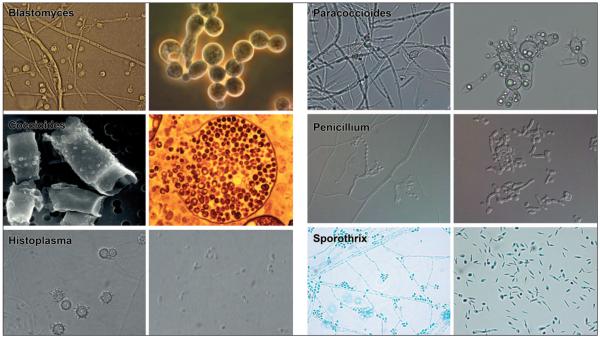
For endemic dimorphic fungi, switching between different morphologies is crucial for pathogenesis. These ascomycetes, which include Blastomyces dermatitidis, Histoplasma capsulatum, Coccidioides immitis, Coccidioides posadasii, Paracoccidioides brasiliensis, Sporothrix schenckii, and Penicillium marneffei, grow as mycelia that produce conidia or, in the case of Coccidioides spp., arthroconidia, when in soil or comparable environments (Fig. 1).
FIGURE 1.
Morphologic forms of the endemic dimorphic fungi. The mold form of each organism is on the left and the yeast or spherule form is on the right are depicted. The infectious particles acquired from the environment are shown on the left and tissue-based form on the right. One exception is the tuberculate macroconidia of Histoplasma, which is not believed to be the infectious particle. Microscopic images were provided by Garry Cole (Coccidiodes); George Deepe and George Smulian (Histoplasma); Gustavo Goldman (Paracoccidiodes); Chester Cooper (Penicillium); and Nuri Rodriguez (Sporothrix).
Dimorphic fungi typically are restricted to specific ecological niches. For example, Coccidioides spp. thrive in the alkaline-rich lower sonoran zone in the U.S. Southwest, whereas H. capsulatum and B. dermatitidis dwell in moist, acidic soils of the Midwest and Southeast. When those environments are disrupted, the conidia and mycelial fragments can be aerosolized, making infectious particles available for mammalian hosts to inhale. Once situated in lungs, the fungi become pathogenic yeast or, in the case of Coccidioides spp., spherules that contain endospores (Fig. 1).
These dimorphic fungi are primary pathogens that infect both immunocompetent and immuncompromised hosts. Although infections sometimes remain subclinical, symptoms may develop to include pneumonia, acute respiratory distress syndrome (ARDS), and disseminated disease, which can affect multiple organ systems. The extent and severity of infection are influenced by the inoculum size and the integrity of the host immune system.
The endemic dimorphic fungi must switch from a mold to a yeast form to survive in human and other mammalian hosts, in part because this change enables them to evade host immune defenses. This phase transition comes with characteristic changes in the cell wall, membrane lipids, intracellular signaling, and gene expression. When locked in the mycelial phase by biochemical or genetic means, these would-be pathogens fail to cause disease. The mechanisms that enable endemic dimorphic fungi to adapt and survive within the mammalian host are of particular interest to us.
Morphogenesis
External stimuli change intracellular signaling, metabolism, and gene expression in dimorphic fungi, triggering phase transition. Temperature is believed to be the major environmental trigger for morphogenesis. At 37°C, endemic dimorphic fungi grow as yeast or spherules, whereas at 22–25°C they grow as mycelia that produce conidia or arthroconidia. Changes in membrane fluidity and lipid composition may contribute to this morphological transition. Lower temperatures decrease membrane fluidity, which is compensated by a decrease in the ratio of saturated fatty acids to unsaturated fatty acids (SFA:UFA). The opposite occurs at higher temperatures.
