Abstract
Parasite differentiation from proliferating tachyzoites into latent bradyzoites is central to pathogenesis and transmission of the intracellular protozoan pathogen Toxoplasma gondii. The presence of bradyzoite-containing cysts in human hosts and their subsequent rupture can cause life-threatening recrudescence of acute infection in the immuno-compromised and cyst formation in other animals contributes to zoonotic transmission and widespread dissemination of the parasite. In this review, we discuss the evidence showing how the clinically relevant process of bradyzoite differentiation is regulated at both transcriptional and post-transcriptional levels. Specific regulatory factors implicated in modulating bradyzoite differentiation include promoter-based cis-elements, epigenetic modifications and protein translation control through eukaryotic initiation factor -2 (eIF2). In addition to a summary of the current state of knowledge in these areas we discuss the pharmacological ramifications and pose some questions for future research.
Keywords: parasite, development, stress, bradyzoite, epigenetics, Apicomplexa
Developmental transitions from one cellular form to another do not require changes in genomic DNA; rather, the transition is due to a reprogramming of the genome. In other words, it is the assortment and quantity of expressed messenger RNAs - referred to as the “transcriptome” - that more directly impacts phenotype. The key to understanding the developmental biology of a protozoan parasite like Toxoplasma gondii lies in deciphering how cellular signals alter the transcriptome.
The impact of bradyzoites on human health
The apicomplexan parasite T. gondii has a complex life cycle centered on felines, the definitive hosts that shed infectious oocysts into the environment during infection (Frenkel et al. 1970). Oocysts, which can be stable in the environment for up to a year (Dubey 1998), contaminate the food and water supply, disseminating the parasite into additional felines, as well as an astonishing number of other warm-blooded vertebrates. Upon infection, the sporozoites inside the oocysts are released and establish new infection in the enterocytes of both intermediate and definitive hosts.
Another critical component of the pathogenesis and transmission of Toxoplasma is the differentiation into latent cysts containing bradyzoites. Nearly 50 years after the initial discovery of the parasite, evidence was being marshaled that demonstrated undercooked meat as a route of infection (Weinman & Chandler 1954, Jacobs et al. 1960a). By 1973, the descriptive terms for the tachyzoite (rapidly growing) and bradyzoite (slowly growing) life cycle stages were coined by Frenkel (Frenkel 1973). Bradyzoites were also called cystozoites (Hoare 1972); to clarify the terminology, Dubey and Beattie (1988) suggested that “tissue cyst” refer to encysted Toxoplasma while oocysts referred to Toxoplasma in feces. The clinical significance of the bradyzoite became apparent during instances of immunosuppression, such as in tissue transplantation and cancer chemotherapy (Cohen 1970, Ruskin & Remington 1976); during the advent of AIDS, in the 1980s, Toxoplasma emerged as a major opportunistic pathogen (Wong & Remington 1993). On occasion, tissue cysts rupture and the released bradyzoites default to tachyzoites, which will proliferate indiscriminately in the absence of an adequate immune response (Ferguson et al. 1989). Since bradyzoite cysts have a proclivity to form in tissues of the heart and the central nervous system, recrudescence of infection can lead to severe cardiovascular and neurological complications. The medical relevance of understanding bradyzoite physiology and Toxoplasma differentiation was now abundantly clear. Consequently, an intense research effort was launched to illuminate the molecular mechanisms driving tachyzoite and bradyzoite inter-conversion in hopes of identifying critical regulatory factors in the process that could be subverted for therapeutic advantage. While many notable advances have been made, the processes modulating bradyzoite differentiation remain unresolved.
Toxoplasma has developed a unique ability to bypass sexual reproduction in the feline (definitive) host to infect directly intermediate hosts after oral ingestion of tissue cysts (Su et al. 2003). Bradyzoite cysts present in livestock or prey are therefore an important source of infection for the carnivorous. While the dissemination of environmentally stable oocysts is a primary factor accounting for the worldwide distribution of Toxoplasma, the ability to form highly transmissible tissue cysts augments the transmission capacity of the parasite. In addition to becoming infected through oocysts, transmission of Toxoplasma to humans through consumption of raw or rare meat remains a significant means of transmission (Lee 2000, Slifko et al. 2000, Dubey & Jones 2008). In short, many important reasons exist that provide the impetus for intensive research into the mechanisms of bradyzoite cyst development and maintenance, as well as the impact of tissue cysts on human physical and mental health.
Bradyzoite biology
Given the importance of bradyzoite differentiation in the pathogenesis, persistence and transmission of Toxoplasma, many research groups have investigated parasite development and the biology of bradyzoites. There are several major ways that bradyzoites differ from the rapidly growing tachyzoite stage (Weiss & Kim 2000). Morphologically, bradyzoites are more slender with a nucleus relocated from the center of the parasite to the posterior. Changes in subcellular organelles include more electron dense rhoptries, fewer dense granules and more abundant micronemes. Bradyzoites also accumulate numerous, large amylopectin granules and dramatically reduce cell division, eventually becoming non-replicative with a DNA content of 1N (Radke et al. 2003). Bradyzoites are contained in a thin, but rigid cyst wall that is retained within a viable host cell, which helps to further shield the parasite from immune responses as well as current drug treatments. The development of the cyst wall appears to be initiated at the time of entry into the host cell (Ferguson & Hutchison 1987); additionally, parasites can express bradyzoite markers prior to invasion in lesions where stage conversion is occurring (Ferguson 2004).
