Abstract
Background:
Measurement of cardiac output and extravascular lung water in critically ill patients using femoral artery double-indicator dilution involves femoral artery catheterization. The potential risk of vascular compromise to the limb may be exacerbated in patients receiving vasopressors. The utility of scanning laser Doppler flowmetry to measure changes in pedal perfusion following catheterization was assessed.
Results:
There were no significant changes in mean occlusion pressures or in cutaneous perfusion between either leg or between measurement time points, immediately after or 24 h following insertion of the catheters.
Conclusions:
Scanning laser Doppler flowmetry is easily used to assess changes in foot perfusion and the effect of interventions that may reduce blood flow to the skin of the foot. Femoral artery catheterization for double-indicator dilution measurements does not reduce calf occlusion pressures or foot skin perfusion in patients receiving vasopressor drugs.
Keywords: arterial catheterisation, laser Doppler, scanning laser Doppler, digital ischaemia, intrathoracic blood volume
Introduction
Fluid restriction and diuresis are frequently used in the management of the acute respiratory distress syndrome (ARDS), and a strategy of reduction in extravascular lung water (EVLW) has been shown to reduce both ventilator and intensive care unit (ICU) days [1].
Intrathoracic blood volume (ITBV) comprises the left and right heart blood volumes and the pulmonary blood volume, and has been shown to be a better indicator of cardiac filling than the pulmonary artery occlusion pressure (PAOP) [2]. Both EVLW and ITBV are measured using double-indicator dilution and the COLD Z-021 system (Pulsion Medical Systems, Munich, Germany) which requires the insertion of a catheter into the femoral artery through a 4.5 F sheath. The technique also measures cardiac output by analysis of the femoral artery thermodilution curve. Digital ischaemia, although rare, is a well known complication of arterial cannulation [3,4,5] and this problem may be exacerbated in patients with sepsis who have microcirculatory disturbances including slowing of capillary blood flow and local arteriolar constriction [6], and who also often require vasopressors for hypotension. A clinical concern exists, therefore, about the safety of femoral artery cannulation in the critically ill patients who may potentially benefit from this sophisticated monitoring.
Following insertion of the femoral artery catheter, peripheral tissue perfusion is usually assessed by regular clinical examination of skin colour, capillary return and temperature, all of which are subjective measures. The presence or absence of pedal pulses and measurement of maximum calf occlusion pressures assess only gross changes in large vessel flow. Single point laser Doppler flowmetry has been employed successfully as a technique for measuring skin blood flow [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21], but there are several methodological drawbacks associated with the technique. These include the potential influence of local perfusion by contact with traditional laser Doppler probes [18], the small sampling area of single point measurements which, because of the spatial heterogeneity of the cutaneous microvasculature, might not be representative of overall flow [22,23], and the long time needed to make multiple measurements.
Scanning laser Doppler flowmetry is a recent development [24,25] of the laser Doppler technique that may overcome these drawbacks. A laser beam is directed to scan over a predetermined area and thousands of individual measurements of perfusion are made. These data are stored electronically and converted into both greyscale photographic and colour-coded perfusion images. Subsequently, regions of interest can be outlined on these images and mean perfusion within them calculated.
This new technique was used to assess the influence upon pedal cutaneous perfusion of the insertion of a femoral artery catheter for the purpose of measuring ITBV, EVLW and cardiac output (CO) using the Pulsion COLD system.
Materials and methods
Following hospital ethical committee approval, and after obtaining informed consent, 10 patients in a single intensive care unit were studied. All were intubated, ventilated and sedated and required cardiovascular support with vasoactive drugs. Following the clinical decision to insert a femoral artery catheter the patients were randomized to right or left leg catheterization. None had either historical nor current clinical evidence of peripheral arterial disease. Room temperature was kept constant at 22°C and both legs were uncovered for a 15 min equilibrium period. All measurements were made immediately before and after insertion of the femoral artery catheter (Pulsiocath 2024 L, Pulsion, Munich, Germany) and 24 h subsequently. All catheterizations were performed by a single individual and involved a standard Seldinger technique and insertion of a 4.0 F catheter through a 4.5 F sheath.
