Abstract
O-Specific polysaccharides of Vibrio cholerae O1, serotype Inaba and Ogawa, consist of α-(1→2)-linked N-(3-deoxy-L-glycero-tetronyl)perosamine (4-amino-4,6-dideoxy-D-mannose). The blockwise synthesis of larger fragments of such O-PSs involves oligosaccharide glycosyl donors that contain a nonparticipating 2-O-glycosyl group at the position vicinal to the anomeric center where the new glycosidic linkage is formed. Such glycosyl donors may bear at C-4 either a latent acylamino (e.g. azido) or the 3-deoxy-L-glycero-tetronamido group. While monosaccharide glycosyl donors, even those bearing a nonparticipating group at O-2 (e.g. methyl) and the 4-N-(3-deoxy-L-glycero-tetronyl) side chain form α-linked oligosaccharides with excellent stereoselectivity, α-mannosylation with analogous oligosaccharide donors in this series is adversely affected by the presence of the side chain. Consequently, the unwanted β-product is formed in a considerable amount. Conducting the reaction at elevated temperature under thermodynamic control substantially enhances formation of the α-linked oligosaccharide. This effect is much more pronounced when glycosyl trichloroacetimidates, rather than thioglycosides or glycosyl chlorides, are used as glycosyl donors.
Keywords: O-Specific polysaccharide, cholera, Vibrio cholerae O1, oligosaccharide synthesis, glycosylation, β-mannosylation
1. Introduction
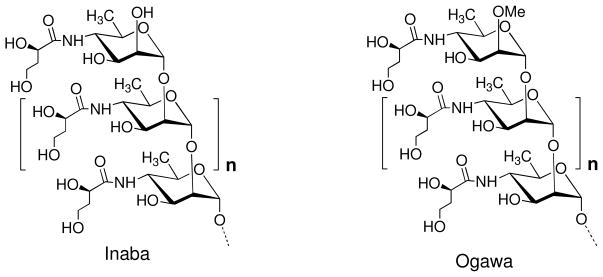
Cholera in humans is caused by three strains of Vibrio cholerae – O1 Inaba, O1 Ogawa, and O139.1 Progress in the development and practical relevance of a conjugate vaccine for cholera from a synthetic antigen depends, among other things, on the availability of oligosaccharides that mimic the O-specific polysaccharides (O-PSs) of the bacterial pathogens involved. The O-PSs of O1 Inaba and O1 Ogawa are very similar and consist of a (1→2)-α-linked perosamine (4-amino-4,6-dideoxy-D-mannose) whose amino group is acylated with 3-deoxy-L-glycero-tetronic acid. They differ2,3 in that the Ogawa O-PS has a methyl group at O-2 of the upstream,4 terminal perosamine residue (Fig. 1). We have been involved in the synthesis of oligosaccharides that mimic the O-PS of Vibrio cholerae O1 and O139 for more than a decade.5 The chemical syntheses are challenging and, despite several attempts to improve early approaches,6–9 there is still a need to optimize the synthetic strategy. We have shown10 that immunization of mice with a conjugate made from the synthetic hexasaccharide that mimics the upstream terminus of the Ogawa O-PS conferred protection. Therefore, we have recently focused our efforts on improving the synthesis of that segment in the V. cholerae O1 series.
Fig 1.
Structure of the O-PSs of the two strains of Vibrio cholerae O1
Our recent blockwise synthesis11 of the Ogawa hexasaccharide from disaccharide glycosyl donors bearing the 4-(3-deoxy-L-glycero-tetronamido) group in place (fully assembled glycosyl donors, as opposed to donors bearing 4-azido groups) has definite advantages over previous approaches, despite less than optimum stereoselectivity in the formation of the α-(1→2)-interglycosidic linkages. There,11 we were able to improve the yield of the desired, α-linked product by reacting a disaccharide thioglycoside donor under thermodynamic control. Here we report on further improvement of the synthesis of the hexasaccharide sequence, which was accomplished by the use of relevant mono- and disaccharide trichloroacetimidates as glycosyl donors. Under thermodynamic control, the stereoselectivity of formation of the α-mannosyl linkage markedly increased, compared to the use of thioglycosides as donors.11
2. Results and Discussion
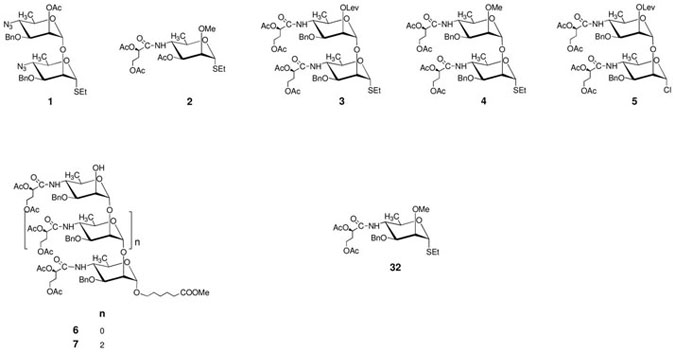
Generally, it is more efficient to synthesize higher oligosaccharides using a convergent (block-wise) strategy than by a linear (step-wise) approach. Also, in the assembly of large N-acyl-hexosamine-containing oligosaccharides, it is preferable to use intermediates where the N-acyl group is already installed (e.g. 3–5), than those containing latent acylamino groups (e.g. 1, where the azido group can be converted to an acylamino function at a later stage of the synthesis). Examples of such strategies can be found in the two different approaches to the tetrasaccharide side chain of the major glycoprotein of the Bacillus anthracis exosporium.12,13 In our initial attempt to synthesize oligosaccharides in the Vibrio cholerae O1 series,14 we explored the feasibility of using fully assembled intermediates (cf., our synthesis of the Inaba disaccharide).14 There, the formation of the α-mannopyranosyl linkage was highly stereoselective due to anchimeric assistance from a participating acyl group at O-2 in the monosaccharide glycosyl donor. That approach,14 however, could not be extended to making higher α-(1→2)-linked oligosaccharides because of the absence of a selectively removable protecting group in the product disaccharide. We have subsequently made a series of Inaba oligosaccharides by a stepwise approach from a fully assembled monosaccharide donor.15 There, again, the stereoselectivity of glycosylation was not an issue because of presence of the participating 2-C-acetyloxy group in the donor.
Seminal work by Peters and Bundle, within their synthetic work toward oligosaccharides that mimic the Brucella A polysaccharide, showed that large α-linked, perosamine-containing oligosaccharides can by synthesized in very good yields from donors that lack a participating moiety at C-2.16,17 They used the C-4 azido group-containing (1→2)-linked disaccharide glycosyl donor 1 to synthesize α-mannopyranosyl linkages with high stereoselectivity (the pure α-products were obtained in >80% yields). Following their strategy, we have been able to prepare various oligosaccharides in the Vibrio cholerae O1 series, including a dodecasaccharide.7,18–20
Encouraged by the high stereoselectivity of formation of the α-mannopyranosyl linkage in the absence of anchimeric assistance,7,16,17 and by the precedence21 for highly stereoselective formation of α-mannosyl linkage from the fully assembled donor 2, we used11 donors 3–5 for glycosylation in the assembly of the Ogawa hexasaccharide 27. It turned out11 that, unlike with glycosyl donors 1 and 2, the presence of the side chain in the oligosaccharide donors 3–5 resulted in loss of the ability of these donors to form α-mannosyl linkage with high stereoselectivity. For example,11 the reaction of thioglycoside 4 with methyl 6-hydroxyhexanoate in DCM at −20°C gave mainly the unwanted β glycoside. Thus, paradoxically, although the synthesis of the β-mannosyl linkage is one of the most difficult glycosidic linkages to synthesize, we faced the uncommon task to minimize formation of that linkage. Conducting the glycosidation of methyl 6-hydroxyhexanoate at higher temperatures changed the situation considerably, as it resulted in increased relative amount of the desired α product formed, with the later slightly predominating when the reaction was conducted at the temperature of refluxing toluene.11 With oligosaccharide glycosyl acceptors 6 and 7 and donor 4, the α:β ratio of products could be increased from 2:1 (DCM as solvent, room temp) to 5:1 (refluxing toluene).11 A high yield of the α product is the prerequisite for efficient syntheses of Vibrio cholerae O1 antigens. Guided by the increase of α-stereoselectivity of glycosylation under thermodynamic control using thioglycosides as glycosyl donors, we deemed it important to examine how trichloroacetimidate 14, which is analogous to thioglycoside 3,11 would perform under similar conditions. To our knowledge, glycosylation under thermodynamic control with trichloroacetimidates has not been attempted, lest decomposition of the highly reactive donor might preclude glycosylation.
