I. Introduction: Some History and Other Vectors
For many years it has been virtually impossible to transfer genes into brain cells either to study or manipulate molecular, cellular, or, in vivo, behavioral processes. In addition to the physical barriers that protect the brain (bone and three layers of meninges), the earliest gene delivery systems, the retroviral vectors, require cell division to integrate the transgenes into their genomes to express any transgenes. Thus they limited transduction to dividing cells of the nervous system, e.g., astrocytes and oligodendrocytes, neurons in the neonatal brain undergoing cell division, or non-neural cells such as fibroblasts that could then be transplanted to the brain. Because neurons in the adult brain do not divide, retroviruses were of limited use to neuro-biologists wanting to manipulate the molecular makeup of neurons in vivo (Fisher and Gage, 1993; Suhr and Gage, 1999; Lowenstein and Castro, 2002; Hsich et al., 2002; see Kageyama et al., this volume, for further discussion).
Far greater transduction efficiencies in the nervous system can now be achieved with the recently developed lentiviral vectors. Lentiviruses are a subclass of retroviruses whose genomes contain additional viral proteins. Two of these virion proteins, matrix (MA) and virion protein R (VPR), are used to transport the cDNA/integrase complex (also called preintegration complex) across the nuclear membrane in the absence of mitosis. This allows lentiviruses not only to infect, but (at least in theory) also to express transgenes in both proliferating, e.g., astrocytes, and nonproliferating cells, such as neurons. In vivo experiments with lentiviral vectors, however, have demonstrated that only neurons express trans-genes, presumably due to a not yet characterized selectivity of lentiviral vectors to infect neuronal cells (Kay et al., 2001; Consiglio et al., 2001; Brooks et al., 2002).
The first lentiviral vectors were derived from human immunodeficiency virus-1 (HIV-1) but were pseudotyped by using the envelope glycoproteins from other viruses such as the vesicular stomatitis virus G protein (VSV-G), a fusion protein used to improve infection efficiency. The cell line 293 is used to generate these vectors using a three-plasmid cotransfection system. Three separate plasmids encode for the pseudotyped env gene, the transgene cassette, and a packaging construct respectively supplying the structural and regulatory genes in trans (Naldini et al., 1996). Currently other lentivurses, e.g., feline immune deficiency virus and equine infectious anemia virus, have also been engineered as gene transfer vectors. An important attraction of these latter systems is the fact that they are derived from animal, rather than human lentiviruses (Brooks et al., 2002; Curran and Nolan, 2002).
Lentivirus vectors have all the desirable properties of the early Moloney murine leukemia virus (Mo-MLV)-based vectors, but, in addition, can infect both dividing and quiescent cells and induce a limited inflammatory response even in the CNS (Blomer et al., 1997; unpublished observations). Lentiviruses have been used very successfully infecting and transducing the central nervous system (CNS), with almost negligible inflammatory responses (Deglon and Aebischer 2002; Hsich et al., 2002; Tate et al., 2002). Lentiviral vectors are currently being evaluated for safety, with a view to removing all nonessential regulatory genes to facilitate and accelerate approval for clinical trials. If the issues of increased production and safety can be overcome, higher titers are achieved, and public fears of some lentiviral vectors being HIV-1 derived are addressed, these vectors hold great promise for clinical applications within the nervous system (Ailles and Naldini, 2002; De Palma and Naldini, 2002; Galimi and Verma, 2002; Huentelman et al., 2002). Novel and/or improved vector systems are constantly being developed to provide a highly effective technology with which to explore the molecular basis of neuronal gene therapy (see Jakobsson et al., for further discussion).
Having briefly reviewed the current status of vectors available for gene transfer into the brain, we will now explore the potential application of replication-deficient adenovirus vectors as vehicles for gene delivery into the CNS (Table I). A significant feature of adenovirus as a potential vector for DNA delivery in human gene therapy protocols has been that a live (replication-competent) vaccine has been safely administered to many million human beings (U.S. military recruits) over several decades to provide protection against natural adenovirus infections (Rubin and Rorke, 1994). More recently, similar replication-competent adenovirus recombinants encoding defined immunogens have also been used in human vaccine trials, or as replication-competent vectors with or without additional anticancer genes to induce tumor cell killing. Although most adenovirus vaccine development has been based on exploiting replication-competent systems, replication-deficient adenovirus vectors have also proved to be highly effective agents with which to generate both humoral and cell-mediated immune responses to expressed transgenes. These vectors have definite attributes as agents for immunization. There is therefore considerable experience in using live adenovirus isolates, replication-competent adenovirus recombinants, and replication-deficient recombinant adenovirus as immunizing agents (Top et al., 1971a, b; Schwartz et al., 1974; Morin et al., 1987; Prevec et al., 1989; Eloit et al., 1990; Jacobs et al., 1992; Gallichan et al., 1993; Rubin, 1994; Wilkinson, 1994a,b; Wilkinson and Borysiewicz, 1995; Amalfitano and Parks, 2002; Nicklin and Baker, 2002; Wickham, 2000).
TABLE I.
A Comparison between Adenovirus Vectors and Other Gene Transfer Methodsa
| Vectors |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ads | HC-Ad | HSV1/r | HSV1/a | AAV | Retro/ lentivirus |
Vaccinia | SFV | Micro injection |
|
| Size (kb) | 36 | 36 | 152 | 5–30 | 4.68 | 7–10 | 185 | 11.51 | 10–30 |
| Cloning capacity (kb) |
7.5 | ~30 | 10–30 | ~150 | 4.5 | 7–10 | 30 | 9–20 | 10–30 |
| Neuronal transduction |
|||||||||
| In vivo? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No |
| In vitro? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Long-term gene expression (≥12 months) |
Yes | Yes | Yes | Yes | Yes | Yes | No | No | N/A |
| Gene therapy? | Yes | Yes | Yes | Yes | Yes | Yes | No | No | No |
| Vaccination | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No |
HSV-1/r, recombinant HSV1-based vectors; HSV-1/a, amplicon HSV1-based vectors; Ad, adenovirus vectors; HC-Ad, high-capacity, helper-dependent adenovirus vectors; SFV, Semiliki Forest virus vectors.
Furthermore, human gene therapy programs have been launched by a number of groups in the United States and Europe, resulting in the application of replication-deficient adenovirus recombinants in the treatment of patients suffering cystic fibrosis, coronary artery restenosis postpercutaneous angioplasty, brain tumors, head and neck carcinomas, ovarian carcinomas, melanoma, and metabolic disorders among others (Rosenfeld et al., 1992; Engelhardt et al., 1993; Mastrangeli et al., 1993; Simon et al., 1993; Boucher et al., 1994; Bout et al., 1994a,b; Brody et al., 1994a,b; Mittereder et al., 1994; Welsh et al., 1994; Wilson et al., 1994; Yei et al., 1994a,b; Zabner et al., 1994; Kirn et al., 2001; Rutanen et al., 2002; Bauknecht and Meinhold-Heerlein, 2002; Raper et al., 2002). Thus, a wealth of data has accumulated and continues to grow concerning the therapeutic administration of adenovirus and their effectiveness as well as their side effects in human patients.
Interest in exploiting the new capabilities offered by adenoviruses as gene therapy vectors has been very high. The key features that make adenovirus potentially a credible vector for neurological gene therapy specifically are that (1) sufficiently high titers can be easily produced as to allow their administration in vivo, (2) they can transduce many different differentiated cell types, including postmitotic neurons, (3) expression in the target cell can be restricted to the transgene only (reviewed in Legrand et al., 2002; Nicklin and Baker, 2002), and (4) they can be scaled up to very high titres, i.e., 1012–1013 IU/ml. So far adenovirus recombinants have been used to transduce cells of the lung (see above for CFTR transfer; Gilardi et al., 1990; Rosenfeld et al., 1991; Yei et al., 1994a,b), liver (Herz and Gerard, 1993; Ishibashi et al., 1993; Engelhardt et al., 1994; Hayashi et al., 1994; Kozarsky et al., 1994), arteries and other blood vessels (Lemarchand et al., 1992; Roessler et al., 1993; Kingston et al., 2001), joints, bone marrow cells, and various differentiated circulating cells of the immune system (Haddada et al., 1993), heart (Stratford-Perricaudet et al., 1992; Kass-Eisler et al., 1993), skeletal muscle (Quantin et al., 1992; Stratford-Perricaudet et al., 1990, 1992; Acsadi et al., 1994a,b), brain (Akli et al., 1993; Bajocchi et al., 1993; Davidson et al., 1993; Le Gal La Salle et al., 1993; Ambar et al., 1999; Dewey et al., 1999; Thomas et al., 2000a,b, 2001a,b), and spinal cord (Lisovoski et al., 1994), and to release factor IX into the bloodstream (Smith et al., 1993). The widespread potential applications of adenovirus systems has promoted further developments of the adenovirus vectors, in particular, efficient strategies have been devised for the insertion of transgenes into recombinant adenovirus. Also, the further developments have concentrated on two main limitations of the adenoviral vectors, namely, the limited transgene capacity of first-generation adenoviral vectors and the inflammatory and immune responses caused by the adenoviral virions and its genome. Inserts of up to 8 kbp are feasible in replication-deficient recombinants, which can be grown without helper virus on trans-complementing 293 cells (Graham et al., 1977; Berkner, 1988; Wilkinson, 1994a,b), gutless vectors, and the new helper-dependent high-capacity vectors have a theoretical capacity of up to 30–36 kbp (Lowenstein et al., 2002; Hartigan-O’Connor et al., 2002; Zhou et al., 2002).
Recent reviews have provided detailed protocols and methodologies involved in constructing and utilizing adenovirus vectors for gene transfer to cells of the brain both in vitro and in vivo (Lowenstein, 1995; Southgate et al., 2001; Thomas et al., 2001a,b; see Phillips, 2002). In this chapter, we will consequently concentrate on progress that has been made in the application of such vectors within the context for their exploitation for neurobiology and discuss potential future developments in this field.
II. The Vector System
Adenoviruses have the ability to infect a wide range of different tissues and cell types. It has been shown convincingly that adenovirus type 5 serotype initiates infection by binding to the coxsackievirus and adenovirus receptor (CAR) through the knob domain located at the distal extreme of the fiber protein (Bergelson et al., 1997; Roelvink et al., 1999). CAR is a 46-kDa integral membrane protein with a typical transmembrane region and an extracellular region composed of two immunoglobulin (Ig)-like domains (Tomko et al., 1997). The C-terminal knob of the fiber protein confers the specificity of the cellular receptor recognition. Following its binding to CAR, adenovirus is subsequently internalized through an interaction with integrins on the cell surface of the target cells, especially αvβ5 (Wickham et al., 1993). Entry into the cell is by receptor-mediated endocytosis (Wickham et al., 1994), after which the viral nucleocapsid is released from endosomes into the cytoplasm, and transported to the nucleus (Greber et al., 1993; Trotman et al., 2001). The double-stranded DNA genome (~36 kb; Fig. 1) is then released into the nucleus of the infected cell (Trotman et al., 2001). Upon infection of a permissive cell, the replication cycle can be divided into three phases of gene expression: immediate early (E1 genes), early (E1–E4 genes), and finally the late events, which follow the onset of viral DNA replication. The very first region of the genome to become active is the left-hand region of the left strand, E1, which encodes a series of transcriptional activators, which will promote progression into the early phase of gene expression. These phases of virus replication are associated with host cell cycle progression to S1, onset of viral DNA replication, as well as the production of gene products associated with evasion of host antivirus defenses. Five late transcriptionally active regions are found at the right of the left strand, and are produced from a single major late promoter by alternative splicing (L1, L2, L3, L4, and L5). Expression of late viral genes becomes activated after the start of viral DNA replication and will eventually lead to the assembly of progeny virions, in excess of 105 new virions per infected cell (Shenk, 1995).
Fig. 1.
(A) Schematic representation of an adenovirus particle based on the current understanding of its polypeptide constituents and genome. (B) Genome is 100 map units (mu) in length (1 mu = 360 bp). Early primary mRNA transcripts are designated in bold. Certain polypeptides are identified by conventional numbering system (roman numerals). Modified from Flint et al. (2000). (See Color Insert.)
Most available recombinant adenoviruses for use as vectors for gene transfer are rendered replication defective through deletions in the E1, E2, E3, or E4 regions, or through combinations thereof. These vectors, have been termed “first (and sometimes second and third)-generation” adenovirus vectors, depending on the authors, and the particular deletions to increase the cloning capacity (Gilardi et al., 1990; Davidson et al., 1993; Brody et al., 1994b; Wilkinson, 1994a,b; see Philips, 2002). The regions deleted comprise some genes that are necessary for replication (e.g., those located in the E2 region), to others that do not appear to be necessary for replication (e.g., the E3 and E4 region) and that encode genes associated with modulating the host immune response and transcriptional control. No deletions are neutral, and they will have varying effects on the production, replication, expression, and toxicity of the particular viral vectors. Replication-deficient adenovirus recombinants deleted in various genes or genomic regions are commonly grown on trans-complementing 293 cells that express an integrated copy of the Ad5 E1 gene, complemented by other regions in the case of deletions in the E2 region (Graham et al., 1977). Transcomplementation with the E3 or E4 region has usually not been necessary.
Efficient systems to construct adenovirus recombinants are now available both from a number of different laboratories, and more recently also from various commercial sources (e.g., Microbix Biosystems Inc., Toronto, Canada; QBio Gene, Montreal, Canada). These allow the construction of both replication-competent and replication-deficient recombinant adenoviruses for gene transfer (Prevec et al., 1989), using conventional and well-established DNA cloning and transfection procedures (Sambrook, 1989). Most published reports utilize adenovirus (Ad) recombinant derived from either serotypes 5 or 2 (see Table II), although there are some 47 human serotypes identified and many more animal isolates, some of which have also been used within the context of gene transfer (Cotten et al., 1993). The exploration of other adenoviral serotypes has substantially increased since 1995 as some of their distinct properties concerning the receptors used for entry, the consequent cell types transduced, have been discovered to differ in important ways from adenovirus type 2 and 5 (Zabner et al., 1999; Segerman et al., 2000; Shayakhmetov et al., 2000). In Ad E1-deleted vectors, transgenes are usually inserted into the E1 region under the control of exogenous viral promoters (e.g., RSV-LTR, IE-hCMV). Cell specific alone, or combined with inducible promoters, or other elements that increase expression levels can also be used effectively to target expression to specific cell types, to regulate its expression, and to increase expression levels (Babiss et al., 1986; Bessereau et al., 1994; Grubb et al., 1994; Smith-Arica et al., 2000, 2001; Southgate et al., 2001; Gerdes et al., 2000; Harding et al., 1998; Ralph et al., 2000; Glover et al., 2002). Most recently the use of replication-competent adenoviruses for the treatment of turmors has also begun to be explored (Nanda et al., 2001; Doronin et al., 2000, 2001).
TABLE II.
