Abstract
There are many reports that cross-linking antibodies (Abs) bound to the surface of B-lymphoma cells can induce apoptosis and/or cell death, especially with anti-CD20 Abs. This study was intended to extend our understanding of these effects. To determine if CD20 is a unique target in this respect, or whether Abs to other antigens would have similar effects, six Abs were tested, with and without cross-linking with a secondary Ab, on three target cell lines. We utilized assays that distinguish between apoptotic, dead, and viable cells. Two assays were used: Annexin V plus propidium iodide, and JC-1 plus SYTOX® green (Molecular Probes, Eugene, OR). Most of the Abs tested induced a low level of apoptosis and cell death in Ramos cells, but not in the other two cell lines (Raji and RL). In general, the level of toxicity was correlated with the level of antigen expression, with Abs to high-density antigens having the strongest effects. However, since the majority of Ramos cells continued to multiply, it is questionable whether toxicity at this level can provide a significant clinical benefit. Unexpectedly, there was also a population of cells that stained weakly with Annexin V. These cells were distinct from classical apoptotic cells, and appeared to belong to the viable cell population. In these cells, Annexin V stained the region of the Ab cap, in contrast to the ringed staining of classical apoptotic cells. In conclusion: 1) Low-level induction of apoptosis was not unique for anti-CD20 Abs, but occurred similarly with other Abs, and 2) results of Annexin V staining experiments may need to be reevaluated. Further studies are required to explain why Annexin V binding sites are exposed in the region of an Ab cap.
Key words: antibody therapy, apoptosis, Annexin V, antibody capping, CD20
Introduction
Antibodies (Abs) have recently become established agents in the therapy of cancer and other diseases. Unconjugated Abs have been most successful in the clinic, but there have also been many attempts to use Ab conjugates with radionuclides, drugs, and toxins, as well as a wide variety of Ab variants produced by genetic engineering.1,2 There is strong evidence that Fc-dependent effector mechanisms, ADCC (Ab-dependent cell-mediated cytotoxicity), and/or complement activation are required in order for unconjugated Abs to have their maximal therapeutic effect.3–6 But, there is also considerable evidence that Ab binding alone, without accessory functions, can kill cells, based mainly on in-vitro data.7–10 The importance of various effector mechanisms will depend on the particular antigen targeted, the form of the Ab used, the particular cell line targeted, and other experimental (or clinical) conditions. If it is possible to kill targeted cells via Abs alone, without any accessory functions, then this approach would appear to have the advantage of simplicity. Therefore, in this study, we evaluated the cytotoxic effect of Abs, binding to B-cell lymphomas, with or without additional cross-linking. Most of the previous work was done with anti-CD20 Abs, with limited investigation of Abs to other antigens. But, to understand the physiology of the response, it is essential to test Abs to other antigens to determine if the function of the antigen recognized plays a role in the response observed. Therefore, Abs to six different antigens were tested, on three cell lines.
In previous studies with similar Abs and target cells, primary Abs alone were generally found to have no significant toxic effect, and substantial levels of toxicity could be demonstrated only by increased cross-linking, which was obtained in various ways. This was first reported by Vitetta et al.,11 using Ab dimers produced biochemically, and there have since been many similar reports, most frequently with anti-CD20 Abs binding to B-lymphoma cells.7,8,10,12,13 Most commonly, the method used to increase cross-linking has been to add a secondary Ab (such as goat antimouse IgG). While the in-vivo relevance of this method may be questioned, we would argue that it can provide useful preliminary data. There are many possible ways in which the level of cross-linking can be increased in vivo,13–17 and in vitro experiments can be useful to establish the rationale for this approach. Zhang et al.13 used polymers of rituximab, both in vitro and in vivo. In this study, cross-linking was obtained with a secondary Ab to mouse IgG.
While the most relevant endpoint in these experiments is cell death, it is also important to determine the mechanism of cell death (if it occurs). The most likely mechanism is apoptosis, since this is the mechanism of cell “suicide” after certain signaling pathways are activated. It is well established that certain Abs can efficiently induce apoptosis after binding to the cell surface,18,19 but such Abs appear to be rare, and it remains uncertain whether Abs to most cell-surface antigens are able to induce apoptosis, with or without cross-linking. There are a great variety of assays used to detect apoptosis, and results will depend, to some extent, on the assays used. Some of the assays that have been widely used to detect apoptosis were shown recently to be unreliable in some situations. Thus, necrotic cells (killed in various ways not involving apoptosis, such as heating or freeze-thawing) may have sub-G1 levels of DNA and nonfunctional mitochondria, both of which have been considered characteristic of apoptotic cells.20 DNA strand breakage is another widely used marker of apoptosis. Although not examined in these recent studies with necrotic cells, it is evident that if necrotic cells have sub-G1 levels of DNA, DNA degradation must have occurred, and broken strands are likely to be present. Another methodologic problem is that cells killed via apoptosis may generate subcellular vesicles that are stained by Annexin V, which may be mistaken for apoptotic cells, since some of them are only slightly smaller in size.20 Counting these objects as apoptotic cells would distort the calculated percentage of apoptotic cells. In this study, two reliable assays for apoptosis were used, and the size of the objects identified was monitored. The number of apoptotic cells, in itself, is an ephemeral value, since apoptotic cells become dead cells. Therefore, dead cells and healthy cells were enumerated, as well as apoptotic cells, in order to obtain a more complete picture of the effects that occurred. The results described below revealed another significant limitation of the Annexin V assay for apoptotic cells.
Materials and Methods
Cell lines
Raji and Ramos are Burkitt lymphomas that were obtained from the American Type Culture Collection (ATCC; Manassas, VA). RL is a diffuse, large-cell lymphoma also obtained from ATCC. Cells were cultured by standard methods that have been described.21,22 Cells were tested routinely for mycoplasma contamination, with the Mycotect kit (Invitrogen, Grand Island, NY), and were found to be negative. During the course of these studies, the identity of these cell lines was confirmed by DNA “fingerprinting” performed by DSMZ (Braunschweig, Germany).
