Abstract
Objective
Auditory mismatch negativity (MMN) and P3a index preattentive detection of rare stimuli. Their amplitudes normally decrease with age. Previous studies have reported generally smaller than normal MMN and P3a in schizophrenia patients. We aimed to further characterize the course of these deficits over schizophrenia patients’ lifespan.
Methods
In 253 schizophrenia patients and 147 normal comparison participants (NCPs) encompassing a wide age range (18–65), event-related potentials were recorded while participants watched a silent video and were presented binaurally with 1-kHz tones 500 ms apart, including standards (P=.90, 50-ms duration) and deviants (P=0.10, 100-ms).
Results
Over the entire age range, MMN and P3a were smaller in schizophrenia patients than NCPs. MMN amplitude declined with age in both groups, though slightly less steeply in schizophrenia patients than NCPs. P3a amplitude declined with age in NCPs but not in schizophrenia patients.
Conclusions
In our cohort of schizophrenia patients, MMN and P3a deficits were already present at the youngest ages. MMN declined further with age, whereas P3a amplitude remained stable.
Significance
This knowledge about how MMN and P3a amplitudes vary with age in schizophrenia patients compared to NCPs can help improve the utility of these indices as clinical endophenotypes or biomarkers.
Keywords: Event-related potentials, electroencephalography, mismatch negativity, preattentional processes, neurophysiology, aging
Introduction
Patients with schizophrenia exhibit neurocognitive deficits across multiple domains, from the earliest levels of sensory information processing, through progressively higher-order faculties such as attention, memory, language, and social functioning. Scalp-recorded electroencephalographic event-related brain potentials (ERPs) allow investigators to probe these cognitive operations with millisecond precision. For example, the auditory mismatch negativity (MMN) ERP waveform indexes an automatic, preattentive form of sensory information processing. MMN is a negative deflection in the ERP occurring when a sequence of frequent, repetitive “standard” stimuli is interrupted by infrequent or “deviant” stimuli, which differ from standards in some characteristic such as duration or frequency. MMN begins as early as 50 ms after the onset of deviant stimuli, and peaks after an additional 100–150 ms. MMN requires no overt behavioral response, and can be elicited even in the absence of directed attention (Naatanen, 1992, Rinne et al., 2001, Sussman et al., 2003). As such, it can be obtained even in fetuses (Draganova et al., 2005), sleeping infants and adults (Alho et al., 1990, Huotilainen et al., 2003, Nashida et al., 2000), and comatose individuals (Kane et al., 1996, Morlet et al., 2000). Thus, it is presumed to reflect an automatic comparison process between the deviant stimulus and a sensory-memory trace (Naatanen et al., 1989).
At frontocentral electrodes, the MMN wave is often followed by a positive-going ERP deflection peaking at 250–300 msec after stimulus onset (Alho et al., 1997, Paavilainen et al., 1989). This “P3a” component is thought to reflect an automatic re-orienting or shifting of attention (Friedman et al., 2001a). The P3a was originally described in response to occasional novel “distractor” stimuli occurring when the individual is actively trying to detect infrequent “target” stimuli (e.g., of a different pitch) embedded in a stream of frequent standard stimuli (Courchesne et al., 1975, Squires et al., 1975). In this situation, the target stimuli elicit a “P3b” (or P300) waveform, thought to reflect comparison with previous stimuli in working memory, and distinguishable from the P3a by longer latency and more posterior topography (see Polich, 2007, for review); both P3b and P3a amplitudes have been found in many studies to be smaller than normal in schizophrenia (Ford, 1999). The corresponding cognitive processes associated with the P3a waveform are not as well understood, however, in the context of a passive MMN paradigm where attention is directed away to some other task such as watching a silent video.
Since MMN occurs even in the absence of conscious attention, investigators can use it to examine the earliest stages of information processing in schizophrenia patients, without interference from attentional difficulties that can confound assessment of higher cognitive operations in this clinical population (Braff and Light, 2004). Indeed, MMN amplitude has consistently been found to be smaller than normal in schizophrenia patients (Michie, 2001, Turetsky et al., 2007, Umbricht and Krljes, 2005). Reduced MMN in schizophrenia fulfills many criteria of an endophenotypic marker (Gottesman and Gould, 2003) of the disease (Molholm et al., 2005). Namely, it is: (a) associated with schizophrenia, with robust effect sizes (Light and Braff, 2005a); (b) specific relative to other psychiatric disorders (Umbricht et al., 2003); (c) heritable (Hall et al., 2006, Price et al., 2005); (d) independent of clinical state and symptoms (Shinozaki et al., 2002); and (e) present in individuals at genetic risk for developing schizophrenia (Baker et al., 2005, Jessen et al., 2001, Michie et al., 2002, Schreiber et al., 1992). Furthermore, MMN amplitude may potentially be a useful biomarker (Green et al., 2004) of treatment response in clinical trials in schizophrenia (Javitt et al., 2008, Salisbury et al., 2007, Umbricht et al., 2003); because it is: (a) extremely reliable in both normal individuals and schizophrenia patients tested over a 1-year interval (Light and Braff, 2005a); (b) insensitive to order or practice effects (Kathmann et al., 1999, Pekkonen et al., 1995); (c) robustly related to level of everyday functioning in schizophrenia patients (Light and Braff, 2005a, b); (d) responsive to pharmacologic models of schizophrenia (e.g., ketamine challenge in healthy volunteers (Umbricht et al., 2002, Umbricht et al., 2000); nicotine- (Baldeweg et al., 2006) and glutathione-induced improvement (Lavoie et al., 2008) in schizophrenia patients); and (e) easily assessed in patients with a broad range of function, due to tolerability and low task demands of the testing procedure.
Compared to the MMN, the P3a has not been studied as extensively in schizophrenia patients. A few studies, however, have found its amplitude to be subnormal in schizophrenia patients, both in response to occasional novel distractors during a target-detection task (Grillon et al., 1990, Turetsky et al., 1998), and following the MMN in the context of a passive auditory paradigm (Mathalon et al., 2000). Thus, it may potentially also be useful as a biomarker in clinical research studies in schizophrenia (Javitt et al., 2008).
The clinical utility of many potential neurophysiological endophenotypes or biomarkers, including MMN and P3a, would be increased with characterization of their normative psychometric properties (see Cho et al., 2005, for discussion), as is the case with traditional neuropsychological tests. For example, knowledge of how a biomarker or endophenotype typically varies with age both in normal individuals and in schizophrenia patients is essential to characterizing its degree of abnormality in a particular clinical study.
Numerous cross-sectional studies of age effects on MMN in healthy individuals (Cooper et al., 2006, Czigler et al., 1992, Kisley et al., 2005, Pekkonen et al., 1996, Woods, 1992) indicate an age-related diminution of MMN, although there is evidence that the degree of this decline depends on stimulus parameters (Pekkonen, 2000, Todd et al., 2008). In contrast, the natural history of MMN amplitude across schizophrenia patients’ lifespan and stage of illness is not well understood. Is MMN amplitude in schizophrenia patients already reduced at the onset of symptomatic illness; and how does its subsequent rate of decline compare to that observed in normal individuals?