Review of Publications Using Recombinant Adenovirus Vectors for Gene Transfer into the Brain or Constituent Cells
| Referencesa | Recombinant adenovirus E1 vector |
Target in vitro and in vivo |
Therapeutic efficacy/type of experiment |
|---|---|---|---|
| (1) “Early” Adenoviral Gene Transfer into the Brain (1993–1994) | |||
| Akli et al. (1993) | Ad-RSVlacZ, Ad type 5 | Brain in vivo; rats | Gene transfer into the rodent brain in vivo |
| Bajocchi et al. (1993) | Ad-RSVlacZ, Ad-α1AT, Ad type 5 |
Intraventricular or intrastriatal administration; 5 × 108–109 pfu/animal |
Gene transfer of marker enzymes and transgenes into the rodent ventricles and brain in vivo |
| Bennett et al. (1994) | Ad-CMVlacZ; Ad5 | Adult mouse mammalian retina; subretinal injection of 102–109 pfu/retina in 1–10 μl |
Gene transfer into retina in vivo |
| Caillaud et al. (1993) | Ad-RSVlacZ; Ad5 | Primary cultures of neurons and glial cells |
Gene transfer into neural cells in vitro |
| Davidson et al. (1993) | Ad-CMVlacZ Ad type 5 E1; E3 | In vivo: intrastriatal, mice | Gene transfer into rodent brain in vivo |
| Jomary et al. (1994) | Ad2/CMV lacZ | Retinal cells | Gene transfer of marker cells into retina |
| Le Gal La Salle et al. (1993) | Ad-RSVlacZ, Ad type 5 | SCG and astrocytes in culture. Hippocampus and substantia nigra (SN) in vivo: 3–5×107 pfu/injection |
Gene transfer into brain cells in vivo and in vitro |
| Li et al. (1994) | Ad-CMV β-Actin-nls lacZ; Ad5 | Subretinal injection in normal and mutant mice of 4 × 104–4 × 107 pfu in 0.3–0.5 μl/retina |
Gene transfer into retina in vivo |
| Ridoux et al. (1994b) | Ad-RSVlacZ | Astrocytes in vitro; 2 × 107 pfu/confluent dish |
Gene transfer into astrocytes prior to intracranial transplantation |
| (2) Ex Vivo Gene Transfer and Transplantation of Transduced Cells | |||
| Hughes et al. (2002) | Ad-lacZ or eGFP |
In vitro: embryonic murine neural progenitor cells |
FIV-transduced progenitors retain potential for differentiation; Ad induces differentiation into astrocytes |
| Corti et al. (1999) | Ad-tet-TH | In vitro: human neural progenitor cells | Ad encoding TH under negative control of the tetracycline regulatory system produces high levels of TH in neural progenitor cells |
| Boer et al. (1997) | Ad-lacZ | In vivo: third ventricle of rats |
Ex vivo Ad delivered transgene expression of neurografts implanted in the third ventricle is compared to explant cultures |
| Yoon et al. (1996) | Ad-lacZ or p75 | In vivo: intraventricular injection in mice | Ad-mediated reporter genes are intro- duced into progenitor cells to monitor their migration and assess stability of transgene expression |
| (3) Recent Vector Developments | |||
| Umana et al. (2001) | HD-Ad-lacZ | In vivo: rats | Use of FLPe recombinase to improve quality and quantitiy of HD Ad production |
| Xia et al. (2000) | Ad-GFP, Ad5GFPBxHI, where “x” is the epitope number |
In vitro: engineered cell lines and human brain microcapillary endothelia |
The Ad HI loop is modified to achieve binding to a specific receptor; this is used to achieve a disseminated pattern of transduction |
| Cregan et al. (2000) | HD-Ad-lacZ |
In vitro: primary cerebellar neuronal cultures |
Efficacy and cytotoxicity of an HD Ad is compared to a first-generation Ad |
| Zheng et al. (2000) | Chimaeric Ad-retroviral vector |
In vitro: in vivo: submandibular gland, cortex, and caudate nucleus |
Examination of the characteristics of an Ad construct containing LTR sequences of a retroviral vector |
| Beer et al. (1998) | Encapsulated RAds | In vitro; in vivo: mice | Administration of PLGA encapsulated Ad to diminish immunogenicity of the viral vectors |
| Sato et al. (1998) | Ad-bc12; regulatable by cre |
In vitro: PC12, a hybrid motoneuronal cell line and primary chicken motoneurons |
A new strategy for delivery of Bcl-2 mediated by Cre/loxP recombination using Ad vectors |
| Peltekian et al. (2002) | CAV2 | In vivo: rat | Selective transduction of neuronal cells by CAV2 vectors introduced as a new tool for targeting key structures of neurodegeneration |
| Soudais et al. (2001) | CAV2 | Rodent CNS neurons in vitro, and in vivo | Neurotropism of CAV2 and its efficient retrograde transport are demonstrated |
| (4) Transcriptional Targeting | |||
| Kugler et al. (2001) | Ad-NSE, tubulin α1, synapsin promoters-lacZ or Bcl-X(L) |
In vitro: primary neuronal cultures; in vivo: rats |
Different cellular promoters are investi- gated in an adenoviral context to determine their capability of neuron- restrictive transgene expression |
| Smith et al. (2000) | Ad-CMV, RSV, E1A promoters-lacZ or eGFP |
In vitro: hippocampal brain slices; in vivo: rat hippocampus |
Cytotoxicity and transgene expression from three viral promoters in the same Ad backbone are compared |
| Ralph et al. (2000) | Ad-synapsin 1, GFAP-tet-eGFP |
In vitro: primary hippocampal cultures; in vivo: rat hippocampus |
Ad system employing regulatable, cell-specific transgene expression |
|
Navarro et al. (1999); Nillecamps (1999) |
Ad-NSE, RSV promoters-LacZ | In vivo: rat | NSE and RSV promoters driving LacZ in an Ad are compared |
| Ad-xNRSE-PK-luc, x denotes ≤ 12 NRSE elements |
In vitro: different nonneuronal and neuronal cell lines and primary SCG neurons; in vivo: mouse tongue injection, rats |
More specific neuronal transgene expres- sion is achieved with an Ad vector construct encoding luciferase with in- creasing numbers of NRSEs upstream of the phosphoglycerate promoter |
|
| Miyaguchi et al. (1999) | Ad-SCG10-NRSE-lacZ | In vitro: rat hippocampal slice cultures | NRSE in an Ad-encoding LacZ to achieve selective neuronal expression |
| Smith-Arica et al. (2000) | Ad-tet-GFAP, NSE, β-Actin- CMV promoters |
In vitro: different cell lines and primary neocortical cultures; in vitro: rats |
A dual Ad system encoding for cell-type- specific and regulatable transcription units is tested |
| Morelli et al. (1999) | Ad-NSE, GFAP, hCMV promoters—FasL |
In vitro: primary, neuronal, glial, fibroblastic, and epithelial cell lines; in vivo: systemic administration in mice |
Ads encoding FasL controlled by non- specific and cell-type specific promo- ters are tested in different cell types and in vivo |
| Shering et al. (1997) | Ad, HSV-1, HSV-1 amplicon vectors—hCMV |
In vitro: rat nervous system cell cultures | Cell-specific expression from a short HCMV major IE promoter enhancer is assessed in Ad and HSV-1 viral vectors |
| (5) Targeting through the Blood–Brain Barrier | |||
| Nilaver et al. (1995) | Ad-RSVlacZ, HSV-1-lacZ |
In vivo: intracerebral xenografts of human LX-1 small cell lung carcinoma in nude rats |
Distribution of LacZ delivered by Ad and HSV-1 vectors after intratumor or intracarotid administration +/− osmotic disruption of the BBB |
| Muldoon et al. (1995) | Ad, HSV | In vivo: rat and cat | Delivery of Ad, HSV, and MION into normal brain or intraarterially is compared in animals with intact or disrupted BBB |
| Doran et al. (1995) | Ad-lacZ | In vivo: rats | Short-term gene expression is evaluated after intracarotid administration of an Ad encoding lacZ after or without osmotic BBB disruption |
| (6) Brain Immunity/Inflammation | |||
| Zermansky et al. (2001) | Ad-HSV1-TK | In vivo: rats | 12 months persisting high-level and widespread expression of Ad delivered HSV1-TK is transgene dependent |
| Thomas et al. (2001a) | Ad-hCMV-lacZ or HPRT | In vivo: rats | Mechanisms contributing to the decline in transgene expression delivered by first generation Ad vectors are examined |
| Thomas et al. (2002) | Ad-hCMV-lacZ, luc with different capsid modifications |
In vivo: rats | Engineered viral capsids ablated for CAR and/or integrin and their in- flammatory potential are examined |
| Thomas et al. (2001b) | Ad-hCMV-lacZ or HPRT, HD-Ad-hCMV-lacZ |
In vivo: rats | Effects of preexisting antiadenoviral im- munity on transduction of brain cells with HD Ad and first-generation Ad are compared |
| Zou et al. (2001) | Ad-lacZ, HD-Ad-lacZ |
In vivo: intraventricular and intrahippocampal administration in rats |
Toxicity and immunogenicity of HD and first-generation Ad vectors and their effect on transgene expression are compared |
| Cua et al. (2001) | Ad-IL10 |
In vivo: animal model of autoimmune encephalomyelitis |
Therapeutic effects of intracranial and systemic administration of an Ad encoding an antiinflammatory cyto- kine are compared |
| Gerdes et al. (2000) | Ad-hCMV, mCMV promoters- lacZ |
In vivo: rats | Ad-delivered MIEmCMV promoter per- formance is characterized |
| Driesse et al. (2000) | Ad-TK or lacZ | In vivo: rats and nonhuman primates | Analysis of immune response to CSF administration of Ad encoding TK with subsequent GCV or lacZ |
| Zou et al. (2000) | Ad and HD-Ad-β-Geo | In vivo: hippocampus of rats | Immunogenicity and its effects on trans- gene expression and stability are com- pared in HD and first-generation Ad |
| Kajiwara et al. (2000) | Ad-lacZ | In vivo: mice | Investigation of the humoral immune response to a first-generation Ad and its transgene delivered into the brain |
| Dewey et al. (1999) | Ad-TK | In vivo: rat glioma model | Characterization of long-term effects of Ad-mediated conditional cytotoxic gene therapy for glioma |
| Ohmoto et al. (1999) | Ad-lacZ | In vivo: different mouse strains | Influence of environmental conditions and animal priming with Ad on quality and duration of immune response to Ad gene delivery is examined |
| Cartmell et al. (1999) | Ad-hCMV-lacZ, Ad-0, Ad-TK |
In vivo: intrastriatal and intraventricular administration in rats |
Role of TNF-α and IL-β in mediating an early inflammatory response to Ad administered to different CNS targets is delineated |
| Proescholdt et al. (1999) | Ad-CMV-VEGF, Ad-CMV-lacZ | In vivo | Inflammatory response to Ad-mediated VEGF versus pump-injected protein and its effects on the integrity of the BBB are studied |
| Shine et al. (1997) | Ad-RSV-TK | In vivo: cotton rat | Toxicity of Ad-mediated TK +/− GCV at different doses in naive and pre- immunized rats is tested |
| Kajiwara et al. (1997) | Ad-lacZ | In vivo: mice | Quantitative analysis of the immune response to an Ad-encoding lacZ and transgene longevity |
| Byrnes et al. (1996) | Ad-lacZ | In vivo: mice | Immune response to Ad-delivered lacZ is examined |
| Byrnes et al. (1996a) | Ad-RSV-lacZ, HSV-1 RSV- lacZ, HSV-1 tsK |
In vivo: caudate nucleus of rats | Duration of Ad-mediated transgene ex- pression and related inflammation in immune-naive animals and subse- quent peripheral exposure to the vector are compared |
| Byrnes et al. (1995) | AdR1, Ad0, Ad5 | Brain, in vivo; 3 × 106 pfu/0.5 μl, injection site |
Study of the immune response to the direct administration of adenovirus recombinants into the brain. |
| Davidson et al. (1994) | Ad5-RSV-lacZ and RSV-rHPRT | Striatum and cortex in vivo | Gene therapy into primate brain; im- mune response to adenovirus vectors and encoded transgenes |
| (7) Neuroprotection | |||
| Wang et al. (2001) | Ad-WldS | In vitro: rat DRG neurons | Ad-mediated WldS protein utilized against axonal degeneration |
| Tominaga-Yoshino et al. (2001) | Ad-APP, Ad-lacZ | In vitro: cultured hippocampal neurons | Adenoviral introduction of APP cDNA to enhance neurotoxic and neuro- protective effects of glutamate |
| Yuan et al. (1999) | Ad-CMV-APP or lacZ | In vitro: central neuron cultures | Ad is used as a vector for protein trafficking and processing studies to elucidate pathogenesis of AD |
| Horellou et al. (1994) | Ad-RSV-hTH, Ad-RSVlacZ | Brain in vivo | Gene therapy of Parkinson’s disease; significant reduction in abnormal behavior of intrastriatal AdRSVhTH vs AdRSVlacZ |
| Apoptosis Zhu et al. (2002) |
Ad-TGF-β1 | In vivo: mouse brains | Transfer of an antiapoptotic gene to the CNS for neuroprotection |
| In vitro: rat primary hippocampal cells | |||
| Araki et al. (2001) | Ad-(wt or mutant) PS2, Ad-lac | In vitro: rat primary cortical neurons | The proapoptotic effect of Ad-mediated PS2 overexpression is examined in vitro |
| Xia et al. (2001) | Ad-EGFP, Ad-JBD |
In vitro: human neuroblastoma cells; in vivo: mouse MPTP model of PD |
Ad-induced inhibition of the JNK path- way protects MPTP lesioned DA neurons |
| Neurotrophic factors Dorsey et al. (2002) |
Ad-trkB |
In vitro: cultured primary hippocampal neurons |
Correction of dysregulated receptor expression with an adenoviral vector relating to an animal model of Down’s syndrome |
| Benraiss et al. (2001) | AdCMV-BDNF-IRES-hGFP |
In vivo: intraventricular administration in rats |
Ad delivered BDNF to induce neuronal recruitment from endogenous progeni- tor cells in the adult forebrain |
| Connor et al. (2001) | Ad-lacZ, Ad-GDNF |
In vivo: intrastriatal and intra-SN administration in a 6-OHDA rat model of PD |
Neuroprotective mechanisms of Ad- mediated GDNF are elucidated |
| Kozlowski et al. (2001) | Ad-hGDNF | In vivo: monkey caudate nucleus | Quantitative assessment of Ad-mediated hGDNF expression, using ELISA and (RT-)PCR techniques |
| Kozlowski et al. (2000) | Ad-lacZ, Ad-GDNF |
In vivo: intrastriatal and SN injection in a 6-OHDA rat model |
Behavioral testing of Ad-mediated GDNF therapy into the striatum or SN is compared |
| Connor et al. (1999) | Ad-LacZ, Ad-GDNF, Ad-mu GDNF |
In vivo: 6-OHDA PD model in aged rats | Effects of Ad-mediated GDNF delivery to the striatum and SN are compared |
| Kitagawa et al. (1999) | Ad-LacZ, Ad-GDNF | In vivo: rats | Effects of Ad-mediated GDNF on is- chemic brain injury are studied |
| Bemelmans et al. (1999) | Ad-LacZ, Ad-BDNF | In vivo: rat model of Huntington’s disease | Therapeutic value of an intrastriatal injection of Ad-mediated BDNF in Huntington’s disease is assessed |
| Choi-Lundberg et al. (1998) | Ad-lacZ, Ad-mGDNF, Ad-GDNF |
In vivo: intrastriatal injection in 6-OHDA rat model of PD |
Effects of intrastriatal administration of an Ad encoding GDNF on dopami- nergic cell survival are examined |
| Choi-Lundberg et al. (1997) | Ad-lacZ, Ad-mGDNF, Ad-GDNF | In vivo: 6-OHDA rat model of PD | Neuroprotective effect of Ad-mediated GDNF injected near the SN is exam- ined |
| (8) Traumatic or Ischemic Brain Injury/Excitotoxicity | |||
| Qiu et al. (2002) | Ad-FasL |
In vivo: mouse parietal cortex and contused human cortical tissue samples |
Role of Fas receptor in traumatic brain injury is addressed with an Ad encod- ing FasL |
| In vitro: embryonal cortical neurons | |||
| Kim et al. (2002) | Ad-Cu/Zn SOD | In vivo: rats | Intracisternal administration of an Ad encoding human Cu/Zn SOD to restore CBF autoregulation after fluid percussion injury |
| Matsuoka et al. (2002) | Ad-bFGF, Ad-Bcl-xL | In vitro: rat neuronal cells | Synergistic neuroprotective effect through Ad-mediated bFGF and Bcl- xl against EAA toxicity |
| Aoki et al. (2001) | Ad-GRP78 |
In vitro: rat hippocampal neurons; in vivo: hippocampus of gerbils |
Ad-mediated overexpression of GRP78 preserves rat neurons; hypothermic treatment is neuroprotective by restor- ing GRP78 expression in ischemic brain |
| Masada et al. (2001) | Ad.RSVlacZ, Ad-IL-1ra |
In vivo: cerebral intraventric-ular injection in rats |
Ad-mediated overexpression of IL-1ra reduces ischemic brain injury |
| Yang et al. (2001) | Ad-CMV-lacZ, Ad-CMV- mSAG, Ad-CMV-SAG |
In vivo: mouse | Overexpression of SAG, an antioxidant protein, via an Ad vector to prevent ischemic injury of brain cells |
| Gupta et al. (2001) | Ad-RSV-LacZ, Ad-RSV-GT | In vitro: hippocampal cultures | Ad-induced overexpression of the Glut-1 glucose transporter in vitro enhances neuronal survival after exposure to the excitotoxin KA |
| Ralph et al. (2001) | Ad-TRE-pep2m, Ad-TRE- pep4c, Ad-TRE-EGFP, Ad-Syn-tTA |
In vitro: primary hippocampal neurons | AMPA receptors convey postischemic neurotoxicity; Ad-mediated reduction of AMPA receptor expression provides neuroprotection |
| Ooboshi et al. (2001) | Ad-lacZ | In vivo: spontaneously hypertensive rats | Effects of brain ischemia on expression of an Ad-delivered marker gene are examined |
