Abstract
Repeated intermittent exposure to stimulants progressively increases a drug's effect, with stressors capable of producing cross-sensitization to stimulants. Studies examining such sensitization during development are few, however, with results mixed. In Experiment 1, juvenile (P22) and adult (P64) female Sprague-Dawley rats were administered (daily for 4 days) 1.5 mg/kg or 3.0 mg/kg amphetamine (1.5A and 3.0A groups), or saline (SAL group). In a second experiment, rats were exposed to either repeated restraint (60 min/day for 4 days; RS group) or were left non-manipulated in the home cage (NM group). Animals from both experiments were then challenged with 1.5 mg/kg of amphetamine and sensitization assessed via locomotion and stereotypy after a 2-day and 3-wk washout period. When compared to SAL animals, 3.0A juveniles and adults exhibited evidence of locomotor sensitization 2 days post-drug exposure, but this sensitization did not persist to the 3-week challenge. Compared to NM animals, RS animals showed stress-induced locomotor sensitization both 2 days and 3 weeks post-stress exposure, regardless of age. These results demonstrate that repeated drug/stress exposures prior to stimulant challenge are sufficient to induce behavioral sensitization among both juveniles and adults, with these effects particularly long-lasting following repeated stressor exposure.
Keywords: juvenile, adult, rat, restraint, amphetamine, behavioral sensitization, locomotor sensitization, stereotypy
Behavioral sensitization is defined as a progressive increase in the behavioral response to a drug following repeated administration (for review, see Robinson and Becker, 1986; Robinson and Berridge, 1993), and is evident following exposure to stimulants such as amphetamine (Battisti et al., 1999; Robinson et al., 1998; for review see Robinson and Becker, 1986) and cocaine (Erb and Brown, 2006; Frantz et al., 2007; Laviola et al., 1995), as well as other drugs such as ethanol (Cunningham and Noble, 1992; Masur and Boerngen, 1980; Masur et al., 1986) and nicotine (Benwell and Balfour, 1992; Kita et al., 1992). Sensitization has been argued to be an important contributor to the development of drug addiction (Robinson and Berridge, 1993). Locomotor sensitization in particular has been associated with alterations in neural reward substrates and hence thought to serve as a marker for increased rewarding value of drugs (Robinson and Berridge, 2003, 2008).
While numerous studies have reported the development of cocaine and amphetamine sensitization in preweanling, juvenile, adolescent, and adult animals (for review see Tirelli et al., 2003), differences in the expression and longevity of such sensitization have been observed across ontogeny. For instance, repeated exposure to amphetamine or other stimulants during the late preweanling period induced context-dependent sensitization of both locomotor activity and stereotypy at post-drug intervals of approximately 7-9 days following drug termination (see Tirelli et al., 2003 for review and references). In contrast, repeated exposure to stimulants during adolescence has often been found to induce relatively long-lasting sensitization (e.g. see Tirelli et al., 2003). Such sensitization, however, was most often expressed in terms of locomotion, with little evidence of sensitization of stereotypy (e.g., see Adriani et al., 1998). Yet, evidence for the relatively early emergence of sensitization is not ubiquitous, with some researchers reporting little evidence of sensitization following repeated exposure to stimulants during development (e.g. Collins and Izenwasser, 2002; Kolta et al., 1990; Laviola et al., 2001; Niculescu et al., 2005). For example, when P1, P7, P21 and P49 rats were exposed to amphetamine two times daily for 5 days and challenged 15 days later, Kolta and colleagues (1992) observed amphetamine sensitization only in the oldest group.
Methodological differences across labs likely play a role in some of these contrasting developmental findings. One potentially important contributor could be differences across laboratories in basal stressor conditions or the amount of stress associated with the repeated exposure protocol, given evidence that sensitization can be induced not only by prior exposure to drugs, but also by exposure to stress. Animals subjected to a variety of stressors often exhibit an enhanced behavioral response to a later stimulant challenge, at least in adult rodents (for review see Sorg and Kalivas, 1995). For example, both acute and repeated restraint stress (Deroche et al., 1992; Diaz-Otanez et al., 1997; Reid et al., 1998; Robinson et al., 1985), as well as repeated footshock (Herman et al., 1984; MacLennan and Maier, 1983), tail-pinch (Antelman et al., 1980; Piazza et al., 1990) and social defeat (Nikulina et al., 2004) have been found to augment later responsiveness to an acute amphetamine challenge in adult rats.
The role of prior stressors in inducing sensitization to drugs during development has received limited attention, and there is little consensus in the findings to date. For instance, evidence for stress cross-sensitization to the locomotor stimulant effects of cocaine was seen in adolescent male rats exposed to either chronic variable stress or restraint (Lepsch et al., 2005). Likewise, both male and female adolescent rats exposed to chronic social stress (Mathews et al., 2008) showed augmented locomotor sensitization to amphetamine when tested in either late adolescence or adulthood. Conversely, however, another study reported that a social but not a physical stressor was found to inhibit later emergence of amphetamine sensitization in adulthood (Kabbaj et al., 2002). These few studies suggest that, under some circumstances, exposure to stressors during development may potentiate later emergence of stimulant sensitization. These studies, however, have largely focused on stressor exposures at only one age, precluding conclusions regarding potential age differences in stress/drug cross-sensitization.
The purpose of this study, therefore, was to investigate potential age-related differences in both drug- and stress-induced sensitization to an amphetamine challenge, with sensitization examined following repeated exposure to amphetamine or restraint during either the post-weanling/juvenile period or in adulthood. Sensitization of both locomotor behavior and stereotypy were examined, given evidence discussed above suggesting potential developmental differences in behavioral mode of expression of sensitization (Adriani et al., 1998). Assessment of sensitization was conducted 48 hrs after the exposure period, as well as 3 weeks later, in order to explore possible age differences in the time-course of drug- and stress-induced sensitization. Evidence for behavioral sensitization among adults has been seen following a 48-hr drug-free phase (Kolta et al., 1985; Leith and Kuczenski, 1982; McPherson and Lawrence, 2006; Robinson, 1984; Schmidt et al., 1999; Vanderschuren et al., 1999) as well as 3 or more weeks later (Kolta et al., 1985; Leith and Kuczenski, 1982; McPherson and Lawrence, 2006; Robinson, 1984; Schmidt et al., 1999; Vanderschuren et al., 1999)—and even up to a year in some cases in rodents (Paulson et al., 1991). Female rats were used in this study given evidence that both adult and adolescent females may be more likely than males to express behavioral sensitization to drugs or stressors (McCormick et al., 2004; McCormick et al., 2005)
General Methods
Subjects
80 female Sprague-Dawley rats (n = 8 per group) used in these experiments were bred and reared in our colony. Rats were maintained in a temperature-controlled vivarium on a 14:10 hr light:dark cycle (lights on at 0700 hr), with ad libitum food (Purina Rat Chow, Lowell, MA) and water. On the day after birth, P1, litters were culled to 8 - 10 pups, with 6 animals of one sex and 4 animals of the other retained whenever possible. Female offspring were weaned at P21 and housed in same-sex littermate pairs, with male animals used in other studies in our laboratory. At all times, rats used in these experiments were maintained and treated in accordance with the National Institutes of Health Guide for Animal Care (1996), using protocols approved by the Binghamton University Institutional Animal Care and Use Committee (IACUC). In order to minimize litter effects (see Holson and Pearce, 1992), no more than one animal per litter was placed into any given experimental group.