Javitt et al. (2000) found that chronic schizophrenia patients had significantly smaller MMN to duration and frequency deviants than did age-matched healthy controls, whereas recent-onset patients exhibited only a trend toward smaller MMN than the same control group. However, the recent-onset group was younger than the controls; had they been compared with age-matched controls (who likely would have larger MMN amplitudes than the study controls), a significant difference might have been found. Umbricht et al. (2006) found that both recent-onset and chronic schizophrenia patients, but not first-episode patients, had smaller duration and frequency MMN than did controls. In this study, recent-onset patients and controls were matched for age, but first-episode patients were younger, and chronic patients were older. Again, this age difference would likely underestimate MMN reduction in the first-episode group. Nevertheless, in a study in which all groups were age-matched, only chronic schizophrenia patients, but not first-episode patients, had smaller MMN to frequency deviants than did controls (Salisbury et al., 2002). In another study (Brockhaus-Dumke et al., 2005), unmedicated schizophrenia patients, but not prodromal patients, had smaller duration MMN than age-matched controls (although frequency MMN did not differ among the groups). A limitation of these studies was their cross-sectional nature; in contrast, Salisbury et al. (2007) longitudinally examined frequency MMN in schizophrenia patients, at first hospitalization and one and a half years later. Compared to age-matched controls, patients had normal MMN amplitudes at the first assessment, but significant reductions at the second assessment. Moreover, MMN amplitude decreases in patients correlated with reductions in left Heschl’s gyrus gray matter volume. These results provided more direct evidence that frequency MMN deficits increase with disease progression.
Overall, the results of these studies suggest that MMN amplitudes are either normal or minimally reduced in patients early in their illness, but become more abnormal as the disease progresses (Umbricht et al., 2000) – implying that, at least at some point, MMN decreases more rapidly with age in schizophrenia patients than in healthy individuals. (The results of studies which did not detect MMN abnormalities in patients in the earliest stages of schizophrenia (Salisbury et al., 2007, Salisbury et al., 2002) seemingly contradict evidence that healthy individuals at genetic risk for schizophrenia have smaller than average MMN (Baker et al., 2005, Jessen et al., 2001, Michie et al., 2002, Schreiber et al., 1992). However, such individuals have not been directly compared with first-episode schizophrenia patients; thus, the apparent discrepancy could stem from differences in the experimental paradigms employed.)
Characterization of the natural history of MMN deficits in schizophrenia may be complicated by variation in age-related MMN decline in healthy individuals and schizophrenia patients according to the acoustic parameter (e.g., duration, frequency, intensity) differentiating standard and deviant stimuli. Todd et al. (2008) found that early-stage schizophrenia patients (<5 years post-onset) had smaller duration and intensity MMN than did age-matched controls, but the two groups did not differ significantly in frequency MMN amplitudes. In contrast, in later-stage patients (>5 years post-onset), duration and frequency MMN were smaller than in controls, but intensity MMN did not differ between the groups. These results reflected the fact that duration MMN declined at a similar rate with age in both groups, whereas frequency MMN declined more quickly in patients than in controls, and intensity MMN declined more quickly in controls than in patients. The results also suggested that duration and intensity MMN may be more sensitive than frequency MMN for distinguishing schizophrenia patients from controls early in the course of illness.
Compared to age effects on MMN, the effect of age on P3a amplitude has not been as widely studied, in either normal individuals or patients with schizophrenia. In normal individuals, a few studies have found age-related reductions in P3a amplitude, both following distractors in actively-attended target-detection paradigms (Gaal et al., 2007, Knight, 1987), and following unattended deviants in an MMN paradigm (Czigler et al., 1992). Meanwhile, the course of P3a amplitude deficits over the lifespan of persons with schizophrenia is unclear.
The present study aimed to contribute to the characterization of age effects on MMN and P3a in schizophrenia by: comparing age effects on duration MMN and its accompanying P3a in schizophrenia patients and normal comparison participants (NCPs), in a cross-sectional sample that covered a wide age range and was substantially larger than those of previous studies.
Methods
Participants
253 schizophrenia patients and 147 NCPs were tested. All participants were assessed for capacity to provide informed consent and, after receiving a detailed description of study procedures, gave written informed consent per University of California, San Diego (UCSD) IRB-approved forms (#030510, 040564, and 071831). All participants received a urine toxicology screen to rule out recent drug use. Participants were assessed diagnostically using the Structured Clinical Interview for DSM-IV (First et al., 1995). Patients did not have an Axis I diagnosis other than schizophrenia, and NCPs did not have any Axis I diagnosis; participants in both groups had not experienced a neurologic insult such as significant head trauma and/or loss of consciousness. Audiometric testing was used to ensure that all participants could detect 40-dB tones at 1000 Hz.
NCPs were recruited through newspaper advertisements, postings on the Internet, and fliers posted at UCSD Medical Center. Schizophrenia patients were recruited from community residential facilities and via physician referral. Table 1 shows demographic characteristics of the sample and clinical characteristics of the patient group.
Table 1.
Demographic and clinical characteristics of the study sample (means ± SD given where applicable)
| Schizophrenia Patients (n=253) | Normal Comparison Participants (n=147) | |
|---|---|---|
| Age, yearsa | 44.3±9.7 (range 19–65) | 39.9±11.9 (range 18–64) |
| Sexb | 73% male, 27% female | 51% male, 49 female |
| Years of educationc | 12.1±2.1 | 15.0±2.2 |
| Onset of illness, age in years | 22.3±7.7 | - |
| Duration of illness, years | 21.8±10.5 | - |
| Number of previous | 9.3±14.4 | - |
| hospitalizations | ||
| SANS Total | 13.3±4.9 | - |
| SAPS Total | 8.4±4.1 | - |
Patients differed significantly from NCPs, p=.001
Patients differed significantly from NCPs, p=.04
Patients differed significantly from NCPs, p<.0001
Stimuli
Participants were presented with binaural tones (1-kHz 85-dB, with 1-millisecond increase/decrease) with stimulus onset-to-onset asynchrony of 500 milliseconds. Standard (P=.90, 50-ms duration) and deviant (P=.10, 100-ms duration) tones were presented in pseudorandom order through foam insert earphones (Model 3A, Aearo Auditory Systems). During the approximately 20-minute session, participants watched a silent, benign, cartoon video to divert attention from the tones.
Electroencephalographic (EEG) Recording
EEG recordings were acquired with a NuAmps system (Neuroscan Labs). The EEG was recorded from the scalp, through sintered Ag/AgCl electrodes embedded in an electrode cap (EasyCap, Falk Minow Services) at the following 34 positions: Fp1-Fp2-Fz-F3-F4-F7-F8-FC1-FC2-FC5-FC6-Cz-C3-C4-CP1-CP2-CP5-CP6-Pz-P3-P4-P7-P8-O1-O2-PO9-PO10-Iz-T1-T2-T7-T8-TP9-TP10. Electrodes placed at the nose tip and at Fpz served as reference and ground, respectively. Four additional electrodes placed above and below the left eye and at the outer canthi of both eyes were used for monitoring blinks and eye movements. All impedances were below 4 kΩ. Signals were digitized at 1 kHz with system acquisition filter settings at 0.5–100 Hz.