Desaturase enzymes encoded by OLE1 in H. capsulatum and Candida albicans catalyze the conversion of saturated fatty acids (SFA) to unsaturated fatty acids (UFA). Exposing H. capsulatum mycelia to UFA prolongs mold-to-yeast conversion to 19 days from about 8 days following a temperature shift from 25 to 37°C. In contrast, exposing such cells to SFA reduces phase transition to 3 days following the same temperature shift. C. albicans conditional OLE1 null mutants fail to convert from yeast to hyphae when put into inducing medium; supplementing the medium with UFA overcomes this defect. Thus, changes in SFA:UFA ratios influence phase transition.
In addition to temperature, several other stimuli regulate morphogenesis, including oxidative stress, changes in carbon dioxide tension, and steroid hormones. To establish infection, dimorphic fungi not only need to convert to yeast, they also must resist reactive oxygen and nitrogen species such as hydrogen peroxide (H2O2), superoxide radical (O 2·−), hydroxyl radical (HO·), and nitric oxide (NO). In response to infection, host macrophages and neutrophils produce such reactive oxygen and nitrogen species, which damage the lipids, proteins, and nucleic acids of invading pathogens, eventually killing them.
Exposing B. dermatitidis and C. immitis cells to reactive oxygen species impairs conidia germination and inhibits phase transition. How such fungi combat reactive oxygen and nitrogen species is poorly understood. However, analysis of gene sequence information pertaining to H. capsulatum, B. dermatitidis, and P. brasiliensis points to several possible mechanisms. For instance, H. capsulatum and P. brasiliensis encode three catalase enzymes and a nitric oxide reductase that can neutralize the damaging effects of H2O2 and NO, respectively. In H. capsulatum, at least 153 transcripts respond to reactive nitrogen species, including those that are involved in acquiring iron, producing energy, responding to stress, folding and degrading proteins, repairing DNA, and detoxifying NO.
Carbon dioxide tension is 150-fold higher inside than outside mammalian lungs. The conversion of C. immitis arthroconidia to spherules requires not only a temperature shift to 37°C, but also a rise in CO2 tension. In the absence of high CO2, arthroconidia germinate into mycelia rather than spherules at 37°C.
Steroid hormones, specifically estrogens, modulate phase transition and fungal growth. The incidence of paracoccidioidomycosis, the systemic mycosis caused by P. brasiliensis, is similar in prepubescent males and females. Following puberty, however, paracoccidioidomycosis almost exclusively affects males. This difference is attributed to increased 17β-estradiol levels in adult females, which inhibits the changing of mycelia into yeast forms. In contrast, C. immitis spherules mature more rapidly in the presence of 17β-estradiol, perhaps explaining the increased risk of disseminated disease during pregnancy. These hormonal influences have not been observed for other endemic dimorphic fungi.
Morphogenesis and Intracellular Signaling
The cyclic adenosine monophosphate–protein kinase A (cAMP-PKA) and mitogen-activated protein kinase (MAPK) pathways are postulated to be the major signaling cascades involved in regulating phase transition in endemic dimorphic fungi. These pathways have been studied in fungi such as Saccharomyces cerevisiae and Neurospora crassa and in fungal pathogens such as C. albicans and Cryptococcus neoformans. When S. cerevisiae yeast cells detect high glucose or low nitrogen concentrations through G-protein-coupled membrane receptors, adenylate cyclase is activated, triggering increases in intracellular cAMP concentrations. In turn, cAMP stimulates PKA, activating transcription factors that promote pseudohyphal differentiation, adhesion, and invasion of agar in vitro. For C. albicans to become virulent, yeast must convert to hyphae. The cAMP-PKA pathway plays a part in regulating this shift, responding to stimuli such as serum. Changes that inhibit the generation of cAMP impair phase transition and attenuate virulence.
The cAMP-PKA signaling cascade is postulated to regulate phase transition in the endemic dimorphic fungi. cAMP concentrations are higher in B. dermatitidis and H. capsulatum mycelia than in their respective yeast forms. When theophylline is added to H. capsulatum cells to inhibit phosphodiesterase, intracellular cAMP levels rise, inducing yeast to convert to mycelia at 37°C. In addition, exposing H. capsulatum yeast cells to cAMP at 37°C promotes mycelial development. In contrast, during the temperature-induced mycelial-to-yeast transition in P. brasiliensis, intracellular cAMP concentrations eventually become higher in yeast than mycelia—again consistent with intracellular cAMP concentrations affecting phase transition.
