Abstract
The thermodynamic preference for the trans isomer of prolyl peptide bonds arises almost entirely from enthalpy in aqueous buffer and in toluene.
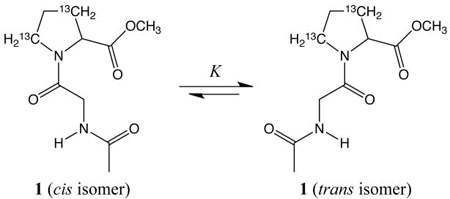
The trans (Z) isomer of a typical peptide bond is favored greatly over the cis (E) isomer. In contrast, a trans bond involving the nitrogen atom of a proline residue is favored only slightly, and both isomers are common in folded proteins.1 Knowing the thermodynamic origin for the relative stability of peptide bond isomers is essential for understanding the thermodynamic basis of protein stability.2 The difference in enthalpy for the cis and trans isomers of X–Pro bonds in aqueous solution has been reported to be zero for model peptides,3 or small (ca. 1.2 kcal/mol) for poly(Pro–Gly).4 The difference in free energy for the cis and trans isomers of amides has been calculated with the 6-31G** basis set of the Gaussian 82 ab initio program to be largely enthalpic in the gas phase.5 We have synthesized a peptide containing 13C-labeled proline, and used 13C NMR spectroscopy to determine the precise difference in enthalpy and entropy between the X–Pro bond isomers in protic and aprotic solvents.
Racemic Ac–Gly–[β,γ-13C]Pro–OMe (1) was synthesized by using standard methods.6 The N-and C-termini of 1 were capped so as to minimize intramolecular electrostatic interactions, which have been shown to alter the relative stability of the cis and trans isomers of X–Pro bonds.7 The equilibrium constant (K) for the isomerization of 1 was determined by integration of the Cβ resonances observed with 13C NMR spectroscopy at temperatures relevant for the study of protein stability.8
The effect of temperature on the value of K in aqueous buffer and in toluene is shown in Fig. 1. Van’t Hoff analysis of these results (assuming ΔCp° = 0) indicates that the difference in free energy for the X-Pro isomers of 1 originates almost entirely from enthalpic differences between these isomers. Further, the similarity of the enthalpies determined in aqueous buffer [ΔH° = − (1.27 ± 0.04) kcal/mol] and in toluene [ΔH° = − (1.27 ± 0.06) kcal/mol] suggests that the enthalpic forces that differentiate the cis and trans isomers of prolyl peptide bonds are similar in protic and aprotic environments. Differences in entropy, though small, favor the cis isomer in both aqueous buffer and toluene. The entropy difference is, however, less in water [ΔS° = − (0.25 ± 0.11) cal·mol/K] than in toluene [ΔS° = − (0.71 ± 0.18) cal·mol/K]. This result is consistent with the lower solvent accessibility of the amide C=O group in the trans isomer of 1, which diminishes the ability of this group to restrict H2O molecules through hydrogen bonding.9
Fig. 1.
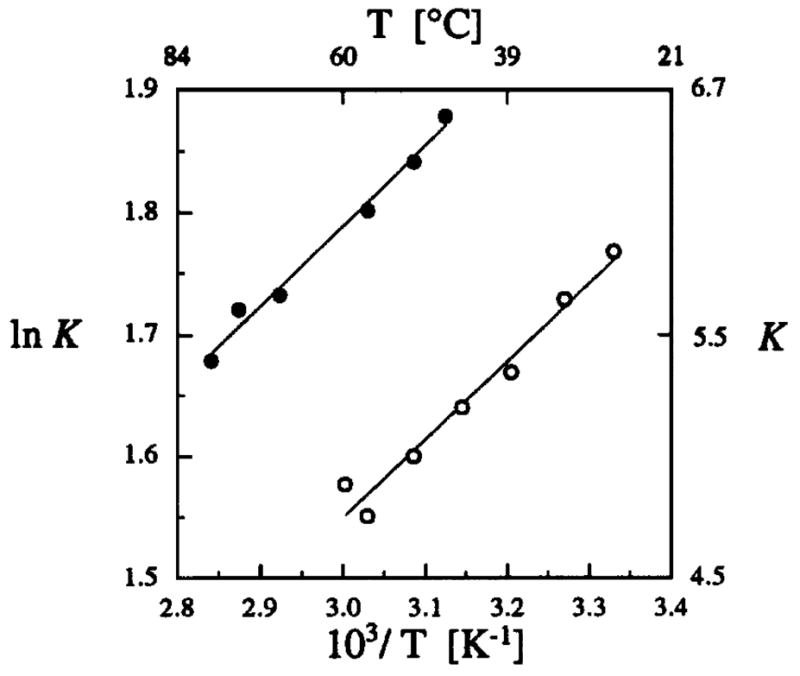
Van’t Hoff plot for the cis to trans isomerization of 1.
