Abstract
To investigate the role of C5a generated on complement activation in brain, the lupus model, MRL/lpr mice were treated with C5a receptor(R) antagonist (ant). Neutrophil infiltration, ICAM, TNF-α and iNOS mRNA expression, neuronal apoptosis and the expression of p-JNK, pSTAT1 and p-Erk were reduced and p-Akt increased on C5aR inhibition in MRL/lpr brains. MRL/lpr serum caused increased apoptosis in neurons showing that lupus had a direct effect on these cells. C5aRant pretreatment prevented the lupus serum induced loss of neuronal cells. Our findings demonstrate for the first time that C5a/C5aR signaling plays an important role in the pathogenesis of CNS lupus.
Keywords: Inflammation, Complement system, Anaphylatoxins, Brain, Systemic lupus erythematosus
1. Introduction
The complement system is a powerful arm of the innate immune system and plays a key role in infection and inflammation. It is a ‘double edged’ sword playing an important role in health and disease (Muller-Eberhard, 1988; Johnston, 1993; Carroll and Fischer, 1997; van Beek et al., 2003; Alexander et al., 2008a; Zipfel, 2009). Although inroads have been made to decipher the role of the complement cascade in different settings such as stroke and lupus, yet it is not completely understood. The complement proteins have a fairly ubiquitous distribution both in the CNS and systemically (Barnum, 1995; Morgan and Gasque, 1996; Gasque et al., 1997). Complement activation generates the anaphylatoxin, C5a, which produces both inflammatory and immune effects by binding to the G-coupled receptor, C5aR (Huber-Lang et al., 2002) and a second receptor, the C5a-like receptor 2 (C5L2). Although both receptors are expressed in the CNS, little is currently known about the potential role of the receptor, C5L2. Several studies have shown that generation of C5 plays a role in the pathology of neuroinflammatory diseases (Niculescu et al., 2003; Niculescu et al., 2004b). Conversely, other studies have raised the tantalizing possibility that in some settings C5a could be beneficial (Weerth et al., 2003; Guo et al., 2004; Fonseca et al., 2009). In this study we hypothesized that C5a acting through its receptor C5aR could cause neuroinflammation and could be a potential target for therapy, in CNS lupus.
The anaphylatoxin, C5a is a 74-aa fragment of C5 generated on complement activation and binds to C5aR inducing potent inflammatory effects (Sayah et al., 1997). They mediate a number of biological processes, including chemotaxis and degranulation of mast cells and basophils, vascular permeability, increased production of reactive oxygen radical species and cytokines (Langkabel et al., 1999; Guo et al., 2004). They could cause the intravascular aggregation of neutrophils with subsequent leukostatic occlusion of the cerebral arteries, contributing to vascular injury. This could alter release of cytokines and cause cell necrosis or apoptosis (Guo et al., 2004). Neuronal apoptosis is a key event in CNS lupus (Sakic et al., 2000; Ballok et al., 2003; Alexander et al., 2005a) and could occur through a number of signaling mechanisms, including p-Erk and JNK pathways (Gabai et al., 1998). Signaling pathways behave differently in various settings. For example, STAT1 has been implicated in modulating pro- and anti-apoptotic genes following several stress-induced responses, depending on STAT1 phosphorylation (Wesemann et al., 2004; Wang et al., 2008). Therefore, elucidation of the signal transduction pathways and the genes activated during this process may provide new insights concerning the prevention and treatment of CNS lupus.
Systemic lupus erythematosus (SLE) or lupus is a devastating disorder (Diamond and Volpe, 2003; Diamond et al., 2006) in which complement activation is an integral event (Alexander and Quigg, 2007). Our earlier studies demonstrated that by inhibition of the complement cascade with the pan complement inhibitor, Crry (Alexander et al., 2005a, b) or by deletion of factor B (Alexander et al., 2007), the disease could be alleviated. However, these inhibitors will shut down the complement cascade or the alternative pathway respectively. This would open the door to infections and may not be therapeutically viable. Therefore, this study examined the role of downstream complement proteins, C5a/C5aR in the well established lupus rodent model, MRL/MpJ-Tnfrsf6lpr (MRL/lpr) (Brey et al., 1997). MRL/lpr mice are felt to accurately reflect that which occurs in human SLE, including the neuropsychiatric manifestations (Sakic et al., 1997; Ballok et al., 2003). MRL/lpr mice differ from the congenic MRL/MpJ (MRL+/+) strain by the nearly complete absence of the proapoptotic membrane Fas protein, due to a retroviral insertion in the Tnfrsf6 gene (Adachi et al., 1993; Watanabe-Fukunaga et al., 1992).
We demonstrate that signaling through C5aR induces neuronal apoptosis in CNS lupus. In parallel, the expression of proinflammatory mediators ICAM, TNF-α, MIP2 and iNOS were significantly increased. Further, our results suggest that the apoptosis occurs through the JNK and STAT1 signaling pathways. In addition, to determine whether neuronal apoptosis was a primary or secondary event in CNS lupus, the role of C5a/C5aR signaling on neuronal cells in culture, was studied. The results obtained indicate that C5a/C5aR signaling plays an important role in lupus setting and may be a viable location for therapeutic intervention in neuroinflammatory and neurodegenerative diseases.
