Abstract
Tianeptine is a clinically used antidepressant that has drawn much attention, because this compound challenges traditional monoaminergic hypotheses of depression. It is now acknowledged that the antidepressant actions of tianeptine, together with its remarkable clinical tolerance, can be attributed to its particular neurobiological properties. The involvement of glutamate in the mechanism of action of the antidepressant tianeptine is consistent with a well-developed preclinical literature demonstrating the key role of glutamate in the mechanism of altered neuroplasticity that underlies the symptoms of depression. This article reviews the latest evidence on tianeptine’s mechanism of action with a focus on the glutamatergic system which could provide a key pathway for its antidepressant action. Converging lines of evidences demonstrate actions of tianeptine on the glutamatergic system, and therefore offer new insights into how tianeptine may be useful in the treatment of depressive disorders.
Index terms: Tianeptine, hippocampus, amygdala, stress, antidepressant
1. Introduction
Depression is a complex, heterogeneous disorder, and the mechanisms underlying its pathogenesis are not that clear and are the subject of intensive investigation using pharmacological and genetic tools and animal models. The “monoamine hypothesis” of depression, which involves imbalances in serotonergic, noradrenergic and possibly dopaminergic functions, has dominated notions and explanations of the pathophysiology of depression since the empirical discovery of the antidepressant properties of monoamine oxidase inhibitors (MAOIs) and tricyclics about fifty years ago. Although the monoaminergic neurotransmitters serotonin (5-HT), noradrenaline (NA) and dopamine (DA) are undoubtedly involved, it is now recognized that monoamine deficits are only part of the story and are not sufficient on their own to explain the mechanism of action of antidepressants.
In extension to the chemical hypothesis of depression, contemporary theories suggest that major depressive disorders may be associated not only with an imbalance of neurotransmitters and neuromodulators but also with an impairment of neuroplasticity and cellular resilience, and that antidepressant medications act by normalizing this impairment (1–3). The term neuroplasticity describes the ability of the adult and differentiated brain to adapt functionally and structurally to internal and external stimuli and is considered today as a feature of depressive illness. Brain regions that exhibit neuroplastic processes include the hippocampus, amygdala and prefrontal cortex (4), as they are reported to undergo structural changes in depression (5,6) and alterations in these brain regions affect emotions, perceptions, memory, and cognitive function.
The concept of a serotonergic deficit in depression is particularly challenged by the drug tianeptine, (S 1574, [3-chloro-6-methyl-5,5-dioxo-6,11-dihydro-(c,f)-dibenzo- (1,2-thiazepine)-11-yl) amino]-7 heptanoic acid, sodium salt), an antidepressant with structural similarities to the tricyclic antidepressant agents but with different pharmacological properties.
The efficacy and tolerability of tianeptine are clearly demonstrated in depressed patients (7). However, the monoamine hypothesis cannot explain these properties. In fact, tianeptine has contributed greatly to our realization of the complexity of the etiology of depression, and to the complexity of central mechanisms triggered by antidepressants. Its mechanisms of action clearly challenge the hypothesis of an immediate modulation of monoamine axes to support the antidepressant actions. Rather, tianeptine triggers a cascade of cellular adaptations that ultimately will lead to the antidepressant efficacy. Among those sustained adaptations, increased phosphorylation of glutamate receptors subtypes in circumscribed brain region appears particularly interesting (8,9). Glutamate is the major excitatory neurotransmitter in the brain controlling synaptic excitability and plasticity in most brain circuits, including limbic pathways. Glutamatergic mechanisms play crucial roles in virtually all key functions perturbed in depressed states (10–15). In addition, glutamate is an essential participant in many forms of adaptive plasticity, including learning and memory. The actions of tianeptine on the glutamatergic system offer new insights into how this compound may be useful in the treatment of depressive disorders. The goal of this review is to summarize neurobiological studies on the antidepressant tianeptine. In an attempt to provide a possible explanation for the observed beneficial clinical profile of tianeptine, this article will review the latest evidence on its mechanisms of action with a focus on the glutamatergic system.
2. The actions of tianeptine
2.1. Non clinical and clinical antidepressant and anxiolytic features
There is a number of experimental studies demonstrating robust efficacies of tianeptine in rodent paradigms of antidepressant properties (16–20). Further, tianeptine opposes not only the affective, but also the cognitive and structural changes that characterize depressive states—at least in experimental models of depression based on chronic stress (20,21). Moreover, in line with these observations, compelling body of clinical data has demonstrated that the clinical efficacy of tianeptine in the treatment of depression is at least equivalent to those of selective serotonin reuptake inhibitors (SSRIs) (22–30). Tianeptine affords rapid relief of depressive symptoms as the analysis of MADRS individual items shows that decreased ability of concentration and inner tension are more rapidly improved in tianeptine-treated than in fluoxetine-treated patients (difference from baseline of MADRS individual scores “ability of concentration” at day 7: −0.5 ; “inner tension” at day 14: −1.3, P <0.05) (31). Tianeptine is effective in reducing symptoms of depression in mild to moderate-to-severe major depression, while it alleviates anxious symptoms associated with depression without the need for concomitant anxiolytic therapy (24,26,32–35). The good tolerability of tianeptine is also established as the antidepressant lacks the sedative (36) autonomic, cardiovascular, and undesirable side effects on attention and memory of tricyclics (19,37) and shows a low propensity to provoke sexual dysfunction and nausea as compared to SSRIs (38,39).
2.2. Neuroplasticity
One of the most thoroughly studied hypotheses of tianeptine’s antidepressant efficacy is the effects it has on central neuroplasticity (20). The relationship between stress and neuronal remodeling, especially in CA3 pyramidal neurons of the hippocampus (40,41), has been described and reviewed extensively. This is of particular relevance given the recent evidence from literature that depression is associated with hippocampal volume loss and that the disorder may present a degenerative component (6,42). Recent neurobiological evidence suggests that mood disorders, such as major depressive disorder, are characterised by neuron dendrite shrinkage, glial cell loss, and/or impairments in neuroplasticity and cellular resilience (43).
Stress paradigms have been used as models for depression in animal studies to investigate the actions of antidepressants on brain structure and neuroplasticity. In such models, tianeptine opposes the effects of chronic stress on brain structure and plasticity. For example, tianeptine prevented structural changes and modified neuronal metabolism and function in the hippocampus (44). In tree shrews subjected to psychosocial stress, tianeptine reversed the stress-induced decreases in hippocampal volume, concentrations of cerebral metabolites such as N-acetyl-aspartate, and proliferation of the granule precursor cells in the dentate gyrus (44). In an investigation of glucocorticoid induced remodeling of hippocampal CA3 dendrites, the therapeutic effects of tianeptine administration was further demonstrated after the shrinkage of dendrites had occurred, since tianeptine treatment was able to reverse these changes even while glucocorticoid administration was continuing (40).
From the literature it seems that, amongst the antidepressants, tianeptine has been the most extensively investigated for its prominent and consistent protective effect against stress induced neuronal remodeling. The most pronounced effects of tianeptine are exerted in the dentate granule cell layer and in the adjacent subgranular zone within the hippocampal formation, which gain special significance when considering the study of Czéh and co-workers (44) in an animal model of depressive disorders. Thus, not only cytogenesis but also cell death, and therefore the entire process of adult dentate gyrus neuronal turnover, is positively affected by tianeptine treatment. In line with the anti-apoptotic effect observed post mortem by Lucassen and colleagues (45), tianeptine prevented the stress-induced reduction of the in vivo brain metabolite levels of N-acetylaspartate, which can be understood as the reversion of a stress-induced reduction of neuroaxonal cellular density and/or dysfunction (44). One can assume that the reversibility is due to alterations in glia cells, as well as in the dendritic, axonal, and synaptic components of the hippocampal neural network.
Studies of the role of intracellular signal transduction and regulation of gene expression in impaired neuroplasticity in depression has led to a neurotrophic hypothesis of depression, with brain-derived neurotrophic factor (BDNF) as an important mediator of neuronal plasticity and a potential target for antidepressant drug development (4). Stress has been shown to decrease BDNF expression in the hippocampus, which may contribute to the neuronal atrophy and sometimes neural cell loss in key limbic regions in the brain seen in patients with depression. Conversely, the efficacy of some classes of antidepressants is proposed to involve increased phosphorylation of cAMP response element binding protein (CREB), leading to increased expression of neurotrophic factors, such as BDNF (4). Data suggest that tianeptine may promote neuroplasticity by increasing expression of genes of neuroplastic factors that are decreased in animal models of stress. These include the genes for BDNF and nerve growth factor in the hippocampus (46) and amygdala (47).
A number of other critical molecules in neurotrophic signalling cascades (most notably cyclic adenosine monophosphate –cAMP- response element binding protein, bcl-2, and mitogen activated protein –MAP- kinases) are also potential targets for tianeptine potentiating modalities. At the present time, the contribution of these molecules to the mechanism of action of tianeptine remains to be demonstrated but the novel signal transduction mechanisms that have been recognized so far may provide a mechanistic resolution for the neuroprotective properties of tianeptine and, moreover, suggest a pharmacological trajectory for the antidepressant and/or memory enhancing activity of tianeptine.
Depressive illness is associated with changes in amygdalar volume, and stressful life events are known to precipitate depressive episodes in this patient population. Anatomically, the amygdala is connected both directly (amygdalo-hippocampal bundles arise from the basolateral amygdala and terminate in the CA1, the CA3 and the subiculum) and indirectly (through the entorhinal cortex) to several hippocampal regions (48).
The effects of tianeptine on brain plasticity extend to the amygdala. Using morphometric techniques, Vyas et al (49) have demonstrated that chronic stress induce contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. Chronic immobilization stress elicited significant dendritic atrophy in hippocampal CA3 pyramidal neurons, as previously reported but, in striking contrast, chronic immobilization stress increased dendritic arborization of neurons in the basolateral nucleus of the amygdala (BLA). This stress-induced enhancement in dendritic arborization did not represent a generalized increase in all classes of BLA neurons, but was restricted only to BLA pyramidal and stellate neurons, which are presumably excitatory projection neurons.