| Yang et al. (1999) | Ad-RSV-lacZ, AdRSV-IL-1ra | In vivo: CD-1 mouse | Ad-mediated overexpression of IL-1ra is used to assess the role of interleukin-1 in cerebral ischemia |
| Yang et al. (1999) | Ad-RSV-lacZ, AdRSV-IL-1ra |
In vivo: intraventricular administration in CD-1 mice |
Effects of Ad-delivered IL-1ra on ICAM- 1 protein in cerebral ischemia |
| Yang et al. (1997) | Ad-RSV-lacZ, AdRSV-IL-1ra |
In vivo: intraventricular administration in mice |
Effect of Ad-mediated IL-1ra on cerebral ischemia is assessed |
| Hagan et al. (1996) | Ad-lacZ, Ad-IL-1ra | In vivo: perinatal rats | Impact of Ad-mediated overexpression of IL-1ra is assessed in a model of excitotoxicity |
| Betz et al. (1995) | Ad-RSV-lacZ, AdRSV-IL-1ra | In vivo: intraventricular injection in rats | Suitability of Ad-mediated IL-1ra in reducing ischemic brain damage is tested |
| (9) Brain Tumors | |||
| Abe et al. (2002) | Ad-lacZ, Ad-p53 | In vivo: athymic rat brains | Intraarterial infusion of p53-containing Ad |
| Shinoura et al. (2002) | Ad-APAF1, Ad-Casp9, Ad-Bax, Ad-Fas, Ad-p53 |
In vivo: glioma cells harboring a mutated p53 |
Cotransduction of Apaf-1 and caspase-9 via Ad to increase p53-mediated apoptosis in glioma cells |
| Miller et al. (2002) | Ad-CMV-CD |
In vitro: different human tumor cell lines; in vivo: immunodeficient mouse model of glioma |
Glioma cell lines can be as sensitive as GI tumor cells to the antineoplastic effects of 5-FU; efficacy and specificity of Ad- mediated CD and subsequent systemi- cally delivered 5-FC are tested in a mouse model of human glioma |
| Adachi et al. (2002) | Ad-uPAR/p16 |
Ex vivo intracerebral tumor model and in vivo subcutaneous tumor model |
Ad-mediated delivery of a bicistronic construct containing antisense uPAR and sense p16 genes to suppress glioma invasion and growth |
| Nanda et al. (2001) | IG.Ad5E1(+); E3Luc, HSV1- TK gene [IG.Ad5E1(+).E3TK] |
In vitro: human glioma cell lines, rat gliosarcoma cell line, other tumor cell lines; in vivo: a mouse s.c. glioma xenograft model |
Combination of replication-competent Ad and the HSV1-TK/GCV suicide system to improve glioma treatment outcome |
| Maleniak et al. (2001) | AdhCMV-mFasL, Ad-CMV-TK |
In vitro: primary human glioma-derived cell cultures |
Ad delivered FasL or HSV1-TK/GCV therapy to induce cell death in some glioma cell lines resistant to the chemotherapeutic agent CCNU |
| Okada et al. (2001) | Ad-TK | In vivo: rat glioma model | s.c. tumor injection of Ad expressing HSV1-TK/GCV shows vaccination effect by inducing a CTL response |
| Galanis et al. (2001) | Ad-MV-F/H |
In vitro: cultured glioma cell lines; in vivo: glioma xenografts in nude mice |
Assessment of two fusogenic membrane glycoproteins as adenovirally delivered transgenes for glioma therapy |
| Lammering et al. (2001) | Ad-EGFR-CD533 |
In vitro: human glioma cell lines; in vivo: glioma xenografts |
Ad-mediated overexpression of a dominant- negative epidermal growth factor receptor to achieve radiosensitization of glioma cells |
| Glaser et al. (2001) | Ad-TK | Human malignant glioma cells | Molecular pathways mediating TK/ GCV-induced cell death are eluci- dated |
| Eck et al. (2001) | Ad-CMV-hIFN-β | Phase I trial in humans | Assessment of toxicity and efficacy of hIFN-β intralesional gene therapy for patients with recurrent or progressive malignant glioma with tumor resection |
| Sandmair et al. (2000) | Ad-CMV-TK | Phase I trial in humans | Evaluation of safety and efficacy of TK gene therapy by using retrovirus packaging cells and Ad |
| Trask et al. (2000) | Ad-RSV-TK | Phase I clinical study | Patients with recurrent malignant brain tumors are treated with HSV TK/ GCV |
| Lawinger et al. (2000) | Ad-REST-VP16 |
In vitro: three types of human medulloblastoma cells |
Effects of expression of REST-VP16 mediated by an Ad vector are studied in medulloblastoma cells |
| Brust et al. (2000) | Ad-TK |
In vitro: RT2 rat glioma cells; in vivo: soft tissue RT2 cell tumors in rats |
Combination of Ad-delivered TK/GCV therapy and subsequent radiosensitiza- tion with BrdC |
| Shinoura et al. (2000) | Ad-FasL, Ad-p53 |
In vitro: A-172 and U251 glioma cell lines |
Coinfection with Ad-mediated p53 and FasL to enhance apoptosis in glioma cells |
| Kurihara et al. (2000) | AxCALNLNZK, Ax2iNPNCre |
In vitro: seven human glioma cell lines, HeLa and COS-7 cells |
Glioma-cell specific Ad-mediated expres- sion is compared in different glioma and nonglioma cell lines |
| Cowsill et al. (2000) | Ad-TK, AdΔTK |
In vitro: primary neuronal and glial cultures; in vitro: rat striatum |
Ad encoding TK or its truncated form +/− GCV is compared |
| Adachi et al. (2000) | Ad-lacZ, Ad-CAG-UPRT, Ad-CD | In vivo: rat brain tumor model | Ad-mediated combined CD/5-FC and UPRT therapy is evaluated |
| Fathallah-Shaykh et al. (2000) | Ad-lacZ, AdIFNγ |
In vivo: mouse model of metastatic brain tumor |
Therapeutic effect of Ad-mediated IFN- γ is examined |
| Biroccio et al. (1999) | Ad-p53, Ad-0 | In vitro: different glioblastoma cell lines | wt-p53 gene transfer enhances BCNU sensitivity in glioblastoma cells depen- ding on the administration sequence |
| Harada et al. (1999) | Ad-p16 | Evaluation of the effect of restoring Ad- mediated p16 on tumor angiogenesis in p16-deleted glioma cells |
|
| Shinoura et al. (1999) | Adv-lacZ-F/K20 and Adv-lacZ-F/wt |
In vitro U-373 MG glioma cells; in vivo | Therapeutic effect of replication- competent Ad with a fiber mutation is compared to its corresponding Ad with wt fiber |
| Wagenknech et al. (1999) | Ad-XIAP | In vitro: different human glioma cell lines | Expression and biological activity of XIAP in human malignant glioma are examined using Ad as a delivery tool |
| Driesse et al. (1998) | Ad-TK | In vivo: nonhuman primates | Toxicity study of Ad-mediated TK with GCV therapy |
| Puumalainen et al. (1998) | Ad-lacZ, BAG retrovirus-lacZ | In vivo: humans | Safety and efficiency of retrovirus and Ad-mediated β-galactosidase in human malignant glioma are examined |
| Gomez-Manzano et al. (1997) | Ad-p53/p21 | In vitro: human glioma cell lines | Ad-mediated p53 and p21 is examined to characterize their role and timing in p53-induced apoptosis |
| Smith et al. (1997) | Ad-GFP, Ad-GFP/5-HT3 | In vivo: rats and monkeys | Toxicity and dose profiles are presented for Ad-mediated TK and systemic GCV administration in healthy animals |
| Tanaka et al. (1997) | Ad-sPF4 | RT2 glioma cells in vitro and in vivo, injected into the subrenal capsule or the caudate nucleus of nude mice |
Analysis of the activity of retroviral and Ad vectors expressing a secretable form of PF4, an antiangiogenic protein |
| Eck et al. (1996) | Ad-TK |
In vivo: human phase I trial in patients with recurrent glioma |
Evaluation of safety and efficiency of Ad-mediated TK followed by systemic GCV therapy |
| Vincent et al. (1996a) | Ad-MLP-luc, Ad-MLP-TK |
In vitro: several human cell lines; in vivo: rats |
Efficiency of Ad-mediated TK and systemic GCV administration is as- sessedin a rat model of leptomenin- geal metastases |
| Goodman et al. (1996) | Ad-RSV-TK | In vivo: baboons | Toxicity studies of Ad-mediated TK and systemic GCV in nonhuman primates |
| Vincent et al. (1996b) | Ad-TK | In vivo: 9L tumors in rats | Ad-mediated TK transfer is compared to retroviral mediation followed by sys- temic GCV in a rat gliosarcoma model |
| Viola et al. (1995) | Ad-lacZ |
In vitro: human glioma cell cultures; in vivo: rat models of solid brain tumors and meningeal cancer |
Transduction profiles of Ad encoding β-galactosidase in solid and leptomi- nengeal tumor models are assessed |
| Badie et al. (1994) | AdLacZ | Rat C6 glioma cells | Delivery of marker genes to the CNS and brain tumors |
| Boviatsis et al. (1994) | Ad5MLP lacZ | Rat 9L gliosarcoma cells |
β-Galactosidase expression in tumors and neurons; tumor necrosis |
| Chen et al. (1994) | Ad5, Ad/RSV-TK | C6 glioma | Tumor treatment/significant tumor de- crease/long-term tumor regrowth |
| Perez-Cruet et al. (1994) | Adv-tl | In vivo: 9L in Fischer rats | Tumor treatment effective for >120 days |
| (10) Metabolic Brain Disease | |||
| Kosuga et al., 2001a | Ad-lacZ, Ad-GUSB | In vivo: monkey |
In vitro enzyme deficiency of MPSVII reduced by transduction with an Ad-expressing GUSB; adenovirally transduced mAEC transplanted into monkey brain to determine their migration and distribution |
| Shen et al., 2001 | Ad-GALC |
In vivo: intraventricular administration in a murine model of human globoid cell leukodystrophy |
Effects of Ad-mediated replacement of GALC in Krabbe disease |
| Kosuga et al., 2001b | Ad-GUSB | In vivo: mice | Adenovirally transduced mAEC overexpressing GUSB transplanted into mice with MPSVII |
| Ghodsi et al., 1998 | Ad-GUSB |
In vivo: intraventricular or intrastriatal injection in MPSVII or wt mice |
Activity of Ad-delivered GUSB is examined up to 84 days postadministration |
| Peltola et al., 1998 | Ad-AGA, Ad-AGU(Fin) |
In vitro and in vivo: primary neuronal cultures from/and mouse model of AGU |
Effects of systemic and intraventricular administration of AD encoding for the deficient enzyme AGA |
| Plumb et al., 1994 | Ad-RSV-rHPRT | In vivo: mice | Utility of Ad encoding HPRT in HPRT-deficient mice is examined |
| (11) Other Applications: Brain Imaging, Pathway Tracing, Substance Abuse | |||
| Umegaki et al. (2002) | Ad-CMV-DopD(2)R | In vivo: rats | PET is shown to be useful for longi- tudinal in vivo assessment of D(2)R expression mediated by an Ad vector in rat brain |
| Ross et al. (1995) | Ad-TK | In vivo: 9L gliosarcoma rat model | Ad-mediated TK and GCV therapy is evaluated with MRI and localized H magnetic resonance spectroscopy |
| Mackler et al. (2000) | Ad-NAC-1 | In vivo: rats | Ad-mediated overexpression of NAC-1 protein affects long-term behavior in psychostimulant abuse |
| Lisovoski et al. (1994) | Ad-RSV-LacZ, Ad type 5 | Spinal cord | Neuroanatomy: cell labeling for morpho- logical studies |
| Kuo et al. (1995) | Ad-RSVlacZ |
In vivo; injection into the laterodorsal striatum |
Suitability of an Ad vector encoding β-galactosidase for neuronal pathways tracing is tested |
| Ridoux et al. (1994a) | Ad-RSVLacZ | CNS, basal ganglia | Pathway tracing in the CNS |
| Abbreviations | |
|---|---|
| Ad | Adenovirus |
| AD | Alzheimer’s disease |
| AGA | Aspartylglucosaminidase |
| AGU | Aspartylglucosaminuria |
| APP | Amyloid precursor protein |
| α1-AT | α1-Antitrypsin |
| BBB | Blood–brain barrier |
| BCNU | Carmustine |
| BDNF | Brain-derived neurotrophic factor |
| bFGF | Basic fibroblastic growth factor |
| BrdC | 5-Bromo-2′-deoxycytidine |
| CAR | Coxsackie and adenovirus receptor |
| CAV2 | Canine adenovirus type 2 |
| CBF | Cerebral blood flow |
| CCNU | 1-(2-Chloroethyl)-3-cyclohexyl-1-nitrosourea |
| CD | Cytosine deaminase |
| CMV | Human cytomegalovirus |
| CTL | Cytotoxic T-lymphocyte |
| DA | Dopaminergic |
| DRG | Dorsal root ganglion |
| D(2)R | Dopamine D(2) receptors |
| EAA | Excitatory amino acid |
| FasL | Fas lingand |
| 5-FC | 5-Fluorocytosine |
| FIV | Feline immunodeficiency virus |
| FPI | Fluid percussion injury |
| 5-FU | 5-Fluorouracil |
| GALC | Galactocerebrosidase |
| GI | Gastrointestinal |
| GRP78 | Glucose-regulated protein 78 |
| hGDNF | Human glial cell-line-derived neurotrophic factor |
| GUSB | β-Glucuronidase |
| HCMV major IE | Human cytomegalovirus major immediate early |
| HD | Helper dependent |
| HPRT | Hypoxanthine-guanine-phosphoribosyltransferase |
| HSV1 | Herpes simplex virus type 1 |
| ICAM-1 | Intercellular adhesion molecule-1 |
| IL-1β | Interleukin-1β |
| IL-1ra | Interleukin-1 receptor antagonist |
| IRES | Internal ribosomal entry site |
| JBD | JNK-binding domain |
| JNK | c-Jun N-terminal kinase |
| KA | Kainic acid |
| LTR | Long terminal repeat |
| Luc | Luciferase |
| MIEmCMV | Major immediate early murine CMV |
| MION | Monocrystalline iron oxide nanoparticles |
| MLP | Major late promoter |
| MPSVII | Mucopolysaccharidosis type VII |
| MPTP | 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine/1-methyl-4-phenylpyridinium |
| MRI | Magnetic resonance imaging |
| Nls | Nuclear localization signal |
| NSE | Neuron-specific enolase |
| NRSE | Neuron-restrictive silencer element |
| 6-OHDA | 6-Hydroxydopamine |
| PD | Parkinson’s disease |
| PET | Positron emission tomography |
| PF4 | Platelet factor 4 |
| PK | Phosphoglycerate kinase |
| PLGA | Polylactic glycolic acid |
| PS2 | Presenilin 2 |
| RAd | Recombinant adenovirus |
| REST | Repressor element 1 silencing transcription factor |
| RPE | Retinal pigment epithelium |
| RSV-LTR | Rous sarcoma virus-long terminal repeat |
| SCG | Superior cervical ganglia |
| SN | Substantia nigra |
| TGF-β1 | Transforming growth factor-β1 |
| TH | Tyrosine hydroxylase |
| TK | Thymidine kinase |
| TNF-α | Tumor necrosis factor-α |
| trkB | Tyrosine receptor kinase B |
| UPAR | Urokinase-type plasminogen activator receptor |
| UPRT | 5-Fluorouridine 5′-monophosphate |
| VEGF | Vascular endothelial growth factor |
| VP16 | Activation domain of a viral protein |
| WldS protein | Unique protein derived from a spontaneous mutant mouse, characterized by slow Wallerian degeneration |
| Wt | Wild type |
III. Developments in the Use of Adenovirus Recombinants as Vectors
The deletion of the Ad E1 gene region results in a failure of the replication-deficient Ad vector to activate both early and late phase transcription from the viral genome. Consequently expression of only a transgene under the control of a constitutive or cell-type-specific or inducible promoter is achieved in the infected target cell. Although this genetic barrier to adenovirus gene expression is efficient, it is not absolute. Even limited breakthrough of Ad gene expression does occur in many if not most tissues, and can thus be problematic, it may either by itself affect the physiology of the target cell, induce cytotoxic responses, a cellular immune response, as well as provide antigenic epitopes recognized by the adaptive arm of the immune response (Engelhardt et al., 1994; Yang et al., 1994a,b; Thomas et al., 2000b, 2001b; Lowenstein and Castro, 2002; Mahr and Gooding, 1999; Wold et al., 1994, 1999; Hackett et al., 2000; Wood et al., 1996a). Although infections at low multiplicity with E1-deleted vectors are unlikely to lead to high level adenoviral gene expression, the same is not true at high multiplicity of infections such as used in gene therapy applications. This is likely to remain the case, even if deletion of Ela, Elb, and other regions of the adenovirus genomes provides a better block than simply the Ela deletion (Imperiale et al., 1984; Spergel and Chen-Kiang, 1991). Nevertheless extremely high input multiplicities of infection (in excess of 100 or even higher infectious units per cell regularly achieved in in vivo experimental designs) can result in breakthrough of low level expression of the Ad genome (Engelhardt et al., 1994; Kass-Eisler et al., 1994; Yang et al., 1994a,b). A further modification to current vectors is therefore of interest, i.e., the supplementation of deletions in E1, E3, with an additional conditional mutation in E2a, the so-called “second-generation vectors” (Engelhardt et al., 1994; Yang et al., 1994a,b). The E2a region plays a role in activating late phase gene expression. Recombinants carrying this additional mutation have proved to be less reactogenic and permit prolonged expression of the transgene (Engelhardt et al., 1994; Yang et al., 1994a,b). Expression of late genes from the adenoviral genome that are normally expressed only following adenoviral DNA replication suggests that limited replication of the adenoviral genome may occur in some cells. This remains to be proven. In view of the fact that the liver contains proteins that can substitute for E1a function, this topic ought to be investigated carefully.