2. Materials and methods
2.1. Mice and treatment
MRL/lpr mice were bought from Jackson lab and maintained at the University of Chicago. To determine the role of C5aR in lupus brain, the MRL/lpr mice were treated with a cyclic hexapeptide antagonist of C5aR (C5aRant; acetyl-Phe-(Orn-Pro-d-cyclohexylalanine)-Trp-Arg) obtained from Dr Lambris (U. Pennsylvania). C5aRant was administered continuously using osmotic pumps (Alzet model 2001; Durect) that were inserted subcutaneously using sterile surgical techniques. They were chosen for weekly delivery based on the solubility, stability and effectiveness of C5aRant from earlier studies (Bao et al., 2005b). Eighteen male MRL/lpr mice (The Jackson Laboratory) were randomly divided into two groups to receive C5aRant (n=9) or vehicle alone (n=9). Starting at 13 week of age, mice were implanted with osmotic pumps containing 0.42 g/kg C5aRant in 0.2 ml of 50% DMSO to deliver 60 mg/kg/day C5aRant, which was the effective dose chosen based on earlier studies (Bao et al., 2005a). Osmotic pumps containing C5aRant were replaced weekly. Control animals were treated identically, except the 50% DMSO solution did not include C5aRant in the osmotic pump. At 19 week of age, all surviving animals were sacrificed for tissue harvest. These studies were approved by the University of Chicago Animal Care and Use Committee.
2.2. Reagents
Unless otherwise stated, all chemicals were purchased from Sigma (St. Louis, MO, USA). Antibodies were chicken anti-mouse C5aR (Dr Scott Barnum, Birmingham); rabbit anti-mouse p-Akt, anti-mouse p-Erk, anti-mouse p-JNK, anti-mouse STAT1 (Cell Signaling), rabbit anti-CD54 (Pharmingen), rabbit anti-Actin (Sigma), rabbit anti-C3 (Bethyl Laboratories, Montgomery, TX, USA). All primary antibodies were used at a dilution of 1:1000.
2.3. Tissue processing
Brains were isolated, and brain stem and cerebellum were discarded. Cerebral cortex was processed for immunofluorescence (IF), RNA, DNA and protein. For IF, brains were snap frozen in OCT compound placed in precooled 2-methylbutane and kept at −80 °C until use. The rest of the brains were frozen at −80 °C and processed accordingly for RNA, DNA and protein.
2.4. Immunofluorescence microscopy
To stain for neutrophils, 4 µm cryosections were fixed in ether/ethanol and then blocked with 10% normal goat serum. Slides were then sequentially incubated in rat anti-mouse neutrophil antibody (Serotec), followed by Alexa 488-conjugated goat anti-rat IgG (absorbed with mouse Ig; Serotec). To quantify cells in each section, neutrophils were counted in at least 20 low-power fields (lpf, 200×) in a blinded manner as described by others (Jacob et al., 2007). To assess ICAM expression, brain sections were stained with anti-CD54 and Alexa 547-labeled anti-rabbit antibody (Molecular Probes). The sections were observed at 20× using a Zeiss microscope.
2.5. Apoptosis determination
2.5.1. Ligase-mediated (LM)-PCR
Brains were harvested and immediately frozen at −80 °C DNA was purified using the DNeasy DNA purification system (Qiagen, Valencia, CA). DNA laddering was detected by LM-PCR (Clontech Laboratories, Palo Alto, CA) according to the manufacturer's instructions. In brief, DNA isolated from each animal or culture dish was incubated with the supplied primer targets and T4 DNA ligase for 18 h at 16 °C. Twenty milligrams of this ligated DNA was then used as substrate for PCR, using supplied primers and Advantage DNA polymerase (Clontech Laboratories) for 23 cycles at 94 °C for 1 min and at 72 °C for 3 min. The reaction product for each animal was electrophoresed through a 1.2% agarose gel with ethidium bromide and visualized with UV light.
2.6. Immunoblotting
Frozen cortical tissue was homogenized in RIPA buffer and protein estimated using BCA reagent (Hill and Straka, 1988). Equal amounts of samples were separated by SDS-PAGE and electrophoretically transferred to a polyvinylidene difluoride membrane (Millipore). Membranes were blocked with 5% nonfat milk for 1 h at room temperature and incubated with antibodies to C5aR, phosphorylated JNK, STAT1 or Erk (Cell Signaling Technology). Membranes were then incubated with peroxidase-conjugated anti-chicken IgY (Sigma-Aldrich) or anti-rabbit antibody (Pierce). Chemiluminescent substrate (Pierce) was used to develop signals. Membranes were then stripped followed by probing with anti-actin (Sigma-Aldrich), anti-PTEN, or anti-Akt antibodies (Cell Signaling Technology). Controls in which the primary antibody was omitted were negative. X-ray films were scanned and optical density of corresponding bands was determined with ImageJ software from NIH (http://rsb.info.nih.gov/ij/).
2.7. Quantitative RT-PCR
Total RNA was extracted from brains using TriZol and cDNA produced as described previously (Alexander et al., 2008b). qRT-PCR was performed on brain cortical RNA using QuantiTect SYBR Green RT-PCR kit. Data were normalized to GAPDH RNA. The primers used are provided in Table 1.
Table 1.
Primers used in qRT-PCR.
| Genes | Primer 1 (Forward) | Primer 2 (Reverse) |
|---|---|---|
| C5aR | 5′-gatgccaccgcctgtatagt-3′ | 5′-acgaaggatggaatggtgag-3′ |
| MIP-2 | 5′-caccaaccaccaggctac-3′ | 5′-gcccttgagagtggctatga-3′ |
| ICAM-1 | 5′-cgcaagtccaattcacactga-3′ | 5′-cagagcggcagagcaaaag-3′ |
| TNF-α | 5′-ccgatgggttgtaccttgtc-3′ | 5′-gtgggtgaggagcacgtagt-3′ |
| GAPDH | 5′-gcaaattcaacggcacagt-3′ | 5′-agatggtgatgggcttccc-3′ |
2.8. Cortical neuronal cultures
Cultures were grown from fetal mouse brains (E E16.5 pooled mouse embryos from one litter as previously described (Li et al., 2005). Cells were plated on poly-l-lysine-coated 15- or 25-mm cover slips at a density of 8×104 cells/cm2 and maintained in serum-free medium (Neurobasal A/B-27; Invitrogen Corp., Grand Island, NY) supplemented with 0.5 mM l-glutamine. Under these conditions neuronal purity is >95%. Cells were treated for 3 h with serum isolated from 20 week old mice (control serum from MRL+/+ mice, lupus serum from MRL/lpr mice). The activity of C5a was inhibited in the serum-treated cells by pretreatment with 1 µM C5aRant. Cells were assessed for apoptosis by two methods, TUNEL (Trevigen, MD) and Annexin V staining (Southern Biotech). Some cultures were fixed with 4% paraformaldehyde and immunostained for C5aR.