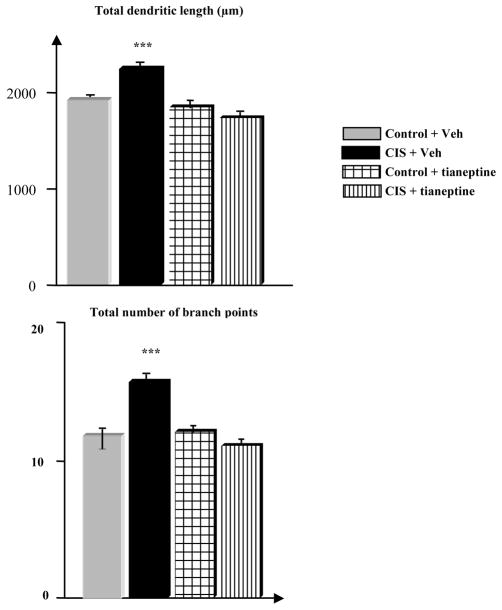
As stress-induced dendritic remodeling in the amygdala may provide a potential cellular substrate for depression caused by chronic immobilization stress (for 10 days), the effects of tianeptine (10 mg/kg, daily) on stress-induced dendritic remodeling in the BLA has been studied. Stress causes a significant increase in the dendritic length of BLA spiny neurons (p<0.05 vs. control animals), but this stress-induced enhancement in dendritic arborization in BLA neurons was prevented by tianeptine (50). There was no significant difference in total dendritic length of BLA neurons from tianeptine-treated animals, stressed or not. However, there was a significant decrease in the total dendritic length of neurons from stressed animals treated by tianeptine when compared to stressed animals receiving the vehicle (Figure 1). Moreover, prevention of dendritic hypertrophy in the BLA by tianeptine was associated with a preventive effect of the antidepressant on potentiation of anxiety-like behavior in male rats (assessed in the elevated plus maze). As Conrad and coworkers (51) reported repeated restraint stress enhanced freezing to context and tone and decreased open field exploration irrespective of whether tianeptine was given or not; such results would suggest no association between morphological and behavioral effects. However, more recently Wood and coworkers (52) have shown that chronic tianeptine prevented stress-induced potentiation of aggressive conflicts, while Burghardt and colleagues (53) reported that chronic tianeptine given for 21 days before training can reduce fear conditioning. Taken together, these findings provide some convergence on the potential efficacy of tianeptine in terms of its actions in the amygdala, most of the data suggesting that tianeptine inhibits stress-induced behavioral changes and/or enhances appropriate responses of the amygdala.
Figure 1. Tianeptine regulates neuronal plasticity in the amygdala.
Rat dendritic arborization in the basolateral amygdale (BLA) increases after repeated chronic (10 days) immobilization stress. This stress-induced enhancement in dendritic arborization in BLA neurons is prevented by daily application of tianeptine (10mg/kg, i.p.). There is a significant decrease in the total dendritic length of BLA neurons from stressed animals treated by tianeptine (1770 ± 107μm) when compared to stressed animals the vehicle (2213 ± 59μm, p<0.001). A similar effect is seen in the number of branch points (15.6±0.9 number of branch points in stressed animals receiving vehicle versus 12 ± 1 in stressed animals treated by tianeptine).CIS:chronic immobilization stress ; Veh: vehicle. ***: p<0.001. Data are means ± SEM.
Conrad and colleagues (51) suggest that dendritic shrinkage is not a form of permanent hippocampal damage, but a type of structural plasticity, or “remodeling,” which could be an adaptation to chronic stress. Although these findings do not exclude the possibility that the stress-induced hippocampal CA3 loss affects some aspects of conditioned fear, they do indicate that repeated restraint stress over 21 days has a powerful enhancing effect on emotionality that may be attributable to the overriding effects of chronic stress on other brain regions such as the amygdala. There is evidence for a critical role for the amygdala in the stress circuitry, which comes from behavioral studies of learning and memory. Repeated stress that produces dendritic remodeling in the CA3 region impairs hippocampal-dependent learning but stress might also impair memory through non-hippocampal mechanisms, such as enhanced emotionality (51). The group of Wood recently reported that chronic stress increases not only fear but also aggression and learned aspects of fear (i.e., fear conditioning) and inescapable stress (i.e., struggling and helplessness) (54).
Diamond and colleagues have studied the effects of tianeptine and stress on hippocampal and amygdala plasticity (55,56). Rats were stressed by a cat intruder for 1 h and recordings were performed in CA1 and BLA. Long term potentiation (LTP) stimulation of low intensity (primed burst, PB) was delivered to the hippocampal commissure for CA1 and BLA recordings or to the entorhinal cortex for BLA recordings. Tianeptine enhanced CA1 PB and increased baseline excitability in the non-stress group, and reversed the stress-induced suppression of CA1 PB. Stress, alone, enhanced LTP in the BLA of vehicle-treated rats, as well as in the stressed tianeptine-treated rats. Thus, these findings indicate that tianeptine can reverse the adverse effects of stress on hippocampal processing without adversely affecting amygdala synaptic function in animal models of stress.
More recent studies by Reagan and coworkers (57) further support a role for tianeptine in the modulation of glutamatergic tone in the amygdala. Specifically, acute restraint stress increases extracellular levels of glutamate in the BLA as measured by in vivo microdialysis, an effect that was inhibited by tianeptine. Interestingly, the SSRI fluoxetine increased extracellular glutamate levels in the BLA irrespective of stress conditions. These data demonstrate that the mechanism of action of tianeptine is distinct from SSRIs and support the hypothesis that the mechanism of action of tianeptine involves normalization of glutamatergic tone in the amygdala and the hippocampus. Additionally, these results provide evidence that stress-induced modulation of glutamate neurotransmission in amygdala reflects a fundamental pathological change that may contribute to the aetiology and progression of depressive illness, and that tianeptine may elicit their clinical effects by modulation of glutamatergic neurotransmission.
Learning and memory can be considered as a form of neuroplasticity response, so that examining the relationship between stress and LTP, and the effects of antidepressants on acquisition and recall of new information,, is mandatory. Remarkably, when administered several hours after stress exposure, tianeptine overcomes the block of hippocampal LTP induction by inescapable stress at a dose level that did not affect LTP in nonstressed animals (58). This finding is consistent with the report that tianeptine can reverse stress-suppressed exploration of a novel environment when injected after the stress (59).
A comprehensive study examining the effect of stress on LTP and the efficiency of antidepressants on LTP was conducted by the group of Jay (60). These authors have shown that severe acute platform stress in rats caused a long-lasting inhibition of LTP in the frontal cortex evoked by stimulation of hippocampal outflow. In agreement with the observed effects of tianeptine in intrinsic hippocampal circuits (58), tianeptine rapidly reverses the inhibitory effects of stress on LTP at hippocampal-prefrontal synapses (60). Thus, while tianeptine has been shown to have a strong impact on the deleterious effects of stress in the hippocampus, Rocher’s data reinforce this outcome to another brain region of interest for depression, the frontal cortex. This study forms a basis for explaining stress-induced cognitive impairments with LTP disturbances in neuron networks between the hippocampus and prefrontal cortex. Recently, Vouimba and coworkers (56) reported that tianeptine blocked the stress-induced suppression of primed burst potentiation in CA1 of rat hippocampus without affecting the stress induced enhancement of LTP in the BLA. They also observed that tianeptine administered under non-stress conditions enhanced primed burst potentiation in the hippocampus and LTP in amygdala. These data suggest that tianeptine should enhance hippocampal and prefrontal cortex memory-related processing in individuals under stress (55).
Collectively the improvement in memory function by tianeptine may represent the partial restoration of normal functional plasticity within hippocampal and hippocampal-cortical networks that may be obtained with tianeptine by accelerating neural adaptive mechanisms that may be deficient.
2.3. Anxiolytic effects
Analyses peformed in large-scale epidemiologic surveys have identified that the anxiety disorders, individually and as a group, exhibit remarkably high rates of comorbidity with each other and with major depression. Such frequent coexistence of an anxiety disorder with depression makes an antidepressant with anxiolytic properties particularly attractive.
Yet, there is evidence for anxiety-reduction by tianeptine. When administered acutely, tianeptine counteracted the anxiogenic effect of benzodiazepine and alcohol withdrawal in the social interaction test, whereas no effects were observed in the stress-induced hyperthermia, elevated plus-maze and social interaction test (61,62). More recent data also provide some convergence on the potential efficacy of tianeptine in terms of its action on the amygdala. Wood and coworkers (52) have shown that chronic tianeptine prevented stress-induced potentiation of aggressive conflicts, such that there was an interaction between chronic stress and tianeptine treatment throughout the study. Tianeptine also significantly reduced the incidence of aggressive conflicts in stressed and unstressed control rats during periods when aggression is high. Moreover, Burghardt and coworkers (53) reported that chronic tianeptine given for 21 days before training can reduce fear conditioning (a strong form of emotional learning) very much as chronic SSRI treatment is able to do; however, tianeptine was devoid of anxiogenic effects after acute administration, whereas acute SSRI treatment increased anxiety. Vouimba and colleagues (56) also showed that a stress-related amygdalar LTP increase that may be regarded as a reflection of fear conditioning is not changed by acute tianeptine treatment.
2.4. Cytoprotective effects
In addition to normalising the rate of cytogenesis in the brain, Lucassen and coworkers (63) have shown that tianeptine may have putative cytoprotective effects in chronically stressed animals and they investigated the effect of tianeptine treatment on apoptosis in the hippocampus and temporal cortex of adult tree shrews. Both stress and tianeptine had region-specific effects and tianeptine treatment reduced apoptosis in the dentate granule cell layer and subgranular zone, most likely on non-neuronal cells, but had no effect in the Ammon′s Horn. These effects were not restricted to the hippocampus alone, as in the temporal cortex, chronic stress alone increased the numbers of apoptotic cells (64), while tianeptine treatment had an antiapoptotic effect both in the stressed and unchallenged animals (63). Furthermore, the chronic administration of tianeptine exhibits cytoprotective effects against the deleterious effects of pro-inflammatory cytokines in both cortex and white matter, and abrogates the negative influence upon mood (65–68). Thus, interference with the deleterious actions of cytokines provides a further potential mechanism for explaining the ability of tianeptine to improve depressive states and, more generally, to counteract the adverse effects of chronic stress.
2.5. Procognitive effects
Cognitive deficits, such as an impairment of attention, memory and problem solving, have often been reported in patients with depressive disorders (69). Cognitive deficits and memory impairments in patients with depression may arise via disruption of the hypothalamic-pituitary adrenal (HPA) axis through hippocampal volume loss and changes in the amygdala. The magnitude of the hippocampal shrinkage reported in certain experimental conditions may partly underlie some of cognitive deficits that accompany major depression. Conversely, any prevention or restoration of these morphological changes in the hippocampus should be parallel to procognitive/promnesiant effects. Accordingly, tianeptine has particularly favorable effects on cognitive functions and the positive effect of tianeptine may be mediated through its upregulation of neurogenesis, but of course, the impact of neurogenesis on cognitive functions remains a matter of controversial debate.
