Abstract
The integration of multisensory information takes place in the optic tectum where visual and auditory/mechanosensory inputs converge and regulate motor outputs. The circuits which integrate multisensory information are poorly understood. In an effort to identify the basic components of a multisensory integrative circuit, we determined the projections of the mechanosensory input from the periphery to the optic tectum and compared their distribution to the retinotectal inputs in Xenopus laevis tadpoles using dye-labelling methods. The peripheral ganglia of the lateral line system project to the ipsilateral hindbrain and the axons representing mechanosensory inputs along the anterior/posterior body axis are mapped along the ventrodorsal axis in the axon tract in the dorsal column of the hindbrain. Hindbrain neurons project axons to the contralateral optic tectum. The neurons from anterior and posterior hindbrain regions project axons to the dorsal and ventral tectum, respectively. While the retinotectal axons project to a superficial lamina in the tectal neuropil, the hindbrain axons project to a deep neuropil layer. Calcium imaging showed that multimodal inputs converge on tectal neurons. The layer specific projections of the hindbrain and retinal axons suggest a functional segregation of sensory inputs to proximal and distal tectal cell dendrites, respectively.
Keywords: optic tectum, hindbrain, mechanosensory, Xenopus, multimodal
Introduction
The structural organization of multiple convergent sensory modalities in the brain is an important determinant in identifying mechanisms of multimodal integration in neural circuits. The superior colliculus (or optic tectum) in the midbrain of vertebrates integrates multimodal sensory information and generates motor responses. In the superior colliculus/optic tectum sensory inputs are topographically organized and aligned with each other and with the motor outputs (Knudsen, 2002; May, 2006; Stein et al., 2009). Two interesting features of the superior colliculus circuit are that multisensory inputs interact nonlinearly to produce more reliable motor responses than unimodal inputs (Rowland et al., 2007; Gingras et al., 2009) and that during development, the different sensory modalities interact in an activity-dependent manner to establish or organize the functional integrative circuit (Stein, 1978; Knudsen, 2002; Wallace et al., 2004). Compared with birds and mammals, non-mammalian vertebrates, including fish and frog tadpoles, accomplish multisensory integrative tasks with relatively small numbers of cells (Precht et al., 1971; Vanegas et al., 1971; Szekely et al., 1973; Matsumoto and Bando, 1980), suggesting that the identification of the components of the integrative circuitry in these animals may reveal the basic circuit units required for the integration of multiple modalities.
In birds and mammals, the superior colliculus is compartmentalized into multiple layers, composed of cell bodies, fibers and neuropil, which are thought to reflect segregation of task-related sensory and motor processing (Huerta and Harting, 1984; Harting and Van Lieshout, 1991; Harting et al., 1992; Stein et al., 2009). By contrast, the structures devoted to multi-sensory integration in amphibian tadpoles are much simpler. The optic tectum and the torus semicircularis, which is homologous to the inferior colliculus of mammals (Edwards and Kelley, 2001), are not yet segregated into different brain regions in amphibian tadpoles. At early tadpole stages, the optic tectum has one major cell body layer and one neuropil layer. Furthermore, dendrites of some tectal neurons span entire the neuropil layer (Lazar, 1973; Wu and Cline, 2003). These data suggest that multiple sensory modalities terminate in the single tectal neuropil layer and that individual tectal neurons may receive and process inputs from multiple modalities. We examined the convergence of mechanosensory and visual inputs to the optic tectum to determine whether the connections from different sensory modalities form distinct projections in the tadpole optic tectum.
A second interest in determining the components of a relatively simple circuit devoted to integrating multimodal inputs concerns the laterality of inputs and information processing. In Xenopus tadpoles, the eyes are located on the sides of the head and each retina project input to the contralateral (monocular) optic tectum. Adult frogs acquire binocular visual information in the tectum as a result of the metamorphic development of an isthmotectal circuit which integrates inputs from both eyes (Udin, 1983; Udin and Scherer, 1990; Rybicka and Udin, 2005). Sound localization requires the comparison of inputs from both ears. In birds and mammals, the bilateral comparison of auditory inputs occurs in the hindbrain (Caird and Klinke, 1983; Harnischfeger et al., 1985; Tsuchitani, 1988), and the signal is transferred to the midbrain for the integration with other modalities and control of motor outputs (Huerta and Harting, 1984; Knudsen, 2002; May, 2006). Although considerable effort has been devoted to identifying the circuit underlying visual and mechanosensory input-mediated escape behavior in non-mammalian vertebrates, particularly fish (Faber et al., 1989; O'Malley et al., 1996) (Fetcho et al., 2008) (Eaton et al., 1991) (Orger et al., 2008) (Douglass et al., 2008) (Sillar and Robertson, 2009), the central projections of mechanosensory inputs to the hindbrain or optic tectum of non-mammalian vertebrates have not been described.
We determined the anatomical circuit from the mechanosensory ganglia through the hindbrain to the optic tectum and compared the distribution of mechanosensory inputs to visual inputs in the optic tectum. The mechanosensory inputs project to the ipsilateral hindbrain and hindbrain neurons located at the target of these axons project their axons directly to the contralateral tectum. We found that the axons of the mechanosensory hindbrain inputs and the retinal inputs form distinct laminae in the tectal neuropil. Calcium imaging data show that tectal neurons respond to converging visual and mechanosensory inputs. We found that the anterior/posterior body axis is mapped along the dorsoventral axis in the axon tract in the hindbrain and that axons from the anterior hindbrain project to the dorsal tectum while neurons in the posterior hindbrain projected to the ventral tectum. We discuss the data in the context of a relatively simple circuit capable of multimodal sensory integration.
Results
Unilateral projection of the hindbrain axons to the tectum
In amphibians, the optic tectum receives input from the retina, as well as other sensory modalities, including mechanosensory and auditory inputs from the hindbrain. To examine the sensory inputs and their organization in Xenopus tadpoles, we used anterograde and retrograde labelling techniques. Retinal ganglion cells (RGCs) project axons to the contralateral tectum (Fig.1a) and generate a monocular topographic projection. To identify mechanosensory (including auditory) inputs from the hindbrain to the tectum, we targeted DiI labelling to the neuropil layer of the optic tectum of stage 47 tadpoles. Retrograde DiI labelling revealed cell bodies exclusively in the contralateral hindbrain (Fig. 1b). To confirm the contralateral labelling pattern, we labeled cells on one side of the hindbrain by electroporating stage 43 tadpoles with plasmids encoding pCMV-EGFP (Fig. 1c). At stage 47 we screened for animals with unilateral EGFP expression in the hindbrain. The hindbrain neurons extend axons across the ventral midline and turn anteriorly, extending towards the midbrain. The axons project through the tegmentum, turn dorsally and terminate in the contralateral optic tectum (Fig. 1c). These results indicate that hindbrain neurons project primarily to the contralateral optic tectum in Xenopus tadpoles, in contrast to the ventral cochlear nucleus axons in mammals that project to both sides of the tectum (Moore, 1991).
Figure 1. Convergent Projections of retinal and mechanossensory inputs to the optic tectum.