The EEG and stimulus markers were recorded continuously. During testing, online ERP averages to standard and deviant tones were monitored to ensure signal quality and to track the number of trials free of gross artifact (±100 μV from −100 to 500 msec relative to stimulus onset). EEG acquisition was terminated when a minimum of 225 artifact-free deviant trials were collected. No participant required presentation of more than 300 deviant tones in order to achieve 225 artifact-free trials. Data processing was performed offline and blind to group membership using automated procedures. First, the continuous EEG recordings were mathematically corrected for eye movement artifact using established methods (Semlitsch et al., 1986). Continuous data were epoched relative to the onset of stimuli (−100–500 msec), with mean voltage of the prestimulus interval used as baseline. Following blink correction, epochs containing > ±50 μV in frontal recording sites (F7/F8/Fp1/Fp2/F3/F4/Fz) were rejected. ERP waveforms were generated by averaging responses to standard and deviant tones, respectively. MMN/P3a waveforms were generated by subtracting ERPs elicited by standard tones from ERPs elicited by deviant tones (Figure 1). The resultant difference waves were low-pass filtered at 20 Hz (zero-phase shift, 24 dB/octave rolloff) to remove any residual high-frequency artifact. MMN and P3a amplitudes were measured as the mean voltage from 135 to 205 msec, and 250 to 300 msec, respectively, consistent with established methods (Kiang et al., 2007, Light and Braff, 2005a, b, Light et al., 2007, Michie et al., 2002).
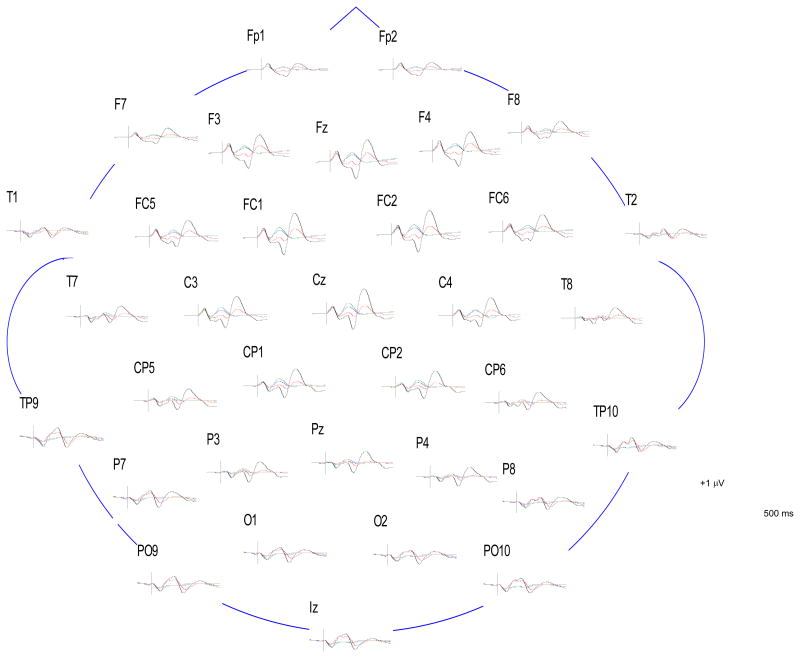
Figure 1.
Grand average ERPs of schizophrenia patients (standards in blue; deviants in red) and NCPs (standards in green; deviants in black), at all electrode sites. ERPs are time-locked to tone onset.
Statistical Analyses
To determine whether ERP amplitudes differed between the two groups in specific age ranges, separate analyses of variance (ANOVA) were conducted on MMN and P3a amplitudes, with Group (schizophrenia vs. NCP) and Age (≤30 years vs. 31–40 vs. 41–50 vs. >50) as between-subjects factors, and Electrode site (Fz vs. Cz vs. Pz) as within-subject factor. Within each of the four age categories, mean age did not differ between schizophrenia patients and NCPs (Kruskal-Wallis test for non-normally distributed samples, p>0.05). P-values for the within-subject factor are reported after Greenhouse-Geisser Epsilon correction. For significant effects, Tukey simultaneous pairwise comparisons of factor-level means were performed, with a family confidence co-efficient of 0.95.
To examine relationships of MMN and P3a amplitudes to age, for schizophrenia patients and NCPs, linear regression analyses were conducted separately for each group, with MMN and P3a amplitudes at Fz as dependent variables, and age as independent variable. To test the hypotheses that the slopes of each regression line differed between patients and NCPs, the method described by Zar (Zar, 1999) was used.
For both patients and NCPs, to determine whether a higher-order polynomial function was a better fit than a linear regression for each of the relationships of MMN and P3a amplitude to age, we applied a quadratic regression and tested whether this significantly improved r2 compared to the linear regression (Zar, 1999). If so, then the next higher-order (cubic) regression would be fit, the improvement in r2 would again be tested for significance, and so on, until no significant additional improvement was found.
To explore whether educational attainment was associated with MMN and P3a amplitudes in the schizophrenia and NCP groups, linear regression analyses were conducted separately for each group, with MMN and P3a amplitudes at Fz as dependent variables, and both age and years of education as independent variables.
Results
Grand average ERPs
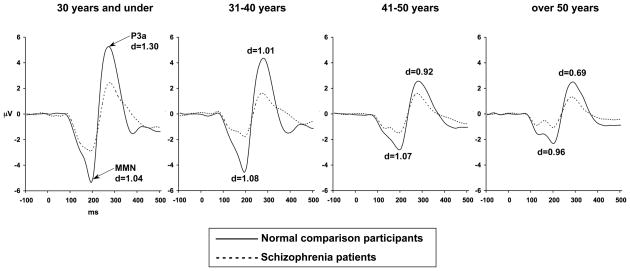
Figure 2 shows grand average difference ERPs, formed by subtracting the ERP elicited by standard tones from the ERP elicited by deviant tones, for schizophrenia patients and NCPs, at all electrodes. Figure 3 shows the difference ERPs for schizophrenia patients and NCPs in each age category, at Fz. The MMN is seen in the difference ERP as a negative deflection from approximately 100 to 200 ms after stimulus onset. The P3a is seen as a positive deflection from approximately 250 to 350 ms after stimulus onset.
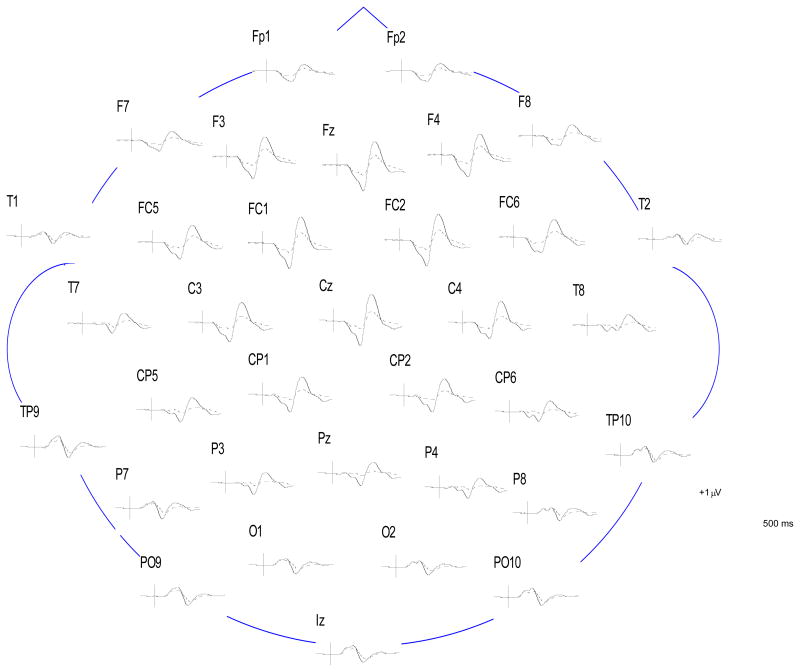
Figure 2.