Tianeptine prevents and reverses stress-induced glucocorticoid-mediated dendritic remodeling in CA3 pyramidal neurons in the hippocampus (40,41) and stress-induced increases in dendritic length and branching in the amygdala (50). Tianeptine blocks the dendritic remodeling caused by stress or glucocorticoids (41), blocks stress-induced impairments of spatial memory performance in radial and Y-maze (70,71) and antagonizes the deleterious effects of alcohol (72).
In a validated model of hippocampal-dependent memory impairment and synaptic plasticity changes by predator stress, acute tianeptine can prevent the deleterious effects of stress on spatial memory, an effect that does not depend on corticosterone levels (73). Tianeptine also facilitates focused attention behavior in the cat in response to its environment or towards a significant stimulus (74). It was shown to exert improving effects on learning as well as on working memory and on reference memory in rodents (72) and to exhibit vigilance-enhancing effects in rats (75) and monkeys (76).
Morris and coworkers (77) found that tianeptine can enhance memory retention in animals whose rate of forgetting of spatial memory in the watermaze has been increased through partial lesions of the diagonal band of Broca which mainly supply the dentate gyrus and the adjacent CA3 and CA4 subfields of Ammon’s horn (78) and provide direct and indirect (disinhibitory) excitatory inputs to the hippocampus. The behavioral findings of Morris and colleagues (77) are consistent with findings that revealed that tianeptine prevents vesicular reorganization in mossy fibres caused by stress (41,79). Morris’ findings (77) indicate that the input into CA3 and dentate gyrus are key sites for the procognitive efficacy of tianeptine.
3. The emerging pharmacological profile of tianeptine
3.1. From serotonin…
Although tianeptine possesses a heterocyclic structure, it is chemically dissimilar to tricyclic agents in that it (1) incorporates 2 heteroatoms (S and N, rather than C) in the central ring and (2) bears an extended amino-heptanoic chain. Tianeptine differs from other antidepressants in its pharmacological and neurochemical properties (80,81). Tianeptine shows no affinity for known neurotransmitter receptors and does not inhibit the uptake of serotonin or noradrenaline in the central nervous system (8,81). Chronic tianeptine administration did not alterthe concentration and affinity of α1A, α1B, α2A, α2B, α2C, β1, β2, 5-HT1, 5-HT2, 5-HT3, 5-HT4, 5-HT5A, 5-HT6, 5-HT7, NMDA, AMPA, kainate, benzodiazepine or GABA-B receptors (8,81) but increased the responsiveness of the α1-adrenergic system (82). Tianeptine does not inhibit MAOa and MAOb activity in the cortex, hippocampus, and hypothalamus. In contrast to SSRIs and tricyclic agents, systemic administration of tianeptine modestly enhanced the mesolimbic release of dopamine (DA) (83) but it is unclear how tianeptine strengthens dopaminergic transmission because the antidepressant recognizes neither DA transporters nor D1, D2, D3, D4 and D5 receptors (8). The uptake of dopamine or noradrenaline from rat cortical or hippocampal synaptosomes remains unchanged after acute and chronic administration. Presumably, actions upstream of dopaminergic neurons are involved, such as tonic GABAergic and glycinergic inhibition of dopaminergic perikarya (84,85).
Apart from its unusual structure, the first indication that tianeptine possesses a mechanism of action different to that of other classes of antidepressant was the finding that, upon acute and sustained administration, tianeptine decreased the extracellular levels of serotonin (5-HT) (86,87). At that time, this finding was hypothesized to be the consequence of a 5-HT re-uptake enhancement. It has been demonstrated that tianeptine also reduced both the number of transporter sites and their mRNA levels in the dorsal raphe nucleus (88). However, any facilitatory influence of tianeptine upon 5-HT re-uptake may be exerted indirectly rather than directly at 5-HT transporters, for which its affinity is low. Further, the validity of older data has been contested on the basis of technical limitations that could not be circumvented at that time (89). More recent investigations have shown that acute and long-term administration of tianeptine does not elicit any marked alterations (neither increases nor decreases) in extracellular levels of 5-HT in corticolimbic structures of conscious rats (89,90). From an electrophysiological point of view, sustained administration of tianeptine did not modify the spontaneous firing rate of serotonergic neurons in the dorsal raphe, nor did it modify the activity of postsynaptic 5-HT1A receptors nor the effectiveness of the terminal 5-HT autoreceptor antagonist in increasing the efficacy of the stimulation of the 5-HT pathway, despite prolonged treatment (90). Thus, the role of 5-HT in the mechanism of antidepressant efficacy of tianeptine is doubtful.
3.2. … to glutamate
The past decade has seen a steady accumulation of evidence supporting a role for the excitatory amino acid neurotransmitter, glutamate, and its receptors (several classes of ionotropic -ion channel-coupled- and metabotropic -G-protein-coupled- receptors) in depression and antidepressant activity. The release of glutamate is highly sensitive to “stress”, and both depressive and anxious states are characterized by a complex pattern of regionally variable alterations (generally increases) in the activity of glutamatergic pathways (5,13).
In contrast, there is some evidence that antidepressants attenuate glutamate release in corticolimbic structures (13,15,91). Normalising and stabilizing glutamate neurotransmission is a potential target of drugs treating depressive disorders (91). Long-term administration of antidepressants modifies the density and dampens the functional status of corticohippocampal populations of NMDA receptors in rodents: in contrast, chronic stress and depressive states are accompanied by in general enhanced activity at NMDA receptors (12–15).
Glutamate is the natural agonist for the ionotropic glutamate receptors, a family of ligand-gated ion channels including the N-methyl-D-aspartate (NMDA), alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA), kainate, and metabotropic receptors. Many receptor subtypes exist and allow heterogeneity in function and expression patterns, but the interplay between antidepressants and the different subtypes of receptors has not been systematically addressed, except for NMDA. NMDA receptors are oligomers composed of one NR1 subunit that is associated with some of the four other NR2A-D subunits to arrange a functional channel receptor. Antidepressants such as citalopram and imipramine were shown to alter the levels of mRNA encoding the NR1 subunit in mice brains and, additionally to produce distinct, region-specific effects on mRNA levels encoding NR2A-D subunits. These chronic antidepressant effects presumably affect the NMDA receptor composition, which in turn can change the physiological properties of these receptors (92). The picture is, however, not that simple as mice lacking the NMDA receptor NR2A subunit display antidepressant-like profiles in the forced swim test and tail suspension test, relative to wild-type littermates (93). Lower synaptic expression of NR1 subunit of the NMDA receptor were also recently observed in an animal model of depression, the Flinders Sensitive Line rats (94)
However, in line with the notion that a reduction in transmission at NMDA receptors may improve mood, both acute and chronic treatment with NMDA receptor antagonists is accompanied by antidepressant efficacies in rats. Recently, the NMDA receptor antagonist ketamine has demonstrated clinical efficacy in a randomized clinical trial of depressed patients. Furthermore, there is also evidence implicating disturbances in metabotropic glutamate mGluR1,5 receptors in depression and suicidality (12). The metabotropic glutamate (mGlu) receptors function to regulate glutamate neuronal transmission by altering the release of neurotransmitter or modulating the post-synaptic responses to glutamate.
Accumulating evidence from biochemical and behavioral studies support the idea that these receptors may serve as novel targets for the discovery of small molecule modulators with unique antidepressant properties (95). For example, mGlu receptor modulation can facilitate neuronal stem cell proliferation (neurogenesis) in the dentate gyrus of the hippocampus and the release of neurotransmitters that are associated with treatment response to depression in humans (serotonin, noradrenaline, dopamine). Long-term administration of antidepressants consistently elicits adaptive changes in the functional status of metabotropic receptors (12,96). Recent studies revealed antidepressant efficacies of Group III agonists in several rodent models (97,98), although interpretation of their roles is complicated by an apparently contradictory report that mice lacking the mGluR7 subtype of Group III metabotropic receptors display an antidepressant and anxiolytic phenotype (99).
Group II antagonists possess antidepressant properties by virtue of their induction of glutamate release in the dorsal raphe nucleus: this leads to the engagement of AMPA receptors facilitatory to serotonergic perikarya (100–103). In fact, both NMDA and Group I mGluR receptors fulfil common and synergistic roles in certain, long term adaptive processes involved in the control of mood and mnemonic function: these actions are mediated in the cortex and limbic structures such as the amygdala (104–106). Finally, drugs that enhance AMPA receptor mediated transmission by acting at allosteric sites possess antidepressant properties in experimental models, notably the forced-swim procedure in rodents and can enhances the antidepressant potency of fluoxetine and other antidepressants (107–109).
Tianeptine has been shown to inhibit the pathological stress-induced changes in glutamatergic neurotransmission in the hippocampus and amygdala in animal models (57,110). Using a combined approach of repeated stress and electrophysiological recording, Kole and coworkers (110) found that restraint stress for 21 days persistently enhances the NMDA-receptor component of excitatory postsynaptic currents (EPSCs) of the commissural/associational synapses onto CA3 pyramidal neurons. When rats were concomitantly treated with tianeptine, the antidepressant normalizes the stress-induced changes in the amplitude ratio of NMDA receptor to AMPA/kainate receptor-mediated currents, which may contribute to its neuroprotective properties. Moreover, tianeptine appears to facilitate signal transduction at the CA3 commissural associational synapse by altering the phosphorylation state of glutamate receptors as the enhancement of EPSCs could be blocked by the intracellular presence of the kinase inhibitor, staurosporine. In rats, tianeptine inhibited stress-induced re-scaling of the ratio of NMDA receptor- to AMPA/kainate receptor-mediated excitatory postsynaptic currents (110).