Mitogen-activated, protein-kinase signaling also can regulate several processes, including morphogenesis. Fungal MAPK signaling cascades consist of several kinases, often shared among different pathways, that regulate genetic programs in response to specific stimuli. In S. cerevisiae and C. albicans, for example, MAPK pathways govern cell wall integrity, pheromone-induced mating, responses to osmotic and oxidative stress, and yeast-to-hyphal transitions. The pheromone response system and filamentation pathway alter S. cerevisiae morphology, resulting in schmoo cells and hyphal growth, respectively. As in the case of the cAMP-PKA pathway, low nitrogen or high glucose concentrations activate the MAPK filamentation cascade. In C. albicans, the Cek1 MAPK pathway promotes yeast-to-hyphal transition.
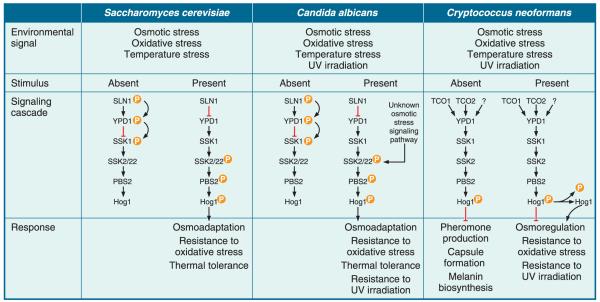
In endemic dimorphic fungi such as B. dermatatidis and H. capsulatum, MAPK signaling is postulated to regulate phase transition through DRK1. This gene encodes a histidine kinase having homology to S. cerevisiae Sln1, C. albicans Sln1, and C. neoformans Tco1, which regulate stress responses through the high-osmolarity glycerol (HOG) pathway (Fig. 2). When Hog1 is activated, it moves from the cytoplasm to the nucleus where it activates several transcription factors that regulate genetic programs protecting these fungi from osmotic, oxidative, thermal, and ultraviolet stresses.
FIGURE 2.
High-osmolarity glycerol (HOG) pathway in S. cerevisiae, C. albicans, and C. neoformans. (A) Sln1 undergoes autophosphorylation in the absence of osmotic, oxidative, or temperature stress. This phosphate is transferred to a phosphotransfer protein, Ypd1, which relays it to and inactivates the response regulator protein, Ssk1. The presence of osmotic, oxidative, or temperature (heat or cold) stress, inhibits the phosphorelay between Sln1, Ypd1, and Ssk1, resulting in unphosphorylated and active Skk1, which can then interact with Ssk2/22. This interaction promotes Ssk2/22 autophosphorylation, which triggers the MAPK cascade resulting in phosphorylation of Pbs2 and Hog1. Phosphorylated Hog1 translocates from the cytoplasm to the nucleus to activate transcription factors involved with osmoadaption, resistance to oxidative stress, and thermal tolerance. (B) The phosphorelay between Sln1, Ypd1, Ssk1 and activation of the MAPK cascade in the human pathogen C. albicans is postulated to be similar to that in S. cerevisiae. Phosphorylated Hog1 translocates from the cytoplasm to the nucleus to activate transcription factors that contribute to C. albicans adaptation and resistance to osmotic, oxidative, and temperature stress, as well as UV irradiation. In addition to Sln1, there is an unknown osmotic stress response pathway that bypasses Ssk1. (C) In the absence of environmental stress, C. neoformans Hog1 is constitutively phosphorylated. This has been demonstrated in most, but not all, C. neoformans isolates. Phosphorylated Hog1 negatively regulates pheromone production, capsule formation, and melanin biosynthesis. In the presence of osmotic, oxidative, and temperature stress, as well as UV irradiation, Hog1 becomes dephosphorylated by the action of phosphatases facilitating C. neoformans adaptation to these stresses. It is unknown if the regulation of the phosphorelay between Tco1/2, Ypd1, and Ssk1 is similar to S. cerevisiae. (Figure adapted with permission from Bahn YS et al., Nature Rev. Microbiol.5:57–69, 2007).