2.8.1. In situ apoptotic cells detection
Cells were treated for 3 h and fixed in 4% paraformaldehyde and examined for the degree of apoptosis using the TACS Fluorescent label in situ apoptosis detection kit (Trevigen) according to the manufacturer's instructions. Briefly, the free 3′-OH ends of nuclear DNA fragments were labeled with BrdU using TdT. Cryosections were then incubated with biotinylated anti-BrdU Ab and streptavidin-594. A positive control treated with nuclease and unlabeled negative experimental controls were also included. A different set of cultured cells were treated and stained with fluorescently labeled Annexin V. Nuclei were stained with DAPI. Apoptotic cells, which were positively labeled for Annexin (green) or TUNEL (red), were counted in a blinded manner from at least 5 lpf (×200)/cover slip. The total numbers of cells were based on counts obtained for DAPI staining. Results are given as mean of three different experiments.
2.9. Statistical analyses
Data are expressed as mean±SEM and were analyzed using Minitab (version 12; Minitab) software. For the comparison between two groups at one time point, t testing was used for parametric data, and Mann–Whitney testing was used for nonparametric data. Potential correlations among variables were determined by calculating Pearson product moment correlation coefficients and their p values. p<0.05 was considered statistically significant.
3. Results
3.1. C5aR mRNA and protein expression is up-regulated in brains of MRL/lpr mice
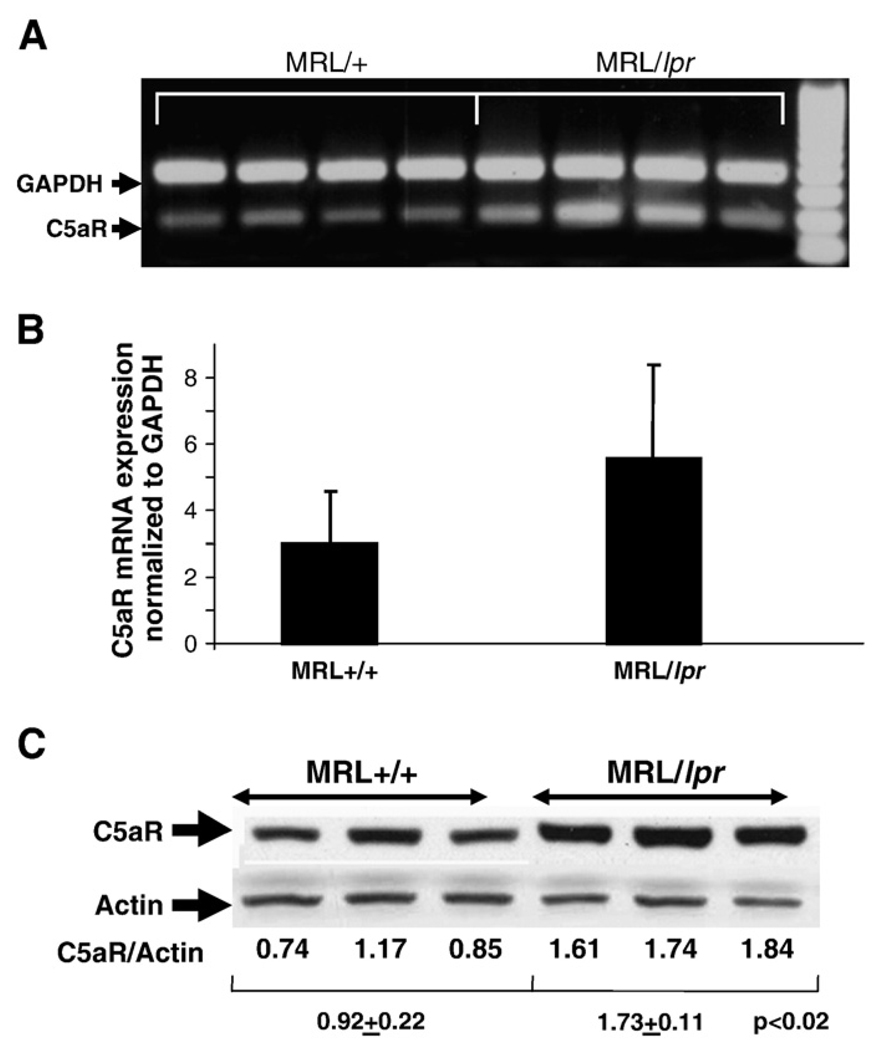
The expression of C5aR in brain was significantly up-regulated in MRL/lpr mice compared to MRL/+ mice (0.92±0.22 vs 1.73±0.11; p<0.02) as assessed by western blotting. In line with the protein expression, the mRNA for C5aR was found to be increased in MRL/lpr mouse brain compared to the control MRL/+ mice, although they did not reach statistical significance (Fig. 1). The up-regulation of C5aR protein expression could make the brain more susceptible to the effects of C5a.
Fig. 1.
Expression of C5aR is up-regulated in lupus brain. Expression of C5aR was up-regulated in the brains of MRL/lpr lupus mice (n=7), both at the mRNA level as determined by qRT-PCR (A and B), and protein as determined by western blotting analysis (C, p<0.02) compared to brains from MRL+/+ controls (n=7). mRNA expression was normalized to GAPDH and the protein expression to actin in each sample. Values are expressed as Mean±S.D. *p<0.05.