Antibodies and other ligands
The Abs used were described previously in detail.23–25 They included Ab to human leukocyte antigen-DR (HLA-DR) (L243), Major histocapability complex (MHC) class I (W6/32), CD19 (HD37), CD20 (2B8), CD22 (LL2), and CD147 (MA103). All are mouse IgG2as except for 2B8, which is a mouse IgG1. Purified LL2 was provided by Immunomedics, Inc. (Morris Plains, NJ), and HD37 was provided by Ellen Vitetta, Ph.D. (UT Southwestern Medical Center, Dallas, TX). The other Abs were produced by hybridoma cells either in tissue culture or growing as ascites in mice. Abs were purified by affinity chromatography on protein A-Sepharose (GE/Pharmacia, Piscataway, NJ). The secondary Abs used were rabbit antimouse IgG (IgG fraction, DAKO Z109), fluorescein-isothiocyanate (FITC)-conjugated goat antimouse IgG (ICN/Cappel Labs, Costa Mesa, CA), or rhodamine-red-X conjugated goat antimouse IgG (Jackson ImmunoResearch, West Grove, PA). Annexin V conjugated to AlexaFluor-488 was obtained from Molecular Probes (Eugene, OR).
Ab treatment of cells
Cells were incubated with the primary Ab at 10 μg/mL for 1 hour at 37°C, then washed twice. Samples were then incubated with the secondary Ab, rabbit antimouse IgG, at 100 μg/mL, or control medium, for 1 hour at 37°C. After 2 washes, cells were plated in 24-well plates, in 1.5 mL of tissue culture medium. At various times, routinely 0 (just after plating), 21, and 45 hour, the contents of some of the wells were transferred to centrifuge tubes, cells were pelleted, and various assays for apoptosis were performed. One well was used for each time point, for each assay, and 5.0 × 105 cells were plated in each well. For the Ab incubations, the volume of each reagent used was 0.1 mL per 106 cells. In some experiments, the method was varied such that cells were plated after coating with the primary Ab only, and the secondary Ab was added to the medium in which the cells were incubated. The concentration of the secondary Ab in these experiments was 40, 10, or 2 μg/mL, as indicated.
JC-1 assay for mitochondrial membrane potential20
JC-1 (Sigma Chemicals, St. Louis, MO) was dissolved at 1.0% in dimethyl sulfoxide (DMSO) and stored at −20°C. One-hundreth volume was added to approximately 5 × 105 cells in approximately 130 μL of tissue-culture medium. After 10 minutes at 37°C, cells were washed twice with tissue-culture medium, the second time without phenol red, then suspended in 0.5 mL. The nuclear stain, SYTOX® green was obtained from Molecular Probes, at 5 mM in DMSO, and stored at −20°C. The stock was prepared by diluting 2 μL into 1.0 mL of tissue-culture medium without phenol red, and a one-hundreth volume of this was added to the cell suspension. After 5 minutes at room temperature, samples were analyzed on a FACSCalibur (BD Bioscience, San Jose, CA), and were also examined on an Olympus (Melville, NY) BH2 fluorescent microscope with a 100-W mercury bulb. SYTOX green stains the cells with disrupted membranes (dead cells), while JC-1 identifies cells with decreased mitochondrial membrane potential, which includes both dead and apoptotic cells. Thus, this assay enumerates apoptotic, dead, and viable cells.
Annexin V staining20
Cells were washed once with tissue-culture medium without phenol red, supplemented with 2 mM of CaCl2. After discarding the supernatant, 5 μL of the Annexin V conjugate was added to the residual volume (approximately 130 μL). After 45 minutes at room temperature, cells were washed twice, then suspended in the same medium, containing 1 μg/mL of propidium iodide, and analyzed as above. Photographs were taken with a Microfire camera (Olympus America, Melville, NY). This assay also enumerates apoptotic, dead, and viable cells.
Results
Cell death and apoptosis induced by Ab cross-linking
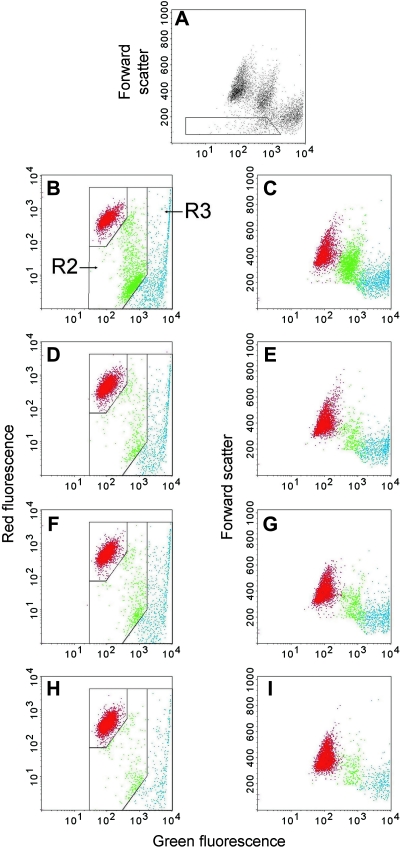
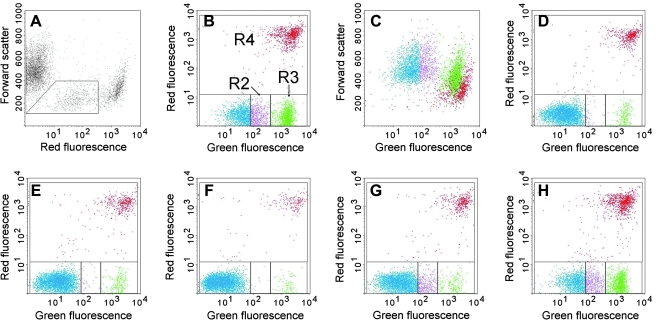
Quantification of dead or apoptotic cells was done by FACS analysis, with confirmation by microscopic observation. Figures 1 and 2 show examples of the data obtained with the JC-1 and the Annexin V assays, respectively. In both figures, panel A shows a dot plot of the total, ungated cells, with a low forward-scatter threshold (set at 52), thus showing essentially all objects. This dot plot has forward scatter on the y-axis and fluorescence on the x-axis, with the fluorescence color that identifies dead cells (green for the JC-1 assay and red for the Annexin V assay). Panel A also shows the gate that was used to exclude subcellular debris. This is an important decision in experiments of this type, since dead and apoptotic cells degenerate to produce subcellular fragments, which may mistakenly be counted as dead or apoptotic cells.20,26 The basic approach was to gate out objects that had low forward scatter and low DNA fluorescence and did not belong to the distinct spots formed by the viable, apoptotic, or dead cells. Microscopic observation confirmed that these faintly fluorescent objects were, in fact, subcellular fragments of varied morphologies. Inasmuch as this gating method is somewhat arbitrary, two points should be noted: 1) Dead cells have less forward scatter than viable cells,27 so gating based on forward scatter only would exclude many dead cells, and 2) the key results of these experiments, namely the induction of apoptosis and death by Abs, would not change regardless of the gates used.