In B. dermatitidis and H. capsulatum, DRK1 appears to control a signaling cascade that resembles the SLN1-regulated HOG pathway in S. cerevisiae. DRK1 is viewed as a “global regulator” with pleotropic effects on cell wall composition and integrity, sporulation, morphogenesis, control of yeast-phase specific genes, and virulence. DRK1 null mutants are avirulent, grow as mycelia at 37°C, cannot sporulate, fail to convert to the pathogenic yeast form, and are avirulent in a murine model of infection. In addition, these mutants have reduced levels of the virulence factor α-1,3-glucan in their cell wall, and decreased expression of the yeast-phase specific proteins BAD1 (B. dermatitidis) and CBP1 (H. capsulatum), which also are indispensable in pathogenesis. The inability of B. dermatitidis and H. capsulatum to upregulate expression of yeast phase-specific genes and convert to a yeast form when shifted from 22 to 37°C suggests that, like S. cerevisiae Sln1, Drk1 functions as sensor of environmental changes such as temperature.
Similar to DRK1, H. capsulatum RYP1 governs the transition of mold to yeast. Following a shift from 25 to 37°C, RYP1 insertional mutants or RYP1-silenced strains remain locked in the mycelial form. Such strains also fail to express 98% of the genes that are differentially expressed in the yeast phase, including two virulence factors, CBP1 and YPS3. These mutants also are unable to downregulate genes preferentially expressed in the mycelial phase. Ryp1 is a member of a family of fungal proteins that includes Wor1, a master transcriptional regulator of the white-opaque transition required for mating in C. albicans.
Host Response and Immune Evasion
Cell-mediated immunity (CMI), encompassing cells of the innate and adaptive immune systems, enables hosts to combat systemic fungal infections. CMI may become impaired in some individuals, either through drugs that suppress immune responses or through other conditions such as acquired immunodeficiency syndrome (AIDS), chronic granulomatous disease, diabetes, and pregnancy. When such individuals are infected with fungi, they are predisposed to develop severe complications, including acute respiratory distress syndrome (ARDS), and disseminated infections that spread to sites such as the central nervous system.
Individuals who are immune competent generally can control such infections once the adaptive immune response is activated. The early stages of this process typically require that macrophages and dendritic cells present fungal antigens to T-lymphocytes. An effective adaptive immune response entails the generation of Th1 cytokines, including tumor necrosis factor (TNF-α) and interferon gamma (IFN-γ). They activate macrophages to produce reactive oxygen and nitrogen species that kill fungi or inhibit their growth.
The dimorphic fungi draw on several strategies to evade innate and adaptive immunity. After entering the lungs, conidia encounter cells of the host innate immune system, including neutrophils, monocytes, and macrophages. Unless activated by Th1 cytokines, however, these cells do little to combat inhaled conidia. In non-activated cells, H. capsulatum, P. brasiliensis, and P. marneffei conidia convert to yeast, and can use these cells to disseminate through the host. Similarly, when host phagocytes take up C. immitis arthroconidia, the endospores typically survive and develop into spherules that eventually lyse the host cells. Nonactivated macrophages also are ineffectual at killing S. schenckii conidia. Perhaps one reason conidia survive inside phagocytes is that such particles fail to induce the generation of reactive oxygen species.