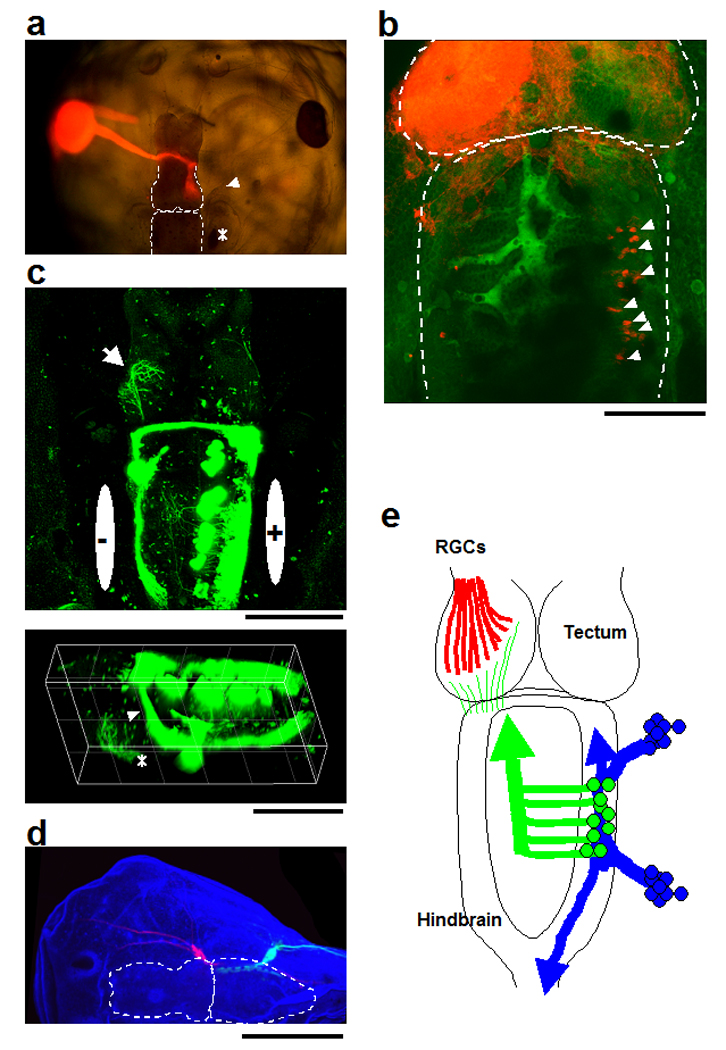
a. Photograph of the tadpole head in which the left eye was labeled with DiI. (red). Retinal gangion cell (RGC) axons project to the contralateral optic tectum. The midbrain and the hindbrain are outlined in dotted lines. The otic vesicle is indicated by the asterisk. Anterior mechanosensory ganglia are marked by the arrowhead. b. Retrograde labeling of the hindbrain neurons that project to the tectum. The left tectum was labeled with DiI (red). The retrogradely labeled hindbrain neurons (arrowheads) are detected contralateral to the labeled tectum. Hindbrain is outlined in dotted lines. The weak DiI signal on the right tectum is in the skin after the labelling. The tectum itself is not labelled. c. Axons of the hindbrain neurons project to the contralateral optic tectum (arrow). Upper: Dorsal view, Lower: dorso-lateral view. Neurons in the right side of the hindbrain were electroporated with pCMV-EGFP. The polarity of the electrodes for unilateral electroporation is indicated. Cerebellar neurons, which also express EGFP, extend axons through a dorsal commissure to the contralateral hindbrain (arrowhead). d. Mechanosensory neurons project to the hindbrain. The ganglia of the anterior lateral line system (trigeminal nerve) were labeled with DiI (red) and the posterior lateral line ganglia were labeled with DiD (blue). The sensory processes projecting to the skin were also labeled. e: Schematic summary of the sensory axon projections to the optic tectum. All images were from stage 47 tadpoles (a–d). Tissues were labeled with NBD-ceramide (b: green, d: blue). Scale bars b, c: 200µm, d: 400µm
Mechanosensory ganglia clustered at the anterior and the posterior sides of the otic vesicle are called the anterior and posterior lateral line systems, respectively (Ghysen and Dambly-Chaudiere, 2004) and include ganglia of the vestibular organ and auditory systems. We labeled the anterior ganglia with DiI and the posterior ganglia with DiD (Fig. 1d). The axonal projections of the mechanosensory ganglia were ipsilateral, similar to the projection of the auditory nerve to the cochlear ganglia in mammals.
Bimodal inputs to the tectum
To test whether tectal neurons respond to multimodal inputs, we determined whether somatic calcium levels change in response to visual and mechanical stimulation. The otic vesicle was vibrated with piezo plate at a frequency of 10 Hz. This stimulus was interleaved with a visual stimuli of 10 Hz full field flicker (Fig. 2a) for 5 trials. The calcium responses in tectal neurons were imaged with 2-photon microscopy (Fig. 2b). Subsets of the tectal cells showed stronger calcium responses to mechanical stimuli or visual stimuli or both (Fig. 2b, c). Since the retrograde labelling showed the dorso-lateral hindbrain neurons are the only hindbrain neurons projecting to the tectum (Fig. 1b), it is likely that these neurons mediate mechanosensory/auditory inputs to the tectum.
Figure 2. Calcium imaging of tectal cell responses to the multimodal inputs.
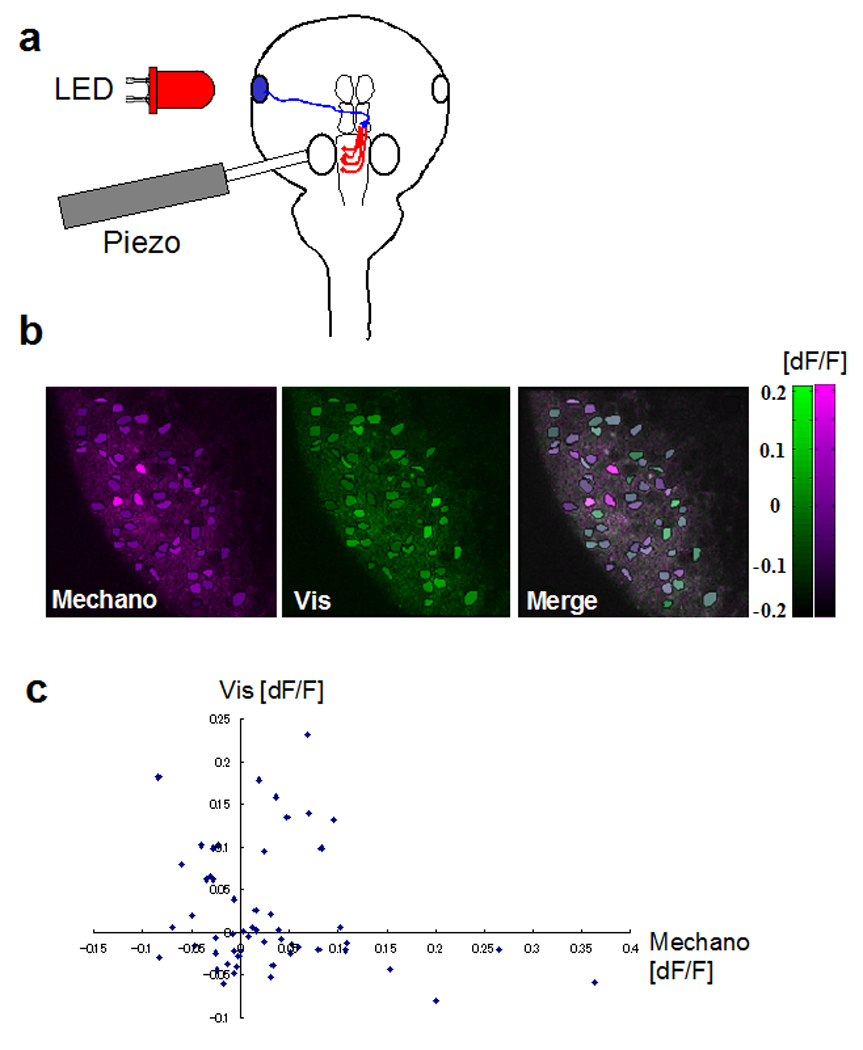
a. Visual and mechanical stimulation were applied by LED or a flexible plastic rod (10mm) attached to a piezo and placed on the otic vesicle. 10Hz vibration was applied for 1.5 seconds to the rod. For the visual stimulation, 10Hz flicker light was applied with an LED for 1.5 seconds. b. Responses (dF/F) for the mechanical (mechano) and the visual (Vis) stimulation. dF/F was calculated from the frames before and the after the stimulation. c. Plot of visual responses versus mechanosensory responses, where each dot is a cell. Cells respond to either or both stimuli and show differential responses to the two types of stimuli.
Layer specific axonal projection of hindbrain axons and retinal axons into the tectum
Tectal cells extend dendrites into the neuropil where they contact afferent inputs (Lazar, 1973; Wu and Cline, 2003). Although lamination of the tectal neuropil has been demonstrated in other species and in adult amphibians (Potter, 1972; Lazar, 1973; Gruberg and Udin, 1978; Masino and Grobstein, 1990; Yamagata et al., 2006; Xiao and Baier, 2007), lamination of the tectal neuropil has not been examined in tadpoles. Since the optic tectum integrates multiple sensory modalities, we were interested in determining how axonal inputs from different modalities are organized in the tectal neuropil. We double-labeled the hindbrain neurons and the retinal axons with DiI and DiD, respectively (Fig. 3a, b, lower). The labeling showed that while RGC axons enter the tectal neuropil structure from its dorsolateral pole and terminate in the intermediate layers of the tectum, hindbrain axons terminate in the layer closest to the cell bodies of the tectal neurons, corresponding to the proximal dendrite of the tectal cells (Fig. 3b upper, c). Consistent with the GFP labelling described above, the DiI labelling from the hindbrain showed that the hindbrain axons that project to the tectum come from tegmentum, which is located ventral to the tectum. The axons from the hindbrain neurons first extend along the ventralmost region of the brain to the anterior direction and cross the hindbrain-midbrain boundary. When the axons reach the tegmentum, they turn dorsally and enter the tectum at the rostrolateral pole (see also Fig. 7e). After entering the tectum, the axons turn to orient in the posterior direction and spread out to fill a deep layer of the neuropil (Fig. 3d). Therefore, the hindbrain axons and the RGC axons segregate into laminae in the tectal neuropil. We always observe a gap between the laminae of the axon projections (Fig. 3b, white bars), suggesting that there are additional afferent layers in the tectal neuropil.