Testing Apparatus
The test context consisted of Plexiglas chambers (Binghamton Plate Glass, Binghamton, NY) (30 × 20 × 20 cm for juveniles; 45 × 30 × 30 cm for adults) containing clean pine shavings. Each apparatus was divided into two compartments by a clear Plexiglas partition containing an aperture (7 × 5 cm for juveniles; 9 × 7 cm for adults) to allow movement of animals between compartments. Exposure to this context was conducted under low light, with a white noise generator used to reduce extraneous sounds and the experimenter not present in the room.
Behavioral Analysis
Cameras mounted directly above each apparatus were used to monitor and record the behavior of the animals. An experimenter blind to the treatment of the subjects scored behavioral data. Crossovers between the two compartments (defined by an animal's two hind feet passing through the aperture) were scored from the videotapes as an index of general locomotion, and were assessed in six consecutive 10-min bins. Measures of stereotypy included repetitive vertical or horizontal weaving or bobbing movements of the head, sniffing at the sides or floor of the chamber, and repetitive focus on the aperture (included sniffing and head scanning focused towards the aperture). These behaviors were quantified using a time-sampling procedure whereby each animal's behavior was scored continuously for 30 sec at 5, 15, 25, 35, 45 and 55 min into the session. Each of these 30-sec observation periods was divided into ten 3-sec blocks during which the presence or absence of each measure of stereotypy was noted, with each measure receiving a score ranging from 0 (denoting that the behavior never occurred) to 10 (reflecting that the behavior occurred in all 10 blocks) for each 30 sec observation period. These scores were summed to provide an overall stereotypy score for each observation period.
Experiment 1
The design of this project was a 2 age [juvenile (P21-22) or adult (P64-66)] × 3 exposure [repeated saline exposure (SAL), repeated 1.5 mg/kg amphetamine (1.5A), or repeated 3.0 mg/kg amphetamine (3.0A)] factorial, with 8 animals tested in each of the 6 experimental groups.
Procedure
Daily for 4 days (P22-26 for juveniles; P65-68 for adults), animals received an intraperitoneal (i.p.) injection of either saline (0.9% NaCl w/v), 1.5 or 3.0 mg/kg/ml D-amphetamine sulfate (Sigma, St. Louis, MO) between 0900 and 1100 hrs, immediately followed by placement into the test context for 60 min. Two days following the last of the daily injections (P28 and P71 for juveniles and adults, respectively) and approximately 3 weeks later (i.e., P49-51 or P92-94) all animals were challenged with amphetamine. On these challenge days, animals were weighed, injected with 1.5 mg/kg amphetamine and immediately placed in the test apparatus for 60 min. A 1.5 mg/kg dose was used given evidence that sensitization is more robustly expressed when the challenge dose is the same or lower than the inducing dose (see Robinson and Becker, 1986). Given that animals exposed as juveniles had reached a relatively mature size by the second challenge day, this test was conducted in adult-sized chambers for all animals.
Experiment 2
The design of this project was a 2 age [juvenile (P21-22) or adult (P64-66)] × 2 exposure [repeated restraint stress (RS) or non-manipulated (NM)] factorial, with 8 animals tested in each of the 4 experimental groups.
Procedure
Animals in the RS group were restrained for 4 consecutive days (P22-26 for juveniles; P65-68 for adults) for 60 min daily in adjustable Plexiglas cylinders [18 × 4.7 cm for juveniles; 20.5 × 7.0 cm for adults] (Braintree Scientific, Braintree, MA). Rats in the NM group were left undisturbed during this stress phase. Animals were then given two amphetamine challenges as outlined in Experiment 1: one 2 days post-stress exposure, and the other approximately 3 weeks later. For these challenges, animals were weighed, injected with 1.5 mg/kg amphetamine and immediately placed in the test apparatus for 60 min.
Data Analysis
Before analysis, behavioral measures were checked for homogeneity of variance. Behaviors were analyzed using analysis of variance (ANOVA) procedures organized around specific questions of interest, with targeted ANOVAs and Fisher's Least Significant (LSD) post hoc tests used to identify the locus of significant main effects and interactions arising from these analyses.
Two animals (one 1.5A and one 3.0A adult) were not included in the analysis of Experiment 1 because their video records were lost on two of the four days of behavioral observation. Data from the 3-week challenge in Experiment 1 from one juvenile (in the SAL group) and five adults (1 SAL, 2 1.5A, 2 3.0A) were lost due to experimenter error and technical problems; 3-week challenge data from 3 animals at each age were similarly lost in Experiment 2 (juveniles: 2 NM; 1 RS: adults: 1 NM; 2 RS).
Results
Body Weight Gain (Table 1)
Table 1.
Percent Body Weight Gain of Juvenile and Adult Rats (mean ± standard error)
| Condition1 | ||||||
|---|---|---|---|---|---|---|
| NM | RS | SAL | 1.5A | 3.0A | ||
| Juveniles | 2-day2 | 68.1 ± 3.8 | 57.6 ± 3.6 | 58.3 ± 3.5 | 62.5 ± 2.7 | 62.2 ± 4.0 |
| 3-week3 | 231.0 ± 11.0 | 254.4 ± 13.4 | 249.9 ± 10.6 | 247.0 ± 9.5 | 269.7 ± 17.3 | |
| Adults | 2-day | 5.4 ± 1.1 | 4.1 ± 1.7 | 5.8 ± 1.7 | 7.5 ± .8 | 6.4 ± 1.5 |
| 3-week | 15.8 ± 2.1 | 14.1 ± 1.8 | 15.0 ± 2.2 | 17.5 ± 1.2 | 15.4 ± 3.5 | |
Conditions were as follows: animals remained non-manipulated (NM); animals were exposed to daily 60-min sessions of restraint stress (for 4 days; RS); rats were given a saline injection each day for 4 days (SAL); rats were administered 1.5 mg/kg (1.5A) or 3.0 mg/kg (3.0A) of amphetamine once a day over 4 days.
