Abstract
Aromatization of testosterone to estradiol by neural tissue has classically been associated with the regulation of sexual differentiation, gonadotropin secretion, and copulatory behavior. However, new data indicate that the capacity for aromatization is not restricted to the endocrine brain and demonstrate roles for locally-formed estrogens in neurogenesis and in responses of brain tissue to injury. This manuscript summaries our current understanding of the distribution and regulation of aromatase in the brain and discusses the classical and novel roles it plays. A better understanding of brain aromatization could shed new light on its physiologic and pathologic functions and someday lead to new centrally acting drug therapies.
Keywords: Cytochrome P450 aromatase, CYP 19, LH, sexual behavior, sexual partner preference, brain ischemia
1. Introduction
The idea that estrogens are synthesized locally in the brain and act to amplify and diversify the action of circulating testosterone is considered axiomatic. The evidence that the brain was capable of converting androgen to estrogen, albeit at very low levels, was crucial evidence used in originally formulating the aromatization hypothesis 1. According to the aromatization hypothesis testosterone synthesized by the fetal testis diffuses into the male brain where it is locally aromatized to estradiol and then initiates the process of masculinization. Originally the aromatization hypothesis was advanced to explain the mechanism whereby testosterone acts to organize and sexually differentiate brain structure and chemistry. However, over the intervening 30+ years since it was first proposed, the aromatization hypothesis has been examined from many experimental perspectives in several neural tissues and species. Today, it is well established that specific areas and cell types of the central nervous system synthesize estrogens from precursor androgen. The local synthesis of estrogen in the brain is a dynamic and regulated process that varies with age, sex, and physiologic status. Moreover, it has become increasingly evident that aromatase has more general roles to play in development, protection, and repair of the brain. The current review will present a synthesis of the physiologic importance of aromatase in the central nervous system control of reproduction and neuroprotection.
2. Distribution of brain aromatase
It is generally accepted that under normal conditions the expression of aromatase is restricted to certain neuronal populations in the central nervous system 2–4. The predominant sites of aromatase activity are within brain regions involved in reproductive functions and behaviors such as the hypothalamus and amygdala 5–7. Neuroanatomic studies using immunohistochemistry and in situ hybridization have confirmed the general pattern of aromatase distribution and further refined it to encompass an arc of interconnected nuclei that includes the nucleus of the posteromedial amygdala, encapsulated region of the bed nucleus of the stria terminalis, ventrolateral portion of the ventromedial hypothalamic nucleus, and central component of the medial preoptic nucleus. This endocrine brain circuit contains an overlapping distribution of androgen- and estrogen-receptor containing cells 8. A number of other brain structures contain measurable aromatase activity and/or immunoreactive cells including the excitatory CA1–3 pyramidal cells and dentate gyrus granule cells of the hippocampus, layer II–VI pyramidal cells of the cerebral cortex, midbrain neurons, sensory afferent neurons, as well as cells in the spinal cord and cerebellum 9–14. Studies on hippocampus demonstrate that locally synthesized estrogen regulates synaptic plasticity to affect the function of excitatory neural circuits 15 and may play a role in neurogenesis within the dentate gyrus of the adult 16.
Aromatase is distributed within several cellular compartments of neurons, including the cell bodies, processes, and pre-synaptic terminals 17, 18. This pattern of localization strongly suggests that not only can aromatase generate estrogens to act within target cells, but that the estrogens produced can also act in a paracrine manor on adjacent cells. This latter possibility is supported by reports showing that the incidence of aromatase and estrogen receptor localization is not absolute but ranges from 5% to 80% depending on specie and brain area 19–21. The issue is further complicated by the mounting evidence that, besides their well-known hormonal mode of action at the genetic level, estrogens such as 17β-estradiol also influence brain function by direct effects on neuronal membranes 22. The observation that aromatase is expressed in presynaptic terminals together with the demonstration that aromatase activity can be modulated within minutes by Ca2+-dependent phosphorylation eventuating in rapid behavioral responses, has been marshaled to support the hypothesis that brain estrogens display many, if not all, functional characteristics of neuromodulators or even neurotransmitters 23.
For a long time aromatase was only reported in neurons, notably of the diencephalon and limbic system of mammals. However, recent studies have demonstrated that aromatase is expressed in radial glial and intermediate progenitor cells of the developing cortex in the mouse 24. Radial glial cells are considered to be a major source of neurons during vertebrate brain development 25. It was recently demonstrated that in fish aromatase immunoreactivity is present in actively dividing cells derived from radial glial that differentiate into neurons 26.
Although aromatase has been detected within astrocytes of the human temporal cortex 27, it appears to be the more general rule that glial cells in the mammalian brain do not express aromatase under normal conditions 28, 29. However, cultured astrocytes express aromatase in vitro and reactive astrocytes express aromatase following brain injury and ischemia 29–32. This induction of aromatase decreases areas of gliosis 33, 34, and apoptotic degeneration 35–37, and may mediate neuroprotection and/or enhance neuronal recovery from injury 29, 30. Aromatase-expressing reactive astrocytes are observed in all injured brain areas including, cortex, corpus collosum, striatum, hippocampus, thalamus, and hypothalamus indicating astrocytes from most brain areas has the potential for producing estrogen in response to injury 29.
3. Regulation of brain aromatase
The conversion of androgens into estrogens in the brain is a key mechanism by which testosterone regulates many physiological and behavioral processes throughout an animal’s life. Because of the diverse age- and region-specific actions of testosterone, it is not surprising that the regulation of aromatase in the brain is complex and not completely understood. Recent studies in several species have led to new perspectives on the control of this enzyme by both transcriptional and posttranscriptional mechanisms 38.
a. Ontogeny of brain aromatase
Aromatase plays an essential role in the development and sexual differentiation of the brain in many mammalian species 39. In short gestation species (i.e. rodents), there is a peak in both activity and mRNA expression of aromatase in the preoptic area/hypothalamus that occurs late in gestation or early neonatal life and corresponds to the critical period for sexual differentiation 3. Transiently higher levels of aromatase activity and greater circulating levels of testosterone are present in perinatal males suggesting that males are exposed to higher levels of estrogen than females at these critical times 3. More detailed studies in rats show that the temporal pattern of aromatase mRNA expression varies in different subregions of the limbic brain suggesting a more complex system of regulation 40, 41.
In long gestation species including non-human primates, sheep, cows, and guinea pigs, the highest levels of aromatase are observed during the first half of gestation, a time when circulating testosterone is also elevated 42–45. Although abundant evidence exists for a role of aromatase in sexual differentiation of the brain in short gestation mammals, the evidence in long gestation species in mixed and thought not to be essential in humans and non-human primates 46. Nonetheless, high levels of aromatase have been measured in the developing brain of all species studied. This finding suggests that local estrogen production could play important developmental roles not limited to sexual differentiation. For instance, estradiol act in the brain, in part, to restrain cell death during brain development 47 and to stimulate the extension of neuronal processes 48 leading to the establishment of important neural pathways. While it is not understood what factor(s) regulate the expression of aromatase in the fetal brain, it does not appear to be androgen dependent in most species 3. In mid-gestation non-human primate fetuses, castration and testosterone treatment did not alter brain aromatase activity 43.
b. Sex steroid regulation of brain aromatase
Aromatase expression in the preoptic area/hypothalamus declines to low levels after birth and into adulthood. In contrast to the fetus, aromatase activity in the adult brain is regulated primarily by androgens in mammals and by estrogens in birds and fish 49–51. Our experiments in rats and nonhuman primates demonstrated that within the preoptic area and hypothalamus, androgens regulate aromatase mRNA via androgen receptor-mediated transcription 6, 52. Extensive co-localization of androgen receptor and aromatase is observed in the neuroendocrine brain 53 and a potential androgen responsive element and SF-1 site is present at the 5′-end of the human brain specific exon lf of aromatase 54. Estrogen has also been found to regulate hypothalamic aromatase in mammals under certain experimental condition and has been shown to synergize with androgen, but it is not clear whether this effect involves direct transcriptional modulation through the estrogen receptor 41, 55, 56. In fish species, estrogen-responsive element (ERE) and androgen-responsive element (ARE) have been identified upstream of the brain AromP450 gene promoter 57.