When endemic dimorphic fungi convert to yeast, they express yeast-phase-specific genes, altering their cell wall composition and enabling them to evade the immune system. For instance, the Blastomyces adhesion-1 (BAD1) virulence factor is a 120-kDa multifunctional, yeast-phase-specific protein that attaches yeast to cells lining the lung alveoli, inhibits complement deposition on the yeast cell surface, scavenges calcium in low-calcium environments, and suppresses TNF-α generation from macrophages and neutrophils by binding complement receptor 3 (CR3). TNF-α is crucial for host defense against fungal pathogens since it enhances IFN-γ production, induces reactive nitrogen species, promotes granulomas that contain infections, and contributes to the development of immunological memory. Cell surface BAD1 also stimulates transforming growth factor β1 (TGF-β1), which down-regulates TNF-α. Conversely, soluble BAD1 undergoes receptor-mediated en-docytosis after binding CR3 on macrophages and likely also alters intracellular signaling to suppress TNF-α.
Whereas BAD1 dysregulates cytokine production, other yeast-phase virulence genes have different modes of action. For instance, H. capsulatum calcium-binding protein (CBP) is a 7.8-kDa secreted protein that allows this pathogen to survive within macrophages. CBP1 null mutants grow poorly under calcium-restricted conditions and within macrophages, and they are severely attenuated when they infect the lungs of mice. B. dermatitidis BAD1 also binds calcium and fosters survival under calcium-limited conditions. The functional role of calcium binding in vivo remains to be elucidated.
H. capsulatum YPS3 encodes a 20-kDa protein that shares structural features with BAD1, particularly its C-terminus EGF-like domain, which attaches to chitin on the yeast cell surface. Silencing YPS3 expression by RNA interference impairs the ability of H. capsulatum to colonize lungs, liver, and spleen, without altering its survival in macrophages. The mechanism behind the virulence-promoting effect of Yps3 is unknown.
Three spherule-specific genes, BLG2, SOWgp, and MEP1, contribute to virulence of Coccidioides spp. BLG2 encodes a 120-kDa β-glucosidase that cleaves β-1,3-glucan, enabling the cell wall to expand during spherule growth. Spherule outer-wall glycoprotein encoded by SOWgp is an adhesin that binds host extracellular matrix proteins such as laminin, fibronectin, and collagen. Although the cellular and humoral immune systems recognize SOWgp, it promotes a Th2 cytokine response that favors pathogen survival. During spherule maturation, the developing endospores become coated with SOWgp, which MEP1 degrades, retarding immune recognition.
Proteins and carbohydrates of the cell wall change during phase transition, contributing to virulence and immune evasion in the yeast phase. For example, α-(1,3)-glucan increases, while β-(1,3)-glucan decreases, and this shift in the yeast cell wall correlates with pathogenicity for dimorphic fungi such as Histoplasma, Blastomyces, and Paracoccidiodes. Deleting the H. capsulatum α-(1,3)-glucan synthase (AGS1) eliminates cell wall α-(1,3)-glucan, retards fungal growth in macrophages following phagocytosis, and attenuates virulence. Similarly, deleting the H. capsulatum α-(1,4)-amylase (AMY1), which contributes to glucan synthesis, reduces cell wall α-(1,3)-glucan and yields a phenotype similar to that of AGS1 null mutants.
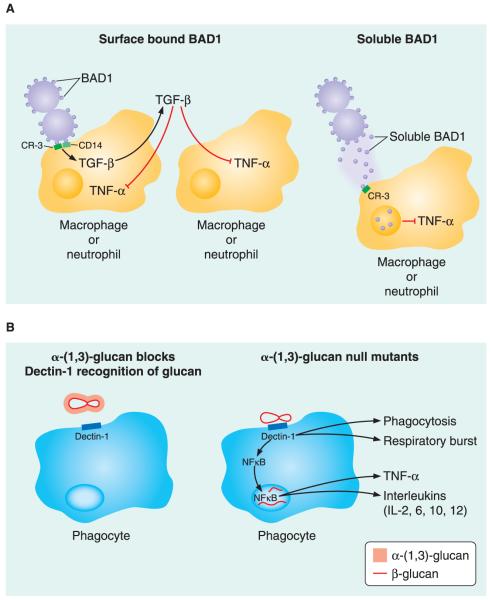
Because α-(1,3)-glucan appears to play a role in the virulence of H. capsulatum, B. dermatitidis, and P. brasiliensis, it may provide a target for novel therapeutics. Because α-(1,3)-glucan is in the outermost layer of the cell wall of H. capsulatum yeast, it may shield β-(1,3)-glucan from pattern recognition receptors (PRR) found on cells of the innate immune system (Fig. 3). Several other cell wall components, including β-(1,3)-glucan, chitin and mannosylated proteins, can act as pathogen-associated molecular patterns (PAMPs) that PRRs on immune cells recognize.