Further, forskolin can increase the breakthrough to late gene expression. Forskolin results in enhanced levels of intracellular calcium and activation of the transcription factor cAMP responsive element binding protein (CREB or ATF), a factor that is known to bind and stimulate transcription from the Ad early promoter (Muchardt et al., 1990). It is therefore not surprising that forskolin treatment of cells infected with a replication-deficient Ad recombinant can enhance breakthrough to late phase gene expression. Both a high input multiplicity of infection (around the site of virus inoculation or instillation) and elevated calcium levels are likely to occur in transduced cells following in vivo gene transfer. Both these phenomena are very likely to coincide in neurons surrounding the site of adenovirus direct injection into the brain. Physiological conditions at the site of gene transfer could thus further regulate transgene expression from viral vectors.
The induction of an immune response to elements of the Ad vector is now perceived as a significant limitation of the vector in long-term gene therapy protocols, especially when systemic administration is needed. Several groups have characterized the effects of the virions on brain inflammation, and the effects of peripheral injections on the stimulation of adaptive immune responses (Byrnes et al., 1995, 1996a,b; Matyszak and Perry, 1996; Wood et al., 1996a; Wood, 1996; Morral et al., 1997; Matyszak 1998; Perry, 1998; Gerdes et al., 2000; Thomas et al., 2000b, 2001a,b, 2002). The effects of each of these on transgene expression are reviewed in detail elsewhere (Lowenstein and Castro, 2002). Although gene transduction remains possible in seropositive animal models and thus possibly in human clinical trials, repeated exposure to viral antigens will be expected to reduce the efficiency of gene transfer. The lack of need to utilize adjuvant when immunizing against adenovirus indicates that even small amounts of vectors leaking into the peripheral immune compartments could activate such immune responses. In the nervous system elements of the adaptive immune response, but not innate inflammatory processes and cells, are able to completely shut-off transgene expression, apparently in the absence of cytotoxicity (unpublished observations). Whether similar phenomena occur in other organs remains to be determined.
Utilizing different Ad serotypes as vectors in sequential treatment administrations could to some extent circumvent this problem. Breakthrough to early phase transcription was demonstrated by the identification of a humoral immune response to early phase proteins. In a mouse model breakthrough has been strongly correlated with the induction of CD8+ cytotoxic T-lymphocyte (CTL) response to adenovirus proteins, which is responsible both for an inflammatory response and an elimination of target cells expressing a transgene (Yang et al., 1994a,b). It has, however, been difficult to conclusively demonstrate that such CTLs are effectively cytotoxic in vivo, and thus some of these results and their implications for immune-mediated killing of target cells have been challenged (Wadsworth et al., 1997). Many experiments have been performed using extremely high doses of input virus. If lower doses of adenovirus recombinant are inoculated into a mouse, major histocompatibility complex-I (MHC-I) responses specific for a viral transgene in the absence of a detectable response to the vector can be induced (Schadeck et al., 1999). However, high input doses of recombinant may be essential for efficient in vivo gene transfer.
It is also important to consider that many experiments are performed in non-permissive or semipermissive species (Ginsberg et al., 1991; Ross and Ziff, 1992), e.g., mice, rats, and nonhuman primates. In such systems, Ad E1 recombinants are unable to replicate due to a “species block.” It would thus be useful to test recombinants in a more relevant species, such as “permissive” cotton rat (Sigmoidon hispidus), to determine whether cell-specific factors in addition to species-specific factors regulate the capacity of adenovirus to replicate in particular target organs (Oualikene et al., 1994).
Many researchers see the current cloning capacity of ~7–8 kb as a limitation toward the more general applicability of adenoviral vectors. There is a stringent limitation exerted by the adenovirus particle, which will package only an additional 3% ( ~1 kb) in addition to the full-length viral genome (Shenk, 1995). Thus, a new generation of high-capacity helper-dependent adenoviral vectors (also known as “gutless” or “gutted” vectors; HD-Ad) has been developed that is devoid of all viral coding sequences (Fig. 2) (Kochanek et al., 1996; Mitani et al., 1995; Chen et al., 1997, 1999; Morral et al., 1998; Morsy et al., 1998; Schiedner et al., 1998; Burcin et al., 1999; Sandig et al., 2000; Akagi et al., 1997; Maione et al., 2001; Parks et al., 1996, 1999a,b; Cregan et al., 2000; Ng et al., 2001, 2002; Umana et al., 2001; Lowenstein et al., 2002). These vectors have a minimum requirement for the extreme termini of the linear adenovirus genome, containing only those cis-acting elements for viral DNA replication and packaging, mainly the inverted terminal repeat (ITR) sequences and packaging signal. Because these elements are contained ~500 bp from the ends of the genome (Grable and Hearing, 1992), helper-dependent vectors have the potential encode range of few hundred base pairs to up to ~30 kb of foreign DNA, which is close to the size of the native genome.
Fig. 2.
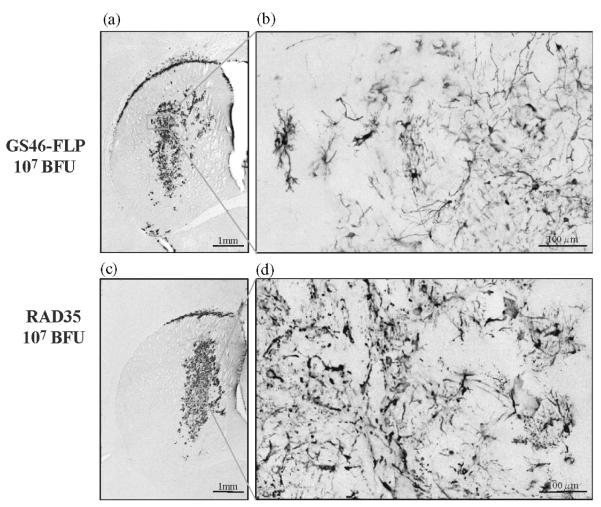
An E1-, E3-deleted helper adenovirus (FL helper) with a packaging signal sensitive to FLP3-mediated excision. (A) FL helper was generated by homologous recombination in 293 cells after cotransfection with plasmid pΔE1sp1A-2xFRT and pBHG10-CMVluc-I. The bottom half of the figure indicates the left-end region of the helper virus genome, before and after FLP3-mediated recombination; the figure also indicates the location of the PCR primers used to determine excision of the packaging signal, ψ (flanked by black arrows). (B) Production of gutless adenoviral vectors. Diagram of an ideal gene delivery vector for neurological gene therapy using the “gutless” adenoviral vector. The packaging signal of the helper virus, flanked by FRT sites, is excised after growth in 293 cells expressing FLPe recombinase. The cloning capacity of this system (N30 kbp) will enable the simultaneous use of two or more therapeutic genes and also includes inducible promoter systems. (See Color Insert.)
HD-Ad are copropagated with an E1-deleted helper virus, which provides in trans all of the proteins required for packaging the vector. Up to now, several systems have been developed to prevent packaging of the helper virus. The Cre/loxP-based system for the generation of HD-Ad involves the use of a first-generation helper virus, where the packaging signal is flanked by loxP recognition sites (Hardy et al., 1997). Infection of Cre-expressing 293 cells with the helper virus results in excision of the viral packaging signal, rendering the helper virus DNA unpackagable but still able to replicate and provide helper functions for HD-Ad vector propagation (Chen et al., 1996). Purification by cesium chloride centrifugation is necessary to reduce the titer of the helper virus to negligible levels, typically ranging from 0.1 to 0.01% of the HD-Ad titer (Morsy et al., 1998). Recently, another Flp/frt-base system has been developed. The Flp recombinase was used in place of Cre, and shown to excise the frt-flanked packaging signal in helper virus efficiently (Lowenstein and Castro, 2001; Ng et al., 2001; Umana et al., 2001). The most recent improvement to this system is the development a new Cre-expressing cell line based on E2T, an E1 and E2a complementary cell line (Zhou et al., 2001). Thus an E1 and E2a double-deleted helper virus can be used with the new cell line to produce HD-Ad vector with low helper contamination, further improving HD vector safety (Zhou et al., 2001). Compared with first-generation adenovirus vectors, the HD-Ad vector can efficiently transduce a wide variety of cell types from numerous species in a cell cycle-independent manner as first generation, but HD-Ad vector has the added advantage of increased cloning capacity, reduced toxicity and immune responses, and prolonged stable transgene expression in vivo (Schiedner et al., 1998; Thomas et al., 2000a,b; Thomas et al., 2001a,b). This system is essentially analogous to herpes simplex virus (HSV1) amplicons and shares some of their limitations (Lowenstein, 1994, 1995), e.g., low titers and the presence of variable amounts of helper virus in viral stocks, although novel systems that produce vectors in a helper-free fashion are now available (Wade-Martins et al., 2001; Logvinoff and Epstein, 2001; Wang et al., 2002). Eventually, it should be possible to develop this system so that the recombinants can be produced in the absence of helper virus to generate a safer vector with markedly reduced potential to be reactogenic.
IV. Adenoviral Recombinant Vectors: Applications to Basic Neuroscience
The technology for gene transfer to the brain has progressed substantially during the past 5 years, and new improved systems are being rapidly developed. Table I compares the major systems for gene transfer into neurons in vitro and into the brain in vivo. Vectors vary dramatically in their efficiency of gene transfer, longevity of expression, associate toxicity, and size of the transgene they can harbor. Some vectors mainly direct short-term expression of transgenes, either because of their intrinsic toxicity [e.g., Semliki Forest virus (SFV), vaccinia], or promoter shut-off, a poorly understood phenomenon by which promoters within viral vectors eventually stop being active in spite of the vector genomes being present. Adenoviruses mediate long-term transgene expression in the central nervous system (up to over 1 year) in naive (nonimmunized) animals. Immunization completely eliminates expression from first-generation vectors but not high-capacity helper-dependent adenoviral vectors, whereas in preimmunized animals, transgene expression mediated by first-generation adenoviruses declines within 2–4 weeks postinjection, but expression from the high-capacity helper-dependent adenoviral vectors is reduced only to approximately 50% and stays stable thereafter (Thomas et al., 2000a,b; Thomas et al., 2001b).
In 1993 four groups independently reported for the first time in vivo gene transfer into several types of brain cells using adenovirus recombinant encoding β-galactosidase (Akli et al., 1993; Bajocchi et al., 1993; Davidson et al., 1993; Le Gal La Salle et al., 1993). Since these initial breakthroughs, there has been a logarithmic increase of original papers using adenovirus recombinants to transduce brain cells (see Table II). Although there is some specificity in the capacity of different adenovirus serotypes to infect/express in different target cells (Acsadi et al., 1994a,b; Bessereau et al., 1994; Grubb et al., 1994; Millecamps et al., 1999) a recombinant adenovirus-derived vector can indeed express trans-genes in all brain cells, e.g., neurons, astrocytes, oligodendrocytes, ependymal cells, fibroblasts, macrophages, endothelial blood vessel cells, retinal pigment epithelium, photoreceptors, as well as retinal neurons proper, and peripheral nerve Schwann cells either in vivo or in vitro (Table II; Akli et al., 1993; Bajocchi et al., 1993; Caillaud et al., 1993; Davidson et al., 1993; Le Gal La Salle et al., 1993; Bain et al., 1994; Bennett et al., 1994; Jomary et al., 1994; Li et al., 1994; Byrnes et al., 1995; Lowenstein, 1995; Shering et al., 1997; Morelli et al., 1999; Smith-Arica et al., 2000; Thomas et al., 2000a,b; Umana et al., 2001; Thomas et al., 2000b, 2001a,b, 2002).
While initial experiments were performed using recombinant adenovirus to transfer marker proteins into the brains of both rodents and primates, recombinant vectors encoding therapeutic genes for treatment of neurological diseases have now been generated (Table III) (Chen et al., 1994; Davidson et al., 1994; Perez-Cruet et al., 1994; Shewach et al., 1994; Lowenstein, 1995; Geddes et al., 1997; Ambar et al., 1999; Dewey et al., 1999; Gupta et al., 2001; Bohn et al., 1999; Lawrence et al., 1999; Bohn, 2000; Connor et al., 1999, 2001; Kozlowski et al., 2000, 2001; Amalfitano and Parks, 2002). Expression of transgenes after in vivo administration of vectors to the brain was detected up to 5–6 months and even after 18 months postinoculation (Geddes et al., 1997; Thomas et al., 2000a,b; Thomas et al., 2000b, 2001a,b; Zermansky et al., 2001).
TABLE III.
Neurological Gene Therapy:What Can Be Achieved with Adenovirus Vectors?a
| Noninherited |
Inherited |
||||||
|---|---|---|---|---|---|---|---|
| Disease | Tumors | PDc | HD | CMT | Leukodystrophies | Lesch–Nyhan | Frax-A |
| Outcome | Fatal | Fatal | Fatal | Nonfatal | Fatal | Fatal | Nonfatal |
| Distribution | Focal | Focal | Diffuse | Diffuse | Diffuse | Diffuse | Diffuse |
| Age at manifestation | Variable | Late | Variable | Variable | Early | Early | Prenatal |
| Presymptomatic detectionb | No | No | Yes | Yesd | Yesd | Yesd | Yesd |
| Genotype predicts disease severity |
ND | ND | Yes | Yes | Yes/noe | Yes | No |
| Gene cloned | Yesf | Yesg | Yes | Yes | Yes | Yes | Yes |
| Expression needed | Short term | Long term | Long term | Long term | Long term | Long term | Long term |
| Experimental | Tumor | Dopamine | Reduce | Normalize | BMT | Express | Express |
| Gene therapy | Cell killing | Replacement, GDNF, AADC |
Expression of expanded allele |
PMP22 expression |
Expressing missing genes |
Brain | Brain |
| Clinically relevant available therapeutic protocols |
Yes | Yes | No | No | Yes | No | No |
| Available adenoviral vectors | Yes | Yes | No | No | No | Yes | No |
HD, Huntington’s disease; CMT, Charcot–Marie–Tooth disease (the commonest genetic alteration is duplication of the region of chromosome 17p11.2 containing the PMP 22 gene); Frax-A, fragile X syndrome type A; PD, Parkinson’s disease (idiopathic); BMT, bone marrow transplant. This table is modified from MacMillan and Lowenstein (1996).
Even if presymptomatic detection is available, it is not done routinely during pregnancies; it is performed only if there is any suspicion due to a positive family history.
The primary pathology is loss of dopaminergic neurons from the pars compacta of the substantia nigra, but other lesions within PD are distributed throughout the brain.
Molecular genetic diagnosis is possible in the fetus, but it is arguable whether this would be prior to the occurrence of pathological changes.
Genotype predicts disease severity in metachromatic and Krabbe’s leukodystrophy, but not yet in adrenoleukodystrophy.
The genes mutated in inherited brain tumors have been cloned. However, therapeutic gene therapy is more likely to utilize the transduction of cytotoxic genes to eliminate tumor cells.
The genes for tyrosine hyroxylase and dopa decarboxylase have been cloned. These are not, however, mutated in either familial or sporadic PD.
Other examples of adenoviruses encoding therapeutic transgenes for preclinical applications include recombinant adenoviral vectors encoding hypoxanthine phosphoribosyltransferase (HPRT), which could be utilized in the treatment of the Lesch–Nyhan syndrome. Although these vectors have mainly been utilized to transduce HPRT in primate (Davidson et al., 1994) and rodent brains (Davidson et al., 1994; Lowenstein, 1995; Southgate et al., 2001), there is yet no published account of their capacity to complement an HPRT deficiency in animal models for the Lesch–Nyhan syndrome. Adenovirus vectors expressing tyrosine hyroxylase, and their therapeutic efficacy in a rat model of Parkinson’s disease, have been reported (Horellou et al., 1994; Corti et al., 1999a,b; Hida et al., 1999). Recombinant adenoviruses encoding neuroprotrective factors such as glial cell line-derived neurotrophic factor (GDNF), aimed to prevent dopaminergic neuron degeneration in a rat model of Parkinson disease, have also been successfully developed (Bilang-Bleuel et al., 1997; Choi-Lundberg et al., 1997, 1998; Bohn et al., 1999; Lawrence et al., 1999; Bohn, 2000; Connor et al., 1999, 2001; Kozlowski et al., 2000, 2001).
Because adenovirus recombinants can be used to transduce essentially all brain cells, they have also been postulated to be applicable to gene therapy protocols for the treatment of brain tumors, by utilizing them to deliver cytotoxic genes directly into the tumors. Thus, a number of groups have utilized several adenovirus recombinants, e.g., expressing HSV1 thymidine kinase or Fas ligand, Fas receptor, or p53, under the control of viral promoters. In experimental paradigms the use of these viruses appears to be promising (Badie et al., 1994; Boviatsis et al., 1994; Brody et al., 1994a; Chen et al., 1994; Perez-Cruet et al., 1994; Shewach et al., 1994; Ambar et al., 1999; Dewey et al., 1999; Morelli et al., 1999; Shinoura et al., 2000a,b; Shono et al., 2002; see George et al., this volume).