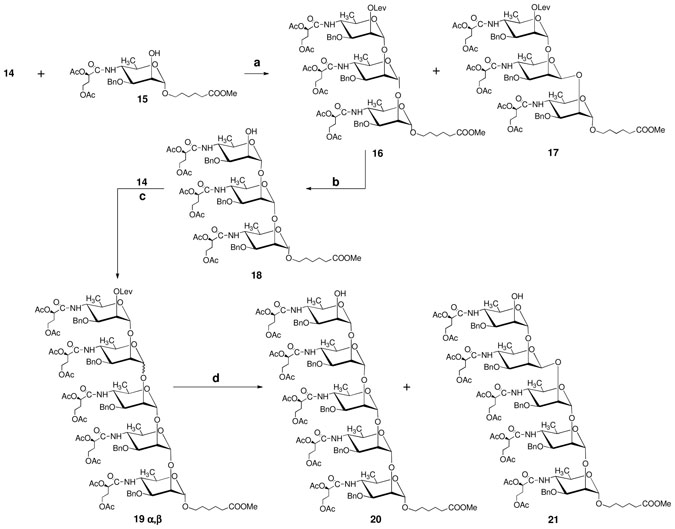
To compare the stereoselectivity of formation of an α-perosaminyl linkage from thioglycoside 311 with that using the corresponding trichloroacetimidate, we synthesized imidate 14 (Scheme 1) and treated it with alcohols 15 and 18 (Scheme 2). When the reaction of 14 and 15 was carried out in DCM at −35°C, a considerable amount of the β-linked trisaccharide 17 was formed (α:β~2.5:1, NMR, unpublished results). The proclivity of this transformation to thermodynamic control became evident when the same synthons were allowed to react either at room temperature or at 100°C, in toluene. Thus, while at room temperature the trisaccharides were formed in the α:β ratio of 10:1, the reaction in hot toluene led to compounds 16 and 17 in the ratio of 20:1. It should be noted that the glycosylation yields in these three reactions were high (86–95%). Reaction of 14 and alcohol 18 under thermodynamic control gave pentasaccharide 19 α,β with the same high stereoselectivity (α: β ratio, ~20:1, NMR). Separation of the two pentasaccharides was difficult and the mixture of anomers was resolved after removal of the levulinoyl group, to give 20 and 21.
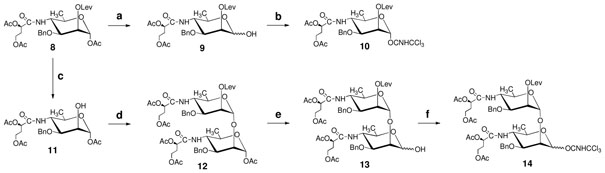
Scheme 1.
(a) Piperidine, THF, rt, 95%; (b) DBU, Cl3CCN, DCM, 0 °C, 71%; (c) hydrazine acetate, DCM–MeOH (10:1, v/v), rt, 86%; (d) 10, TMSOTf, 4 Å MS, toluene–DCM (3:1, v/v), rt, 76%; (e) piperidine, THF, 0 °C to rt, 75%; (f) DBU, Cl3CCN, DCM, 0 °C to rt, 84%.
Scheme 2.
(a) TMSOTf, 4 Å MS, toluene, 100 °C, 86%; (b) hydrazine acetate, DCM–MeOH (10:1, v/v), rt, 87%; (c) 14,TMSOTf, 4 Å MS, toluene, 100 °C, 75%; (d) hydrazine acetate, DCM–MeOH (10:1, v/v), rt, 91%.
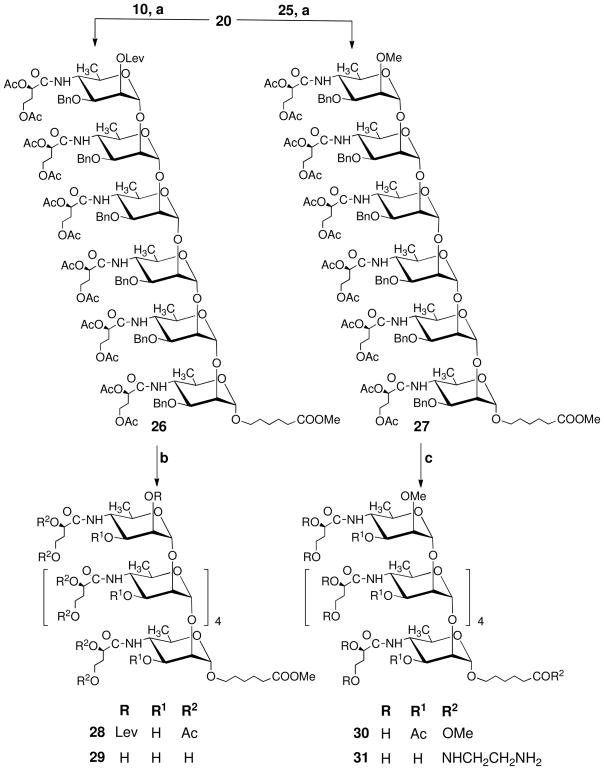
Synthesis of the hexasaccharide fragments of the O-PS of Vibrio cholerae O1, serotype Inaba and Ogawa required extension of pentasaccharide 20 at O-2 with glycosyl donor 10 and its 2-O-methyl analog 25, respectively.
To obtain imidate 25, the known8 glycoside 22 was subjected sequentially to acetolysis (→23), anomeric deacetylation, and conversion of the free sugar 24 thus formed to trichloroacetimidate 25. We have previously observed21 high stereoselectivity of formation of the α-perosaminyl linkage under conventional, not thermodynamically controlled conditions (room temperature, CH2Cl2 as solvent) using a different fully assembled monosaccharide glycosyl donor, namely thioglycoside 32. Therefore, alcohol 20 was treated with each of imidates 10 and 25 (Scheme 4) under the same conditions. Both reactions were very slow, and some unchanged glycosyl donor was still present in both reaction mixtures after 16 h of reaction time. The β-linked hexasaccharides could not be found among the reaction products (NMR spectra of isolated, minor by-products), indicating excellent stereoselectivity of formation of 26 and 27, which were isolated in 73 and 69% yield, respectively. Hydrogenolysis of the foregoing fully protected intermediates gave alcohols 28 and 30, respectively. Treatment of the former with NaOMe in MeOH effected simultaneous removal of protecting acetyl and levulinoyl groups, to give the fully deprotected hexasaccharide 29. Treatment of the Ogawa compound 30 with ethylenediamine effected simultaneous deacetylation of the side chains and amidation of the spacer, to give the final hexasaccharide 31, which is amenable for conjugation by squaric acid chemistry.22
Scheme 4.
(a) TMSOTf, 4 Å MS, DCM, rt, 73% for 27 and 69% for 28; (b) (i) 5% Pd/C, H2, DCM–MeOH (1:10, v/v), rt; (ii) NaOMe, MeOH, rt. 89% over two steps; (c)(i) 5% Pd/C, H2, DCM–MeOH (1:10, v/v), rt, 87%; (ii) H2NCH2CH2NH2, 50 °C, 75%.