Full-scale gene expression profiling between the two stages is now possible with the completion of the Toxoplasma genome sequence and availability of the ToxoGeneChip microarray. But to date, only a handful of stage-specific genes have been characterized that include cell surface antigens, cyst wall proteins, metabolic enzymes (some of which reflect the shift in glucose metabolism and utilization of amylopectin) and a small heat shock protein (HSP30/BAG1). Additional heat shock proteins are up-regulated and, in the case of HSP90, translocated to the parasite nucleus during bradyzoite development (Echeverria et al. 2005).
The main theme that emerges from studies of Toxoplasma differentiation is that the process has much in common with other cellular developmental transitions. The tachyzoite and bradyzoite are two distinct phases of the parasite life cycle, each formed by expressing a subset of genes defining that stage. Therefore, studies designed to illuminate how these stage-specific genes are turned on and off in response to developmental stimuli are essential in order to understand the mechanisms of Toxoplasma differentiation. Moreover, a better understanding of the control of gene expression during tachyzoite-bradyzoite differentiation may provide information on how to unlock the problem of being able to trigger the genes responsible for coccidian development in vitro.
The key to success is learning how to deal with stress
The bradyzoite cyst form contributes to the success of Toxoplasma as a parasite in four main ways: (i) the cyst survives ingestion and passage through the stomach to invade the small intestine (Jacobs et al. 1960b); (ii) the cyst is impervious to the host immune response and current drug treatments; (iii) the virtually quiescent bradyzoites within the cysts prevent Toxoplasma from killing its host, allowing the parasite to persist in a presumably benign manner; (iv) bradyzoites in tissue cysts remain infectious, thus enhancing transmission of the parasite should the host be consumed by a predator.
The molecular mechanisms governing the stage conversion from tachyzoite to bradyzoite are far from resolved, but it is clear that cellular stress can trigger the developmental change. Well-characterized bradyzoite inducing agents in vitro include pH stress, heat shock, chemical stress (e.g., sodium arsenite) (Soete et al. 1994), nutrient deprivation (Fox et al. 2004), the exogenous nitric oxide donor sodium nitrosprusside, mitochondrial inhibitors (Bohne et al. 1994) and endoplasmic reticulum (ER) stress (e.g., tunicamycin) (Narasimhan et al. 2008). In vivo, it is possible that fever (heat shock) and various factors in the host immune response contribute to the formation of bradyzoite cysts.
There is also evidence that differentiation may be a programmed response to slowed growth, independent of cellular stress. A slowed growth rate is intimately associated with the propensity of Toxoplasma tachyzoites to develop into bradyzoites (Bohne et al. 1994, Jerome et al. 1998). Additionally, type I hypervirulent strains of Toxoplasma that grow faster are much less efficient at forming cysts than the slower growing type II and III strains (Soete et al. 1994). The environment of the host cell is also a critical determinant of Toxoplasma differentiation. Certain types of host cells seem to have a greater ability to trigger spontaneous differentiation of Toxoplasma without need for exogenous insult (Ferreira da Silva et al. 2008). Altering host cell gene expression with a trisubstituted pyrrole has been shown to slow tachyzoite replication and induce bradyzoite-specific gene expression (Radke et al. 2006). It should be emphasized that many of the cellular stress agents mentioned above cause growth retardation of the parasite or may alter the host cell environment. However, extracellular tachyzoites subjected to cellular stress exhibit an increased frequency to convert into bradyzoites upon infection of host cells in vitro, supporting the idea that stress can directly act on the parasite and trigger a differentiation program (Weiss et al. 1998).
Extreme makeover: bradyzoite edition
Development is a highly regulated process in eukaryotic organisms and the differentiation of Toxoplasma is no exception. Here we will discuss evidence that suggests conversion between tachyzoites and bradyzoites involves regulatory mechanisms operating at the transcriptional as well as post-transcriptional level (Fig. 1). At the transcriptional level, evidence has been presented that shows cis-elements exist in Toxoplasma promoters and work together with chromatin remodeling events to control gene expression pertinent to stage conversion (Behnke et al. 2008). Recently, new evidence has surfaced demonstrating that post-transcriptional control may also be involved in differentiation, namely through the regulation of translation (Vonlaufen et al. 2008).
Fig. 1.
