Abstract
The entry of herpes simplex virus (HSV) into cells was once thought to be a general process. It is now understood that the virus is able to use multiple mechanisms for entry and spread, including the use of receptors and co-receptors that have been determined to be cell-type specific. This is certainly true for ocular cell types, which is important as the virus may use different mechanisms to gain access to multiple anatomic structures in close proximity, leading to various ocular diseases. There are some patterns that may be utilized by the virus in the eye and elsewhere, including surfing along filopodia in moving from cell to cell. There are common themes as well as intriguing differences in the entry mechanisms of HSV into ocular cells. We discuss these issues in the context of conjunctivitis, keratitis, acute retinal necrosis and other ocular diseases.
Keywords: herpes simplex virus, entry, ocular, fusion, endocytosis
Introduction
Herpes simplex virus (HSV) type-1 and -2 infections in humans can cause a variety of health problems, including diseases of the genitalia, ocular and central nervous systems. Although HSV-1 and HSV-2 have been mainly associated with infections above and below the waist, respectively, both are able to enter virtually all of the same cell types. This includes ocular cells, and infection of the various anatomic regions of the eye leads to a number of disease manifestations including conjunctivitis, keratitis, iridocyclitis and acute retinal necrosis. HSV-1 has been responsible for a majority of these, and is believed to be the leading cause of infectious blindness in developed nations. HSV-2 has been theorized to be causing an increasing proportion of acute retinal necrosis cases.1 An estimated 400,000 people in the United States have ocular herpes, while treatment of the disease is estimated to cost the nation 17.7 million dollars annually.2,3
The basic structure of the herpesviruses includes an icosahedral capsid containing a linear, double-stranded viral DNA genome. Surrounding the capsid is a layer of mRNAs and proteins known as the tegument, which in turn is covered by a lipid bilayer envelope containing various glycoproteins and proteins (Figure). HSV-1 and -2 are highly-related members of the alphaherpesvirus subfamily, and are able to develop life-long infections or latency in sensory ganglia. Recurrent episodes leading to ocular disease may occur more frequently and be more resistant to treatment in HIV/AIDS patients.4,5 For immunocompetent individuals many episodes of recurrence are asymptomatic, which may facilitate the high seropositivity to HSV-1 via transmission of the virus through saliva and tears.6 Recent polymerase chain reaction (PCR) studies suggest that nearly all adult humans are exposed to the virus.7 It is not clearly understood why some individuals go on to develop clinically significant ocular pathology as a rare sequel and others do not.
FIGURE 1.
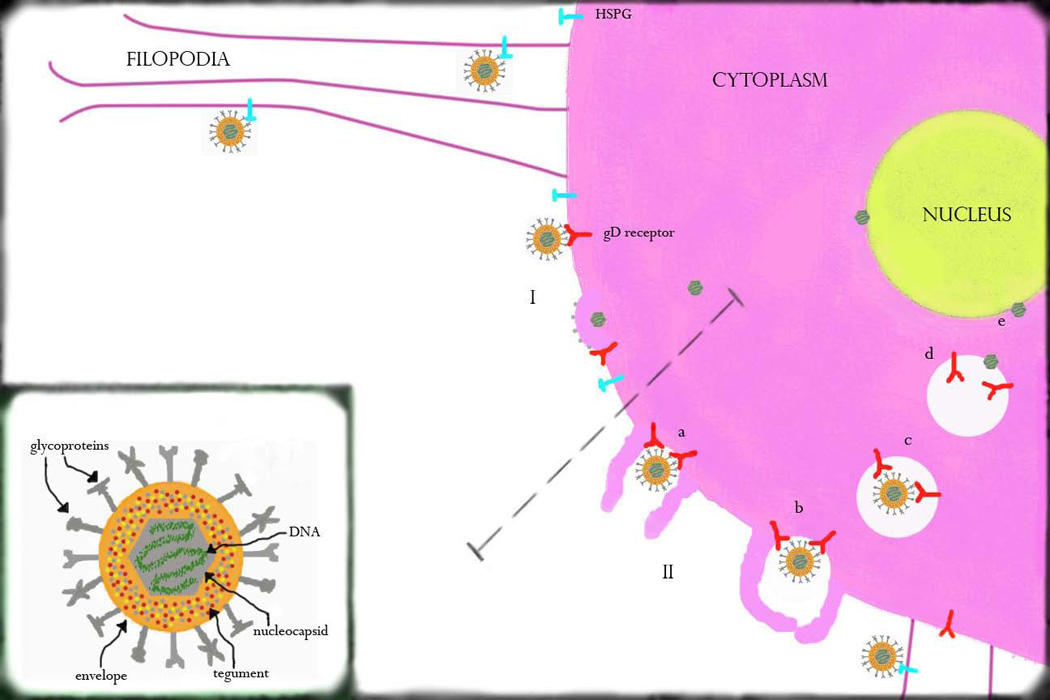