The mean systemic arterial blood pressure was measured using a 20-gauge cannula positioned within the radial artery. Calf occlusion pressure was measured using a hand held sonic Doppler and a standard sphygmomanometer cuff, the maximum in either the dorsalis pedis or posterior tibial artery being recorded as the value. Vasoactive drug doses were recorded at the time of each measurement.
Laser Doppler scans (Moor LDI, Moor Instruments Ltd, Axminster, Devon, UK; approximate cost £30 000) of the plantar aspect of both feet were made from a distance of 38 cm. This distance was chosen as the closest from which a scan of the entire sole of the foot could always be included. In each case, the laser Doppler scanner was positioned to ensure that the laser beam was perpendicular to the patient's feet. From each set of data, two images were simultaneously produced and stored electronically: a greyscale photographic image and a colour-coded perfusion map. On the photographic image, the sole of the foot was outlined, which equated to a mean of over 17 000 individual perfusion measurements on the corresponding blood flow image, allowing calculation of mean perfusion units (PUs) for each foot for comparison with the other side. The results were analysed statistically using the Mann-Whitney U-test.
Results
The laser Doppler scans were easy to make (Fig. 1) and the computer software supplied with the scanner allowed simple measurement of mean perfusion within the outlined foot. There were no complications associated with catheter insertion.
Figure 1.
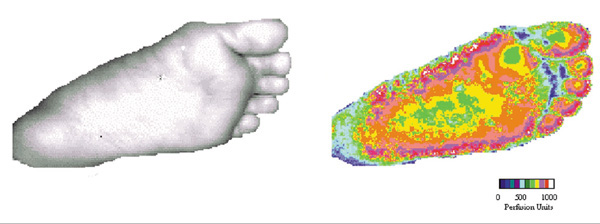
Laser Doppler scans of the sole of the foot. The photographic image and colour coded perfusion map were made simultaneously.
There were no significant changes in mean systemic arterial blood pressure or maximal occlusion pressures between preinsertion and postinsertion measurements (Table 1). Furthermore, there were no significant changes in any of these parameters when they were measured 24 h following catheterization.
Table 1.
Systemic arterial and calf occlusion pressures (mmHg) prior to, immediately after and 24 h following arterial catheterization
| Preinsertion | Postinsertion | P value | 24 h | P value | |
| Mean systemic arterial pressure | 76 ± 11 | 80 ± 12 | 0.11 | 79 ± 11 | 0.44 |
| Catheter leg maximum occlusion pressure | 112 ± 21 | 108 ± 27 | 0.41 | 117 ± 26 | 0.65 |
| Noncatheter leg maximum occlusion pressure | 111 ± 33 | 111 ± 28 | 0.89 | 114 ± 18 | 0.82 |
Values are shown as mean± SD. P values of postcatheterization and 24 h measurements are with regard to preinsertion values.
All the patients were receiving vasopressor agents over the 24 h study period (norepinephrine, 0.08–0.78 μg/kg per min; and epinephrine, 0.47–0.92 μg/kg per min). Dobutamine (0.03–16.6 μg/kg per min) was also administered to seven patients. No alterations to the patients' doses were made between arterial catheterization and the first post-catheterisation measurement. There was no significant change in any of these drug dosages 24 h following catheter insertion (P values for norepinephrine, epinephrine and dobutamine 0.65, 0.89 and 0.11, respectively).
There was no significant reduction in foot cutaneous perfusion measured with the laser Doppler scanner either between the first two or between the second and final measurements following insertion of the catheters (Table 2). Prior to catheter insertion, the mean difference in foot perfusion (insertion minus noninsertion legs) was 12.5 PUs; following insertion this value was 16.8 PUs (P = 0.22) and, at 24 h, -2.9 PUs (P = 0.8).
Table 2.