Grand average difference waves formed by subtracting average ERPs to standard tones from average ERPs to deviant tones, for schizophrenia patients (dashed line) and NCPs (solid line), at all electrode sites.
Figure 3.
Grand average difference waves formed by subtracting average ERPs to standard tones from average ERPs to deviant tones, for schizophrenia patients and NCPs in each age category, at electrode site Fz.
Relationship between MMN and age in participant groups
ANOVA of MMN amplitude showed a Group effect [F(1,392)=134.8, p<0.001], with schizophrenia patients exhibiting smaller (less negative) amplitudes than NCPs (Figure 4). There was also an Age effect [F(3,392)=27.9, p<0.001]; overall, amplitudes were largest for the ≥30 category, and progressively smaller for the 31–40, 41–50 and >50 categories. There was an Electrode effect [F(2,784)=601.4, ε=0.64, p<0.001], with MMN being largest (most negative) at Fz, intermediate at Cz, and smallest at Pz. There was an Age × Electrode interaction [F(6,784)=14.2, ε=0.64, p<0.001], indicating that MMN amplitude declined more with age at Fz (where it was larger to begin with) than at Pz. This was true of both schizophrenia patients and NCPs, as reflected in the absence of a Group × Age × Electrode interaction [F(6,784)=0.87, ε=0.64, p=0.48].
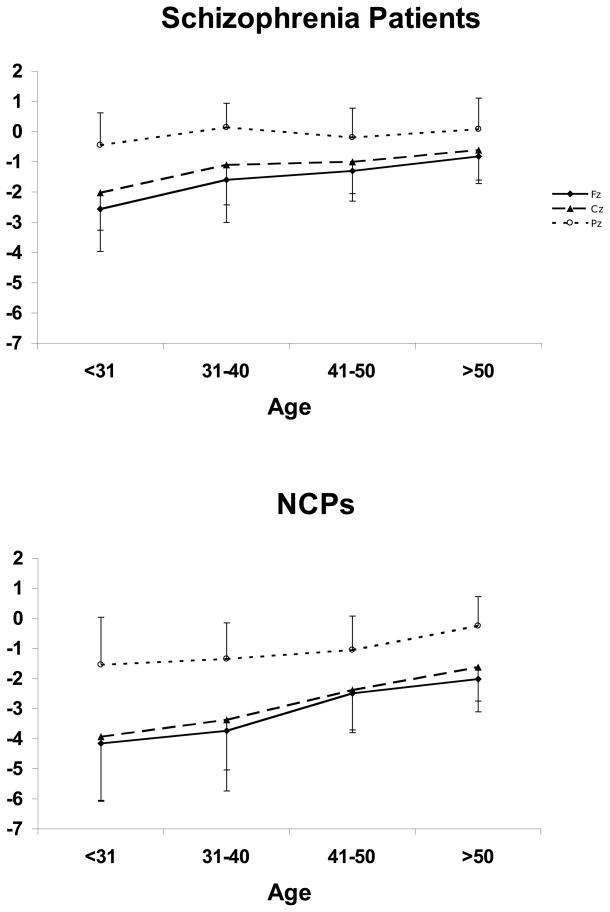
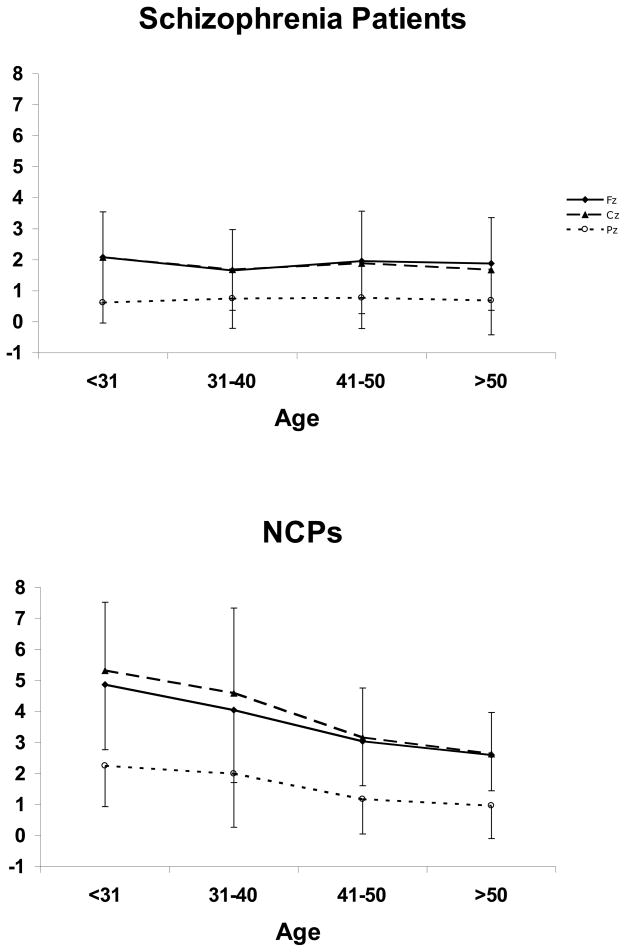
Figure 4.
Mean MMN amplitudes by age and electrode site, for (a) schizophrenia patients and (b) NCPs.
Mean MMN amplitudes for each combination of diagnostic group and age category are shown in Table 2 for Fz. In every age category, MMN was significantly smaller in patients than in NCPs, and the effect size of the difference was large (Cohen, 1988).
Table 2.