c. Region-specific regulation of brain aromatase
The expression of aromatase gene, Cyp19, in various tissue is regulated by the use of tissue-specific promoters, that are, in turn, regulated by different transcription factors and signaling pathways 58. Regulation of aromatase expression in the brain is region-specific 7, 41. In contrast to aromatase expression in the preoptic area and hypothalamus, gonadal steroids do not regulate aromatase in most other brain areas, including the amygdala and hippocampus 6. This observation suggests that there are at least two populations of aromatase-positive cells in the adult brain, a steroid-dependent and steroid-independent population. The finding that aromatase exhibits different patterns of promoter usage in different brain areas 27, 59, 60 supports the idea that region specific regulation could be depend on differential promoter usage, but does not exclude the possibility that other modes of transcriptional and post-transcriptional regulation are involved.
d. Rapid phosphorylation regulation of brain aromatase
Accumulating evidence suggests that brain aromatase may be rapidly regulated through nongenomic mechanisms involving direct phosphorylation of the aromatase enzyme 50. Aromatase activity in hypothalamic homogenates is rapidly (within minutes) down-regulated by exposure to conditions that enhance protein phosphorylation such as the presence of high concentrations of calcium, magnesium and ATP. Similarly, pharmacological manipulations such as treatment with thapsigargin or stimulation of various neurotransmitter receptors (alpha-amino-3-hydroxy-methyl-4-isoxazole propionic acid (AMPA), kainate, and N-methyl-d-aspartate (NMDA)) leading to enhanced intracellular calcium concentrations depress within minutes the aromatase activity measured in quail preoptic explants. The effects of receptor stimulation are presumably direct: electrophysiological data confirm the presence of these receptors in the membrane of aromatase-expressing cells. Inhibitors of protein kinases interfere with these processes and Western blotting experiments on brain aromatase purified by immunoprecipitation confirm that the phosphorylations regulating aromatase activity directly affect the enzyme rather than another regulatory protein. Accordingly, several phosphorylation consensus sites are present on the deduced amino acid sequence of the recently cloned quail aromatase. Fast changes in the local availability of estrogens in the brain can thus be caused by aromatase phosphorylation so that estrogen could rapidly regulate neuronal physiology and behavior 61.
4. Reproductive functions of aromatase in the brain
a. Aromatase and sexual differentiation of the brain
The classic view of sexual differentiation in mammalian species is that sex differences in the brain structure and function are programmed by exposure to testosterone produced by the fetal testis acting during a critical period in perinatal development 62. This is most profoundly true in brain regions directly involved in reproductive functions. The brain develops as male after exposure to testosterone produced by the developing testis and as female largely in the absence of such exposure 63. In most species, masculinization of the brain is evidenced in adults by a capacity to express male-typical sexual behaviors and high levels of aggression. In contrast, the feminized brain is capable of supporting female-typical responses such as ovulation, female-typical receptivity and maternal behavior. Masculinization is usually accompanied by defeminization, and although masculinization can be disrupted by various means it is not a natural part of feminization. There are interesting examples that illustrate the dichotomy between masculinization and defeminization. For instance, adult male non-human primates exhibit tonic gonadotropin secretion but are still capable of responding to estradiol with an LH surge 64 demonstrating that behavior is masculinized without gonadotropin secretion becoming defeminized.
The paradoxical observation that estradiol was just as effective as testosterone in the masculinization of rat behavior and the reduced nonaromatizable androgen dihydrotestosterone was not, led investigators to ask whether testosterone acts in brain after it is aromatized to estradiol 65. We now understand that most sexually dimorphic areas of the rat brain contain substantial levels of both aromatase and high concentrations of estrogen receptors lending strong indirect support to the aromatization hypothesis of sexual differentiation 1. However, the importance of aromatase for brain sexual differentiation differs depending on the specific dimorphic endpoint examined and the species considered. A preponderance of evidence in rats suggests that testosterone must be aromatized to completely masculinize and defeminize male brain structure, function, and behavior 65, 66. In ferrets, masculinization is initiated by aromatization prenatally and completed by testosterone itself acting through androgen receptors postnatally 67. In contrast, androgen receptor mechanisms appear to more exclusively regulate aspects of brain differentiation in non-human primates and guinea pigs, whereas both molecular pathways appear to play roles in mice and sheep 46, 68. Much less is known about psychosexual differentiation in humans, but there is little evidence that aromatization is involved 46.
The concept that in situ estrogen synthesis influences neural structures is well illustrated by the development of the sexually dimorphic nucleus (SDN) of the preoptic area of the rat 69. The SDN is larger in males than in females and forms part of the circuitry that processes sexually relevant sensory cues and formulates appropriate male-typical sexual behavior responses. High levels of aromatase mRNA expression have been observed in the nascent SDN of fetal rats and sheep 40. In rats, neonatal administration of estrogen or testosterone to genetic females masculinizes the SDN 70. Direct evidence that neural aromatization is normally essential for the masculinization of SDN was provided by the demonstration that SDN size is significantly reduced compared to controls in males that were treated with an aromatase inhibitor neonatally 71. Sexually dimorphic nuclei exist in other brain regions and analogous structures are found in other species, including humans 62. In addition to the size of particular brain regions, there are a number of other structural sexual dimorphisms in the brain that are due to the effect of aromatization; such as the extent of dendritic aborization, differences in the density and pattern of synaptic connections, size, number and phenotype of neurons in a particular region and astrocyte morphology 72, 73. All of these structural differences are believed to underlie adult sex differences in behavior and neural responsiveness to gonadal hormones in ways yet to be completely understood.
Although the SDN was originally hypothesized to play a role in the expression of copulatory behavior, no strong experimental evidence exists to support this view 74, 75. More recent lesion studies suggest that the SDN is critical for male-typical sexual partner preferences in rats and ferrets 76, 77. It is well documented that a minority of males in some species are attracted to same-sex mating partner 78. The domestic ram is one model species currently being studied to understand the relationship between prenatal hormone exposure, brain structure, and sexual attraction because ~8% of domestic rams are sexually attracted to other rams (i.e. male-oriented rams), whereas the majority of rams are attracted to estrous ewes (female-oriented rams) 79. A SDN (ovine SDN) exists in the sheep preoptic area and is larger in female-oriented rams than in male-oriented rams and ewes 80. The ovine SDN is organized prenatally by testosterone, but the role of aromatase is not yet known 81.
Structural sexual dimorphisms in the human brain have been described, but many of the reports are controversial and their functional significance is less well established than in animal models. In 1991, LeVay 82, reported that homosexual men, like heterosexual women, have a smaller interstitial nucleus in the anterior hypothalamus (INAH3) than heterosexual men. Nothing is yet known about the ontogeny of the INAH3 in humans.
Sexual partner preferences, like other aspects of reproductive behavior are highly sexually dimorphic 83. Aromatization in perinatal rat brain has been shown to be involved in differentiation of male-typical female-directed sexual partner preferences 71, 84. Targeted disruption of the aromatase gene in mice results in a loss of male sexual behavior, including male-typical sexual partner preferences 85. Low levels of aromatase activity and mRNA expression in adult rams are correlated with a male-oriented sexual preference 80, 86. Taken together these studies indicate that there may be a relationship between aromatase expression in the developing preoptic/anterior hypothalamic and male-typical sexual preferences. However, a recent attempt to prove this causal relationship in sheep was unsuccessful 68. Moreover, men with congenital aromatase deficiencies are uniformly heterosexual 87, 88 and gene linkage studies in humans failed to suggest a role for aromatase variation in the development of sexual orientation in men 89. Thus, the data in humans and sheep appear to conflict with rodent studies emphasizing that the causal factors involved in establishing sexual partner preferences remain to be established.
b. Aromatase and regulation of sexual behaviors
There is clear evidence for the involvement of the aromatization hypothesis in the central activation of sexual behavior in adult male rodents 90. As noted above, aromatase, androgen receptors, and estrogen receptors are abundant in the brain circuitry that regulates male copulatory behaviors. Estradiol is both necessary and sufficient to maintain or restore most elements of copulatory behavior except for ejaculatory patterns in male rats 91. Estradiol administration reverses the effects of castration on copulatory behavior in male rats, while treatment with aromatase inhibitors and estrogen antagonists inhibits the restoration of copulatory behavior by testosterone administration to castrates 92–94. Administration of the aromatase inhibitor fadrozole to testosterone-clamped male rats, decreased anticipatory level changing (i.e. sexual motivation), increased the latencies to mount, intromit, and ejaculate, and decreased the numbers of these behaviors 95. Replacement with estradiol restored anticipatory behavior, mounts and intromissions, but not ejaculations.