3.2. Effect of C5aRant on brain expression of inflammatory mediators
Expression of ICAM mRNA was significantly increased in brains of MRL/lpr mice. Immunostaining demonstrates increase in ICAM expression especially around the microvasculature (Fig. 2). Since ICAM along with other inflammatory mediators facilitates recruitment of mononuclear cells into inflamed tissue, infiltration of neutrophils into the lupus brain was assessed. In line with ICAM expression, significant number of neutrophils was present in lupus brain compared to controls (Fig. 3). The neutrophils were concentrated around the lumina of blood vessels and extravascularly throughout the brain parenchyma. Blockade of C5aR reduced ICAM expression (Fig. 2) and neutrophil infiltration (Fig. 3) in brains of MRL/lpr mice. Since neutrophils are recruited to sites of inflammation and can release proinflammatory molecules, we studied the brain expression of mRNAs for TNF-α, CXCL2/MIP-2 and iNOS by qRT-PCR to provide insight into potential mechanism(s) causing pathology. As shown in Fig. 4, the expression of all three mediators were significantly reduced (p<0.05) by inhibition of C5aR, confirming that the expression of these cytokines appears to be mediated, at least in part, through signals delivered through C5aR.
Fig. 2.
C5a alters ICAM expression in lupus brain. Immunofluorescence staining using anti-mouse CD54 antibody and detection with Alexa-594 labeled anti-rabbit antibody demonstrates a significant up-regulation of ICAM expression in MRL/lpr brain compared to control MRL+/+ brains. Shown are representative sections from each group. The staining is mainly present around the microvasculature as shown in the inset. The increase in expression of ICAM was reduced on C5aRant treatment. mRNA expression of ICAM as assessed by qRT-PCR was altered in brain in line with the observed protein changes. Values are expressed as Mean±S.D. *p<0.05.
Fig. 3.
Neutrophil accumulation in lupus brain is C5aR-dependent. Cortical sections from MRL/lpr mouse brain stained with anti-neutrophil antibody had significant infiltration of neutrophils compared to MRL/+ mice (8.05±3.2 vs 0.5±0.6; *p<0.05). In contrast, substantial reduction to no neutrophils was observed in MRL/lpr mice that were treated with C5aRa (1.6±1.1 vs 8.05±3.2; =p<0.05). Neutrophils were counted from 20 different fields each, per high-power field.
Fig. 4.
Brain expression of inflammatory mediators is regulated by C5a in lupus. Expression of proinflammatory mediators, MIP2, iNOS and TNF-α was significantly up-regulated in the brains of lupus mice, as determined by qRT-PCR, normalized to 18 S expression. These alterations were complement-dependent and were prevented by treatment with C5aRant. Values are shown as Means±S.D.; *p<0.05 vs saline-treated.
3.3. Inhibition of C5aR reduces neuronal apoptosis in MRL/lpr mouse brain
Our earlier studies demonstrate complement-dependent neuronal apoptosis is a key event in MRL/lpr brain (Alexander et al., 2005a). The increase in expression of inflammatory molecules could be one of the factors that induce apoptosis. In addition, C5a was previously shown to induce or reduce apoptosis, depending on the setting in which complement activation occurs. Therefore, we assessed the role of C5a/C5aR signaling in apoptosis by DNA fragmentation, in CNS lupus. As seen in our earlier experiments, significant DNA fragmentation was observed in control MRL/lpr mouse brain cortex, which was localized to the neurons. In contrast, the brains of mice treated with C5aRant had substantially less DNA fragmentation (Fig. 5). The changes in neurons could occur due to events in the brain per se or due to stimuli that obtain access to the brain through a disturbed BBB. The ability of the C5aRant to reduce neuronal apoptosis in vivo prompted us to determine effects in an in vitro system. Therefore we assessed the effect of lupus serum on neurons in culture.
Fig. 5.
C5aR inhibition reduces apoptosis in lupus brain. DNA was isolated from brains and subjected to LM-PCR to assess apoptotic DNA laddering. Significant apoptosis was observed in the brains of the MRL/lpr mice, which was considerably reduced by C5aR inhibition. Given are representative samples from the saline-treated and C5aRa-treated MRL/lpr groups. Each lane contains brain tissue harvested from an individual animal.
3.4. Signaling pathways involved in C5aR induced neuronal apoptosis
To obtain insights as to the pathways and proteins involved in neuronal apoptosis in CNS lupus, expression of phosphorylated Akt, Erk, JNK and STAT1 were assessed by western blotting and normalized to actin to circumvent any loading efficiency errors. The decrease in p-Akt expression and increase in expression of p-Erk, p-JNK and pSTAT1 that occurred in the brains of MRL/lpr mice and assessed by western blotting was reduced by treatment with C5aRant (Fig. 6, p<0.05). The changes in expression of these proteins were calculated using the ImageJ software. Further studies are required to determine the effect of the altered expression of these proteins on their activity and their downstream targets.
Fig. 6.
C5a/C5aR signaling regulates the expression of signaling proteins in lupus brain. The expression of survival factors p-Akt and other signaling molecules, p-Erk, p-JNK, p-STAT1 was assessed by western blotting with equal amounts of protein. They were normalized to the housekeeping protein, actin. The expression of p-Akt was significantly reduced while all the other molecules were up-regulated in MRL/lpr brain. The alteration in expression was alleviated by C5aR inhibition. All changes of *p<0.05 were considered significant. Shown are representative blots from three separate experiments.
3.5. C5a/C5aR signaling regulates apoptosis in neurons in vitro
Treatment of cultured neuronal cells with lupus serum enhanced C5aR expression in these cells (Fig. 7). In addition, cells treated with lupus serum as assessed by TUNEL and Annexin V (Fig. 8) staining underwent significant apoptosis compared to cells treated with serum from control mice. These results suggest that in a setting where the BBB is compromised, C5a is one of the factors in the circulating serum that cause neuronal apoptosis.
