Abstract
A resistant and capable fungal strain in removing hexavalent chromium was isolated from an environment near of Chemical Science Faculty, located in the city of San Luis Potosí, Mexico. The strain was identified as Paecilomyces sp., by macro- and microscopic characteristics. Strain resistance of the strain to high Cr (VI) concentrations and its ability to reduce chromium were studied. When it was incubated in minimal medium with glucose, another inexpensive commercial carbon source like unrefined and brown sugar or glycerol, in the presence of 50 mg/L of Cr (VI), the strain caused complete disappearance of Cr (VI), with the concomitant production of Cr (III) in the growth medium after 7 days of incubation, at 28°C, pH 4.0, 100 rpm, and an inoculum of 38 mg of dry weight. Decrease of Cr (VI) levels from industrial wastes was also induced by Paecilomyces biomass. These results indicate that reducing capacity of chromate resistant filamentous fungus Cr (VI) could be useful for the removal of Cr (VI) pollution.
1. Introduction
Chromium (Cr) toxicity is one of the major causes of environmental pollution emanating from tannery effluents. This metal is used in the tanning of hides and leather, the manufacture of stainless steel, electroplating, textile dyeing, and as a biocide in the cooling waters of nuclear power plants, resulting chromium discharges causing environmental concerns [1]. Cr exists in nine valence states ranging from −2 to +6. Of these states, only hexavalent [Cr (VI)] and trivalent chromium [Cr (III)] have primary environmental significance because they are the most stable oxidation forms in the environment [2]. Both are found in various bodies of water and wastewaters [3]. Cr (VI) typically exists in one of these two forms: chromate (CrO4 −2) or dichromate (Cr2O7 −2), depending on the pH of the solution [3]. These two divalent oxyanions are very water soluble and poorly adsorbed by soil and organic matter, making them mobile in soil and groundwater [2]. Both chromate anions represent acute and chronic risks to animals and human health, since they are extremely toxic, mutagenic, carcinogenic, and teratogenic [4]. In contrast to Cr (VI) forms, the Cr (III) species: predominantly hydroxides, oxides, or sulphates, are less water soluble, mobile (100 times less toxic) [5], and (1000 times less) mutagenic [6]. The principal techniques for recovering or removing Cr (VI), from wastewater are chemical reduction and precipitation, adsorption on activated carbon, ion Exchange, and reverse osmosis, in a basic medium [7]. These methods present high cost, low efficieny and the toxic sludge generation, which could require to imply disposal complex operations [8].
The ability of some microorganisms to interact with different Cr forms makes them attractive in the context of environmental biotechnology. In this sense, the use of microbial biomass for the removal of Cr from industrial wastewater and polluted water has already been recognized [9]. The properties of some microorganisms to both tolerate and reduce Cr (VI) enable their application in biotechnological process focusing on detoxification of Cr (VI). Cr resistance has been described in bacteria and fungi isolated from Cr-polluted environments. Yeast strains isolated include Candida and Rhodosporidium genera, but in these, the general mechanism of chromate resistance is related to limited ion uptake, rather than to chemical reduction of the toxic species [10]. However, other yeasts such as Candida utilis [11] and Candida maltosa [12] showed partial ability to reduce Cr (VI) and also the capability to accumulate Cr in the biomass. Recent reports have also examined Cr (III) and Cr (VI) uptake and accumulation by different filamentous fungi [13, 14]. The present study reports the isolation and identification of a Paecilomyces sp. fungal strain that exhibits high level Cr (VI) resistance and reduction potential.
2. Experimental
2.1. Microorganism and Chromate Resistance Test
A chromate-resistant filamentous fungus was isolated from polluted air with industrial vapors, near of Chemical Science Faculty, located in the city of San Luis Potosí, Mexico, in Petri dishes containing modified Lee's minimal medium [LMM, 15] [with 0.25% KH2PO4, 0.20% MgSO4, 0.50% (NH4)2SO4, 0.50% NaCl, 0.25% glucose] supplemented with 500 mg/L K2CrO4; the pH of the medium was adjusted and maintained at 5.3 with 100 mMol/L citrate-phosphate buffer. The cultures were incubated at 28°C for 7 days. The strain was identified based on their morphological structures such as color, diameter of the mycelia, and microscopic observation of formation of spores [15]. Chromate-resistant tests of the isolated strain, filamentous fungus Paecilomyces sp., were performed on liquid LMM containing the appropriate nutritional requirements and different concentrations of Cr (VI) (as potassium chromate), and determining the dry weight.
