response. Supplemental Table 3 summarizes power estimates (each > 99%) for detecting a 30% difference in total or bioavailable T concentrations by age.
Discussion
The novelty of these studies is documentation of comparable GnRH dose-dependencies of gonadotropin secretion in young and older men subjected to a transdermal T clamp. Variable T levels in young and older groups had previously precluded definitive statements about GnRH dose-response functions. The clamp paradigm was successful in enforcing clinically and statistically similar systemic concentrations of total, bioavailable, and free T as well as SHBG. Under these experimental conditions, all four complementary measures of stimulated LH secretion were strongly determined by GnRH dose (P < 0.001) assessed over a 300-fold range, but not by age. In particular, GnRH-stimulated mean, peak, and incremental LH concentrations and summed LH secretory-burst mass were all controlled by GnRH dose but unaffected by age. The demonstration of good statistical power (range of medians, 90–99% to detect a 30% or greater age contrast for these various LH-response models) strongly supports the inference that, in a euandrogenemic milieu, older and young men respond to exogenous GnRH pulses with highly comparable LH output. Inasmuch as prior studies did not enforce similar sex-steroid levels in young and older subjects at the time of GnRH stimulation (see introductory section), the present outcomes may provide an explanation for conflicting reports of increased, decreased, and unchanged GnRH actions in aging men.
Although we are unaware of any other analysis of GnRH dose-responsiveness during an experimentally controlled eugonadal T milieu, one prior study was conducted under T-deplete conditions (27). The model comprised pharmacological inhibition of steroidogenesis, which resulted in nearly castrate levels of T. In this setting, older men exhibited enhanced gonadotrope sensitivity and GnRH potency compared with young men, but comparable GnRH efficacy. A simple inference would be that the hypogonadal context contributed to age-associated enhancement of low-dose GnRH action.
Other investigations have used a single maximally effective dose of GnRH (e.g. 100 μg iv bolus), precluding dose-response assessments. In one assessment, GnRH (100 μg) evoked greater LH release during high-dose transdermal dihydrotestosterone delivery than at baseline in older men. In the same study, the effect of 100 μg GnRH was blunted during high-dose transdermal E2 delivery compared with baseline in older men (28). In another report, T and E2 injections failed to suppress (100 μg) GnRH-stimulated LH release comparably in older vis-à-vis young men (11). The relationship of these models to the present dose-response outcomes in a eugonadal sex-steroid milieu is not clear. However, the accompanying analyses clearly indicate that, in a young adult sex-steroid milieu, age does not have defining effects on either LH or FSH secretion in healthy men stimulated with a 300-fold range of GnRH doses.
In the healthy cohort of euandrogenemic men studied here, estimated basal (nonpulsatile) LH secretion rates were independent of both age and GnRH dose. In contrast, basal FSH secretion was higher in the older than the young cohort after saline injection at all GnRH doses. In both age groups, the waveform (shape) of LH, but not FSH, secretory bursts was abbreviated by the highest GnRH dose. The gonadotropin selectivity of the latter effect would suggest that the exocytotic kinetics of GnRH-stimulated LH and FSH release differ in healthy men.
Only a few clinical investigations have evaluated the dose-dependent effects of GnRH on LH and FSH secretion in aging men (8,12,14,15,17). One study reported a decrease, two studies an increase, and two others no effect of age on incremental LH responses in men. One investigation, albeit without the use of validated deconvolution analysis, suggested an age difference in LH half-lives (17). None attempted to achieve eugonadal T concentrations independently of age. Thus, reported accentuation of LH responses to GnRH in aging men may reflect reduced androgen availability for feedback rather than true potentiation of gonadotrope responsiveness (13,15,16,28,29). Inferred normal incremental LH responses to maximal GnRH stimulation would be consistent with the age-comparable effects of 750 ng/kg GnRH observed in the current paradigm (5,10,14,15,17,30). Conversely, lower fractional (percentage ratio of incremental peak to baseline) LH responses to GnRH in elderly men in some studies might be due to higher baseline LH and α-subunit measurements with age and/or relatively infrequent sample collection before and after GnRH injection (12,13,16,31).