Recombinant retroviruses have been used in the treatment of brain tumors (Ram et al., 1993; Palu et al., 1999; Tamura et al., 2001), and replication-competent and conditional HSV1 vectors as well as HSV1 amplicon vectors have also been tried (Martuza et al., 1991; Markert et al., 1993; Kramm et al., 1997; Pechan et al., 1999; Herrlinger et al., 2000; Papanastassiou et al., 2002; see Hu and Coffin, this volume). In a recent phase I clinical trial the efficacy and safety profiles of retrovirus and adenovirus expressing HSVI TK have been compared (Sandmair et al., 2000). Adenoviruses showed very promising results, both in terms of their safety and also their efficacy in glioma growth control (Sandmair et al., 2000). However, the only vector system that has progressed to a phase III clinical trial has been a retroviral vector expressing HSV-1 thymidine kinase. The trial was halted because of the absence of positive effects in the gene therapy arm of patients treated (Rainov, 2000; Rainov and Kramm, 2001). With Phase I and II being essentially toxicity trials, Phase III efficacy clinical trials are crucial in order to determine whether any of the new therapies are indeed effective, because such results can be obtained only rarely from the much smaller Phase I and II trials.
Two groups have also made interesting observations on the use of adenovirus recombinants that should be of interest to neuroanatomists, namely the use of adenovirus for pathway tracing and cell labeling. Ridoux and colleagues (1994a) observed retrograde labeling of substantia nigra neurons after injections of recombinants expressing β-galactosidase into the striatum; similar results were also observed by Byrnes et al. (1995). Thus, replication-defective adenovirus could be used for retrograde labeling of neuronal pathways. Interestingly, HSV1-derived recombinants can also be used to trace neuronal pathways (Ugolini et al., 1989), with some recombinants being specific for anterograde transport and others for retrograde transport (Zemanick et al., 1991), while certain pseudorabies recombinants have been shown to be specific markers for individual neuronal circuits and capable of retrograde axonal transport (Card et al., 1991, 1992; Mazarakis et al., 2001; Enquist et al., 1998; DeFalco et al., 2001). Lisovoski et al. (1994) utilized adenovirus recombinants to label neurons of the spinal cord at different stages of development to study their morphological development. The staining they obtained after in vivo administration filled the dendritic arbors of neurons, thus allowing for their morphological and morphometric examination during spinal cord development.
An effective way of delivering gene products to the brain, rather than by introducing it into constituent cells, is by transplanting genetically engineered cells directly into the CNS. So far, most genetically engineered cells expressing trangenes for transplantation have been transduced with recombinant retroviral vectors. Ridoux et al., (1994b) have used adenovirus to tranduce primary cultures of rat astrocytes in vitro and then transplanted these into the CNS of host rats. Expression of transgene was detected for at least 5 months. Thus, adenovirus might constitute an alternative to retrovirus for transducing cells in preparation for transplantation into the brain. In many cases expression from retroviral vectors ceases after a few days to a few weeks; thus, it will be interesting to compare both systems vis-à-vis length of transgene expression.
Expression of transgenes after the administration of adenovirus recombinants into adult brains has been seen by some groups to last from 6 to 18 months. It also was reported that injection of recombinants into neonates has allowed expression to be sustained over periods up to a year. Long-term modulation of the immune response and the elucidation of its exact role in the regulation of long-term expression from adenoviral recombinants administered into the CNS will be crucial to achieve stable expression over a long period of time, which will be needed to implement gene therapy for chronic neurological disorders in humans. (Stratford-Perricaudet et al., 1990; Kass-Eisler et al., 1994; Geddes et al., 1997; Thomas et al., 2000a,b; Thomas et al., 2000b, 2001a,b; Zermansky et al., 2001).
Scant information is available on the interactions of adenovirus with the brain. Thus it will be of great importance to explore this field, e.g., adenovirus entry into brain cells, transport to the nucleus, and viral replication in neurons of permissive species. In addition, the effect of adenovirus recombinants on the electrophysiology of specific neuronal populations will have to be investigated. It also will be critical to assess the effects of adenoviral delivery into different brain regions on animal behavior. It is surprising that not much work on these topics has been published in the past 5 years.
V. Adenoviral Recombinant Vectors: Applications to Neurological Gene Therapy
Essentially there are two types of applications for which vectors for gene transfer into the brain could be used. Short-term expression could be used, for example, to express cytotoxic products to kill tumor cells, or to provide drugs to block ischemia-induced neurotoxicity. Ideally, the area of brain tissue to be targeted should be focal, and therapeutic benefit should be predicted after short-term transgene expression (Table III). Long term expression would be needed to treat chronic neurodegenerative disorders, such as Parkinson’s disease, Alzheimer’s disease, or amyotrophic lateral dystrophy. In this case, the area of brain tissue will be larger, and the results of the therapeutic intervention may not be seen until after several years.
The availability of viral vectors that express short term at high levels of expression could lead to important new treatments for brain diseases. A case in point are brain tumors, which are now being treated experimentally with adenovirus recombinants expressing conditional cytotoxic gene products (Badie et al., 1994; Boviatsis et al., 1994; Brody et al., 1994a,b; Chen et al., 1994; Perez-Cruet et al., 1994; Shewach et al., 1994; Takamiya et al., 1992; Barba et al., 1994; Benedetti et al., 1997; Ram et al., 1997; Bansal and Engelhard, 2000; Ikeda et al., 2000; Trask et al., 2000; Jacobs et al., 2001; Rainov and Kramm, 2001). Brain tumors constitute a good target disease for gene therapy for the following reasons: (1) the disease is focal; (2) the therapeutic objective—the destruction of tumor cells— needs to be achieved within the short term, and this can be done using cytotoxic or conditionally cytotoxic gene products; (3) the disease is life threatening within 6–12 months of diagnosis; (4) no effective treatments are available; and (5) adenovirus-particle-induced inflammation could be a beneficial adjunct to tumor cell elimination. Similarly, replication-deficient and replication-competent HSV1-derived vectors have also been also developed (Martuza et al., 1991; Markert et al., 1993) and these vectors have already been tested in Phase I–III clinical trials (Martuza et al., 1991; Markert et al., 1993, 2000; Rampling et al., 1998, 2000; Rainov, 2000).
Achieving long-term expression in brain following the administration of recombinant vectors will open up the development of gene therapy clinical trials for human neurological diseases where long-term expression is paramount, such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, and the metabolic and degenerative brain disorders (Amalfitano and Parks, 2002; Hsich et al., 2002; Lowenstein, 2002; Lowenstein and Castro, 2002). What makes it difficult to treat such diseases is that they affect large areas of the brain, many extending throughout large anatomical regions of the human CNS, and they progress relatively slowly over years or decades. For Parkinson’s diseases there are as yet no presymptomatic diagnostic methods available, so treatment can be instituted only when symptoms appear. In families suffering from Huntington’s disease, presymptomatic diagnosis is now possible (Holloway et al., 1994) and this might also soon become possible in Alzheimer’s disease (Saunders et al., 1993; Nalbantoglu et al., 1994; Scinto et al., 1994). The diffuse nature of these diseases has led to the development of other therapeutic interventions that could target genes and their products to different areas located throughout the brain, such as transplantation of genetically engineered cells (Fisher, 1993), the direct intracranial administration of neuronal growth factors (NGF), (Seiger et al., 1993), or more recently the development of embryonic and or neural stem cells. HSV1 (Lokensgard et al., 1994; Burton et al., 2001; Kay et al., 2001; Latchman and Coffin, 2001; Lilley et al., 2001) and adeno-associated virus vectors (Kaplitt et al., 1994; Sanlioglu et al., 2001; Fu et al., 2002; Muramatsu et al., 2002) might also provide alternative vectors to achieve long-term transgene expression in brain for clinical applications in humans.
Although human brain infections caused by adenovirus are extremely rare, it has been reported that adenovirus can cause central nervous system disease, especially in children and immuncompromised individuals, although rare cases have also been described in nonimmunocompromised patients (Ginsberg and Prince, 1994; Engel, 1995; Chirmule et al., 1999; Ginsberg, 1999). Meningoencephalitis, encephalitis, and cerebellar ataxia have been reported in children in association with adenovirus infection of the CNS, mostly adenovirus type 7, 1, 2, or 32 (Chou et al., 1973; Kelsey, 1978; Davis et al., 1988; Osamura et al., 1993). Direct inoculation of adenovirus into the brain of rhesus monkeys has demonstrated that several adenovirus serotypes can cause neuropathology, although adenovirus did not appear to replicate to a great extent in the primate brain (Ginsberg and Prince, 1994; Engel, 1995; Chirmule et al., 1999; Ginsberg, 1999). Also, neuropathology due to an anamnestic reponse of the immune system following the injection of the adenovirus type 5 into the primate brain was recently described (Davidson et al., 1994).
The possibility that latent adenovirus infection might be localized to the human brain, and that adenovirus could enter the brain through infected macrophages from the periphery, has not yet been examined in enough detail, although widespread neuronal inclusions diagnostic of adenovirus particles have been seen in some fatal encephalitis cases (Chou et al., 1973). However, all these data taken together with our observations that wild-type adenovirus can express endogenous proteins in neurons, and brain glial cells, strongly suggest that adenovirus could replicate, or at last express many of its genes after infecting human neurons or glial cells and that expression might proceed for a longer time than predicted, even in the absence of symptoms of encephalitis. If there is a risk of adenoviral encephalitis it will have to be carefully considered at a time adenovirus vector is directly administered into the brain. It is clear that many more studies will be required to clarify the origins of neurotoxicity after direct injections of adenovirus virions into the brain. The use of the completely deleted high-capacity helper-dependent adenoviral vectors will completely avoid these potential complications.
VI. Immune Responses to Recombinant Adenoviral Vectors Delivered into the Brain
As stated above, recombinant adenoviruses are very attractive vectors for gene transfer and therapy within the CNS, however, they still cause acute inflammation, and any of their expressed genes can be the target of adaptive immune responses. Local inflammation is elicited in the brain in response to the injection of either a first-generation, or a high-capacity helper-dependent adenoviral vector. This acute inflammation is dose dependent, self-limited (disappears within 30 days), adenoviral-capsid dependent, and does not affect long-term adenoviral-mediated transgene expression (Byrnes et al., 1995, 1996a; Kajiwara et al., 1997; Gerdes et al., 2000; Thomas et al., 2000b, 2001a,b, 2002). Injection of adenoviral vectors into the brain leads to persistent expression (e.g., up to 18 months). This is thought to be due to the failure to stimulate an effective antiadenoviral T cell response following the careful injection of these vectors into the brain parenchyma (Byrnes et al., 1996b; Wood et al., 1996a; Kajiwara et al., 1997; Gerdes et al., 2000; Thomas et al. 2000b, 2001a,b, 2002). Activation of antiadenoviral immune response by peripheral immunization with adenovirus leads to massive lymphocyte infiltration of the brain parenchyma, macrophage/microglial activation, up-regulation of MHCI and II, and loss of vector-mediated transgene expression (Byrnes et al., 1996a; Gerdes et al., 2000; Thomas et al., 2000b, 2001a,b, 2002). Mechanisms of loss of transgene expression have not been completely elucidated. Either cytotoxicity of transduced cells or downregulation of mRNA expression could explain it. Thus, loss of transgene expression from first-generation adenoviral vectors in the brain following systemic immunization against adenovirus limits their utility for long-term neurological gene therapy applications (Lowenstein and Castro, 2002).
Recent work from our laboratory indicates that high doses of E1/E3-deleted viral vectors (>108 IU) cause direct acute cytotoxicity, and chronic inflammation, which lead to reduced transgene expression and substantial tissue damage (Thomas et al., 2001a,b). We thus reasoned that an absolute reduction in the dose of vectors would be necessary to eliminate both the acute cytotoxicity and chronic inflammation. The use of the powerful mCMV promoter in adenoviral vectors has recently uncovered the capacity to transfer transgenes into the brain, and achieving similar results in the levels of transgene expression while using 2–3 logs lower doses of vectors when compared to vectors employing the hCMV promoter. The important reduction in total viral dose needed thus allows transduction in the absence of acute cytotoxicity or chronic brain inflammation (Gerdes et al., 2000).
Additionally, E1/E3-deleted adenoviruses do express adenoviral proteins encoded within their genomes (Yang et al., 1994a,b). These can either generate specific immune responses or provide target epitopes recognized by the activated adaptive immune system. We thus studied transgene expression and inflammatory responses elicited by the intrastriatal injection of E1–E3-deleted or HC-Adv vectors in four different paradigms: (1) acutely in naive rats, (2) long term, in naive animals, (3) long term, in naive animals, followed by a peripheral immunization against adenovirus type 5, and (4) medium term, in animals immunized against adenovirus type 5 preceding intracranial vector injection (Thomas et al., 2000b, 2001a,b, 2002).
These studies have shown the following. (1) Injection of 1 × 107 IU of either E1/E3-deleted vectors or HC-Adv into the brain causes an acute, transitory inflammatory response, with both cellular (e.g., activation of microglia) and molecular components (e.g., upregulation of MHCI-I). This acute inflammation does not affect long-term transgene expression (see Figs. 3–6). (2) Within 12 months the expression of a marker transgene from first generation vectors decreases. This decrease is less when expression is directed by an HC-Adv. (3) Following peripheral immunization against adenovirus type 5, transgene expression from an E1/E3-deleted adenovirus vector is completely abolished within 45 days, whereas expression from a HC-Adv remains unaffected (Fig. 4). (4) Down-regulation of transgene expression appears to be mediated at the transcriptional level (specific reduction of transgene mRNA levels), rather than cytotoxicity (unpublished data). (5) Expression from an E1/E3-deleted adenovirus in brains of animals previously immunized against adenovirus type 5 is almost completely eliminated by 14 days postinjection, whereas expression from an HC-Adv is reduced to only 50%, and remains stable for at least 2 months (Thomas et al., 2000b, 2001a,b, 2002; Lowenstein and Castro, 2002). The complexities, causes, and consequences of using viral vectors as gene transfer vectors in the brain have been explored elsewhere (Lowenstein, 2002).
Fig. 3.
Transgene expression (β-galactosidase) and inflammation in the brains of animals injected with either the E1/E3-deleted vector RAd35 or the HC-Adv AdGS46. Rows a and b show β-galactosidase expression (columns 1 and 2), CD8+ cell infiltration (column 3), MHC class I upregulation (column 4), and microglial cell activation (ED1, column 5), 3 days after injection of 1 × 107 IU of RAd35 (row a) or AdGS46 (row b) into the brains of naive animals. Levels of transgene expression and inflammation mediated by the E1/E3-deleted, or the HC-Adv vector, were indistinguishable in naive animals. Transgene expression from 1 × 107 IU of both RAd35 and AdGS46 is stable for at least 30 days in naive animals and inflammation resolves within this time frame (shown in row c only for AdGS46). However, transgene expression from E1/E3-deleted vectors in the CNS is rapidly eliminated and accompanied by severe brain inflammation if animals receive a subsequent peripheral infection with adenovirus [row d; RAd35 injected in the brain at Day 0, RAdHPRT (Southgate et al., 1999) injected in the skin at Day 60. See also the quanitative analysis of all markers, in panel f]. In contrast, transgene expression from HC-Adv remains stable, and no brain inflammation is elicited when animals are subsequently injected with RAdHPRT in the skin (row e; AdGS46 injected in the brain at Day 0, RAdHPRT injected in the skin at Day 60).
Fig. 6.
The mCMV promoter allows high-level expression at low doses of vector, without inflammation. Increasing doses of RAd36 (mCMV-βgal) (104–106) were injected into the striatum of adult Sprague–Dawley rats in a total volume of 3 μl, and animals were perfused 5 days later. Brains were processed for immunohistochemistry to detect β-galactosidase (transgene expression; first two left hand side columns), ED1 (macrophages-microglial cells), and CD8 (infiltrating lymphocytes and NK cells). Low-power view of the striatum is shown in the left-hand side column, and a higher power view is shown in the column next to it. Scale bar shown in the upper left = 1 mm; all others = 100 μm. RAd36 allows strong transduction of the striatum, in the absence of recruitment of inflammatory cells, compared to animals injected with saline controls. This image illustrates three doses of animals injected with RAd36. The quantification of this experiment is shown in Fig. 4, above.
Fig. 4.
Preimmunization against adenovirus eliminates rapidly transgene expression from E1/E3-deleted vectors, but not from high-capacity vectors. Quantification of the area within 40-μm-thick brain sections occupied by β-galactosidase and ED1. Error bars show the SEM value from the five animals in each experimental group. Student’s t test was used to calculate the degree of significance of differences between levels of transgene expression and inflammation in the brains of nonimmunized animals (black bars) and immunized animals (hatched bars) after intrastriatal injection of RAd35 (right) or AdGS46 (left). The x-axis indicates the days after vector injection; vector was injected on day 0, and animals were immunized 14 days before the brain injection.
VII. Summary: Targeting the Brain with Adenovirus-Derived Vectors
The early generations of replication-deficient adenovirus vectors, as well as the most recently developed high-capacity helper-dependent adenovirus vectors are extremely versatile vehicles for gene transfer into the brain, and have thus become invaluable reagents to neuroscientists. Current applications cover wide areas from helping to address basic neurobiological problems to clinical trials for the treatment of human diseases. The main advantages of adenovirus-derived vectors are as follows:
the existence of well-characterized methods to produce high titer GMP grade replication-defective vectors for human clinical trials, as well as methods to produce high titers of high-capacity helper-dependent vectors with contamination levels of helper virus < 0.01%. Although these last ones have yet to be used in clinical trials, the production of high levels of such vectors is being pursued by several groups;
a capacity to infect, in the adult brain, both terminally differentiated neurons and dividing brain astrocytes, both in vivo and in vitro;
a capacity to transduce both immature neonatal and mature neurons;
the potential to achieve cell type-specific and regulated transgene expression using appropriate promoters and transcriptional regulatory elements;
an ability to achieve multiple infections of a single target cell with separate vector virions;
their use to elucidate neuronal morphology and connectivity;
long-term high-level expression in vivo in naive nonimmunized animals;
low incidence of adenovirus-induced brain infections in humans;
large experience in the use of adenovirus to immunize humans against natural infections.