Aromatase knockout (ArKO) mice have been generated by several investigators independently and used to test whether neural aromatization of testosterone contributes to copulatory behavior in male mice 96–99. The ArKO mouse model is ideal for studying the contribution of aromatization to both sexual differentiation of the brain and adult behavior because they have functional estrogen receptors. Male copulatory behavior is severely impaired in male ArKO mice consistent with the role for aromatization in both the organizational and activational effects of testosterone. Testosterone administration did not improve behavior in castrated ArKO adults, whereas combined treatment with estradiol and dihydrotestosterone almost completely restored copulatory behavior to levels observed in wildtype males 100. These results suggest that estrogens derived from aromatization of testosterone exert major activational effects on male coital behavior in male C57Bl6 mice.
Species differences exist in the extent to which neural aromatization of testosterone and accompanying activation of neural estrogen receptors are required to maintain male sexual behavior. Nonaromatizable dihydrotestosterone is able to maintain or restore copulation in guinea pigs 101 and monkeys 102 suggesting that androgens are sufficient in these species. However, studies in non-human primates demonstrated that sexual motivation and copulatory behaviors also depend in part on aromatized testosterone 103.
The extent to which testosterone in men acts through aromatization to estradiol is not clear. Gooren 104 found that, in eugonadal men, the estrogen receptor antagonist, tamoxifen, and the aromatase inhibitor, testolactone, had no adverse sexual effects, and that dihydrotestosterone was as effective as testosterone in maintaining sexuality in hypogonadal men arguing that aromatase was not involved. The comparison of two men with congenital aromatase deficiencies, one with accompanying hypogonadism, suggested that testosterone alone allows for a normal sexual activity, but that there is a synergistic effect between testosterone and estradiol derived from aromatization 87, 88. These findings suggest that aromatization may be required in men for completely normal sexual behavior, but androgens are the main steroids involved.
c. Aromatase and regulation of gonadotropin secretion
It is well accepted that the secretion of the gonadotropin, LH, is tonically inhibited by testosterone (i.e. negative feedback) in males of all species, including man. However, the importance of aromatase in this action of testosterone differs across species. A preponderance of evidence in men, non-human primates, sheep, and mice suggests that testosterone must be aromatized in the hypothalamus and periphery to completely exert negative feedback control over LH secretion, but no role for aromatase has been demonstrated for rats and guinea pigs 52, 105–108. The most direct evidence for a role of central aromatization in testosterone negative feedback comes from studies in sheep. In sheep, infusion of the aromatase inhibitor, fadrozole, intracerebrally increased LH pulse frequency without effecting plasma estradiol concentrations 105.
Inhibition of aromatase in non-human primates causes a marked increase in LH secretion despite a concomitant increase in endogenous testosterone levels that normally serve to restrain LH secretion 109. Testosterone action in non-human primates is thought to occur in part through central aromatization because it was found that aromatase activity and GnRH concentrations are positively correlated in micropunch dissections of the medial basal hypothalamus 110.
A number of studies in men, have demonstrated that aromatization is needed for testosterone negative feedback, whereas other studies provide evidence that testosterone can act independent of aromatization 111–114. Discrepancies in this field are due to several issues including, the use of pharmacological sex steroid regimes and aromatase inhibitors with androgenic activity, as well as the difficulty in isolating the hypothalamic and pituitary components that control LH secretion. More recently, a clever human investigative model that employs sex steroid ablation with physiologic sex steroid add back in normal men and GnRH-deficient men treated with fixed GnRH pulses was used to determine the importance of central and peripheral aromatization in the control of gonadotropin secretion 106, 115. This model represents a hypothalamic clamp because the doses and frequency of exogenous GnRH are experimentally controlled. Thus, any effect on LH secretion of altering gonadal steroids can reflect only a pituitary site in such men, and can be used to detect a hypothalamic effect when experimental responses differ from normal men. Inhibition of aromatase and comparison of gonadotropin secretion between normal men and hypogonadotropic men revealed that estrogen acts within the hypothalamus to exert negative feedback in men. Moreover, it was shown that testosterone can slow LH pulse frequency in chemically castrated normal men while estradiol levels remain suppressed indicating that testosterone feedback at the hypothalamus can occur through direct androgen action not requiring aromatization. In contrast, testosterone treatment failed to suppress either mean LH levels or LH amplitude in the hypogonadotropic men given pulsatile GnRH indicating that testosterone’s negative feedback at the pituitary is mediated by aromatization. These data argue that circulating testosterone together with circulating estradiol generated by peripheral aromatization and estrogen formed locally in the brain all contribute to negative feedback in men.
5. A role for the aromatase in neuroprotection
Emerging data suggest that enhanced aromatase activity near the site of an ischemic or traumatic insult can favorably impact injury outcome, presumably by producing neuroprotective estradiol. The background for this concept arises from two sets of experimental observations. First, it has long been noted that injury outcomes after experimental stroke and trauma are sex-specific. Female rats and mice of various inbred and outbred strains experience smaller tissue damage for an equivalent insult from cerebral ischemia 116–120 and improved functional outcome 121. Similarly, male animals sustain greater injury than do age-matched females after traumatic brain injury 122. Data from these studies suggest that, in part, the female “benefit” is mediated through ovarian steroid availability. Second, exogenous estradiol administration provides robust cerebroprotection in a wide range of neurological disease models, and it is equally effective in both sexes; for reviews, see 123–126. These observations set the stage for investigations into how changes in aromatase expression and endogenous estradiol production alter the environment for evolving brain damage.
Early reports confirmed that mechanical trauma or an excitotoxic challenge by intra-cerebral kainic or domoic acid injection leads to increased local aromatase expression and activity 29, 127, 128. At the cellular level, aromatase induction after injury occurs in the reactive astrocyte rather than in the neuron as ordinarily occurs under physiological conditions. Treatment with the specific aromatase inhibitor, fadrozole, enhances kainic acid-induced neuronal loss, confirming that aromatase induction has functional consequences 127. Using a similar paradigm, Veiga et al. 129 implicated extragonadal estradiol in protecting hippocampal neurons in rodents of both sexes. In addition to aromatase induction in the zone-of-injury, local increases in androgen receptor and estrogen receptor α expression have also been documented 130. Accordingly, many of the essential mechanisms for neuroprotective signalling by estradiol are engaged after injury in a coordinate manner.
Others have borne out these original observations using more complex, but clinically relevant, models of experimental stroke. Immunohistochemistry in spontaneously hypertensive rats demonstrates that the aromatase protein is elevated at 24 h and at 8 days after focal cerebral ischemia, specifically in peri-infarct zones known as the penumbra 31. Penumbral regions include tissue which has been injured by ischemia but remains viable. Therefore, the localization pattern for the enzyme likely has significance. Double labeling studies indicated that post-ischemic aromatase expression is associated with astrocytic processes 31.
We speculated that the female brain is strongly dependent on estradiol production in the ischemic penumbra for favorable outcomes. To provide initial proof of this concept, ischemic injury was evaluated in mice with targeted deletion of the CYP19 gene (ArKOs) 32. ArKOs of both sexes have abnormal reproductive phenotypes with plasma estradiol levels below detection by radioimmunoassay and elevated testosterone and gonadotropin levels 96, 131–133. ArKOs also demonstrate abnormalities in bone formation, both early and late in life, and heavy accumulation of adipose tissue. In McCullough et al.32, female ArKOs were treated with a standard focal cerebral ischemic insult: reversible middle cerebral artery occlusion followed by 22 hours of reperfusion. ArKOs sustained larger tissue damage than did their wild- type littermates, a result that was replicated by treating wild type female mice with fadrozole, a well-known aromatase inhibitor. Loss of aromatase activity resulted in greater ischemic damage than observed with ovariectomy, suggesting that extragonadal estradiol is vital to female stroke.
In vitro, aromatase is constitutively expressed in astrocytes 35, and plays a role in cell viability after oxygen and glucose deprivation (OGD, a commonly used model of “ischemic injury”). In addition, there are significant sex differences in how the aromatase is engaged after OGD in male vs. female astrocytes. For example, we have shown that female astrocytes are more resistant to OGD than male cells. In part, this is due to the higher aromatase mRNA and protein and higher enzyme activity than occurs in female cells under ischemic stress 134. Furthermore, the aromatase inhibitor, Arimidex, markedly increases cell death in female astrocytes and abolishes the sexual dimorphism in outcome from OGD. Using a bio-assay approach, we observed that astrocyte-conditioned media from female cultures conferred protection against OGD-induced cell death in male cultures. However, when pretreated with Arimidex, conditioned medium from female astrocytes was unable to protect male cells 134.