2.2. Culture Conditions in Liquid Media
Cultures in 100 mL of sterile LMM [amended with 50 mg/L Cr (VI)] inoculated with 5 × 105 spores/mL were incubated at 28°C for 48 hours. Then, cells were aseptically separated by centrifugation at 2000 rpm (4°C) for 10 minutes, and washed twice with sterile trideionized water to eliminate culture medium components and cell debris. The cell pellet was resuspended in 3 mL of sterile trideionized water by shaking in a vortex mixer for 30 seconds, and was then transferred to 100 mL of fresh LMM amended with 50 mg/L Cr (VI). At various times during the course of incubation, 1 mL aliquots were removed and centrifuged at 5000 rpm for 10 minutes to sediment the cells; the supernatant fluid was used to determine the concentration of Cr (VI) or total Cr.
2.3. Determination of Hexavalent, Trivalent, and Total Cr
Hexavalent Cr and trivalent chromium were quantified by a spectrophotometric method employing diphenylcarbazide and chromazurol S, respectively [16, 17], total Cr was determined by electrothermal atomic absorption spectroscopy [16].
The values shown in Section 3 are the mean from three experiments carried out by triplicate.
3. Results and Discussion
3.1. Isolation and Identification of a Fungal Strain Capable of Removing Cr (VI)
The fungal strain isolated was able to grow on LMM supplemented with 2000 mg/L of Cr (VI) (Figure 1). This indicates that this fungus developed the Cr (VI) resistance and probably the Cr (VI) is being reduced in the polluted air. A variety of microorganisms with the Cr (VI) resistance and Cr (VI) reducing ability have been isolated from effluents of tanneries [3, 7, 14]. Colonies of the isolated fungal strain grew rapidly and mature within 3 days. Paecilomyces sp. are thermopile and can grow well at temperatures as high as 50° and 60°C. The colonies are flat, powdery, or velvety in texture. The initial color is white, and becomes yellow, yellow-green, pink, or violet. The reverse is dirty white or buff. A sweet aromatic color may be associated with older cultures. Septate hyaline hyphae, conidiophores, phialides, conidia, and chlamidospores are observed. Conidiophores (3-4 μm wide and 400–600 μm long) are often branched and carry the phialides at their tips. The phialides are swollen at their bases and taper towards their apices. They are usually grouped in pair or brush-like clusters. Conidia are unicellular, hyaline to darkly colored, smooth or rough, oval to fusoid, and form long chains. Chlamidospores are occasionally present [15].
Figure 1.
Growth in dry weight of Paecilomyces sp. with different concentrations of Cr (VI). 1 × 105 spores/mL, 28°C, 7 days of incubation, 100 rpm.
3.2. Effect of pH
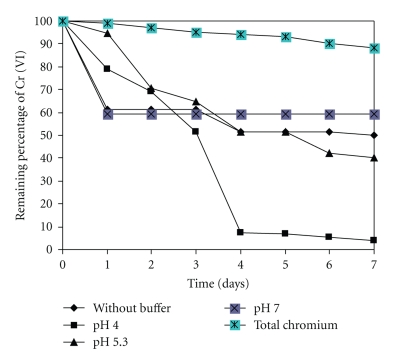
Figure 2 shows the effect of varying pH (4.0, 5.3, and 7.0, maintained with 100 mmol/L citrate-phosphate buffer.) on the rate of Cr (VI) removal. The rate of chromium uptake and the extent of that capture were enhanced as the pH falls from 7.0 to 4.0. The maximum uptake was observed at pH 4.0 (96% at 7 days), 96%, Liu et al. and Bai and Abraham [18, 19] reported maximum removal at 100 mg/L Cr (VI) solution using Mucor racemosus and Rhizopus nigricans with pH optimum of 0.5–1.0 and 2.0, respectively, Sandana Mala et al. [20], at pH 5.0 for Cr (VI) with Aspergillus niger MTCC 2594, Rodríguez et al. [21], at pH 3.0–5.0 for Pb+2, Cd+2 and Cr+3 with the yeast Saccharomyces cerevisiae, Park et al. [22], at pH 1–5 for Cr (VI) with brown seaweed Ecklonia, Higuera cobos et al. [23], at pH 5.0 for Cr (VI) with the brown algae Sargassum sp, and Fukuda et al. [14], at pH 3.0 for Cr (VI) with Penicillium sp. In contrast to our observations, Prasenjit and Sumathi [24] reported maximum uptake of Cr (VI) at pH 7.0 with Aspergillus foetidus, Puranik and Paknikar [25] reported an enhanced uptake of lead, cadmium, and zinc, with a shift in pH from 2.0 to 7.0 using a Citrobacter strain, and a decrease at higher pH values. Al-Asheh and Duvnjak [26] also demonstrated a positive effect of increasing pH in the range 4.0–7.0 on Cr (III) uptake using Aspergillus carbonarius. At low pH, the negligible removal of chromium may be due to the competition between hydrogen (H+) and metal ions [27]. At higher pH (7.0), the increased metal removal may be due to the ionization of functional groups and the increase in the negative charge density on the cell surface. At alkaline pH values (8.0 or higher), a reduction in the solubility of metals may contribute to lower uptake rates.