Mean MMN amplitudes (with SD) at Fz in V for participant groups, and Cohen’s effect size d of the difference in MMN amplitude between groups, by age category.
| Schizophrenia Patients | Normal Comparison Participants | Effect Size d | |||
|---|---|---|---|---|---|
| n | MMN amplitude (μV) | n | MMN amplitude (μV) | ||
| ≤30 years old | 27 | −2.48 (1.26) | 36 | −4.21 (2.00) | 1.04 |
| 31–40 years old | 48 | −1.78 (1.36) | 40 | −3.61 (2.00) | 1.08 |
| 41–50 years old | 111 | −1.25 (0.96) | 41 | −2.51 (1.35) | 1.07 |
| >50 years old | 67 | −0.91 (0.98) | 30 | −1.88 (1.04) | 0.96 |
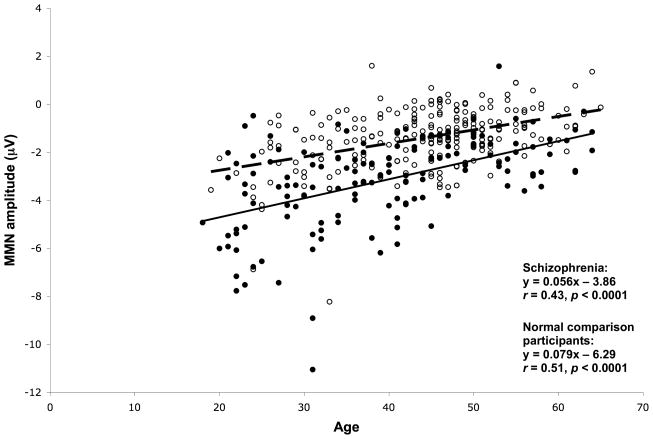
Figure 5 shows scatterplots of MMN amplitude versus age, showing regression line and correlation co-efficients, for schizophrenia and NCP groups. Older age was significantly correlated with reduced (less negative) MMN amplitudes in both groups, but the slope of this decline was smaller in schizophrenia patients than in NCPs. The slopes differed significantly between the schizophrenia and NCP groups (t=20.9, df=396, p<0.001).
Figure 5.
Scatterplot of MMN amplitude at Fz versus age, for schizophrenia patients (open circles; dashed regression line) and NCPs (solid circles; solid regression line).
Fitting a quadratic regression function to the relationship of MMN amplitude versus age did not result in any significant improvement in r2, compared to the linear regression, for either patients [r2=0.158 vs. 0.155, F(1,221)=0.98, p=0.32] or NCPs [r2=0.262 vs. 0.256, F(1,149)=1.15, p=0.29].
Relationship between MMN amplitude and education in participant groups
For both the schizophrenia and NCP groups, when linear regression was performed with MMN amplitude at Fz as dependent variable, and age and years of education as independent variables, years of education was not a significant predictor of MMN amplitude.
Relationship between P3a and age in participant groups
ANOVA of P3a amplitude showed a Group effect [F(1,372)=88.5, p<0.001], with schizophrenia patients exhibiting smaller (less positive) amplitudes than NCPs (Figure 6). There was also an Age effect [F(3,372)=7.76, p<0.001]; overall, amplitudes were largest for the ≤30 category, intermediate for the 31–40 category, and smallest for the 41–50 and >50 categories which did not significantly differ from one another. There was a Group × Age interaction [F(3,372)=7.29, p<0.001] – reflecting the fact that, in NCPs, there was a significant decrement in P3a amplitudes between the 31–40 and 41–50 categories, whereas in patients, P3a amplitudes were not significantly different between any of the age categories. There was an Electrode effect [F(2,744)=330.3, ε=0.79, p<0.001], with P3a being larger at Fz and Cz than at Pz. There was an Age × Electrode interaction [F(6,744)=3.27, ε=0.79, p<0.001], indicating that P3a amplitude declined more with age at Fz and Cz (where it was larger to begin with) than at Pz. This was true of both schizophrenia patients and NCPs, as reflected in the absence of a Group × Age × Electrode interaction [F(6,744)=1.55, ε=0.64, p=0.16].
Figure 6.
Mean P3a amplitudes by age and electrode site, for (a) schizophrenia patients and (b) NCPs.
Mean P3a amplitudes for each combination of diagnostic group and age category are shown in Table 3 for Fz. In every age category, P3a was significantly smaller in patients than in NCPs, and the effect size of the difference was large (Cohen, 1988).
Table 3.
Mean P3a amplitudes (with SD) at Fz in V for participant groups, and Cohen’s effect size d of the difference in P3a amplitude between groups, by age category.
| Schizophrenia Patients | Normal Comparison Participants | Effect Size d | |||
|---|---|---|---|---|---|
| n | P3a amplitude (μV) | n | P3a amplitude (μV) | ||
| ≤30 years old | 23 | 2.33 (1.62) | 32 | 4.79 (2.13) | 1.30 |
| 31–40 years old | 47 | 2.19 (1.45) | 38 | 4.21 (2.43) | 1.01 |
| 41–50 years old | 108 | 1.78 (1.25) | 40 | 3.00 (1.38) | 0.92 |
| >50 years old | 62 | 1.67 (1.24) | 30 | 2.52 (1.24) | 0.69 |
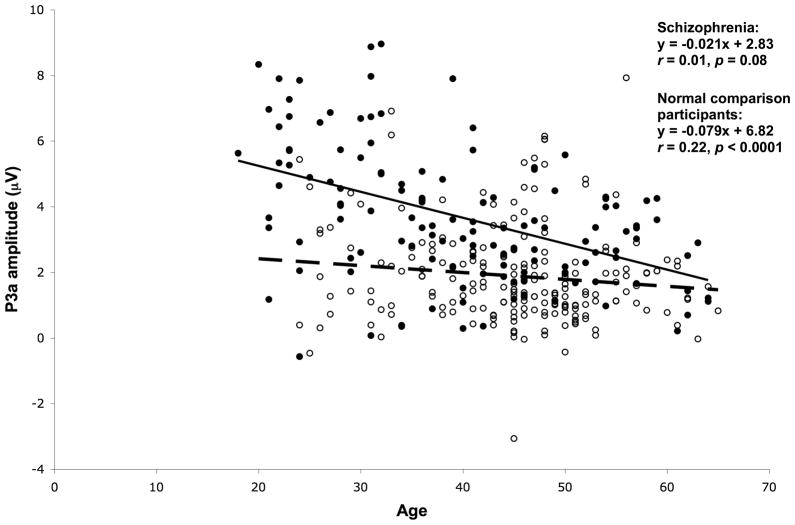
Figure 7 shows scatterplots of P3a amplitude versus age, showing regression line and correlation co-efficients, for schizophrenia and NCP groups. Older age was significantly correlated with reduced P3a amplitudes in NCPs, but P3a amplitude and age were not significantly correlated in schizophrenia patients.
Figure 7.
Scatterplot of P3a amplitude at Fz versus age, for schizophrenia patients (open circles; dashed regression line) and NCPs (solid circles; solid regression line).
Fitting a quadratic regression function to the relationship of P3a amplitude versus age did not result in significant improvement in r2 compared to the linear regression, for either patients [r2=0.015 vs. 0.015, F(1,200)=0.05, p=0.83] or NCPs [r2=0.231 vs. 0.223, F(1,127)=1.42, p=0.23].
Relationship between P3a amplitude and education in participant groups
For both the schizophrenia and NCP groups, when linear regression was performed with P3a amplitude at Fz as dependent variable, and age and years of education as independent variables, years of education was not a significant predictor of P3a amplitude.