3. Conclusions
Results of this study confirm our previous11 observation that the presence of the 3-deoxy-L-glycero-tetronamido side chain in glycosyl donors derived from perosamine significantly affect the stereoselectivity of glycosylation. When such glycosyl donors bear a nonparticipating group (O-alkyl or O-glycosyl) at the position vicinal to the active glycosidic center, the stereochemical outcome of perosaminylations – conducted by us7,11,15,21,23 and elsewhere16,17,24 – can be summarized as follows. Firstly, the formation of α-glycosidic linkage is highly favored when mono- and oligosaccharide glycosyl donors having azido group at O-4 are employed. Secondly, the α glycosidic linkage can also be stereoselectively formed from monosaccharide glycosyl donors where the 4-N-side chain is already in place (e.g. 2 or 32), regardless of the O-2 substituent. Thirdly, and in contrast to their monosaccharide counterparts, the stereoselectivity of glycosylation conducted at conventional reaction conditions is poor when the glycosyl donor, a thioglycoside, glycosyl chloride or trichloroacetimidate, is an oligosaccharide made from α-(1→2)-linked perosamine having the 4-(3-deoxy-L-glycero-tetronamido) side chain already in place. In such situations, the α stereoselectivity can be markedly improved by conducting the reaction under thermodynamic control. The benefit of the thermodynamic control is more pronounced when glycosylation is effected by the use of trichloroacetimidate, which are preferred for this purpose, to the corresponding thioglycosides or glycosyl chlorides.11
4. Experimental
4.1 General Methods
Unless stated otherwise, optical rotations were measured at ambient temperature with a Perkin–Elmer automatic polarimeter, Model 341. All reactions were monitored by thin-layer chromatography (TLC) on silica gel 60 coated glass slides. Column chromatography was performed by elution from columns of silica gel with the CombiFlash Companion Chromatograph (Isco, Inc.) or Isolera Flash Chromatograph (Biotage). Solvent mixtures less polar than those used for TLC were used at the onset of separation. Nuclear Magnetic Resonance (NMR) spectra were measured at 300 MHz (1H) and 75 MHz (13C) with a Varian Gemini or Varian Mercury spectrometer, or at 600 MHz (1H) and 150 MHz (13C) with a Bruker Avance 600 spectrometer. Assignments of NMR signals were made by homonuclear and heteronuclear 2-dimensional correlation spectroscopy, run with the software supplied with the spectrometers. Assignment of 13C NMR spectra of some higher oligosaccharides was aided by comparison with spectra of related substances reported previously from this laboratory. When the latter approach was used, to aid in the 13C NMR signal-nuclei assignments, advantage was taken of variations of line intensity expected for oligosaccharides belonging to the same homologous series.25,26 Thus, spectra showed close similarity of chemical shifts of equivalent carbon atoms of the internal residues, and an increase in the relative intensity of these signals with the increasing number of D-perosamine residues in the molecule. Nevertheless, often only incomplete assignment was achieved because of the overlap of resonances. When reporting assignment of NMR signals, nuclei associated with the 4-amido side chain are denoted with a prime and those with the spacer (linker) are denoted with a double prime. When reporting assignments of NMR signals, sugar residues in oligosaccharides are serially numbered, beginning with the one bearing the aglycon, and are identified by a Roman numeral superscript in listings of signal assignments. Signals for sugar ring nuclei lacking Roman numeral assignment may belong to any of the rings. Only resonances that could be confidently assigned are listed in signal assignments. When reporting preparation of known substances, their identity was confirmed by comparison of NMR characteristics with those published previously. Liquid Chromatography–Electron Spray-Ionization Mass Spectrometry (ESI-MS) was performed with a Hewlett–Packard 1100 MSD spectrometer. Attempts have been made to obtain correct combustion analysis data for all new compounds. However, some compounds tenaciously retained traces of solvents, despite exhaustive drying, and analytical figures for carbon could not be obtained within 0.4%. Structures of these compounds follow unequivocally from the mode of synthesis, NMR spectroscopic data and m/z values found in their mass spectra, and their purity was verified by TLC and NMR spectroscopy. 5% Palladium-on-charcoal catalyst (Escat| 103) was purchased from Engelhard Industries. 1-(3-Dimethylaminopropyl)-3-ethyl-carbodiimide (EDAC) was purchased from ACROS Organics. Rubber septa used to close reaction flasks containing organic solvents were protected with a thin Teflon| sheet, to avoid leaching. Solutions in organic solvents were dried with anhydrous Na2SO4, and concentrated at 40 °C/2 kPa.
4.2 1-O-Acetyl-3-O-benzyl-4-(2,4-di-O-acetyl-3-deoxy-L-glycero-tetronamido)-4,6-dideoxy-α-D-mannopyranose (11)
A solution of hydrazine acetate (4.10 g, 44.6 mmol) in MeOH (50 ml) was added to a stirred mixture of 811 (18.4 g, 31.9 mmol) in DCM (500 ml). The stirring was continued until TLC (3:1 toluene–acetone) showed that the starting material was completely consumed (~6 h). After concentration, chromatography (3:1 hexane–EtOAc) afforded 11 (13.2 g, 86%), [α]D +16 (c 0.5, CHCl3); 1H NMR (600 MHz, CDCl3) δ: 7.40–7.26 (m, 5 H, Ph), 6.14 (d, J 1,2 = 1.2 Hz, H-1), 6.03 (d, J = 8.3 Hz, 1 H, NH), 5.14 (dd, J = 8.0 Hz and 4.9 Hz, 1 H, H-2′), 4.67–4.51 (ABq, J = 12.0 Hz, 2 H, PhCH2), 4.18–3.99 (m, 6 H, 2-OH, H-3, H-4, H-5, H-4′), 3.85 (m, 1 H, H-2), 2.12–2.02 (m, 11 H, 3 COCH3, H-4′), 1.24 (d, J5,6 = 6.4 Hz, 3 H, H-6); 13C NMR (150 MHz, CDCl3) δ: 95.4 (C-1), 137.2 (Cq), 92.8 (C-1), 74.9 (C-3), 71.4 (PhCH2), 71.1 (C-2′), 68.9 (C-5), 66.3 (C-4), 59.9 (C-4′), 52.7 (C-2), 30.9 (C-3′), 21.0, 20.9, 20.8 (3 CH3CO), 17.9 (C-6); TOF–MS m/z: 504 [M+Na]+; Anal. Calad. for C23H31NO10: C, 57.37; H, 6.49. Found C, 57.11; H, 6.55.
4.3 1-O-Acetyl-3-O-benzyl-4-(2,4-di-O-acetyl-3-deoxy-L-glycero-tetronamido)-4,6-dideoxy-2-O-levulinoyl-α-D-mannopyranosyl-(1→2)-3-O-benzyl-4-(2,4-di-O-acetyl-3-deoxy-L-glycero-tetronamido)-4,6-dideoxy-α-D-mannopyranose (12)
Piperidine (54 ml, 549 mmol) was added with stirring to a solution of 827 (15.7 g, 27.1 mmol) in THF (250 ml) and the stirring was continued at room temperature until TLC (2:1 hexane–acetone) showed that the reaction was complete. The mixture was diluted with DCM (1000 ml) and washed successively with ice-cooled 0.5 M HCl (2 × 250 mL), satd NaHCO3 (200 ml) and brine (200 ml), the organic phase was dried and concentrated. Chromatography (2:1, hexane–acetone) gave 3-O-benzyl-4-(2,4-di-O-acetyl-3-deoxy-L-glycero-tetronamido)-4,6-dideoxy-2-O-levulinoyl-α,β-D-mannopyranose (9, 13.8 g, 95%) as a mixture of anomers (α:β ~6:1). 1H NMR (α product, 300 MHz, CDCl3) δ: 7.35–7.26 (m, 5 H, Ph), 5.82 (d, J = 7.9 Hz, 1 H, NH), 5.40 (t, J = 2.06 Hz, 1 H, H-2), 5.20–5.16 (m, 2 H, H-1 and H-2′), 4.66–4.33 (ABq, J = 12.0 Hz, 2 H, PhCH2), 4.16–3.90 (m, 5 H, H-3, H-4, H-5, H-4′), 2.76–2.64 (m, 4 H, CH2CH2), 2.20–2.00 (m, 11 H, 3 COCH3 and H-3′), 1.21 (d, J5,6 = 6.0 Hz, H-6); 13C NMR (75 MHz, CDCl3) δ: 172.2 (CO), 169.8 (CO), 169.6 (CO), 137.9 (Cq), 92.5 (C-1), 73.2 (C-3), 71.3 (C-2′), 70.8 (PhCH2), 67.8 (C-2 and C-5), 60.2 (C-4′), 53.0 (C-4), 38.2 (CH2CO), 31.1 (C-3′), 30.0 (OCOCH2), 28.3 (COCH3), 21.0 (2 CH3CO), 18.3 (C-6); TOF-HRMS m/z: [M+H]+ Calcd for C26H36NO11: 538.2288. Found 538.2278.