FIG. 1.
Example of data from the JC-1 assay, with Ramos cells treated for 1 day with antibodies (Abs). JC-1 emits both red and green fluorescence while SYTOX® green (Molecular Probes, Eugene, OR), at the concentration used, is brighter green. Panels A–C show cells that had been treated with L243 plus a secondary Ab. (A) A plot of forward scatter versus green fluorescence, which was the plot used to gate out cell debris. The gate shows the region that was excluded in the subsequent analysis. Left panels: plots of red fluorescence versus green fluorescence, which was used to enumerate apoptotic cells (region R2, green dots) and dead cells (R3, blue dots). Right panels: plots of forward scatter versus green fluorescence, using the color of the dots defined in the left panels, to show another characteristic of the cells. Cells were treated with: (D–E) L243 without a secondary Ab; (F–G) medium; (H and I) untreated cells, meaning cells that were not incubated and centrifuged. (Note: To view this figure in color, see online version of this article.)
FIG. 2.
Example of data from the Annexin V assay, with Ramos cells treated for 1 day with antibodies (Abs). Panels A–C show cells that had been treated with W6/32 plus a secondary Ab. (A) A plot of forward scatter versus red fluorescence (propidium iodide), which was the plot used to gate out cell debris. The gate shows the region that was excluded in the subsequent analysis. (B) A plot of red fluorescence versus green fluorescence (Annexin V), which was used to enumerate apoptotic cells (region R3, green dots) and dead cells (R4, red dots). R2 (purple dots) contains cells that have faint green fluorescence, but are distinct from the apoptotic population. The nature of these cells is discussed in the text. (C) A plot of forward scatter versus green fluorescence, using the color of the dots defined in panel B, to show another characteristic of the cells. The other panels are similar to (B), except with cells treated with different Abs. (D) W6/32 without a secondary Ab; (E) medium; (F) untreated cells, meaning cells that were not incubated and centrifuged; (G) L243 without a secondary Ab; (H) L243 plus a secondary Ab. (Note: To view this figure in color, see online version of this article.)
Figures 1 and 2 show results with Abs L243 (anti-HLA-DR) and W6/32 (anti-HLA class I), both Abs that induced relatively high levels of apoptosis and death. Results are shown for the primary Ab only, the primary plus a secondary Ab, and a medium control, which was handled in the same way as the other samples. Also shown are results with control cells that were not handled, since it was apparent that some cell death resulted simply from the handling of the cells, in the absence of Ab binding. In the protocol used, cells are incubated twice for 45 minutes and washed twice after each Ab incubation. In addition, cells are pelleted twice before the first Ab incubation, to remove spent medium. It is not unexpected that such handling would kill some fraction of the cells, due to some type of nonspecific damage, and this effect accounts, in part, for the background level of cell death. The level of cell kill due to handling was generally low (<10%), but approached 20% in some experiments. The level observed was clearly related to the cell viability at the start of the experiment: When viability was relatively low (80–90%), the cells were more susceptible to this type of damage. In addition, it appeared to vary, depending on the particular cell lines used. The highest level of nonspecific killing was observed with Ramos cells, and no killing was obtained with Raji or RL cells.
In Figure 1, showing results of the JC-1 assay, region 2 (R2) contains the apoptotic cells, which have lost some red fluorescence and gained some green fluorescence, due to the loss of mitochondrial membrane potential. R3 contains dead cells that stain bright green with SYTOX green. It is evident that treatment with L243 plus a secondary Ab induced a large increase in apoptotic cells and a smaller increase in dead cells at day 1. The primary Ab alone had no effect. The standard analysis of the results is shown in the panels on the left, but we also show a different analysis of the same samples on the right, a plot of forward scatter versus green fluorescence. These graphs are included because, in some respects, they show the three distinct cell populations more clearly than the standard plot. In these plots, the colors of the dots are defined by the gates shown in the panels on the left. This plot, of course, does not show the bright red mitochondrial fluorescence of JC-1 in viable cells, but it does show the increase in green fluorescence from the JC-1 as the drug moves from the mitochondria to the cytoplasm in apoptotic cells.28
In Figure 2, showing results of the Annexin V assay, R3 (green dots) contains the classical apoptotic cells, with bright green Annexin V staining, and R4 (red dots) contains dead cells, with bright red staining. R2 in Figure 2 (purple dots) includes cells that have faint green staining by Annexin V. R2 and R3 are clearly distinct populations and, therefore, were enumerated separately. Cells in the R2 region are frequently counted as apoptotic cells, but they are certainly not classical apoptotic cells, and we suggest that they are not apoptotic cells at all, as discussed below. The data shown in Figure 2 demonstrate that: 1) W6/32 alone had essentially no effect (D), but when combined with a secondary Ab, induced a substantial increase in both apoptotic and dead cells (B), and an increase in R2, and 2) L243 alone produced a small increase in cells in R2 (G), but no increase in classical apoptotic or dead cells. When the secondary Ab was added to L243, both apoptotic and dead cells increased (H). Panel 2C shows a different analysis of the same sample shown in panel 2B, using a plot of forward scatter versus green fluorescence. The colors of the dots in this plot are based on the gates shown in panel 2B. The purpose of this plot is to demonstrate that the cells in R2 appear to be part of the viable cell population and are clearly distinct from either the classical apoptotic or dead cells.