To further verify sex-dependent differences in aromatase-mediated mechanisms of protection against cell death, we developed a novel method that employs sex-specific and genotype-specific single pup primary astrocyte cultures from wild-type and ArKO mice. While wild-type female astrocytes are predictably more resistant to OGD than wildtype male cells, this sex difference disappears in ArKO cells. Specifically, post-OGD cell death is increased in ArKO female astrocytes to a level indistinguishable from male ArKO cells 135. These findings confirm that the aromatase is instrumental in mediating astrocyte survival following OGD and again emphasize that activation of the enzyme leads to cytoprotection. Furthermore, aromatase activity appears to be essential in endogenous neuroprotection in female astrocytes.
The precise mechanisms by which aromatization of steroids protect brain have not been directly studied. However, the prevailing hypothesis is that it is estrogen production is responsible, rather than metabolism and reduction of tissue androgen levels. It has been exhaustively demonstrated that exogenous estradiol reduces ischemic brain injury even in complex animal models with co-morbidities such as hypertension, genetic predisposition to stroke 117, diabetes136, 137 and in aging 138. Estradiol’s protective mechanisms are thought to be multi-factorial and this is the subject of other reviews in this series.
6. Conclusions
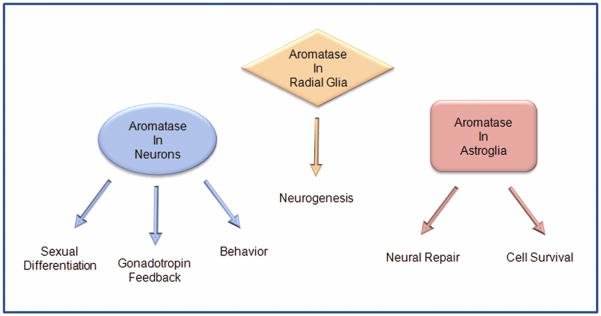
Aromatase plays a critical role in brain function under physiological and pathological conditions (Fig. 1). Classically, aromatase has been implicated in the control of reproductive status, sexual development, neuroendocrine function, and sexual behavior. As such, the predominant center of aromatization is within limbic neural circuits that regulate these functions. The regulation of aromatase within these circuits is complex and not uniform in all brain regions. Evidence exists for post-transcriptional control, but sex steroids exert the major transcriptional control. Brain-derived estrogen is necessary for the effects that testosterone exerts on sexual differentiation, gonadotropin secretion and male-typical reproductive behavior, however, its role varies among species playing more essential roles in rodents than in primates. More recently, brain aromatase has been shown to play unanticipated roles in neurogenesis, neural plasticity, neuroprotection, and repair suggesting that local estrogen synthesis has broader roles in brain function than previously appreciated. These exciting new observations hold the promise that someday pharmacologic regulation of brain aromatase could offer therapeutic opportunities for the treatment neurologic disorders and cerebrovascular disease.
Figure 1.
Summary of classical reproductive and novel nonreproductive actions of brain aromatase. Under physiological conditions aromatase is predominantly expressed in mammalian neurons and radial glia, but under pathological conditions can be expressed in astroglia. Locally formed estrogen acts to alter synaptic activity, stimulate neuronal connectivity, and enhance cell survival. Acting through these mechanisms, brain aromatase affects development, reproduction, behavior, and repair.
Acknowledgments
Support for this research was provided by NIH grants RR14270, NS049210, and American Heart Association grant 0535284N.
References
- 1.Naftolin F, Ryan KJ, Davies IJ, et al. The formation of estrogens by central neuroendocrine tissues. Rec Prog Horm Res. 1975;31:295–319. doi: 10.1016/b978-0-12-571131-9.50012-8. [DOI] [PubMed] [Google Scholar]
- 2.Naftolin F. Brain aromatization of androgens. J Rep Med. 1994;39:257–261. [PubMed] [Google Scholar]
- 3.Lephart ED. A review of brain aromatase cytochrome P450. Brain Res Rev. 1996;22:1–26. [PubMed] [Google Scholar]
- 4.Roselli CE, Abdelgadir SE, Ronnekleiv OK, Klosterman SA. Anatomic distribution and regulation of aromatase gene expression in the rat brain. Biol Reprod. 1998;58:79–87. doi: 10.1095/biolreprod58.1.79. [DOI] [PubMed] [Google Scholar]
- 5.Naftolin F, Ryan KJ, Davies IJ, et al. The formation of estrogens by central neuroendocrine tissues. Rec Prog Horm Res. 1974;31:295–319. doi: 10.1016/b978-0-12-571131-9.50012-8. [DOI] [PubMed] [Google Scholar]
- 6.Abdelgadir SE, Resko JA, Ojeda SR, Lephart ED, McPhaul MJ, Roselli CE. Androgens regulate aromatase cytochrome P450 messenger ribonucleic acid in rat brain. Endocrinology. 1994;135:395–401. doi: 10.1210/endo.135.1.8013375. [DOI] [PubMed] [Google Scholar]
- 7.Roselli CE, Horton LE, Resko JA. Distribution and regulation of aromatase activity in the rat hypothalamus and limbic system. Endocrinology. 1985;117:2471–2477. doi: 10.1210/endo-117-6-2471. [DOI] [PubMed] [Google Scholar]
- 8.Simerly RB, Chang C, Muramatsu M, Swanson LW. The distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: An in situ hybridization study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- 9.Hojo Y, Hattori TA, Enami T, et al. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017α and P450 aromatase localized in neurons. Proc Natl Acad Sci U S A. 2004;101:865–870. doi: 10.1073/pnas.2630225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacLusky NJ, Walters MJ, Clark AS, Toran-Allerand CD. Aromatase in the cerebral cortex, hippocampus, and mid-brain: ontogeny and developmental implications. Mol Cell Neurosci. 1994;5:691–698. doi: 10.1006/mcne.1994.1083. [DOI] [PubMed] [Google Scholar]
- 11.Evrard HC. Estrogen synthesis in the spinal dorsal horn: a new central mechanism for the hormonal regulation of pain. Am J Physiol Regul Integr Comp Physiol. 2006;291(2):R291–R299. doi: 10.1152/ajpregu.00930.2005. [DOI] [PubMed] [Google Scholar]
- 12.Stoffel-Wagner B, Watzka M, Schramm J, Bidlingmaier F, Klingmuller D. Expression of CYP19 (aromatase) mRNA in different areas of the human brain. J Steroid Biochem Mol Biol. 1999;70(4–6):237–241. doi: 10.1016/s0960-0760(99)00114-4. [DOI] [PubMed] [Google Scholar]
- 13.Horvath TL, Wikler KC. Aromatase in developing sensory systems of the rat brain. J Neuroendocrinol. 1999;11:77–84. doi: 10.1046/j.1365-2826.1999.00285.x. [DOI] [PubMed] [Google Scholar]
- 14.Lavaque E, Mayen A, Azcoitia I, Tena-Sempere M, Garcia-Segura LM. Sex differences, developmental changes, response to injury and cAMP regulation of the mRNA levels of steroidogenic acute regulatory protein, cytochrome p450scc, and aromatase in the olivocerebellar system. J Neurobiol. 2006;66(3):308–318. doi: 10.1002/neu.20221. [DOI] [PubMed] [Google Scholar]
- 15.Kretz O, Fester L, Wehrenberg U, et al. Hippocampal Synapses Depend on Hippocampal Estrogen Synthesis. J Neurosci. 2004;24(26):5913–5921. doi: 10.1523/JNEUROSCI.5186-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanapat P, Hastings NB, Gould E. Ovarian steroids influence cell proliferation in the dentate gyrus of the adult female rat in a dose- and time-dependent manner. J Comp Neurol. 2005;481(3):252–265. doi: 10.1002/cne.20385. [DOI] [PubMed] [Google Scholar]
- 17.Naftolin F, Horvath TL, Jakab RL, Leranth C, Harada N, Balthazart J. Aromatase immunoreactivity in axon terminals of the vertebrate brain - An immunocytochemical study on quail, rat, monkey and human tissues. Neuroendocrinology. 1996;63(2):149–155. doi: 10.1159/000126951. [DOI] [PubMed] [Google Scholar]