Fig. 7.
C5aR expression is up-regulated on neurons, when treated with lupus serum. Immunofluorescence using chicken anti-mouse C5aR antibody, clearly demonstrates the increased expression of C5aR when primary neuronal cultures were treated with lupus serum compared to cells treated with control MRL+/+ serum. C5aR was observed using a Zeiss microscope and was present both on the surface and in the cytosol.
Fig. 8.
C5aR inhibition reduces apoptosis in neurons. Cells treated with serum were processed for Annexin V (A) and TUNEL (B) staining as described in the Methods. Positive cells (Annexin and TUNEL) and total number of cells (DAPI) were counted in a blinded manner from 5 different fields of each sample using a Zeiss microscope and the values are given as Mean±S.D.
4. Discussion
Lupus is a complex disease of unknown etiology in which complement activation plays an important role (Belmont et al., 1986; Abramson et al., 1987; Arora et al., 2004). Our earlier studies, using Crry and complement factor B located upstream in the complement cascade is protective in lupus, but to date no one has examined the role of downstream complement protein, C5a in this disease. Our present data clearly demonstrate that treatment with the C5aRant, inhibiting C5a/C5aR signaling results in significant and substantial decreases in brain pathology in MRL/lpr, lupus mouse model, thereby leaving the upstream potentially protective complement activation events intact. Our results provide novel evidence for a relationship between complement activation, inflammation and neuronal viability in lupus brain.
Complement activation product, C5a, is thought to play a role in the pathogenesis of numerous neurological diseases, although its precise role remains an enigma. Transcriptional and translational analysis revealed up-regulation of C5aRs on CNS cells in CNS lupus. C5aR expression is increased on cortical endothelia (van Beek et al., 2003), neurons and astrocytes (Gasque et al., 1997) during inflammation. The dual role of C5 in increasing inflammation (Weerth et al., 2003) and reducing apoptosis (Niculescu et al., 2004a), makes it important to decipher its role in CNS lupus. In this study we utilized a C5aR antagonist to study the role of C5a in CNS lupus. C5a dependent increase in the expression of the proinflammatory cytokines, ICAM-1, MIP-2, TNF-α and iNOS were observed in lupus brain. A similarity between the human disease and the mouse model is the increased ICAM-1 expression and a statistically significant positive correlation between ICAM-1 levels and SLE disease activity index (SLEDAI) score (Baraczka et al., 2001; Norman et al., 2008). In line with the fact that ICAM-1 recruits cells into tissues undergoing inflammatory responses, we observed increased infiltration of neutrophils into MRL/lpr brain. Infiltrating cells along with resident cells could result in complement activation and generation of proinflammatory molecules in the tissues that could cause the cells to apoptose. MRL/lpr mice treated with C5aRant had significantly reduced neutrophil infiltration and associated apoptosis. Both these events could be partially caused by other factors such as C3a, generated by complement activation and increased during inflammation in ischemia, sepsis and CNS lupus (Gasque et al., 1998; Nadeau and Rivest, 2001; Jacob et al., 2009).
Protein kinase cascades are emerging as important modulators of the apoptotic response, by phosphorylating apoptotic proteins or regulating the transcription of pro- and anti-apoptotic genes (Tournier et al., 2000; Kuan et al., 2003). In lupus, a number of protein kinases could be activated and cause apoptosis. Altered phosphorylation of p-Akt, p-Erk, p-JNK and pSTAT1 in our studies suggest that in CNS lupus, apoptosis could occur through these signaling pathways. Since inhibition of C5aR allows MAC complex formation to continue unhindered, the Akt activation observed could be induced by the MAC as shown earlier (Soane et al., 2001; Fosbrink et al., 2006). Phosphorylation of STAT1 occurs through Erk and JNK in response to stress signals and inflammation (Zykova et al., 2005). All these proteins interface with each other and downstream targets to regulate the apoptotic machinery. Apoptosis could occur through caspase-dependent and independent mechanisms, since caspase 3 activity is increased in CNS lupus. Since inhibition of C5aR reduced the changes in expression and activity of these signaling proteins, these signaling pathways are at least partially regulated by C5a/C5aR signaling in CNS lupus. However, further studies are necessary to determine the role and contribution of each of these molecules in CNS lupus.
Since the effect of C5aR inhibition was hypothesized to reduce inflammation globally in brain in CNS lupus, the cellular environment and mechanism by which this occurs remain to be unequivocally established, particularly since C5a receptors are expressed on other CNS cells such as glia as well (Gasque et al., 1998). In vitro studies using primary cultures of neurons indicate C5aR up-regulation on these cells when treated with lupus serum making them more susceptible to C5a. Lupus serum caused apoptosis of neuronal cells in culture, which was significantly reduced by C5aR inhibition, indicating that C5a caused neuronal apoptosis through C5a/C5aR signaling. In lupus, it is possible that blood–brain barrier disruption (Abbott et al., 2003; Alexander et al., 2003; Diamond et al., 2006) could result in accumulation of factors not normally present in the CNS which may subsequently contribute to neuronal apoptosis. Neuronal C5aR activation has been suggested to be involved in the induction of neuronal apoptosis (Guo et al., 2004), although this remains controversial. Therefore, further experimentation is required to define the precise sequence of events as to how C5a causes the pathology in lupus brain. However, our results in this study provide compelling evidence that C5a plays a crucial proinflammatory role in this model.
In summary, these studies provide the first evidence of a neuroprotective role for C5aR antagonist in the rodent lupus model, MRL/lpr mice. The therapeutic effects of C5aR antagonists in this model suggest that neuroinflammation in the form of complement activation and C5a generation plays a deleterious role in CNS lupus, and indicates a potential future role in the treatment of a variety of human neurodegenerative diseases.