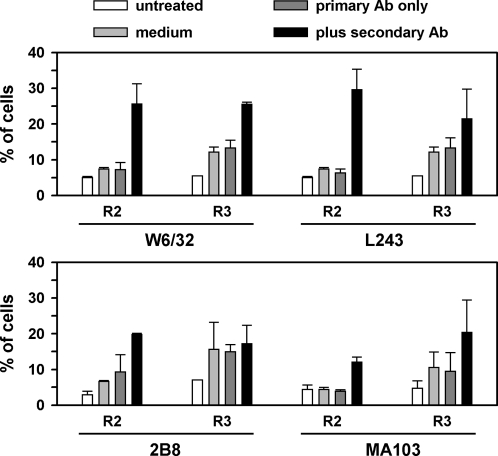
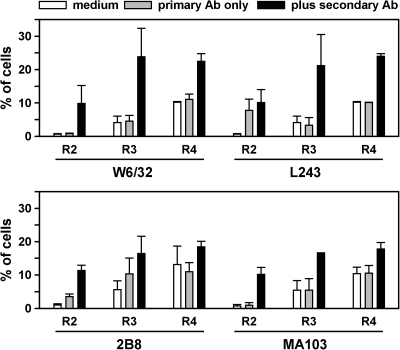
Figures 3 and 4 provide a summary of the results with Ramos cells, with the assay for JC-1 or Annexin V, respectively, for the four Abs that were significantly toxic, which were W6/32, L243, 2B8, and MA103 (anti-CD147). LL2 and HD37 were also tested, but had little, if any, toxic effect. The most potent Abs were L243 and W6/32, but toxicity also was induced, to a lesser extent, with 2B8 and MA103. Toxicity peaked at day 1, which is shown in the figures, and decreased at later time points, as viable cells proliferated. The regions are as defined in Figures 1 and 2. The results of the JC-1 and Annexin assays were entirely concordant, except that the cells in R2 with the Annexin V assay did not appear to be apoptotic by the JC-1 assay, which supports the other evidence that these faint green cells are not, in fact, apoptotic.
FIG. 3.
A summary of data from the JC-1 assay, with four antibodies (Abs) tested on Ramos cells. Results are shown for cells that were untreated, or treated with medium, primary Ab only, or both Abs, as indicated in the figure. R2 are the cells in the apoptotic region of the dot plot, and R3 are the cells in the dead region of the dot plot. Means and standard deviations of two experiments are shown.
FIG. 4.
A summary of data from the Annexin V assay, with four antibodies (Abs) tested on Ramos cells. Results are shown for cells that were treated with medium, primary Ab only, or both Abs, as indicated in the figure. R3 are the cells in the classical apoptotic region of the dot plot, and R4 are the cells in the dead region of the dot plot. R2 are the cells in the slightly green region of the dot plot, and the nature of these cells is discussed in the text. Means and standard deviations of two experiments are shown.
The Abs that were most potent on Ramos cells—L243, W6/32, and 2B8—were also tested on Raji and RL: There was no significant effect on these two cell lines. Moreover, the handling-induced toxicity that was observed with Ramos cells also did not occur with Raji and RL. Thus, it appears that Ramos cells are unusually sensitive to these treatments.
The nature of the cells faintly stained with Annexin
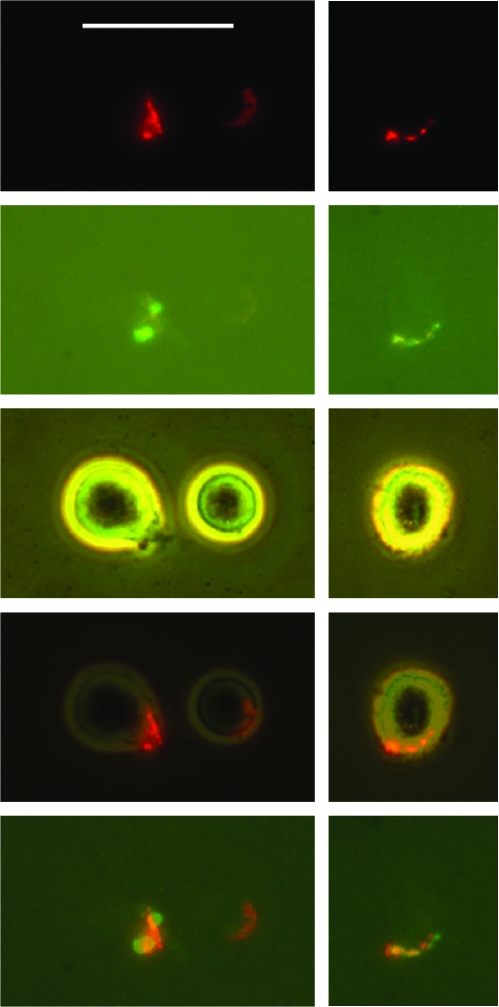
These cells were seen at day 1, but much less—or not at all—at time 0 after Ab treatment. However, there was one exception to this: Ramos cells treated with L243 had a significant number of cells in R2 at time 0, and this number decreased slightly by day 1. Although these cells are frequently counted as apoptotic cells, the following factors must be considered: 1) These cells comprise a population that was distinct from the population of bright green cells, the classical apoptotic cells, in the dot-plots; 2) these cells appeared to be part of the healthy cell population in the dot plots; 3) when these cells were present, the entire healthy cell population was invariably shifted slightly to the right (compare Figs. 2B and 2D), suggesting that all of the healthy cells had slight Annexin staining—that is, the location of these cells in R2 seems to be a consequence of shifting the entire cell population to the right, rather than staining a distinct subpopulation of the cells; 4) microscopic observation demonstrated that the green staining of these cells was usually in the form of a small cluster of faint green dots on the cell surface, which might be described as an apparent small cap of fluorescence (Fig. 5); these cells appeared viable, by phase-contrast observation, with no morphologic difference between them and healthy cells; and 5) Ramos cells occasionally form uropods29 of varying size, which are present on approximately 10–20% of the cells. The Annexin V caps were invariably present on the uropods, if uropods were present.