- 18.Peterson RS, Yarram L, Schlinger BA, Saldanha CJ. Aromatase is pre-synaptic and sexually dimorphic in the adult zebra finch brain. Proc R Soc Lond B Biol Sci. 2005;272(1576):2089–2096. doi: 10.1098/rspb.2005.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veney SL, Rissman EF. Co-localization of estrogen receptor and aromatase enzyme immunoreactivities in adult musk shrew brain. Horm Behav. 1998;33:151–162. doi: 10.1006/hbeh.1998.1446. [DOI] [PubMed] [Google Scholar]
- 20.Tsuruo Y, Ishimura K, Hayashi S, Osawa Y. Immunohistochemical localization of estrogen receptors within aromatase-ummunoreactive neurons in the fetal and neonatal rat brain. Anat Embryol. 1996;193:115–121. doi: 10.1007/BF00214702. [DOI] [PubMed] [Google Scholar]
- 21.Balthazart J, Foidart A, Surlemont C, Harada N. Neuroanatomical specificity in the co-localization of aromatase and estrogen receptors. J Neurobiol. 1991;22:143–157. doi: 10.1002/neu.480220205. [DOI] [PubMed] [Google Scholar]
- 22.Ronnekleiv OK, Malyala A, Kelly MJ. Membrane-initiated signaling of estrogen in the brain. Semin Reprod Med. 2007;25(3):165–177. doi: 10.1055/s-2007-973429. [DOI] [PubMed] [Google Scholar]
- 23.Balthazart J, Ball GF. Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci. 2006;29(5):241–249. doi: 10.1016/j.tins.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Martinez-Cerdeno V, Noctor SC, Kriegstein AR. Estradiol stimulates progenitor cell division in the ventricular and subventricular zones of the embryonic neocortex. Eur J Neurosci. 2006;24(12):3475–3488. doi: 10.1111/j.1460-9568.2006.05239.x. [DOI] [PubMed] [Google Scholar]
- 25.Götz M, Barde YA. Radial Glial Cells: Defined and major intermediates between embryonic stem cells and CNS neurons. Neuron. 2005;46(3):369–372. doi: 10.1016/j.neuron.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 26.Mouriec K, Pellegrini E, Anglade I, et al. Synthesis of estrogens in progenitor cells of adult fish brain: Evolutive novelty or exaggeration of a more general mechanism implicating estrogens in neurogenesis? Brain Res Bull. 2008;75(2–4):274–280. doi: 10.1016/j.brainresbull.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 27.Yague JG, Munoz A, Monasterio-Schrader P, DeFelipe J, Garcia-Segura LM, Azcoitia I. Aromatase expression in the human temporal cortex. Neurosci. 2006;138(2):389–401. doi: 10.1016/j.neuroscience.2005.11.054. [DOI] [PubMed] [Google Scholar]
- 28.Yague JG, Wang ACJ, Janssen WGM, et al. Aromatase distribution in the monkey temporal neocortex and hippocampus. Brain Res. 2008;1209:115–127. doi: 10.1016/j.brainres.2008.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia-Segura LM, Wozniak A, Azcoitia I, Rodriguez JR, Hutchison RE, Hutchison JB. Aromatase expression by astrocytes after brain injury: Implications for local estrogen formation in brain repair. Neurosci. 1999;89:567–578. doi: 10.1016/s0306-4522(98)00340-6. [DOI] [PubMed] [Google Scholar]
- 30.Peterson RS, Saldanha CJ, Schlinger BA. Rapid upregulation of aromatase mRNA and protein following neural injury in the zebra finch (Taeniopygia guttata) J Neuroendocrinol. 2001;13(4):317–323. doi: 10.1046/j.1365-2826.2001.00647.x. [DOI] [PubMed] [Google Scholar]
- 31.Carswell HVO, Dominiczak AF, Garcia-Segura LM, Harada N, Hutchison JB, Macrae IM. Brain aromatase expression after experimental stroke: Topography and time course. J Steroid Biochem Mol Biol. 2005;96:89–96. doi: 10.1016/j.jsbmb.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 32.McCullough LD, Blizzard K, Simpson ER, Oz OK, Hurn PD. Aromatase cytochrome P450 and extragonadal estrogen play a role in ischemic neuroprotection. J Neurosci. 2003;23(25):8701–8705. doi: 10.1523/JNEUROSCI.23-25-08701.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wynne RD, Walters BJ, Bailey DJ, Saldanha CJ. Inhibition of injury-induced glial aromatase reveals a wave of secondary degeneration in the songbird brain. Glia. 2008;56(1):97–105. doi: 10.1002/glia.20594. [DOI] [PubMed] [Google Scholar]
- 34.Hoyk Z, Parducz A, Garcia-Segura LM. Dehydroepiandrosterone regulates astroglia reaction to denervation of olfactory glomeruli. Glia. 2004;48(3):207–216. doi: 10.1002/glia.20070. [DOI] [PubMed] [Google Scholar]
- 35.Azcoitia I, Sierra AE, Garcia-Segura LM. Aromatase expression by reactive astroglia Is neuroprotective. Ann N Y Acad Sci. 2003;1007(1):298–305. doi: 10.1196/annals.1286.028. [DOI] [PubMed] [Google Scholar]
- 36.Garcia-Segura LM, Veiga S, Sierra A, Melcangi RC, Azcoitia I. Aromatase: a neuroprotective enzyme. Prog Neurobiol. 2003;71(1):31–41. doi: 10.1016/j.pneurobio.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 37.Wynne RD, Saldanha CJ. Glial aromatization decreases neural injury in the zebra finch (Taeniopygia guttata): influence on apoptosis. J Neuroendocrinol. 2004;16(8):676–683. doi: 10.1111/j.1365-2826.2004.01217.x. [DOI] [PubMed] [Google Scholar]
- 38.Balthazart J, Ball GF. New insights into the regulation and function of brain estrogen synthetase (aromatase) Trends Neurosci. 1998;21:243–249. doi: 10.1016/s0166-2236(97)01221-6. [DOI] [PubMed] [Google Scholar]
- 39.MacLusky NJ, Naftolin F. Sexual differentiation of the central nervous system. Science. 1981;211:1294–1302. doi: 10.1126/science.6163211. [DOI] [PubMed] [Google Scholar]
- 40.Lauber ME, Lichtensteiger W. Pre- and postnatal ontogeny of aromatase cytochrome P450 messenger ribonucleic acid expression in the male rat brain studied by in situ hybridization. Endocrinology. 1994;135:1661–1668. doi: 10.1210/endo.135.4.7925130. [DOI] [PubMed] [Google Scholar]
- 41.Zhao C, Fujinaga R, Tanaka M, Yanai A, Nakahama K, Shinoda K. Region-specific expression and sex-steroidal regulation on aromatase and its mRNA in the male rat brain: immunohistochemical and in situ hybridization analyses. J Comp Neurol. 2007;500(3):557–573. doi: 10.1002/cne.21193. [DOI] [PubMed] [Google Scholar]
- 42.Connolly PB, Roselli CE, Resko JA. Aromatase activity in developing guinea pig brain: Ontogeny and effects of exogenous androgens. Biol Reprod. 1994;50:436–441. doi: 10.1095/biolreprod50.2.436. [DOI] [PubMed] [Google Scholar]
- 43.Roselli CE, Resko JA. Effects of gonadectomy and androgen treatment on aromatase acitivity in the fetal monkey brain. Biol Reprod. 1986;35:106–112. doi: 10.1095/biolreprod35.1.106. [DOI] [PubMed] [Google Scholar]
- 44.Roselli CE, Resko JA, Stormshak F. Estrogen synthesis in fetal sheep brain: effect of maternal treatment with an aromatase inhibitor. Biol Reprod. 2003;68(2):370–374. doi: 10.1095/biolreprod.102.007633. [DOI] [PubMed] [Google Scholar]
- 45.Peruffo A, Cozzi B, Ballarin C. Ontogenesis of brain aromatase P450 expression in the bovine hypothalamus. Brain Res Bull. 2008;75(1):60–65. doi: 10.1016/j.brainresbull.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 46.Wallen K, Baum MJ. Masculinization and defeminization in altricial and precocial mammals: comparative aspects of steroid hormone action. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. San Diego: Elsevier Science (USA); 2002. pp. 385–423. [Google Scholar]