Figure 2.
The effect of pH on Chromium remotion by Paecilomyces sp. 50 mg/L Cr (VI), 100 rpm, 28°C.
3.3. Effect of Cell Concentration
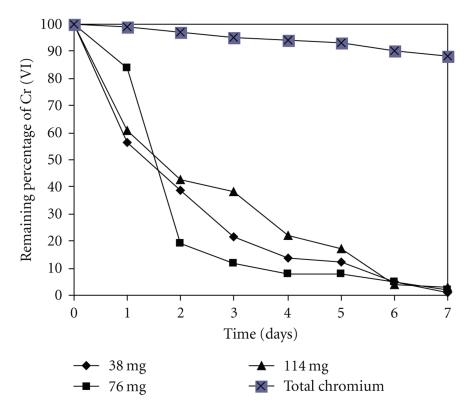
The influence biomass in the removal capacity of Cr (VI) was depicted in Figure 3. From the analyzed 38, 76, and 114 mg of dry weight the removal capacity was in the order of 99.17%, 97.95%, and 97.25%, respectively. In contrast to our observations, most of the reports in the literature observe at higher biomass dose resulting in an increase in the percentage removal [1, 3, 7, 8, 18, 21]. The higher the biomass dose, the more binding sites for complex of Cr (VI) (e.g., HCrO4 − and Cr2O7 −2 ions) [3, 9]. However, it did not show in our observations.
Figure 3.
The effect of cell concentration on the removal of Cr (VI). 50 mg/L Cr (VI). 100 rpm, 28°C, pH 1.0.
3.4. Effect of Initial Cr (VI) Concentration
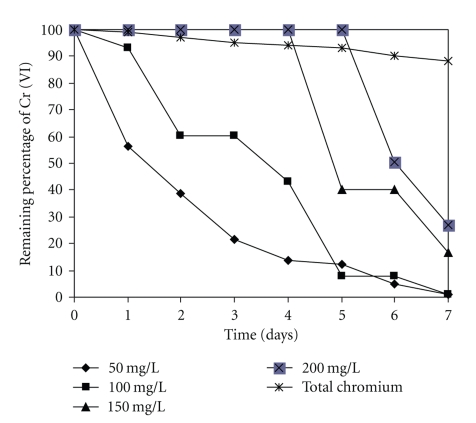
As seen in Figure 4, when the initial Cr (VI) ions concentration increased from 50 mg/L to 200 mg/L, the percentage removal of metal ions decreased. This was due to the increase in the number of ions competing for the available functions groups on the surface of biomass. Our observations are like most of the reports in the literature [1, 3, 5, 7, 8, 18, 21–24].
Figure 4.
The effect of the concentration of Cr (VI) in solution on the removal. 100 rpm, 28°C, pH 4.0.