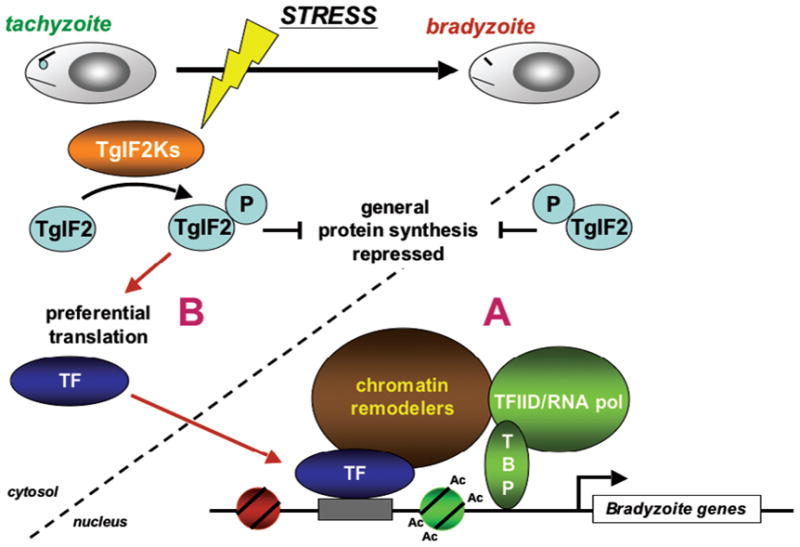
Multiple levels of regulation control stress-induced differentiation in Toxoplasma. The developmental transition from proliferating tachyzoites to encysted bradyzoites, which can be induced by cellular stress, is regulated by transcriptional (A) and post-transcriptional mechanisms (B). At least one response pathway that has been linked to stress-induced bradyzoite differentiation involves the eIF2 kinases, which lead to the phosphorylation of eIF2α in response to stress. In turn, this decreases general protein synthesis in favor of a select subset of mRNAs that may encode transcription factors (TF). Other post-transcriptional modes of regulation, such as RNA transport or splicing, may also be involved (not shown). While no transcription factor has been directly implicated in Toxoplasma developmental transitions, cis-elements have been identified in bradyzoite promoters (gray box). There is also evidence that epigenetic events, such as chromatin remodeling, are associated with gene expression changes that are required for bradyzoite differentiation. An example of histone acetylation (Ac), which activates gene expression, is shown (see Fig. 2). It has yet to be determined if similar mechanisms of regulation occur in parasites that “spontaneously” differentiate, i.e. convert to bradyzoites without a stress application. TBP: TATA-binding protein.
Post-transcriptional regulation
Translation control
It has been noted previously that stress-induced differentiation in Toxoplasma is very likely to share features common to hyphae formation in fungi or spore formation in Dictyostelium (Weiss & Kim 2000). A well characterized pathway that is responsive to numerous different stress conditions involves eukaryotic initiation factor-2 (eIF2). When the alpha subunit of eIF2 is phosphorylated, it becomes an inhibitor of its own guanine nucleotide exchange factor, eIF2B, thereby dampening protein translation. In the yeast Saccharomyces cerevisiae, an eIF2 kinase called GCN2 phosphorylates eIF2α during nutritional stress (Hinnebusch 2000, Wek et al. 2004). While general protein synthesis is reduced, there is a preferential translation of GCN4 by a mechanism involving short open reading frames (ORFs) in the 5′-noncoding portion of the GCN4 mRNA (Hinnebusch 1997). GCN4 is a basic-zipper transcriptional activator of genes involved in the metabolism of amino acids, nucleotides and vitamins, all of which remedy nutrient deprivation (Natarajan et al. 2001, Hinnebusch & Natarajan 2002). By way of comparison, the GCN2 orthologue in Dictyostelium is involved in triggering a developmental program resulting in the formation of fruiting bodies in response to nutrient starvation (Fang et al. 2003). In short, the eIF2 kinase-mediated stress response begins by altering gene expression via translation control and culminates in the reprogramming of the genome to survive and cope with the insult.
We sought to determine if such a pathway existed in Toxoplasma and, if so, if it participated in parasite differentiation. Evident in the Toxoplasma genome is an eIF2α orthologue (TgIF2α) and four putative TgIF2 kinases (TgIF2K-A through D). Using antibodies that we had generated against unmodified and phosphorylated TgIF2α, we were able to determine that stresses triggering bradyzoite differentiation in vitro also induced TgIF2α phosphorylation (Sullivan Jr et al. 2004). Moreover, as in other species, when TgIF2α is phosphorylated in response to stress, protein translation is greatly diminished (Narasimhan et al. 2008). In the context of Toxoplasma differentiation, the rapid reduction in general protein translation would serve to help the parasite cease to be a tachyzoite while it reprograms the genome to express bradyzoite genes. Importantly, we also found that TgIF2α remains phosphorylated in the cyst stage, which helps explain how bradyzoites maintain their quiescent state (phosphorylated TgIF2α dramatically reduces translation, consistent with the dormant nature of the cyst stage). There are no pharmacological inhibitors of eIF2α phosphorylation, but an inhibitor of eIF2α dephosphorylation, salubrinal, has been characterized (Boyce et al. 2005). We have verified that salubrinal inhibits TgIF2α dephosphorylation; consequently, it also induces tachyzoites to develop into bradyzoite cysts (Narasimhan et al. 2008). These findings indicated that the phosphorylation status of TgIF2α is a major determinant of developmental transitions in Toxoplasma; moreover, they were the first to demonstrate that the pathogenically relevant process of bradyzoite differentiation may be modulated by post-transcriptional mechanisms such as translation control. Other post-transcriptional mechanisms may be involved in Toxoplasma development, such as differential mRNA splicing or stability, but these are presently understudied.