Acknowledgements
This work was supported by National Institutes of Health Grant R01DK055357 (to RJQ) and American Heart Association Grant No. 0855717G (to JRB).
Abbreviations
- SLE
System lupus erythematosus
- IC
Immune complex
- qRT-PCR
Quantitative RT-PCR
- C5aRant
Antagonist of C5aR
- PKB
Protein kinase B
- CNS
Central nervous system
- MAC
Membrane attack complex
- Crry
CR1-related protein y
- CSF
Cerebrospinal fluid
- MRL/lpr
MRL/MpJ-Tnfrsf6lpr/lpr
- MRL+/+
MRL/MpJ-Tnfrsf6+/+
- ERK
Extracellular signal-regulated kinase
- JNK
c-Jun N-terminal kinase
References
- Abbott NJ, Mendonca LL, Dolman DE. The blood-brain barrier in systemic lupus erythematosus. Lupus. 2003;12:908–915. doi: 10.1191/0961203303lu501oa. [DOI] [PubMed] [Google Scholar]
- Abramson S, Belmont HM, Hopkins P, Buyon J, Winchester R, Weissmann G. Complement activation and vascular injury in systemic lupus erythematosus. J. Rheumatol. Suppl. 1987;14 Suppl 13:43–46. [PubMed] [Google Scholar]
- Adachi M, Watanabe-Fukunaga R, Nagata S. Aberrant transcription caused by the insertion of an early transposable element in an intron of the Fas antigen gene of lpr mice. Proc. Natl. Acad. Sci. U. S. A. 1993;90:1756–1760. doi: 10.1073/pnas.90.5.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander JJ, Quigg RJ. Systemic lupus erythematosus and the brain: what mice are telling us. Neurochem. Int. 2007;50:5–11. doi: 10.1016/j.neuint.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Alexander JJ, Bao L, Jacob A, Kraus DM, Holers VM, Quigg RJ. Administration of the soluble complement inhibitor, Crry-Ig, reduces inflammation and aquaporin 4 expression in lupus cerebritis. Biochim. Biophys. Acta. 2003;1639:169–176. doi: 10.1016/j.bbadis.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Alexander JJ, Jacob A, Bao L, MacDonald RL, Quigg RJ. Complement-dependent apoptosis and inflammatory gene changes in murine lupus cerebritis. J. Immunol. 2005a;175:8312–8319. doi: 10.4049/jimmunol.175.12.8312. [DOI] [PubMed] [Google Scholar]
- Alexander JJ, Zwingmann C, Quigg R. MRL/lpr mice have alterations in brain metabolism as shown with [(1)H-(13)C] NMR spectroscopy. Neurochem. Int. 2005b;47:143–151. doi: 10.1016/j.neuint.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Alexander JJ, Jacob A, Vezina P, Sekine H, Gilkeson GS, Quigg RJ. Absence of functional alternative complement pathway alleviates lupus cerebritis. Eur. J. Immunol. 2007;37:1691–1701. doi: 10.1002/eji.200636638. [DOI] [PubMed] [Google Scholar]
- Alexander JJ, Anderson AJ, Barnum SR, Stevens B, Tenner AJ. The complement cascade: Yin-Yang in neuroinflammation--neuro-protection and -degeneration. J. Neurochem. 2008a;107:1169–1187. doi: 10.1111/j.1471-4159.2008.05668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander JJ, Jacob A, Cunningham P, Hensley L, Quigg RJ. TNF is a key mediator of septic encephalopathy acting through its receptor, TNF receptor-1. Neurochem. Int. 2008b;52:447–456. doi: 10.1016/j.neuint.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora V, Verma J, Dutta R, Marwah V, Kumar A, Das N. Reduced complement receptor 1 (CR1, CD35) transcription in systemic lupus erythematosus. Mol. Immunol. 2004;41:449–456. doi: 10.1016/j.molimm.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Ballok DA, Millward JM, Sakic B. Neurodegeneration in autoimmune MRL-lpr mice as revealed by Fluoro Jade B staining. Brain Res. 2003;964:200–210. doi: 10.1016/s0006-8993(02)03980-x. [DOI] [PubMed] [Google Scholar]
- Bao L, Osawe I, Puri T, Lambris JD, Haas M, Quigg RJ. C5a promotes development of experimental lupus nephritis which can be blocked with a specific receptor antagonist. Eur. J. Immunol. 2005a;35:2496–2506. doi: 10.1002/eji.200526327. [DOI] [PubMed] [Google Scholar]
- Bao L, Osawe I, Puri T, Lambris JD, Haas M, Quigg RJ. C5a promotes development of experimental lupus nephritis which can be blocked with a specific receptor antagonist. Eur. J. Immunol. 2005b;35:2496–2506. doi: 10.1002/eji.200526327. [DOI] [PubMed] [Google Scholar]
- Baraczka K, Nekam K, Pozsonyi T, Jakab L, Szongoth M, Sesztak M. Concentration of soluble adhesion molecules (sVCAM-1, sICAM-1 and sL-selectin) in the cerebrospinal fluid and serum of patients with multiple sclerosis and systemic lupus erythematosus with central nervous involvement. Neuroimmunomodulation. 2001;9:49–54. doi: 10.1159/000049007. [DOI] [PubMed] [Google Scholar]
- Barnum SR. Complement biosynthesis in the central nervous system. Crit. Rev. Oral Biol. Med. 1995;6:132–146. doi: 10.1177/10454411950060020301. [DOI] [PubMed] [Google Scholar]