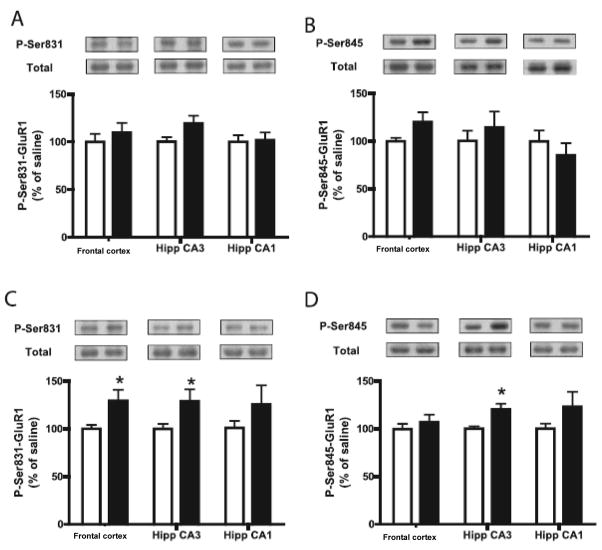
Chronic (19 day), but not acute treatment with tianeptine caused increased phosphorylation of the CaMK II-PKC site (Ser831) on GluR1 subunit of AMPA receptors in hippocampus (CA3 and dentate gyrus) and frontal cortex of mice (8) (Figure 2). Chronic tianeptine also decreases P-Ser133-CREB in the CA3 region of the hippocampus and increases it in the CA1 region, indicating that the tianeptine-mediated effects on phosphorylation of CREB is differentially regulated depending on the brain region (8). In a recent study (9), it was also found that acute administration of tianeptine increased phosphor-Ser831-GluR1 in frontal cortex from rats. This effect paralleled the ability of tianeptine to restore stress-impaired LTP in hippocampal to frontal cortex synapses. No similar effects were found with imipramine. These findings are in good agreement with the electrophysiological effects of tianeptine described by Kole and colleagues (110). As phosphorylation of GluR1 subunits is a way of potentiating AMPA receptor function, the latter mechanism seems to be implicated in the mechanism of action of tianeptine, and may represent a proximal target in the pathway leading to the antidepressant response.
Figure 2. Regulation of AMPA receptor phosphorylation by tianeptine.
The amounts of (A and C) phospho-Ser831-GluR1 and (B and D) phospho-Ser845-GluR1 were measured in the frontal cortex and in the CA3 and CA1 regions of hippocampus from animals treated (A and B) acutely or (C and D) chronically with saline (white bars) or tianeptine (black bars). Chronic treatment with tianeptine increased phospho-Ser831-GluR1 in both the frontal cortex and the CA3 region of hippocampus and phospho-Ser845-GluR1 in the CA3 region of hippocampus. Data are means ± SEM for n = 8–16 animals. Anova followed by Newman Keul’s test for pairwaise comparisons; *P < 0.05 compared with saline-treated animals. From Svenningsson et al. (2007).
In the amygdala Reagan and coworkers (57) have examined the stress modulation of extracellular glutamate levels in the basolateral nucleus and the central nucleus of the amygdala by in vivo microdialysis. Acute stress increased extracellular glutamate levels in the basolateral and central nuclei of the amygdala and tianeptine normalises synaptic concentrations of glutamate in the rat basolateral nucleus of the amygdala (Figure 3). Tianeptine also modulates the stress-induced changes observed in the expression of glial glutamate transporters that represent the sole mechanism through which the activity of glutamate is terminated in excitatory synapses (111).
Figure 3. Tianeptine, but not fluoxetine, attenuated stress-induced glutamate release in amygdala.
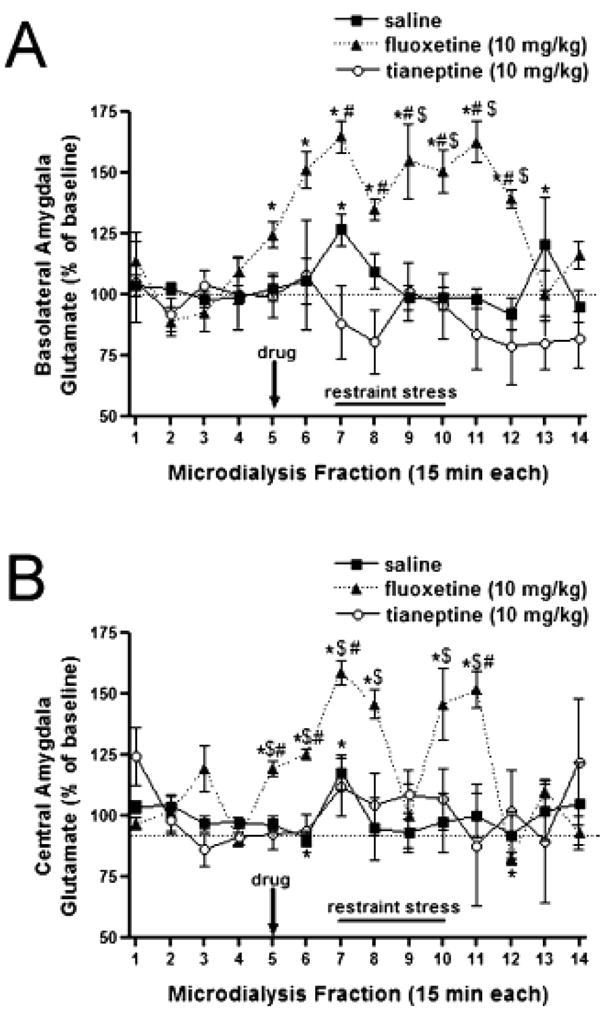
(A) Administration of tianeptine 30 min prior to the acute restraint stress session inhibited the stress-induced increases in extracellular glutamate levels in the basolateral amygdala (BLA); conversely, fluoxetine treatment increased basal BLA glutamate efflux and did not modulate the increases elicited by stress. (B) Tianeptine did not inhibit stress-mediated increases in extracellular glutamate levels in the central amygdala (CeA). Similar to observations in the BLA, fluoxetine administration increased glutamate efflux in the CeA in the prestress period, increases that were potentiated during stress. Data (means ± SEM) are based upon six rats per amygdalar nucleus for each drug. *P < 0.05 vs. baseline; $P < 0.05 vs. saline; #P < 0.05 vs. tianeptine. From Reznikov et al. (2007).
In fact, the chronic action of tianeptine that can target the phosphorylation-state of glutamate receptors and increase phosphorylation of intracellular kinases is of particular interest to explain the effects of tianeptine on neurogenesis. Indeed, kinase phosphorylation can in turn provide a priming signal for the activity-dependent structural shaping of dendrites, either by promoting dendritic outgrowth or providing enhanced structural stability. For example the calcium-calmodulin-dependent protein kinase II (CaMK II) is involved in stabilizing structural rearrangements (112).
An increasing number of studies suggest that it is the ability to modify synaptic plasticity that is the crucial feature of clinically effective antidepressants, rather than the enhancement of neuronal survival alone. For example, there is evidence that antidepressants may regulate the excitatory amino acid systems that underlie changes in synaptic connection strength (in addition to enhancing BDNF expression). Glutamate is an essential participant in many forms of adaptive plasticity. In fact, glutamate has been shown to suppress neurogenesis in dentate gyrus of hippocampus and have a key role in stress-induced dendritic retraction and remodeling. Animal studies have demonstrated that in stress conditions, extracellular glutamate levels are modestly increased in the hippocampus (113–115) as well as in the amygdala (57). These abnormal glutamate levels can lead to changes in reductions in neuronal size and density and hippocampal volume. Evidence that glutamate is involved in the regulation of neuroplasticity comes from animal studies where blockade of NMDA receptors (40,116) prevents deleterious effects of stress, whereas activation of NMDA receptors decreased neurogenesis (117).
Tianeptine prevents the stress-induced reorganization of glutamatergic synaptic vesicles in the mossy fibers abutting CA3 neurons (21,40,79). Regulation of the extracellular glutamate levels may be a potential cause of remodeling because of the dendritic disruption and breakage of CA3 region hippocampal neurons induced by chronic stress. Thus, regulation of glutamatergic transmission could be an important component of neuroplasticity (50) and indeed, altered neuroplasticity via disturbed glutamatergic neurotransmission has been shown to result in the type of neuronal dysfunction associated with depression (15).
Regulation and distribution of glia glutamate transporter (GLT-1) and its isoforms may give an idea about glutamate activity as well as structural disruptions of dendrite and suppression of neurogenesis. A detailed study testing this hypothesis was conducted recently by Reagan and coworkers (111) who have explored the regulation of GLT-1 under conditions that produce hippocampal dendritic remodeling (chronic restrain stress for 21 days). Chronic stress increases GLT-1 in the dentate gyrus and the CA3 regions of the rat hippocampus. Only circumscribed regions of the hippocampus are more sensitive to chronic stress and to the following changes in glutamate activity.
Indeed, the significant increases in GLT-1 mRNA levels and protein expression appeared only in the hippocampal CA3 region. When the effects of stress in the CA3 region are evaluated in detail, stress-induced increases were seen to be limited to the stratum oriens and stratum radium. No stress-induced change occurred for GLT-1 either in the CA3 region of the stratum pyramidal or in the granular layer of the dentate gyrus or hilus. The most important finding of this study is that stress-induced increases in GLT-1 expression can be suppressed by tianeptine. Increases in GLT-1 may result from stress-mediated increases in glutamate (113,114) and therefore normalization of synaptic concentrations of glutamate by tianeptine would eliminate the stimulus for increased GLT-1 expression. Such results would suggest that modulation of GLT-1 expression does not result from changes in hippocampal morphology, but rather reflects fundamental changes in the underlying neurochemical or molecular activities of the hippocampus in response to stress. In support of this hypothesis, recent studies demonstrate that chronic stress mediated changes in glutamate neurochemistry in the hippocampus are attenuated by tianeptine administration. Collectively, these findings thus provide additional support for the hypothesis that stress-induced changes in the hippocampus involves a critical role in glutamate neurotransmission, disposition and plasticity and a dynamic interplay between numerous neurotransmitter systems, including at least excitatory amino acids (21).
A modification of glutamatergic mechanisms by tianeptine may therefore be implicated in its ability to oppose the negative influence of chronic stress upon hippocampal neurogenesis, cell proliferation, and dendritic remodeling, processes profoundly disrupted in depressive states (21,44,63). The emerging pharmacological profile of tianeptine suggests that this antidepressant may serve to ‘normalize’ synaptic function, thereby allowing the chemical signal to reinstate the optimal functioning of critical circuits necessary for normal affective functioning.