Schematic for HSV entry into ocular cells. HSV virion and its components are shown in the lower left portion of the image. There are two major modes used by the virus in entering ocular cells, both of which may be preceded by attachment to heparan sulfate proteoglycans (HSPG) and surfing of HSV along filopodia toward the cell body. HSV can enter via an attachment/fusion mechanism (I) in which viral contents enter directly into the cell cytoplasm through the formation of a fusion pore. It may also enter through an endocytic pathway, such as the phagocytosis-like mechanism shown here (II). This begins as the virus reaches the cell surface (a), where it associates with membrane protrusions. These protrusions engulf the virion, causing clustering of viral receptors within the phagosome (b). The phagosome travels toward the cell nucleus, during which time the viral envelope associates and fuses with the phagosomal membrane (c). The naked viral nucleocapsid is then released into the cytoplasm (d) leading to attachment and subsequent entry into the nucleus (e).
Development of latency relies on access to sensory nerves, which may occur after initial entry and replication in epithelial cells. The cornea in particular represents one of the most highly innervated tissues in humans due to a dense arrangement of branches of the ophthalmic division of the 5th cranial nerve. After retrograde transport along these branches to the trigeminal ganglion, reactivation may be triggered by numerous environmental factors including stress and ultraviolet radiation.8 Latency associated transcripts (LATs) have a role in the modulation of latency and reactivation through anti-apoptotic activity protecting against CD8+ T cells and regulation of ICP0 gene expression.9–12 It is believed that HSV may also develop latency in the cornea, an immune-privileged site. This may contribute to the ability of the virus to be transmitted by corneal transplant and cause disease in the recipient, although this is exceedingly rare.13 The entry of HSV into ocular cells may therefore occur after: exogenous exposure to the virus (“front-door” entry), locally reactivated virus in the cornea, or reactivated virus that travels anterograde along the ophthalmic division of the 5th cranial nerve (“back-door” entry).14 A significant percentage of cases may involve superinfection, in which there is reexposure to the same viral strain or exposure to a different strain after a previous infection.15
Although ocular HSV can occur through several pathways and is modulated by multiple factors including the host immune response, its entry into various ocular structures facilitates replication and is therefore a key step in pathogenesis. This includes the development of stromal keratitis, which is the major cause of visual morbidity and is likely mediated by CD4+ T cells in the inflammatory response to stromal infection in addition to direct viral effects.16 The close proximity of several structures in the eye may cause overlapping categories of clinically evident infection (e.g. keratouveitis). While previously the entry of HSV into cells was believed to be a general process, it is now understood that the virus is able to make use of viral and host factors that makes entry cell type-specific and in some cases viral subtype-specific. The mechanisms used by the virus for entry may also be conserved in its methods of spread.17 Furthermore, a viral strain that initially caused one disease process could produce a different disease upon reactivation (e.g. epithelial versus stromal keratitis). These issues make an understanding of the ways by which HSV enters ocular cells highly significant.