FIGURE 3.
Selected mechanisms of immune evasion in B. dermatitidis and H. capsulatum. (A) Blastomyces BAD1 downregulates TNF-α production by phagocytes. Yeast surface BAD1 binds to CR3 and CD14 receptors on macrophages and neutrophils stimulating the production of TGF-β, which acts in an autocrine and paracrine fashion to inhibit TNF-α production. Soluble BAD1 also binds CR3, facilitating receptor-mediated endocytosis. Internalized BAD1 is postulated to alter intracellular signaling to inhibit TNF-α production. (B) Histoplasma α-(1,3)-glucan blocks dectin-1 recognition of β-glucan. H. capsulatum α-(1,3)-glucan shields β-(1,3)-glucan in the cell wall from phagocyte Dectin-1 receptor recognition. The absence of α-(1,3)-glucan in the H. capsulatum cell wall leaves β-(1,3)-glucan exposed and accessible for phagocyte recognition and binding by Dectin-1 receptors. Engagement of the Dectin-1 receptor promotes phagocytosis, generation of the respiratory burst, and activation of NF-κB resulting in TNF-α and interleukin (IL-2, IL-6, IL-10, IL-12) production.
One such PRR, Dectin-1, on myeloid cells such as neutrophils, macrophages, and dendritic cells recognizes β-(1,3)-glucan in the cell walls of several fungal species, including H. capsulatum, Aspergillus spp., Candida spp., and Pneumocystis. Engagement of Dectin-1 by β-glucan triggers several events, including phagocytosis, a respiratory burst, and release of cytokines such as TNF-α, IL-12, and other interleukins (Fig. 3). Yeast α-(1,3)-glucan may actively interfere with these events. For instance, H. capsulatum AGS1 null mutants stimulate macrophage TNF-α release to a greater extent than wild-type isolates that have α-(1,3)-glucan on their surface. Silencing macrophage Dectin-1 gene expression blunts this TNF-α response, suggesting that α-(1,3)-glucan effectively shields β-(1,3)-glucan from Dectin-1 on myeloid cells. This may explain how α-(1,3)-glucan promotes virulence in dimorphic fungi such as H. capsulatum.
The concept of shielding β-(1,3)-glucan is not limited to dimorphic fungi. C. albicans displays β-(1,3)-glucan only on the bud scars of dividing yeast, and not on the more pathogenic hyphae. The large capsule of C. neoformans also shields β-(1,3)-glucan.
In B. dermatatidis and P. brasiliensis, the concentration of cell-wall β-(1,3)-glucan drops from 40–50% in mycelia to less than 5% in yeast, suggesting that α-(1,3)-glucan shielding of this polymer may be less important in these fungi. In addition, exposed β-(1,3)-glucan in B. dermatitidis fixes mannose-binding lectins (MBL-A and MBL-C), preventing recognition by Dectin-1. During fungal recognition by immune cells, Dectin-1 may cooperate with other PRR, for example toll-like receptor 2 (TLR2). β-(1,3)-glucan in the cell wall of C. posadasii, induces macrophage production of IL-6, IL-12, TNF-α, and MIP-2 in a manner that requires TLR2. It is therefore likely that additional PRRs, alone and together with Dectin-1, enable the host to sense fungal invaders and eliminate them. Investigation of this host-pathogen interface, and the tension between recognition and evasion, is likely to shed light on the pathogenesis of fungal infections.
Summary.