A solution of compound 9 (600 mg, 1.19 mmol) in DCM (10 ml) was treated at 0 °C with Cl3CCN (1.2 ml, 12 mmol) in the presence of DBU (0.09 ml, 0.6 mmol) for 2 h, and concentrated. Chromatography (3:2 hexane–EtOAc) gave 3-O-benzyl-4-(2,4-di-O-acetyl-3-deoxy-L-glycero-tetronamido)-4,6-dideoxy-2-O-levulinoyl-α-D-mannopyranose 1-O-trichloroacetimidate (10, 540 mg, 71%). 1H NMR (300 MHz, CDCl3) δ: 8.71 (s, 1 H, NH), 7.35–7.28 (m, 5 H, Ph), 6.23 (d, J 1,2 = 1.92 Hz, H-1), 5.80 (d, J = 7.4 Hz, 1 H, NH), 5.51 (t, J = 2.2 Hz, 1 H, H-2), 5.17 (dd, J = 7.9 Hz and 4.8 Hz, 1 H, H-2′), 4.66–4.37 (ABq, J = 12.0 Hz, 2 H, PhCH2), 4.15–3.93 (m, 5 H, H-3, H-4, H-5, H-4′), 2.79–2.71 (m, 4 H, CH2CH2), 2.18–2.05 (m, 11 H, 3 COCH3 and H-3′), 1.25 (d, J5,6 = 5.6 Hz, H-6); 13C NMR (75 MHz, CDCl3) δ: 95.4 (C-1), 72.5 (C-3), 71.3 (C-2′), 71.09, 70.6 (PhCH2), 66.25 (C-2 and C-5), 60.1 (C-4′), 52.7 (C-4), 38.1 (CH2CO), 31.1 (C-3′), 30.0 (OCOCH2), 28.2 (COCH3), 21.0 (2 CH3CO), 18.3 (C-6); TOF-HRMS m/z: [M+H]+ Calcd for C28H36N2O11Cl3: 681.1385. Found 681.1388.
A mixture of 10 (9.8 g, 14.4 mmol), 11 (6.29 g, 13.0 mmol) and 4 Å MS (2.1 g) in toluene-DCM (3:1, v/v 150 ml) was stirred under N2 at room temperature for 30 min. TMSOTf (16 μl, 0.65 mmol) was added, and the stirring was continued for 4 h when TLC (3:2 hexane–acetone) showed that the donor was completely consumed. After addition of Et3N (1.0 ml), the mixture was filtered through Celite pad, the filtrate was concentrated, and chromatography (3:1→3:2 hexane–acetone) gave 12 (10.0 g, 76%). [α]D −120 (c 0.92, CHCl3); 1H NMR (600 MHz, CDCl3) δ: 7.30–7.17 (m, 10 H, 2 Ph), 6.02 (d, J = 8.7 Hz, 1 H, NHI), 5.99 (d, J1,2 = 2.2 Hz, 1 H, H-1I), 5.68 (d, J = 9.0 Hz, 1 H, NHII), 5.41 (t, J = 2.5 Hz, H-2II), 5.09 (m, 2 H, H-2′I,II), 4.85 (d, J1,2 = 1.8 Hz, H-1II), 4.60–4.30 (m, 4 H, 2 PhCH2), 4.04–3.95 (m, 7 H, H-3I, H-5I, H-4II, H-4′I,II), 3.85 (t, J = 2.5 Hz, 1 H, H-2I), 3.71–3.67 (m, 3 H, H-4I, 3II, H-5II), 2.62 (m, 4 H, CH2CH2), 2.10–1.92 (m, 22 H, 6 COCH3, H-3′I,II), 1.15 (m, 6 H, H-6I,II); 13C NMR (150 MHz, CDCl3) δ: 137.6 (Cq), 137.5 (Cq), 99.4 (C-1II), 92.5 (C-1I), 74.2 (C-3I), 73.3 (C-3II), 72.6 (C-2I), 71.9 (PhCH2), 71.3 and 71.2 (C-2′I,II), 70.7 (PhCH2), 69.6 (C-5II), 69.0 (C-5I), 67.2 (C-2II), 60.1 and 60.0 (C-4′I,II), 53.4 (C-4I), 51.9 (C-4II), 38.0 (CH2CO), 31.0 and 30.9 (C-3′I,II), 29.8 (COCH3), 28.2 (OCOCH2), 21.0, 20.9, 20.8 (5 C, 5 COCH3), 18.1 and 17.9 (C-6I,II); TOF-HRMS m/z: [M+H]+ Calcd for C49H65N2O20: 1001.4131. Found 1001.4139. Anal. Calad. for C49H64N2O20: C, 58.79; H, 6.44. Found C, 58.60; H, 6.48.
4.4 5-(Methoxycarbonyl)pentyl 3-O-benzyl-4-(2,4-di-O-acetyl-3-deoxy-L-glycero-tetronamido)-4,6-dideoxy-2-O-levulinoyl-α-D-mannopyranosyl-(1→2)-3-O-benzyl-4-(2,4-di-O-acetyl-3-deoxy-L-glycero-tetronamido)-4,6-dideoxy-α-D-mannopyranosyl-(1→2)-3-O-benzyl-4-(2,4-di-O-acetyl-3-deoxy-L-glycero-tetronamido)-4,6-dideoxy-α-D-mannopyranoside (16)
Piperidine (3.6 ml, 36 mmol) was added with stirring at 0 °C to a solution of 12 (1.32 g, 1.3 mmol) in THF (20 ml) and the mixture was allowed to warm up to room temperature. After 24 h, the mixture was diluted with DCM (200 ml) and washed with 0.5 M HCl (50 ml) and saturated NaHCO3 aq (50 ml), dried, concentrated, and chromatography (3:1→95:5 EtOAc–Hexane) gave 3-O-benzyl-4-(2,4-di-O-acetyl-3-deoxy-L-glycero-tetronamido)-4,6-dideoxy-2-O-levulinoyl-α-D-mannopyranosyl-(1→2)-3-O-benzyl-4-(2,4-di-O-acetyl-3-deoxy-L-glycero-tetronamido)-4,6-dideoxy-α-D-mannopyranose (13, 940 mg, 75%). 1H NMR (600 MHz, CDCl3) δ: 7.36–7.27 (m, 10 H, 2 Ph), 5.91 (d, J = 8.3 Hz, 1 H, NHI), 5.82 (d, J = 9.2 Hz, 1 H, NHII), 5.49 (t, J = 2.5 Hz, H-2II), 5.20–5.15 (m, 3 H, H-1I, H-2′I,II), 4.87 (d, J1,2 = 1.9 Hz, H-1II), 4.65–4.38 (m, 4 H, 2 PhCH2), 4.20–3.90 (m, 9 H, H-2I, H-3I, H-4I, H-5I, H-4II, H-4′I,II), 3.81–3.74 (m, 2 H, 3II, H-5II), 3.10 (brs, 1 H, 1-OH), 2.76–2.60 (m, 4 H, CH2CH2), 2.10–1.92 (m, 19 H, 5 COCH3, H-3′I,II), 1.18 (d, J5,6 = 5.8 Hz, 3 H, H-6I), 1.15 (d, J5,6 = 6.3 Hz, 3 H, H-6II); 13C NMR (150 MHz, CDCl3) δ: 137.8 (Cq), 137.6 (Cq), 99.5 (C-1II), 93.4 (C-1I), 74.5 (C-2I, C-3I), 72.9 (C-3II), 71.3 (PhCH2), 71.2 and 71.0 (C-2′I,II), 70.4 (PhCH2), 68.5 (C-5II), 67.9 (C-5I), 67.1 (C-2II), 60.0 (C-4′I,II), 52.6 (C-4I), 51.9 (C-4II), 38.0 (CH2CO), 31.0 and 30.9 (C-3′I,II), 29.8 (COCH3), 28.2 (OCOCH2), 20.9 (2 COCH3), 20.8 (2 COCH3), 18.1 and 17.9 (C-6I,II); TOF-HRMS m/z: [M+H]+ Calcd for C47H63N2O19: 959.4052. Found 959.4014.