Discussion
In this study, we assessed the relationship of auditory MMN and P3a amplitudes to age in schizophrenia patients and NCPs. We measured MMN and P3a to duration deviants, in a large cross-sectional sample of 253 patients and 147 NCPs who ranged widely in age (18–65 years). Over the entire age range, as expected, MMN amplitude was significantly smaller in schizophrenia patients than in NCPs, with large between-group effect sizes (d 0.95) at all ages. Moreover, MMN amplitude declined as a function of age in both schizophrenia patients and NCPs, though slightly less steeply in patients. For both patients and NCPs, a quadratic regression function did not fit this relationship significantly better than did a linear function. In contrast to MMN amplitude, although P3a amplitude was also smaller in schizophrenia patients than in NCPs across all ages examined, it declined as a function of age in NCPs but not in schizophrenia patients.
Although Todd et al. (2008) did not quantify the slope of the MMN-age relationship in their study, they noted that visual inspection of MMN-age scatterplots revealed that duration MMN declined more slowly with age in schizophrenia patients than in controls – consistent with what we found across our entire sample. This slightly slower decline in MMN amplitude in schizophrenia patients as compared to NCPs could be due to a floor effect. It is also consistent with a situation in which schizophrenia patients experience an early disease-related deterioration of MMN, in the initial stages of symptomatic illness or prior to its onset; and subsequently exhibit a decline in MMN only as a result of normal aging. Such a pattern would parallel the trajectory that some researchers have hypothesized for neuropsychological deficits in schizophrenia (Heaton et al., 2001, Hoff et al., 2005).
Like MMN, P3a was smaller than normal in schizophrenia patients over the entire age span we examined, indicating that it too is already impaired relatively early in the course of symptomatic disease. In contrast to MMN, however, and unlike in NCPs, P3a amplitude did not decline significantly with age in the patients. This finding is consistent with other evidence that MMN and P3a reflect separate cognitive processes (Horvath et al., 2008).
Importantly, our sample included outpatients with an established diagnosis of schizophrenia, and not first-episode or prodromal patients. Thus, our data do not allow us to address the question of whether MMN and/or P3a amplitudes are already reduced at illness onset or earlier. We also did not include individuals over 65 years old in our study, and thus could not examine for evidence of an accelerated decline in MMN and/or P3a amplitude in late life in schizophrenia, analogous to findings for some other cognitive indices (Friedman et al., 2001b). In addition, because of the cross-sectional nature of our study, extrapolation of the results could yield an inaccurate picture of the course of MMN and P3a amplitude in individual patients, if patients with different levels of impairment were not equally represented at all age levels (Umbricht and Krljes, 2005). Additional cross-sectional and longitudinal studies across the lifespan in schizophrenia patients and at-risk individuals, using a range of stimulus characteristics, are necessary to further characterize the course of MMN and P3a impairment in schizophrenia.
Acknowledgments
Funding: This work was supported by the Bowman Family Foundation research partnership with the National Alliance for Research on Schizophrenia and Depression; and by grants from the Department of Veteran Affairs (VISN 22 Mental Illness Research, Education, and Clinical Center); and the National Institute of Mental Health (MH79777, MH18399, MH042228 and MH065571).
The authors thank Dr. Neal Swerdlow, Dr. Kristin Cadenhead, Sheldrick Holmes, Dr. Barbara Haugeland, Kari Tweedale, Richard Sharp, and Emmeline Crowley for their assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alho K, Escera C, Diaz R, Yago E, Serra JM. Effects of involuntary auditory attention on visual task performance and brain activity. Neuroreport. 1997;8(15):3233–7. doi: 10.1097/00001756-199710200-00010. [DOI] [PubMed] [Google Scholar]
- Alho K, Sainio K, Sajaniemi N, Reinikainen K, Naatanen R. Event-related brain potential of human newborns to pitch change of an acoustic stimulus. Electroencephalogr Clin Neurophysiol. 1990;77(2):151–5. doi: 10.1016/0168-5597(90)90031-8. [DOI] [PubMed] [Google Scholar]
- Baker K, Baldeweg T, Sivagnanasundaram S, Scambler P, Skuse D. COMT Val108/158 Met modifies mismatch negativity and cognitive function in 22q11 deletion syndrome. Biol Psychiatry. 2005;58(1):23–31. doi: 10.1016/j.biopsych.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Baldeweg T, Wong D, Stephan KE. Nicotinic modulation of human auditory sensory memory: Evidence from mismatch negativity potentials. Int J Psychophysiol. 2006;59(1):49–58. doi: 10.1016/j.ijpsycho.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Braff DL, Light GA. Preattentional and attentional cognitive deficits as targets for treating schizophrenia. Psychopharmacology (Berl) 2004;174(1):75–85. doi: 10.1007/s00213-004-1848-0. [DOI] [PubMed] [Google Scholar]
- Brockhaus-Dumke A, Tendolkar I, Pukrop R, Schultze-Lutter F, Klosterkotter J, Ruhrmann S. Impaired mismatch negativity generation in prodromal subjects and patients with schizophrenia. Schizophr Res. 2005;73(2–3):297–310. doi: 10.1016/j.schres.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Cho RY, Ford JM, Krystal JH, Laruelle M, Cuthbert B, Carter CS. Functional neuroimaging and electrophysiology biomarkers for clinical trials for cognition in schizophrenia. Schizophr Bull. 2005;31(4):865–9. doi: 10.1093/schbul/sbi050. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: L. Erlbaum Associates; 1988. [Google Scholar]
- Cooper RJ, Todd J, McGill K, Michie PT. Auditory sensory memory and the aging brain: A mismatch negativity study. Neurobiol Aging. 2006;27(5):752–62. doi: 10.1016/j.neurobiolaging.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Hillyard SA, Galambos R. Stimulus novelty, task relevance and the visual evoked potential in man. Electroencephalogr Clin Neurophysiol. 1975;39(2):131–43. doi: 10.1016/0013-4694(75)90003-6. [DOI] [PubMed] [Google Scholar]
- Czigler I, Csibra G, Csontos A. Age and inter-stimulus interval effects on event-related potentials to frequent and infrequent auditory stimuli. Biol Psychol. 1992;33(2–3):195–206. doi: 10.1016/0301-0511(92)90031-o. [DOI] [PubMed] [Google Scholar]