3.5. Effect of Carbon Source
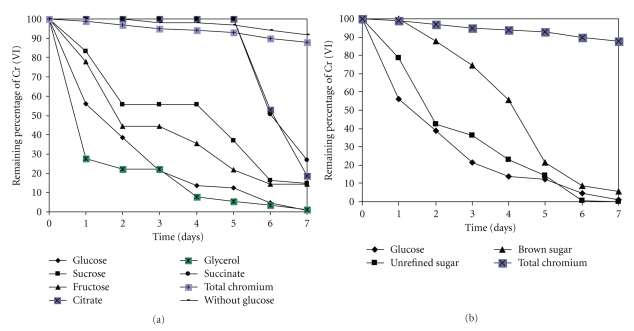
Figures 5(a) and 5(b) show that the decrease of Cr (VI) level in culture medium of Paecilomyces sp. occurred exclusively in the presence of a carbon source, either fermentable (glucose sucrose, fructose, citrate) or oxidable (glycerol). In the presence of glucose, another inexpensive commercial carbon sources like unrefined sugar and brown sugar or glycerol, the decrease in Cr (VI) levels occurred at a similar rate, at 7 days of incubation, are of 99.17%, 100%, 94.28%, 81.5, and 99%, respectively, and the other carbon sugar were less effectives. On the other hand, incubation of the biomass in the absence of a carbon source did not produce any noticeable change in the initial Cr (VI) concentration in the growth medium. These observations indicated that in culture of the fungus a carbon source is required to provide the reducing power needed to decrease Cr (VI) in the growth medium. Our observations are like to the report of Acevedo Aguilar et al., Prasenjit and Sumathi [13, 24] with glucose like carbon source, and are different from the observations of Srivasta and Thakur [28] with Aspergillus sp, and Acinetobater sp, who observed how the main carbon source is the sodium acetate.
Figure 5.
Influence of carbon source on the capability of Paecilomyces sp. to decrease Cr (VI) levels in the growth medium. 100 rpm, 28°C, pH 4.0. Influence of commercial carbon sources and salt on the capability of Paecilomyces sp. to decrease Cr (VI) levels in the growth medium. 100 rpm, 28°C, pH 4.0.
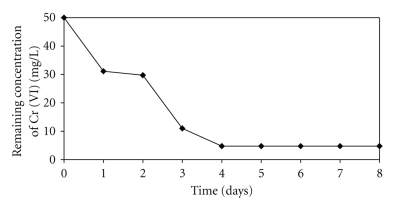
3.6. Time Course of Cr (VI) Decrease and Cr (III) Production
The ability of the isolated strain to lower the initial Cr (VI) of 50 mg/L and Cr (III) production in culture medium was analyzed. Figure 6 shows that Paecilomyces sp. exhibited a remarkable efficiency to diminish Cr (VI) level with the concomitant production of Cr (III) in the growth medium. Thus, after 7 days of incubation, the fungus strain caused a drop in Cr (VI) from its initial concentration of 50 mg/L to almost undetectable levels, and the decreased level occurred without significant change in total Cr content. As expected, total Cr concentration remained constant over time, in medium without inoculum. These observations indicate that Paecilomyces sp. strain is able to reduce Cr (VI) to Cr (III) in growth medium amended with chromate. There are two mechanisms by which chromate could be reduced to a lower toxic oxidation state by an enzymatic reaction. Currently, we do not know whether the fungal strain used in this study expresses Cr (VI) reducing enzyme(s). Further studies are necessary to extend our understanding of the effects of coexisting ions on the Cr (VI) reducing activity of the strain reported in this study. Cr (VI) reducing capability has been described in some reports in the literature [2, 8, 11–14, 19, 20, 22, 24, 27]. Biosorption is the second mechanism by which the chromate concentration could be reduced, and 1 g of fungal biomass of Paecilomyces sp. is able to remove 1000 mg/L of Cr (VI) at 60°C, at 3 hours of incubation (date not shown), because the fungal cell wall can be regarded as a mosaic of different groups that could form coordination complexes with metals, and our observations are like most of the reports in the literature [1, 3, 5, 7–9, 18, 21–24].
Figure 6.
Time-course of Cr (VI) decrease and Cr (III) production in the spent medium of culture initiated in Lee's minimal medium, amended with 50 mg/L Cr (VI). 100 rpm, 28°C, pH 4.0.
3.7. Removal of Cr (VI) in Industrial Wastes with Fungal Biomass
We adapted a water-phase bioremediation assay to explore possible usefulness of strain of Paecilomyces sp., for eliminating Cr (VI) from industrial wastes, the mycelium biomass was incubated with nonsterilized contaminated soil containing 50 mg Cr (VI)/g, suspended in LMM, pH 4.0. It was observed that after eight days of incubation with the Paecilomyces sp. biomass, the Cr (VI) concentration of soil sample decreased fully (Figure 7), and the decreased level occurred without significant change in total Cr content, during the experiments. In the experiment carried out in the absence of the fungal strain, the Cr (VI) concentration of the soil samples decreased by about of 18% (date not shown); this might be caused by indigenous microflora and (or) reducing components present in the soil. The chromium removal abilities of Paecilomyces sp. are equal or better than those of other reported strains, for example, Candida maltose RR1 [12]. In particular, this strain was superior to the other strains because it has the capacity for efficient chromium reduction under acidic conditions. Most other Cr (VI) reduction studies were carried out at neutral pH [14, 15]. Aspergillus niger also has the ability to reduce and adsorb Cr (VI) [14]. When the initial concentration of Cr (VI) was 500 ppm, A. niger mycelium removed 8.9 mg of chromium/g dry weight of mycelium in 7 days. In the present study, Paecilomyces sp., remove 50 mg/g, (pH, 4.0 and 8 days).