Foot cutaneous perfusion prior to, immediately following and 24 h after arterial catheterization
| Preinsertion | Postinsertion | 24 h | |
| Insertion leg | 230.5 ± 173 | 205.7 ± 178 | 217.1 ± 146 |
| Noninsertion leg | 218.1 ± 170 | 188.8 ± 176 | 219.9 ± 171 |
| Insertion minus noninsertion legs | 12.5 ± 72 | 16.8 ± 51 | -2.9 ± 77 |
| P value | — | 0.22 | 0.80 |
Values are shown as mean ± SD. P values of postcatheterization and 24 h measurements are with regard to preinsertion values.
Discussion
ARDS describes a pathological syndrome characterized by an immunological reaction to various insults that results in diffuse alveolar damage. It is commonly associated with sepsis and has a mortality of 50–70%. Mitchell et al [1] showed that patients with ARDS managed with diuresis/ fluid restriction and guided by the measurement of EVLW had significantly less EVLW than those managed conventionally using the PAOP; furthermore, a >15% reduction in EVLW was associated with a reduction in ventilator and ICU days. However, fluid management in patients with ARDS and septic shock can be difficult. Fluid restriction in patients with septic shock may exacerbate haemodynamic instability, increase inotropic requirement and lead to vital organ hypoperfusion. Thus, if this strategy is to be used, circulatory volume status and end-organ function must be carefully monitored.
Following insertion of a femoral artery catheter prior to the measurement of EVLW and ITBV using double-indicator dilution, peripheral perfusion has usually been assessed by regular clinical examination for capillary return, warmth, presence of pedal pulses and measurement of maximum calf occlusion pressures. More objective techniques of measuring cutaneous perfusion are available [26], but these do not lend themselves to everyday clinical use. Conventional single-point laser Doppler flowmetry (LDF) has been successfully used to assess the cutaneous microcirculation in a variety of clinical applications. Several studies have demonstrated the ability of LDF to distinguish patients with established peripheral vascular disease from normal patients [7,8], and it has been used to assess the viability of tissue flaps [10,11,12]; LDF has also been useful in the measurement of cutaneous perfusion following percutaneous transluminal angioplasty [13] in patients with peripheral vascular disease and following the administration of vasoactive agents [14].
Several comparative studies have validated LDF with other methods of measuring skin blood flow [15,16], but the failure of the technique to gain widespread acceptance in clinical practice is secondary to the methodological drawbacks associated with it. Variation in the number of perfused capillaries and local differences in the vasodilator response characteristics of vessels cause local differences in blood flow of up to sixfold [17]. The sampling area of single-point laser Doppler probes is dependent upon their geometry, but is usually between 1 and 3 mm3 and, because of the spatial heterogeneity of tissue blood flow, single measurements may not be representative of overall local cutaneous blood flow [22,23]. It has been suggested that calculation of the mean of up to six measurements each taken over 30 s will reduce the error, but clearly this is time consuming [22]. Direct contact with the skin is necessary with traditional laser Doppler probes, and there are frequent difficulties in maintaining the same point of contact, making sequential measurements open to inaccuracy [27] furthermore, pressure may influence local perfusion itself [18].
The recent development of the scanning laser Doppler technique [24,25] allows the rapid measurement of flow over a large predetermined area and, by collecting reflected light via mirrors, the previously requisite probe and optic cables are dispensed with and there is no contact with the skin itself. The scanner is mounted on a portable stand and can be positioned to allow imaging around a 360° axis (Figs 2 and 3). It is run by a portable notebook computer which allows the operator to predetermine the area in which flow is to be sampled. The laser is then scanned in a continuous rasta pattern over the tissue. A measurement is made every 4 ms, and up to 65 500 individual measurements can be made in less than 5 min. The perfusion data is transformed instantaneously into a greyscale photographic image and a corresponding colour perfusion image is generated, with different colours representing different levels of flow. The associated computer software allows either the whole image or smaller regions of interest on either the colour or photographic images to be outlined and mean perfusion calculated within this area.
Figure 2.