FIG. 5.
Annexin V stains the antibody (Ab) cap in Ramos cells. Cells were treated with L243 antihuman leukocyte antigen-DR (anti-HLA-DR) plus a rhodamine-conjugated secondary Ab, to induce a red Ab cap. Cells were then stained with Annexin V–Alexa-488 (green). Two fields are shown, for (top to bottom) red fluorescence, green fluorescence, phase contrast, a superposition of red fluorescence and phase contrast, or a superposition of red fluorescence and green fluorescence. Bar = 25 μm. (Note: To view this figure in color, see online version of this article.)
Ab caps also typically form on uropods.29 Thus, it seemed possible that Annexin was staining the Ab-induced caps. To investigate this possibility, we determined whether the Annexin V staining colocalized with the Ab cap, by using a rhodamine-conjugated secondary Ab (red), and Annexin V conjugated to Alexa-488 (green). Following the standard protocol, cells were incubated sequentially with both Abs, washed, incubated overnight, then stained with Annexin V. As shown in Figure 5, which shows representative cells, both labels were colocalized. While the two colors were in the same region of the cell, the cap, there were slight differences in the distribution of the colors, indicating that the structures stained were not exactly the same. In one of the cells shown, staining was localized to the uropod, but in the other cell, no uropod was evident. Another cell had an Ab cap (relatively faint) but no evident staining with Annexin V. In general, nearly all of the cells with a bright red Ab cap had green staining within the capped region, but often the green staining was very faint, consisting of a few small spots, and was difficult to photograph. A small number of classical apoptotic cells were present: These cells were stained in a bright ring pattern with Annexin V, as expected. We conclude that the faint green staining appears to represent staining of Ab caps, rather than apoptotic cells, although the reason that Annexin V stains Ab caps is not yet understood.
Cells in R2 were observed not only with Ramos cells, but also with Raji and RL cells treated with Abs, but not with untreated cells, despite the fact that classical apoptotic cells or dead cells were not induced by this treatment in these cells lines. R2 cells were found at time 0 (just after the Ab incubations and washes) and/or at 24 hour, depending on the particular Ab used. R2 cells were observed after treatment of Raji or RL cells with Abs L243, W6/32, and 2B8. In some cases, especially with Abs L243 or 2B8, R2 cells were induced with the primary Ab only, in the absence the secondary Ab (although they were also present when the secondary Ab was added). In all cases, the cells in R2 appeared to be a part of the viable cell population, as described above. Again, the R2 cells were examined by microscope and always appeared to be viable cells with a small Annexin cap. Double staining experiments were also performed with RL and Raji, as described for Ramos cells, to determine if the Annexin V was staining the Ab cap. Only L243 induced a sufficient number of Annexin V–stained caps to allow definite conclusions; but in this case, the annexin caps did colocalize with the Ab caps, which was similar to the results seen with Ramos cells. Since Ab treatment of Raji and RL cells caused an increase in the cells in R2, without any increase in apoptotic or dead cells, these results can be considered to support the conclusion that the cells in region R2 are not apoptotic cells.
Is toxicity a result of mechanical damage due to the handling of aggregated cells?
Inasmuch as Ab cross-linking typically induced some aggregation of the cells, we considered the possibility that cells might be damaged as a consequence of the aggregation, together with handling. That is, cells aggregated by Abs might be compared to balloons taped together: The tape does not damage the balloons, but pulling them apart (which would be a consequence of handling procedures) will break them. To investigate the possible role of a process of this type, the standard protocol was modified: After treatment with the primary Ab, cells were washed and plated, and the secondary Ab was added to the medium in which the cells were cultured overnight. Three concentrations of the secondary Ab were tested: 40, 10, and 2 μg/mL. Two primary Abs were tested: L243 and W6/32. After overnight incubation, the cells were collected and assayed by both the JC-1 and Annexin V assays. Since the cells were handled minimally after adding the secondary Ab (which is the reagent that induces most of the aggregation), any toxicity observed cannot be attributed to mechanical damage to the cells. Results demonstrated that apoptosis and cell death still occurred, which was very similar to the levels observed in the previous experiments (data not shown). The higher concentrations of the secondary Ab were more potent than the lowest concentration, but there was little difference between 40 and 10 μg/mL. These data suggest that the damage to cells in these experiments cannot be attributed to the handling of aggregated cells.
Discussion
This study confirmed the previous findings that apoptosis and cell death are induced by Ab cross-linking. All of the Abs to high-density antigens were active, and the level of activity was generally correlated with the density of antigen expression. Although antigen density was not determined as a part of this study, it is well established that, in general, HLA-class I, HLA-DR, CD147, and CD20 are high-density antigens on B-lymphoma cells, while CD19 and CD22 are usually expressed at a lower level.30–32 This statement is supported by the fluorescent intensity of cells stained with saturating concentrations of fluorescent Abs reacting with these antigens (MJM; unpublished data). Therefore, the results overall suggest that the physiologic role of the antigen recognized is not an important variable, although the data are not conclusive on this point. These results are consistent with those of Ghettie et al.,11 who demonstrated that essentially all Abs (to various antigens) became toxic after dimerization. As reported previously by others, primary Abs alone had essentially no toxic effect, and cross-linking with the secondary Ab was required.