DBU (0.18 ml, 0.9 mmol) was added with stirring at 0 °C to a mixture of 13 (9.0 g, 9.4 mmol), CNCCl3 (4.7 ml, 47.0 mmol) and DCM (150 ml), the mixture was allowed to warm up to temperature and stirred overnight. After concentration and chromatography (3:1→3:2 hexane–acetone) gave 3-O-benzyl-4-(2,4-di-O-acetyl-3-deoxy-L-glycero-tetronamido)-4,6-dideoxy-2-O-levulinoyl-α-D-mannopyranosyl-(1→2)-3-O-benzyl-4-(2,4-di-O-acetyl-3-deoxy-L-glycero-tetronamido)-4,6-dideoxy-α-D-mannopyranose-1-O-trichloroacetimidate (14, 8.7 g, 84%). 1H NMR (600 MHz, CDCl3) δ: 8.62 (s, 1 H, OCNHCCl3), 7.37–7.27 (m, 10 H, 2 Ph), 6.15 (d, J1,2 = 2.0 Hz, H-1I), 5.89 (d, J = 7.8 Hz, 1 H, NHI), 5.74 (d, J = 9.0 Hz, 1 H, NHII), 5.51 (bt, J = ~2.4 Hz, H-2II), 5.23–5.17 (m, 2 H, H-2′I,II), 4.91 (d, J1,2 = 1.7 Hz, H-1II), 4.68–4.40 (m, 4 H, 2 PhCH2), 4.20–3.97 (m, 9 H, H-4′I,II, H-4II, H-2I, H-5I, H-3I, H-4I), 3.78–3.76 (m, 2 H, 3II, H-5II), 2.75–2.65 (m, 4 H, CH2CH2), 2.10–1.92 (m, 19 H, 5 COCH3, H-3′I,II), 1.24 (d, J5,6 = 6.3 Hz, 3 H, H-6I), 1.19 (d, J5,6 = 6.3 Hz, 3 H, H-6II); 13C NMR (150 MHz, CDCl3) δ: 137.7 (Cq), 137.4 (Cq), 99.8 (C-1II), 96.5 (C-1I), 74.2 (C-3I), 73.1 (C-3II), 72.6 (C-2I), 71.9 (PhCH2), 71.3 and 71.2 (C-2′I,II), 70.8 (C-5I), 70.7 (PhCH2), 69.1 (C-5II), 67.2 (C-2II), 60.1 (C-4′I,II), 52.7 (C-4II), 51.9 (C-4I), 38.2 (CH2CO), 31.2 and 31.0 (C-3′I,II), 30.0 (COCH3), 28.3 (OCOCH2), 21.1 (2 COCH3), 21.0 (2 COCH3), 18.3 and 18.0 (C-6I,II); TOF–MS m/z: 1127 [M+Na]+.
A solution of TMSOTf in toluene (0.03 M, 10 ml) was added at 100 °C under N2 to a stirred mixture of 14 (7.5 g, 6.79 mmol), 5-(methoxycarbonyl)pentyl 3-O-benzyl-4-(2,4-di-O-acetyl-3-deoxy-L-glycero-tetronamido)-4,6-dideoxy-α-D-mannopyranoside (15,11 3.5 g, 6.17 mmol), 4 Å molecular sieves (10 g) and toluene (300 ml). After 5 h, when TLC (3:2 hexane–acetone) showed that donor 14 was consumed, the mixture was cooled to room temperature and Et3N (0.8 ml) was added. The mixture was filtered through a Celite pad, the filtrate was concentrated, and chromatography (3:1→3:2 hexane–acetone) gave the unchanged glycosyl acceptor 15 (0.83 g) and a mixture of known8 α-linked compound 16 and 5-(methoxycarbonyl)pentyl 3-O-benzyl-4-(2,4-di-O-acetyl-3-deoxy-L-glycero-tetronamido)-4,6-dideoxy-2-O-levulinoyl-α-D-mannopyranosyl-(1→2)-3-O-benzyl-4-(2,4-di-O-acetyl-3-deoxy-L-glycero-tetronamido)-4,6-dideoxy-β-D-mannopyranosyl-(1→2)-3-O-benzyl-4-(2,4-di-O-acetyl-3-deoxy-L-glycero-tetronamido)-4,6-dideoxy-α-D-mannopyranoside (17) (combined yield, 7.8 g, glycosylation yield 84%, 16:17 ~20:1). The mixture was rechromatographed (12:1 toluene–2-propanol), to give 16 (6.22 g) and 17 (0.21 g). A small amount of unresolved mixture of 16 and 17 was also obtained.
17: 1H NMR (600 MHz, CDCl3) δ: 7.92 (d, J = 9.0 Hz, 1 H, NHIII), 7.36–7.08 (m, 15 H, 3 Ph), 6.43 (d, J = 10.0 Hz, 1 H, NHI), 6.25 (d, J = 7.4 Hz, 1 H, NHII), 5.47 (d, J1,2 = 1.3 Hz, 1 H, H-1III), 5.44 (dd, J1,2 = 1.3 Hz, J2,3 = 3.1 Hz, 1 H, H-2III), 5.26 (dd, J = 5.4 Hz, J = 7.6 Hz, 1 H, H-2′), 5.02 (m, 2 H, H-2′), 4.80 (d, J =10.3 Hz, 1 H, PhCH2), 4.75 (d, J1,2 = 1.6 Hz, 1 H, H-1I), 4.67 (d, J =11.3 Hz, 1 H, PhCH2), 4.56 (s, 1 H, H-1II), 4.36 (d, J2,3 =2.2 Hz, H-2II), 4.28 (d, J =11.3 Hz, 1 H, PhCH2), 4.24–4.21 (m, 2 H, H-2I, H-4III), 4.10–3.98 (m, 13 H, PhCH2, H-4I, H-3II, H-5II, H-5III, H-4′I,II,III), 3.77 (dd, J2,3 = 3.1 Hz, J3.4 = 10.9 Hz, H-3III), 3.64–3.61 (m, 6 H, OCH3, H-1″a, H-3I and H-5I), 3.37–3.34 (m, 2 H, H-4II, H-1″b), 2.56–2.51 (m, 4 H, CH2CH2), 2.29 (t, J = 7.5 Hz, 2 H, H-5″a,b), 2.02–1.97 (m, 27 H, 5 COCH3, H-3′I,II,III), 1.62–1.54 (m, 4 H, H-2″a,b, H-4″a,b), 1.28 (m, 2 H, H-3″a,b), 1.26 (d, J5,6 = 6.3 Hz, 3 H, H-6I), 1.19 (d, J5,6 = 6.3 Hz, 3 H, H-6II); 13C NMR (150 MHz, CDCl3) δ: 97.4 (2 C, C-1I, C-1II), 96.9 (C-1III), 77.3 (C-3III), 74.2 (C-3I), 71.7 (C-2′), 71.6 (PhCH2), 71.5 (PhCH2), 71.1 (C-2′), 70.8 (C-2′), 69.6 (C-2I, C-5III), 69.4 (PhCH2), 69.3 (C-5II), 68.8 (C-2III), 67.2 (C-5I), 76.1 (C-1″), 66.9 (C-2II), 60.9 (C-4′), 60.1 (C-4′), 59.9 (C-4′), 55.7 (C-4II), 51.8 (C-4I, C-4II), 38.4 (2 C, CH2CH2), 34.0 (C-5″), 31.3(C-3′), 31.2 (C-3′), 30.6 (C-3′), 28.8 (C-2″), 25.6 (C-3″), 24.4 (C-4″), 21.0 (2 COCH3), 20.9 (2 COCH3), 20.8 (2 COCH3), 18.4, 18.1 and 18.0 (C-6I,II,III); TOF-HRMS m/z: [M+Na]+ Calcd for C75H101N3O29Na: 1530.6418. Found 1530.6324.
4.5 5-(Methoxycarbonyl)pentyl 3-O-benzyl-4-(2,4-di-O-acetyl-3-deoxy-L-glycero-tetronamido)-4,6-dideoxy-α-D-mannopyranosyl-(1→2)-3-O-benzyl-4-(2,4-di-O-acetyl-3-deoxy-L-glycerotetronamido)-4,6-dideoxy-α-D-mannopyranosyl-(1→2)-3-O-benzyl-4-(2,4-di-O-acetyl-3-deoxy-L-glycero-tetronamido)-4,6-dideoxy-α-D-mannopyranoside (18)
Compound 16 (6.2 g, 4.1 mmol) was treated with hydrazine acetate, as described for preparation of 11, to give trisaccharide 18 (5.0 g, 87%), which was identical (TLC, NMR) with the known, independently synthesized11 substance.