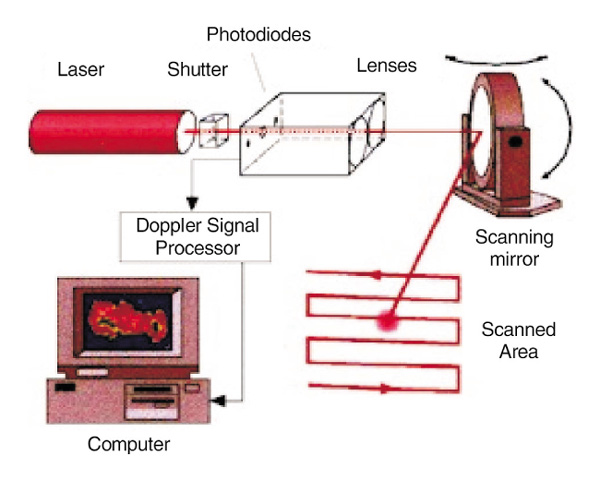
Schematic diagram of scanning laser Doppler technique. The computer directs the mirror to scan the laser beam across the predesignated area. Reflected Doppler shifted light is decoded by the signal processor which then creates a colour-coded perfusion image on the computer screen. The whole image or areas within it can be outlined and perfusion within them automatically calculated.
Figure 3.
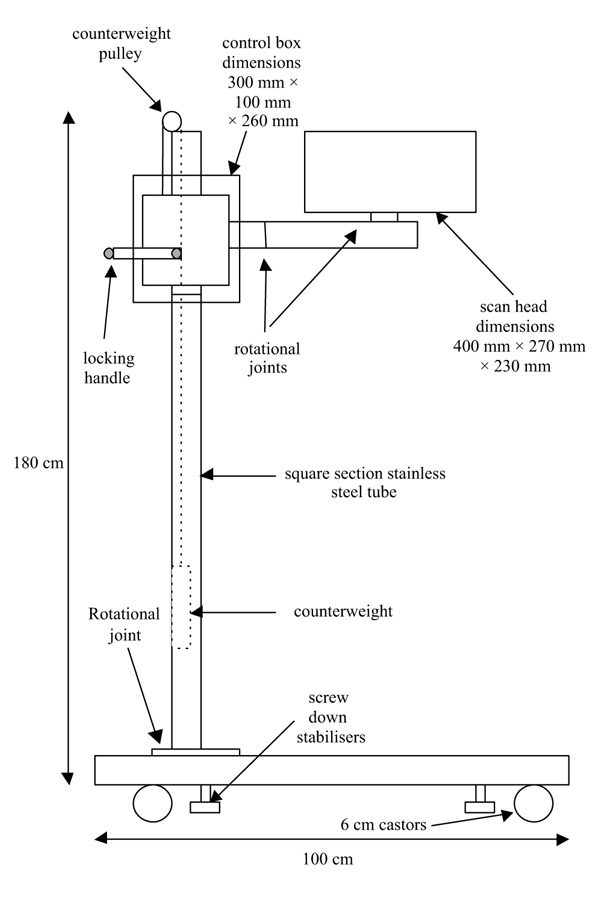
Schematic drawing of scanning laser Doppler assembly on its mobile stand.
The technique is noninvasive and, because of the multiple measurements made over a wide area, the error associated with the spatial heterogeneity of cutaneous vessels when making sequential measurements from the same point is removed. The technique has been validated against flow models, conventional single-point laser Doppler flowmetry and other established methods of assessing skin perfusion [28,29,30,31], and has been used to measure perfusion in hepatic [32] and colonic [33] tissue. Amongst cutaneous applications it has been successfully used to assess burn depth [34], skin flap viability [18], the cutaneous response to inflammation [35] and the therapeutic response of psoriatic plaques to psoralen and long-wave ultraviolet-A radiation therapy (PUVA) treatment [35]. Other clinical applications of laser Doppler imaging to the measurement of cutaneous blood flow include the effects of postural changes in blood flow in venous leg ulcers [20], the assessment of digital perfusion following arterial repair [21], skin perfusion overlying benign and malignant breast disease [36] and the effect of prostaglandins on ischaemic ulcers [37].