Figure 3. Lamina specific projections of hindbrain axons and retinal axons in optic tectum.
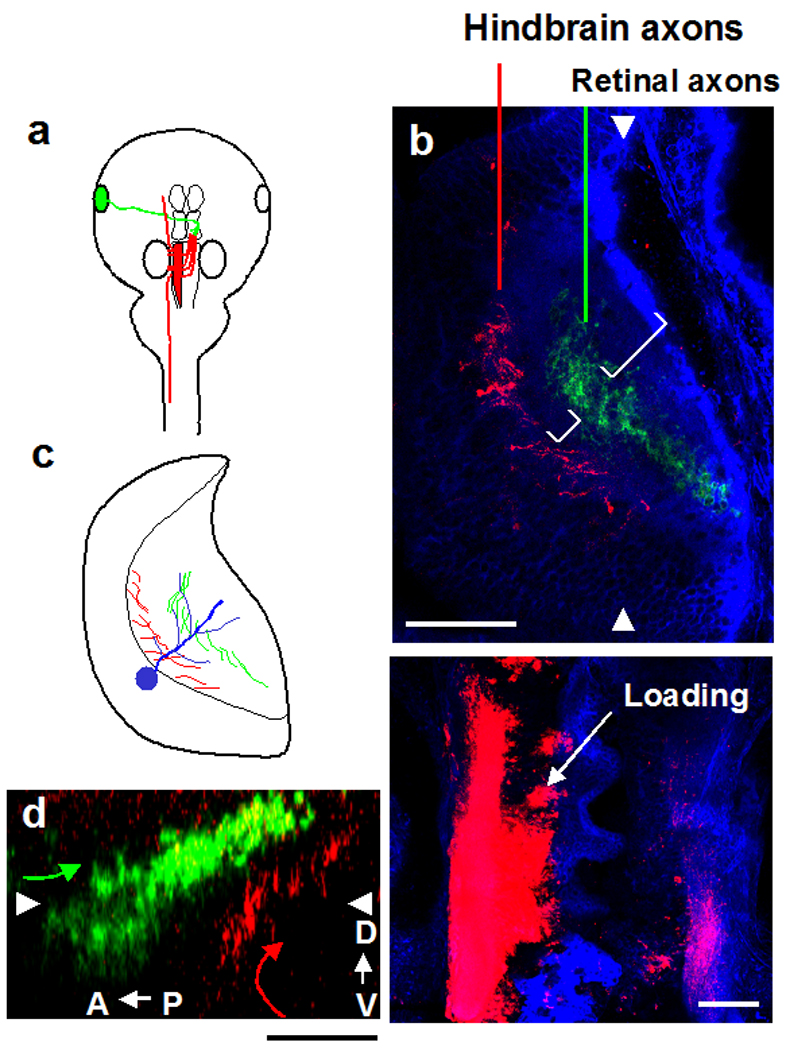
a. Labeling method. The right eye was labeled with DiD (green) and right side of the hindbrain was labeled with DiI (red). Retinal and hindbrain axons project to the left side of the tectum. b. Upper panel: RGC axons (green) and hindbrain axons (red) project to non-overlapping layers in the tectal neuropil. Single horizontal optical section 19.5µm from the dorsal surface of the tectum. Hindbrain axons project to the layer closest to the cell body layer of the tectum. The boundary between the neuropil and cell body layer is indicated by the dotted line. RGC axons project to a neuropil layer superficial to the hindbrain axons. Spaces between the laminae occupied by the retinal axons and hindbrain axons and superficial to the retinal axons are marked by brackets. Cell membranes were labeled with NBD-ceramide (blue). Arrowheads indicate the position of the sagital section shown in (d). Lower panel: DiI Injection site. The dorso-lateral region of the hindbrain is filled with DiI (red) to label most or all of the hindbrain projection neurons shown in Fig. 1b. c. Schematic drawing of the retinal and hindbrain axon projections in the tectum. Dendrites of tectal neurons (blue) penetrate the neuropil. Inputs from hindbrain (red) terminate on the proximal dendrite and the retinal inputs (green) terminate on more distal dendritic locations. d. Distribution of retinal and hindbrain axons in a sagital section through the tectum. Arrowheads indicate the position of the horizontal section shown in (b). Scale bar, b: 100µm, c: 50µm.
Figure 7. Regional projection of axons from hindbrain to tectum.
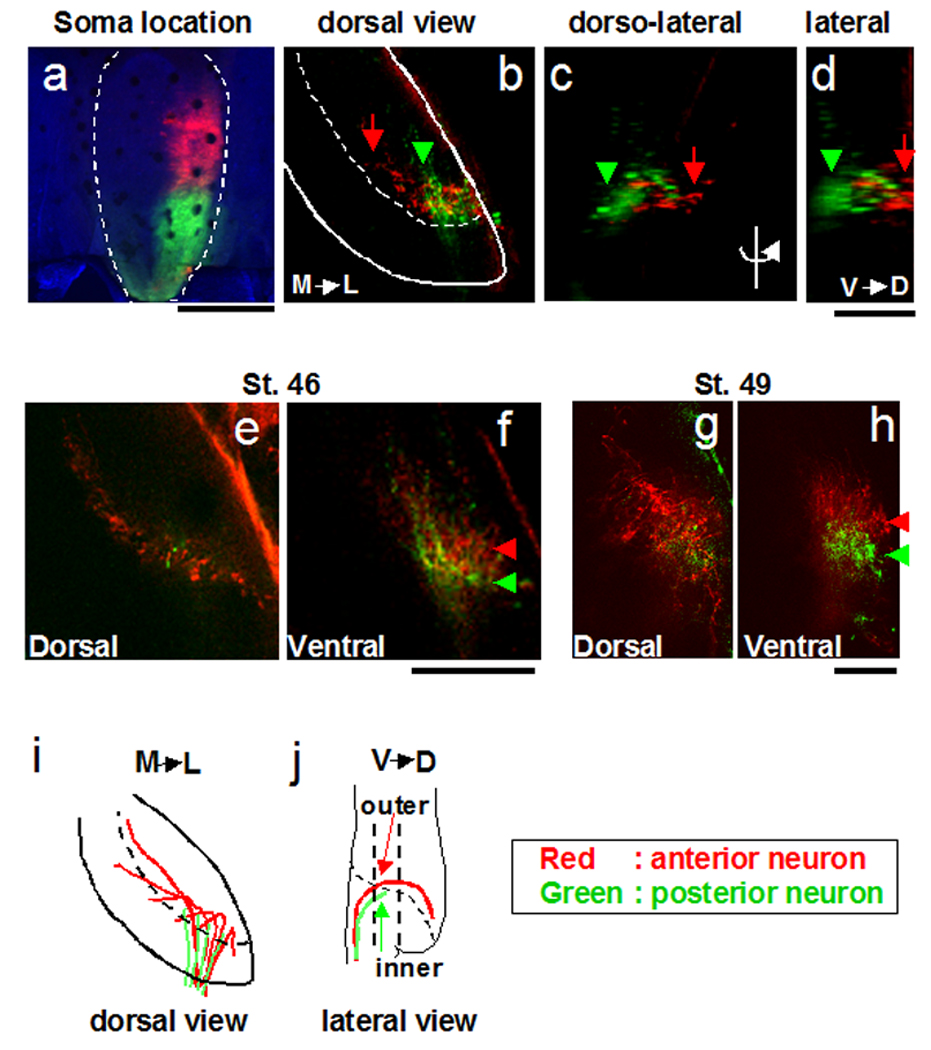
a. Anterior and posterior hindbrain (outlined) were labeled with DiI and DiD respectively. b–d. retrograde label in hindbrain axon terminals in the tectal neuropil. Red and green label show the axons from anterior and posterior hindbrain, respectively, in all panels. b: dorsal view (Tectum is outlined; dotted line shows the boundary of the neuropil and cell body layer) c: dorsolateral view d: lateral view. Axons from the anterior hindbrain projected to dorsal tectum (b–d: red arrows). Axons from the posterior hindbrain terminated in ventral tectum (b–d: green arrowheads). e–h: Horizontal optical section of the hindbrain axons deep (67µm, 110µm, 63µm or 94µm from the dorsal-most tip of the hindbrain axons in the tectal neuropil, respectively) at stage 46 or stage 49. The optical section was taken at the position of the straight dotted lines in j). At this position (f, h), axons from the anterior hindbrain (red) follow the pathway outside of the posterior hindbrain axons’ pathway. i, j. Schematic drawing of projections. See text for details. Scale bars a: 400µm, b–h: 100µm
Concomitant innervation of the optic tectum by retina and hindbrain axons
In birds and mammals, the formation of integrative multisensory circuits is an experience-dependent process that requires inputs from the multiple sensory modalities (Knudsen, 2002) (Wallace and Stein, 2007). In birds, the organization of the auditory map in the external nucleus of the inferior colliculus is instructed by topographically organized visual inputs from in the optic tectum (Knudsen, 2002). In mammals, aberrant sensory input activity during development resulted in disorganized multisensory integration (Wallace and Stein, 2007). These studies of inter-modal interactions of visual and auditory inputs prompted us to investigate whether visual and mechanosensory inputs innervate the tadpole optic tectum simultaneously during development. If the input from one modality develops earlier than the other, the organization of the delayed input could be instructed by the earlier input. To investigate whether the retinal and hindbrain inputs form in a sequential manner, we labelled the retina and hindbrain with DiD and DiI, respectively at stages 37–48. The innervation of the optic tectum by hindbrain neurons occurs simultaneously with RGC axon development (Fig. 4). The RGC axons first arrive at the anterior end of the tectum at stage 37 (not shown) and axon arbors are clearly seen in dorsal tectum at stage 40 (Fig. 4. a–b: green), consistent with previous electrophysiological recordings (Holt, 1983). At stage 40, a few hindbrain axons have reached the tectal neuropil and the arbors were not extensive (Fig. 4. a–b: red). At stage 43, RGC axon arbors spread along the entire length of the tectal neuropil. Likewise, the axon terminals of the hindbrain neurons occupy most of the area of the layer just superficial to the cell body layer (Fig. 4. c–d). At stage 48, the axonal projections from both RGCs and hindbrain are well developed (Fig. 4. e–f). This shows that the retinal and hindbrain innervation of the tectal neuropil develop concomitantly, suggesting that one sensory modality does not instruct the organization of the other, but rather that they may interact reciprocally during the development of the inputs.