Transcriptional regulation
Cis-elements and trans-acting factors
A great deal of evidence supports that Toxoplasma differentiation is also governed by transcriptional regulation. Studies of bradyzoite development using Toxoplasma cDNA microarrays show that changes in mRNA levels correlate with the expression of known bradyzoite-specific proteins and suggest that a hierarchy of gene induction directs stage conversion (Cleary et al. 2002, Singh et al. 2002). Analysis of the Toxoplasma transcriptome during different developmental stages by serial analysis of gene expression further supports that transcriptional mechanisms play a key role in governing parasite development (Radke et al. 2005). Genomic sequencing of Toxoplasma has also revealed that many coordinately expressed genes are scattered across the chromosomes, which indicates that they are likely to be transcriptionally regulated by transacting factors that bind to cis-regulatory elements in gene promoters (Radke et al. 2005). Recently, cis-elements have been identified in Toxoplasma promoters that are capable of binding proteins in parasite lysate; moreover, minimal cis-elements were mapped that are capable of converting a constitutive promoter to one that is induced by bradyzoite conditions (Behnke et al. 2008). Collectively, these studies argue that DNA-binding transcription factors operate in Toxoplasma as they do in higher eukaryotes, but are likely to be divergent. Consistent with this idea is the recent validation that novel parasite proteins containing plant-like Apetala2 (AP2) domains constitute a lineage-specific set of specific transcription factors in Apicomplexa (Balaji et al. 2005, De Silva et al. 2008, Yuda et al. 2009).
Epigenetics
Another aspect of transcriptional regulation that has been linked to the control of gene expression pertinent to bradyzoite differentiation involves epigenetics and chromatin modifications (Sullivan & Hakimi 2006). Chromatin can be fashioned to be transcriptionally repressive or permissive, dependent in part on post-translational modifications made to histones, a phenomenon referred to as the “histone code” (Strahl & Allis 2000). Histone acetylation is a mark of gene activation in other species and we have shown this to be true in Toxoplasma. Chromatin immunoprecipitation (ChIP) performed on tachyzoites show that histones in the upstream sequence of bradyzoite-specific genes such as LDH2 are hypoacetylated, but in stressed parasite populations enriched for developing bradyzoites, the sequence upstream of the LDH2 gene contains acetylated histones (Fig. 2). Constitutively expressed genes like tubulin have upstream regions containing histones that are acetylated in both of these life cycle stages. Similar ChIP analyses have been performed for other stage-specific genes with the same results, strongly implicating histone acetylation as well as methylation as modulators of gene expression during differentiation (Saksouk et al. 2005, Gissot et al. 2007). Attention has turned to the “writers” of the Toxoplasma histone code, which are numerous based on a bioinformatics survey of the genome (Sullivan & Hakimi 2006). To date, several histone acetyltransferases and deacetylases have been cloned from Toxoplasma, as well as a SWI/SNF ATPase (Sullivan et al. 2003, Saksouk et al. 2005, Smith et al. 2005, Bhatti et al. 2006). Consistent with our observations linking histone acetylation to differentiation, Toxoplasma cDNA arrays used to analyze parasites exposed to apicidin - an HDAC inhibitor - reveal that transcriptional activity during bradyzoite development may be regulated through gene-specific acetylation and deacetylation of histones (Boyle et al. 2006). These experiments have also shown that histone acetylation status may play a significant role in the regulation of groups of genes important in the Toxoplasma life cycle (Boyle et al. 2006). Histone methylases have also been cloned, including SET8-related modifiers whose activities mark silent heterochromatic domains in apicomplexan genomes (Sautel et al. 2007). Additional evidence that histone methylation is critical to tachyzoite-bradyzoite conversion comes from a study showing that inhibition of a histone methylase, TgCARM1, induces differentiation (Saksouk et al. 2005).
Fig. 2.
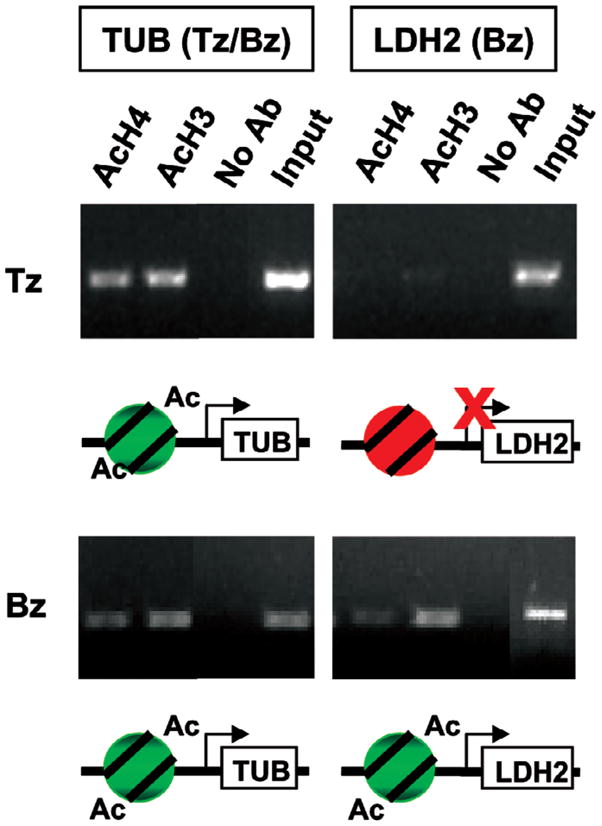
Histones upstream of bradyzoite-specific genes become acetylated during stress-induced differentiation in vitro. Chromatin immunoprecipitation using anti-acetylated histone H3 (AcH3) or H4 (AcH4) was performed on Toxoplasma tachyzoites (Tz) or a population enriched in bradyzoites (Bz) due to alkaline pH exposure for three days. Upstream regions of constitutively expressed genes like tubulin (TUB) are acetylated regardless of life cycle stage. However, those of Bz-specific genes (LDH2) become hyperacetylated after subjecting parasites to a pH stress that induces cyst development. “No Ab” refers to a no antibody control ChIP and “input” is the PCR of the DNA prior to immunoprecipitation. Methods used have been described previously (Saksouk et al. 2005).