Body weight gain was calculated as a percentage change from the first day of the experiment to the first 1.5 mg/kg amphetamine challenge at 2 days post-exposure (2-day) with juveniles and adults analyzed separately at each age.
Body weight gain was calculated as a percentage change from the first day of the experiment to the second 1.5 mg/kg amphetamine challenge at 3 weeks post-exposure (3-week), with juveniles and adults again analyzed separately at each age.
Percent body weight gains from the first day of the experiment, as well as the 2-day and 3-week amphetamine challenge days were analyzed separately at each age, because of the marked age differences in body weight gain. No significant differences among the exposure conditions emerged in these analyses.
Experiment 1
Amphetamine-Induced Sensitization
Exposure period analyses
Significant baseline age differences in activity were present among animals injected with saline, with juveniles exhibiting fewer crosses overall [25.0 ± 2.55 (mean ± SEM)] than adults (79.88 ± 8.93) [F(1,14) = 34.89, p ≤ .0001] (Figure 1). Baseline indices of the behavioral measure collectively defined as stereotypy also differed with age among saline animals, with juveniles again exhibiting significantly less stereotypy (12.25 ± 2.68) than adults (44.38 ± 3.50) [F(1,14) = 53.05, p ≤ .00001] (data not shown). Because of these baseline differences in both measures, as well as the persistent lack of activity in saline-exposed animals during the latter half of the testing sessions, responses to amphetamine during the exposure period were examined using difference scores from the saline group. In these and all subsequent analyses, the focus was on data summed across the final 4 bins of the session (i.e., from 20-60 min. post-injection) during which drug exposure-related effects were most pronounced.
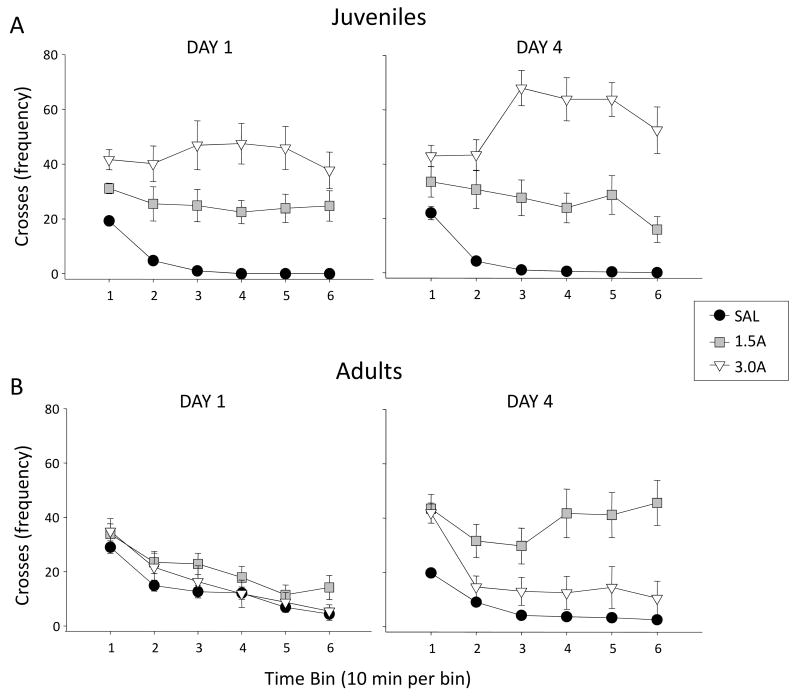
Figure 1.
Crossovers exhibited during the 60-min observation period in the test chamber were examined across six 10-min bins in (A) juveniles and (B) adults given saline (SAL), 1.5 mg/kg amphetamine (1.5A), or 3.0 mg/kg amphetamine (3.0A) on the first (day 1) and fourth day (day 4) of amphetamine injections.
Analysis of the activity data on days 1 and 4 of the exposure period (Figure 2a) revealed a significant day × age × exposure interaction [F(2, 40) = 5.69, p ≤ .01], as well as significant main effects of age [F(1,40) = 32.48, p ≤ .000001], exposure [F(1,40) = 38.40, p ≤ .000001], and day [F(1,40) = 13.76, p ≤ .001]. The three-way interaction of these variables showed that, on Day 1, juveniles in both the 1.5A and 3.0A groups exhibited significant elevations in number of crosses following an acute administration of amphetamine. In contrast, 1.5A and 3.0A adults failed to show an elevation in crossing behavior beyond that of saline control adults. Indeed, on this day juveniles showed significantly greater elevations in crosses over saline control animals in response to 1.5A and 3.0A than adults, with their response to 3.0A also greater than adults on Day 4 as well. Post-hoc analyses conducted to compare increases in the amphetamine response from day 1 to 4 of the exposure period as an index of sensitization revealed significant sensitization of number of crosses only in juveniles in the 3.0A group and adults in the 1.5A group.
Figure 2.