Figure 4. Simultaneous innervation of the tectum by hindbrain axons and retinal axons.
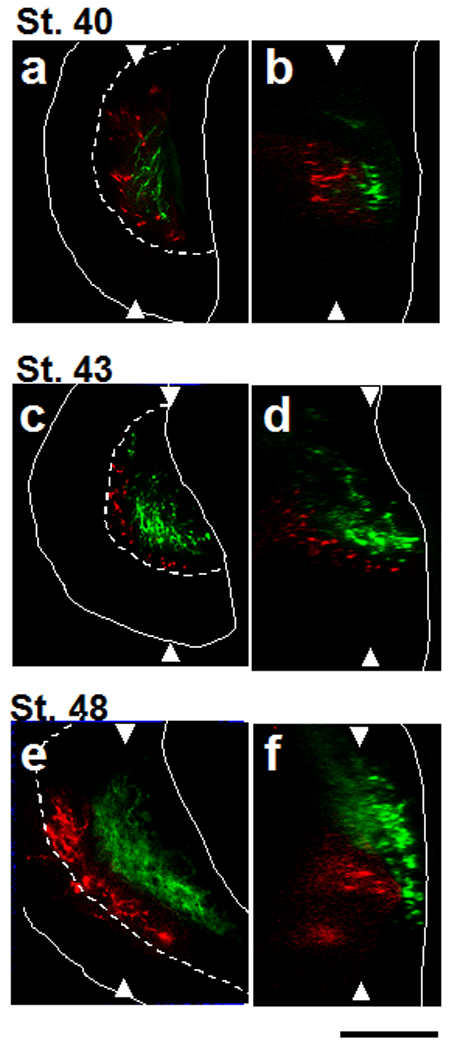
a, c, e: horizontal sections. b, d, f: vertical sections. For each pair of images, arrowheads indicate the plane of section in the neighboring image. a, b. Hindbrain axons (red) and RGC axons (green) both innervate dorsal tectum at stage 40. c, d. At stage 43, RGC and hindbrain axons elaborate in distinct laminae in the tectal neuropil. e, f. RGC and hindbrain axons are separated by a label-free zone. Dashed lines: boundaries between the neuropil and the cell body layers. Solid lines: Outline of the tecta. Scale bar, 100µm
Somatotopic organization of the mechanosensory input to the hindbrain
Mechanosensory inputs from the anterior and posterior lateral line ganglia, which are clusters of cranial ganglia also known as the trigeminal, facial and acoustic ganglia, project to the hindbrain (Wilson et al., 2007). To investigate the somatotopic arrangement of mechanosensory inputs to the hindbrain, we labelled the anterior and posterior lateral line ganglia with DiI and DiD, respectively. Mechanosensory neurons receive input from distinct receptive fields in the skin (Fig. 5a, b). The axons from the anterior and posterior ganglia enter the hindbrain from the anterior and the posterior sides of the otic vesicle respectively through the Vth and VII/VIIIth nerve bundles (Fig. 5c, asterisks). Once in the hindbrain the axon bundle from the anterior ganglia remains segregated from and is located more ventrally than the axon bundle from the posterior ganglion (Fig. 5d), consistent with reports from Zebrafish (Ghysen and Dambly-Chaudiere, 2004). To determine whether there is further somatotopic organization of mechanosensory inputs along the dorsoventral axis in the hindbrain, we labelled 2 ganglia within the same anterior cluster with DiD and DiI (Fig. 5e). Their projections label different receptive fields in the periphery; one located anterior to the other (Fig. 5b). We find that the axons of neurons with the anterior somatosensory receptive field were located ventral to the axons of neurons with the more posterior somatosensory receptive fields. These data indicate that mechanosensory information pertaining to the anterior/posterior body axis is transformed into the ventrodorsal axis in the organization of axons terminating in the dorsal column of the hindbrain. When Synaptophysin-CFP, the presynaptic marker was introduced by DNA electroporation in either anterior or posterior mechanosensory ganglia, synaptophysin-CFP puncta distributed in the axons along entire length of the hindbrain (Fig. 5g, h). This suggests that each hindbrain segment has the potential to process the mechanosensory information from the entire anterior/posterior body axis.
Figure 5. Conversion of the anterior posterior axis of mechanosensory input to a ventrodorsal axis of axon tracts within the hindbrain.
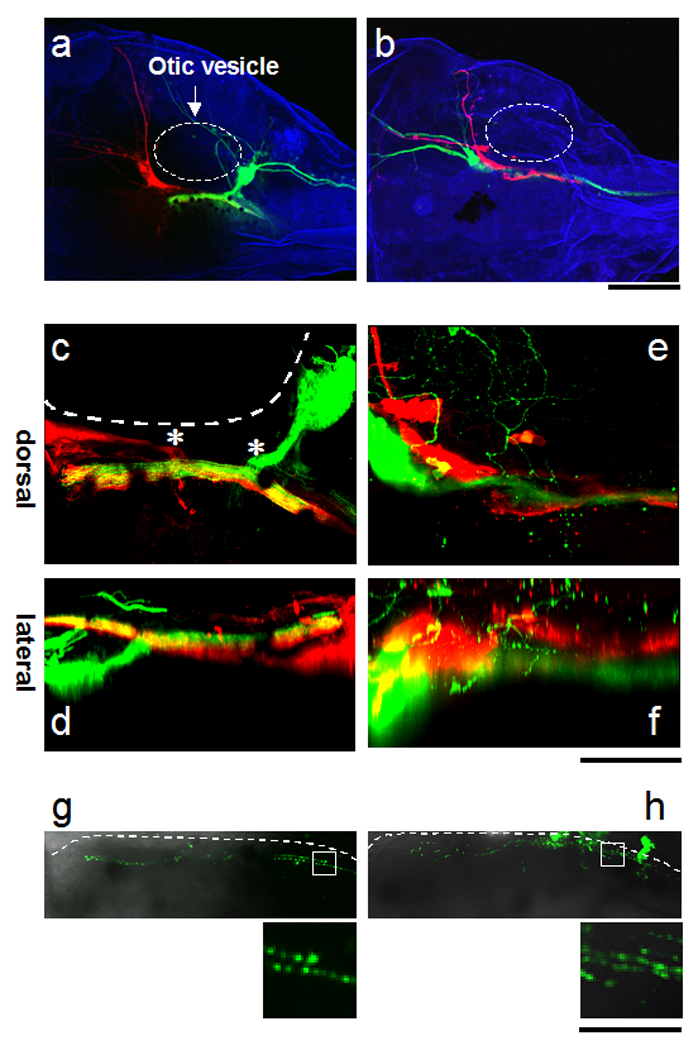
a,c,d. Distribution of axon projections from an anterior (red) and posterior (green) mechanosensory ganglia to the hindbrain. a. Label in the ganglia shows projections from the periphery and into the hindbrain. c, d Distribution of axons in the hindbrain from the dorsal (c) and lateral (d) views. The axons from the posterior ganglion are dorsal to the axons from the anterior ganglion. b, e, f. Distribution of axons from two ganglia located anterior to the otic vesicle b. Label in the 2 ganglia shows their spatial relation. e, f. The axons from the anterior ganglia (green) extend along tracts located ventral to the posteriorly-located ganglion (red) (e: dorsal view, f: side view). g, h. Synaptophysin-CFP was expressed in the anterior (g) and posterior ganglia (h) of stage 46 tadpoles. Lower panels show punctate labelling of synaptophysin-CFP at 2× magnification. Transmission images of the hindbrain are superimposed. Outline of the hindbrain is traced with the dotted lines. The images are projections of 50µm optical sections. Dotted line in a, b and c: otic vesicle. Anterior is to the left. Scale bars a, b: 400µm, c–f: 200µm, g, h: 200µm (upper), 100µm (lower).