Figure 7.
Removal of Chromium (VI) in industrial wastes incubated with the fungal biomass. 100 rpm, 28°C, pH 4.0, 50 g of contaminated soil (50 mg Cr (VI)/g soil).
4. Conclusion
The Paecilomyces sp. fungal strain was isolated from polluted air with industrial vapors. This strain showed the capacity at complete concentrations reduction of 50 mg/L Cr (VI) in the growth medium after 7 days of incubation, at 28°C, pH, 4.0, 100 rpm, and an inoculum of 38 mg of dry weight. These results suggest the potential applicability of Paecilomyces sp. for the remediation of Cr (VI) from polluted soils in the fields.
References
- 1.Bai RS, Abraham TE. Biosorption of Cr (VI) from aqueous solution by Rhizopus nigricans . Bioresource Technology. 2001;79(1):73–81. doi: 10.1016/s0960-8524(00)00107-3. [DOI] [PubMed] [Google Scholar]
- 2.Smith WA, Apel WA, Petersen JN, Peyton BM. Effect of carbon and energy source on bacterial chromate reduction. Bioremediation Journal. 2002;6:205–215. [Google Scholar]
- 3.Shen H, Wang YT. Biological reduction of chromium by E. coli . Journal of Environmental Engineering. 1994;120(3):560–572. [Google Scholar]
- 4.Marsh TL, McInerney MJ. Relationship of hydrogen bioavailability to chromate reduction in aquifer sediments. Applied and Environmental Microbiology. 2001;67(4):1517–1521. doi: 10.1128/AEM.67.4.1517-1521.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beszedits S. Chromium removal from industrial wastewaters. In: Nriagu JO, Nieboer E, editors. Chromium in the Natural and Human Environments. New York, NY, USA: John Wiley & Sons; 1988. pp. 232–263. [Google Scholar]
- 6.Lofroth G, Ames BN. Mutagenicity of inorganic compounds in Salmonella typhimurium: arsenic, chromium and selenium. Mutation Research. 1978;53(1):65–66. [Google Scholar]
- 7.Park D, Yun YS, Cho HY, Park JM. Chromium biosorption by thermally treated biomass of the brown seaweed, Ecklonia sp. Industrial and Engineering Chemistry Research. 2004;43(26):8226–8232. [Google Scholar]
- 8.Sahin Y, Öztürk A. Biosorption of chromium(VI) ions from aqueous solution by the bacterium Bacillus thuringiensis . Process Biochemistry. 2005;40(5):1895–1901. [Google Scholar]
- 9.Cervantes C, Campos-García J, Devars S, et al. Interactions of chromium with microorganisms and plants. FEMS Microbiology Reviews. 2001;25(3):335–347. doi: 10.1111/j.1574-6976.2001.tb00581.x. [DOI] [PubMed] [Google Scholar]
- 10.Pas M, Milacic R, Draslar K, Pollak N, Raspor P. Uptake of chromium(III) and chromium(VI) compounds in the yeast cell structure. BioMetals. 2004;17(1):25–33. doi: 10.1023/a:1024437802914. [DOI] [PubMed] [Google Scholar]
- 11.Muter O, Patmalnieks A, Rapoport A. Interrelations of the yeast Candida utilis and Cr(VI): metal reduction and its distribution in the cell and medium. Process Biochemistry. 2001;36(10):963–970. [Google Scholar]
- 12.Ramírez-Ramírez R, Calvo-Méndez C, Avila-Rodríguez M, et al. Cr(VI) reduction in a chromate-resistant strain of Candida maltosa isolated from the leather industry. Antonie van Leeuwenhoek. 2004;85(1):63–68. doi: 10.1023/B:ANTO.0000020151.22858.7f. [DOI] [PubMed] [Google Scholar]
- 13.Acevedo Aguilar FJ, Wrobel K, Lokits K, et al. Analytical speciation of chromium in in-vitro cultures of chromate-resistant filamentous fungi. Analytical and Bioanalytical Chemistry. 2008;392(1-2):269–276. doi: 10.1007/s00216-008-2270-y. [DOI] [PubMed] [Google Scholar]