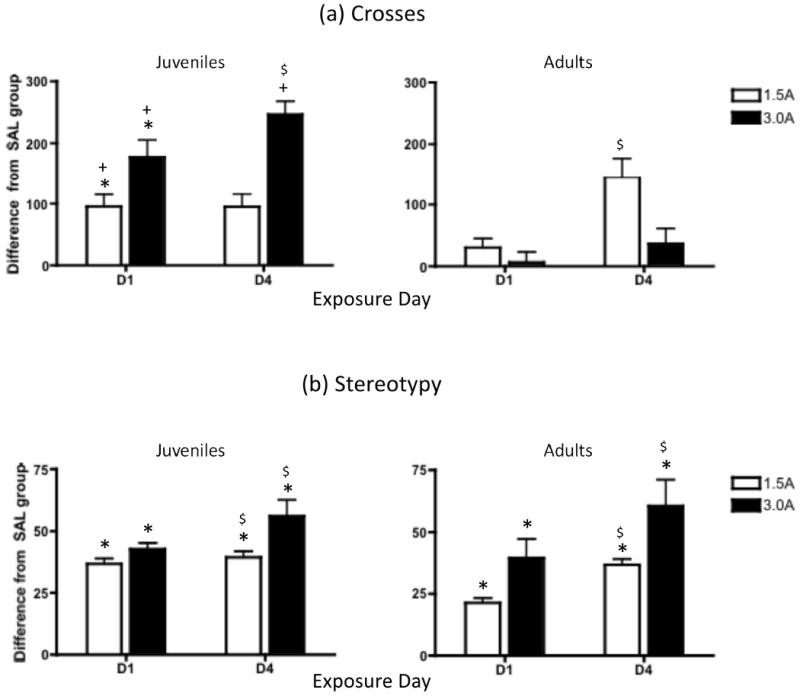
(a) Number of crosses and (b) stereotypy exhibited during the final 40 minutes of a 60-min observation period in juveniles and adults given saline, 1.5 mg/kg amphetamine (1.5A), or 3.0 mg/kg amphetamine (3.0A) on the first (D1) and fourth (D4) day of amphetamine exposure. Data are expressed as the mean difference score from the saline group at each age, with bars representing standard error of the mean. Asterisks (*) indicate a significant difference from the saline controls (p ≤ .05); Plus signs (+) denote a significant age difference within exposure condition and day; dollar signs ($) signify a significant increase from day 1 to day 4 of drug exposure within a particular age and dose condition. For figure (b), asterisks and dollar signs denote significant changes, collapsed across age group.
For the stereotypy data (Figure 2b), main effects of day [F(1,40) = 17.33, p ≤ .001] and exposure [F(2, 40) = 100.97, p ≤ .000001] were observed, as well as a significant interaction of these two variables [F(2,40) = 5.62, p ≤ .01]. At both ages and on both test days, animals given 1.5A and 3.0A showed significantly more stereotypy than saline control animals. Significant sensitization of stereotypy also emerged at both doses of amphetamine, with both 1.5A and 3.0A animals showing significantly greater levels of stereotypy on day 4 compared to day 1. Although this effect was seemingly driven by adults (especially at the 1.5A exposure dose – see Figure 2b), no significant interactions with age emerged in the analysis of these data.
Amphetamine challenge 2 days and 3 weeks post-exposure
Data from the SAL, 1.5A and 3.0A groups were analyzed with repeated measures ANOVAs for both locomotor activity (Figure 3a) and stereotypy (Figure 3b), with day (2-day and 3-week challenge) as the repeated measure. When all animals were challenged with 1.5 mg/kg amphetamine on these days, animals in the 3.0A group showed significantly more crosses than the 1.5A and SAL groups two days following the last exposure day, regardless of test age [day × exposure interaction: F(2, 36) = 4.73, p ≤ .05]. Three weeks post exposure, however, these significant group differences in amphetamine-induced activity were no longer apparent. Although similar trends were seen for stereotypy (see Figure 3b), no significant main effects or interactions involving age, day or drug exposure emerged in the analysis of this behavior.
Figure 3.
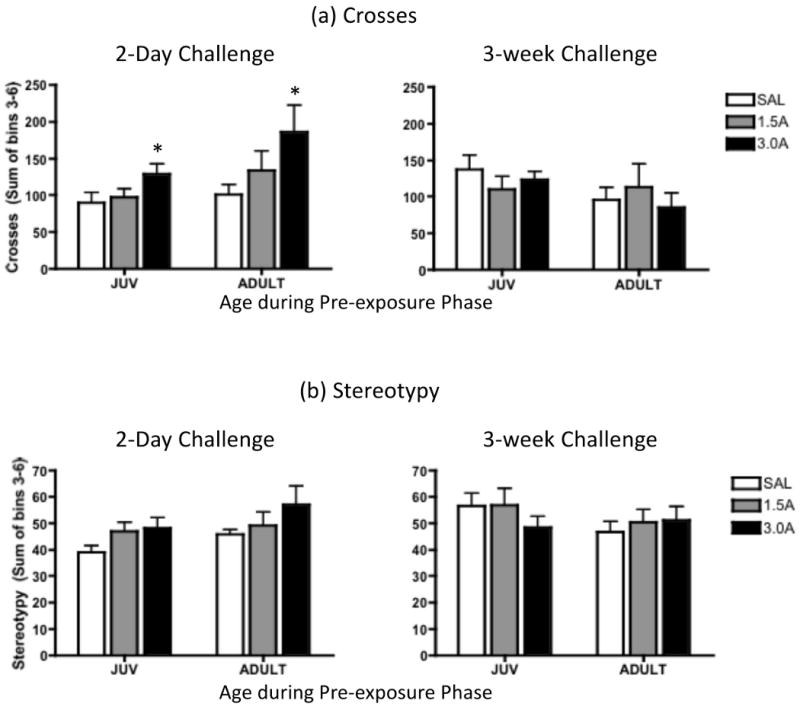
(a) Number of crosses and (b) stereotypy behavior exhibited in response to challenge with 1.5 mg/kg amphetamine two days and 3 weeks after juveniles (JUV) or adults were exposed to repeated saline (SAL), 1.5 mg/kg amphetamine (1.5A), or 3.0 mg/kg amphetamine (3.0A). Data represent group means of the sum of behaviors exhibited during the final 40 minutes of a 60-min observation period, with bars depicting the standard error of the mean. In this figure, asterisks (*) indicate a significant increase in the 3.0A condition (collapsed across age) compared to the 1.5A and SAL groups (p ≤.05).
Experiment 2
Stress-Induced Sensitization
Possible evidence of stress-drug cross sensitization was analyzed for locomotor crosses and stereotypy via repeated measure ANOVAs of data from the RS and NM groups, with day (2-day and 3-week amphetamine challenges) as the repeated measure. Analysis of number of crosses (Figure 4a) revealed significant stress sensitization, with RS animals showing significantly more crosses than the NM controls (main effect of pre-exposure: F(1,24) = 4.45, p ≤ .05). No main effects or interactions involving day or age were observed in this analysis, suggesting that stress/amphetamine cross-sensitization to the locomotor stimulant effects of amphetamine was present at both ages, and when tested either 2 days or 3 weeks following stressor termination. In contrast, only a main effect of day [F(1,22) = 22.45, p ≤ .001] emerged in the analysis of stereotypy (Figure 4b), with the overall amount of stereotypy exhibited in response to challenge with 1.5A being slightly but significantly higher at the 3-week compared to the 2-day post-stressor challenge.
Figure 4.