Femoral artery catheterization has an associated limb threatening complication rate of up to 1% and can jeopardise peripheral perfusion [5]. Femoral arterial trauma with associated thrombosis and limb threatening ischaemia following catheterization will be clinically obvious. Standard postcatheterization observations usually involve measurement of calf occlusion pressures and, in this study, these were not influenced by insertion of the catheter.
More subtle changes in cutaneous flow may be more difficult to establish using clinical observation, particularly in patients who are receiving vasoactive drugs secondary to their septic condition; this group of patients may be particularly at risk of critical peripheral ischaemia. Furthermore, by their very nature, these techniques are wholly subjective. Scanning laser Doppler flowmetry allows objective measurement of cutaneous blood flow and is able to detect changes in cutaneous perfusion that are not detected by the human eye. We were not able to demonstrate any significant alteration in pedal cutaneous perfusion either immediately following catheterization or 24 h later. Despite the vasoactive drugs that the patients were receiving, the presence of the femoral catheter did not appear to reduce the blood supply to the skin.
Femoral artery catheterization for the purpose of measuring ITBW, EVLW and CO does not compromise peripheral cutaneous perfusion in critically ill patients. Scanning laser Doppler flowmetry is easily used to assess cutaneous perfusion in the ICU and provides an objective alternative to standard clinical evaluation of limb blood flow. It also has the potential to assess cutaneous perfusion following other interventions, either mechanical or pharmacological.
References
- Mitchell JP, Schuller D, Calandrino FS, Schuster DP. Improved outcome based on fluid management in critically ill patients requiring pulmonary artery catheterization. Am Rev Respir Dis. 1992;145:990–998. doi: 10.1164/ajrccm/145.5.990. [DOI] [PubMed] [Google Scholar]
- Lichtwarck-Aschoff M, Zeravik J, Pfeiffer UJ. Intrathoracic blood volume accurately reflects circulatory volume status in critically ill patients with mechanical ventilation. Intensive Care Med. 1992;18:142–147. doi: 10.1007/BF01709237. [DOI] [PubMed] [Google Scholar]
- Russell JA, Joel M, Hudson RJ, Mangano DT, Schlobohm RM. Prospective evaluation of radial and femoral artery catheterization sites in critically ill adults. . Crit Care Med. 1983;11:936–939. doi: 10.1097/00003246-198312000-00007. [DOI] [PubMed] [Google Scholar]
- Katz SG, Kohl RD. Angiographic induced arterial occlusion. . J Am Coll Surg. 1994;178:439–442. [PubMed] [Google Scholar]
- Oweida SW, Roubin GS, Smith RB, Salam AA. Postcatheterisation vascular complications associated with percutaneous transluminal coronary angioplasty. J Vasc Surg. 1990;12:310–315. [PubMed] [Google Scholar]
- Hinshaw LB. Sepsis/septic shock: participation of the microcirculation: an abbreviated review. Crit Care Med. 1996;24:1072–1078. doi: 10.1097/00003246-199606000-00031. [DOI] [PubMed] [Google Scholar]
- Kvernebo K, Slagsvold CE, Strandon E, Kroese A, Larsen S. Laser Doppler flowmetry in evaluation of lower limb resting skin circulation. A study in healthy controls and atherosclerotic patients. Scand J Clin Invest. 1988;48:621–626. doi: 10.1080/00365518809085781. [DOI] [PubMed] [Google Scholar]