Projection of the mechanosensory inputs to the hindbrain neurons that innervate the tectum
Experiments described above indicate that hindbrain neurons project to the optic tectum and that mechanosensory ganglia project to the hindbrain. Here, we double-labeled the mechanosensory ganglia in the periphery and the cell bodies of the hindbrain neurons that project to the tectum. The hindbrain neurons were labeled retrogradely by injecting DiI into the tectal neuropil. The axons of the mechanosensory neurons in peripheral ganglia were labeled anterogradely with DiD (Fig. 6a). Axons from the mechanosensory ganglia span the hindbrain longitudinally and extend along the entire length of the spinal cord (Fig. 6b, green), implying that the mechanosensory inputs modify motor outputs throughout the length of the spinal cord. The cell bodies of the hindbrain neurons and the axon bundle of the mechanosensory neurons were located in the dorsal column in the hindbrain and were apposed each other (Fig. 6b: 3D view, Fig. 6c: sagital optical section), and projected processes into the axon bundle (Fig. 6c arrows), suggesting that mechanosensory information is transferred to the tectum through one hindbrain connection, similar to the connection from dorsal cochlear nucleus to inferior colliculus in birds and mammals, which mainly processes mono-aural information (Moore, 1991).
Figure 6. Axonal projections of the mechanosensory neurons to the hindbrain neurons.
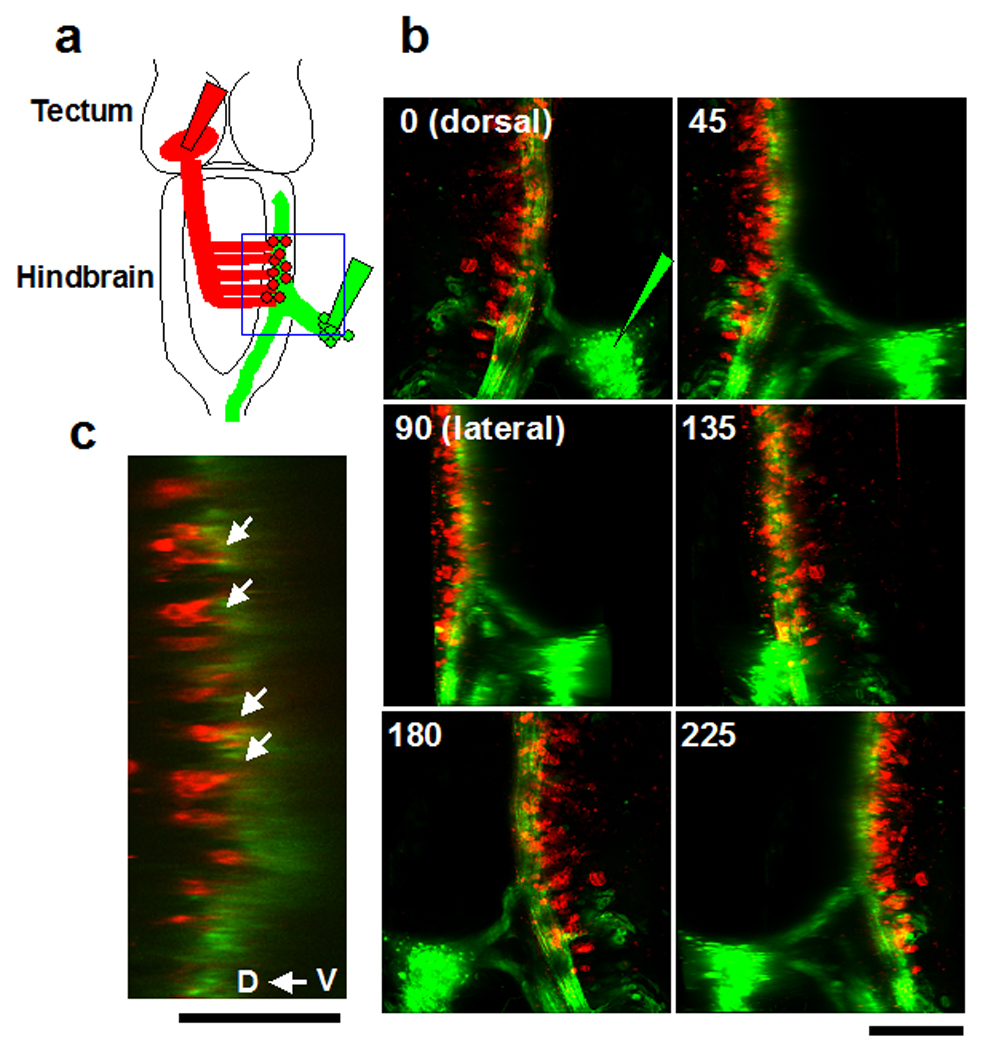
a: Hindbrain neurons that project to the contralateral tectum were retrogradely labeled by DiI injection into the tectal neuropil. Mechanosensory axons were labeled anterogradely by DiD injection into the ganglia posterior to the otic vesicle. The mechanosensory axons project to the hindbrain (green). Boxed area (blue) is shown in b. b: Images of a 3D projection of the boxed area in a shown at different angles, indicated at the top of each projected image. The cell bodies of the neurons projecting to the tectum (red) are located dorsomedial to the mechanosensory axon bundle (green; see 45deg and 90deg). c: Sagital section. Hindbrain neurons are apposed to the axon bundle of the mechanosensory neurons. The processes of the hindbrain neurons extend into the axon bundle (arrows). Scale bar, b: 200µm, c: 100µm
Organization of the axon projection from hindbrain to the tectum
To determine whether the projection from the hindbrain to the tectal neuropil shows spatial organization, we labeled the anterior and posterior hindbrain with DiI and DiD, respectively (Fig. 7a, red: DiI, green: DiD). Axons from the anterior hindbrain terminate in the dorsal tectum (Fig. 7b–h, red), while axons from the posterior hindbrain terminate in the ventral tectum (Fig. 7f, h, green), suggesting that the anterior/posterior axis of the hindbrain is transformed into a dorsoventral axis in the tectum (Fig. 7i–j). The axons from the posterior hindbrain neurons terminate in the ventral tectum even at stage 49, indicating that the distribution of hindbrain axons in tectum does not change with development (Fig. 7 g, h). Axons from the posterior hindbrain neurons project along a pathway closer to the cell body layer of the tectum, distinct from the pathway taken by the axons of the anterior hindbrain neurons (Fig. 7f, g: arrowheads). This separation of the axon tracts of the hindbrain anterior and posterior neurons becomes more pronounced as development proceeds (compare Fig. 7f and Fig. 7h). The hindbrain afferents enter from the posterior pole of the tectum and fan out as they extend to the dorsal tectum. As this occurs, axons from anterior hindbrain neurons extend into more anterior tectum than the axons from posterior hindbrain neurons (Fig. 7 e–h), indicating that anterior/posterior axis of the hindbrain is maintained as an anterior/posterior axis of the tectum.