Although the results so far have been highly encouraging (Tables II and III), adenovirus vector systems continue to be optimized for application to somatic cell gene therapy and specifically for neurological gene transfer. Currently, progress in clinical neurological gene therapy is both dependent on and limited by the technology for gene transfer. Selection of the most appropriate vector system for each application is crucial. Adenoviral vectors clearly have distinct advantages for efficient gene delivery into nervous system both in vitro and in vivo. Vector development will undoubtedly further enhance the utility of these vectors and allow their implementation for gene therapy applications to treat neurological diseases.
Fig. 5.
In vivo β-galactosidase expression in rat brain infected with either GS46-FLP HC-Adv vector or E1/E3-deleted adenovirus (RAd35). 7 × 107 BFU of either virus (in 3 μl) was injected into the striatum of male Sprague–Dawley rats. Six days after virus inoculation, β-galactosidase expression was analyzed by immunohistochemistry. (a, b) GS46-FLP HD virus. (c, d) RAd35 virus. β-Galactosidase is expressed under the control of the hCMV promoter in both viruses. From Umana et al. (2001b).
Acknowledgments
Work in the Gene Therapeutics Research Institute at Cedars Sinai Medical Center is funded by R01 NS42893.01 and R01 NS44556.01 grants from the National Institutes of Health, National Institute for Neurological Disorders and Stroke. We also thank the funding our Institute receives from the Board of Governors at Cedars Sinai Medical Center and the encouragement and support of every one of its members. We also wish to thank Dr. Shlomo Melmed for unparalleled support and academic leadership. We are grateful to Ms. Cheryl Cathcart for her superb administrative and organizational skills and to Mr. Danny Malaniak for his encouragement, support, and commitment. Ms. Juanita Gutierrez was responsible for the editing and preparation of this manuscript for publication and we are grateful for her dedication and superb skills.
References
- Acsadi G, Jani A, et al. Cultured human myoblasts and myotubes show markedly different transducibility by replication-defective adenovirus recombinants. Gene Ther. 1994a;1(5):338–340. [PubMed] [Google Scholar]
- Acsadi G, Jani A, et al. A differential efficiency of adenovirus-mediated in vivo gene transfer into skeletal muscle cells of different maturity. Hum. Mol. Genet. 1994b;3(4):579–584. doi: 10.1093/hmg/3.4.579. [DOI] [PubMed] [Google Scholar]
- Ailles LE, Naldini L. HIV-1-derived lentiviral vectors. Curr. Top. Microbiol. Immunol. 2002;261:31–52. doi: 10.1007/978-3-642-56114-6_2. [DOI] [PubMed] [Google Scholar]
- Akagi K, Sandig V, et al. Cre-mediated somatic site-specific recombination in mice. Nucleic Acids Res. 1997;25(9):1766–1773. doi: 10.1093/nar/25.9.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akli S, Caillaud C, et al. Transfer of a foreign gene into the brain using adenovirus vectors. Nat. Genet. 1993;3(3):224–228. doi: 10.1038/ng0393-224. [DOI] [PubMed] [Google Scholar]
- Amalfitano A, Parks RJ. Separating fact from fiction: Assessing the potential of modified adenovirus vectors for use in human gene therapy. Curr. Gene Ther. 2002;2(2):111–133. doi: 10.2174/1566523024605618. [DOI] [PubMed] [Google Scholar]
- Ambar BB, Frei K, et al. Treatment of experimental glioma by administration of adenoviral vectors expressing Fas ligand. Hum. Gene Ther. 1999;10(10):1641–1648. doi: 10.1089/10430349950017644. [DOI] [PubMed] [Google Scholar]
- Babiss LE, Friedman JM, et al. Cellular promoters incorporated into the adenovirus genome: Effects of viral regulatory elements on transcription rates and cell specificity of albumin and beta-globin promoters. Mol. Cell. Biol. 1986;6(11):3798–3806. doi: 10.1128/mcb.6.11.3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badie B, Hunt K, et al. Stereotactic delivery of a recombinant adenovirus into a C6 glioma cell line in a rat brain tumor model. Neurosurgery. 1994;35(5):910–915. doi: 10.1227/00006123-199411000-00016. discussion 915–916. [DOI] [PubMed] [Google Scholar]
- Bain D, Wilkinson GW, et al. Adenovirus vectors to transfer genes into neurones: Implications for gene therapy of neurological disorders. Gene Ther. 1994;1(Suppl. 1):S68. [PubMed] [Google Scholar]
- Bajocchi G, Feldman SH, et al. Direct in vivo gene transfer to ependymal cells in the central nervous system using recombinant adenovirus vectors. Nat. Genet. 1993;3(3):229–234. doi: 10.1038/ng0393-229. [DOI] [PubMed] [Google Scholar]
- Bansal K, Engelhard HH. Gene therapy for brain tumors. Curr. Oncol. Rep. 2000;2(5):463–472. doi: 10.1007/s11912-000-0067-z. [DOI] [PubMed] [Google Scholar]
- Barba D, Hardin J, et al. Development of anti-tumor immunity following thymidine kinase-mediated killing of experimental brain tumors. Proc. Natl. Acad. Sci. USA. 1994;91(10):4348–4352. doi: 10.1073/pnas.91.10.4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauknecht T, Meinhold-Heerlein I. Gene therapy of ovarian cancer. Curr. Womens Health Rep. 2002;2(1):39–46. [PubMed] [Google Scholar]
- Benedetti S, Dimeco F, et al. Limited efficacy of the HSV-TK/GCV system for gene therapy of malignant gliomas and perspectives for the combined transduction of the interleukin-4 gene. Hum. Gene Ther. 1997;8(11):1345–1353. doi: 10.1089/hum.1997.8.11-1345. [DOI] [PubMed] [Google Scholar]
- Bennett J, Wilson J, et al. Adenovirus vector-mediated in vivo gene transfer into adult murine retina. Invest. Ophthalmol. Vis. Sci. 1994;35(5):2535–2542. [PubMed] [Google Scholar]
- Bergelson JM, Cunningham JA, et al. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275(5304):1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- Berkner KL. Development of adenovirus vectors for the expression of heterologous genes. Biotechniques. 1988;6(7):616–629. [PubMed] [Google Scholar]
- Bessereau JL, Stratford-Perricaudet LD, et al. In vivo and in vitro analysis of electrical activity-dependent expression of muscle acetylcholine receptor genes using adenovirus. Proc. Natl. Acad. Sci. USA. 1994;91(4):1304–1308. doi: 10.1073/pnas.91.4.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilang-Bleuel A, Revah F, et al. Intrastriatal injection of an adenoviral vector expressing glial-cell-line-derived neurotrophic factor prevents dopaminergic neuron degeneration and behavioral impairment in a rat model of Parkinson disease. Proc. Natl. Acad. Sci. USA. 1997;94(16):8818–8823. doi: 10.1073/pnas.94.16.8818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomer U, Naldini L, et al. Highly efficient and sustained gene transfer in adult neurons with a lentivirus vector. J. Virol. 1997;71(9):6641–6649. doi: 10.1128/jvi.71.9.6641-6649.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn MC. Parkinson’s disease: A neurodegenerative disease particularly amenable to gene therapy. Mol. Ther. 2000;1(6):494–496. doi: 10.1006/mthe.2000.0078. [DOI] [PubMed] [Google Scholar]
- Bohn MC, Choi-Lundberg DL, et al. Adenovirus-mediated transgene expression in nonhuman primate brain. Hum. Gene Ther. 1999;10(7):1175–1184. doi: 10.1089/10430349950018166. [DOI] [PubMed] [Google Scholar]
- Boucher RC, Knowles MR, et al. Gene therapy for cystic fibrosis using E1-deleted adenovirus: A phase I trial in the nasal cavity. The University of North Carolina at Chapel Hill. Hum. Gene Ther. 1994;5(5):615–639. doi: 10.1089/hum.1994.5.5-615. [DOI] [PubMed] [Google Scholar]
- Bout A, Imler JL, et al. In vivo adenovirus-mediated transfer of human CFTR cDNA to rhesus monkey airway epithelium: Efficacy, toxicity and safety. Gene Ther. 1994a;1(6):385–394. [PubMed] [Google Scholar]
- Bout A, Perricaudet M, et al. Lung gene therapy: In vivo adenovirus-mediated gene transfer to rhesus monkey airway epithelium. Hum. Gene Ther. 1994b;5(1):3–10. doi: 10.1089/hum.1994.5.1-3. [DOI] [PubMed] [Google Scholar]
- Boviatsis EJ, Chase M, et al. Gene transfer into experimental brain tumors mediated by adenovirus, herpes simplex virus, and retrovirus vectors. Hum. Gene Ther. 1994c;5(2):183–191. doi: 10.1089/hum.1994.5.2-183. [DOI] [PubMed] [Google Scholar]
- Brody SL, Jaffe HA, et al. Direct in vivo gene transfer and expression in malignant cells using adenovirus vectors. Hum. Gene Ther. 1994a;5(4):437–447. doi: 10.1089/hum.1994.5.4-437. [DOI] [PubMed] [Google Scholar]
- Brody SL, Metzger M, et al. Acute responses of non-human primates to airway delivery of an adenovirus vector containing the human cystic fibrosis transmembrane conductance regulator cDNA. Hum. Gene Ther. 1994b;5(7):821–836. doi: 10.1089/hum.1994.5.7-821. [DOI] [PubMed] [Google Scholar]
- Brooks AI, Stein CS, et al. Functional correction of established central nervous system deficits in an animal model of lysosomal storage disease with feline immunodeficiency virus-based vectors. Proc. Natl. Acad. Sci. USA. 2002;99(9):6216–6221. doi: 10.1073/pnas.082011999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burcin MM, Schiedner G, et al. Adenovirus-mediated regulable target gene expression in vivo. Proc. Natl. Acad. Sci. USA. 1999;96(2):355–360. doi: 10.1073/pnas.96.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton EA, Bai Q, et al. Targeting gene expression using HSV vectors. Adv. Drug Deliv. Rev. 2001;53(2):155–170. doi: 10.1016/s0169-409x(01)00226-5. [DOI] [PubMed] [Google Scholar]
- Byrnes AP, Rusby JE, et al. Adenovirus gene transfer causes inflammation in the brain. Neuroscience. 1995;66(4):1015–1024. doi: 10.1016/0306-4522(95)00068-t. [DOI] [PubMed] [Google Scholar]
- Byrnes AP, MacLaren RE, et al. Immunological instability of persistent adenovirus vectors in the brain: Peripheral exposure to vector leads to renewed inflammation, reduced gene expression, and demyelination. J. Neurosci. 1996a;16(9):3045–3055. doi: 10.1523/JNEUROSCI.16-09-03045.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes AP, Wood MJ, et al. Role of T cells in inflammation caused by adenovirus vectors in the brain. Gene Ther. 1996b;3(7):644–651. [PubMed] [Google Scholar]
- Caillaud C, Akli S, et al. Adenoviral vector as a gene delivery system into cultured rat neuronal and glial cells. Eur. J. Neurosci. 1993;5(10):1287–1291. doi: 10.1111/j.1460-9568.1993.tb00914.x. [DOI] [PubMed] [Google Scholar]
- Card JP, Whealy ME, et al. Two alpha-herpesvirus strains are transported differentially in the rodent visual system. Neuron. 1991;6(6):957–969. doi: 10.1016/0896-6273(91)90236-s. [DOI] [PubMed] [Google Scholar]
- Card JP, Whealy ME, et al. Pseudorabies virus envelope glycoprotein gI influences both neurotropism and virulence during infection of the rat visual system. J. Virol. 1992;66(5):3032–3041. doi: 10.1128/jvi.66.5.3032-3041.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HH, Mack LM, et al. Persistence in muscle of an adenoviral vector that lacks all viral genes. Proc. Natl. Acad. Sci. USA. 1997;94(5):1645–1650. doi: 10.1073/pnas.94.5.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HH, Mack LM, et al. DNA from both high-capacity and first-generation adenoviral vectors remains intact in skeletal muscle. Hum. Gene. Ther. 1999;10(3):365–373. doi: 10.1089/10430349950018814. [DOI] [PubMed] [Google Scholar]
- Chen SH, Shine HD, et al. Gene therapy for brain tumors: Regression of experimental gliomas by adenovirus-mediated gene transfer in vivo. Proc. Natl. Acad. Sci. USA. 1994;91(8):3054–3057. doi: 10.1073/pnas.91.8.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SH, Kosai K, et al. Combination suicide and cytokine gene therapy for hepatic metastases of colon carcinoma: Sustained antitumor immunity prolongs animal survival. Cancer Res. 1996;56(16):3758–3762. [PubMed] [Google Scholar]
- Chirmule N, Propert K, et al. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther. 1999;6(9):1574–1583. doi: 10.1038/sj.gt.3300994. [DOI] [PubMed] [Google Scholar]
- Choi-Lundberg DL, Lin Q, et al. Dopaminergic neurons protected from degeneration by GDNF gene therapy. Science. 1997;275(5301):838–841. doi: 10.1126/science.275.5301.838. [DOI] [PubMed] [Google Scholar]
- Choi-Lundberg DL, Lin Q, et al. Behavioral and cellular protection of rat dopaminergic neurons by an adenoviral vector encoding glial cell line-derived neurotrophic factor. Exp. Neurol. 1998;154(2):261–275. doi: 10.1006/exnr.1998.6887. [DOI] [PubMed] [Google Scholar]
- Chou SM, Roos R, et al. Subacute focal adenovirus encephalitis. J. Neuropathol. Exp. Neurol. 1973;32(1):34–50. doi: 10.1097/00005072-197301000-00003. [DOI] [PubMed] [Google Scholar]
- Connor B, Kozlowski DA, et al. Differential effects of glial cell line-derived neurotrophic factor (GDNF) in the striatum and substantia nigra of the aged Parkinsonian rat. Gene Ther. 1999;6(12):1936–1951. doi: 10.1038/sj.gt.3301033. [DOI] [PubMed] [Google Scholar]
- Connor B, Kozlowski DA, et al. Glial cell line-derived neurotrophic factor (GDNF) gene delivery protects dopaminergic terminals from degeneration. Exp. Neurol. 2001;169(1):83–95. doi: 10.1006/exnr.2001.7638. [DOI] [PubMed] [Google Scholar]
- Consiglio A, Quattrini A, et al. In vivo gene therapy of metachromatic leukodystrophy by lentiviral vectors: Correction of neuropathology and protection against learning impairments in affected mice. Nat. Med. 2001;7(3):310–316. doi: 10.1038/85454. [DOI] [PubMed] [Google Scholar]
- Corti O, Sabate O, et al. A single adenovirus vector mediates doxycycline-controlled expression of tyrosine hydroxylase in brain grafts of human neural progenitors. Nat. Biotechnol. 1999a;17(4):349–354. doi: 10.1038/7901. [DOI] [PubMed] [Google Scholar]
- Corti O, Sanchez-Capelo A, et al. Long-term doxycycline-controlled expression of human tyrosine hydroxylase after direct adenovirus-mediated gene transfer to a rat model of Parkinson’s disease. Proc. Natl. Acad. Sci. USA. 1999b;96(21):12120–12125. doi: 10.1073/pnas.96.21.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotten M, Wagner E, et al. Chicken adenovirus (CELO virus) particles augment receptor-mediated DNA delivery to mammalian cells and yield exceptional levels of stable transformants. J. Virol. 1993;67(7):3777–3785. doi: 10.1128/jvi.67.7.3777-3785.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cregan SP, MacLaurin J, et al. Helper-dependent adenovirus vectors: Their use as a gene delivery system to neurons. Gene Ther. 2000;7(14):1200–1209. doi: 10.1038/sj.gt.3301208. [DOI] [PubMed] [Google Scholar]
- Curran MA, Nolan GP. Nonprimate lentiviral vectors. Curr. Top. Microbiol. Immunol. 2002;261:75–105. doi: 10.1007/978-3-642-56114-6_4. [DOI] [PubMed] [Google Scholar]
- Davidson BL, Allen ED, et al. A model system for in vivo gene transfer into the central nervous system using an adenoviral vector. Nat. Genet. 1993;3(3):219–223. doi: 10.1038/ng0393-219. [DOI] [PubMed] [Google Scholar]
- Davidson BL, Doran SE, et al. Expression of Escherichia coli beta-galactosidase and rat HPRT in the CNS of Macaca mulatta following adenoviral mediated gene transfer. Exp. Neurol. 1994;125(2):258–267. doi: 10.1006/exnr.1994.1028. [DOI] [PubMed] [Google Scholar]