4.6 5-(Methoxycarbonyl)pentyl 3-O-benzyl-4-(2,4-di-O-acetyl-3-deoxy-L-glycero-tetronamido)-4,6-dideoxy-α-D-mannopyranosyl-(1→2)-3-O-benzyl-4-(2,4-di-O-acetyl-3-deoxy-L-glycero-tetronamido)-4,6-dideoxy-α-D-mannopyranosyl-(1→2)-3-O-benzyl-4-(2,4-di-O-acetyl-3-deoxy-L-glycero-tetronamido)-4,6-dideoxy-α-D-mannopyranosyl-(1→2)-3-O-benzyl-4-(2,4-di-O-acetyl-3-deoxy-L-glycero-tetronamido)4,6-dideoxy-α-D-mannopyranosyl-(1→2)-3-O-benzyl-4-(2,4-di-O-acetyl-3-deoxy-L-glycero-tetronamido)-4,6-dideoxy-α-D-mannopyranoside (20)
A solution of TMSOTf in toluene (0.03 M, 6 mL) was added with stirring at 100 °C under N2 to a mixture of 1811 (5.0 g, 3.5 mmol), 14 (4.69 g, 4.25 mmol), and 4 Å molecular sieves (5.8 g) in toluene (110 mL). After 3 h, when TLC (1:1 hexane–acetone) showed that the reaction was virtually complete, the mixture was cooled to room temperature and Et3N (0.8 ml) was added. The mixture was filtered through Celite pad, the filtrate was concentrated, and chromatography (6:1 →3:1 hexane–acetone) gave an anomeric mixture of fully protected pentasaccharides 19α, β (6.0 g, total yield 75%). TOF–MS m/z: 2374 [M+Na]+. The foregoing anomeric mixture (6.0 g, 2.5 mmol) was treated with hydrazine acetate (202 mg, 3.0 mmol) in MeOH (400 ml) overnight. Work-up, as described above for a similar reaction, and chromatography (5:1 DCM–acetone), gave first the β-linked product, 5-(methoxycarbonyl)pentyl 3-O-benzyl-4-(2,4-di-O-acetyl-3-deoxy-L-glycero-tetronamido)-4,6-dideoxy-α-D-mannopyranosyl-(1→2)-3-O-benzyl-4-(2,4-di-O-acetyl-3-deoxy-L-glycero-tetronamido)-4,6-dideoxy-α-D-mannopyranosyl-(1→2)-3-O-benzyl-4-(2,4-di-O-acetyl-3-deoxy-L-glycero-tetronamido)-4,6-dideoxy-β-D-mannopyranosyl-(1→2)-3-O-benzyl-4-(2,4-di-O-acetyl-3-deoxy-L-glycero-tetronamido)-4,6-dideoxy-α-D-mannopyranosyl-(1→2)-3-O-benzyl-4-(2,4-di-O-acetyl-3-deoxy-L-glycero-tetronamido)-4,6-dideoxy-α-D-mannopyranoside (21, 220 mg, 4%). 1H NMR (600 MHz, CDCl3) δ: 7.74 (brs, 1 H, NH), 7.37–7.21 (m, 25 H, 5 Ph), 6.40–6.12 (m, 4 H, 4 NH), 5.45 (brs, 1 H, H-1), 5.28 (dd, J = 4.8, 8.2 Hz, 1 H, H-2′), 5.18–5.12 (m, 4 H, 4 x H-2′), 5.04 (brs, 1 H, H-1), 4.90 (brs, 1 H, H-1), 4.78 (d, overlapped with CHPh, H-1), ~4.30 (m, H-1IV, overlapped), 2.30 (t, J = 7.2 Hz, H-5″a,b), 2.17–1.92 (m, 40 H, H-3′I–V, 10 COCH3), 1.67–1.63 (m, 4 H, H-2″a,b, H-4″a,b), 1.38 (m, 2 H, H-3″a,b), 1.26–1.08 (m, 15 H, H-6I–V); 13C NMR (150 MHz, CDCl3) δ: 100.9 (C-1, JC-1,H-1 172 Hz), 99.6 (br, C-1, JC-1,H-1 not determined), 98.8(C-1, JC-1,H-1 169.6 Hz), 98.6 (C-1, JC-1,H-1 172 Hz), 98.1(C-1, JC-1,H-1 154 Hz), 20.9 and 20.8 (5 C, C-6I–V); TOF–MS m/z: 2275 [M+Na]+.
Eluted next was the all-α-linked pentasaccharide 20 (5.0 g, 87%), [α]D −9.2 (c 1.08, CHCl3); 1H NMR (600 MHz, CDCl3) δ: 7.40–7.24 (m, 25 H, 5 Ph), 6.53–6.13 (m, 4 H, 4 NH), 5.18 (m, 5 H, H-2′I–V), 5.08 (d, J1,2 = 2.0 Hz, H-1), 4.09 (brs, 2 H, 2 H-1), 4.87 (brs, 1 H, H-1), 4.71 (brs, 1 H, H-1), 4.64–4.58 (m, 10 H, 5 PhCH2), 4.20–4.08 (m, 20 H, H-2I–V, H-4I–V, H-4′I–V), 3.86–3.32 (m, 15 H, H-3I–V, H-5I–V, OCH3, H-1″a,b), 2.46 (brs, 1 H, OH-2V), 2.32 (t, J = 7.2 Hz, H-5″a,b), 2.21–1.95 (m, 40 H, H-3′I–V, 10 COCH3), 1.66–1.53 (m, 4 H, H-2″a,b, H-4″a,b), 1.44–1.32 (m, 2 H, H-3″a,b), 1.14–1.08 (m, 15 H, H-6I–V). 13C NMR (150 MHz, CDCl3) δ: 100.7 (C-1), 99.9 (3 C, 3 C-1), 98.8 (C-1), 18.4 (C-6), 18.3 (C-6), 18.2 (2 C, 2 C-6), 17.9 (C-6). TOF-HRMS m/z: [M+H]+ Calcd for C112H150N5O43: 2252.9705. Found 2252.9724; Anal. Calcd. for C112H149N5O43: C, 59.70; H, 6.66; N, 3.11. Found C, 59.41; H, 6.70; N, 3.08.
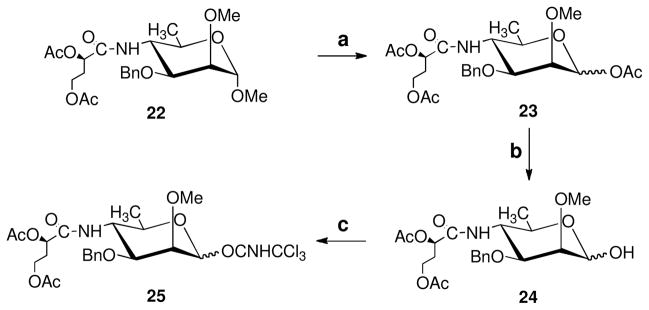
4.7 1-O-Acetyl-3-O-benzyl-4-(2,4-di-O-acetyl-3-deoxy-L-glycero-tetronamido)-4,6-dideoxy-2-O-methyl-α-D-mannopyranose (23)
Methyl 3-O-Benzyl-4-(2,4-di-O-acetyl-3-deoxy-L-glycero-tetronamido)-4,6-dideoxy-2-O-methyl-α-D-mannopyranose (22,8 7.0 g, 15 mmol) was treated at room temperature with Ac2O-HOAc-H2SO4 (10–4–0.1, 140 ml) for 1.5 h, and NaOAc.3H2O (2.0 g) was added to terminate the reaction. After concentration and chromatography (1:1 hexane–EtOAc) gave 23 (6.7 g, 91%), mp 104–105 °C (EtOH); [α]D −2 (c 1.1, CHCl3); 1H NMR (600 MHz, CDCl3) δ: 7.36–7.27 (m, 5 H, Ph), 6.13 (d, J1,2 = 1.9 Hz, 1 H, H-1), 6.08 (d, J = 8.1 Hz, 1 H, NH), 5.16 (dd, J = 8.0 Hz and 4.7 Hz, 1 H, H-2′), 4.65–4.51 (ABq, J = 11.7 Hz, 2 H, PhCH2), 4.18–4.07 (m, 4 H, H-3, H-5 and H-4′), 3.80 (m, 1 H, H-4), 3.50 (brs, 4H, H-2 and OCH3), 2.19 (m, 1 H, H-3a′), 2.11–2.04 (m, 10 H, 3 CH3CO and H-3b′), 1.22 (d, J5,6 = 6.3 Hz, 3 H, H-6). 13C NMR (150 MHz, CDCl3) δ: 91.5 (C-1), 75.7 (C-2), 74.4 (C-3), 71.6 (PhCH2), 71.3 (C-2′), 69.5 (C-5), 60.1 (C-4′), 59.3 (OCH3), 53.8 (C-4), 31.1 (C-3′), 21.2, 21.0, 20.9 (3 COCH3), 18.6 (C-6); TOF-HRMS m/z: [M+H]+ Calcd for C24H34NO10: 496.2183. Found 496.2178; Anal. Calad. for C24H33NO10: C, 58.17; H, 6.71; N, 2.83. Found C, 58.36; H, 6.76; N 2.78.