3.2.1. Glutamatergic mechanisms to explain procognitive effects
Tianeptine has been shown to have beneficial effects on cognitive function and memory function in animal models (71–73). The clinical benefit of tianeptine may relate to its effects on neurogenesis and dendrite remodeling (118,119) as its ability to normalise the neuroplasticity mechanisms (long-term and primed-burst potentiation) can to be responsible for memory formation (55,120). However, the exact mechanism of the protective effect of tianeptine on memory-related neuroplasticity is not yet known. Campbell and colleagues (73) have hypothesised that tianeptine may enable information to be stored in the hippocampus in such a way that it is protected from being impaired by later stress stimuli, possibly by normalising stress-induced changes in the glutamatergic system. Glutamatergic processes are intimately involved in the induction of long-term potentiation and in memory formation, and several studies indicate that tianeptine exerts its protective effects against stress-elicited cognitive disruption, at least partly, via the modulation of glutamatergic transmission. For example, by alteration of the phosphorylation state of postsynaptic populations of AMPA and NMDA receptors, and by alterations in the re-uptake of glutamate by glial transporters (21,60,110,111).
4. Conclusions
Tianeptine has challenged the monoaminergic hypothesis of depression, as well as the proposed monoaminergic mechanisms whereby the action of most known antidepressants was explained. The generally accepted biological basis of depression, e.g. a serotonergic deficit, cannot explain the antidepressant activity of tianeptine. Events beyond the monoaminergic regulation are relevant to its clinical antidepressant efficacy. The neurobiological properties of tianeptine involve a dynamic interplay between numerous neurotransmitter systems and the critical ability 1) to restore normal neuroplasticity in circumscribed limbic brain regions and 2) to reverse stress-induced impairments in synaptic glutamate transmission, which plays crucial roles in virtually all key functions perturbed in depressed states. The effects of tianeptine on the glutamatergic system could provide a key action in the cascade of events triggered by this unique compound, and may represent the most proximal target in the pathway leading to its antidepressant efficacy.
The story of tianeptine is unfolding and this antidepressant is rich in future possibilities for understanding basic mechanisms as well as for its therapeutic applications. For example, it would be valuable to study whether the antidepressant reduces the behavioral and/or biochemical changes that correlate with increased helplessness observed in mice with deletion of the main AMPA receptor subunit GluR-A (121). Moreover, as recounted above, there is now strong evidence that tianeptine affects AMPA receptor function at the molecular level, but there is no detailed information on which kinase/phosphatase signaling cascades are regulated by this antidepressant, and whether it directly binds to any of those enzymes. As tianeptine has a fast onset of antidepressant action, it would be interesting to examine the mechanisms underlying this feature, for example whether it could be related to enhancing AMPA relative to NMDA throughput in critical neuronal circuits, as recently demonstrated for some NMDA antagonists (122). Finally, from a clinical perspective, it would be attractive to study effects of tianeptine in atypical forms of depression such social stress-induced depression (123) that, for example are not adequately treated by SSRIs.
During the past decades, inhibitors of biogenic amine reuptake have been the mainstay for the treatment of major depression, but from a mechanistic perspective, the use of reuptake inhibitors has come full circle. A significant proportion of patients (approx.30%) do not receive clinically meaningful relief from these agents. Among a number of strategies to overcome these shortcomings some of these approaches circumvent the aminergic synapse, whilst others remain grounded in monoaminergic theories of depression. Glutamate modulators such as tianeptine have great promise in this area, and there is a growing body of evidences that many patients with mood disorders will benefit from antidepressants that include, though not exclusively, glutamate-based mode of action. Identifying predictors of the pharmacological response of such broad spectrum antidepressants, using for example tools from brain imaging and genetics, remains one of the important area of future research.
Abbreviations
- HPA
hypothalamic-pituitary-adrenal axis
- BLA
basolateral amygdaloid
- LTP
Long term potentiation
- 5-HT
serotonin
Contributor Information
Bruce S. McEwen, Email: mcewen@mail.rockefeller.edu, Alfred E. Mirsky Professor Head, Harold and Margaret Milliken Hatch Laboratory of Neuroendocrinology. The Rockefeller University 1230 York Avenue New York, NY 10021, USA, Phone: +1 212 327 8624
Sumantra Chattarji, Email: shona@ncbs.res.in, National Center for Biological Sciences, Bangladore 560065, India, Phone: +91 80 23636421.
David M. Diamond, Email: ddiamond@cas.usf.edu, Medical Research Division, Veterans Hospital, 13000 Bruce B. Downs Blvd., Tampa, FL, 33612;Center for Preclinical and Clinical Research on PTSD; Departments of Psychology and Molecular Pharmacology and Physiology, University of South Florida, 4202 E. Fowler Ave., PCD 4118G, Tampa, FL, 33620, USA, Phone: +1 813974048
Thérèse M. Jay, Email: therese.jay@inserm.fr, INSERM, Physiopathologie des Maladies Psychiatriques, U894 and Université Paris Descartes, Faculté de Médecine Paris Descartes, Paris, France, Phone: +33 1 40788631
Lawrence P. Reagan, Email: lreagan@uscmed.sc.edu, Department of Pharmacology, Physiology and Neuroscience, University of South Carolina School of Medicine, Columbia, SC 29208, Phone: 803 733-3237
Per Svenningsson, Email: Per.Svenningsson@ki.se, Center for Molecular Medicine, Department of Physiology and Pharmacology, Karolinska Institutet, 17177 Stockholm, Sweden, Phone: +46 (8) 52 48 79 26.
Eberhard Fuchs, Email: efuchs@gwdg.de, Clinical Neurobiology Laboratory, German Primate Center, Department of Neurology and DFG Research Center Molecular Physiology of the Brain (CMPB), University of Göttingen, 37077 Göttingen, Germany, Phone: +49-551-3851 130.
Bibliography
- 1.Duman RS, Malberg J, Thome J. Neural plasticity to stress and antidepressant treatment. Biol Psychiatry. 1999;46:1181–1191. doi: 10.1016/s0006-3223(99)00177-8. [DOI] [PubMed] [Google Scholar]
- 2.Manji HK, Duman RS. Impairments of neuroplasticity and cellular resilience in severe mood disorders: implications for the development of novel therapeutics. Psychopharmacol Bull. 2001;35:5–49. [PubMed] [Google Scholar]
- 3.Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- 4.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Manji HK, Gottesman II, Gould TD. Signal transduction and genes-to-behaviors pathways in psychiatric diseases. Sci STKE. 2003;2003:e49. doi: 10.1126/stke.2003.207.pe49. [DOI] [PubMed] [Google Scholar]
- 6.Sheline YI. Neuroimaging studies of mood disorder effects on the brain. Biol Psychiatry. 2003;54:338–352. doi: 10.1016/s0006-3223(03)00347-0. [DOI] [PubMed] [Google Scholar]
- 7.Kasper S, McEwen BS. Neurobiological and clinical effects of the antidepressant tianeptine. CNS Drugs. 2008;22:15–26. doi: 10.2165/00023210-200822010-00002. [DOI] [PubMed] [Google Scholar]
- 8.Svenningsson P, Bateup H, Qi H, Takamiya K, Huganir RL, Spedding M, Roth BL, McEwen BS, Greengard P. Involvement of AMPA receptor phosphorylation in antidepressant actions with special reference to tianeptine. Eur J Neurosci. 2007;26:3509–3517. doi: 10.1111/j.1460-9568.2007.05952.x. [DOI] [PubMed] [Google Scholar]
- 9.Qi H, Mailliet F, Spedding M, Rocher C, Zhang X, Delagrange P, McEwen B, Jay TM, Svenningsson P. Antidepressants reverse the attenuation of the neurotrophic MEK/MAPK cascade in frontal cortex by elevated platform stress; reversal of effects on LTP is associated with GluA1 phosphorylation. Neuropharmacology. 2008 doi: 10.1016/j.neuropharm.2008.06.068. [DOI] [PubMed] [Google Scholar]
- 10.Bergink V, van Megen HJ, Westenberg HG. Glutamate and anxiety. Eur Neuropsychopharmacol. 2004;14:175–183. doi: 10.1016/S0924-977X(03)00100-7. [DOI] [PubMed] [Google Scholar]
- 11.Lowy MT, Wittenberg L, Yamamoto BK. Effect of acute stress on hippocampal glutamate levels and spectrin proteolysis in young and aged rats. J Neurochem. 1995;65:268–274. doi: 10.1046/j.1471-4159.1995.65010268.x. [DOI] [PubMed] [Google Scholar]
- 12.Paul IA, Skolnick P. Glutamate and depression: clinical and preclinical studies. Ann N Y Acad Sci. 2003;1003:250–272. doi: 10.1196/annals.1300.016. [DOI] [PubMed] [Google Scholar]
- 13.Sanacora G, Rothman DL, Mason G, Krystal JH. Clinical studies implementing glutamate neurotransmission in mood disorders. Ann N Y Acad Sci. 2003;1003:292–308. doi: 10.1196/annals.1300.018. [DOI] [PubMed] [Google Scholar]
- 14.Skolnick P, Legutko B, Li X, Bymaster FP. Current perspectives on the development of non-biogenic amine-based antidepressants. Pharmacol Res. 2001;43:411–423. doi: 10.1006/phrs.2000.0806. [DOI] [PubMed] [Google Scholar]