- Karanfilian RG, Lynch TG, Lee BC, Hobson RW. The assessment of skin blood flow in peripheral vascular diseae by laser Doppler flowmetry. . AmSurg. 1984;50:641–644. [PubMed] [Google Scholar]
- Winsor T, Haumschild DJ, Winsor DW, Wang Y, Luong TN. Clinical application of laser Doppler flowmetry for measurement of cutaneous circulation in health and disease. Angiology. 1987;38:727–736. doi: 10.1177/000331978703801001. [DOI] [PubMed] [Google Scholar]
- Clinton MS, Sepka RS, Bristol D, Pederson WC, Barwick WJ, Serafin D, Klitzman B. Establishment of normal ranges of laser-Doppler blood flow in autologous tissue transplants. Plast Reconstr Surg. 1991;87:299–309. doi: 10.1097/00006534-199102000-00012. [DOI] [PubMed] [Google Scholar]
- Heden P, Arnander C. Temperature load test to increase the accuracy of laser Doppler monitoring of flaps. Scand J Plast Reconstr Surg. 1992;26:29–32. doi: 10.3109/02844319209035179. [DOI] [PubMed] [Google Scholar]
- Jones BM. Predicting the fate of free tissue transfers. . Ann R Coll Surg Engl. 1985;67:63–70. [PMC free article] [PubMed] [Google Scholar]
- Moneta Gl, Schneider E, Jäger K, Brülisauer M, Thüring-Vollenweider U, Bollinger A. Laser Doppler flux and vasomotion in patients before and after transluminal angioplasty for limb salvage. Vasa. 1988;17:26–31. [PubMed] [Google Scholar]
- Creutzig A, Caspary L, Hertel RF, Alexander K. Temperature-dependent laser Doppler fluxmetry in healthy and patients with peripheral arterial occlusive disease. Int J Microcirc Clin Exp. 1987;6:381–390. [PubMed] [Google Scholar]
- Johnson JM, Taylor WF, Shepherd AP, Park MK. Laser Doppler measurement of skin blood flow; comparison with plethysmography. J Appl Physiol. 1984;56:798–803. doi: 10.1152/jappl.1984.56.3.798. [DOI] [PubMed] [Google Scholar]
- Holloway GA Jr, Watkins DW. Laser Doppler measurement of cutaneous blood flow. J Invest Dermatol. 1977;69:306–309. doi: 10.1111/1523-1747.ep12507665. [DOI] [PubMed] [Google Scholar]
- Tenland T, Salerud LG, Nilsson GE, Öberg PÅ. Spatial and temporal variations in human skin blood flow. Int J Microcirc Clin Exp. 1983;2:81–90. [PubMed] [Google Scholar]
- Arnold F, He CF, Jia CY, Cherry GW. Perfusion imaging of skin island flap blood flow by a scanning laser-Doppler technique. Br J Past Surg. 1995;48:280–287. doi: 10.1016/0007-1226(95)90065-9. [DOI] [PubMed] [Google Scholar]
- Speight EL, Farr PM. Erythemal and therapeutic response of psoriasis to PUVA using high-dose UVA. Br J Derm. 1994;131:667–672. doi: 10.1111/j.1365-2133.1994.tb04980.x. [DOI] [PubMed] [Google Scholar]
- Svedman C, Cherry GW, Ryan TJ. Postural changes in circulation of venous leg ulcer patients studied with laser Doppler imager. J Invest Dermatol. 1992;98:604. [Google Scholar]
- Bornmyr S, Arner M, Svensson H. Laser Doppler imaging of finger skin blood flow in patients after microvascular repair of the Ulnar artery at the wrist. J Hand Surg. 1994;19B:295–300. doi: 10.1016/0266-7681(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Line PD, Mowinckel P, Lien B, Kvernebo K. Repeated measurement variation and precision of laser Doppler flowmetry measurements. . Microvasc Res. 1992;43:285–293. doi: 10.1016/0026-2862(92)90026-l. [DOI] [PubMed] [Google Scholar]
- Krohg-Sørensen K, Line PD, Kvernebo K. The significance of probe design in evaluation of colonic perfusion with laser Doppler flowmetry. . Scand J Gastroenterol. 1993;28:381–386. doi: 10.3109/00365529309098236. [DOI] [PubMed] [Google Scholar]