An important inference from evidently normal gonadotrope secretory responses to exogenous GnRH in older men subjected to a T clamp is that attenuated incremental LH pulse amplitude, area, and mass in aging would reflect impoverished hypothalamic GnRH secretion, as suggested initially by Kaufman et al. (17) and subsequently by others (3,5,20). This postulate is supported but not proven by experimental data in the rat, mouse, dog, and human (5,7,17,20,27,32,33,34,35,36,37) and by recent analytical models of GnRH secretion (18,19). The latter suggest that neuronal GnRH outflow in healthy men is reduced by 33–50% between the second and eighth decades of life. Establishing the GnRH-deficiency hypothesis would require direct measurements of GnRH in hypothalamo-pituitary portal blood or anterior pituitary interstitial fluid.
Caveats include the need to verify these outcomes in larger cohorts of healthy subjects (here, n = 16) in a longitudinal context using additional doses of GnRH. This study was powered to detect a 30% age-related difference, corresponding to a biologically relevant interclass difference for many hormones, including gonadotropins (38,39,40). In addition, one could not exclude the possibility of an unknown confounding effect of KTCZ, DEX, or slightly different E2 concentrations (32 vs. 26 pg/ml) on GnRH action during the T clamp. However, T replacement reversed KTCZ and DEX effects in two earlier studies (6,21), and normalization of individual LH responses for E2 concentrations here (Results) did not alter conclusions.
In summary, the present investigation demonstrates comparability of GnRH's dose-dependent stimulation of LH and FSH secretion in young and older men studied under an experimental eugonadal T clamp. Multiple complementary measures of LH (and FSH) secretion did not differ by age at good statistical power but were markedly GnRH dose-dependent. GnRH dose shortened LH but not FSH secretory bursts, whereas age elevated basal FSH but not LH secretion. The collective outcomes provide a possible explanatory basis for discrepant earlier studies performed in the face of inconsistent androgen levels.
Supplementary Material
Footnotes
These studies were supported in part by Grant M01 RR00585 from the National Center for Research Resources (Rockville, MD) to the Clinical Translational-Research Unit of Mayo Clinic and Foundation and by Grants RO1 AG31763 and R21 AG23777 from the National Institutes of Health (Bethesda, MD).
Disclosure Summary: The authors have nothing to declare.
First Published Online March 31, 2010
Abbreviations: ANCOVA, Analysis of covariance; DEX, dexamethasone; E2, estradiol; KTCZ, ketoconazole; T, testosterone.
References
- Liu PY, Swerdloff RS, Veldhuis JD 2004 The rationale, efficacy and safety of androgen therapy in older men: future research and current practice recommendations. J Clin Endocrinol Metab 89:4789–4796 [DOI] [PubMed] [Google Scholar]
- Liu PY, Veldhuis JD 2009 The hypothalamo-pituitary unit, testis and male accessory organs. In: Barbieri R, Strauss J, eds. Yen and Jaffe's reproductive endocrinology: physiology, pathophysiology, and clinical management. Vol. 6. Philadelphia: Elsevier; 283–298 [Google Scholar]
- Veldhuis JD, Urban RJ, Lizarralde G, Johnson ML, Iranmanesh A 1992 Attenuation of luteinizing hormone secretory burst amplitude as a proximate basis for the hypoandrogenism of healthy aging in men. J Clin Endocrinol Metab 75:707–713 [DOI] [PubMed] [Google Scholar]
- Mulligan T, Iranmanesh A, Gheorghiu S, Godschalk M, Veldhuis JD 1995 Amplified nocturnal luteinizing hormone (LH) secretory burst frequency with selective attenuation of pulsatile (but not basal) testosterone secretion in healthy aged men: possible Leydig cell desensitization to endogenous LH signaling—a clinical research center study. J Clin Endocrinol Metab 80:3025–3031 [DOI] [PubMed] [Google Scholar]
- Mulligan T, Iranmanesh A, Kerzner R, Demers LW, Veldhuis JD 1999 Two-week pulsatile gonadotropin releasing hormone infusion unmasks dual (hypothalamic and Leydig-cell) defects in the healthy aging male gonadotropic axis. Eur J Endocrinol 141:257–266 [DOI] [PubMed] [Google Scholar]