●, aqueous buffer:
ΔH° = − (1.27 ± 0.04) kcal/mol
ΔS° = − (0.25 ± 0.11) cal·mol/K
○, toluene:
ΔH° = − (1.27 ± 0.06) kcal/mol
ΔS° = − (0.71 ± 0.18) cal·mol/K
At 25°C in aqueous buffer:
ΔG° = − (1.34 ± 0.05) kcal/mol
At 25°C in toluene:
ΔG° = − (1.48 ± 0.08) kcal/mol
Acknowledgments
E.S.E. was a Wharton Predoctoral Fellow. S.N.L. was supported by Cellular and Molecular Biology Training Grant GM07215 (NIH). R.T.R. is a Presidential Young Investigator (NSF), Searle Scholar (Chicago Community Trust), and Shaw Scientist (Milwaukee Foundation). The National Magnetic Resonance Facility at Madison is supported by Grant RR02301 (NIH).
REFERENCES AND NOTES
- 1.(a) Thomas WA, Williams MK. J Chem Soc, Chem Commun. 1972:994. [Google Scholar]; (b) Evans CA, Rabenstein DL. J Am Chem Soc. 1974;96:7312–7317. doi: 10.1021/ja00830a023. [DOI] [PubMed] [Google Scholar]; (c) Stewart DE, Sarkar A, Wampler JE. J Mol Biol. 1990;214:253–260. doi: 10.1016/0022-2836(90)90159-J. [DOI] [PubMed] [Google Scholar]
- 2.(a) Evans PA, Kautz RA, Fox RO, Dobson CM. Biochemistry. 1989;28:362–370. doi: 10.1021/bi00427a050. [DOI] [PubMed] [Google Scholar]; (b) Alexandrescu AT, Hinck AP, Markley JL. Biochemistry. 1990;29:4516–4525. doi: 10.1021/bi00471a003. [DOI] [PubMed] [Google Scholar]; (c) Schultz DA, Baldwin RL. Protein Sci. 1992;1:910–916. doi: 10.1002/pro.5560010709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Madison V, Schellman J. Biopolymers. 1970;9:511–567. doi: 10.1002/bip.1970.360090502. [DOI] [PubMed] [Google Scholar]; (b) Maia HL, Orrell KG, Rydon HN. J Chem Soc, Chem Commun. 1971:1209–1210. [Google Scholar]; (c) Raleigh DP, Evans PA, Pitkeathly M, Dobson CM. J Mol Biol. 1992;228:338–342. doi: 10.1016/0022-2836(92)90822-2. [DOI] [PubMed] [Google Scholar]
- 4.Torchia DA. Biochemistry. 1972;11:1462–1468. doi: 10.1021/bi00758a021. [DOI] [PubMed] [Google Scholar]
- 5.Radzicka A, Pedersen L, Wolfenden R. Biochemistry. 1988;27:4538–4541. doi: 10.1021/bi00412a047. [DOI] [PubMed] [Google Scholar]
- 6.(a) Eberhardt ES, Loh SN, Hinck AP, Raines RT. J Am Chem Soc. 1992;14:5437–5439. doi: 10.1021/ja00039a072. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hinck AP, Eberhardt ES, Markley JL. submitted. [Google Scholar]
- 7.Grathwohl C, Wuthrich K. Biopolymers. 1981;20:2623–2633. [Google Scholar]
- 8.NMR experiments were performed on a Bruker AM500 instrument. Samples contained 0.1 M 1 in 100 mM sodium phosphate buffer, pH 7.2, containing 20% (v/v) D2O, or in dry toluene-d8. 13C NMR of 1 (125.77 MHz, CDCl3, 25 °C) δ 29.01 (Cβ, trans), 31.26 (Cβ, cis), 45.96 (Cδ, trans), 46.61 (Cδ, cis). δ was essentially independent of solvent or temperature.
- 9.Loh SN, Eberhardt ES, Edison AS, Weinhold F, Raines RT, Markley JL. submitted. [Google Scholar]