Discussion
This study maps the projections of mechanosensory inputs to the optic tectum and shows their relation to retinotectal inputs in the developing Xenopus tadpole. We found that axonal projections to the optic tectum from the retina and hindbrain have laminar specificity in the tectal neuropil prior to the establishment of distinct cell body layers seen after metamorphosis. The hindbrain axons project to a deep neuropil layer closest to the tectal cell bodies. The RGC axons project to a separate lamina located superficially in the neuropil. The mechanosensory and retinal inputs develop concomitantly suggesting that the two inputs may interact during development. The mechanosensory inputs from the periphery have cell bodies in cranial lateral line ganglia, which project axons to the ipsilateral hindbrain. We demonstrate the organization of anterior/posterior mechanosensory inputs in the hindbrain. Hindbrain neurons, which are the target of the mechanosensory axons, project to the contralateral optic tectum. The projection from hindbrain to the tectum is somatotopic: the axons from the anterior hindbrain project to the dorsal tectum, while posterior hindbrain axons project to the ventral tectum (Fig.8).
Figure 8. Summary of axonal projections of the visual and mechanosensory systems.
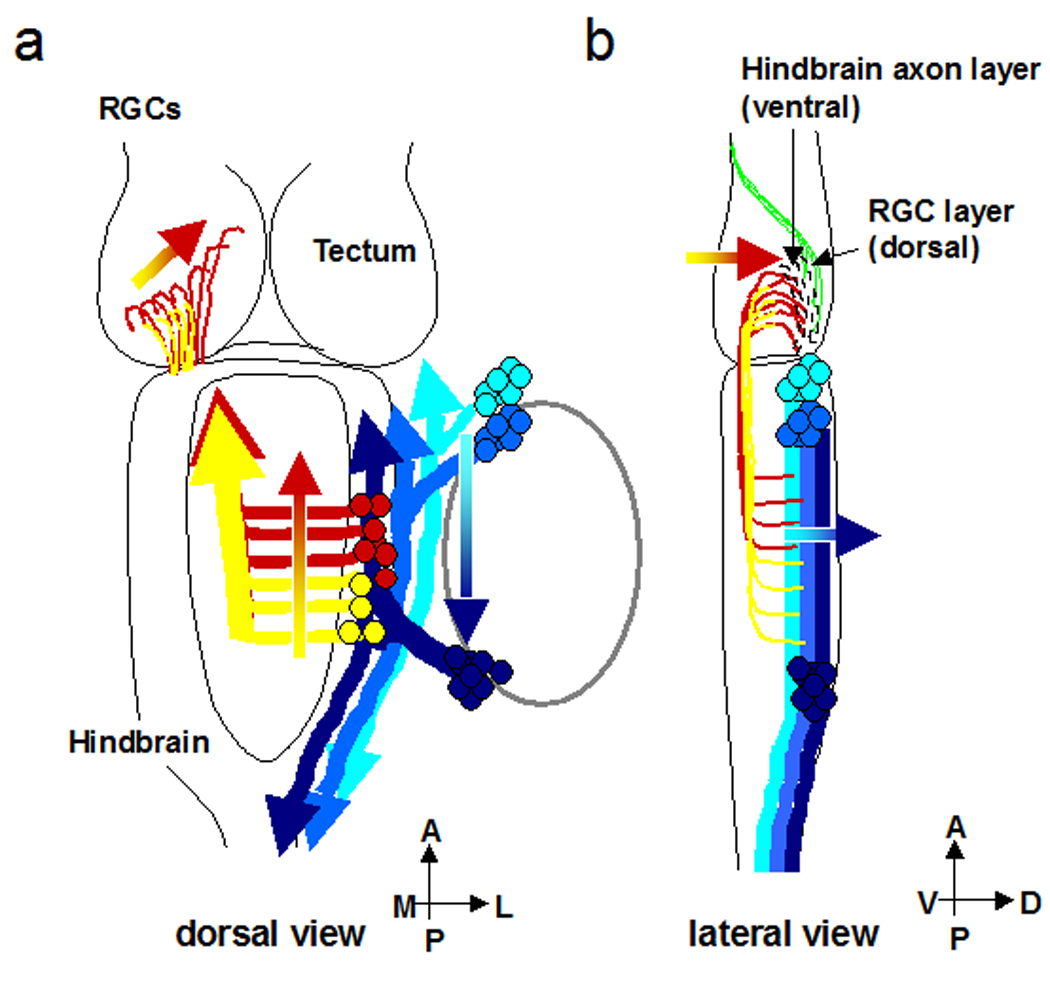
a: dorsal view. b: lateral view. Mechanosensory neurons project to the hindbrain neurons. Mechanosensory axons carrying anterior somatosensory information (light blue) extend along the ventral axon tract of the dorsal column, while mechanosensory axons carrying posterior somatosensory information project along the dorsal axon tract of the dorsal column (dark blue). Hindbrain neurons from axons to the contralateral optic tectum. The hindbrain neurons and retinal inputs project to distinct laminae in the tectum (outlined by dotted lines). Axons from the anterior hindbrain (red) extend to the dorsal tectum, which also processes information from the dorsal visual field. Axons from the posterior hindbrain (yellow) terminate in the ventral tectum, which processes information from the ventral visual field.
Laminar specificity and intermodal integration
Our results show that retinal axons and hindbrain axons project to different laminae of the tectal neuropil. Together with observations that tectal cell dendrites extend through the neuropil (Lazar, 1973; Wu and Cline, 2003), this laminar organization suggests that inputs from the two sensory modalities terminate on different dendritic regions of the same optic tectal cells. Indeed, a recent study suggests that tectal neurons in stage 47/48 Xenopus tadpoles receive and process both visual and mechanosensory inputs (Pratt and Aizenman, 2009). A laminar arrangement of multimodal sensory inputs terminating on pyramidal cell dendrites occurs throughout the vertebrate CNS including hippocampus and cortex (Lee et al., 2004) (Takahashi and Magee, 2009). It is interesting to note that such laminar segregation of multiple inputs often correlates with the hierarchical integration of the different inputs to control the output of the neuron (Lee et al., 2004; Spruston, 2008; Stein and Stanford, 2008; Takahashi and Magee, 2009). The adult frog tectum has modality specific lamina (Setalo and Szekely, 1967; Potter, 1972; Lazar, 1973; Szekely et al., 1973; Matsumoto and Bando, 1980), where different sensory modalities are thought to be processed by distinct cell types and then integrated prior to contact with motor outputs. This complex laminar circuit develops during metamorphosis, partially as a result of significant cell proliferation in the optic tectum and the establishment of new sensory inputs (Straznicky and Gaze, 1972; Udin and Fisher, 1985). Our data suggest that the tadpole tectum contains a relatively simple circuit for multisensory integration and control of motor output.
Organization of connections from the hindbrain to the tectum
The axon projection from the hindbrain to the tectum is predominantly contralateral in Xenopus tadpoles. This resembles the projection of the dorsal cochlear nucleus to the inferior colliculus in mammals (Strutz, 1987). Since the dorsal cochlear nucleus is mainly involved in the monaural processing (Sutherland et al., 1998; May, 2000) and the ventral cochlear nucleus, whose axons project to both sides of inferior colliculus, processes binaural integration, the tadpole hindbrain neurons are likely to be involved in the monaural/unilateral processing. We did not find a candidate circuit that would serve to compare bilateral mechanosensory inputs from the hindbrain and pass this information to the tectum. Such bilateral comparisons are made by Mauthner cells in the escape circuit (Faber et al., 1989; O'Malley et al., 1996) through feed-forward inhibition of the motor system. A similar mechanism for comparison of mechanosensory stimuli in Xenopus tadpoles could in principle operate at a synapse close to the tectal motor output.
The entire anterior/posterior extent of the hindbrain received input from mechanosensory neurons at each location along the anterior/posterior body axis, however inputs along the anterior/posterior body axis are segregated along the dorsoventral axis of the axon tract in the hindbrain. This suggests that hindbrain neurons in each segment may process information from all along anterior/posterior body axis. On the other hand, anterior hindbrain neurons project axons to the dorsal tectum and fan out to occupy the entire A/P axis of the tectum while posterior hindbrain neurons project axons to the ventral tectum and are confined to the posterior pole. This shows that the somatotopic map is largely co-linear between the hindbrain and the tectum, with an extension along the curvature of the dorsovental tectal axis.