HSV attachment and fusion: role of glycoproteins
Embedded in the lipid bilayer envelope that surrounds the virus capsid are several glycoproteins, of which gB, gC, gD, gH and gL are important for entry.18,19 The initial interaction between virus and cell occurs as attachment between gB and/or gC and heparan sulfate proteoglycans on the cell surface. This may also occur on filopodia, extensions of the cellular membrane that may be used by the virus for unilateral spread to the cell body.20,21 Viral penetration into the cell may require a pH independent fusion mechanism in which the lipid bilayer envelope is merged with the cellular membrane or an intracellular vesicle that travels to the surface, creating a hemifusion intermediate.19 Several viral glycoproteins play a role in fusion, including gB and gH which have multiple fusogenic domains, as well as gD and gL. It is believed that the role of gD as a catalyst for entry involves interactions with specific receptors on the cellular surface, inducing a conformational change that allows it to form a complex with the other fusion-related glycoproteins.18,22 These receptors include nectin-1 and -2, herpesvirus entry mediator (HVEM) and 3-O sulfated heparan sulfate (3-OS HS), most of which are expressed on several ocular cell types and are therefore implicated in entry (Table).18,19 The end result is entry of viral capsids and tegument proteins into the cellular cytoplasm through the creation of a fusion pore (Figure).
TABLE 1.
Importance for Entry of HSV-1/HSV-2 Receptors and Co-Receptors by Ocular Cell Type
| HCjE | HCorE | SF | TM | RPE | |
|---|---|---|---|---|---|
| Nectin-1 | +++/unknown | +++/unknown | −/− | −/− | +++/+++ |
| HVEM | ++/unknown | ++/unknown | −/+++ | +++/unknown | ++/++ |
| 3O-S HS | +/− | −/− | +++/− | unknown/− | −/− |
| PILR-α | −/unknown | +/unknown | unknown/unknown | unknown/unknown | +/− |
Note: The importance of receptors for entry are given for HSV-1 followed by HSV-2 for each ocular cell type. +++ means primary receptor, ++ means secondary receptor, + means co-receptor or otherwise less important for entry. A minus sign (−) denotes that either the receptor is not important for entry or it is not expressed. The unknowns are primarily for HSV-2 entry and PILR-α. HCjE = human conjunctival epithelial cells, HCorE = human corneal epithelial cells, SF = human corneal stromal fibroblasts, TM = human trabecular meshwork cells, RPE = human retinal pigment epithelial cells, HVEM = herpes virus entry mediator, 3O-S HS = 3-O sulfated heparan sulfate, PILR-α = paired immunoglobulin-like 2 receptor.
Nectin-1
Nectin-1 is a member of the immunoglobulin superfamily, and one of four calcium-independent immunoglobulin-like cell-cell adhesion molecules that have been described.23 Nectin-1 is also known to be an important entry receptor for HSV.18,19 The importance of nectin-1 as a gD receptor that facilitates entry has been well-established for a number of non-ocular cell types, and it is also involved in entry of HSV by endocytosis.19,24 Within the murine eye it is known to be expressed in the corneal epithelium and endothelium, ciliary body, iris, lens epithelium, retina and choroid.25 In ocular infections, it has been demonstrated that nectin-1 is the primary receptor for entry of HSV-1 into human conjunctival epithelial (HCjE) cells, retinal pigment epithelial (RPE) cells, and human corneal epithelial (HCorE) cells (unpublished data).20,26 Corneal fibroblasts, in contrast, do not seem to express nectin-1.27 HSV-2 also relies on nectin-1 as the primary receptor for entry into RPE cells.28 A study of HSV-1 and -2 entry into polarized human epithelial cells, including an RPE cell line (ARPE-19), showed that there was preferential infection at the apical surface that correlated with access to nectin-1.29 Therefore, nectin-1 may be strongly implicated as a host factor in the development of conjunctivitis and epithelial keratitis due to HSV-1 and also acute retinal necrosis due to both viral subtypes.