- Draganova R, Eswaran H, Murphy P, Huotilainen M, Lowery C, Preissl H. Sound frequency change detection in fetuses and newborns, a magnetoencephalographic study. Neuroimage. 2005;28(2):354–61. doi: 10.1016/j.neuroimage.2005.06.011. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders -- Patient Edition. New York: New York State Psychiatric Institute; 1995. [Google Scholar]
- Ford JM. Schizophrenia: the broken P300 and beyond. Psychophysiology. 1999;36(6):667–82. [PubMed] [Google Scholar]
- Friedman D, Cycowicz YM, Gaeta H. The novelty P3: an event-related brain potential (ERP) sign of the brain’s evaluation of novelty. Neurosci Biobehav Rev. 2001a;25(4):355–73. doi: 10.1016/s0149-7634(01)00019-7. [DOI] [PubMed] [Google Scholar]
- Friedman JI, Harvey PD, Coleman T, Moriarty PJ, Bowie C, Parrella M, et al. Six-year follow-up study of cognitive and functional status across the lifespan in schizophrenia: a comparison with Alzheimer’s disease and normal aging. Am J Psychiatry. 2001b;158(9):1441–8. doi: 10.1176/appi.ajp.158.9.1441. [DOI] [PubMed] [Google Scholar]
- Gaal ZA, Csuhaj R, Molnar M. Age-dependent changes of auditory evoked potentials--effect of task difficulty. Biol Psychol. 2007;76(3):196–208. doi: 10.1016/j.biopsycho.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Gottesman, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160(4):636–45. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Gold JM, Barch DM, Cohen J, Essock S, et al. Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biol Psychiatry. 2004;56(5):301–7. doi: 10.1016/j.biopsych.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Grillon C, Courchesne E, Ameli R, Geyer MA, Braff DL. Increased distractibility in schizophrenic patients. Electrophysiologic and behavioral evidence. Arch Gen Psychiatry. 1990;47(2):171–9. doi: 10.1001/archpsyc.1990.01810140071010. [DOI] [PubMed] [Google Scholar]
- Hall MH, Schulze K, Rijsdijk F, Picchioni M, Ettinger U, Bramon E, et al. Heritability and Reliability of P300, P50 and Duration Mismatch Negativity. Behav Genet. 2006 doi: 10.1007/s10519-006-9091-6. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Gladsjo JA, Palmer BW, Kuck J, Marcotte TD, Jeste DV. Stability and course of neuropsychological deficits in schizophrenia. Arch Gen Psychiatry. 2001;58(1):24–32. doi: 10.1001/archpsyc.58.1.24. [DOI] [PubMed] [Google Scholar]
- Hoff AL, Svetina C, Shields G, Stewart J, DeLisi LE. Ten year longitudinal study of neuropsychological functioning subsequent to a first episode of schizophrenia. Schizophr Res. 2005;78(1):27–34. doi: 10.1016/j.schres.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Horvath J, Winkler I, Bendixen A. Do N1/MMN, P3a, and RON form a strongly coupled chain reflecting the three stages of auditory distraction? Biol Psychol. 2008;79(2):139–47. doi: 10.1016/j.biopsycho.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Huotilainen M, Kujala A, Hotakainen M, Shestakova A, Kushnerenko E, Parkkonen L, et al. Auditory magnetic responses of healthy newborns. Neuroreport. 2003;14(14):1871–5. doi: 10.1097/00001756-200310060-00023. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Shelley AM, Silipo G, Lieberman JA. Deficits in auditory and visual context-dependent processing in schizophrenia: defining the pattern. Arch Gen Psychiatry. 2000;57(12):1131–7. doi: 10.1001/archpsyc.57.12.1131. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Spencer KM, Thaker GK, Winterer G, Hajos M. Neurophysiological biomarkers for drug development in schizophrenia. Nat Rev Drug Discov. 2008;7(1):68–83. doi: 10.1038/nrd2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen F, Fries T, Kucharski C, Nishimura T, Hoenig K, Maier W, et al. Amplitude reduction of the mismatch negativity in first-degree relatives of patients with schizophrenia. Neurosci Lett. 2001;309(3):185–8. doi: 10.1016/s0304-3940(01)02072-9. [DOI] [PubMed] [Google Scholar]
- Kane NM, Curry SH, Rowlands CA, Manara AR, Lewis T, Moss T, et al. Event-related potentials--neurophysiological tools for predicting emergence and early outcome from traumatic coma. Intensive Care Med. 1996;22(1):39–46. doi: 10.1007/BF01728329. [DOI] [PubMed] [Google Scholar]
- Kathmann N, Frodl-Bauch T, Hegerl U. Stability of the mismatch negativity under different stimulus and attention conditions. Clin Neurophysiol. 1999;110(2):317–23. doi: 10.1016/s1388-2457(98)00011-x. [DOI] [PubMed] [Google Scholar]
- Kiang M, Light GA, Prugh J, Coulson S, Braff DL, Kutas M. Cognitive, neurophysiological, and functional correlates of proverb interpretation abnormalities in schizophrenia. J Int Neuropsychol Soc. 2007;13(4):653–63. doi: 10.1017/S1355617707070816. [DOI] [PubMed] [Google Scholar]
- Kisley MA, Davalos DB, Engleman LL, Guinther PM, Davis HP. Age-related change in neural processing of time-dependent stimulus features. Brain Res Cogn Brain Res. 2005;25(3):913–25. doi: 10.1016/j.cogbrainres.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Knight RT. Aging decreases auditory event-related potentials to unexpected stimuli in humans. Neurobiol Aging. 1987;8(2):109–13. doi: 10.1016/0197-4580(87)90019-4. [DOI] [PubMed] [Google Scholar]
- Lavoie S, Murray MM, Deppen P, Knyazeva MG, Berk M, Boulat O, et al. Glutathione precursor, N-acetyl-cysteine, improves mismatch negativity in schizophrenia patients. Neuropsychopharmacology. 2008;33(9):2187–99. doi: 10.1038/sj.npp.1301624. [DOI] [PubMed] [Google Scholar]
- Light GA, Braff DL. Mismatch negativity deficits are associated with poor functioning in schizophrenia patients. Arch Gen Psychiatry. 2005a;62(2):127–36. doi: 10.1001/archpsyc.62.2.127. [DOI] [PubMed] [Google Scholar]
- Light GA, Braff DL. Stability of mismatch negativity deficits and their relationship to functional impairments in chronic schizophrenia. Am J Psychiatry. 2005b;162(9):1741–3. doi: 10.1176/appi.ajp.162.9.1741. [DOI] [PubMed] [Google Scholar]
- Light GA, Swerdlow NR, Braff DL. Preattentive sensory processing as indexed by the MMN and P3a brain responses is associated with cognitive and psychosocial functioning in healthy adults. J Cogn Neurosci. 2007;19(10):1624–32. doi: 10.1162/jocn.2007.19.10.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathalon DH, Ford JM, Pfefferbaum A. Trait and state aspects of P300 amplitude reduction in schizophrenia: a retrospective longitudinal study. Biol Psychiatry. 2000;47(5):434–49. doi: 10.1016/s0006-3223(99)00277-2. [DOI] [PubMed] [Google Scholar]
- Michie PT. What has MMN revealed about the auditory system in schizophrenia? Int J Psychophysiol. 2001;42(2):177–94. doi: 10.1016/s0167-8760(01)00166-0. [DOI] [PubMed] [Google Scholar]
- Michie PT, Innes-Brown H, Todd J, Jablensky AV. Duration mismatch negativity in biological relatives of patients with schizophrenia spectrum disorders. Biol Psychiatry. 2002;52(7):749–58. doi: 10.1016/s0006-3223(02)01379-3. [DOI] [PubMed] [Google Scholar]