Although these data provide some support for the idea that this approach may be useful clinically, the apparent limitations of this method should be emphasized. Despite the fact that saturating concentrations of Abs were used, and that these in-vitro experiments did not have to overcome obstacles, such as tumor penetration that are important in vivo, the level of cell death was relatively low, with a maximum of approximately 25%. The remaining cells appeared to be entirely healthy and continued to multiply normally. Other laboratories have similarly reported a limited level of toxicity, in contrast to the high levels of killing that are obtained by therapeutic drugs, external beam irradiation, or some radiolabeled Abs.32 Such a low level of toxicity, in itself, would not be clinically important. Therefore, it is important to understand why only a subpopulation of cells was sensitive to this type of toxicity. One possible explanation is that susceptibility may be related to cell-cycle phase, but this suggestion is purely speculative, and further investigation of this question is required. It may yet be possible to enhance the level of killing, by modifying some of the experimental conditions, and the results of Zhang et al.13 with a rituximab polymer are encouraging, but if near 100% kill cannot be obtained in vitro, where all conditions can be manipulated at will, it is unlikely that this approach will provide effective therapy clinically.
Further, apoptosis and death occurred only with Ramos cells, of the three cell lines tested. The susceptibility of Ramos to apoptosis induction is consistent with many publications in which Ramos was used as a “typical” target cell in apoptosis experiments,7–9,20 although the difference between Ramos and other cell lines was usually not emphasized. The susceptibility of Ramos cells to apoptosis, compared to the other two cell lines tested, is likely to be due, in part, to two factors: 1) Ramos is one of the few Burkitt's lymphoma lines that is not infected by Epstein-Barr (EB) virus; the EB virus inhibits apoptosis by the down regulation of the proapoptotic protein, Bim33; and 2) unlike RL cells (which also are not infected by EB virus), Ramos does not normally have a high expression of the antiapoptotic protein, Bcl-2.34 Although Raji and RL did not generate any classical apoptotic cells or dead cells, they did have a subpopulation that was weakly stained by Annexin V after Ab cross-linking and capping. This result supports the other data indicating that these weakly stained cells are not apoptotic cells.
It should be noted that other investigators reported that cross-linked rituximab did induce apoptosis in either Raji or RL cells,10,12,13 while the results of Chan et al.8, consistent with ours, had the opposite conclusion. This discrepancy cannot currently be explained, but might be due to differences in the Ab derivatives used, the assays employed, or other experimental conditions. It might be due, in part, to problems with some of the assays used to measure apoptosis, as described in this paper and previously.20 The possibility of cell line cross-contamination must always be considered.35 The identity of the cell lines used in our experiments was confirmed by DNA “fingerprinting,” as described under Material and Methods. Also, we note that other Abs to CD20 were reported to more potently induce apoptosis than rituximab8 (which was derived from the 2B8 Ab used in this study).
One aspect of these types of experiments that has not been sufficiently considered is the role of cell aggregation, since cell aggregation is a possible consequence of Ab cross-linking. If cells are tightly aggregated by Abs, it may be impossible to produce a single cell suspension, which is required for FACS analysis, without killing the cells via mechanical trauma. The role of this factor will depend on the tightness of the aggregates induced. Many B-lymphoma cell lines spontaneously form clusters in vitro, but these typically are loose clusters that can be readily dispersed by shaking, vortexing, or pipetting, without evident damage to the cells. However, for Abs that induce very tight clusters, such as anti-HLA-DR Abs, this can present a major problem and might be largely responsible for the cell death that results from Ab binding,36 as suggested previously.20 In this study, the role of mechanical damage was investigated by varying the experimental conditions: in some cases, cells were coated with the primary Ab, washed, and plated in the presence of the secondary Ab to minimize handling after the secondary Ab was applied. Results in this case were no different from the results of experiments in which cells were coated with both Abs before plating. Therefore, under the conditions tested, it appears that mechanical damage to cell aggregates was not responsible for the toxicity that was observed.
An important result of this study was the finding that Annexin V bound weakly to Ab caps, but not to cells untreated with Ab, and that the cells having such binding did not appear to be apoptotic. Cells faintly stained with Annexin V have frequently been considered to be apoptotic,37–39 but the evidence presented strongly suggests that this is not likely to be the case. Further, results of Annexin V staining experiments are frequently reported only in terms of the percent of positive cells, without the raw data, so it is uncertain whether the positive cells were Ab-capped viable cells or true apoptotic cells. We recently reported the presence of Annexin V binding sites associated with Ab caps, using a fluorochrome-conjugated primary Ab,40 but are not aware of earlier descriptions of this effect. Dillon et al.41 reported the capping of Annexin V binding sites on viable cells, but their studies were different from those reported by us in important ways, as we discussed previously in detail.40 Briefly, they used mouse spleen cells, which spontaneously expressed Annexin V binding sites and, therefore, are different from human B-lymphoma cells in this respect. Thus, in their case, the Annexin V binding sites were not induced by Ab binding, although they were cocapped with Abs to cell-surface IgM. Further investigation is required in order to determine the relationship between these two sets of observations. The mechanism by which Annexin V binding sites are exposed in Ab caps remains to be determined, but a role for lipid rafts must be considered.8,42
It might be argued that newly apoptotic cells also might have faint Annexin V staining, if the phosphatidyl serine (PS) is only partially exposed on the cell surface. Such cells would be expected to be ringed, not capped, and, therefore, could be identified by microscopic observation. However, such cells were rarely observed in these experiments, probably because the movement of PS to the exterior face of the membrane is rapid once it is induced. Further, it is important to emphasize that apoptotic cells and viable cells formed two distinct populations in the dot plots, with few intermediate cells, which further supports the idea that the change in PS expression is rapid. Despite these results, Annexin V staining remains an excellent, reliable assay for apoptosis, as long as the stained objects are well characterized, either by microscopic observation or by FACS. The Annexin V assay (used conservatively) was entirely concordant with the modified JC-1 assay, which further establishes the value of the JC-1 assay, which is simpler and faster to perform than the Annexin V assay, while providing a similar enumeration of apoptotic, dead and healthy cells.
Conclusions
Primary Abs alone did not induce apoptosis of B-lymphoma cell lines, but the addition of a secondary Ab, goat antimouse IgG, did induce a low level of apoptosis. This did not appear to depend on the specificity of the primary Ab, since it occurred with all Abs tested that reacted with relatively high-density cell-surface antigens. Apoptosis was induced only in Ramos cells and not in Raji or RL cells. With all three cell lines, there was additional, unexpected staining by Annexin V confined to the region of the Ab cap. The data indicated that the cells stained in this way were viable cells, not apoptotic cells, which had much brighter, ringed staining.