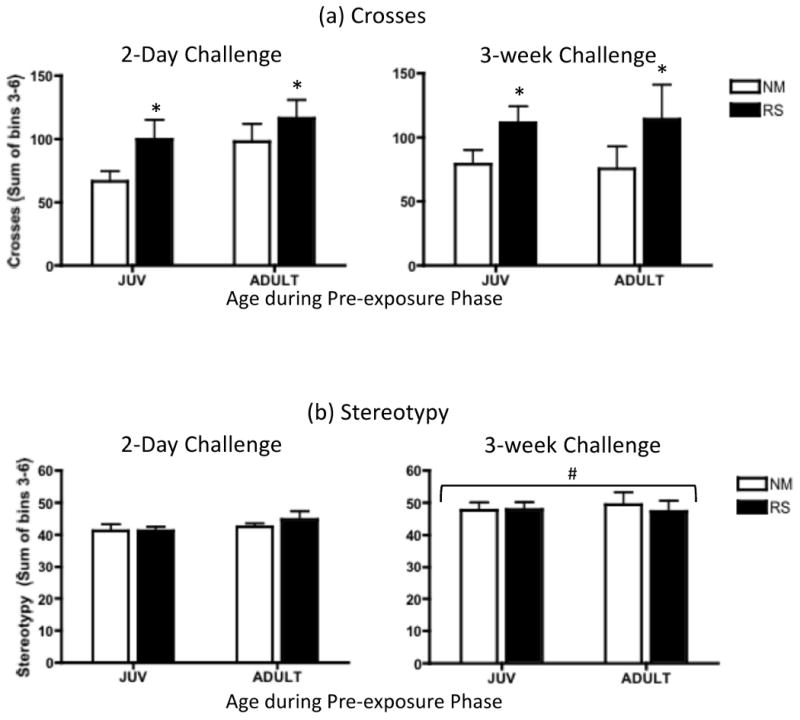
(a) Number of crosses and (b) stereotypy behavior exhibited in response to challenge with 1.5 mg/kg amphetamine two days and 3 weeks after juveniles (JUV) and adults were previously exposed to either repeated restraint sessions (RS) (60 min per day for 5 days) or were left non-manipulated (NM). Data represent group means of the sum of behaviors exhibited during the final 40 minutes of a 60-min observation period, with bars denoting the standard error of the mean. In this figure, asterisks (*) signify a significant increase in the RS groups compared to the NM controls, collapsed across age and day of testing (p ≤ .05).
Discussion
In these experiments, possible age-related differences in the acute effects of amphetamine, as well as the development of amphetamine- and stress-induced sensitization, were examined among juvenile and adult female rats. The results showed that juveniles were more sensitive to the locomotor activating effects of an acute amphetamine challenge compared to adults. Animals of the two ages also differed in the pattern of behavioral sensitization exhibited during the induction phase. Specifically, when comparing the first and last induction days, evidence for locomotor sensitization was seen in adults at the 1.5 mg/kg dose and sensitization of stereotypy at both doses. In contrast, juveniles generally expressed behavioral sensitization during the induction phase only at the 3.0 mg/kg dose. When all animals were challenged with 1.5 mg/kg amphetamine two days later, locomotor sensitization was evident among the 3.0A groups but not the 1.5A groups at both ages. Three weeks later, no evidence of sensitization was seen in response to a second amphetamine challenge in any of the amphetamine exposure or age groups. In contrast, evidence of stress-drug cross-sensitization was seen in terms of amphetamine-induced locomotor stimulation when both juveniles and adults were challenged with amphetamine 2 days as well as 3 weeks following repeated restraint stress.
That adults did not demonstrate locomotor activation when initially exposed to the lower dose of amphetamine during the induction period was unexpected, given previous studies reporting that low to moderate doses of amphetamine result in marked increases in locomotor behavior among adult rodents (e.g. Dietz et al., 2008; Fiorino and Phillips, 1999; McCormick and Ibrahim, 2007; Rasmussen et al., 2010). There are a number of possibilities as to why adult females in the present study did not respond to initial injection of 1.5 mg/kg amphetamine with enhanced locomotion when indexed via crosses from one side of the apparatus. Particularly likely is the nature of the testing apparatus used in the current experiment. Specifically, the aperture between the two sides of the test apparatus was an unusual feature, and animals often appeared to direct their stereotypic behavior to this portion of the chamber. It is possible that the aperture induced more stereotypy than commonly observed, especially among adult animals. Indeed, adults exhibited more stereotypic-type behavior (including aperture sniffing and head scanning of the aperture) than juveniles even under baseline conditions, with this enhanced stereotypy perhaps competing with the expression of locomotor behavior. It is also possible that the scaling of apparatus dimensions to equate relative body-to-apparatus size across age may have somehow fostered greater basal levels of locomotor activity among adults than adolescents, perhaps obscuring further increases in activity when initially challenged with amphetamine. This possibility seems counter-intuitive, though, and is inconsistent with recent data reporting similar age effects on locomotion and novelty-seeking regardless of whether animals were tested in the same apparatus or apparatuses scaled to animal size (Philpot and Wecker, 2008).
Consistent with prior findings, locomotor sensitization emerged in adulthood during the induction phase at the 1.5 mg/kg dose in adults. Previous studies have reported that, while locomotor sensitization is often evident at relatively low doses, at higher doses of psychostimulants, adult rodents are more likely to exhibit sensitization of stereotypy at the expense of locomotor activation (for review see Robinson and Becker, 1986; Tirelli et al., 2003). Indeed, the 3.0A adults in this study showed marked increases in stereotyped behavior relative to 1.5A adults and their SAL controls, perhaps precluding expression of locomotor sensitization. In contrast to the adult data, however, juvenile animals in the 1.5A condition did not exhibit locomotor sensitization during the exposure period. One possibility is that the 1.5 mg/kg dose was not large enough to induce the neural adaptations underlying sensitization among juveniles. While age differences in sensitization of stereotypy were not significant during the exposure phase, the lack of an increase in stereotypy from day 1 to day 4 among 1.5A juveniles further supports this possibility. Alternatively, developmental differences in general responsiveness to amphetamine could be responsible for these results. Since across day (and hence, age) comparisons were necessary to infer emergence of sensitization during the exposure period, differences in the response to the 1.5 mg/kg dose across days could reflect emergence of an adolescent-associated attenuation in response to stimulant drugs that is often (e.g., Bolanos et al., 1998; Laviola et al., 1995; Snyder et al., 1998; Spear and Brake, 1983; Zombeck et al., 2009) but not always (e.g. Badanich et al., 2008; Caster et al., 2005) observed.