Endemic dimorphic fungi switch from an environmental mold to a pathogenic yeast form after inhalation into the lungs because this change enables them to survive inside humans and other mammalian hosts.
Temperature is the key among several stimuli that change intracellular signaling, metabolism, and gene expression in dimorphic fungi, triggering this phase transition that leads to virulence.
The cyclic adenosine monophosphate–protein kinase A and mitogen-activated protein kinase pathways are thought to be the major signaling cascades involved in regulating phase transition.
The phase transition promotes evasion of innate and adaptive immunity in part through the expression of yeast-phase specific genes and alteration of cell wall components.
Early in His Career, Klein Was Smitten with Investigating Disease Outbreaks.
When Bruce Klein was a second-year medical student, he was so taken with the Berton Roueche “Annals of Medicine” narratives in the New Yorker that he decided to become a disease detective himself. “I read about investigations by medical sleuths solving all kinds of cool mysteries,” he says. “I found the stories terribly captivating and romantic.”
In 1981, Klein joined the Epidemic Intelligence Service (EIS) at the Centers for Disease Control and Prevention in Atlanta, Ga., and was sent to Madison, Wis., where he established surveillance for the “fungal problem” then causing concerns throughout the state. His biggest “case” occurred several years later when he was an infectious disease fellow in medicine and pediatrics at the University of Wisconsin. That case revolved around four dozen individuals—all but two, children in the fifth and sixth grade. They developed a similar respiratory infection soon after the school year ended in June.
The common denominator was their recent field trip to an environmental camp in Eagle River, where the children had explored a beaver pond and lodge. The infection among the children “turned out to be a fungal infection of the kind I had been asked to bone up on as a resident expert at the state health department,” Klein says. Specifically, the children were suffering from blastomycosis, caused by Blastomyces dermatitidis, a fungus from soil. Although several children were hospitalized and placed in intensive care, no one died.
“The intellectual gratification that came from cracking this case was overwhelming and intoxicating,” Klein says. “I was smitten! I had never experienced a thrill like this, and was hooked.”
Klein, 56, is the Gerard B. Odell Professor of Pediatrics at the University of Wisconsin Medical School, where he continues to investigate fungi. But the mystery that preoccupies him today is why and how they change, and the reasons they become virulent when they infect humans. “Over the last few decades, we have seen a dramatic upsurge in the frequency and severity of infections due to fungi,” he says. “These are some of the most difficult kinds of infections to treat, and there are no vaccines to prevent them,” he says.
Further, because fungi are eukaryotes, it is difficult to develop drugs that kill or control fungal growth without harming humans. “As a physician who is also a scientist, I see the growing, urgent need for new therapies,” he says. “I have witnessed severe and invasive fungal infections spread wildly in vulnerable children undergoing treatment for cancer, while we watch helplessly.”
Klein, a first-generation American whose parents are survivors of World War 2 concentration camps, was born and raised in Queens, N.Y. His parents did not have the opportunity to attend college “but they had a deep respect for education, hard work, and, of course, family,” he says. He went to high school in Franklin Square, N.Y., where the family moved after his father bought a shoe store there.
Neither of Klein’s parents has a background in science, but “as with most Jewish mothers, mine felt that I should become a doctor,” he says. “While I loved nature and biology, and am a deeply curious person about how things work, I had some ambivalence about medicine per se—although the idea of giving something back to make the world a better place did appeal to me.”
Part of this commitment is tied to personal history. “Many of my family that I never knew perished in Europe,” he says. “Perhaps by contributing something of lasting value on this earth, through me, their lives and our lineage will have greater meaning.”
As an undergraduate, Klein studied political science at Boston University before shifting his attentions to medicine. He received his M.D., also from Boston University, in 1978 and served his residency, in pediatrics, at John Hopkins Hospital in Baltimore between 1978 and 1981.
“As I advanced, I saw and seized opportunities for scientific investigation in parallel with the practice of clinical medicine, and these have added a richness and gratification to my work, both intellectually and in a way that makes me feel that there is a chance to have a lasting impact,” he says. “Not only one patient at a time, but perhaps by improving the practice of medicine for many patients, or for populations.”