- 47.Forger NG, Rosen GJ, Waters EM, Jacob D, Simerly RB, de Vries GJ. Deletion of Bax eliminates sex differences in the mouse forebrain. Proc Natl Acad Sci USA. 2004;101(37):13666–13671. doi: 10.1073/pnas.0404644101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Toran-Allerand CD. Sex steroids and the development of the newborn mouse hypothalamus and preoptic area in vitro: implication for sexual differentiation. Brain Res. 1976;106:407–412. doi: 10.1016/0006-8993(76)91038-6. [DOI] [PubMed] [Google Scholar]
- 49.Roselli CE, Resko JA. Sex differences in adnrogen-regulated expression of cytochrome P450 aromatase in the rat brain. J Steroid Biochem Mol Biol. 1997;61:365–374. [PubMed] [Google Scholar]
- 50.Balthazart J, Baillien M, Charlier TD, Cornil CA, Ball GF. Multiple mechanisms control brain aromatase activity at the genomic and non-genomic level. J Steroid Biochem Mol Biol. 2003;86(3–5):367–379. doi: 10.1016/s0960-0760(03)00346-7. [DOI] [PubMed] [Google Scholar]
- 51.Callard GV, Tchoudakova AV, Kishida M, Wood E. Differential tissue distribution, developmental programming, estrogen regulation and promoter characteristics of cyp19 genes in teleost fish. J Steroid Biochem Mol Biol. 2001;79(1–5):305–314. doi: 10.1016/s0960-0760(01)00147-9. [DOI] [PubMed] [Google Scholar]
- 52.Roselli CE, Resko JA. Cytochrome P450 aromatase (CYP19) in the non-human primate brain: distribution, regulation, and functional significance. J Steroid Biochem Mol Biol. 2001;79(1–5):247–253. doi: 10.1016/s0960-0760(01)00141-8. [DOI] [PubMed] [Google Scholar]
- 53.Veney SL, Rissman EF. Immunolocalization of androgen receptors and aromatase enzyme in the adult musk shrew brain. Neuroendocrinology. 2000;72(1):29–36. doi: 10.1159/000054568. [DOI] [PubMed] [Google Scholar]
- 54.Honda S, Harada N, Takagi Y. Novel exon 1 of the aromatase gene specific for aromatase transcripts in human brain. Biochem Biophys Res Commun. 1994;198:1153–1160. doi: 10.1006/bbrc.1994.1163. [DOI] [PubMed] [Google Scholar]
- 55.Reddy VVR, Naftolin F, Ryan KJ. Aromatization in the central nervous system of rabbits: Effects of castration and hormone treatment. Endocrinology. 1973;92(2):589–594. doi: 10.1210/endo-92-2-589. [DOI] [PubMed] [Google Scholar]
- 56.Roselli CE. Synergistic induction of aromatase activity in the rat brain by estradiol and 5 -dihydrotestosterone. Neuroendocrinology. 1991;53:79–84. doi: 10.1159/000125701. [DOI] [PubMed] [Google Scholar]
- 57.Gardner L, Anderson T, Place AR, Dixon B, Elizur A. Sex change strategy and the aromatase genes. J Steroid Biochem Mol Biol. 2005;94(5):395–404. doi: 10.1016/j.jsbmb.2004.12.045. [DOI] [PubMed] [Google Scholar]
- 58.Bulun SE, Sebastian S, Takayama K, Suzuki T, Sasano H, Shozu M. The human CYP19 (aromatase P450) gene: update on physiologic roles and genomic organization of promoters. J Steroid Biochem Mol Biol. 2003;86(3–5):219–224. doi: 10.1016/s0960-0760(03)00359-5. [DOI] [PubMed] [Google Scholar]
- 59.Sasano H, Takahashi K, Satoh F, Nagura H, Harada N. Aromatase in the human central nervous system. Clin Endocrinol. 1998;48:325–329. doi: 10.1046/j.1365-2265.1998.00390.x. [DOI] [PubMed] [Google Scholar]
- 60.Kato J, Yamada-Mouri N, Hirata S. Structure of aromatase mRNA in the rat brain. J Steroid Biochem Molec Biol. 1997;61:381–385. [PubMed] [Google Scholar]
- 61.Cornil CA, Ball GF, Balthazart J. Functional significance of the rapid regulation of brain estrogen action: where do the estrogens come from? Brain Res. 2006;1126(1):2–26. doi: 10.1016/j.brainres.2006.07.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cooke B, Hegstrom CD, Villeneuve LS, Breedlove SM. Sexual differentiation of the vertebrate brain: principles and mechanisms. Front Neuroendocrinol. 1998;19:253–286. doi: 10.1006/frne.1998.0171. [DOI] [PubMed] [Google Scholar]
- 63.Bakker J, Baum MJ. Role for estradiol in female-typical brain and behavioral sexual differentiation. Front Neuroendocrinol. 2008;29(1):1–16. doi: 10.1016/j.yfrne.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Karsch FJ, Dierschke DJ, Knobil E. Sexual differentiation of pituitary function: apparent difference between primates and rodents. Science. 1973;179:484–486. doi: 10.1126/science.179.4072.484. [DOI] [PubMed] [Google Scholar]
- 65.McEwen BS, Lieberburg I, Chaptal C, Krey LC. Aromatization: important for sexual differentiation of the neonatal rat brain. Horm Behav. 1977;9:249–263. doi: 10.1016/0018-506x(77)90060-5. [DOI] [PubMed] [Google Scholar]
- 66.Vreeburg JTM, van der Vaart PDM, Van Der Schoot P. Prevention of central defeminization but not masculinization in male rats by inhibition neonatally of oestrogen biosynthesis. J Endocr. 1977;74:375–382. doi: 10.1677/joe.0.0740375. [DOI] [PubMed] [Google Scholar]
- 67.Baum MJ, Carroll RS, Tobet SA. Steroidal control of behavioural, neuroendocrine and brain sexual differentiation: studies in a carnivore, the ferret. J Neuroendocrinol. 1990;2:401–418. doi: 10.1111/j.1365-2826.1990.tb00425.x. [DOI] [PubMed] [Google Scholar]
- 68.Roselli CE, Schrunk JM, Stadelman HL, Resko JA, Stormshak F. The effect of aromatase inhibition on the sexual differentiation of the sheep brain. Endocrine. 2006;29(3):501–512. doi: 10.1385/ENDO:29:3:501. [DOI] [PubMed] [Google Scholar]
- 69.Gorski RA, Gordon JH, Shryne JE, Southam AM. Evidence for a morphological sex difference within the medial preoptic area or the rat brain. Brain Res. 1978;148:333–346. doi: 10.1016/0006-8993(78)90723-0. [DOI] [PubMed] [Google Scholar]
- 70.Dohler KD, Coquelin A, Davis F, Hines M, Shryne JE, Gorski RA. Pre- and postnatal influence of testosterone propionate and diethylstilbestrol on differentiation of the sexually dimorphic nucleus of the preoptic area in male and female rats. Brain Res. 1984;302:291–295. doi: 10.1016/0006-8993(84)90242-7. [DOI] [PubMed] [Google Scholar]
- 71.Houtsmuller EJ, Brand T, De Jonge FH, Joosten RNJMA, Van De Poll NE, Slob AK. SDN-POA volume, sexual behavior, and partner preference of male rats affected by perinatal treatment with ATD. Physiol Behav. 1994;56:535–541. doi: 10.1016/0031-9384(94)90298-4. [DOI] [PubMed] [Google Scholar]
- 72.Schwarz JM, McCarthy MM. Cellular mechanisms of estradiol-mediated masculinization of the brain. J Steroid Biochem Mol Biol. 2008;109(3–5):300–306. doi: 10.1016/j.jsbmb.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Simerly RB. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Ann Rev Neurosci. 2002;25(1):507–536. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- 74.Arendash GW, Gorski RA. Effects of discrete lesions of the sexually dimorphic nucleus of the preoptic area or other medial preoptic regions on the sexual behavior of male rats. Brain Res Bull. 1983;10:147–154. doi: 10.1016/0361-9230(83)90086-2. [DOI] [PubMed] [Google Scholar]
- 75.De Jonge FH, Louwerse AL, Ooms MP, Evers P, Endert E, Van De Poll NE. Lesions of the SDN-POA inhibit sexual behavior of male Wistar rats. Brain Res Bull. 1989;23:483–492. doi: 10.1016/0361-9230(89)90194-9. [DOI] [PubMed] [Google Scholar]
- 76.Paredes RG, Baum MJ. Altered sexual partner preference in male ferrets given excitotoxic lesions of the preoptic area anterior hypothalamus. J Neurosci. 1995;15:6619–6630. doi: 10.1523/JNEUROSCI.15-10-06619.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Paredes RG, Nakagawa Y, Nakach N. Lesions of the medial preoptic area/anterior hypothalamus (MPOA/AH) modify partner preference in male rats. Brain Res. 1998;813:1–8. doi: 10.1016/s0006-8993(98)00914-7. [DOI] [PubMed] [Google Scholar]