The similar degree of sensitization seen in both juvenile- and adult-exposed animals when challenged with amphetamine two days following the induction period suggests that age differences in sensitization were not likely under these circumstances. While some experiments have reported no age differences in expression of stimulant sensitization (e.g. Collins and Izenwasser, 2002), others have shown that younger animals (in most cases, adolescents) are more vulnerable to the development of sensitization than comparably exposed adults (for review see Laviola et al., 1999; Tirelli et al., 2003) when indexed via locomotor activity (Adriani et al., 1998; Laviola et al., 1999; Laviola et al., 1995) or stereotypy (Laviola et al., 2001). Methodological differences across studies such as dose, length and pattern of drug exposures, age at the time of exposure, and exposure-to-test intervals have often been invoked as likely contributors to these mixed results (e.g. Collins and Izenwasser, 2002).
The lack of persistence of the sensitized amphetamine response after 3 drug-free weeks was unexpected, at least in adults, given evidence for the persistence of stimulant sensitization for up to a year in adult rats under some test circumstances (e.g. Paulson et al., 1991). In this study, however, relatively low doses of amphetamine were used during the exposure phase. Previous studies have shown that higher doses of amphetamine (e.g. 5.0 mg/kg or more) are likely to induce intense stereotyped behavior that lasts for extended periods of time (for review see Robinson and Becker, 1986). The intent here was to administer relatively low doses of amphetamine over a limited exposure period to avoid a possible ceiling effect that could obscure potential ontogenetic differences. These doses, however, may not have provided sufficient exposure to induce long-lasting sensitization at either age.
It is not simply the case, however, that these test circumstances were insufficient to reveal sensitization over this time interval in females at the two ages, as evidenced by significant stress-induced cross-sensitization to challenge with 1.5 mg/kg amphetamine at both 2 days and 3 weeks following stressor termination. Stress/drug cross-sensitization by restraint stress has previously been observed among adults (Deroche et al., 1992; Reid et al., 1998), and occasionally, but not always in developing animals (Lepsch et al., 2005; Trzcinska et al., 2002). It is intriguing that the stress/stimulant cross-sensitization observed in this study was more robust than that seen following stimulant sensitization per se, with this stress-stimulant cross-sensitization lasting a full 3 weeks post-stressor, regardless of age. In a previous study that directly compared behavioral sensitization induced by stressors versus stimulants, both stimulant- and stress-induced sensitization were shown to persist for 10 days following the induction phase (Yap and Miczek, 2008). In contrast, another report found that stimulant-induced sensitization endured longer than stress-induced sensitization, with repeated amphetamine but not a single exposure to footshock stress resulting in behavioral sensitization to an amphetamine challenge 3 weeks later (Schmidt et al., 1999). While the latter experiment would suggest that stress-induced sensitization does not persist as long as drug-induced sensitization, it is likely that the single stressor exposure used in the above study may have only induced short-term sensitization, with similar results reported elsewhere (de Jong et al., 2005). Instead, repeated application of the same stressor (as in the current study) may be necessary to induce long-lasting sensitization, as other have also demonstrated stress-induced cross-sensitization to amphetamine for up to 2 months after a repeated stress application (Nikulina et al., 2004). The present results add to this diverse literature by demonstrating that under at least some test circumstances, juveniles and adults are equally vulnerable to the effects of repeated stressor exposure on later amphetamine sensitivity, with expression of this cross-sensitization persisting longer among females at both ages than following sensitizing exposures to relatively low doses of amphetamine per se.
Taken together, the results of the present study demonstrate that a history of repeated drug or stressor exposure during the juvenile period or in adulthood can induce sensitization to a later amphetamine challenge, effects that persisted longer under these test conditions following repeated restraint than repeated amphetamine exposure per se. Age-related differences in the current study were not marked, with juveniles differing from adults only upon acute exposure to amphetamine and during the exposure period. While these results suggest that female juveniles and adults do not differ notably in their susceptibility to develop sensitization to amphetamine, they highlight the importance of stress history on drug responsiveness. An individual's stress history may be a particularly important variable when considering a person's vulnerability to addiction, even if that stressor occurs during the juvenile period and long before exposure to drugs.
Acknowledgments
Supported by grants R01 DA019071, R37 AA12525 and R01 AA017355 to Linda Spear.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adriani W, Chiarotti F, Laviola G. Elevated novelty seeking and peculiar d-amphetamine sensitization in periadolescent mice compared with adult mice. Behav Neurosci. 1998;112:1152–66. doi: 10.1037//0735-7044.112.5.1152. [DOI] [PubMed] [Google Scholar]
- Antelman SM, Eichler AJ, Black CA, Kocan D. Interchangeability of stress and amphetamine in sensitization. Science. 1980;207:329–31. doi: 10.1126/science.7188649. [DOI] [PubMed] [Google Scholar]
- Badanich KA, Maldonado AM, Kirstein CL. Early adolescents show enhanced acute cocaine-induced locomotor activity in comparison to late adolescent and adult rats. Dev Psychobiol. 2008;50:127–33. doi: 10.1002/dev.20252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battisti JJ, Chang CH, Uretsky NJ, Wallace LJ. Sensitization of stereotyped behavior to amphetamine is context and response dependent. Pharmacol Biochem Behav. 1999;63:263–9. doi: 10.1016/s0091-3057(98)00259-7. [DOI] [PubMed] [Google Scholar]