HVEM
HVEM is a member of the tumor necrosis factor receptor superfamily that is involved in inflammatory regulation through interaction with the natural ligands lymphotoxin alpha and LIGHT.30 It interacts directly with HSV gD and was found to facilitate entry into previously resistant cells.31 The expression of HVEM in tissues is not well understood, although its expression may increase in response to HSV-1 infection in corneal epithelium and stromal fibroblasts.30 It appears to play an important role in HSV-1 entry into HCjE and HCorE cells (unpublished data) and in entry by both subtypes into RPE cells, although it may not be the primary receptor.20,26,28 HVEM was demonstrated to be the primary receptor for HSV-1 entry into trabecular meshwork cells and for HSV-2 entry into stromal fibroblasts.32,33 It may, therefore, have a supportive role in conjunctivitis and epithelial keratitis due to HSV-1 and acute retinal necrosis due to both subtypes. HVEM appears to be a more critical host factor in trabeculitis due to HSV-1 and stromal keratitis due to HSV-2, which is believed to be rare.
3-OS HS
Heparan sulfate is a polysaccharide of the glycosaminoglycan family. 19 It is found in the vast majority of tissues, either in association with cellular surfaces in the form of heparan sulfate proteoglycan or in the extracellular matrix. 19 Heparan sulfate has a variety of biological functions, which are facilitated by distinct modifications during its biosynthesis.34 The 3-OS HS receptor is a rare modified form of heparan sulfate that is the product of 3-O-sulfotransferases (3-OSTs), including 3-OST-1, 3-OST-2, 3-OST-3A, 3-OST-3B, 3-OST-4 and 3-OST-5. It serves as a binding site for antithrombin in the propagation of anticoagulant activity, and all 3-OST isoforms except 3-OST-1 are able to produce a receptor that can bind to HSV gD for entry.35–37 3-OS HS has been shown to be important for HSV-1 entry into non-ocular cell types and appears to play an important role in viral spread.38, 39 It is also the primary receptor for HSV-1 entry into stromal fibroblasts and may have a supportive role in HSV-1 entry into HCjE cells.26, 27 3-OS HS is therefore implicated as a host factor in stromal keratitis and conjunctivitis due to HSV-1.
PILR-α
Recently it was discovered that paired immunoglobulin-like 2 (PILR) α is a co-receptor that associates with gB and is involved in HSV-1 fusion to host cells.40 Its contribution to HSV-1 entry depends on binding to sialylated O-linked glycans on gB.41 The entry of HSV-1 into certain non-ocular cell types was reduced after treatment with anti-PILR-α antibody, as has been shown previously for the gD receptors.40 The role of PILR-α as a co-receptor has been demonstrated for HSV-1 entry into HCorE cells (unpublished data). PILR-α was also examined for HSV-2 entry into RPE cells but the results did not favor any significant role for it during HSV-2 entry.28 It therefore may have a supportive role in the development of epithelial keratitis due to HSV-1. The importance of PILR-α in ocular herpes and herpes infections in general is yet to be fully determined.
Filopodia as an important means of entry and spread
The expression of filopodia along the leading edge of migrating cells plays a role in wound healing by facilitating cellular motility. The formation of these actin-rich structures is mediated by Rho GTPases, particularly Cdc42, and is induced by a GTPase known as Rif.42 Filopodia can also be exploited by HSV for spread, likely through a myosin-dependent process in which the virus travels from an infected cell to a non-infected cell, similar to a mechanism of surfing used by retroviruses.43 The virus may also attach to filopodia extensions originating from the cellular membrane as a route for entry, mediated by gB and/or gC binding to heparan sulfate proteoglycans. Interestingly, the expression of nectin-1 may be limited to the cell body, whereas heparan sulfate has been detected on filopodia.21 It has also been observed that HSV-1 can induce the formation of filopodia in infected cells.44,45 This may contribute to the ability of the virus to spread to uninfected cells. In addition to non-ocular cell types, this process has been described in HSV-1 infection of HCjE and also RPE cells.20,26 It is currently unknown whether HSV-2 similarly uses filopodia for entry and spread, although it also relies on heparan sulfate for attachment.36