He and his wife Patty have two sons. His wife founded and directed a private nonprofit agency for child abuse prevention until a few years ago. Since retiring, she spends her time “caring for cats, sometimes dogs, and occasionally birds,” Klein says.
In his spare time, Klein likes to read and watch independent films. When the weather permits, Klein rides a bike. He also runs as part of “a group that has been running together every Sunday in Madison for about 25 years,” he says. “We used to train for marathons, but now we run casually, or occasionally bike, with our kids, dogs, and growing waistlines. One thing is for sure—at the end of every Sunday run, a big breakfast reward awaits.”
Marlene Cimons
Marlene Cimons is a freelance writer in Bethesda, Md.
ACKNOWLEDGMENTS
The authors are supported by grants from the National Institutes of Health and the Hartwell Foundation, and by institutional funds from the University of Wisconsin-Madison.
SUGGESTED READING
- Brandhorst TT, Wuthrich M, Finkel-Jimenez B, Warner T, Klein BS. Exploiting type 3 complement receptor for TNF-alpha suppression, immune evasion, and progressive pulmonary infection. J. Immunol. 2004;173:7444–7453. doi: 10.4049/jimmunol.173.12.7444. [DOI] [PubMed] [Google Scholar]
- Hung CY, Yu JJ, Seshan KR, Reichard U, Cole GT. A parasitic phase-specific adhesion of Coccidioides immitis contributes to the virulence of this respiratory fungal pathogen. Infect. Immun. 2002;70:3443–3456. doi: 10.1128/IAI.70.7.3443-3456.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung CY, Seshan KR, Yu JJ, Schaller R, Xue J, Basrur V, Gardner MJ, Cole GT. A metalloproteinase of Coccidioides posadasii contributes to evasion of host detection. Infect. Immun. 2005;73:6689–6703. doi: 10.1128/IAI.73.10.6689-6703.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koneti A, Linke MJ, Brummer E, Stevens DA. Evasion of innate immune responses: evidence for mannose binding lectin inhibition of TNF-α production by macrophages in response to Blastomyces dermatitidis. Infect. Immun. 2008;76:994–1002. doi: 10.1128/IAI.01185-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresca B, Kobayashi GS. Changes in membrane fluidity modulate heat shock gene expression and produced attenuated strains in the dimorphic fungus H. capsulatum. Arch. Med. Res. 1993;24:247–249. [PubMed] [Google Scholar]
- Nemecek JC, Wuthrich M, Klein BS. Global control of dimorphism and virulence in fungi. Science. 2006;312:583–588. doi: 10.1126/science.1124105. [DOI] [PubMed] [Google Scholar]
- Nguyen VQ, A Sil. Temperature-induced switch to the pathogenic yeast form of Histoplasma capsulatum requires Ryp1, a conserved transcriptional regulator. Proc. Natl. Acad. Sci. USA. 2008;105:4880–4885. doi: 10.1073/pnas.0710448105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappleye CA, Engle JT, Goldman WE. RNA interference in Histoplasma capsulatum demonstrates a role for alpha-(1,3)-glucan in virulence. Mol. Microbiol. 2004;53:153–165. doi: 10.1111/j.1365-2958.2004.04131.x. [DOI] [PubMed] [Google Scholar]
- Rappleye CA, Eissenberg LG, Goldman WE. Histoplasma capsulatum alpha-(1,3)-glucan blocks innate immune recognition by the beta-glucan receptor. Proc. Natl. Acad. Sci. 2007;104:1366–1370. doi: 10.1073/pnas.0609848104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebghati TS, Engle JT, Goldman WE. Intracellular parasitism by Histoplasma capsulatum: fungal virulence and calcium dependence. Science. 2000;290:1368–1372. doi: 10.1126/science.290.5495.1368. [DOI] [PubMed] [Google Scholar]