- Zwart AD, Iranmanesh A, Veldhuis JD 1997 Disparate serum free testosterone concentrations and degrees of hypothalamo-pituitary-LH suppression are achieved by continuous versus pulsatile intravenous androgen replacement in men: a clinical experimental model of ketoconazole-induced reversible hypoandrogenemia with controlled testosterone add-back. J Clin Endocrinol Metab 82:2062–2069 [DOI] [PubMed] [Google Scholar]
- Liu PY, Takahashi PY, Roebuck PD, Bailey JN, Keenan DM, Veldhuis JD 2009 Testosterone's short-term positive effect on luteinizing-hormone secretory-burst mass and its negative effect on secretory-burst frequency are attenuated in middle-aged men. J Clin Endocrinol Metab 94:3978–3986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebar R, Yen SS, VandenBerg G, Naftolin F, Ehara Y, Engblom S, Ryan KJ, Amoss M, Guillemin R 1973 Gonadotropin responses to synthetic LRF: dose-response relationship in men. J Clin Endocrinol Metab 36:10–16 [DOI] [PubMed] [Google Scholar]
- Haug E, Aakvaag A, Sand T, Torjesen PA 1974 The gonadotrophin response to synthetic gonadotrophin-releasing hormone in males in relation to age, dose, and basal serum levels of testosterone, oestradiol-17β and gonadotrophins. Acta Endocrinol (Copenh) 77:625–635 [DOI] [PubMed] [Google Scholar]
- Winters SJ, Troen P 1982 Episodic luteinizing hormone (LH) secretion and the response of LH and follicle-stimulating hormone to LH-releasing hormone in aged men: evidence for coexistent primary testicular insufficiency and an impairment in gonadotropin secretion. J Clin Endocrinol Metab 55:560–565 [DOI] [PubMed] [Google Scholar]
- Muta K, Kato K, Akamine Y, Ibayashi H 1981 Age-related changes in the feedback regulation of gonadotrophin secretion by sex steroids in men. Acta Endocrinol (Copenh) 96:154–162 [DOI] [PubMed] [Google Scholar]
- Snyder PJ, Reitano JF, Utiger RD 1975 Serum LH and FSH responses to synthetic gonadotropin-releasing hormone in normal men. J Clin Endocrinol Metab 41:938–945 [DOI] [PubMed] [Google Scholar]
- Harman SM, Tsitouras PD, Costa PT, Blackman MR 1982 Reproductive hormones in aging men. II. Basal pituitary gonadotropins and gonadotropin responses to luteinizing hormone-releasing hormone. J Clin Endocrinol Metab 54:547–551 [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Iranmanesh A, Mulligan T 2005 Age and testosterone feedback jointly control the dose-dependent actions of gonadotropin-releasing hormone in healthy men. J Clin Endocrinol Metab 90:302–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwart AD, Urban RJ, Odell WD, Veldhuis JD 1996 Contrasts in the gonadotropin-releasing dose-response relationships for luteinizing hormone, follicle-stimulating hormone, and α-subunit release in young versus older men: appraisal with high-specificity immunoradiometric assay and deconvolution analysis. Eur J Endocrinol 135:399–406 [DOI] [PubMed] [Google Scholar]
- Celani MF, Montanini V, Baraghini GF, Carani C, Marrama P 1984 Effects of acute stimulation with gonadotropin releasing hormone (GnRH) on biologically active serum luteinizing hormone (LH) in elderly men. J Endocrinol Invest 7:589–595 [DOI] [PubMed] [Google Scholar]
- Kaufman JM, Giri M, Deslypere JM, Thomas G, Vermeulen A 1991 Influence of age on the responsiveness of the gonadotrophs to luteinizing hormone-releasing hormone in males. J Clin Endocrinol Metab 72:1255–1260 [DOI] [PubMed] [Google Scholar]
- Keenan DM, Takahashi PY, Liu PY, Roebuck PD, Nehra AX, Iranmanesh A, Veldhuis JD 2006 An ensemble model of the male gonadal axis: illustrative application in aging men. Endocrinology 147:2817–2828 [DOI] [PubMed] [Google Scholar]
- Keenan DM, Veldhuis JD 2009 Age-dependent regression analysis of male gonadal axis. Am J Physiol Regul Integr Comp Physiol 297:R1215–R1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuis JD, Zwart A, Mulligan T, Iranmanesh A 2001 Muting of androgen negative feedback unveils impoverished gonadotropin-releasing hormone/luteinizing hormone secretory reactivity in healthy older men. J Clin Endocrinol Metab 86:529–535 [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Lizarralde G, Iranmanesh A 1992 Divergent effects of short-term glucocorticoid excess on the gonadotropic and somatotropic axes in normal men. J Clin Endocrinol Metab 74:96–102 [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S, Veldhuis JD, Keenan DM 2008 Probabilistic recovery of pulsatile, secretory and kinetic structure: an alternating discrete and continuous schema. Q Appl Math 66:401–421 [Google Scholar]