- Belmont HM, Hopkins P, Edelson HS, Kaplan HB, Ludewig R, Weissmann G, Abramson S. Complement activation during systemic lupus erythematosus. C3a and C5a anaphylatoxins circulate during exacerbations of disease. Arthritis Rheum. 1986;29:1085–1089. doi: 10.1002/art.1780290905. [DOI] [PubMed] [Google Scholar]
- Brey RL, Sakic B, Szechtman H, Denburg JA. Animal models for nervous system disease in systemic lupus erythematosus. Ann. N. Y. Acad. Sci. 1997;823:97–106. doi: 10.1111/j.1749-6632.1997.tb48382.x. [DOI] [PubMed] [Google Scholar]
- Carroll MC, Fischer MB. Complement and the immune response. Curr. Opin. Immunol. 1997;9:64–69. doi: 10.1016/s0952-7915(97)80160-4. [DOI] [PubMed] [Google Scholar]
- Diamond B, Volpe B. On the track of neuropsychiatric lupus. Arthritis Rheum. 2003;48:2710–2712. doi: 10.1002/art.11278. [DOI] [PubMed] [Google Scholar]
- Diamond B, Kowal C, Huerta PT, Aranow C, Mackay M, DeGiorgio LA, Lee J, Triantafyllopoulou A, Cohen-Solal J, Volpe BT. Immunity and acquired alterations in cognition and emotion: lessons from SLE. Adv. Immunol. 2006;89:289–320. doi: 10.1016/S0065-2776(05)89007-8. [DOI] [PubMed] [Google Scholar]
- Fonseca MI, Ager RR, Chu SH, Yazan O, Sanderson SD, LaFerla FM, Taylor SM, Woodruff TM, Tenner AJ. Treatment with a C5aR antagonist decreases pathology and enhances behavioral performance in murine models of Alzheimer's disease. J. Immunol. 2009;183:1375–1383. doi: 10.4049/jimmunol.0901005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosbrink M, Niculescu F, Rus V, Shin ML, Rus H. C5b-9-induced endothelial cell proliferation and migration are dependent on Akt inactivation of forkhead transcription factor FOXO1. J. Biol. Chem. 2006;281:19009–19018. doi: 10.1074/jbc.M602055200. [DOI] [PubMed] [Google Scholar]
- Gabai VL, Meriin AB, Yaglom JA, Volloch VZ, Sherman MY. Role of Hsp70 in regulation of stress-kinase JNK: implications in apoptosis and aging. FEBS Lett. 1998;438:1–4. doi: 10.1016/s0014-5793(98)01242-3. [DOI] [PubMed] [Google Scholar]
- Gasque P, Singhrao SK, Neal JW, Gotze O, Morgan BP. Expression of the receptor for complement C5a (CD88) is up-regulated on reactive astrocytes, microglia, and endothelial cells in the inflamed human central nervous system. Am. J. Pathol. 1997;150:31–41. [PMC free article] [PubMed] [Google Scholar]
- Gasque P, Singhrao SK, Neal JW, Wang P, Sayah S, Fontaine M, Morgan BP. The receptor for complement anaphylatoxin C3a is expressed by myeloid cells and nonmyeloid cells in inflamed human central nervous system: analysis in multiple sclerosis and bacterial meningitis. J. Immunol. 1998;160:3543–3554. [PubMed] [Google Scholar]
- Guo RF, Riedemann NC, Ward PA. Role of C5a-C5aR interaction in sepsis. Shock. 2004;21:1–7. doi: 10.1097/01.shk.0000105502.75189.5e. [DOI] [PubMed] [Google Scholar]
- Hill HD, Straka JG. Protein determination using bicinchoninic acid in the presence of sulfhydryl reagents. Anal. Biochem. 1988;170:203–208. doi: 10.1016/0003-2697(88)90109-1. [DOI] [PubMed] [Google Scholar]
- Huber-Lang MS, Riedeman NC, Sarma JV, Younkin EM, McGuire SR, Laudes IJ, Lu KT, Guo RF, Neff TA, Padgaonkar VA, Lambris JD, Spruce L, Mastellos D, Zetoune FS, Ward PA. Protection of innate immunity by C5aR antagonist in septic mice. FASEB J. 2002;16:1567–1574. doi: 10.1096/fj.02-0209com. [DOI] [PubMed] [Google Scholar]
- Jacob A, Hensley LK, Safratowich BD, Quigg RJ, Alexander JJ. The role of the complement cascade in endotoxin-induced septic encephalopathy. Lab Invest. 2007;87:1186–1194. doi: 10.1038/labinvest.3700686. [DOI] [PubMed] [Google Scholar]
- Jacob A, Bao L, Brorson J, Quigg RJ, Alexander JJ. C3aR inhibition reduces neurodegeneration in experimental lupus. Lupus. 2009;19:73–82. doi: 10.1177/0961203309348978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston RB., Jr The complement system in host defense and inflammation: the cutting edges of a double edged sword. Pediatr. Infect. Dis. J. 1993;12:933–941. doi: 10.1097/00006454-199311000-00009. [DOI] [PubMed] [Google Scholar]
- Kuan CY, Whitmarsh AJ, Yang DD, Liao G, Schloemer AJ, Dong C, Bao J, Banasiak KJ, Haddad GG, Flavell RA, Davis RJ, Rakic P. A critical role of neural-specific JNK3 for ischemic apoptosis. Proc. Natl. Acad. Sci. U. S. A. 2003;100:15184–15189. doi: 10.1073/pnas.2336254100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langkabel P, Zwirner J, Oppermann M. Ligand-induced phosphorylation of anaphylatoxin receptors C3aR and C5aR is mediated by ″G protein-coupled receptor kinases. Eur. J. Immunol. 1999;29:3035–3046. doi: 10.1002/(SICI)1521-4141(199909)29:09<3035::AID-IMMU3035>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Li D, Marks JD, Schumacker PT, Young RM, Brorson JR. Physiological hypoxia promotes survival of cultured cortical neurons. Eur. J. Neurosci. 2005;22:1319–1326. doi: 10.1111/j.1460-9568.2005.04335.x. [DOI] [PubMed] [Google Scholar]