4.8 (2-Aminoethylamido)carbonylpentyl 4-(3-deoxy-L-glycero-tetronamido)-4,6-dideoxy-α-D-mannopyranosyl-(1→2)-tetrakis[-4-(3-deoxy-L-glycero-tetronamido)-4,6-dideoxy-α-D-mannopyranosyl]-4-(3-deoxy-L-glycero-tetronamido)-4,6-dideoxy-2-O-methyl-α-D-mannopyranoside (31)
Piperidine (4.0 ml, 40 ml) was added dropwise to a solution of 23 (1.0 g, 2.0 mmol) in THF (10 ml). After 3 h, when TLC (3:1 DCM–acetone) showed that the reaction was complete, the mixture was concentrated and chromatography (10:1 DCM–acetone) gave the intermediate 24, 3-O-Benzyl-4-(2,4-di-O-acetyl-3-deoxy-L-glycero-tetronamido)-4,6-dideoxy-2-O-methyl-α,β-D-mannopyranose (800mg, 75%). TOF–MS: m/z: 476.1 [M+Na]+.
DBU (0.08 ml, 0.5 mmol) was added with stirring at 0° C to a solution of 24 (2.2 g, 4.9 mmol) and CCl3CN (2.5 ml, 24 mmol) in DCM (30 ml), and the mixture was allowed to warm up to room temperature. After 3 h, the mixture was concentrated and chromatography (3:1, hexane–acetone with 1% Et3N) afforded 3-O-benzyl-4-(2,4-di-O-acetyl-3-deoxy-L-glycero-tetronamido)-4,6-dideoxy-2-O-methyl-α-D-mannopyranose-1-O-trichloroacetimidate (25, 2.5 g, 86%). 1H NMR (600 MHz, CDCl3) δ: 8.58 (s, 1 H, NHCCl3), 7.36–7.27 (m, 5 H, Ph), 6.27 (d, J1,2 = 2.0 Hz, 1 H, H-1), 5.95 (d, J = 8.3 Hz, 1 H, NH), 5.16 (dd, J = 8.0 Hz and 4.7 Hz, 1 H, H-2′), 4.64–4.54 (ABq, J = 12.0 Hz, 2 H, PhCH2), 4.16–4.06 (m, 4 H, H-3, H-5 and H-4′), 3.93 (m, 1 H, H-4), 3.63 (t, J = 2.5 Hz, 1 H, H-2), 3.53 (s, 3 H, OCH3), 2.18–2.03 (m, 8 H, 2 COCH3 and H-3′), 1.23 (d, J5,6 = 6.4 Hz, 3 H, H-6); 13C NMR (150 MHz, CDCl3) δ: 95.3 (C-1), 74.9 (C-2), 74.1 (C-3), 71.5 (PhCH2), 71.0 (C-2′), 70.2 (C-5), 59.2 (C-4′), 59.2 (OCH3), 52.9 (C-4), 30.8 (C-3′), 20.8 20.7 (2 COCH3), 18.0 (C-6); TOF-HRMS, m/z: Calcd for C24H31N2O9NaCl3 [M+Na]+: 619.0993. Found 619.0969.
A solution of TMSOTf in toluene (0.03 M, 11 ml) was added with exclusion of moisture to a stirred mixture of 25 (1.6 g, 2.68 mmol), 20 (4.0 g, 1.77 mmol), 4 Å molecular sieves (6.5 g) in DCM (100 ml), and the stirring was continued overnight, when TLC (3:1 DCM–acetone) showed that only small amount of the imidate 25 was present. The mixture was neutralized with Et3N (1.0 ml), filtered, the filtrate was concentrated, and chromatography (5:1 DCM–acetone) gave the known11 hexasaccharide, 5-(methoxycarbonyl)pentyl 3-O-benzyl-4-(2,4-di-O-acetyl-3-deoxy-L-glycero-tetronamido)-4,6-dideoxy-α-D-mannopyranosyl-(1→2)-tetrakis[-3-O-benzyl-4-(2,4-di-O-acetyl-3-deoxy-L-glycero-tetronamido)-4,6-dideoxy-α-D-mannopyranosyl]-3-O-benzyl-4-(2,4-di-O-acetyl-3-deoxy-L-glycero-tetronamido)-4,6-dideoxy-2-O-methyl-α-D-mannopyranoside 27 (3.3 g, yield 69%).
A stirred solution of 27 (3.3 g) in DCM-MeOH (1: 10, 70 ml) was treated overnight with hydrogen in the presence 5% Pd/C (2.0 g). After filtration and concentration, the residue was chromatographed (12:1 EtOAc–EtOH) to afford 5-(methoxycarbonyl)pentyl-4-(2,4-di-O-acetyl-3-deoxy-L-glycero-tetronamido)-4,6-dideoxy-α-D-mannopyranosyl-(1→2)-tetrakis[-4-(2,4-di-O-acetyl-3-deoxy-L-glycero-tetronamido)-4,6-dideoxy-α-D-mannopyranosyl]-4-(2,4-di-O-acetyl-3-deoxy-L-glycero-tetronamido)-4,6-dideoxy-2-O-methyl-α-D-mannopyranoside 30 (2.25 g, 87%). TOF–MS m/z: 1074 [M]2+, 2148 [M]+.
The foregoing hexsaccharide 30 (2.23 g, 1.03 mmol) was treated with ethylenediamine (17 ml) at 50 °C overnight. After concentration and chromatography (1:1:0.1 MeOH–DCM–25% NH4OH) afforded the known20 31 (1.3 g, 75%). 1H NMR (400 MHz, D2O) δ: 5.12–4.79 (m, 6 H, H-1I–VI), 4.21–4.18 (m, 6 H, H-2′I–VI), 4.08–3.95 (m, 10 H, H-2II–V, H-3I–VI), 3.86–4.74 (m, 13 H, H-2I, H-4I–VI, H-5I–VI), 3.59–3.54 (m, 1 H, H-2VI, H-4′I–VI, H-1″a), 3.46 (m, 1 H, H-1″b), 3.40 (s, 3 H, OCH3), 3.34 (t, J = 6.0 Hz, H-6″), 2.95 (t, J = 6.0 Hz, H-7″), 2.20 (t, J = 7.6 Hz, H-5″), 1.98–1.92 (m, 6 H, H-3′aI–VI), 1.80–1.72(m, 6 H, H-3′bI–VI), 1.52 (m, 4 H, H-2″ and H-4″), 1.27 (m, 2 H, H-3″), 1.12–1.08 (m, 18 H, H-6I–VI). 13C NMR (100 MHz, D2O) δ: 100.8– 98.3 (6 C, C-1I–VI), 78.8, 77.7, 77.4, 77.1 (2 C), 68.9 (6 C, C-2′I–VI), 57.8 (6 C, C-4′I–VI), 16.9–16.7 (6 C, C-6I–VI); TOF–MS m/z: 1670.8 [M]+.