Endocytosis: an additional mechanism of entry
The use of endocytosis as a mechanism of entry by HSV-1 has been demonstrated to be the dominant pattern for a number of cell types (Figure). In general, endocytic pathways utilized by viruses include caveolae, clathrin-mediated endocytosis, macropinocytosis and novel pathways.46 An electron microscopic study of entry into corneal stromal fibroblasts and nectin-1-Chinese hamster ovary (CHO) cells revealed that the virus appeared to enter through a pH dependent phagocytosis-like mechanism in which protrusions of the plasma membrane helped engulf enveloped virions.44 It was noted that the internalized virions were contained in large vesicles that were not clathrin-coated. This was contrasted with human trabecular meshwork cells, in which few protrusions were seen and there was a lack of virion-containing vesicles. 44 It was also found in nectin-1-CHO cells that nectin-1 co-localized with virion-containing vesicles. The use of this unique phagocytosis-like mechanism in HSV entry into stromal fibroblasts may therefore play a role in the development of stromal keratitis. 44 Recent data suggest that endocytosis may be more common for ocular cell types than previously thought. Entry of HSV into HCjE, HCorE (unpublished data), and RPE cells all seem to be influenced by changes in pH, which is a hallmark of endocytic entry.26,28
Conclusions
HSV-1 and HSV-2 are capable of infecting ocular cells, although HSV-1 is believed to be the more common cause of ocular disease. The main treatments for ocular HSV can be limited in efficacy and rely on inhibition of viral replication, a process that occurs only after entry into host cells. The mechanisms of HSV entry include viral attachment/fusion and endocytosis, both of which may involve gD interaction with host glycoproteins. Filopodia, which are implicated in tissue repair and viral spread from cell to cell, may also provide additional sites for HSV entry. The mechanism of cell-cell fusion, which has been described for non-ocular cells in HSV infection and may be a means for viral propagation to uninfected cells, requires further study in ocular cell types. A review of the general mechanisms of entry, while important, does not lead to a complete understanding of virus-cell interactions leading to ocular disease. The entry of HSV into ocular cells is cell-type specific and perhaps also viral subtype specific. It follows that determining the mode of entry into various cell types may illuminate general trends of infection. This is important as improved treatment methodologies may require knowledge of viral entry and spread, which are linked. Notably, the present mini-review discusses studies that were primarily conducted in human cultured cells as opposed to live animal models. Further studies are required to determine in vivo as well as clinical correlations.
Acknowledgement
This work was supported by NIH grants AI057860 (D.S), AI081869 (D.S.) and a Core Grant EY01792. D.S. is a recipient of the Lew Wasserman Merit award from Research to Prevent Blindness (RPB). A.V.F is supported by an RPB Medical Student Eye Research Fellowship.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Liesegang TJ. Herpes simplex virus epidemiology and ocular importance. Cornea. 2001;20:1–13. doi: 10.1097/00003226-200101000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Liesegang TJ, Melton LJ, Daly PJ, Ilstrup DM. Epidemiology of ocular herpes simplex: incidence in Rochester, Minn, 1950 through 1982. Arch Ophthalmol. 1989;107:1155–1159. doi: 10.1001/archopht.1989.01070020221029. [DOI] [PubMed] [Google Scholar]