- 78.Vasey PL. Same-sex sexual partner preference in hormonally and neurologically unmanipulated animals. Annu Rev Sex Res. 2002;13:141–179. [PubMed] [Google Scholar]
- 79.Perkins A, Roselli CE. The ram as a model for behavioral neuroendocrinology. Horm Behav. 2007;52(1):70–77. doi: 10.1016/j.yhbeh.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Roselli CE, Larkin K, Resko JA, Stellflug JN, Stormshak F. The volume of a sexually dimorphic nucleus in the ovine medial preoptic area/anterior hypothalamus varies with sexual partner preference. Endocrinology. 2004;145:478–483. doi: 10.1210/en.2003-1098. [DOI] [PubMed] [Google Scholar]
- 81.Roselli CE, Stadelman H, Reeve R, Bishop CV, Stormshak F. The ovine sexually dimorphic nucleus of the medial preoptic area is organized prenatally by testosterone. Endocrinology. 2007;148(9):4450–4457. doi: 10.1210/en.2007-0454. [DOI] [PubMed] [Google Scholar]
- 82.LeVay S. A difference in hypothalamic structure between heterosexual and homosexual men. Science. 1991;253:1034–1037. doi: 10.1126/science.1887219. [DOI] [PubMed] [Google Scholar]
- 83.Adkins-Regan E, Mansukhani V, Thompson R, Yang S. Organizational actions of sex hormones on sexual partner preference. Brain Res Bull. 1997;44(4):497–502. doi: 10.1016/s0361-9230(97)00231-1. [DOI] [PubMed] [Google Scholar]
- 84.Brand T, Kroonen J, Mos J, Slob AK. Adult partner preference and sexual behavior of male rats affected by perinatal endocrine manipulations. Horm Behav. 1991;25:323–341. doi: 10.1016/0018-506x(91)90005-3. [DOI] [PubMed] [Google Scholar]
- 85.Bakker J, Honda S, Harada N, Balthazart J. Sexual partner preference requires a functional aromatase (Cyp 19) gene in male mice. Horm Behav. 2002;42:158–171. doi: 10.1006/hbeh.2002.1805. [DOI] [PubMed] [Google Scholar]
- 86.Resko JA, Perkins A, Roselli CE, Fitzgerald JA, Choate JVA, Stormshak F. Endocrine correlates of partner preference behavior in rams. Biol Reprod. 1996;55:120–126. doi: 10.1095/biolreprod55.1.120. [DOI] [PubMed] [Google Scholar]
- 87.Carani C, Qin K, Simoni M, et al. Effect of testosterone and estradiol in a man with aromatase deficiency. N Engl J Med. 1997;337:91–95. doi: 10.1056/NEJM199707103370204. [DOI] [PubMed] [Google Scholar]
- 88.Carani C, Granata ARM, Rochira V, et al. Sex steroids and sexual desire in a man with a novel mutation of aromatase gene and hypogonadism. Psychoneuroendocrinol. 2005;30(5):413–417. doi: 10.1016/j.psyneuen.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 89.DuPree MG, Mustanski BS, Bocklandt S, Nievergelt C, Hamer DH. A candidate gene study of CYP19 (aromatase) and male sexual orientation. Behav Genet. 2004;34(3):243–250. doi: 10.1023/B:BEGE.0000017870.77610.52. [DOI] [PubMed] [Google Scholar]
- 90.Hull EM, Meisel RL, Sachs BD. Male Sexual Behavior. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. San Diego: Academic Press; 2002. pp. 3–137. [Google Scholar]
- 91.Sodersten P. Estrogen-activated sexual behavior in male rats. Horm Behav. 1973;4:247–256. doi: 10.1016/0018-506x(73)90009-3. [DOI] [PubMed] [Google Scholar]
- 92.Bonsall RW, Clancy AN, Michael RP. Effects of the nonsteroidal aromatase inhibitor, Fadrozole, on sexual behavior in male rats. Horm Behav. 1992;26:240–254. doi: 10.1016/0018-506x(92)90045-w. [DOI] [PubMed] [Google Scholar]
- 93.Vagell ME, McGinnis MY. The role of aromatization in the restoration of male rat reproductive behavior. J Neuroendocrinol. 1997;9:415–421. doi: 10.1046/j.1365-2826.1997.00598.x. [DOI] [PubMed] [Google Scholar]
- 94.Beyer C, Morali G, Naftolin F, Larsson K, Perez-Palacios G. Effect of some antiestrogens and aromatase inhibitors on androgen induced sexual behavior in castrated male rats. Horm Behav. 1976;7:353–363. doi: 10.1016/0018-506x(76)90040-4. [DOI] [PubMed] [Google Scholar]
- 95.Roselli CE, Cross E, Poonyagariyagorn HK, Stadelman HL. Role of aromatization in anticipatory and consummatory aspects of sexual behavior in male rats. Horm Behav. 2003;44(2):146–151. doi: 10.1016/s0018-506x(03)00123-5. [DOI] [PubMed] [Google Scholar]
- 96.Fisher CR, Graves KH, Parlow AF, Simpson ER. Characterization of mice deficient in aromatase (ArKO) because of targeted disruption of the cyp 19 gene. Proc Natl Acad Sci USA. 1998;95:6965–6970. doi: 10.1073/pnas.95.12.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Honda S, Harada N, Ito S, Takagi Y, Maeda S. Disruption of sexual behavior in male aromatase-deficient mice lacking exons 1 and 2 of the cyp19 gene. Biochem Biophys Res Commun. 1998;252:445–449. doi: 10.1006/bbrc.1998.9672. [DOI] [PubMed] [Google Scholar]
- 98.Toda K, Okada T, Takeda K, et al. Oestrogen at the neonatal stage is critical for the reproductive ability of male mice as revealed by supplementation with 17beta-oestradiol to aromatase gene (Cyp19) knockout mice. J Endocrinol. 2001;168(3):455–463. doi: 10.1677/joe.0.1680455. [DOI] [PubMed] [Google Scholar]
- 99.Matsumoto T, Honda S, Harada N. Alteration in sex-specific behaviors in male mice lacking the aromatase gene. Neuroendocrinology. 2003;77(6):416–424. doi: 10.1159/000071313. [DOI] [PubMed] [Google Scholar]
- 100.Bakker J, Honda S, Harada N, Balthazart J. Restoration of male sexual behavior by adult exogenous estrogens in male aromatase knockout mice. Horm Behav. 2004;46:1–10. doi: 10.1016/j.yhbeh.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 101.Alsum P, Goy RW. Actions of esters of testosterone, dihydrotestosterone, or estradiol on sexual behavior in castrated male guinea pigs. Horm Behav. 1974;5:207–217. doi: 10.1016/0018-506x(74)90029-4. [DOI] [PubMed] [Google Scholar]
- 102.Phoenix CH. Effect of dihydrotestosterone on sexual behavior of castrated male rhesus monkeys. Physiol Behav. 1974;12:1045–1055. doi: 10.1016/0031-9384(74)90153-x. [DOI] [PubMed] [Google Scholar]
- 103.Zumpe D, Clancy AN, Bonsall RB, Michael RP. Behavioral responses to depo-provera, fadrozole, and estradiol in castrated, testosterone-treated cynomolgus monkeys (Macaca fascicularis): The involvement of progestin receptors. Physiol Behav. 1996;60:531–540. doi: 10.1016/s0031-9384(96)80028-x. [DOI] [PubMed] [Google Scholar]
- 104.Gooren LJ. Human male sexual functions do not require aromatization of testosterone: a study using tamoxifen, testolactone, and dihydrotestosterone. Arch Sexual Behav. 1985;14:539–548. doi: 10.1007/BF01541754. [DOI] [PubMed] [Google Scholar]
- 105.Sharma TP, Blache D, Blackberry MA, Martin GB. Role of peripheral and central aromatization in the control of gonadotrophin secretion in the male sheep. Reprod Fertil Dev. 1999;11(4–5):293–302. doi: 10.1071/rd99084. [DOI] [PubMed] [Google Scholar]
- 106.Hayes FJ, Seminara SB, Decruz S, Boepple PA, Crowley WF., Jr Aromatase inhibition in the human male reveals a hypothalamic site of estrogen feedback [In Process Citation] J Clin Endocrinol Metab. 2000;85(9):3027–3035. doi: 10.1210/jcem.85.9.6795. [DOI] [PubMed] [Google Scholar]
- 107.Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20(3):358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- 108.Jones MEE, Chin BW, Proietto J, Simpson ER. Of mice and men: the evolving phenotype of aromatase deficiency. Trends Endocrinol Metab. 2006;17(2):55–64. doi: 10.1016/j.tem.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 109.Ellinwood WE, Hess DL, Roselli CE, Spies HG, Resko JA. Inhibition of aromatization stimulates luteinizing hormone and testosterone secretion in adult male rhesus monkeys. J Clin Endocrinol Metab. 1984;59:1088–1096. doi: 10.1210/jcem-59-6-1088. [DOI] [PubMed] [Google Scholar]