4.9 5-(Methoxycarbonyl)pentyl 3-O-benzyl-4-(2,4-di-O-acetyl-3-deoxy-L-glycero-tetronamido)-4,6-dideoxy-2-O-levulinoyl-α-D-mannopyranosyl-(1→2)-tetrakis[-3-O-benzyl-4-(2,4-di-O-acetyl-3-deoxy-L-glycero-tetronamido)-4,6-dideoxy-α-D-mannopyranosyl]-3-O-benzyl-4-(2,4-di-O-acetyl-3deoxy-L-glycero-tetronamido)-4,6-dideoxy-α-D-mannopyranoside (26)
A solution of TMSOTf in toluene (0.03 M, 2 ml) was added with stirring and exclusion of moisture to a mixture of 20 (500 mg, 0.22 mmol), 10 (300 mg, 0.44 mmol), 4 Å molecular sieves (300 mg) in dichloromethane (10 ml). The stirring was continued overnight, when TLC (2:1 toluene–acetone) showed that only small amount of the imidate remained unchanged. After neutralization with Et3N (0.2 ml), filtration and concentration of the filtrate, the residue was chromatographed (3:1 toluene–acetone) to give 26 (450 mg, 73%). 1H NMR (600 MHz, CDCl3) δ: 6.24–5.93 (m, 5 H, 5 NH), 5.40 (brs, 1 H, H-2VI), 5.20–5.15 (m, 6 H, H-2′I–VI), 5.05–4.97 (3 s, 4 H, H-1II–V), 4.69 (s, 2 H, H-1I,VI), 4.63–4.43 (m, 12 H, 6 PhCH2), 4.30–4.01 (m, 22 H, H-2II–V, H-4I–VI, H-4′I–VI), 3.86 (brs, 1 H, H-2I), 3.80–3.60 (m, 16 H, H-3I–VI, H-5I–VI, OCH3, H-1″a), 3.35 (m, 1 H, H-1″b), 2.72–2.61 (m, 4 H, CH2CH2), 2.33 (t, J = 7.2 Hz, H-5″a,b), 2.26–1.99 (m, 51 H, 13 COCH3, H-3′I–VI), 1.67–1.55 (m, 4 H, H-2″a,b, H-4″a,b), 1.43–1.31 (m, 2 H, H-3″a,b), 1.17–1.08 (m, 18 H, H-6I–VI); 13C NMR (150 MHz, CDCl3) δ: 137.9 (Cq), 137.8 (Cq), 137.7 (Cq), 137.6 (Cq), 137.5 (Cq), 137.4 (Cq), 128.5, 128.4, 128.3, 128.2, 128.1, 128.0, 127.8, 127.6, 127.4, 100.5–99.6 (4 C, C-1II–V), 98.6 (2 C, C-1I,VI), 18.1–17.8 (6 C, C-6I–VI); TOF–MS m/z: 1387 [M]2+, 2772 [M+H]+, 2795 [M+Na]+.
4.10 5-(Methoxycarbonyl)pentyl 4-(3-deoxy-L-glycero-tetronamido)-4,6-dideoxy-α-D-mannopyranosyl-(1→2)-tetrakis[-4-(3-deoxy-L-glycero-tetronamido)-4,6-dideoxy-α-D-mannopyranosyl]-4-(3-deoxy-L-glycero-tetronamido)-4,6-dideoxy-α-D-manno-pyranoside (29)
A solution of compound 26 (400 mg, 0.14 mmol) in DCM-MeOH (1:10, 10 ml) was stirred under H2 with 5% Pd/C (400 mg) overnight, then filtered and concentrated. Without further purification, the residue was treated with 1.0 M NaOMe (1.0 ml) in MeOH (10 ml), to complete deacylation. After neutralization with Amberlite IR-120, H+-resin, the mixture was filtered, the filtrate was concentrated, and the residue was chromatographed (11 DCM–MeOH) to afford the known7 compound 29 (210 mg, 89%).
Scheme 3.
(a) Ac2O-HOAc-H2SO4 (10:4:0.1, v/v), rt, 91%; (b) piperidine, THF, rt, 75%; (d) DBU, Cl3CCN, DCM, 0 °C to rt, 86%.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, NIDDK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bennish ML. In: Vibrio cholerae and Cholera: Molecular to Global Perspectives. Wachsmuth IK, Blake PA, Olsvik O, editors. American Society for Microbiology; Washington, D.C: 1994. pp. 229–255. [Google Scholar]
- 2.Redmond JW. Biochem Biophys Acta. 1979;584:346–352. doi: 10.1016/0304-4165(79)90280-0. [DOI] [PubMed] [Google Scholar]
- 3.Hisatsune K, Kondo S, Isshiki Y, Iguchi T, Haishima Y. Biochem Biophys Res Commun. 1993;194:584. doi: 10.1006/bbrc.1993.1046. [DOI] [PubMed] [Google Scholar]
- 4.McNaught AD. Carbohydr Res. 1997;297:1–92. doi: 10.1016/s0008-6215(97)83449-0. [DOI] [PubMed] [Google Scholar]
- 5.Kováč P. In: Protein-Carbohydrate Interactions in Infectious Diseases. Bewley C, editor. Royal Society of Chemistry; London: 2006. pp. 175–220. [Google Scholar]
- 6.Lei P-s, Ogawa Y, Kováč P. Carbohydr Res. 1996;281:47–60. doi: 10.1016/0008-6215(95)00331-2. [DOI] [PubMed] [Google Scholar]
- 7.Ogawa Y, Lei P-s, Kováč P. Carbohydr Res. 1996;293:173–194. doi: 10.1016/0008-6215(96)00202-9. [DOI] [PubMed] [Google Scholar]
- 8.Arencibia-Mohar A, Ariosa-Alvarez A, Madrazo-Alonso O, Abreu EG, Garcia-Imia L, Sierra-Gonzalez G, Verez-Bencomo V. Carbohydr Res. 1998;306:163–170. doi: 10.1016/s0008-6215(97)10052-0. [DOI] [PubMed] [Google Scholar]
- 9.Ariosa-Alvarez A, Arencibia-Mohar A, Madrazo-Alonso O, Garcia-Imia L, Siera-Gonzalez G, Verez-Bencomo V. J Carbohydr Chem. 1998;17:1307–1320. doi: 10.1016/s0008-6215(97)10052-0. [DOI] [PubMed] [Google Scholar]
- 10.Chernyak A, Kondo S, Wade TK, Meeks MD, Alzari PM, Fournier JM, Taylor RK, Kováč P, Wade WF. J Infect Dis. 2002;185:950–962. doi: 10.1086/339583. [DOI] [PubMed] [Google Scholar]
- 11.Adamo R, Kováč P. Eur J Org Chem. 2007:988–2000. [Google Scholar]
- 12.Hou S-j, Kováč P. Synthesis. 2009:545–550. [Google Scholar]
- 13.Werz DB, Seeberger PH. Angew Chem, Int Ed Engl. 2005;44:6315–6318. doi: 10.1002/anie.200502615. [DOI] [PubMed] [Google Scholar]
- 14.Gotoh M, Kováč P. J Carbohydr Chem. 1994;13:1193–1213. [Google Scholar]
- 15.Ma X, Saksena R, Chernyak A, Kováč P. Org Biomol Chem. 2003;1:775–784. doi: 10.1039/b211660j. [DOI] [PubMed] [Google Scholar]
- 16.Bundle DR, Gerken M, Peters T. Carbohydr Res. 1988;174:239–251. doi: 10.1016/0008-6215(88)85094-8. [DOI] [PubMed] [Google Scholar]
- 17.Peters T, Bundle DR. Can J Chem. 1989;67:497–502. [Google Scholar]
- 18.Ogawa Y, Lei P-s, Kováč P. Carbohydr Res. 1996;288:85–98. doi: 10.1016/s0008-6215(96)90781-8. [DOI] [PubMed] [Google Scholar]
- 19.Ogawa Y, Kováč P. Glycoconjugate J. 1997;14:433–438. doi: 10.1023/a:1018591132723. [DOI] [PubMed] [Google Scholar]
- 20.Saksena R, Zhang J, Kováč P. Tetrahedron Asymmetry. 2005;16:187–197. [Google Scholar]
- 21.Zhang J, Yergey A, Kowalak J, Kováč P. Tetrahedron. 1998;54:11783–11792. [Google Scholar]
- 22.Hou S-j, Saksena R, Kováč P. Carbohydr Res. 2008;343:196–210. doi: 10.1016/j.carres.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang J, Kováč P. Carbohydr Res. 1997;300:329–339. doi: 10.1016/s0008-6215(97)00070-0. [DOI] [PubMed] [Google Scholar]
- 24.Peters T, Bundle DR. Can J Chem. 1989;67:491–496. [Google Scholar]
- 25.Gast JC, Atalla RH, McKelvey RD. Carbohydr Res. 1980;84:137–146. [Google Scholar]
- 26.Kováč P, Hirsch J. Carbohydr Res. 1982;100:177–193. [Google Scholar]
- 27.Adamo R, Kováč P. Eur J Org Chem. 2006:2803–2809. [Google Scholar]