- Liu PY, Keenan DM, Kok P, Padmanabhan V, O'Byrne KT, Veldhuis JD 2009 Sensitivity and specificity of pulse detection using a new deconvolution method. Am J Physiol Endocrinol Metab 297:E538–E544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan DM, Roelfsema F, Biermasz N, Veldhuis JD 2003 Physiological control of pituitary hormone secretory-burst mass, frequency and waveform: a statistical formulation and analysis. Am J Physiol Regul Integr Comp Physiol 285:R664–R673 [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Fraioli F, Rogol AD, Dufau ML 1986 Metabolic clearance of biologically active luteinizing hormone in man. J Clin Invest 77:1122–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zar JH 1996 Biostatistical analysis. 3rd ed. Upper Saddle River, NJ: Prentice Hall [Google Scholar]
- Veldhuis JD, Iranmanesh A 2005 Short-term aromatase-enzyme blockade unmasks impaired feedback adaptations in luteinizing hormone and testosterone secretion in older men. J Clin Endocrinol Metab 90:211–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deslypere JP, Kaufman JM, Vermeulen T, Vogelaers D, Vandalem JL, Vermeulen A 1987 Influence of age on pulsatile luteinizing hormone release and responsiveness of the gonadotrophs to sex hormone feedback in men. J Clin Endocrinol Metab 64:68–73 [DOI] [PubMed] [Google Scholar]
- Urban RJ, Veldhuis JD, Blizzard RM, Dufau ML 1988 Attenuated release of biologically active luteinizing hormone in healthy aging men. J Clin Invest 81:1020–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontiroli AE, Ruga S, Maffi P, Scaglia L, Perfetti MG, Pozza G 1992 Pituitary reserve after repeated administrations of releasing hormones in young and in elderly men: reproducibility on different days. J Endocrinol Invest 15:559–566 [DOI] [PubMed] [Google Scholar]
- Ceda GP, Denti L, Ceresini G, Torsiglieri W, Hoffman AR, Valenti G 1991 The effects of aging on the secretion of the common α-subunit of the glycoprotein hormones in men. J Am Geriatr Soc 39:353–358 [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Keenan DM, Iranmanesh A, Takahashi PY, Nehra AX 2007 The ensemble male hypothalamo-pituitary-gonadal axis. In: Timiras PS, ed. Physiological basis of aging and geriatrics. 4th ed. New York: Taylor, Francis Group, LLC, Health Science Division; 185–203 [Google Scholar]
- Liu PY, Pincus SM, Takahashi PY, Roebuck PD, Iranmanesh A, Keenan DM, Veldhuis JD 2006 Aging attenuates both the regularity and joint synchrony of LH and testosterone secretion in normal men: analyses via a model of graded GnRH receptor blockade. Am J Physiol Endocrinol Metab 290:E34–E41 [DOI] [PubMed] [Google Scholar]
- Böttner M, Leonhardt S, Wuttke W, Jarry H 2007 Changes of expression of genes related to the activity of the gonadotrophin-releasing hormone pulse generator in young versus middle-aged male rats. J Neuroendocrinol 19:779–787 [DOI] [PubMed] [Google Scholar]
- Coquelin A, Desjardins C 1982 Luteinizing hormone and testosterone secretion in young and old male mice. Am J Physiol 243:E257–E263 [DOI] [PubMed] [Google Scholar]
- Gruenewald DA, Naai MA, Marck BT, Matsumoto AM 2000 Age-related decrease in hypothalamic gonadotropin-releasing hormone (GnRH) gene expression, but not pituitary responsiveness to GnRH, in the male Brown Norway rat. J Androl 21:72–84 [PubMed] [Google Scholar]
- Vermeulen A, Deslypere JP, Kaufman JM 1989 Influence of antiopioids on luteinizing hormone pulsatility in aging men. J Clin Endocrinol Metab 68:68–72 [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Roemmich JN, Richmond EJ, Bowers CY 2006 Somatotropic and gonadotropic axes linkages in infancy, childhood, and the puberty-adult transition. Endocr Rev 27:101–140 [DOI] [PubMed] [Google Scholar]
- Keenan DM, Licinio J, Veldhuis JD 2001 A feedback-controlled ensemble model of the stress-responsive hypothalamo-pituitary-adrenal axis. Proc Natl Acad Sci USA 98:4028–4033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuis JD, Keenan DM, Pincus SM 2008 Motivations and methods for analyzing pulsatile hormone secretion. Endocr Rev 29:823–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.