Differentiation: a working model
Progression to the bradyzoite stage of the life cycle is regulated by a multilayered cascade of steps involving transcriptional and post-transcriptional control. A working model highlighting some of these steps and their interplay is presented in Fig. 1. The first step appears to involve the cessation of general protein translation via phosphorylation of TgIF2α (Narasimhan et al. 2008). During the shutdown of general protein synthesis, we postulate that preferential translation of presumed transcription factors would ensue, possibly through a mechanism involving upstream open ORFs as has been shown in other species (Vattem et al. 2001). These transcription factors would work in concert with chromatin remodeling machinery to facilitate the expression of genes required for building bradyzoite cysts (Saksouk et al. 2005, Behnke et al. 2008). TgIF2α remains phosphorylated during the cyst stage, possibly to minimize protein translation, which would be consistent with the semi-dormant nature of the bradyzoite (Narasimhan et al. 2008).
Bradyzoite interrupted: pharmacological perspectives
Toxoplasma is currently incurable due to the persistence of cysts in host tissues. To achieve a radical cure of Toxoplasma, bradyzoites would have to be eliminated. However, in addition to being intracellular, the cyst wall and quiescent metabolism makes doing so pharmacologically challenging. There may be new hope, however, with the development of arginine oligomers that can act as a carrier of antimicrobial cargo across the cyst wall (Samuel et al. 2003).
As outlined above, new advances are illuminating the mechanisms of differentiation, but does it make sense to disrupt parasite development into bradyzoites in vivo? It could be argued that preventing Toxoplasma from forming cysts in an immunocompromised individual is an unsound therapeutic strategy because then the parasites would be locked into the rapidly growing, tissue destroying tachyzoite phase. Therefore, drugs that block cyst development will most certainly need to be co-administered with one of the currently available drugs that effectively eliminate tachyzoites. Approaches targeting bradyzoite cyst maintenance could also be exploited, which would act to “wake” Toxoplasma from its dormancy so it would no longer be able to evade the effective standard treatment. Alternative strategies could include prophylactic therapies that an infected immunosuppressed patient could take to minimize recrudescence of Toxoplasma. Small molecules like salubrinal block TgIF2α dephosphorylation in vitro (Narasimhan et al. 2008) and thus may be useful in preventing the conversion of bradyzoites into tachyzoites in vivo.
Another important strategy that can develop from learning about Toxoplasma differentiation is the potential to exploit the information to create additional vaccines against the parasite. For example, a type II strain of Toxoplasma could be genetically engineered that no longer has the capability to form cysts. An analogous vaccine strain is already being employed in sheep (Toxovax™) and has been proposed to be useful in helping reduce zoonotic transmission, hence making meat safer for consumption (Buxton & Innes 1995, Buxton et al. 2007). Animals vaccinated with such a strain should acquire immunity to Toxoplasma, yet would not have their tissues contaminated with parasite cysts.
Future directions
The various levels participating in the differentiation process provides multiple opportunities for pharmacological interference. However, much more needs to be learned about each step and its importance in the overall process. The increased tendency for stressed tachyzoites to differentiate into bradyzoites when placed back into host cell culture (Weiss et al. 1998) argues the importance of elucidating how Toxoplasma recognizes and responds to stress or other environmental cues. Deciphering the roles of each of the four TgIF2Ks and learning how each one is activated should provide great insight into the mechanisms of parasite stress recognition. We hypothesize that each one has exquisitely evolved to sense a specific stress(es) in order to phosphorylate TgIF2α. In support of this, the two we have characterized thus far argues that subcellular localization is tied to the type of stress recognized. We have found that TgIF2K-A is present in the parasite ER and TgIF2K-B is present throughout the cytoplasm, suggesting that these eIF2 kinases respond to ER stress (e.g., misfolded proteins) and cytosolic stress (e.g., oxidative stress), respectively (Narasimhan et al. 2008). It would also be important to create parasites that are deficient in TgIF2α phosphorylation to test if they lose the competency to differentiate; such a mutant may make an ideal vaccine candidate.
Another key item that needs to be addressed is the mechanism that bridges TgIF2α phosphorylation and translation control to the transcriptional changes required to build a bradyzoite cyst. By analogy to other species, this would involve preferential translation of a transcription factor, but Apicomplexa exhibit a striking paucity of such factors in their genomes. While the AP2-containing proteins are promising candidates, it is likely that more than one family of transcription factors operate in Toxoplasma. The ability to purify polyribosome fractions from Toxoplasma should provide a novel means to identify gene products that are preferentially translated during stress-induced differentiation (Narasimhan et al. 2008). We have been pursuing a biochemical approach to co-purify transcription factors that are in association with Toxoplasma HAT enzymes. Finally, now that cis-regulatory elements have been mapped in Toxoplasma promoters (Behnke et al. 2008), the motifs could be employed in DNA-affinity chromatography or yeast one-hybrid approaches to isolate the protein factors that bind to these regulatory elements.
It would be of great interest to expand on the initial studies suggesting that pharmacological manipulation of the Toxoplasma histone code can perturb viability and differentiation. Therefore, studies aimed to decipher the Toxoplasma histone code are very likely to reveal novel points of therapeutic intervention. Not only do we need to learn more about the cellular consequences of specific histone modifications in singular and in combination, but we also need to further characterize the writers and readers of this code. The integration of this information with studies of eIF2 kinase-mediated translation control will help provide a more complete picture of the pathways leading to parasite differentiation.