Acknowledgment
This work was supported, in part, by US PHS grant PO1 CA103985 (to DMG) from the National Institutes of Health (Bethesda, MD).
Disclosure Statement
No competing financial interests exist.
References
- 1.Waldmann TA. Morris JC. Development of antibodies and chimeric molecules for cancer immunotherapy. Adv Immunol. 2006;90:83. doi: 10.1016/S0065-2776(06)90003-0. [DOI] [PubMed] [Google Scholar]
- 2.Sharkey RM. Goldenberg DM. Targeted therapy of cancer: New prospects for antibodies and immunoconjugates. CA Cancer J Clin. 2006;56:226. doi: 10.3322/canjclin.56.4.226. [DOI] [PubMed] [Google Scholar]
- 3.Smith MR. Rituximab (monoclonal anti-CD20 antibody): Mechanisms of action and resistance. Oncogene. 2003;22:7359. doi: 10.1038/sj.onc.1206939. [DOI] [PubMed] [Google Scholar]
- 4.Chinn P. Braslawsky G. White C, et al. Antibody therapy of non-Hodgkin's B-cell lymphoma. Cancer Immunol Immunother. 2003;52:257. doi: 10.1007/s00262-002-0347-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Czuczman MS. Weaver R. Alkuzweny B, et al. Prolonged clinical and molecular remission in patients with low-grade or follicular non-Hodgkin's lymphoma treated with rituximab plus CHOP chemotherapy: 9-year follow-up. J Clin Oncol. 2004;22:4711. doi: 10.1200/JCO.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 6.Cittera E. Leidi M. Buracchi C, et al. The CCL3 family of chemokines and innate immunity cooperate in vivo in the eradiation of an established lymphoma xenograft by rituximab. J Immunol. 2007;178:6616. doi: 10.4049/jimmunol.178.10.6616. [DOI] [PubMed] [Google Scholar]
- 7.Shan D. Ledbetter JA. Press OW. Signaling events involved in anti-CD20-induced apoptosis of malignant human B cells. Cancer Immunol Immunother. 2000;48:673. doi: 10.1007/s002620050016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan HTC. Hughes D. French RR, et al. CD20-induced lymphoma cell death is independent of both caspases and its redistribution into Triton X-100 insoluble membrane rafts. Cancer Res. 2003;63:5480. [PubMed] [Google Scholar]
- 9.Jazirehi AR. Gan X-H. De Vos S, et al. Rituximab (anti-CD20) selectively modifies Bcl-xl and apoptosis protease activating factor-1 (Apaf-1) expression and sensitizes human non-Hodgkin's lymphoma B-cell lines to paclitaxel-induced apoptosis. Mol Cancer Ther. 2003;2:1183. [PubMed] [Google Scholar]
- 10.Stein R. Qu Z. Chen S, et al. Characterization of a new humanized anti-CD20 monoclonal antibody, IMMU-106, and its use in combination with the humanized anti-CD22 antibody, epratuzumab, for the therapy of non-Hodgkin's lymphoma. Clin Cancer Res. 2004;10:2868. doi: 10.1158/1078-0432.ccr-03-0493. [DOI] [PubMed] [Google Scholar]
- 11.Ghettie M-A. Podar EM. Ilgen A, et al. Homodimerization of tumor-reactive monoclonal antibodies markedly increases their ability to induce growth arrest or apoptosis of tumor cells. Proc Natl Acad Sci USA. 1997;94:7509. doi: 10.1073/pnas.94.14.7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghetie MA. Bright H. Vitetta ES. Homodimers but not monomers of rituxan (chimeric anti-CD20) induce apoptosis in human B-lymphoma cells and synergize with a chemotherapeutic agent and an immunotoxin. Blood. 2001;97:1392. doi: 10.1182/blood.v97.5.1392. [DOI] [PubMed] [Google Scholar]
- 13.Zhang N. Khawli LA. Hu P, et al. Generation of rituximab polymer may cause hyper-cross-linking-induced apoptosis in non-Hodgkin's lymphomas. Clin Cancer Res. 2005;11:5971. doi: 10.1158/1078-0432.CCR-05-0554. [DOI] [PubMed] [Google Scholar]
- 14.King DJ. Turner A. Farnsworth APH, et al. Improved tumor targeting with chemically cross-linked recombinant antibody fragments. Cancer Res. 1994;54:6176. [PubMed] [Google Scholar]
- 15.Miller K. Meng G. Liu J, et al. Design, construction, and in vitro analyses of multivalent antibodies. J Immunol. 2003;170:4854. doi: 10.4049/jimmunol.170.9.4854. [DOI] [PubMed] [Google Scholar]
- 16.Qu Z. Goldenberg DM. Cardillo TM, et al. Bispecific anti-CD20/22 antibodies inhibit B-cell lymphoma proliferation by a unique mechanism of action. Blood. 2008;111:2211. doi: 10.1182/blood-2007-08-110072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rossi EA. Goldenberg DM. Cardillo TM, et al. Stably tethered multifunctional structures of defined composition made by the dock and lock method for use in cancer targeting. Proc Natl Acad Sci U S A. 2006;103:6841. doi: 10.1073/pnas.0600982103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo Y. Chen C. Zheng Y, et al. A novel antihuman DR5 monoclonal antibody with tumoricidal activity induces caspase-dependent and caspase-independent cell death. J Biol Chem. 2005;280:41940. doi: 10.1074/jbc.M503621200. [DOI] [PubMed] [Google Scholar]
- 19.Motoki K. Mori E. Matsumoto A, et al. Enhanced apoptosis and tumor regression induced by a direct agonist antibody to tumor necrosis factor–related apoptosis-inducing ligand receptor 2. Clin Cancer Res. 2005;11:3126. doi: 10.1158/1078-0432.CCR-04-1867. [DOI] [PubMed] [Google Scholar]