- Morgan BP, Gasque P. Expression of complement in the brain: role in health and disease. Immunol. Today. 1996;17:461–466. doi: 10.1016/0167-5699(96)20028-f. [DOI] [PubMed] [Google Scholar]
- Muller-Eberhard HJ. Molecular organization and function of the complement system. Annu. Rev. Biochem. 1988;57:321–347. doi: 10.1146/annurev.bi.57.070188.001541. [DOI] [PubMed] [Google Scholar]
- Nadeau S, Rivest S. The complement system is an integrated part of the natural innate immune response in the brain. FASEB J. 2001;15:1410–1412. doi: 10.1096/fj.00-0709fje. [DOI] [PubMed] [Google Scholar]
- Niculescu T, Weerth S, Soane L, Niculescu F, Rus V, Raine CS, Shin ML, Rus H. Effects of membrane attack complex of complement on apoptosis in experimental autoimmune encephalomyelitis. Ann. N. Y. Acad. Sci. 2003;1010:530–533. doi: 10.1196/annals.1299.098. [DOI] [PubMed] [Google Scholar]
- Niculescu T, Weerth S, Niculescu F, Cudrici C, Rus V, Raine CS, Shin ML, Rus H. Effects of complement C5 on apoptosis in experimental autoimmune encephalomyelitis. J. Immunol. 2004a;172:5702–5706. doi: 10.4049/jimmunol.172.9.5702. [DOI] [PubMed] [Google Scholar]
- Niculescu T, Weerth S, Niculescu F, Cudrici C, Rus V, Raine CS, Shin ML, Rus H. Effects of complement C5 on apoptosis in experimental autoimmune encephalomyelitis. J. Immunol. 2004b;172:5702–5706. doi: 10.4049/jimmunol.172.9.5702. [DOI] [PubMed] [Google Scholar]
- Norman MU, James WG, Hickey MJ. Differential roles of ICAM-1 and VCAM-1 in leukocyte-endothelial cell interactions in skin and brain of MRL/faslpr mice. J. Leukoc. Biol. 2008;84:68–76. doi: 10.1189/jlb.1107796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakic B, Szechtman H, Denburg JA. Neurobehavioral alterations in autoimmune mice. Neurosci. Biobehav. Rev. 1997;21:327–340. doi: 10.1016/s0149-7634(96)00018-8. [DOI] [PubMed] [Google Scholar]
- Sakic B, Maric I, Koeberle PD, Millward JM, Szechtman H, Maric D, Denburg JA. Increased TUNEL staining in brains of autoimmune Fas-deficient mice. J. Neuroimmunol. 2000;104:147–154. doi: 10.1016/s0165-5728(99)00277-5. [DOI] [PubMed] [Google Scholar]
- Sayah S, Patte C, Gasque P, Chan P, Ischenko A, Vaudry H, Fontaine M. Characterization of rat C5a anaphylatoxin receptor (C5aR): cloning of rat C5aR cDNA and study of C5aR expression by rat astrocytes. Brain Res. Mol. Brain Res. 1997;48:215–222. doi: 10.1016/s0169-328x(97)00094-6. [DOI] [PubMed] [Google Scholar]
- Soane L, Cho HJ, Niculescu F, Rus H, Shin ML. C5b-9 terminal complement complex protects oligodendrocytes from death by regulating Bad through phosphatidylinositol 3-kinase/Akt pathway. J. Immunol. 2001;167:2305–2311. doi: 10.4049/jimmunol.167.4.2305. [DOI] [PubMed] [Google Scholar]
- Tournier C, Hess P, Yang DD, Xu J, Turner TK, Nimnual A, Bar-Sagi D, Jones SN, Flavell RA, Davis RJ. Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science. 2000;288:870–874. doi: 10.1126/science.288.5467.870. [DOI] [PubMed] [Google Scholar]
- van Beek J, Elward K, Gasque P. Activation of complement in the central nervous system: roles in neurodegeneration and neuroprotection. Ann. N. Y. Acad. Sci. 2003;992:56–71. doi: 10.1111/j.1749-6632.2003.tb03138.x. [DOI] [PubMed] [Google Scholar]
- Wang J, Ouyang C, Chen X, Fu B, Lu Y, Hong Q. Effect of Jak2 kinase inhibition on Stat1 and Stat3 activation and apoptosis of tubular epithelial cells induced by ATP depletion/recovery. J. Nephrol. 2008;21:919–923. [PubMed] [Google Scholar]
- Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356:314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- Weerth SH, Rus H, Shin ML, Raine CS. Complement C5 in experimental autoimmune encephalomyelitis (EAE) facilitates remyelination and prevents gliosis. Am. J. Pathol. 2003;163:1069–1080. doi: 10.1016/S0002-9440(10)63466-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesemann DR, Qin H, Kokorina N, Benveniste EN. TRADD interacts with STAT1-alpha and influences interferon-gamma signaling. Nat. Immunol. 2004;5:199–207. doi: 10.1038/ni1025. [DOI] [PubMed] [Google Scholar]
- Zipfel PF. Complement and immune defense: from innate immunity to human diseases. Immunol. Lett. 2009;126:1–7. doi: 10.1016/j.imlet.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Zykova TA, Zhang Y, Zhu F, Bode AM, Dong Z. The signal transduction networks required for phosphorylation of STAT1 at Ser727 in mouse epidermal JB6 cells in the UVB response and inhibitory mechanisms of tea polyphenols. Carcinogenesis. 2005;26:331–342. doi: 10.1093/carcin/bgh334. [DOI] [PubMed] [Google Scholar]