- 110.Roselli CE, Stadelman H, Horton LE, Resko JA. Regulation of androgen metabolism and luteinizing hormone-releasing hormone content in discrete hypothalamic and limbic areas of male rhesus macaques. Endocrinology. 1987;120:97–106. doi: 10.1210/endo-120-1-97. [DOI] [PubMed] [Google Scholar]
- 111.Schnorr JA, Bray MJ, Veldhuis JD. Aromatization mediates testosterone’s short-term feedback restraint of 24-hour endogenously driven and acute exogenous gonadotropin-releasing hormone-stimulated luteinizing hormone and follicle-stimulating hormone secretion in young men. J Clin Endocrinol Metab. 2001;86(6):2600–2606. doi: 10.1210/jcem.86.6.7520. [DOI] [PubMed] [Google Scholar]
- 112.Bagatell CJ, Dahl KD, Bremner WJ. The direct pituitary effect of testosterone to inhibit gonadotropin secretion in men is partially mediated by aromatization to estradiol. J Andr. 1994;15:15–21. [PubMed] [Google Scholar]
- 113.Boyar RM, Moore RJ, Rosner W, et al. Studies of gonadotropin-gonadal dynamics in patients with androgen insensitivity. J Clin Endocrinol Metab. 1978;47:1116–1122. doi: 10.1210/jcem-47-5-1116. [DOI] [PubMed] [Google Scholar]
- 114.Veldhuis JD, Urban RJ, Dufau ML. Evidence that androgen negative feedback regulates hypothalamic gonadotropin-releasing hormone impulse strength and the burst-like secretion of biologically active luteinizing hormone in men. J Clin Endocrinol Metab. 1992;74:1227–1235. doi: 10.1210/jcem.74.6.1592863. [DOI] [PubMed] [Google Scholar]
- 115.Pitteloud N, Dwyer AA, Decruz S, et al. Inhibition of luteinizing hormone secretion by testosterone in men requires aromatization for its pituitary but not its hypothalamic effects: evidence from the tandem study of normal and gonadotropin-releasing hormone-deficient men. J Clin Endocrinol Metab. 2008;93(3):784–791. doi: 10.1210/jc.2007-2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hall ED, Pazara KE, Linseman KL. Sex differences in postischemic neuronal necrosis in gerbils. J Cerebral Blood Flow Metab. 1991;11:292–298. doi: 10.1038/jcbfm.1991.61. [DOI] [PubMed] [Google Scholar]
- 117.Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD. Gender-linked brain injury in experimental stroke. Stroke. 1998;29:159–166. doi: 10.1161/01.str.29.1.159. [DOI] [PubMed] [Google Scholar]
- 118.Alkayed NJ, Murphy SJ, Traystman RJ, Hurn PD. Neuroprotective effects of female gonadal steroids in reproductively senescent female rats. Stroke. 2000;31(1):161–168. doi: 10.1161/01.str.31.1.161. [DOI] [PubMed] [Google Scholar]
- 119.Carswell HV, Anderson NH, Clark JS, et al. Genetic and gender influences on sensitivity to focal cerebral ischemia in the stroke-prone spontaneously hypertensive rat. Hypertension. 1999;33(2):681–685. doi: 10.1161/01.hyp.33.2.681. [DOI] [PubMed] [Google Scholar]
- 120.McCullough LD, Blizzard K, Simpson ER, Oz OK, Hurn PD. Aromatase cytochrome P450 and extragonadal estrogen play a role in ischemic neuroprotection. J Neurosci. 2003;23(25):8701–8705. doi: 10.1523/JNEUROSCI.23-25-08701.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li X, Blizzard KK, Zeng Z, DeVries AC, Hurn PD, McCullough LD. Chronic behavioral testing after focal ischemia in the mouse: functional recovery and the effects of gender. Exp Neurol. 2004;187(1):94–104. doi: 10.1016/j.expneurol.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 122.Bramlett HM, Dietrich WD. Neuropathological protection after traumatic brain injury in intact female rats versus males or ovariectomized females. J Neurotrauma. 2001;18(9):891–900. doi: 10.1089/089771501750451811. [DOI] [PubMed] [Google Scholar]
- 123.Hurn PD, Macrae IM. Estrogen as a neuroprotectant in stroke. J Cereb Blood Flow Metab. 2000;20(4):631–652. doi: 10.1097/00004647-200004000-00001. [DOI] [PubMed] [Google Scholar]
- 124.Morrison JH, Brinton RD, Schmidt PJ, Gore AC. Estrogen, menopause, and the aging brain: how basic neuroscience can inform hormone therapy in women. J Neurosci. 2006;26(41):10332–10348. doi: 10.1523/JNEUROSCI.3369-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bryant DN, Sheldahl LC, Marriott LK, Shapiro RA, Dorsa DM. Multiple pathways transmit neuroprotective effects of gonadal steroids. Endocrine. 2006;29(2):199–207. doi: 10.1385/ENDO:29:2:199. [DOI] [PubMed] [Google Scholar]
- 126.Vagnerova K, Koerner IP, Hurn PD. Gender and the injured brain. Anesth Analg. 2008;107(1):201–214. doi: 10.1213/ane.0b013e31817326a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Azcoitia I, Sierra A, Veiga S, Honda S, Harada N, Garcia-Segura LM. Brain aromatase is neuroprotective. J Neurobiol. 2001;47(4):318–329. doi: 10.1002/neu.1038. [DOI] [PubMed] [Google Scholar]
- 128.Garcia-Segura LM, Azcoitia I, DonCarlos LL. Neuroprotection by estradiol. Prog Neurobiol. 2001;63(1):29–60. doi: 10.1016/s0301-0082(00)00025-3. [DOI] [PubMed] [Google Scholar]
- 129.Veiga S, Azcoitia I, Garcia-Segura LM. Extragonadal synthesis of estradiol is protective against kainic acid excitotoxic damage to the hippocampus. NeuroReport. 2005;16(14):1599–1603. doi: 10.1097/01.wnr.0000179081.39659.7d. [DOI] [PubMed] [Google Scholar]
- 130.Garcia-Ovejero D, Veiga S, Garcia-Segura LM, DonCarlos LL. Glial expression of estrogen and androgen receptors after rat brain injury. J Comp Neurol. 2002;450(3):256–271. doi: 10.1002/cne.10325. [DOI] [PubMed] [Google Scholar]
- 131.Jones MEE, Thornburn AW, Britt KL, et al. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc Natl Acad Sci U S A. 2000;97:12735–12740. doi: 10.1073/pnas.97.23.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Simpson ER, Clyne C, Rubin G, et al. Aromatase--a brief overview. Annu Rev Physiol. 2002;64:93–127. doi: 10.1146/annurev.physiol.64.081601.142703. [DOI] [PubMed] [Google Scholar]
- 133.Oz OK, Zerwekh JE, Fisher C, et al. Bone has a sexually dimorphic response to aromatase deficiency. J Bone Miner Res. 2000;15(3):507–514. doi: 10.1359/jbmr.2000.15.3.507. [DOI] [PubMed] [Google Scholar]
- 134.Liu M, Hurn PD, Roselli CE, Alkayed NJ. Role of P450 aromatase in sex-specific astrocytic cell death. J Cereb Blood Flow Metab. 2007;27(1):135–141. doi: 10.1038/sj.jcbfm.9600331. [DOI] [PubMed] [Google Scholar]
- 135.Liu M, Oyarzabal EA, Yang R, Murphy SJ, Hurn PD. A novel method for assessing sex-specific and genotype-specific response to injury in astrocyte culture. J Neurosci Meth. 2008;171(2):214–217. doi: 10.1016/j.jneumeth.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Toung TK, Hurn PD, Traystman RJ, Sieber FE, Faraci FM. Estrogen decreases infarct size after temporary focal ischemia in a genetic model of type 1 diabetes mellitus: editorial comment. Stroke. 2000;31(11):2701–2706. doi: 10.1161/01.str.31.11.2701. [DOI] [PubMed] [Google Scholar]
- 137.Vannucci SJ, Willing LB, Goto S, et al. Experimental stroke in the female diabetic, db/db, mouse. J Cereb Blood Flow Metab. 2001;21(1):52–60. doi: 10.1097/00004647-200101000-00007. [DOI] [PubMed] [Google Scholar]
- 138.Alkayed NJ, Murphy SJ, Traystman RJ, Hurn PD, Miller VM. Neuroprotective effects of female gonadal steroids in reproductively senescent female rats. Stroke. 2000;31(1):161–168. doi: 10.1161/01.str.31.1.161. [DOI] [PubMed] [Google Scholar]