- Essex TJH, Byrne PO. A laser Doppler scanner for imaging blood flow in skin. J Biomed Eng. 1991;13:189–194. doi: 10.1016/0141-5425(91)90125-q. [DOI] [PubMed] [Google Scholar]
- Wårdell K, Jakobsson A, Nilsson GE. Laser Doppler perfusion imaging by dynamic light scattering. IEEE Trans Biomed Eng. 1993;40:309–316. doi: 10.1109/10.222322. [DOI] [PubMed] [Google Scholar]
- Corbally MT, Brennan MF. Noninvasive measurement of regional blood flow in man. Am J Surg. 1990;160:313–321. doi: 10.1016/s0002-9610(06)80031-0. [DOI] [PubMed] [Google Scholar]
- Marks NJ, Trachy RE, Cummings CW. Dynamic variations in blood flow as measured by laser Doppler velocimetry: a study in rat skin flaps. . Plast Reconstr Surg. 1984;73:804–810. doi: 10.1097/00006534-198405000-00014. [DOI] [PubMed] [Google Scholar]
- Harrison DK, Abbott NC, Beck JC, McCollum PT. A preliminary assessment of laser Doppler perfusion imaging in human skin using the tuberculin reaction as a model. Physiol Meas. 1993;14:241–252. doi: 10.1088/0967-3334/14/3/002. [DOI] [PubMed] [Google Scholar]
- Stucker M, Heese A, Hoffmann K, Rochling A, Altmeyer P. Precision of laser Doppler scanning in clinical use. Clin Exper Derm. 1995;20:371–376. doi: 10.1111/j.1365-2230.1995.tb01352.x. [DOI] [PubMed] [Google Scholar]
- Quinn AG, McLelland J, Essex T, Farr PM. Measurement of cutaneous inflammatory reactions using a scanning laser-Doppler velocimeter. . Br J Dermatol. 1991;125:30–37. doi: 10.1111/j.1365-2133.1991.tb06035.x. [DOI] [PubMed] [Google Scholar]
- Seifalian AM, Stansby G, Jackson A, Howell K, Hamilton G. Comparison of laser Doppler perfusion imaging, laser Doppler flowmetry, and thermographic imaging for assessment of blood flow in human skin. Eur J Vasc Surg. 1994;8:65–69. doi: 10.1016/s0950-821x(05)80123-9. [DOI] [PubMed] [Google Scholar]
- Seifalian AM, Davidson BR, Rolles K. Laser Doppler imaging for the assessment of liver perfusion during transplantation. Eur J Gastroenterol Hepatol. 1993;5:479–482. [Google Scholar]
- Hajivassiliou CA, Greer K, Fisher A, Finlay IG. Non-invasive measurement of colonic blood flow distribution using laser Doppler imaging. . Br J Surg. 1998;85:52–55. doi: 10.1046/j.1365-2168.1998.00555.x. [DOI] [PubMed] [Google Scholar]
- Niazi ZBM, Essex TJH, Papini R, Scott D, McLean NR, Black MJM. New laser Doppler scanner, a valuable adjunct in burn depth assessment. Burns. 1993;19:485–489. doi: 10.1016/0305-4179(93)90004-r. [DOI] [PubMed] [Google Scholar]
- Quinn AG, McLelland J, Essex T, Farr PM. Measurement of cutaneous inflammatory reactions using a scanning laser-Doppler velocimeter. . Br J Derm. 1991;125:30–37. doi: 10.1111/j.1365-2133.1991.tb06035.x. [DOI] [PubMed] [Google Scholar]
- Seifalian AM, Chaloupka K, Parbhoo SP. Laser Doppler perfusion imaging: a new technique for measuring breast skin blood flow. Int J Microcirc Clin Exp. 1995;15:125–130. doi: 10.1159/000178963. [DOI] [PubMed] [Google Scholar]
- Gschwandtner ME, Koppensteiner R, Maca T, Minar E, Schneider B, Schnurer G, Ehringer H. Spontaneous laser Doppler flux distribution in ischaemic ulcers and the effect of prostanoids: a crossover study comparing the acute action of prostaglandin E1 and iloprost vs saline. . Microvasc Res. 1996;51:29–38. doi: 10.1006/mvre.1996.0004. [DOI] [PubMed] [Google Scholar]