- Benwell ME, Balfour DJ. The effects of acute and repeated nicotine treatment on nucleus accumbens dopamine and locomotor activity. Br J Pharmacol. 1992;105:849–56. doi: 10.1111/j.1476-5381.1992.tb09067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolanos CA, Glatt SJ, Jackson D. Subsensitivity to dopaminergic drugs in periadolescent rats: a behavioral and neurochemical analysis. Brain Res Dev Brain Res. 1998;111:25–33. doi: 10.1016/s0165-3806(98)00116-3. [DOI] [PubMed] [Google Scholar]
- Caster JM, Walker QD, Kuhn CM. Enhanced behavioral response to repeated-dose cocaine in adolescent rats. Psychopharmacology (Berl) 2005;183:218–25. doi: 10.1007/s00213-005-0159-4. [DOI] [PubMed] [Google Scholar]
- Collins SL, Izenwasser S. Cocaine differentially alters behavior and neurochemistry in periadolescent versus adult rats. Brain Res Dev Brain Res. 2002;138:27–34. doi: 10.1016/s0165-3806(02)00471-6. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Noble D. Conditioned activation induced by ethanol: role in sensitization and conditioned place preference. Pharmacol Biochem Behav. 1992;43:307–13. doi: 10.1016/0091-3057(92)90673-4. [DOI] [PubMed] [Google Scholar]
- de Jong JG, Wasilewski M, van der Vegt BJ, Buwalda B, Koolhaas JM. A single social defeat induces short-lasting behavioral sensitization to amphetamine. Physiol Behav. 2005;83:805–11. doi: 10.1016/j.physbeh.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Deroche V, Piazza PV, Casolini P, Maccari S, Le Moal M, Simon H. Stress-induced sensitization to amphetamine and morphine psychomotor effects depend on stress-induced corticosterone secretion. Brain Res. 1992;598:343–8. doi: 10.1016/0006-8993(92)90205-n. [DOI] [PubMed] [Google Scholar]
- Diaz-Otanez CS, Capriles NR, Cancela LM. D1 and D2 dopamine and opiate receptors are involved in the restraint stress-induced sensitization to the psychostimulant effects of amphetamine. Pharmacol Biochem Behav. 1997;58:9–14. doi: 10.1016/s0091-3057(96)00344-9. [DOI] [PubMed] [Google Scholar]
- Dietz DM, Dietz KC, Moore S, Ouimet CC, Kabbaj M. Repeated social defeat stress-induced sensitization to the locomotor activating effects of d-amphetamine: role of individual differences. Psychopharmacology (Berl) 2008;198:51–62. doi: 10.1007/s00213-008-1078-y. [DOI] [PubMed] [Google Scholar]
- Erb S, Brown ZJ. A role for corticotropin-releasing factor in the long-term expression of behavioral sensitization to cocaine. Behav Brain Res. 2006;172:360–4. doi: 10.1016/j.bbr.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Fiorino DF, Phillips AG. Facilitation of sexual behavior in male rats following d-amphetamine-induced behavioral sensitization. Psychopharmacology (Berl) 1999;142:200–8. doi: 10.1007/s002130050880. [DOI] [PubMed] [Google Scholar]
- Frantz KJ, O'Dell LE, Parsons LH. Behavioral and neurochemical responses to cocaine in periadolescent and adult rats. Neuropsychopharmacology. 2007;32:625–37. doi: 10.1038/sj.npp.1301130. [DOI] [PubMed] [Google Scholar]
- Herman JP, Stinus L, Le Moal M. Repeated stress increases locomotor response to amphetamine. Psychopharmacology (Berl) 1984;84:431–5. doi: 10.1007/BF00555227. [DOI] [PubMed] [Google Scholar]
- Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol. 1992;14:221–8. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Kabbaj M, Isgor C, Watson SJ, Akil H. Stress during adolescence alters behavioral sensitization to amphetamine. Neuroscience. 2002;113:395–400. doi: 10.1016/s0306-4522(02)00188-4. [DOI] [PubMed] [Google Scholar]
- Kita T, Okamoto M, Nakashima T. Nicotine-induced ambulatory stimulant effect and its reverse tolerance. Yakubutsu Seishin Kodo. 1992;12:17–25. [PubMed] [Google Scholar]
- Kolta MG, Scalzo FM, Ali SF, Holson RR. Ontogeny of the enhanced behavioral response to amphetamine in amphetamine-pretreated rats. Psychopharmacology (Berl) 1990;100:377–82. doi: 10.1007/BF02244610. [DOI] [PubMed] [Google Scholar]
- Kolta MG, Shreve P, De Souza V, Uretsky NJ. Time course of the development of the enhanced behavioral and biochemical responses to amphetamine after pretreatment with amphetamine. Neuropharmacology. 1985;24:823–9. doi: 10.1016/0028-3908(85)90032-2. [DOI] [PubMed] [Google Scholar]
- Laviola G, Adriani W, Terranova ML, Gerra G. Psychobiological risk factors for vulnerability to psychostimulants in human adolescents and animal models. Neurosci Biobehav Rev. 1999;23:993–1010. doi: 10.1016/s0149-7634(99)00032-9. [DOI] [PubMed] [Google Scholar]
- Laviola G, Pascucci T, Pieretti S. Striatal dopamine sensitization to D-amphetamine in periadolescent but not in adult rats. Pharmacol Biochem Behav. 2001;68:115–24. doi: 10.1016/s0091-3057(00)00430-5. [DOI] [PubMed] [Google Scholar]
- Laviola G, Wood RD, Kuhn C, Francis R, Spear LP. Cocaine sensitization in periadolescent and adult rats. J Pharmacol Exp Ther. 1995;275:345–57. [PubMed] [Google Scholar]
- Leith NJ, Kuczenski R. Two dissociable components of behavioral sensitization following repeated amphetamine administration. Psychopharmacology (Berl) 1982;76:310–5. doi: 10.1007/BF00449116. [DOI] [PubMed] [Google Scholar]
- Lepsch LB, Gonzalo LA, Magro FJ, Delucia R, Scavone C, Planeta CS. Exposure to chronic stress increases the locomotor response to cocaine and the basal levels of corticosterone in adolescent rats. Addict Biol. 2005;10:251–6. doi: 10.1080/13556210500269366. [DOI] [PubMed] [Google Scholar]
- MacLennan AJ, Maier SF. Coping and the stress-induced potentiation of stimulant stereotypy in the rat. Science. 1983;219:1091–3. doi: 10.1126/science.6681679. [DOI] [PubMed] [Google Scholar]
- Masur J, Boerngen R. The excitatory component of ethanol in mice: a chronic study. Pharmacol Biochem Behav. 1980;13:777–80. doi: 10.1016/0091-3057(80)90206-3. [DOI] [PubMed] [Google Scholar]
- Masur J, Oliveira de Souza ML, Zwicker AP. The excitatory effect of ethanol: absence in rats, no tolerance and increased sensitivity in mice. Pharmacol Biochem Behav. 1986;24:1225–8. doi: 10.1016/0091-3057(86)90175-9. [DOI] [PubMed] [Google Scholar]