- Davis D, Henslee PJ, et al. Fatal adenovirus meningoencephalitis in a bone marrow transplant patient. Ann. Neurol. 1988;23(4):385–389. doi: 10.1002/ana.410230412. [DOI] [PubMed] [Google Scholar]
- De Palma M, Naldini L. Transduction of a gene expression cassette using advanced generation lentiviral vectors. Methods Enzymol. 2002;346:514–529. doi: 10.1016/s0076-6879(02)46074-0. [DOI] [PubMed] [Google Scholar]
- DeFalco J, Tomishima M, et al. Virus-assisted mapping of neural inputs to a feeding center in the hypothalamus. Science. 2001;291(5513):2608–2613. doi: 10.1126/science.1056602. [DOI] [PubMed] [Google Scholar]
- Deglon N, Aebischer P. Lentiviruses as vectors for CNS diseases. Curr. Top. Microbiol. Immunol. 2002;261:191–209. doi: 10.1007/978-3-642-56114-6_10. [DOI] [PubMed] [Google Scholar]
- Dewey RA, Morrissey G, et al. Chronic brain inflammation and persistent herpes simplex virus 1 thymidine kinase expression in survivors of syngeneic glioma treated by adenovirus-mediated gene therapy: Implications for clinical trials. Nat. Med. 1999;5(11):1256–1263. doi: 10.1038/15207. [DOI] [PubMed] [Google Scholar]
- Doronin K, Toth K, et al. Tumor-specific, replication-competent adenovirus vectors overexpressing the adenovirus death protein. J. Virol. 2000;74(13):6147–6155. doi: 10.1128/jvi.74.13.6147-6155.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doronin K, Kuppuswamy M, et al. Tissue-specific, tumor-selective, replication-competent adenovirus vector for cancer gene therapy. J. Virol. 2001;75(7):3314–3324. doi: 10.1128/JVI.75.7.3314-3324.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eloit M, Gilardi-Hebenstreit P, et al. Construction of a defective adenovirus vector expressing the pseudorabies virus glycoprotein gp50 and its use as a live vaccine. J. Gen. Virol. 1990;71(pt. 10):2425–2431. doi: 10.1099/0022-1317-71-10-2425. [DOI] [PubMed] [Google Scholar]
- Engel JP. Viral upper respiratory infections. Semin. Respir. Infect. 1995;10(1):3–13. [PubMed] [Google Scholar]
- Engelhardt JF, Simon RH, et al. Adenovirus-mediated transfer of the CFTR gene to lung of nonhuman primates: Biological efficacy study. Hum. Gene Ther. 1993;4(6):759–769. doi: 10.1089/hum.1993.4.6-759. [DOI] [PubMed] [Google Scholar]
- Engelhardt JF, Ye X, et al. Ablation of E2A in recombinant adenoviruses improves transgene persistence and decreases inflammatory response in mouse liver. Proc. Natl. Acad. Sci. USA. 1994;91(13):6196–6200. doi: 10.1073/pnas.91.13.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enquist LW, Husak PJ, et al. Infection and spread of alphaherpesviruses in the nervous system. Adv. Virus Res. 1998;51:237–347. doi: 10.1016/s0065-3527(08)60787-3. [DOI] [PubMed] [Google Scholar]
- Fisher LJ, Gage FH. Grafting in the mammalian central nervous system. Physiol. Rev. 1993;73(3):583–616. doi: 10.1152/physrev.1993.73.3.583. [DOI] [PubMed] [Google Scholar]
- Flint SJ, Enquist LW, et al. Principles of Virology. ASM Press; Washington, DC: 2000. Principle of Virology: Molecular Biology, Pathogenesis, and Control. [Google Scholar]
- Fu H, Samulski RJ, et al. Neurological correction of lysosomal storage in a mucopolysaccharidosis IIIB mouse model by adeno-associated virus-mediated gene delivery. Mol. Ther. 2002;5(1):42–49. doi: 10.1006/mthe.2001.0514. [DOI] [PubMed] [Google Scholar]
- Galimi F, Verma IM. Opportunities for the use of lentiviral vectors in human gene therapy. Curr. Top. Microbiol. Immunol. 2002;261:245–254. doi: 10.1007/978-3-642-56114-6_13. [DOI] [PubMed] [Google Scholar]
- Gallichan WS, Johnson DC, et al. Mucosal immunity and protection after intranasal immunization with recombinant adenovirus expressing herpes simplex virus glycoprotein B. J. Infect. Dis. 1993;168(3):622–629. doi: 10.1093/infdis/168.3.622. [DOI] [PubMed] [Google Scholar]
- Geddes BJ, Harding TC, et al. Long-term gene therapy in the CNS: Reversal of hypothalamic diabetes insipidus in the Brattleboro rat by using an adenovirus expressing arginine vasopressin. Nat. Med. 1997;3(12):1402–1404. doi: 10.1038/nm1297-1402. [DOI] [PubMed] [Google Scholar]
- Gerdes CA, Castro MG, et al. Strong promoters are the key to highly efficient, noninflammatory and noncytotoxic adenoviral-mediated transgene delivery into the brain in vivo. Mol. Ther. 2000;2(4):330–338. doi: 10.1006/mthe.2000.0140. [DOI] [PubMed] [Google Scholar]
- Gilardi P, Courtney M, et al. Expression of human alpha 1-antitrypsin using a recombinant adenovirus vector. FEBS Lett. 1990;267(1):60–62. doi: 10.1016/0014-5793(90)80287-s. [DOI] [PubMed] [Google Scholar]
- Ginsberg HS. The life and times of adenoviruses. Adv. Virus Res. 1999;54:1–13. doi: 10.1016/s0065-3527(08)60363-2. [DOI] [PubMed] [Google Scholar]
- Ginsberg HS, Prince GA. The molecular basis of adenovirus pathogenesis. Infect. Agents Dis. 1994;3(1):1–8. [PubMed] [Google Scholar]
- Ginsberg HS, Moldawer LL, et al. A mouse model for investigating the molecular pathogenesis of adenovirus pneumonia. Proc. Natl. Acad. Sci. USA. 1991;88(5):1651–1655. doi: 10.1073/pnas.88.5.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover CP, Bienemann AS, et al. Adenoviral-mediated, high-level, cell-specific transgene expression: A SYN1-WPRE cassette mediates increased transgene expression with no loss of neuron specificity. Mol. Ther. 2002;5(5, pt. 1):509–516. doi: 10.1006/mthe.2002.0588. [DOI] [PubMed] [Google Scholar]
- Grable M, Hearing P. cis and trans requirements for the selective packaging of adenovirus type 5 DNA. J. Virol. 1992;66(2):723–731. doi: 10.1128/jvi.66.2.723-731.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham FL, Smiley J, et al. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol. 1977;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Greber UF, Willetts M, et al. Stepwise dismantling of adenovirus 2 during entry into cells. Cell. 1993;75(3):477–486. doi: 10.1016/0092-8674(93)90382-z. [DOI] [PubMed] [Google Scholar]
- Grubb BR, Pickles RJ, et al. Inefficient gene transfer by adenovirus vector to cystic fibrosis airway epithelia of mice and humans. Nature. 1994;371(6500):802–806. doi: 10.1038/371802a0. [DOI] [PubMed] [Google Scholar]
- Gupta A, Ho DY, et al. Neuroprotective effects of an adenoviral vector expressing the glucose transporter: A detailed description of the mediating cellular events. Brain Res. 2001;908(1):49–57. doi: 10.1016/s0006-8993(01)02572-0. [DOI] [PubMed] [Google Scholar]
- Hackett NR, Kaminsky SM, et al. Antivector and antitransgene host responses in gene therapy. Curr. Opin. Mol. Ther. 2000;2(4):376–382. [PubMed] [Google Scholar]
- Haddada H, Lopez M, et al. Efficient adenovirus-mediated gene transfer into human blood monocyte-derived macrophages. Biochem. Biophys. Res. Commun. 1993;195(3):1174–1783. doi: 10.1006/bbrc.1993.2168. [DOI] [PubMed] [Google Scholar]
- Harding TC, Geddes BJ, et al. Switching transgene expression in the brain using an adenoviral tetracycline-regulatable system. Nat. Biotechnol. 1998;16(6):553–555. doi: 10.1038/nbt0698-553. [DOI] [PubMed] [Google Scholar]
- Hardy S, Kitamura M, et al. Construction of adenovirus vectors through Cre-lox recombination. J. Virol. 1997;71(3):1842–1849. doi: 10.1128/jvi.71.3.1842-1849.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartigan-O’Connor D, Barjot C, et al. Generation and growth of gutted adenoviral vectors. Methods Enzymol. 2002;346:224–246. doi: 10.1016/s0076-6879(02)46058-2. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, DePaoli AM, et al. Expression of a thyroid hormone-responsive recombinant gene introduced into adult mice livers by replication-defective adenovirus can be regulated by endogenous thyroid hormone receptor. J. Biol. Chem. 1994;269(39):23872–23875. [PubMed] [Google Scholar]
- Herrlinger U, Jacobs A, et al. Helper virus-free herpes simplex virus type 1 amplicon vectors for granulocyte-macrophage colony-stimulating factor-enhanced vaccination therapy for experimental glioma. Hum. Gene Ther. 2000;11(10):1429–1438. doi: 10.1089/10430340050057503. [DOI] [PubMed] [Google Scholar]
- Herz J, Gerard RD. Adenovirus-mediated transfer of low density lipoprotein receptor gene acutely accelerates cholesterol clearance in normal mice. Proc. Natl. Acad. Sci. USA. 1993;90(7):2812–2816. doi: 10.1073/pnas.90.7.2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hida H, Hashimoto M, et al. Dopa-producing astrocytes generated by adenoviral transduction of human tyrosine hydroxylase gene: In vitro study and transplantation to hemiparkinsonian model rats. Neurosci. Res. 1999;35(2):101–112. doi: 10.1016/s0168-0102(99)00073-5. [DOI] [PubMed] [Google Scholar]
- Holloway S, Mennie M, et al. Predictive testing for Huntington disease: Social characteristics and knowledge of applicants, attitudes to the test procedure and decisions made after testing. Clin. Genet. 1994;46(2):175–180. doi: 10.1111/j.1399-0004.1994.tb04220.x. [DOI] [PubMed] [Google Scholar]
- Horellou P, Vigne E, et al. Direct intracerebral gene transfer of an adenoviral vector expressing tyrosine hydroxylase in a rat model of Parkinson’s disease. Neuroreport. 1994;6(1):49–53. doi: 10.1097/00001756-199412300-00014. [DOI] [PubMed] [Google Scholar]
- Hsich G, Sena-Esteves M, et al. Critical issues in gene therapy for neurologic disease. Hum. Gene Ther. 2002;13(5):579–604. doi: 10.1089/10430340252837198. [DOI] [PubMed] [Google Scholar]
- Huentelman MJ, Reaves PY, et al. Large-scale production of retroviral vectors for systemic gene delivery. Methods Enzymol. 2002;346:562–573. doi: 10.1016/s0076-6879(02)46077-6. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Wakimoto H, et al. Complement depletion facilitates the infection of multiple brain tumors by an intravascular, replication-conditional herpes simplex virus mutant. J. Virol. 2000;74(10):4765–4775. doi: 10.1128/jvi.74.10.4765-4775.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperiale MJ, Kao HT, et al. Common control of the heat shock gene and early adenovirus genes: Evidence for a cellular E1A-like activity. Mol. Cell. Biol. 1984;4(5):867–874. doi: 10.1128/mcb.4.5.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi S, Brown MSI, et al. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J. Clin. Invest. 1993;92(2):883–893. doi: 10.1172/JCI116663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs A, Tjuvajev JG, et al. Positron emission tomography-based imaging of transgene expression mediated by replication-conditional, oncolytic herpes simplex virus type 1 mutant vectors in vivo. Cancer Res. 2001;61(7):2983–2995. [PubMed] [Google Scholar]
- Jacobs SC, Stephenson JR, et al. High-level expression of the tick-borne encephalitis virus NS1 protein by using an adenovirus-based vector: Protection elicited in a murine model. J. Virol. 1992;66(4):2086–2095. doi: 10.1128/jvi.66.4.2086-2095.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jomary C, Piper TA, et al. Adenovirus-mediated gene transfer to murine retinal cells in vitro and in vivo. FEBS Lett. 1994;347(2–3):117–122. doi: 10.1016/0014-5793(94)00512-5. [DOI] [PubMed] [Google Scholar]
- Kajiwara K, Byrnes AP, et al. Immune responses to adenoviral vectors during gene transfer in the brain. Hum. Gene Ther. 1997;8(3):253–265. doi: 10.1089/hum.1997.8.3-253. [DOI] [PubMed] [Google Scholar]
- Kaplitt MG, Leone P, et al. Long-term gene expression and phenotypic correction using adeno-associated virus vectors in the mammalian brain. Nat. Genet. 1994;8(2):148–154. doi: 10.1038/ng1094-148. [DOI] [PubMed] [Google Scholar]
- Kass-Eisler A, Falck-Pedersen E, et al. Quantitative determination of adenovirus-mediated gene delivery to rat cardiac myocytes in vitro and in vivo. Proc. Natl. Acad. Sci. USA. 1993;90(24):11498–11502. doi: 10.1073/pnas.90.24.11498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass-Eisler A, Falck-Pedersen E, et al. The impact of developmental stage, route of administration and the immune system on adenovirus-mediated gene transfer. Gene Ther. 1994;1(6):395–402. [PubMed] [Google Scholar]
- Kay MA, Glorioso JC, et al. Viral vectors for gene therapy: The art of turning infectious agents into vehicles of therapeutics. Nat. Med. 2001;7(1):33–40. doi: 10.1038/83324. [DOI] [PubMed] [Google Scholar]
- Kelsey DS. Adenovirus meningoencephalitis. Pediatrics. 1978;61(2):291–293. [PubMed] [Google Scholar]
- Kingston PA, Sinha S, et al. Adenovirus-mediated gene transfer of a secreted transforming growth factor-beta type II receptor inhibits luminal loss and constrictive remodeling after coronary angioplasty and enhances adventitial collagen deposition. Circulation. 2001;104(21):2595–2601. doi: 10.1161/hc4601.099405. [DOI] [PubMed] [Google Scholar]
- Kirn D, Martuza RL, et al. Replication-selective virotherapy for cancer: Biological principles, risk management and future directions. Nat. Med. 2001;7(7):781–787. doi: 10.1038/89901. [DOI] [PubMed] [Google Scholar]
- Kochanek S, Clemens PR, et al. A new adenoviral vector: Replacement of all viral coding sequences with 28 kb of DNA independently expressing both full-length dystrophin and beta-galactosidase. Proc. Natl. Acad. Sci. USA. 1996;93(12):5731–5736. doi: 10.1073/pnas.93.12.5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozarsky KF, McKinley DR, et al. In vivo correction of low density lipoprotein receptor deficiency in the Watanabe heritable hyperlipidemic rabbit with recombinant adenoviruses. J. Biol. Chem. 1994;269(18):13695–13702. [PubMed] [Google Scholar]
- Kozlowski DA, Connor B, et al. Delivery of a GDNF gene into the substantia nigra after a progressive 6-OHDA lesion maintains functional nigrostriatal connections. Exp. Neurol. 2000;166(1):1–15. doi: 10.1006/exnr.2000.7463. [DOI] [PubMed] [Google Scholar]
- Kozlowski DA, Bremer E, et al. Quantitative analysis of transgene protein, mRNA, and vector DNA following injection of an adenoviral vector harboring glial cell line-derived neurotrophic factor into the primate caudate nucleus. Mol. Ther. 2001;3(2):256–261. doi: 10.1006/mthe.2000.0256. [DOI] [PubMed] [Google Scholar]
- Kramm CM, Chase M, et al. Therapeutic efficiency and safety of a second-generation replication-conditional HSV1 vector for brain tumor gene therapy. Hum. Gene Ther. 1997;8(17):2057–2068. doi: 10.1089/hum.1997.8.17-2057. [DOI] [PubMed] [Google Scholar]
- Latchman DS, Coffin RS. Viral vectors for gene therapy in Parkinson’s disease. Rev. Neurosci. 2001;12(1):69–78. doi: 10.1515/revneuro.2001.12.1.69. [DOI] [PubMed] [Google Scholar]
- Lawrence MS, Foellmer HG, et al. Inflammatory responses and their impact on beta-galactosidase transgene expression following adenovirus vector delivery to the primate caudate nucleus. Gene Ther. 1999;6(8):1368–1379. doi: 10.1038/sj.gt.3300958. [DOI] [PubMed] [Google Scholar]
- Le Gal La Salle G, Robert JJ, et al. An adenovirus vector for gene transfer into neurons and glia in the brain. Science. 1993;259(5097):988–990. doi: 10.1126/science.8382374. [DOI] [PubMed] [Google Scholar]
- Legrand V, Leissner P, et al. Transductional targeting with recombinant adenovirus vectors. Curr. Gene Ther. 2002;2(3):323–339. doi: 10.2174/1566523023347823. [DOI] [PubMed] [Google Scholar]