- 3.Lairson DR, Begley CE, Reynolds TF, Wilhelmus KR. Prevention of herpes simplex virus eye disease: a cost-effectiveness analysis. Arch Ophthalmol. 2003;121:108–112. doi: 10.1001/archopht.121.1.108. [DOI] [PubMed] [Google Scholar]
- 4.Hodge WG, Margolis TP. Herpes simplex virus keratitis among patients who are positive or negative for human immunodeficiency virus: an epidemiologic study. Ophthalmology. 1997;104(1):120–124. doi: 10.1016/s0161-6420(97)30351-0. [DOI] [PubMed] [Google Scholar]
- 5.Young TL, Robin JB, Holland GN, Hendricks RL, Paschal JF, Engstrom RE, Jr, Sugar J. Herpes simplex keratitis in patients with acquired immune deficiency syndrome. Ophthalmology. 1989;96:1476–1479. doi: 10.1016/s0161-6420(89)32706-0. [DOI] [PubMed] [Google Scholar]
- 6.Kaufman HE, Azcuy AM, Varnell ED, Sloop GD, Thompson HW, Hill JM. HSV-1 DNA in tears and saliva of normal adults. Invest Ophthalmol Vis Sci. 2005;46:241–247. doi: 10.1167/iovs.04-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hill JM, Ball MJ, Neumann DM, Azcuy AM, Bhattacharjee JS, Bouhanik S, Clement C, Lukiw WJ, Foster TP, Kumar M, Kaufman HE, Thompson HW. The high prevalence of herpes simplex virus type 1 DNA in human trigeminal ganglia is not a function of age or gender. J Virol. 2008;82:8230–8234. doi: 10.1128/JVI.00686-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilhelmus KR. Epidemiology of ocular infections. In: Tasman W, Jaeger EA, editors. Duane's Foundations of Clinical Ophthalmology. Philadelphia: Lippincott Williams & Wilkins; 1998. pp. 1–46. [Google Scholar]
- 9.Perng G, Jones C, Ciacci-Zanella J, Stone M, Henderson G, Yukht A, Slanina SM, Hofman FM, Ghiasi H, Nesburn AB, Wechsler SL. Virus-induced neuronal apoptosis blocked by the herpes simplex virus latency-associated transcript. Science. 2000;287(5457):1500–1503. doi: 10.1126/science.287.5457.1500. [DOI] [PubMed] [Google Scholar]
- 10.Branco FJ, Fraser NW. Herpes simplex virus type 1 latency-associated transcript expression protects trigeminal ganglion neurons from apoptosis. J Virol. 2005;79(14):9019–9025. doi: 10.1128/JVI.79.14.9019-9025.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leib DA, Coen DM, Bogard CL, Hicks KA, Yager DR, Knipe DM, Tyler KL, Schaffer PL. Immediate-early regulatory gene mutants define different stages in the establishment and reactivation of herpes simplex virus latency. J Virol. 1989;63:759–768. doi: 10.1128/jvi.63.2.759-768.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burton EA, Hong CS, Glorioso JC. The stable 2.0-kilobase intron of the herpes simplex virus type 1 latency-associated transcript does not function as an antisense repressor of ICP0 in nonneuronal cells. J Virol. 2003;77:3516–3530. doi: 10.1128/JVI.77.6.3516-3530.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Remeijer L, Maertzdorf J, Doomenbal P, Verjans GM, Osterhaus AD. Herpes simplex virus 1 transmission through corneal transplantation. Lancet. 2001;357:442. doi: 10.1016/S0140-6736(00)04011-3. [DOI] [PubMed] [Google Scholar]
- 14.Kaye S, Choudhary A. Herpes simplex keratitis. Prog Retin Eye Res. 2006;25:355–380. doi: 10.1016/j.preteyeres.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Remeijer L, Maertzdorf J, Buitenwerf J, Osterhaus AD, Verjans GM. Corneal herpes simplex virus type 1 superinfection with recrudescent herpetic keratitis. Invest Ophthalmol Vis Sci. 2002;43:358–363. [PubMed] [Google Scholar]
- 16.Newell CK, Martin S, Sendele D, Mercadal CM, Rouse BT. Herpes simplex virus-induced stromal keratitis: role of T-lymphocyte subsets in immunopathology. J Virol. 1989;63(2):769–775. doi: 10.1128/jvi.63.2.769-775.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Even DL, Henley AM, Geraghty RJ. The requirements for herpes simplex virus type 1 cell-cell Spread via nectin-1 parallel those for virus entry. Virus Res. 2006;119:195–207. doi: 10.1016/j.virusres.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 18.Spear PG. Herpes simplex virus: receptors and ligands for cell entry. Cell Microbiol. 2004;6:401–410. doi: 10.1111/j.1462-5822.2004.00389.x. [DOI] [PubMed] [Google Scholar]