- 15.Zarate CA, Jr, Du J, Quiroz J, Gray NA, Denicoff KD, Singh J, Charney DS, Manji HK. Regulation of cellular plasticity cascades in the pathophysiology and treatment of mood disorders: role of the glutamatergic system. Ann N Y Acad Sci. 2003;1003:273–291. doi: 10.1196/annals.1300.017. [DOI] [PubMed] [Google Scholar]
- 16.Curzon G, Kennett GA, Sarna GS, Whitton PS. The effects of tianeptine and other antidepressants on a rat model of depression. Br J Psychiatry Suppl. 1992:51–55. [PubMed] [Google Scholar]
- 17.Thiebot MH, Martin P, Puech AJ. Animal behavioural studies in the evaluation of antidepressant drugs. Br J Psychiatry Suppl. 1992:44–50. [PubMed] [Google Scholar]
- 18.Kelly JP, Leonard BE. The effect of tianeptine and sertraline in three animal models of depression. Neuropharmacology. 1994;33:1011–1016. doi: 10.1016/0028-3908(94)90160-0. [DOI] [PubMed] [Google Scholar]
- 19.Wagstaff AJ, Ormrod D, Spencer CM. Tianeptine: a review of its use in depressive disorders. CNS Drugs. 2001;15:231–259. doi: 10.2165/00023210-200115030-00006. [DOI] [PubMed] [Google Scholar]
- 20.McEwen BS, Olie JP. Neurobiology of mood, anxiety, and emotions as revealed by studies of a unique antidepressant: tianeptine. Mol Psychiatry. 2005;10:525–537. doi: 10.1038/sj.mp.4001648. [DOI] [PubMed] [Google Scholar]
- 21.McEwen BS, Magarinos AM, Reagan LP. Structural plasticity and tianeptine: cellular and molecular targets. Eur Psychiatry. 2002;17 (Suppl 3):318–330. doi: 10.1016/s0924-9338(02)00650-8. [DOI] [PubMed] [Google Scholar]
- 22.Costa e Silva JA, Ruschel SI, Caetano D, Rocha FL, da SL, Jr, Arruda S, Ozun M. Placebo-controlled study of tianeptine in major depressive episodes. Neuropsychobiology. 1997;35:24–29. doi: 10.1159/000119326. [DOI] [PubMed] [Google Scholar]
- 23.Loo H, Saiz-Ruiz J, Costa e Silva JACE, Ansseau M, Herrington R, Vaz-Serra A, Dilling H, De Risio S. Efficacy and safety of tianeptine in the treatment of depressive disorders in comparison with fluoxetine. J Affect Disord. 1999;56:109–118. doi: 10.1016/s0165-0327(99)00009-9. [DOI] [PubMed] [Google Scholar]
- 24.Lepine JP, Altamura C, Ansseau M, Gutierrez JL, Bitter I, Lader M, Waintraub L. Tianeptine and paroxetine in major depressive disorder, with a special focus on the anxious component in depression: an international, 6-week double-blind study dagger. Hum Psychopharmacol. 2001;16:219–227. doi: 10.1002/hup.289. [DOI] [PubMed] [Google Scholar]
- 25.Novotny V, Faltus F. Tianeptine and fluoxetine in major depression: a 6-week randomised double-blind study. Hum Psychopharmacol. 2002;17:299–303. doi: 10.1002/hup.411. [DOI] [PubMed] [Google Scholar]
- 26.Szadoczky E, Furedi J. Efficacy and acceptability of tianeptine and sertraline in the acute treatment phase of depression. Encephale. 2002;28:343–349. [PubMed] [Google Scholar]
- 27.Waintraub L, Septien L, Azoulay P. Efficacy and safety of tianeptine in major depression: evidence from a 3-month controlled clinical trial versus paroxetine. CNS Drugs. 2002;16:65–75. doi: 10.2165/00023210-200216010-00005. [DOI] [PubMed] [Google Scholar]
- 28.Nickel T, Sonntag A, Schill J, Zobel AW, Ackl N, Brunnauer A, Murck H, Ising M, Yassouridis A, Steiger A, Zihl J, Holsboer F. Clinical and neurobiological effects of tianeptine and paroxetine in major depression. J Clin Psychopharmacol. 2003;23:155–168. doi: 10.1097/00004714-200304000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Olie JP, Bayle F, Kasper S. A meta-analysis of randomized controlled trials of tianeptine versus SSRI in the short-term treatment of depression. Encephale. 2003;29:322–328. [PubMed] [Google Scholar]
- 30.Kasper S, Olie JP. A meta-analysis of randomized controlled trials of tianeptine versus SSRI in the short-term treatment of depression. Eur Psychiatry. 2002;17 (Suppl 3):331–340. doi: 10.1016/s0924-9338(02)00651-x. [DOI] [PubMed] [Google Scholar]
- 31.Novotny V, Faltus F. First signs of improvement with tianeptine in the treatment of depression: an analysis of a double-blind study versus fluoxetine. Eur Neuropsychopharmacol. 2003;13(suppl 4):S230. Ref Type: Abstract. [Google Scholar]
- 32.Loo H, Deniker P. Position of tianeptine among antidepressive chemotherapies. Clin Neuropharmacol. 1988;11 (Suppl 2):S97–102. [PubMed] [Google Scholar]
- 33.Guelfi JD, Pichot P, Dreyfus JF. Efficacy of tianeptine in anxious-depressed patients: results of a controlled multicenter trial versus amitriptyline. Neuropsychobiology. 1989;22:41–48. doi: 10.1159/000118590. [DOI] [PubMed] [Google Scholar]
- 34.Invernizzi G, Aguglia E, Bertolino A, Casacchia M, Ciani N, Marchesi GF, Nardini M, Rapisarda V. The efficacy and safety of tianeptine in the treatment of depressive disorder: results of a controlled double-blind multicentre study vs. amitriptyline. Neuropsychobiology. 1994;30:85–93. doi: 10.1159/000119142. [DOI] [PubMed] [Google Scholar]
- 35.Brion S, Audrain S, De Bodinat C. Major depressive episodes in patients over 70 years of age. Evaluation of the efficiency and acceptability of tianeptine and mianserin. Presse Med. 1996;25:461–468. [PubMed] [Google Scholar]
- 36.Ridout F, Hindmarch I. Effects of tianeptine and mianserin on car driving skills. Psychopharmacology (Berl) 2001;154:356–361. doi: 10.1007/s002130000662. [DOI] [PubMed] [Google Scholar]
- 37.Wilde MI, Benfield P. Tianeptine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in depression and coexisting anxiety and depression. Drugs. 1995;49:411–439. doi: 10.2165/00003495-199549030-00007. [DOI] [PubMed] [Google Scholar]
- 38.Atmaca M, Kuloglu M, Tezcan E, Buyukbayram A. Switching to tianeptine in patients with antidepressant-induced sexual dysfunction. Hum Psychopharmacol. 2003;18:277–280. doi: 10.1002/hup.479. [DOI] [PubMed] [Google Scholar]
- 39.Bonierbale M, Lancon C, Tignol J. The ELIXIR study: evaluation of sexual dysfunction in 4557 depressed patients in France. Curr Med Res Opin. 2003;19:114–124. doi: 10.1185/030079902125001461. [DOI] [PubMed] [Google Scholar]
- 40.Magarinos AM, Deslandes A, McEwen BS. Effects of antidepressants and benzodiazepine treatments on the dendritic structure of CA3 pyramidal neurons after chronic stress. Eur J Pharmacol. 1999;371:113–122. doi: 10.1016/s0014-2999(99)00163-6. [DOI] [PubMed] [Google Scholar]
- 41.Watanabe Y, Gould E, Daniels DC, Cameron H, McEwen BS. Tianeptine attenuates stress-induced morphological changes in the hippocampus. Eur J Pharmacol. 1992;222:157–162. doi: 10.1016/0014-2999(92)90830-w. [DOI] [PubMed] [Google Scholar]
- 42.MacQueen GM, Campbell S, McEwen BS, Macdonald K, Amano S, Joffe RT, Nahmias C, Young LT. Course of illness, hippocampal function, and hippocampal volume in major depression. Proc Natl Acad Sci U S A. 2003;100:1387–1392. doi: 10.1073/pnas.0337481100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rajkowska G. Cell pathology in mood disorders. Semin Clin Neuropsychiatry. 2002;7:281–292. doi: 10.1053/scnp.2002.35228. [DOI] [PubMed] [Google Scholar]
- 44.Czeh B, Michaelis T, Watanabe T, Frahm J, de Biurrun G, van Kampen M, Bartolomucci A, Fuchs E. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci U S A. 2001;98:12796–12801. doi: 10.1073/pnas.211427898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lucassen PJ, Muller MB, Holsboer F, Bauer J, Holtrop A, Wouda J, Hoogendijk WJ, De Kloet ER, Swaab DF. Hippocampal apoptosis in major depression is a minor event and absent from subareas at risk for glucocorticoid overexposure. Am J Pathol. 2001;158:453–468. doi: 10.1016/S0002-9440(10)63988-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alfonso J, Frick LR, Silberman DM, Palumbo ML, Genaro AM, Frasch AC. Regulation of hippocampal gene expression is conserved in two species subjected to different stressors and antidepressant treatments. Biol Psychiatry. 2006;59:244–251. doi: 10.1016/j.biopsych.2005.06.036. [DOI] [PubMed] [Google Scholar]
- 47.Reagan LP, Hendry RM, Reznikov LR, Piroli GG, Wood GE, McEwen BS, Grillo CA. Tianeptine increases brain-derived neurotrophic factor expression in the rat amygdala. Eur J Pharmacol. 2007;565:68–75. doi: 10.1016/j.ejphar.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 48.Petrovich GD, Canteras NS, Swanson LW. Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Res Brain Res Rev. 2001;38:247–289. doi: 10.1016/s0165-0173(01)00080-7. [DOI] [PubMed] [Google Scholar]
- 49.Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McEwen BS, Chattarji S. Molecular mechanisms of neuroplasticity and pharmacological implications: the example of tianeptine. Eur Neuropsychopharmacol. 2004;14 (Suppl 5):S497–S502. doi: 10.1016/j.euroneuro.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 51.Conrad CD, LeDoux JE, Magarinos AM, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci. 1999;113:902–913. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- 52.Wood GE, Reagan LP, Grillo CA, Piroli GG, McEwen BS. Chronic antidepressant treatment with tianeptine prevents the stress-induced potentiation of aggressive conflicts. Society for Neuroscience. 2003:217.7. [Google Scholar]