The availability of the sequenced genome coupled with the rapidly expanding set of molecular tools has greatly facilitated Toxoplasma research (Meissner et al. 2007). To help keep members of the research community apprised of new discoveries, reagents and events, we maintain a Toxoplasma news blog that can be accessed at http://toxoplasmaparasite.blogspot.com/. We invite readers to take advantage of this free web site and contact us with their feedback and ideas.
Acknowledgments
To Dr. Michael White (University of Montana), Dr. Sherry Queener (IUSM) and members of the Sullivan laboratory, for a critical reading of the manuscript.
Financial support: National Institutes of Health (AI077502), American Heart Association (0750201Z)
References
- Balaji S, Babu MM, Iyer LM, Aravind L. Discovery of the principal specific transcription factors of Apicomplexa and their implication for the evolution of the AP2-integrase DNA binding domains. Nucleic Acids Res. 2005;33:3994–4006. doi: 10.1093/nar/gki709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnke MS, Radke JB, Smith AT, Sullivan WJ, Jr, White MW. The transcription of bradyzoite genes in Toxoplasma gondii is controlled by autonomous promoter elements. Mol Microbiol. 2008;68:1502–1518. doi: 10.1111/j.1365-2958.2008.06249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatti MM, Livingston M, Mullapudi N, Sullivan WJ., Jr Pair of unusual GCN5 histone acetyltransferases and ADA2 homologues in the protozoan parasite Toxoplasma gondii. Eukaryot Cell. 2006;5:62–76. doi: 10.1128/EC.5.1.62-76.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohne W, Heesemann J, Gross U. Reduced replication of Toxoplasma gondii is necessary for induction of bradyzoite-specific antigens: a possible role for nitric oxide in triggering stage conversion. Infect Immun. 1994;62:1761–1767. doi: 10.1128/iai.62.5.1761-1767.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce M, Bryant KF, Jousse C, Long K, Harding HP, Scheuner D, Kaujman RJ, Ma D, Coen DM, Ron K, Yuan J. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science. 2005;307:935–939. doi: 10.1126/science.1101902. [DOI] [PubMed] [Google Scholar]
- Boyle JP, Saeij JP, Cleary MD, Boothroyd JC. Analysis of gene expression during development: lessons from the Apicomplexa. Microbes Infect. 2006;8:1623–1630. doi: 10.1016/j.micinf.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Buxton D, Innes EA. A commercial vaccine for ovine toxoplasmosis. Parasitology. 1995;110(Suppl):S11–S16. doi: 10.1017/s003118200000144x. [DOI] [PubMed] [Google Scholar]
- Buxton D, Maley SW, Wright SE, Rodger S, Bartley P, Innes EA. Ovine toxoplasmosis: transmission, clinical outcome and control. Parassitologia. 2007;49:219–221. [PubMed] [Google Scholar]
- Cleary MD, Singh U, Blader IJ, Brewer JL, Boothroyd JC. Toxoplasma gondii asexual development: identification of developmentally regulated genes and distinct patterns of gene expression. Eukaryot Cell. 2002;1:329–340. doi: 10.1128/EC.1.3.329-340.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SN. Toxoplasmosis in patients receiving immunosuppressive therapy. JAMA. 1970;211:657–660. [PubMed] [Google Scholar]
- De Silva EK, Gehrke AR, Olszewski K, Leon I, Chahal JS, Bulyk ML, Llinas M. Specific DNA-binding by apicomplexan AP2 transcription factors. Proc Natl Acad Sci USA. 2008;105:8393–8398. doi: 10.1073/pnas.0801993105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey JP. Toxoplasma gondii oocyst survival under defined temperatures. J Parasitol. 1998;84:862–865. [PubMed] [Google Scholar]
- Dubey JP, Beattie CP. Toxoplasmosis of animals and man. Boca Raton: CRC Press; 1988. p. 220. [Google Scholar]
- Dubey JP, Jones JL. Toxoplasma gondii infection in humans and animals in the United States. Int J Parasitol. 2008;38:1257–1278. doi: 10.1016/j.ijpara.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Echeverria PC, Matrajt M, Harb OS, Zappia MP, Costas MA, Roos DS, Dubremetz JF, Angel SO. Toxoplasma gondii Hsp90 is a potential drug target whose expression and subcellular localization are developmentally regulated. J Mol Biol. 2005;350:723–734. doi: 10.1016/j.jmb.2005.05.031. [DOI] [PubMed] [Google Scholar]
- Fang R, Xiong Y, Singleton CK. IfkA, a presumptive eIF2 alpha kinase of Dictyostelium, is required for proper timing of aggregation and regulation of mound size. BMC Dev Biol. 2003;3:3. doi: 10.1186/1471-213X-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson DJ. Use of molecular and ultrastructural markers to evaluate stage conversion of Toxoplasma gondii in both the intermediate and definitive host. Int J Parasitol. 2004;34:347–360. doi: 10.1016/j.ijpara.2003.11.024. [DOI] [PubMed] [Google Scholar]