- 19.Akhtar J, Shukla D. Viral entry mechanisms: cellular and viral mediators of herpes simplex virus entry. FEBS J. 2009;276(24):7228–7236. doi: 10.1111/j.1742-4658.2009.07402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tiwari V, Oh MJ, Kovacs M, Shukla SY, Valyi-Nagy T, Shukla D. Role for nectin-1 in herpes simplex virus 1 entry and spread in human retinal pigment epithelial cells. FEBS J. 2008;275(21):5272–5285. doi: 10.1111/j.1742-4658.2008.06655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oh MJ, Akhtar J, Desai P, Shukla D. A role for heparan sulfate in viral surfing. Biochem Biophys Res Commun. 2010;391:176–181. doi: 10.1016/j.bbrc.2009.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carfi A, Willis SH, Whitbeck JC, Krummenacher C, Cohen GH, Eisenberg RJ, Wiley DC. Herpes simplex virus glycoprotein D bound to the human receptor HveA. Mol Cell. 2001;8:169–179. doi: 10.1016/s1097-2765(01)00298-2. [DOI] [PubMed] [Google Scholar]
- 23.Takai Y, Miyoshi J, Ikeda W, Ogita H. Nectins and nectin-like molecules: roles in contact inhibition of cell movement and proliferation. Nat Rev Mol Cell Biol. 2008;9(8):603–615. doi: 10.1038/nrm2457. [DOI] [PubMed] [Google Scholar]
- 24.Stiles KM, Milne RS, Cohen GH, Eisenberg RJ, Krummenacher C. The herpes simplex virus receptor nectin-1 is down-regulated after trans-interaction with glycoprotein D. Virology. 2008;373(1):98–111. doi: 10.1016/j.virol.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valyi-Nagy T, Sheth V, Clement C, Tiwari V, Scanlan P, Kavouras J, Leach L, Guzman-Hartman G, Dermody T, Shukla D. Herpes simplex virus entry receptor nectin-1 is widely expressed in the murine eye. Curr Eye Res. 2004;29:303–309. doi: 10.1080/02713680490516756. [DOI] [PubMed] [Google Scholar]
- 26.Akhtar J, Tiwari V, Oh M-J, Kovacs M, Jani A, Kovacs SK, Valyi-Nagy T, Shukla D. HVEM and nectin-1 are the major mediators of herpes simplex virus 1 (HSV-1) entry into human conjunctival epithelium. Invest Ophthalmol Vis Sci. 2008;49(9):4026–4035. doi: 10.1167/iovs.08-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tiwari V, Clement C, Xu D, Valyi-Nagy T, Yue BY, Liu J, Shukla D. Role for 3-O-sulfated heparan sulfate as the receptor for herpes simplex virus type 1 entry into primary human corneal fibroblasts. J Virol. 2006;80(18):8970–8980. doi: 10.1128/JVI.00296-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shukla SY, Singh YK, Shukla D. Role of nectin-1, HVEM, and PILR-α in HSV-2 entry into human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2009;50(6):2878–2887. doi: 10.1167/iovs.08-2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galen B, Cheshenko N, Tuyama A, Ramratnam B, Herold BC. Access to nectin favors herpes simplex virus infection at the apical surface of polarized human epithelial cells. J Virol. 2006;80(24):12209–12218. doi: 10.1128/JVI.01503-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kovacs SK, Tiwari V, Prandovszky E, Dosa S, Bacsa S, Valyi-Nagy K, Shukla D, Valyi-Nagy T. Expression of herpes virus entry mediator (HVEM) in the cornea and trigeminal ganglia of normal and HSV-1 infected mice. Curr Eye Res. 2009;34(10):896–904. doi: 10.3109/02713680903184250. [DOI] [PubMed] [Google Scholar]