- 53.Burghardt NS, Sullivan GM, McEwen BS, Gorman JM, LeDoux JE. The selective serotonin reuptake inhibitor citalopram increases fear after acute treatment but reduces fear with chronic treatment: a comparison with tianeptine. Biol Psychiatry. 2004;55:1171–1178. doi: 10.1016/j.biopsych.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 54.Wood GE, Norris EH, Waters E, Stoldt JT, McEwen BS. Chronic immobilization stress alters aspects of emotionality and associative learning in the rat. Behav Neurosci. 2008;122:282–292. doi: 10.1037/0735-7044.122.2.282. [DOI] [PubMed] [Google Scholar]
- 55.Diamond DM, Campbell A, Park CR, Vouimba RM. Preclinical research on stress, memory, and the brain in the development of pharmacotherapy for depression. Eur Neuropsychopharmacol. 2004;14 (Suppl 5):S491–S495. doi: 10.1016/j.euroneuro.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 56.Vouimba RM, Munoz C, Diamond DM. Differential effects of predator stress and the antidepressant tianeptine on physiological plasticity in the hippocampus and basolateral amygdala. Stress. 2006;9:29–40. doi: 10.1080/10253890600610973. [DOI] [PubMed] [Google Scholar]
- 57.Reznikov LR, Grillo CA, Piroli GG, Pasumarthi RK, Reagan LP, Fadel J. Acute stress-mediated increases in extracellular glutamate levels in the rat amygdala: differential effects of antidepressant treatment. Eur J Neurosci. 2007;25:3109–3114. doi: 10.1111/j.1460-9568.2007.05560.x. [DOI] [PubMed] [Google Scholar]
- 58.Shakesby AC, Anwyl R, Rowan MJ. Overcoming the effects of stress on synaptic plasticity in the intact hippocampus: rapid actions of serotonergic and antidepressant agents. J Neurosci. 2002;22:3638–3644. doi: 10.1523/JNEUROSCI.22-09-03638.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Whitton PS, Sarna GS, Datla KP, Curzon G. Effects of tianeptine on stress-induced behavioural deficits and 5-HT dependent behaviour. Psychopharmacology (Berl) 1991;104:81–85. doi: 10.1007/BF02244558. [DOI] [PubMed] [Google Scholar]
- 60.Rocher C, Spedding M, Munoz C, Jay TM. Acute stress-induced changes in hippocampal/prefrontal circuits in rats: effects of antidepressants. Cereb Cortex. 2004;14:224–229. doi: 10.1093/cercor/bhg122. [DOI] [PubMed] [Google Scholar]
- 61.File SE, Andrews N, al Farhan M. Anxiogenic responses of rats on withdrawal from chronic ethanol treatment: effects of tianeptine. Alcohol Alcohol. 1993;28:281–286. [PubMed] [Google Scholar]
- 62.Zethof TJ, van der Heyden JA, Tolboom JT, Olivier B. Stress-induced hyperthermia as a putative anxiety model. Eur J Pharmacol. 1995;294:125–135. doi: 10.1016/0014-2999(95)00520-x. [DOI] [PubMed] [Google Scholar]
- 63.Lucassen PJ, Fuchs E, Czeh B. Antidepressant treatment with tianeptine reduces apoptosis in the hippocampal dentate gyrus and temporal cortex. Biol Psychiatry. 2004;55:789–796. doi: 10.1016/j.biopsych.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 64.Lucassen PJ, Vollmann-Honsdorf GK, Gleisberg M, Czeh B, De Kloet ER, Fuchs E. Chronic psychosocial stress differentially affects apoptosis in hippocampal subregions and cortex of the adult tree shrew. Eur J Neurosci. 2001;14:161–166. doi: 10.1046/j.0953-816x.2001.01629.x. [DOI] [PubMed] [Google Scholar]
- 65.Castanon N, Bluthe RM, Dantzer R. Chronic treatment with the atypical antidepressant tianeptine attenuates sickness behavior induced by peripheral but not central lipopolysaccharide and interleukin-1beta in the rat. Psychopharmacology (Berl) 2001;154:50–60. doi: 10.1007/s002130000595. [DOI] [PubMed] [Google Scholar]
- 66.Castanon N, Konsman JP, Medina C, Chauvet N, Dantzer R. Chronic treatment with the antidepressant tianeptine attenuates lipopolysaccharide-induced Fos expression in the rat paraventricular nucleus and HPA axis activation. Psychoneuroendocrinology. 2003;28:19–34. doi: 10.1016/s0306-4530(02)00005-7. [DOI] [PubMed] [Google Scholar]
- 67.Castanon N, Medina C, Mormede C, Dantzer R. Chronic administration of tianeptine balances lipopolysaccharide-induced expression of cytokines in the spleen and hypothalamus of rats. Psychoneuroendocrinology. 2004;29:778–790. doi: 10.1016/S0306-4530(03)00142-2. [DOI] [PubMed] [Google Scholar]
- 68.Plaisant F, Dommergues MA, Spedding M, Cecchelli R, Brillault J, Kato G, Munoz C, Gressens P. Neuroprotective properties of tianeptine: interactions with cytokines. Neuropharmacology. 2003;44:801–809. doi: 10.1016/s0028-3908(03)00066-2. [DOI] [PubMed] [Google Scholar]
- 69.Zihl J, Gron G, Brunnauer A. Cognitive deficits in schizophrenia and affective disorders: evidence for a final common pathway disorder. Acta Psychiatr Scand. 1998;97:351–357. doi: 10.1111/j.1600-0447.1998.tb10014.x. [DOI] [PubMed] [Google Scholar]
- 70.Luine V, Villegas M, Martinez C, McEwen BS. Repeated stress causes reversible impairments of spatial memory performance. Brain Res. 1994;639:167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- 71.Conrad CD, Galea LA, Kuroda Y, McEwen BS. Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment. Behav Neurosci. 1996;110:1321–1334. doi: 10.1037//0735-7044.110.6.1321. [DOI] [PubMed] [Google Scholar]
- 72.Jaffard R, Mocaer E, Poignant JC, Micheau J, Marighetto A, Meunier M, Beracochea D. Effects of tianeptine on spontaneous alternation, simple and concurrent spatial discrimination learning and on alcohol-induced alternation deficits in mice. Behav Pharmacol. 1991;2:37–46. [PubMed] [Google Scholar]
- 73.Campbell AM, Park CR, Zoladz PR, Munoz C, Fleshner M, Diamond DM. Pre-training administration of tianeptine, but not propranolol, protects hippocampus-dependent memory from being impaired by predator stress. Eur Neuropsychopharmacol. 2008;18:87–98. doi: 10.1016/j.euroneuro.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 74.Delagrange P, Bouyer JJ, Montaron MF, Durand C, Mocaer E, Rougeul A. Action of tianeptine on focalization of attention in cat. Psychopharmacology (Berl) 1990;102:227–233. doi: 10.1007/BF02245926. [DOI] [PubMed] [Google Scholar]
- 75.Lejeune F, Poignant JC, Reure H. Electrophysiological study of tianeptine, a new enhancer of serotonin uptake with antidepressant activity. Neurophysiol Clin. 1988;18:369–381. doi: 10.1016/s0987-7053(88)80093-5. [DOI] [PubMed] [Google Scholar]
- 76.Mocaer E, Rettori MC, Kamoun A. Pharmacological antidepressive effects and tianeptine-induced 5-HT uptake increase. Clin Neuropharmacol. 1988;11 (Suppl 2):S32–S42. [PubMed] [Google Scholar]
- 77.Morris RG, Kelly S, Burney D, Anthony T, Boyer PA, Spedding M. Tianeptine and its enantiomers: effects on spatial memory in rats with medial septum lesions. Neuropharmacology. 2001;41:272–281. doi: 10.1016/s0028-3908(01)00058-2. [DOI] [PubMed] [Google Scholar]
- 78.Amaral DG, Kurz J. An analysis of the origins of the cholinergic and noncholinergic septal projections to the hippocampal formation of the rat. J Comp Neurol. 1985;240:37–59. doi: 10.1002/cne.902400104. [DOI] [PubMed] [Google Scholar]
- 79.Magarinos AM, Verdugo JM, McEwen BS. Chronic stress alters synaptic terminal structure in hippocampus. Proc Natl Acad Sci USA. 1997;94:14002–14008. doi: 10.1073/pnas.94.25.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chamba G, Lemoine P, Flachaire E, Ferry N, Quincy C, Sassard J, Ferber C, Mocaer E, Kamoun A, Renaud B. Increased serotonin platelet uptake after tianeptine administration in depressed patients. Biol Psychiatry. 1991;30:609–617. doi: 10.1016/0006-3223(91)90030-p. [DOI] [PubMed] [Google Scholar]
- 81.Kato G, Weitsch AF. Neurochemical profile of tianeptine, a new antidepressant drug. Clin Neuropharmacol. 1988;11 (Suppl 2):S43–S50. [PubMed] [Google Scholar]
- 82.Rogoz Z, Skuza G, Dlaboga D, Maj J, Dziedzicka-Wasylewska M. Effect of repeated treatment with tianeptine and fluoxetine on the central alpha(1)-adrenergic system. Neuropharmacology. 2001;41:360–368. doi: 10.1016/s0028-3908(01)00079-x. [DOI] [PubMed] [Google Scholar]
- 83.Invernizzi R, Pozzi L, Garattini S, Samanin R. Tianeptine increases the extracellular concentrations of dopamine in the nucleus accumbens by a serotonin-independent mechanism. Neuropharmacology. 1992;31:221–227. doi: 10.1016/0028-3908(92)90171-k. [DOI] [PubMed] [Google Scholar]
- 84.Kim YJ, Shin MC, Kim SA, Chung JH, Kim EH, Kim CJ. Modulation of tianeptine on ion currents induced by inhibitory neurotransmitters in acutely dissociated dorsal raphe neurons of Sprague-Dawley rats. Eur Neuropsychopharmacol. 2002;12:417–425. doi: 10.1016/s0924-977x(02)00054-8. [DOI] [PubMed] [Google Scholar]
- 85.Labrid C, Mocaer E, Kamoun A. Neurochemical and pharmacological properties of tianeptine, a novel antidepressant. Br J Psychiatry Suppl. 1992:56–60. [PubMed] [Google Scholar]
- 86.Mennini T, Mocaer E, Garattini S. Tianeptine, a selective enhancer of serotonin uptake in rat brain. Naunyn Schmiedebergs Arch Pharmacol. 1987;336:478–482. doi: 10.1007/BF00169302. [DOI] [PubMed] [Google Scholar]
- 87.Fattaccini CM, Bolanos-Jimenez F, Gozlan H, Hamon M. Tianeptine stimulates uptake of 5-hydroxytryptamine in vivo in the rat brain. Neuropharmacology. 1990;29:1–8. doi: 10.1016/0028-3908(90)90076-4. [DOI] [PubMed] [Google Scholar]
- 88.Watanabe Y, Sakai RR, McEwen BS, Mendelson S. Stress and antidepressant effects on hippocampal and cortical 5-HT1A and 5-HT2 receptors and transport sites for serotonin. Brain Res. 1993;615:87–94. doi: 10.1016/0006-8993(93)91117-b. [DOI] [PubMed] [Google Scholar]