Methods
Imaging axons with carbocyanine dyes
To control the diffusion of the carbocyanine dyes, we used different fixation and labeling protocols depending on the resolution required in the experiments. We found that brief fixation is suitable for pin-point labeling, and strong fixation prevents non-specific diffusion. 1,1'-dioctadecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate (DiI, Molecular Probes) and 1,1'-dioctadecyl-3,3,3',3'-tetramethylindodicarbocyanine perchlorate (DiD, Molecular Probes) are dissolved into ethanol (5% w/v) for injection. For initial fixation of all experiments, Xenopus tadpoles were anesthetized in 0.02% tricaine methanesulfonate (MS222, Sigma) and submerged into 4% paraformaldehyde (PFA) in 0.1M phosphate buffer (PB: pH 7.4) solution and microwaved at 150W power for 12 seconds. After 1 minute, the samples were microwaved again for 8 seconds at the same power. After this step, different protocols were used as follows: For fine targeted labeling of the mechanosensory ganglia (Fig1.d and Fig. 5), dyes were injected and the tissue fixed in 4% PFA for 48h and then transferred to 1% PFA for 2 weeks for the complete diffusion of dye. For bulk labeling of the hindbrain (Fig. 3b and Fig. 4), samples were fixed in 4% PFA overnight. The hindbrain ventricle was filled with the dye and the tissue was incubated in 0.1MPB for 1 week to label cells in the hindbrain. For labeling of subregions of the hindbrain (Fig. 7), samples were fixed in 4% PFA for 48h (longer than bulk labeling) to prevent non-specific diffusion. Small amounts of the dye were injected into anterior and posterior hindbrain and the sample was incubated in 1% PFA for 1 week for diffusion of the dyes. For retrograde labeling of the hindbrain neurons from the tectum (Fig. 1b, Fig. 6), the tissue was fixed in 4% PFA overnight. The neuropil of the tectum was labeled with dye, and the tissue was incubated for 2 weeks in 1% PFA. For bulk labeling of the eyes (Fig. 1a, Fig. 3b and Fig. 4), the animal was fixed in 4% PFA 0.1M PB for 2 hours and incubated in 0.1M PB for 2 hours. The retina is filled with dye until the eye was swollen. After overnight incubation in 4% PFA, samples were incubated 1% PFA for 1 week for diffusion. The samples were imaged with a laser scanning microscope (Carl Zeiss LSM 510 META, Olympus FV500).
Electroporation of hindbrain neurons
To trace the axon trajectories from the hindbrain to the tectum, we expressed EGFP in a subset of the hindbrain neurons. The hindbrain ventricle of stage 40 tadpoles was injected with 2ug/ul pCMV:EGFP DNA solution dissolved in water with 0.01% Fast Green with a picospritzer. The hindbrain was sandwiched with a bipolar platinum electrode and electroporated with one polarity of current immediately after DNA injection. 46V pulses for 16msec were applied for 5 times (Haas et al., 2002). To introduce Synaptophysin-CFP into the mechanosensory neurons, we electroporated the mechanosesory ganglia with a platinum needle (anode) placed on the ganglia and a platinum plate (cathode) beneath the tadpole body. 38V pulses for 16 sec were applied for 3 times.
Calcium imaging of the tectal cells
Stage 47 tadpoles were anesthetized in 0.01% MS222 (Sigma) in Steinberg's solution. α-Bungarotoxin (Sigma) was dissolved in water at a concentration of10mg/ml. Approximately 1% of the volume of the tadpole's body was injected into the pericardial cavity. The tadpoles were placed in Steinberg Solution to allow the drug to take effect. Oregon Green 488 BAPTA-AM (Invitrogen) was dissolved with 20% Pluronic (Invitrogen) at a concentration of 10mM and diluted 10 times with Ringer’s solution (115mM NaCl, 2.5mM KCl, 10mM HEPES pH7.6). The solution was pressure injected into the tecta through a glass pipette with a Picospritzer II (General Valve). The tadpoles were kept into Steinberg’s solution for 2 hours before imaging. The injected tadpoles were embedded in 1% low melting agarose for the calcium imaging experiments.
Visual stimuli were generated with a red LED (OptoSupply, OSHR5161A-QR, peak at 625 nm, 60deg for 50% Power Angle). The LED (3.3 cd/cm2) was placed 5mm away from the eye contra-lateral to the imaged tectum. Mechanosensory stimuli were generated by piezo plate (Piezo Systems, Inc. T215-A4-103Y) connected to an amplifier (NF, As-904). 10Hz square pulses were generated by NI USB-6009 MATLAB output to the amplifier. A 1cm length of flexible plastic rod (made from the tip of microloader, Eppendorf) was glued to the piezo plate. The amplitude of the vibration at the tip of the flexible rod was adjusted to 100µm when it was no-load. Imaging of Oregon Green 488 BAPTA-1 was performed with a custom built laser-scanning two-photon microscope, based on Mira-900 Ti-Sapphire pulse laser (Coherent) tuned to 920nm with water immersion objective (40×, Olympus). Scanning timing was controlled by Fluoview TIEMPO (Olympus) and MATLAB. Images were taken at the speed of 600msec/frame. 51 frames were scanned in each trial. Stimuli were applied for 1.5 seconds after the 23rd frame. Five repetitions were averaged for stimulus. The images were aligned by the least squares method and checked for the matching of landmarks.
Acknowledgements
We thank Dr. Carlos Aizenmann and members of the Cline laboratory for helpful discussions and comments on the manuscript. The research was supported by DP1 OD 000458.
Literature cited
- Caird D, Klinke R. Processing of binaural stimuli by cat superior olivary complex neurons. Exp Brain Res. 1983;52:385–399. doi: 10.1007/BF00238032. [DOI] [PubMed] [Google Scholar]
- Douglass AD, Kraves S, Deisseroth K, Schier AF, Engert F. Escape behavior elicited by single, channelrhodopsin-2-evoked spikes in zebrafish somatosensory neurons. Curr Biol. 2008;18:1133–1137. doi: 10.1016/j.cub.2008.06.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton RC, DiDomenico R, Nissanov J. Role of the Mauthner cell in sensorimotor integration by the brain stem escape network. Brain Behav Evol. 1991;37:272–285. doi: 10.1159/000114365. [DOI] [PubMed] [Google Scholar]
- Edwards CJ, Kelley DB. Auditory and lateral line inputs to the midbrain of an aquatic anuran: neuroanatomic studies in Xenopus laevis. J Comp Neurol. 2001;438:148–162. doi: 10.1002/cne.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber DS, Fetcho JR, Korn H. Neuronal networks underlying the escape response in goldfish. General implications for motor control. Ann N Y Acad Sci. 1989;563:11–33. doi: 10.1111/j.1749-6632.1989.tb42187.x. [DOI] [PubMed] [Google Scholar]
- Fetcho JR, Higashijima S, McLean DL. Zebrafish and motor control over the last decade. Brain Res Rev. 2008;57:86–93. doi: 10.1016/j.brainresrev.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghysen A, Dambly-Chaudiere C. Development of the zebrafish lateral line. Curr Opin Neurobiol. 2004;14:67–73. doi: 10.1016/j.conb.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Gingras G, Rowland BA, Stein BE. The differing impact of multisensory and unisensory integration on behavior. J Neurosci. 2009;29:4897–4902. doi: 10.1523/JNEUROSCI.4120-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruberg ER, Udin SB. Topographic projections between the nucleus isthmi and the tectum of the frog Rana pipiens. J Comp Neurol. 1978;179:487–500. doi: 10.1002/cne.901790303. [DOI] [PubMed] [Google Scholar]
- Haas K, Jensen K, Sin WC, Foa L, Cline HT. Targeted electroporation in Xenopus tadpoles in vivo--from single cells to the entire brain. Differentiation. 2002;70:148–154. doi: 10.1046/j.1432-0436.2002.700404.x. [DOI] [PubMed] [Google Scholar]
- Harnischfeger G, Neuweiler G, Schlegel P. Interaural time and intensity coding in superior olivary complex and inferior colliculus of the echolocating bat Molossus ater. J Neurophysiol. 1985;53:89–109. doi: 10.1152/jn.1985.53.1.89. [DOI] [PubMed] [Google Scholar]
- Harting JK, Updyke BV, Van Lieshout DP. Corticotectal projections in the cat: anterograde transport studies of twenty-five cortical areas. J Comp Neurol. 1992;324:379–414. doi: 10.1002/cne.903240308. [DOI] [PubMed] [Google Scholar]