- Ferguson DJ, Hutchison WM. An ultrastructural study of the early development and tissue cyst formation of Toxoplasma gondii in the brains of mice. Parasitol Res. 1987;73:483–491. doi: 10.1007/BF00535321. [DOI] [PubMed] [Google Scholar]
- Ferguson DJ, Hutchison WM, Pettersen E. Tissue cyst rupture in mice chronically infected with Toxoplasma gondii. An immunocytochemical and ultrastructural study. Parasitol Res. 1989;75:599–603. doi: 10.1007/BF00930955. [DOI] [PubMed] [Google Scholar]
- Ferreira da Silva MD, Barbosa HS, Gross U, Luder CG. Stress-related and spontaneous stage differentiation of Toxoplasma gondii. Mol Biosyst. 2008;4:824–834. doi: 10.1039/b800520f. [DOI] [PubMed] [Google Scholar]
- Fox BA, Gigley JP, Bzik DJ. Toxoplasma gondii lacks the enzymes required for de novo arginine biosynthesis and arginine starvation triggers cyst formation. Int J Parasitol. 2004;34:323–331. doi: 10.1016/j.ijpara.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Frenkel JK. Toxoplasma in and around us. BioScience. 1973;23:343–352. [Google Scholar]
- Frenkel JK, Dubey JP, Miller NL. Toxoplasma gondii in cats: fecal stages identified as coccidian oocysts. Science. 1970;167:893–896. doi: 10.1126/science.167.3919.893. [DOI] [PubMed] [Google Scholar]
- Gissot M, Kelly KA, Ajioka JW, Greally JM, Kim K. Epigenomic modifications predict active promoters and gene structure in Toxoplasma gondii. PLoS Pathog. 2007;3:e77. doi: 10.1371/journal.ppat.0030077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch AG. Translational regulation of yeast GCN4. A window on factors that control initiator-tRNA binding to the ribosome. J Biol Chem. 1997;272:21661–21664. doi: 10.1074/jbc.272.35.21661. [DOI] [PubMed] [Google Scholar]
- Hinnebusch AG. Mechanism and regulation of initiator methionyl-tRNA binding to ribosomes. In: Sonenberg N, Hershey JWB, Mathews M, editors. Translational control of gene expression. New York: Cold Spring Harbor Laboratory Press; 2000. pp. 185–244. [Google Scholar]
- Hinnebusch AG, Natarajan K. Gcn4p, a master regulator of gene expression, is controlled at multiple levels by diverse signals of starvation and stress. Eukaryot Cell. 2002;1:22–32. doi: 10.1128/EC.01.1.22-32.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoare CA. The developmental stages of Toxoplasma. J Trop Med Hyg. 1972;75:56–58. [PubMed] [Google Scholar]
- Jacobs L, Remington JS, Melton ML. A survey of meat samples from swine, cattle, and sheep for the presence of encysted Toxoplasma. J Parasitol. 1960a;46:23–28. [PubMed] [Google Scholar]
- Jacobs L, Remington JS, Melton ML. The resistance of the encysted form of Toxoplasma gondii. J Parasitol. 1960b;46:11–21. [PubMed] [Google Scholar]
- Jerome ME, Radke JR, Bohne W, Roos DS, White MW. Toxoplasma gondii bradyzoites form spontaneously during sporozoite-initiated development. Infect Immun. 1998;66:4838–4844. doi: 10.1128/iai.66.10.4838-4844.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MB. Everyday and exotic foodborne parasites. Can J Infect Dis. 2000;11:155–158. doi: 10.1155/2000/120498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner M, Agop-Nersesian C, Sullivan WJ., Jr Molecular tools for analysis of gene function in parasitic microorganisms. Appl Microbiol Biotechnol. 2007;75:963–975. doi: 10.1007/s00253-007-0946-4. [DOI] [PubMed] [Google Scholar]
- Narasimhan J, Joyce BR, Naguleswaran A, Smith AT, Livingston MR, Dixon SE, Coppens I, Wek RC, Sullivan WJ., Jr Translation regulation by eukaryotic initiation factor-2 kinases in the development of latent cysts in Toxoplasma gondii. J Biol Chem. 2008;283:16591–16601. doi: 10.1074/jbc.M800681200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan K, Meyer MR, Jackson BM, Slade D, Roberts C, Hinnebusch AG, Marton MJ. Transcriptional profiling shows that Gcn4p is a master regulator of gene expression during amino acid starvation in yeast. Mol Cell Biol. 2001;21:4347–4368. doi: 10.1128/MCB.21.13.4347-4368.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke JR, Behnke MS, Mackey AJ, Radke JB, Roos DS, White MW. The transcriptome of Toxoplasma gondii. BMC Biol. 2005;3:26. doi: 10.1186/1741-7007-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke JR, Donald RG, Eibs A, Jerome ME, Behnke MS, Liberator P, White MW. Changes in the expression of human cell division autoantigen-1 influence Toxoplasma gondii growth and development. PLoS Pathog. 2006;2:e105. doi: 10.1371/journal.ppat.0020105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke JR, Guerini MN, Jerome M, White MW. A change in the premitotic period of the cell cycle is associated with bradyzoite differentiation in Toxoplasma gondii. Mol Biochem Parasitol. 2003;131:119–127. doi: 10.1016/s0166-6851(03)00198-1. [DOI] [PubMed] [Google Scholar]