- 89.Malagie I, Deslandes A, Gardier AM. Effects of acute and chronic tianeptine administration on serotonin outflow in rats: comparison with paroxetine by using in vivo microdialysis. Eur J Pharmacol. 2000;403:55–65. doi: 10.1016/s0014-2999(00)00486-6. [DOI] [PubMed] [Google Scholar]
- 90.Pineyro G, Deveault L, de Montigny C, Blier P. Effect of prolonged administration of tianeptine on 5-HT neurotransmission: an electrophysiological study in the rat hippocampus and dorsal raphe. Naunyn Schmiedebergs Arch Pharmacol. 1995;351:119–125. doi: 10.1007/BF00169325. [DOI] [PubMed] [Google Scholar]
- 91.Bonanno G, Giambelli R, Raiteri L, Tiraboschi E, Zappettini S, Musazzi L, Raiteri M, Racagni G, Popoli M. Chronic antidepressants reduce depolarization-evoked glutamate release and protein interactions favoring formation of SNARE complex in hippocampus. J Neurosci. 2005;25:3270–3279. doi: 10.1523/JNEUROSCI.5033-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Boyer PA, Skolnick P, Fossom LH. Chronic administration of imipramine and citalopram alters the expression of NMDA receptor subunit mRNAs in mouse brain. A quantitative in situ hybridization study. J Mol Neurosci. 1998;10:219–233. doi: 10.1007/BF02761776. [DOI] [PubMed] [Google Scholar]
- 93.Boyce-Rustay JM, Holmes A. Genetic inactivation of the NMDA receptor NR2A subunit has anxiolytic- and antidepressant-like effects in mice. Neuropsychopharmacology. 2006;31:2405–2414. doi: 10.1038/sj.npp.1301039. [DOI] [PubMed] [Google Scholar]
- 94.Ryan B, Musazzi L, Mallei A, Tardito D, Gruber SH, El Khoury A, Anwyl R, Racagni G, Mathe AA, Rowan MJ, Popoli M. Remodelling by early-life stress of NMDA receptor-dependent synaptic plasticity in a gene-environment rat model of depression. Int J Neuropsychopharmacol. 2009;12:553–559. doi: 10.1017/S1461145708009607. [DOI] [PubMed] [Google Scholar]
- 95.Witkin JM, Marek GJ, Johnson BG, Schoepp DD. Metabotropic glutamate receptors in the control of mood disorders. CNS Neurol Disord Drug Targets. 2007;6:87–100. doi: 10.2174/187152707780363302. [DOI] [PubMed] [Google Scholar]
- 96.Palucha A, Pilc A. Metabotropic glutamate receptor ligands as possible anxiolytic and antidepressant drugs. Pharmacol Ther. 2007;115:116–147. doi: 10.1016/j.pharmthera.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 97.Palucha A, Tatarczynska E, Branski P, Szewczyk B, Wieronska JM, Klak K, Chojnacka-Wojcik E, Nowak G, Pilc A. Group III mGlu receptor agonists produce anxiolytic- and antidepressant-like effects after central administration in rats. Neuropharmacology. 2004;46:151–159. doi: 10.1016/j.neuropharm.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 98.Tatarczynska E, Palucha A, Szewczyk B, Chojnacka-Wojcik E, Wieronska J, Pilc A. Anxiolytic- and antidepressant-like effects of group III metabotropic glutamate agonist (1S,3R,4S)-1-aminocyclopentane-1,3,4-tricarboxylic acid (ACPT-I) in rats. Pol J Pharmacol. 2002;54:707–710. [PubMed] [Google Scholar]
- 99.Cryan JF, Kelly PH, Neijt HC, Sansig G, Flor PJ, van Der PH. Antidepressant and anxiolytic-like effects in mice lacking the group III metabotropic glutamate receptor mGluR7. Eur J Neurosci. 2003;17:2409–2417. doi: 10.1046/j.1460-9568.2003.02667.x. [DOI] [PubMed] [Google Scholar]
- 100.Klodzinska A, Chojnacka-Wojcik E, Palucha A, Branski P, Popik P, Pilc A. Potential anti-anxiety, anti-addictive effects of LY 354740, a selective group II glutamate metabotropic receptors agonist in animal models. Neuropharmacology. 1999;38:1831–1839. doi: 10.1016/s0028-3908(99)00066-0. [DOI] [PubMed] [Google Scholar]
- 101.Karasawa J, Shimazaki T, Kawashima N, Chaki S. AMPA receptor stimulation mediates the antidepressant-like effect of a group II metabotropic glutamate receptor antagonist. Brain Res. 2005;1042:92–98. doi: 10.1016/j.brainres.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 102.Kawashima N, Karasawa J, Shimazaki T, Chaki S, Okuyama S, Yasuhara A, Nakazato A. Neuropharmacological profiles of antagonists of group II metabotropic glutamate receptors. Neurosci Lett. 2005;378:131–134. doi: 10.1016/j.neulet.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 103.Matrisciano F, Scaccianoce S, Del Bianco P, Panaccione I, Canudas AM, Battaglia G, Riozzi B, Ngomba RT, Molinaro G, Tatarelli R, Melchiorri D, Nicoletti F. Metabotropic glutamate receptors and neuroadaptation to antidepressants: imipramine-induced down-regulation of beta-adrenergic receptors in mice treated with metabotropic glutamate 2/3 receptor ligands. J Neurochem. 2005;93:1345–1352. doi: 10.1111/j.1471-4159.2005.03141.x. [DOI] [PubMed] [Google Scholar]
- 104.Pietraszek M, Sukhanov I, Maciejak P, Szyndler J, Gravius A, Wislowska A, Plaznik A, Bespalov AY, Danysz W. Anxiolytic-like effects of mGlu1 and mGlu5 receptor antagonists in rats. Eur J Pharmacol. 2005;514:25–34. doi: 10.1016/j.ejphar.2005.03.028. [DOI] [PubMed] [Google Scholar]
- 105.Pin JP, Acher F. The metabotropic glutamate receptors: structure, activation mechanism and pharmacology. Curr Drug Targets CNS Neurol Disord. 2002;1:297–317. doi: 10.2174/1568007023339328. [DOI] [PubMed] [Google Scholar]
- 106.Anwyl R. Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res Brain Res Rev. 1999;29:83–120. doi: 10.1016/s0165-0173(98)00050-2. [DOI] [PubMed] [Google Scholar]
- 107.Li X, Tizzano JP, Griffey K, Clay M, Lindstrom T, Skolnick P. Antidepressant-like actions of an AMPA receptor potentiator (LY392098) Neuropharmacology. 2001;40:1028–1033. doi: 10.1016/s0028-3908(00)00194-5. [DOI] [PubMed] [Google Scholar]
- 108.Li X, Witkin JM, Need AB, Skolnick P. Enhancement of antidepressant potency by a potentiator of AMPA receptors. Cell Mol Neurobiol. 2003;23:419–430. doi: 10.1023/A:1023648923447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.O’Neill MJ, Bleakman D, Zimmerman DM, Nisenbaum ES. AMPA receptor potentiators for the treatment of CNS disorders. Curr Drug Targets CNS Neurol Disord. 2004;3:181–194. doi: 10.2174/1568007043337508. [DOI] [PubMed] [Google Scholar]
- 110.Kole MH, Swan L, Fuchs E. The antidepressant tianeptine persistently modulates glutamate receptor currents of the hippocampal CA3 commissural associational synapse in chronically stressed rats. Eur J Neurosci. 2002;16:807–816. doi: 10.1046/j.1460-9568.2002.02136.x. [DOI] [PubMed] [Google Scholar]
- 111.Reagan LP, Rosell DR, Wood GE, Spedding M, Munoz C, Rothstein J, McEwen BS. Chronic restraint stress up-regulates GLT-1 mRNA and protein expression in the rat hippocampus: reversal by tianeptine. Proc Natl Acad Sci U S A. 2004;101:2179–2184. doi: 10.1073/pnas.0307294101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wu GY, Cline HT. Stabilization of dendritic arbor structure in vivo by CaMKII. Science. 1998;279:222–226. doi: 10.1126/science.279.5348.222. [DOI] [PubMed] [Google Scholar]
- 113.Lowy MT, Wittenberg L, Yamamoto BK. Effect of acute stress on hippocampal glutamate levels and spectrin proteolysis in young and aged rats. J Neurochem. 1995;65:268–274. doi: 10.1046/j.1471-4159.1995.65010268.x. [DOI] [PubMed] [Google Scholar]
- 114.Lowy MT, Gault L, Yamamoto BK. Adrenalectomy attenuates stress-induced elevations in extracellular glutamate concentrations in the hippocampus. J Neurochem. 1993;61:1957–1960. doi: 10.1111/j.1471-4159.1993.tb09839.x. [DOI] [PubMed] [Google Scholar]
- 115.Bagley J, Moghaddam B. Temporal dynamics of glutamate efflux in the prefrontal cortex and in the hippocampus following repeated stress: effects of pretreatment with saline or diazepam. Neuroscience. 1997;77:65–73. doi: 10.1016/s0306-4522(96)00435-6. [DOI] [PubMed] [Google Scholar]
- 116.Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995;69:89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- 117.Cameron HA, McEwen BS, Gould E. Regulation of adult neurogenesis by excitatory input and NMDA receptor activation in the dentate gyrus. J Neurosci. 1995;15:4687–4692. doi: 10.1523/JNEUROSCI.15-06-04687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fuchs E, Czeh B, Kole MH, Michaelis T, Lucassen PJ. Alterations of neuroplasticity in depression: the hippocampus and beyond. Eur Neuropsychopharmacol. 2004;14 (Suppl 5):S481–S490. doi: 10.1016/j.euroneuro.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 119.Fuchs E, Czeh B, Michaelis T, de Biurrun G, Watanabe T, Frahm J. Synaptic plasticity and tianeptine: structural regulation. Eur Psychiatry. 2002;17 (Suppl 3):311–317. doi: 10.1016/s0924-9338(02)00652-1. [DOI] [PubMed] [Google Scholar]
- 120.Lynch G. AMPA receptor modulators as cognitive enhancers. Curr Opin Pharmacol. 2004;4:4–11. doi: 10.1016/j.coph.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 121.Chourbaji S, Vogt MA, Fumagalli F, Sohr R, Frasca A, Brandwein C, Hortnagl H, Riva MA, Sprengel R, Gass P. AMPA receptor subunit 1 (GluR-A) knockout mice model the glutamate hypothesis of depression. FASEB J. 2008;22:3129–3134. doi: 10.1096/fj.08-106450. [DOI] [PubMed] [Google Scholar]
- 122.Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, Manji HK. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 123.Rydmark I, Wahlberg K, Ghatan PH, Modell S, Nygren A, Ingvar M, Asberg M, Heilig M. Neuroendocrine, cognitive and structural imaging characteristics of women on longterm sickleave with job stress-induced depression. Biol Psychiatry. 2006;60:867–873. doi: 10.1016/j.biopsych.2006.04.029. [DOI] [PubMed] [Google Scholar]