- Molholm S, Martinez A, Ritter W, Javitt DC, Foxe JJ. The neural circuitry of pre-attentive auditory change-detection: an fMRI study of pitch and duration mismatch negativity generators. Cereb Cortex. 2005;15(5):545–51. doi: 10.1093/cercor/bhh155. [DOI] [PubMed] [Google Scholar]
- Morlet D, Bouchet P, Fischer C. Mismatch negativity and N100 monitoring: potential clinical value and methodological advances. Audiol Neurootol. 2000;5(3–4):198–206. doi: 10.1159/000013881. [DOI] [PubMed] [Google Scholar]
- Naatanen R. Attention and Brain Function. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1992. [Google Scholar]
- Naatanen R, Paavilainen P, Alho K, Reinikainen K, Sams M. Do event-related potentials reveal the mechanism of the auditory sensory memory in the human brain? Neurosci Lett. 1989;98(2):217–21. doi: 10.1016/0304-3940(89)90513-2. [DOI] [PubMed] [Google Scholar]
- Nashida T, Yabe H, Sato Y, Hiruma T, Sutoh T, Shinozaki N, et al. Automatic auditory information processing in sleep. Sleep. 2000;23(6):821–8. [PubMed] [Google Scholar]
- Paavilainen P, Karlsson ML, Reinikainen K, Naatanen R. Mismatch negativity to change in spatial location of an auditory stimulus. Electroencephalogr Clin Neurophysiol. 1989;73(2):129–41. doi: 10.1016/0013-4694(89)90192-2. [DOI] [PubMed] [Google Scholar]
- Pekkonen E. Mismatch negativity in aging and in Alzheimer’s and Parkinson’s diseases. Audiol Neurootol. 2000;5(3–4):216–24. doi: 10.1159/000013883. [DOI] [PubMed] [Google Scholar]
- Pekkonen E, Rinne T, Naatanen R. Variability and replicability of the mismatch negativity. Electroencephalogr Clin Neurophysiol. 1995;96(6):546–54. doi: 10.1016/0013-4694(95)00148-r. [DOI] [PubMed] [Google Scholar]
- Pekkonen E, Rinne T, Reinikainen K, Kujala T, Alho K, Naatanen R. Aging effects on auditory processing: an event-related potential study. Exp Aging Res. 1996;22(2):171–84. doi: 10.1080/03610739608254005. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol. 2007;118(10):2128–48. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price GW, Michie PT, Johnston J, Innes-Brown H, Kent A, Clissa P, et al. A multivariate electrophysiological endophenotype, from a unitary cohort, shows greater research utility than any single feature in the Western Australian Family Study of Schizophrenia. Biol Psychiatry. 2005 doi: 10.1016/j.biopsych.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Rinne T, Antila S, Winkler I. Mismatch negativity is unaffected by top-down predictive information. Neuroreport. 2001;12(10):2209–13. doi: 10.1097/00001756-200107200-00033. [DOI] [PubMed] [Google Scholar]
- Salisbury DF, Kuroki N, Kasai K, Shenton ME, McCarley RW. Progressive and interrelated functional and structural evidence of post-onset brain reduction in schizophrenia. Arch Gen Psychiatry. 2007;64(5):521–9. doi: 10.1001/archpsyc.64.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury DF, Shenton ME, Griggs CB, Bonner-Jackson A, McCarley RW. Mismatch negativity in chronic schizophrenia and first-episode schizophrenia. Arch Gen Psychiatry. 2002;59(8):686–94. doi: 10.1001/archpsyc.59.8.686. [DOI] [PubMed] [Google Scholar]
- Schreiber H, Stolz-Born G, Kornhuber HH, Born J. Event-related potential correlates of impaired selective attention in children at high risk for schizophrenia. Biol Psychiatry. 1992;32(8):634–51. doi: 10.1016/0006-3223(92)90294-a. [DOI] [PubMed] [Google Scholar]
- Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology. 1986;23(6):695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Shinozaki N, Yabe H, Sato Y, Hiruma T, Sutoh T, Nashida T, et al. The difference in Mismatch negativity between the acute and post-acute phase of schizophrenia. Biol Psychol. 2002;59(2):105–19. doi: 10.1016/s0301-0511(01)00129-6. [DOI] [PubMed] [Google Scholar]
- Squires NK, Squires KC, Hillyard SA. Two varieties of long-latency positive waves evoked by unpredictable auditory stimuli in man. Electroencephalogr Clin Neurophysiol. 1975;38(4):387–401. doi: 10.1016/0013-4694(75)90263-1. [DOI] [PubMed] [Google Scholar]
- Sussman E, Winkler I, Wang W. MMN and attention: competition for deviance detection. Psychophysiology. 2003;40(3):430–5. doi: 10.1111/1469-8986.00045. [DOI] [PubMed] [Google Scholar]
- Todd J, Michie PT, Schall U, Karayanidis F, Yabe H, Naatanen R. Deviant matters: duration, frequency, and intensity deviants reveal different patterns of mismatch negativity reduction in early and late schizophrenia. Biol Psychiatry. 2008;63:58–64. doi: 10.1016/j.biopsych.2007.02.016. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Calkins ME, Light GA, Olincy A, Radant AD, Swerdlow NR. Neurophysiological endophenotypes of schizophrenia: the viability of selected candidate measures. Schizophr Bull. 2007;33(1):69–94. doi: 10.1093/schbul/sbl060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turetsky BI, Colbath EA, Gur RE. P300 subcomponent abnormalities in schizophrenia: I. Physiological evidence for gender and subtype specific differences in regional pathology. Biol Psychiatry. 1998;43(2):84–96. doi: 10.1016/S0006-3223(97)00258-8. [DOI] [PubMed] [Google Scholar]
- Umbricht D, Bates JA, Lieberman JA, Kane JM, Javitt DC. Electrophysiological indices of automatic and controlled auditory information processing in first-episode, recent-onset and chronic schizophrenia. Biol Psychiatry. 2006;59(8):762–72. doi: 10.1016/j.biopsych.2005.08.030. [DOI] [PubMed] [Google Scholar]
- Umbricht D, Koller R, Schmid L, Skrabo A, Grubel C, Huber T, et al. How specific are deficits in mismatch negativity generation to schizophrenia? Biol Psychiatry. 2003;53(12):1120–31. doi: 10.1016/s0006-3223(02)01642-6. [DOI] [PubMed] [Google Scholar]
- Umbricht D, Koller R, Vollenweider FX, Schmid L. Mismatch negativity predicts psychotic experiences induced by NMDA receptor antagonist in healthy volunteers. Biol Psychiatry. 2002;51(5):400–6. doi: 10.1016/s0006-3223(01)01242-2. [DOI] [PubMed] [Google Scholar]
- Umbricht D, Krljes S. Mismatch negativity in schizophrenia: a meta-analysis. Schizophr Res. 2005;76(1):1–23. doi: 10.1016/j.schres.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Umbricht D, Schmid L, Koller R, Vollenweider FX, Hell D, Javitt DC. Ketamine-induced deficits in auditory and visual context-dependent processing in healthy volunteers: implications for models of cognitive deficits in schizophrenia. Arch Gen Psychiatry. 2000;57(12):1139–47. doi: 10.1001/archpsyc.57.12.1139. [DOI] [PubMed] [Google Scholar]
- Woods DL. Auditory selective attention in middle-aged and elderly subjects: an event-related brain potential study. Electroencephalogr Clin Neurophysiol. 1992;84(5):456–68. doi: 10.1016/0168-5597(92)90033-8. [DOI] [PubMed] [Google Scholar]
- Zar JH. Biostatistical Analysis. 4. Upper Saddle River, NJ: Prentice Hall; 1999. [Google Scholar]