- 14.Fukuda T, Ishino Y, Ogawa A, Tsutsumi K, Morita H. Cr(VI) reduction from contaminated soils by Aspergillus sp. N2 and Penicillium sp. N3 isolated from chromium deposits. Journal of General and Applied Microbiology. 2008;54(5):295–303. doi: 10.2323/jgam.54.295. [DOI] [PubMed] [Google Scholar]
- 15.Kirk MP, Cannon FP, David CJ, Stalpers AJ. Dictionary of the Fungi. Oxford, UK: CABI; 2001. [Google Scholar]
- 16.Greenberg AE, Clesceri LS, Eaton AD. Standard Methods for the Examination of Water and Wastewater. 18th edition. Washington, DC, USA: American Public Health Association; 1992. [Google Scholar]
- 17.Pantaler RP, Pulyaeva IV. A spectrophotometric study of complexation between chromium and chromazurol S. Journal of Analytical Chemistry. 1985;40:1634–1639. [Google Scholar]
- 18.Liu T, Li H, Li Z, Xiao X, Chen L, Deng L. Removal of hexavalent chromium by fungal biomass of Mucor racemosus: influencing factors and removal mechanism. World Journal of Microbiology and Biotechnology. 2007;23(12):1685–1693. doi: 10.1007/s11274-007-9416-5. [DOI] [PubMed] [Google Scholar]
- 19.Bai RS, Abraham TE. Biosorption of Cr (VI) from aqueous solution by Rhizopus nigricans . Bioresource Technology. 2001;79(1):73–81. doi: 10.1016/s0960-8524(00)00107-3. [DOI] [PubMed] [Google Scholar]
- 20.Sandana Mala JG, Nair BU, Puvanakrishnan R. Bioaccumulation and biosorption of chromium by Aspergillus niger MTCC 2594. Journal of General and Applied Microbiology. 2006;52(3):179–186. doi: 10.2323/jgam.52.179. [DOI] [PubMed] [Google Scholar]
- 21.Rodríguez ME, Miranda RC, Olivas R, Sosa CA. Efectos de las condiciones de operación sobre la biosorción de Pb++, Cd++ and Cr+++, en solución por Saccharomyces cerevisiae residual. Informacion Tecnologica. 2008;19(6):47–55. [Google Scholar]
- 22.Park D, Yun YS, Park JM. Reduction of Hexavalent chromium with the brown seaweed Ecklonia biomass. Environmental Science and Technology. 2004;38(18):4860–4864. doi: 10.1021/es035329+. [DOI] [PubMed] [Google Scholar]
- 23.Higuera Cobos OF, Escalante Hernández H, Laverde D. Reducción del cromo contenido en efluentes líquidos de la industria del cuero, mediante un proceso adsorción-desorción con algas marinas. Scientia et Técnica. 2005;12(29):115–120. [Google Scholar]
- 24.Prasenjit B, Sumathi S. Uptake of chromium by Aspergillus foetidus . Journal of Material Cycles and Waste Management. 2005;7(2):88–92. [Google Scholar]
- 25.Puranik PR, Paknikar KM. Biosorption of lead, cadmium, and zinc by Citrobacter strain MCM B-181: characterization studies. Biotechnology Progress. 1999;15(2):228–237. doi: 10.1021/bp990002r. [DOI] [PubMed] [Google Scholar]
- 26.Al-Asheh S, Duvnjak Z. Adsorption of copper and chromium by Aspergillus carbonarius . Biotechnology Progress. 1995;11(6):638–642. doi: 10.1021/bp00036a006. [DOI] [PubMed] [Google Scholar]
- 27.Gadd GM. Accumulation of metals by microorganisms and algae. In: Rhem HJ, Reed G, editors. Biotechnology: A Comprehensive Treatise. 6B. Weinheim, Germany: VCH; 1981. pp. 401–433. [Google Scholar]
- 28.Srivastava S, Thakur IS. Evaluation of biosorption potency of Acinetobacter sp. for removal of hexavalent chromium from tannery effluent. Biodegradation. 2007;18(5):637–646. doi: 10.1007/s10532-006-9096-0. [DOI] [PubMed] [Google Scholar]