- Ruskin J, Remington JS. Toxoplasmosis in the compromised host. Ann Intern Med. 1976;84:193–199. doi: 10.7326/0003-4819-84-2-193. [DOI] [PubMed] [Google Scholar]
- Saksouk N, Bhatti MM, Kieffer S, Smith AT, Musset K, Garin J, Sullivan WJ, Jr, Cesbron-Delauw MF, Hakimi MA. Histone-modifying complexes regulate gene expression pertinent to the differentiation of the protozoan parasite Toxoplasma gondii. Mol Cell Biol. 2005;25:10301–10314. doi: 10.1128/MCB.25.23.10301-10314.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel BU, Hearn B, Mack D, Wender P, Rothbard J, Kirisits MJ, Mui E, Wernimont S, Roberts CW, Muench SP, Rice DW, Prigge ST, Law AN, McLeod R. Delivery of antimicrobials into parasites. Proc Natl Acad Sci USA. 2003;100:14281–14286. doi: 10.1073/pnas.2436169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sautel CF, Cannella D, Bastien O, Kieffer S, Aldebert D, Garin J, Tardieux I, Belrhali H, Hakimi MA. SET8-mediated methylations of histone H4 lysine 20 mark silent heterochromatic domains in apicomplexan genomes. Mol Cell Biol. 2007;27:5711–5724. doi: 10.1128/MCB.00482-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh U, Brewer JL, Boothroyd JC. Genetic analysis of tachyzoite to bradyzoite differentiation mutants in Toxoplasma gondii reveals a hierarchy of gene induction. Mol Microbiol. 2002;44:721–733. doi: 10.1046/j.1365-2958.2002.02903.x. [DOI] [PubMed] [Google Scholar]
- Slifko TR, Smith HV, Rose JB. Emerging parasite zoonoses associated with water and food. Int J Parasitol. 2000;30:1379–1393. doi: 10.1016/s0020-7519(00)00128-4. [DOI] [PubMed] [Google Scholar]
- Smith AT, Tucker-Samaras SD, Fairlamb AH, Sullivan WJ., Jr MYST family histone acetyltransferases in the protozoan parasite Toxoplasma gondii. Eukaryot Cell. 2005;4:2057–2065. doi: 10.1128/EC.4.12.2057-2065.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soete M, Camus D, Dubremetz JF. Experimental induction of bradyzoite-specific antigen expression and cyst formation by the RH strain of Toxoplasma gondii in vitro. Exp Parasitol. 1994;78:361–370. doi: 10.1006/expr.1994.1039. [DOI] [PubMed] [Google Scholar]
- Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- Su C, Evans D, Cole RH, Kissinger JC, Ajioka JW, Sibley LD. Recent expansion of Toxoplasma through enhanced oral transmission. Science. 2003;299:414–416. doi: 10.1126/science.1078035. [DOI] [PubMed] [Google Scholar]
- Sullivan WJ, Jr, Hakimi MA. Histone mediated gene activation in Toxoplasma gondii. Mol Biochem Parasitol. 2006;148:109–116. doi: 10.1016/j.molbiopara.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Sullivan WJ, Jr, Monroy MA, Bohne W, Nallani KC, Chrivia J, Yaciuk P, Smith CK, 2nd, Queener SF. Molecular cloning and characterization of an SRCAP chromatin remodeling homologue in Toxoplasma gondii. Parasitol Res. 2003;90:1–8. doi: 10.1007/s00436-002-0814-1. [DOI] [PubMed] [Google Scholar]
- Sullivan WJ, Jr, Narasimhan J, Bhatti MM, Wek RC. Parasite-specific eukaryotic initiation factor -2 (eIF2. kinase required for stress-induced translation control. Biochem J. 2004;380:523–531. doi: 10.1042/BJ20040262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vattem KM, Staschke KA, Zhu S, Wek RC. Inhibitory sequences in the amino terminus of the double-stranded-RNA-dependent protein kinase, PKR, are important for regulating phosphorylation of eukaryotic initiation factor -2α (eIF2α) Eur J Biochem. 2001;268:1143–1153. doi: 10.1046/j.1432-1327.2001.01979.x. [DOI] [PubMed] [Google Scholar]
- Vonlaufen N, Kanzok SM, Wek RC, Sullivan WJ., Jr Stress response pathways in protozoan parasites. Cell Microbiol. 2008;10:2387–2399. doi: 10.1111/j.1462-5822.2008.01210.x. [DOI] [PubMed] [Google Scholar]
- Weinman D, Chandler AH. Toxoplasmosis in swine and rodents; reciprocal oral infection and potential human hazard. Proc Soc Exp Biol Med. 1954;87:211–216. doi: 10.3181/00379727-87-21337. [DOI] [PubMed] [Google Scholar]
- Weiss LM, Kim K. The development and biology of bradyzoites of Toxoplasma gondii. Front Biosci. 2000;5:D391–405. doi: 10.2741/weiss. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss LM, Ma YF, Takvorian PM, Tanowitz HB, Wittner M. Bradyzoite development in Toxoplasma gondii and the hsp70 stress response. Infect Immun. 1998;66:3295–3302. doi: 10.1128/iai.66.7.3295-3302.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wek RC, Staschke KA, Narasimhan J. Regulation of the yeast general amino acid control pathway in response to nutrient stress. In: Winderickx J, Taylor PM, editors. Nutrient-induced responses in eukaryotic cells. Topics in current genetics. Springer-Verlab; Berlin: 2004. –7.pp. 171–199. [Google Scholar]
- Wong SY, Remington JS. Biology of Toxoplasma gondii. AIDS. 1993;7:299–316. doi: 10.1097/00002030-199303000-00001. [DOI] [PubMed] [Google Scholar]