- 20.Mattes MJ. Apoptosis assays with lymphoma cell lines: Problems and pitfalls. Br J Cancer. 2007;96:928. doi: 10.1038/sj.bjc.6603663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansen HJ. Ong GL. Diril H, et al. Internalization and catabolism of radiolabeled antibodies to the MHC class II invariant chain by B-cell lymphomas. Biochem J. 1996;320:293. doi: 10.1042/bj3200293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michel RB. Mattes MJ. Intracellular accumulation of the anti-CD20 antibody 1F5 in B-lymphoma cells. Clin Cancer Res. 2002;8:2701. [PubMed] [Google Scholar]
- 23.Vangeepuram N. Ong GL. Mattes MJ. Processing of antibodies bound to B-cell lymphomas and lymphoblastoid cell lines. Cancer (Suppl) 1997;80:2425. doi: 10.1002/(sici)1097-0142(19971215)80:12+<2425::aid-cncr14>3.3.co;2-d. [DOI] [PubMed] [Google Scholar]
- 24.Hanna R. Ong GL. Mattes MJ. Processing of antibodies bound to B-cell lymphomas and other hematological malignancies. [Correction in 1998; 58: 375] Cancer Res. 1996;56:3062. [PubMed] [Google Scholar]
- 25.Michel RB. Mattes MJ. Antibodies to CD20 and MHC class II antigen bound to B-lymphoma cells accumulate in shed cytoplasmic fragments. Br J Cancer. 2004;91:1500. doi: 10.1038/sj.bjc.6602131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mattes MJ. The mechanism of killing of B-lymphoma cells by 111In-conjugated antibodies. Int J Radiat Biol. 2008;84:389. doi: 10.1080/09553000801998867. [DOI] [PubMed] [Google Scholar]
- 27.Shapiro HM. Practical Flow Cytometry. 2nd. New York: Alan R. Liss, Inc.; 1988. [Google Scholar]
- 28.Reers M. Smiley ST. Mottola-Hartshorn C, et al. Mitochondrial membrane potential monitored by JC-1 dye. Metho Enzymol. 1995;260:406. doi: 10.1016/0076-6879(95)60154-6. [DOI] [PubMed] [Google Scholar]
- 29.Braun J. Unanue ER. The lymphocyte cytoskeleton and its control of surface receptor functions. Semin Hematol. 1983;20:322. [PubMed] [Google Scholar]
- 30.Press OW. Farr AG. Borroz KI, et al. Endocytosis and degradation of monoclonal antibodies targeting human B-cell malignancies. Cancer Res. 1989;49:4906. [PubMed] [Google Scholar]
- 31.Vervoordeldonk SF. Merle PA. van Leeuwen EF, et al. Preclinical studies with radiolabeled monoclonal antibodies for treatment of patients with B-cell malignancies. Cancer. 1994;73:1006. doi: 10.1002/1097-0142(19940201)73:3+<1006::aid-cncr2820731339>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 32.Ong GL. Elsamra SE. Goldenberg DM, et al. Single-cell cytotoxicity with radiolabeled antibodies. Clin Cancer Res. 2001;7:192. [PubMed] [Google Scholar]
- 33.Clybouw C. Mchichi B. Mouhamad S, et al. EBV infection of human B-lymphocytes leads to downregulation of Bim expression: Relationship to resistance to apoptosis. J Immunol. 2005;175:2968. doi: 10.4049/jimmunol.175.5.2968. [DOI] [PubMed] [Google Scholar]
- 34.Ning ZQ. Norton JD. Johnson D, et al. Early gene signalling-dependent and -independent induction of apoptosis in Ramos human B-cells can be inhibited by over expression of Bcl-2. Biochem Biophys Res Commun. 1995;215:23. doi: 10.1006/bbrc.1995.2429. [DOI] [PubMed] [Google Scholar]
- 35.Drexler HG. Dirks WG. Matsuo Y, et al. False leukemia-lymphoma cell lines: An update on over 500 cell lines. Leukemia. 2003;17:416. doi: 10.1038/sj.leu.2402799. [DOI] [PubMed] [Google Scholar]
- 36.Nagy ZA. Mooney NA. A novel, alternative pathway of apoptosis triggered through class II major histocompatibility complex molecules. J Mol Med. 2003;81:757. doi: 10.1007/s00109-003-0489-9. [DOI] [PubMed] [Google Scholar]
- 37.Rose AL. Smith BE. Maloney DG. Glucocorticoids and rituximab in vitro: Synergistic direct antiproliferative and apoptotic effects. Blood. 2002;100:1765. [PubMed] [Google Scholar]
- 38.Cragg MS. Glennie MJ. Antibody specificity controls in vivo effector mechanisms of anti-CD20 reagents. Blood. 2004;103:2738. doi: 10.1182/blood-2003-06-2031. [DOI] [PubMed] [Google Scholar]
- 39.Cardarelli PM. Quinn M. Buckman D, et al. Binding to CD20 by anti-B1 antibody or F(ab′)2 is sufficient for induction of apoptosis in B-cell lines. Cancer Immunol Immunother. 2002;51:15. doi: 10.1007/s00262-001-0247-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Michel RB. Abu-Asab M. Tsokos M, et al. Characterization of antibody-containing vesicles shed from B-lymphoma cell lines: Exposure of Annexin V binding sites. Leuk Lymph. 2006;47:2388. doi: 10.1080/10428190600783494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dillon SR. Mancini M. Rosen A, et al. Annexin V binds to viable B-cells and colocalizes with a marker of lipid rafts upon B-cell receptor activation. J Immunol. 2000;164:1322. doi: 10.4049/jimmunol.164.3.1322. [DOI] [PubMed] [Google Scholar]
- 42.Unruh TL. Li H. Mutch CM, et al. Cholesterol depletion inhibits src family kinase-dependent calcium mobilization and apoptosis induced by rituximab cross-linking. Immunology. 2005;116:223. doi: 10.1111/j.1365-2567.2005.02213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]