- 31.Whitbeck JC, Peng C, Lou H, Xu R, Willis SH, Ponce de Leon M, Peng T, Nicola AV, Montgomery RI, Warner MS, Soulika AM, Spruce LA, Moore WT, Lambris JD, Spear PG, Cohen GH, Eisenberg RJ. Glycoprotein D of herpes simplex virus (HSV) binds directly to HVEM, a member of the tumor necrosis factor receptor superfamily and a mediator of HSV entry. J Virol. 1997;71(8):6083–6093. doi: 10.1128/jvi.71.8.6083-6093.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tiwari V, Clement C, Scanlan PM. A role for herpesvirus entry mediator as the receptor for herpes simplex virus 1 entry into primary human trabecular meshwork cells. J Virol. 2005;79(20):13173–13179. doi: 10.1128/JVI.79.20.13173-13179.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tiwari V, Shukla SY, Yue BY. Herpes simplex virus type 2 entry into cultured human corneal fibroblasts is mediated by herpesvirus entry mediator. J Gen Virol. 2007;88:2106–2110. doi: 10.1099/vir.0.82830-0. [DOI] [PubMed] [Google Scholar]
- 34.O’Donnell CD, Shukla D. A novel function of heparan sulfate in the regulation of cell-cell fusion. J Biol Chem. 2009;284(43):29654–29665. doi: 10.1074/jbc.M109.037960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shukla D, Liu J, Blaiklock P, Shworak NW, Bai X, Esko JD, Cohen GH, Eisenberg RJ, Rosenberg RD, Spear PG. A novel role for 3-O sulfated heparan sulfate in herpes simplex virus 1 entry. Cell. 1999;99(1):13–22. doi: 10.1016/s0092-8674(00)80058-6. [DOI] [PubMed] [Google Scholar]
- 36.Shukla D, Spear PG. Herpesviruses and heparan sulfate: An intimate relationship in aid of viral entry. J Clin Invest. 2001;108:503–510. doi: 10.1172/JCI13799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xia G, Chen J, Tiwari V, Ju W, Li JP, Malmstrom A, Shukla D, Liu J. Heparan sulfate 3-O-sulfotransferase isoform 5 generates both an antithrombin-binding site and an entry receptor for herpes simplex virus, type 1. J Biol Chem. 2002;277:37912–37919. doi: 10.1074/jbc.M204209200. [DOI] [PubMed] [Google Scholar]
- 38.O’Donnell CD, Tiwari V, Oh MJ, Shukla D. A role for heparan sulfate 3-O-sulfotransferase isoform 2 in herpes simplex virus type 1 entry and spread. Virology. 2006;346:452–459. doi: 10.1016/j.virol.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 39.O’Donnell CD, Kovacs M, Akhtar J, Valyi-Nagy T, Shukla D. Expanding the role of 3-O sulfated heparan sulfate in herpes simplex virus type-1 entry. Virology. 2009 doi: 10.1016/j.virol.2009.11.011. E-pub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Satoh T, Arii J, Suenaga T, Wang J, Kogure A, Uehori J, Arase N, Shiratori I, Tanaka S, Kawaguchi Y, Spear PG, Lanier LL, Arase H. PILRα is a Herpes simplex virus-1 entry co-receptor that associates with glycoprotein B. Cell. 2008;132(6):935–944. doi: 10.1016/j.cell.2008.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang J, Fan Q, Satoh T, Arii J, Lanier LL, Spear PG, Kawaguchi Y, Arase H. Binding of herpes simplex virus glycoprotein B (gB) to paired immunoglobulin-like Type 2 receptor alpha depends on specific sialylated O-linked glycans on gB. J Virol. 2009;83(24):13042–13045. doi: 10.1128/JVI.00792-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pellegrin S, Mellor H. The Rho family GTPase Rif induces filopodia through mDia2. Curr Biol. 2005;15(2):129–133. doi: 10.1016/j.cub.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 43.Lehmann MJ, Sherer NM, Marks CB, Pypaert M, Mothes W. Actin- and myosin-driven movement of viruses along filopodia precedes their entry into cells. J Cell Biol. 2005;170:317–325. doi: 10.1083/jcb.200503059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clement C, Tiwari V, Scanlan PM, Valyi-Nagy T, Yue BY, Shukla D. A novel role for phagocytosis-like uptake in herpes simplex virus entry. J Cell Biol. 2006;174(7):1009–1021. doi: 10.1083/jcb.200509155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dixit R, Tiwari V, Shukla D. Herpes simplex virus type 1 induces filopodia in differentiated P19 neural cells to facilitate viral spread. Neurosci Lett. 2008;440:113–118. doi: 10.1016/j.neulet.2008.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sieczkarski SB, Whittaker GR. Dissecting virus entry via endocytosis. J Gen Virol. 2002;83:1535–1545. doi: 10.1099/0022-1317-83-7-1535. [DOI] [PubMed] [Google Scholar]