- Mathews IZ, Mills RG, McCormick CM. Chronic social stress in adolescence influenced both amphetamine conditioned place preference and locomotor sensitization. Dev Psychobiol. 2008;50:451–9. doi: 10.1002/dev.20299. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Ibrahim FN. Locomotor activity to nicotine and Fos immunoreactivity in the paraventricular nucleus of the hypothalamus in adolescent socially-stressed rats. Pharmacol Biochem Behav. 2007;86:92–102. doi: 10.1016/j.pbb.2006.12.012. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Robarts D, Gleason E, Kelsey JE. Stress during adolescence enhances locomotor sensitization to nicotine in adulthood in female, but not male, rats. Horm Behav. 2004;46:458–66. doi: 10.1016/j.yhbeh.2004.05.004. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Robarts D, Kopeikina K, Kelsey JE. Long-lasting, sex- and age-specific effects of social stressors on corticosterone responses to restraint and on locomotor responses to psychostimulants in rats. Horm Behav. 2005;48:64–74. doi: 10.1016/j.yhbeh.2005.01.008. [DOI] [PubMed] [Google Scholar]
- McPherson CS, Lawrence AJ. Exposure to amphetamine in rats during periadolescence establishes behavioural and extrastriatal neural sensitization in adulthood. Int J Neuropsychopharmacol. 2006;9:377–92. doi: 10.1017/S1461145705005845. [DOI] [PubMed] [Google Scholar]
- Niculescu M, Ehrlich ME, Unterwald EM. Age-specific behavioral responses to psychostimulants in mice. Pharmacol Biochem Behav. 2005;82:280–8. doi: 10.1016/j.pbb.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Nikulina EM, Covington HE, 3rd, Ganschow L, Hammer RP, Jr, Miczek KA. Long-term behavioral and neuronal cross-sensitization to amphetamine induced by repeated brief social defeat stress: Fos in the ventral tegmental area and amygdala. Neuroscience. 2004;123:857–65. doi: 10.1016/j.neuroscience.2003.10.029. [DOI] [PubMed] [Google Scholar]
- Paulson PE, Camp DM, Robinson TE. Time course of transient behavioral depression and persistent behavioral sensitization in relation to regional brain monoamine concentrations during amphetamine withdrawal in rats. Psychopharmacology (Berl) 1991;103:480–92. doi: 10.1007/BF02244248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpot RM, Wecker L. Dependence of adolescent novelty-seeking behavior on response phenotype and effects of apparatus scaling. Behav Neurosci. 2008;122:861–75. doi: 10.1037/0735-7044.122.4.861. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, le Moal M, Simon H. Stress- and pharmacologically-induced behavioral sensitization increases vulnerability to acquisition of amphetamine self-administration. Brain Res. 1990;514:22–6. doi: 10.1016/0006-8993(90)90431-a. [DOI] [PubMed] [Google Scholar]
- Rasmussen BA, Unterwald EM, Kim JK, Rawls SM. Methanandamide blocks amphetamine-induced behavioral sensitization in rats. Eur J Pharmacol. 2010;627:150–5. doi: 10.1016/j.ejphar.2009.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MS, Ho LB, Tolliver BK, Wolkowitz OM, Berger SP. Partial reversal of stress-induced behavioral sensitization to amphetamine following metyrapone treatment. Brain Res. 1998;783:133–42. doi: 10.1016/s0006-8993(97)01319-x. [DOI] [PubMed] [Google Scholar]
- Robinson TE. Behavioral sensitization: characterization of enduring changes in rotational behavior produced by intermittent injections of amphetamine in male and female rats. Psychopharmacology (Berl) 1984;84:466–75. doi: 10.1007/BF00431451. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Angus AL, Becker JB. Sensitization to stress: the enduring effects of prior stress on amphetamine-induced rotational behavior. Life Sci. 1985;37:1039–42. doi: 10.1016/0024-3205(85)90594-6. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. 1986;396:157–98. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci. 2008;363:3137–46. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–91. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Browman KE, Crombag HS, Badiani A. Modulation of the induction or expression of psychostimulant sensitization by the circumstances surrounding drug administration. Neurosci Biobehav Rev. 1998;22:347–54. doi: 10.1016/s0149-7634(97)00020-1. [DOI] [PubMed] [Google Scholar]
- Schmidt ED, Tilders FJ, Binnekade R, Schoffelmeer AN, De Vries TJ. Stressor- or drug-induced sensitization of the corticosterone response is not critically involved in the long-term expression of behavioural sensitization to amphetamine. Neuroscience. 1999;92:343–52. doi: 10.1016/s0306-4522(98)00725-8. [DOI] [PubMed] [Google Scholar]
- Snyder KJ, Katovic NM, Spear LP. Longevity of the expression of behavioral sensitization to cocaine in preweanling rats. Pharmacol Biochem Behav. 1998;60:909–14. doi: 10.1016/s0091-3057(98)00078-1. [DOI] [PubMed] [Google Scholar]
- Sorg BA, Kalivas PW. Stress and Neuronal Sensitization. In: Friedman ML, Charney DS, Deutch AY, editors. Neurobiological and Clinical Consequences of Stress: From Normal Adaptations ot PTSD. Philadelphia: Lippincott-Raven; 1995. pp. 83–102. [Google Scholar]
- Spear LP, Brake SC. Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Dev Psychobiol. 1983;16:83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- Tirelli E, Laviola G, Adriani W. Ontogenesis of behavioral sensitization and conditioned place preference induced by psychostimulants in laboratory rodents. Neurosci Biobehav Rev. 2003;27:163–78. doi: 10.1016/s0149-7634(03)00018-6. [DOI] [PubMed] [Google Scholar]
- Trzcinska M, Bergh J, DeLeon K, Stellar JR, Melloni RH., Jr Social stress does not alter the expression of sensitization to cocaine. Physiol Behav. 2002;76:457–63. doi: 10.1016/s0031-9384(02)00727-8. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Schmidt ED, De Vries TJ, Van Moorsel CA, Tilders FJ, Schoffelmeer AN. A single exposure to amphetamine is sufficient to induce long-term behavioral, neuroendocrine, and neurochemical sensitization in rats. J Neurosci. 1999;19:9579–86. doi: 10.1523/JNEUROSCI.19-21-09579.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap JJ, Miczek KA. Stress and Rodent Models of Drug Addiction: Role of VTA-Accumbens-PFC-Amygdala Circuit. Drug Discov Today Dis Models. 2008;5:259–70. doi: 10.1016/j.ddmod.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zombeck JA, Gupta T, Rhodes JS. Evaluation of a pharmacokinetic hypothesis for reduced locomotor stimulation from methamphetamine and cocaine in adolescent versus adult male C57BL/6J mice. Psychopharmacology (Berl) 2009;201:589–99. doi: 10.1007/s00213-008-1327-0. [DOI] [PubMed] [Google Scholar]