- Harting JK, Van Lieshout DP. Spatial relationships of axons arising from the substantia nigra, spinal trigeminal nucleus, and pedunculopontine tegmental nucleus within the intermediate gray of the cat superior colliculus. J Comp Neurol. 1991;305:543–558. doi: 10.1002/cne.903050403. [DOI] [PubMed] [Google Scholar]
- Holt CE. The topography of the initial retinotectal projection. Prog Brain Res. 1983;58:339–345. doi: 10.1016/S0079-6123(08)60035-7. [DOI] [PubMed] [Google Scholar]
- Huerta MF, Harting JK. Connectional organization of the superior colliculus. Trends Neurosci. 1984;7:286–289. [Google Scholar]
- Knudsen EI. Instructed learning in the auditory localization pathway of the barn owl. Nature. 2002;417:322–328. doi: 10.1038/417322a. [DOI] [PubMed] [Google Scholar]
- Lazar G. The development of the optic tectum in Xenopus laevis: a Golgi study. J Anat. 1973;116:347–355. [PMC free article] [PubMed] [Google Scholar]
- Lee I, Yoganarasimha D, Rao G, Knierim JJ. Comparison of population coherence of place cells in hippocampal subfields CA1 and CA3. Nature. 2004;430:456–459. doi: 10.1038/nature02739. [DOI] [PubMed] [Google Scholar]
- Masino T, Grobstein P. Tectal connectivity in the frog Rana pipiens: tectotegmental projections and a general analysis of topographic organization. J Comp Neurol. 1990;291:103–127. doi: 10.1002/cne.902910108. [DOI] [PubMed] [Google Scholar]
- Matsumoto N, Bando T. Excitatory synaptic potentials and morphological classification of tectal neurons of the frog. Brain Res. 1980;192:39–48. doi: 10.1016/0006-8993(80)91006-9. [DOI] [PubMed] [Google Scholar]
- May BJ. Role of the dorsal cochlear nucleus in the sound localization behavior of cats. Hear Res. 2000;148:74–87. doi: 10.1016/s0378-5955(00)00142-8. [DOI] [PubMed] [Google Scholar]
- May PJ. The mammalian superior colliculus: laminar structure and connections. Prog Brain Res. 2006;151:321–378. doi: 10.1016/S0079-6123(05)51011-2. [DOI] [PubMed] [Google Scholar]
- Moore DR. Anatomy and physiology of binaural hearing. Audiology. 1991;30:125–134. doi: 10.3109/00206099109072878. [DOI] [PubMed] [Google Scholar]
- O'Malley DM, Kao YH, Fetcho JR. Imaging the functional organization of zebrafish hindbrain segments during escape behaviors. Neuron. 1996;17:1145–1155. doi: 10.1016/s0896-6273(00)80246-9. [DOI] [PubMed] [Google Scholar]
- Orger MB, Kampff AR, Severi KE, Bollmann JH, Engert F. Control of visually guided behavior by distinct populations of spinal projection neurons. Nat Neurosci. 2008;11:327–333. doi: 10.1038/nn2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter HD. Terminal arborizations of retinotectal axons in the bullfrog. J Comp Neurol. 1972;144:269–284. doi: 10.1002/cne.901440303. [DOI] [PubMed] [Google Scholar]
- Pratt KG, Aizenman CD. Multisensory integration in mesencephalic trigeminal neurons in Xenopus tadpoles. J Neurophysiol. 2009;102:399–412. doi: 10.1152/jn.91317.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Precht W, Llinas R, Clarke M. Physiological responses of frog vestibular fibers to horizontal angular rotation. Exp Brain Res. 1971;13:378–407. doi: 10.1007/BF00234338. [DOI] [PubMed] [Google Scholar]
- Rowland BA, Stanford TR, Stein BE. A model of the neural mechanisms underlying multisensory integration in the superior colliculus. Perception. 2007;36:1431–1443. doi: 10.1068/p5842. [DOI] [PubMed] [Google Scholar]
- Rybicka KK, Udin SB. Connections of contralaterally projecting isthmotectal axons and GABA-immunoreactive neurons in Xenopus tectum: an ultrastructural study. Vis Neurosci. 2005;22:305–315. doi: 10.1017/S0952523805223064. [DOI] [PubMed] [Google Scholar]
- Setalo G, Szekely G. The presence of membrane specializations indicative of somato-dendritic synaptic junctions in the optic tectum of the frog. Exp Brain Res. 1967;4:237–242. doi: 10.1007/BF00248024. [DOI] [PubMed] [Google Scholar]
- Sillar KT, Robertson RM. Thermal activation of escape swimming in post-hatching Xenopus laevis frog larvae. J Exp Biol. 2009;212:2356–2364. doi: 10.1242/jeb.029892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruston N. Pyramidal neurons: dendritic structure and synaptic integration. Nat Rev Neurosci. 2008;9:206–221. doi: 10.1038/nrn2286. [DOI] [PubMed] [Google Scholar]
- Stein BE. Development and organization of multimodal representation in cat superior colliculus. Fed Proc. 1978;37:2240–2245. [PubMed] [Google Scholar]
- Stein BE, Stanford TR. Multisensory integration: current issues from the perspective of the single neuron. Nat Rev Neurosci. 2008;9:255–266. doi: 10.1038/nrn2331. [DOI] [PubMed] [Google Scholar]
- Stein BE, Stanford TR, Rowland BA. The neural basis of multisensory integration in the midbrain: Its organization and maturation. Hear Res. 2009 doi: 10.1016/j.heares.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straznicky K, Gaze RM. The development of the tectum in Xenopus laevis: an autoradiographic study. J Embryol Exp Morphol. 1972;28:87–115. [PubMed] [Google Scholar]
- Strutz J. [Anatomy of the central auditory pathway. Demonstration with horseradish peroxidase in the guinea pig] HNO. 1987;35:407–415. [PubMed] [Google Scholar]
- Sutherland DP, Masterton RB, Glendenning KK. Role of acoustic striae in hearing: reflexive responses to elevated sound-sources. Behav Brain Res. 1998;97:1–12. doi: 10.1016/s0166-4328(98)00008-4. [DOI] [PubMed] [Google Scholar]
- Szekely G, Setalo G, Lazar G. Fine structure of the frog's optic tectum: optic fibre termination layers. J Hirnforsch. 1973;14:189–225. [PubMed] [Google Scholar]
- Takahashi H, Magee JC. Pathway interactions and synaptic plasticity in the dendritic tuft regions of CA1 pyramidal neurons. Neuron. 2009;62:102–111. doi: 10.1016/j.neuron.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Tsuchitani C. The inhibition of cat lateral superior olive unit excitatory responses to binaural tone bursts. I. The transient chopper response. J Neurophysiol. 1988;59:164–183. doi: 10.1152/jn.1988.59.1.164. [DOI] [PubMed] [Google Scholar]
- Udin SB. Abnormal visual input leads to development of abnormal axon trajectories in frogs. Nature. 1983;301:336–338. doi: 10.1038/301336a0. [DOI] [PubMed] [Google Scholar]
- Udin SB, Fisher MD. The development of the nucleus isthmi in Xenopus laevis. I. Cell genesis and the formation of connections with the tectum. J Comp Neurol. 1985;232:25–35. doi: 10.1002/cne.902320103. [DOI] [PubMed] [Google Scholar]
- Udin SB, Scherer WJ. Restoration of the plasticity of binocular maps by NMDA after the critical period in Xenopus. Science. 1990;249:669–672. doi: 10.1126/science.2166343. [DOI] [PubMed] [Google Scholar]
- Vanegas H, Essayag-Millan E, Laufer M. Excitability characteristics of field and unitary potentials in optic tectum of fish. Acta Cient Venez. 1971;22:85–88. [PubMed] [Google Scholar]
- Wallace MT, Perrault TJ, Jr, Hairston WD, Stein BE. Visual experience is necessary for the development of multisensory integration. J Neurosci. 2004;24:9580–9584. doi: 10.1523/JNEUROSCI.2535-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace MT, Stein BE. Early experience determines how the senses will interact. J Neurophysiol. 2007;97:921–926. doi: 10.1152/jn.00497.2006. [DOI] [PubMed] [Google Scholar]
- Wilson AL, Shen YC, Babb-Clendenon SG, Rostedt J, Liu B, Barald KF, Marrs JA, Liu Q. Cadherin-4 plays a role in the development of zebrafish cranial ganglia and lateral line system. Dev Dyn. 2007;236:893–902. doi: 10.1002/dvdy.21085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GY, Cline HT. Time-lapse in vivo imaging of the morphological development of Xenopus optic tectal interneurons. J Comp Neurol. 2003;459:392–406. doi: 10.1002/cne.10618. [DOI] [PubMed] [Google Scholar]
- Xiao T, Baier H. Lamina-specific axonal projections in the zebrafish tectum require the type IV collagen Dragnet. Nat Neurosci. 2007;10:1529–1537. doi: 10.1038/nn2002. [DOI] [PubMed] [Google Scholar]
- Yamagata M, Weiner JA, Dulac C, Roth KA, Sanes JR. Labeled lines in the retinotectal system: markers for retinorecipient sublaminae and the retinal ganglion cell subsets that innervate them. Mol Cell Neurosci. 2006;33:296–310. doi: 10.1016/j.mcn.2006.08.001. [DOI] [PubMed] [Google Scholar]