- Lemarchand P, Jaffe HA, et al. Adenovirus-mediated transfer of a recombinant human alpha 1-antitrypsin cDNA to human endothelial cells. Proc. Natl. Acad. Sci. USA. 1992;89(14):6482–6486. doi: 10.1073/pnas.89.14.6482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Adamian M, et al. In vivo transfer of a reporter gene to the retina mediated by an adenoviral vector. Invest. Ophthalmol. Vis. Sci. 1994;35(5):2543–2549. [PubMed] [Google Scholar]
- Lilley CE, Branston RH, et al. Herpes simplex virus vectors for the nervous system. Curr. Gene Ther. 2001;1(4):339–358. doi: 10.2174/1566523013348346. [DOI] [PubMed] [Google Scholar]
- Lisovoski F, Cadusseau J, et al. In vivo transfer of a marker gene to study motoneuronal development. Neuroreport. 1994;5(9):1069–1072. doi: 10.1097/00001756-199405000-00013. [DOI] [PubMed] [Google Scholar]
- Logvinoff C, Epstein AL. A novel approach for herpes simplex virus type 1 amplicon vector production, using the Cre-loxP recombination system to remove helper virus. Hum. Gene Ther. 2001;12(2):161–167. doi: 10.1089/104303401750061221. [DOI] [PubMed] [Google Scholar]
- Lokensgard JR, Bloom DC, et al. Long-term promoter activity during herpes simplex virus latency. J. Virol. 1994;68(11):7148–7158. doi: 10.1128/jvi.68.11.7148-7158.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein PR. Molecular neurosurgery: Mending the broken brain. Biotechnology. 1994;12(11):1075–1080. doi: 10.1038/nbt1194-1075. [DOI] [PubMed] [Google Scholar]
- Lowenstein PR. Degenerative and inherited neurological disorders. In: Dickson G, editor. Molecular and Cell Biology of Human Gene Therapeutics. Chapman and Hall; London: 1995. pp. 301–349. [PubMed] [Google Scholar]
- Lowenstein PR. Immunology of viral-vector-mediated gene transfer into the brain: An evolutionary and developmental perspective. Trends Immunol. 2002;23(1):23–30. doi: 10.1016/s1471-4906(01)02063-4. [DOI] [PubMed] [Google Scholar]
- Lowenstein PR, Castro MG. Genetic engineering within the adult brain: Implications for molecular approaches to behavioral neuroscience. Physiol. Behav. 2001;73(5):833–839. doi: 10.1016/s0031-9384(01)00520-0. [DOI] [PubMed] [Google Scholar]
- Lowenstein PR, Castro MG. Progress and challenges in viral vector-mediated gene transfer to the brain. Curr. Opin. Mol. Ther. 2002;4(4):359–371. [PubMed] [Google Scholar]
- Lowenstein PR, Enquist LW. Protocols for Gene Transfer into Neurons: Towards Gene Therapy of Neurological Disorders. John Wiley & Sons; Chichester, UK: 1996. [Google Scholar]
- Lowenstein PR, Thomas CE, et al. High-capacity, helper-dependent, gutless adenoviral vectors for gene transfer into brain. Methods Enzymol. 2002;346:292–311. doi: 10.1016/s0076-6879(02)46062-4. [DOI] [PubMed] [Google Scholar]
- Macmillan JC, Lowenstein PR. Gene Therapy Approaches to the Treatment of Human Neurological Disease: The Future of Neurological Therapies? In: Lowenstein PR, Enquist LW, editors. Protocols for Gene Transfer in Nueroscience: Towards Gene Therapy of Neurological Disorders. John Wiley and Sons; Chichester, UK: 1994. [Google Scholar]
- Mahr JA, Gooding LR. Immune evasion by adenoviruses. Immunol. Rev. 1999;168:121–130. doi: 10.1111/j.1600-065x.1999.tb01287.x. [DOI] [PubMed] [Google Scholar]
- Maione D, Della Rocca C, et al. An improved helper-dependent adenoviral vector allows persistent gene expression after intramuscular delivery and overcomes preexisting immunity to adenovirus. Proc. Natl. Acad. Sci. USA. 2001;98(11):5986–5991. doi: 10.1073/pnas.101122498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markert JM, Malick A, et al. Reduction and elimination of encephalitis in an experimental glioma therapy model with attenuated herpes simplex mutants that retain susceptibility to acyclovir. Neurosurgery. 1993;32(4):597–603. doi: 10.1227/00006123-199304000-00016. [DOI] [PubMed] [Google Scholar]
- Markert JM, Medlock MD, et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: Results of a phase I trial. Gene Ther. 2000;7(10):867–874. doi: 10.1038/sj.gt.3301205. [DOI] [PubMed] [Google Scholar]
- Martuza RL, Malick A, et al. Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science. 1991;252(5007):854–856. doi: 10.1126/science.1851332. [DOI] [PubMed] [Google Scholar]
- Mastrangeli A, Danel C, et al. Diversity of airway epithelial cell targets for in vivo recombinant adenovirus-mediated gene transfer. J. Clin. Invest. 1993;91(1):225–234. doi: 10.1172/JCI116175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matyszak MK. Inflammation in the CNS: Balance between immunological privilege and immune responses. Prog. Neurobiol. 1998;56(1):19–35. doi: 10.1016/s0301-0082(98)00014-8. [DOI] [PubMed] [Google Scholar]
- Matyszak MK, Perry VH. A comparison of leucocyte responses to heat-killed bacillus Calmette-Guerin in different CNS compartments. Neuropathol. Appl. Neurobiol. 1996;22(1):44–53. [PubMed] [Google Scholar]
- Mazarakis ND, Azzouz M, et al. Rabies virus glycoprotein pseudotyping of lentiviral vectors enables retrograde axonal transport and access to the nervous system after peripheral delivery. Hum. Mol. Genet. 2001;10(19):2109–2121. doi: 10.1093/hmg/10.19.2109. [DOI] [PubMed] [Google Scholar]
- Millecamps S, Kiefer H, et al. Neuron-restrictive silencer elements mediate neuron specificity of adenoviral gene expression. Nat. Biotechnol. 1999;17(9):865–869. doi: 10.1038/12849. [DOI] [PubMed] [Google Scholar]
- Mitani K, Graham FL, et al. Rescue, propagation, and partial purification of a helper virus-dependent adenovirus vector. Proc. Natl. Acad. Sci. USA. 1995;92(9):3854–3858. doi: 10.1073/pnas.92.9.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittereder N, Yei S, et al. Evaluation of the efficacy and safety of in vitro, adenovirus-mediated transfer of the human cystic fibrosis transmembrane conductance regulator cDNA. Hum. Gene Ther. 1994;5(6):717–729. doi: 10.1089/hum.1994.5.6-717. [DOI] [PubMed] [Google Scholar]
- Morelli AE, Larregina AT, et al. Neuronal and glial cell type-specific promoters within adenovirus recombinants restrict the expression of the apoptosis-inducing molecule Fas ligand to predetermined brain cell types, and abolish peripheral liver toxicity. J. Gen. Virol. 1999;80(pt. 3):571–583. doi: 10.1099/0022-1317-80-3-571. [DOI] [PubMed] [Google Scholar]
- Morin JE, Lubeck MD, et al. Recombinant adenovirus induces antibody response to hepatitis B virus surface antigen in hamsters. Proc. Natl. Acad. Sci. USA. 1987;84(13):4626–4630. doi: 10.1073/pnas.84.13.4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morral N, O’Neal W, et al. Immune responses to reporter proteins and high viral dose limit duration of expression with adenoviral vectors: Comparison of E2a wild type and E2a deleted vectors. Hum. Gene Ther. 1997;8(10):1275–1286. doi: 10.1089/hum.1997.8.10-1275. [DOI] [PubMed] [Google Scholar]
- Morral N, Parks RJ, et al. High doses of a helper-dependent adenoviral vector yield supraphysiological levels of alpha1-antitrypsin with negligible toxicity. Hum. Gene Ther. 1998;9(18):2709–2716. doi: 10.1089/hum.1998.9.18-2709. [DOI] [PubMed] [Google Scholar]
- Morsy MA, Gu M, et al. An adenoviral vector deleted for all viral coding sequences results in enhanced safety and extended expression of a leptin transgene. Proc. Natl. Acad. Sci. USA. 1998;95(14):7866–7871. doi: 10.1073/pnas.95.14.7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchardt C, Li C, et al. CREB regulation of cellular cyclic AMP-responsive and adenovirus early promoters. J. Virol. 1990;64(9):4296–4305. doi: 10.1128/jvi.64.9.4296-4305.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu S, Fujimoto K, et al. Behavioral recovery in a primate model of Parkinson’s disease by triple transduction of striatal cells with adeno-associated viral vectors expressing dopamine-synthesizing enzymes. Hum. Gene Ther. 2002;13(3):345–354. doi: 10.1089/10430340252792486. [DOI] [PubMed] [Google Scholar]
- Nalbantoglu J, Gilfix BM, et al. Predictive value of apolipoprotein E genotyping in Alzheimer’s disease: Results of an autopsy series and an analysis of several combined studies. Ann. Neurol. 1994;36(6):889–895. doi: 10.1002/ana.410360614. [DOI] [PubMed] [Google Scholar]
- Naldini L, Blomer U, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272(5259):263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- Nanda D, Vogels R, et al. Treatment of malignant gliomas with a replicating adenoviral vector expressing herpes simplex virus-thymidine kinase. Cancer Res. 2001;61(24):8743–8750. [PubMed] [Google Scholar]
- Ng P, Beauchamp C, et al. Development of a FLP/frt system for generating helper-dependent adenoviral vectors. Mol. Ther. 2001;3(5 pt. 1):809–815. doi: 10.1006/mthe.2001.0323. [DOI] [PubMed] [Google Scholar]
- Ng P, Parks RJ, et al. Preparation of helper-dependent adenoviral vectors. Methods Mol. Med. 2002;69:371–388. doi: 10.1385/1-59259-141-8:371. [DOI] [PubMed] [Google Scholar]
- Nicklin SA, Baker AH. Tropism-modified adenoviral and adeno-associated viral vectors for gene therapy. Curr. Gene Ther. 2002;2(3):273–293. doi: 10.2174/1566523023347797. [DOI] [PubMed] [Google Scholar]
- Osamura T, Mizuta R, et al. Isolation of adenovirus type 11 from the brain of a neonate with pneumonia and encephalitis. Eur. J. Pediatr. 1993;152(6):496–499. doi: 10.1007/BF01955058. [DOI] [PubMed] [Google Scholar]
- Oualikene W, Gonin P, et al. Short and long term dissemination of deletion mutants of adenovirus in permissive (cotton rat) and non-permissive (mouse) species. J. Gen. Virol. 1994;75(pt. 10):2765–2768. doi: 10.1099/0022-1317-75-10-2765. [DOI] [PubMed] [Google Scholar]
- Palu G, Cavaggioni A, et al. Gene therapy of glioblastoma multiforme via combined expression of suicide and cytokine genes: A pilot study in humans. Gene Ther. 1999;6(3):330–337. doi: 10.1038/sj.gt.3300805. [DOI] [PubMed] [Google Scholar]
- Papanastassiou V, Rampling R, et al. The potential for efficacy of the modified (ICP 34.5(−)) herpes simplex virus HSV1716 following intratumoural injection into human malignant glioma: A proof of principle study. Gene Ther. 2002;9(6):398–406. doi: 10.1038/sj.gt.3301664. [DOI] [PubMed] [Google Scholar]
- Parks RJ, Chen L, et al. A helper-dependent adenovirus vector system: Removal of helper virus by Cre-mediated excision of the viral packaging signal. Proc. Natl. Acad. Sci. USA. 1996;93(24):13565–13570. doi: 10.1073/pnas.93.24.13565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks R, Evelegh C, et al. Use of helper-dependent adenoviral vectors of alternative serotypes permits repeat vector administration. Gene Ther. 1999a;6(9):1565–1573. doi: 10.1038/sj.gt.3300995. [DOI] [PubMed] [Google Scholar]
- Parks RJ, Bramson JL, et al. Effects of stuffer DNA on transgene expression from helper-dependent adenovirus vectors. J. Virol. 1999b;73(10):8027–8034. doi: 10.1128/jvi.73.10.8027-8034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechan PA, Herrlinger U, et al. Combined HSV-1 recombinant and amplicon piggyback vectors: Replication-competent and defective forms, and therapeutic efficacy for experimental gliomas. J. Gene Med. 1999;1(3):176–185. doi: 10.1002/(SICI)1521-2254(199905/06)1:3<176::AID-JGM35>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Perez-Cruet MJ, Trask TW, et al. Adenovirus-mediated gene therapy of experimental gliomas. J. Neurosci. Res. 1994;39(4):506–511. doi: 10.1002/jnr.490390417. [DOI] [PubMed] [Google Scholar]
- Perry VH. A revised view of the central nervous system microenvironment and major histocompatibility complex class II antigen presentation. J. Neuroimmunol. 1998;90(2):113–121. doi: 10.1016/s0165-5728(98)00145-3. [DOI] [PubMed] [Google Scholar]
- Phillips MI, editor. Gene therapy methods. Methods Enzymol. 2002;346:1–728. doi: 10.1016/s0076-6879(02)46046-6. [DOI] [PubMed] [Google Scholar]
- Prevec L, Schneider M, et al. Use of human adenovirus-based vectors for antigen expression in animals. J. Gen. Virol. 1989;70(pt. 2):429–434. doi: 10.1099/0022-1317-70-2-429. [DOI] [PubMed] [Google Scholar]
- Quantin B, Perricaudet LD, et al. Adenovirus as an expression vector in muscle cells in vivo. Proc. Natl. Acad. Sci. USA. 1992;89(7):2581–2584. doi: 10.1073/pnas.89.7.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainov NG. A phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastoma multiforme. Hum. Gene Ther. 2000;11(17):2389–2401. doi: 10.1089/104303400750038499. [DOI] [PubMed] [Google Scholar]
- Rainov NG, Kramm CM. Vector delivery methods and targeting strategies for gene therapy of brain tumors. Curr. Gene Ther. 2001;1(4):367–383. doi: 10.2174/1566523013348445. [DOI] [PubMed] [Google Scholar]
- Ralph GS, Bienemann A, et al. Targeting of tetracycline-regulatable transgene expression specifically to neuronal and glial cell populations using adenoviral vectors. Neuroreport. 2000;11(9):2051–2055. doi: 10.1097/00001756-200006260-00048. [DOI] [PubMed] [Google Scholar]
- Ram Z, Culver KW, et al. In situ retroviral-mediated gene transfer for the treatment of brain tumors in rats. Cancer Res. 1993;53(1):83–88. [PubMed] [Google Scholar]
- Ram Z, Culver KW, et al. Therapy of malignant brain tumors by intratumoral implantation of retroviral vector-producing cells. Nat. Med. 1997;3(12):1354–1361. doi: 10.1038/nm1297-1354. [DOI] [PubMed] [Google Scholar]
- Rampling R, Cruickshank G, et al. Therapeutic replication-competent herpes virus. Nat. Med. 1998;4(2):133. doi: 10.1038/nm0298-133c. [DOI] [PubMed] [Google Scholar]
- Rampling R, Cruickshank G, et al. Toxicity evaluation of replication-competent herpes simplex virus (ICP 34.5 null mutant 1716) in patients with recurrent malignant glioma. Gene Ther. 2000;7(10):859–866. doi: 10.1038/sj.gt.3301184. [DOI] [PubMed] [Google Scholar]
- Raper SE, Yudkoff M, et al. A pilot study of in vivo liver-directed gene transfer with an adenoviral vector in partial ornithine transcarbamylase deficiency. Hum. Gene Ther. 2002;13(1):163–175. doi: 10.1089/10430340152712719. [DOI] [PubMed] [Google Scholar]
- Ridoux V, Robert JJ, et al. Adenoviral vectors as functional retrograde neuronal tracers. Brain Res. 1994a;648(1):171–175. doi: 10.1016/0006-8993(94)91919-4. [DOI] [PubMed] [Google Scholar]
- Ridoux V, Robert JJ, et al. The use of adenovirus vectors for intracerebral grafting of transfected nervous cells. Neuroreport. 1994;5(7):801–804. doi: 10.1097/00001756-199403000-00016. [DOI] [PubMed] [Google Scholar]
- Roelvink PW, Mi Lee G, et al. Identification of a conserved receptor-binding site on the fiber proteins of CAR-recognizing adenoviridae. Science. 1999;286(5444):1568–1571. doi: 10.1126/science.286.5444.1568. [DOI] [PubMed] [Google Scholar]
- Roessler BJ, Allen ED, et al. Adenoviral-mediated gene transfer to rabbit synovium in vivo. J. Clin. Invest. 1993;92(2):1085–1092. doi: 10.1172/JCI116614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld MA, Siegfried W, et al. Adenovirus-mediated transfer of a recombinant alpha 1-antitrypsin gene to the lung epithelium in vivo. Science. 1991;252(5004):431–434. doi: 10.1126/science.2017680. [DOI] [PubMed] [Google Scholar]
- Rosenfeld MA, Yoshimura K, et al. In vivo transfer of the human cystic fibrosis transmembrane conductance regulator gene to the airway epithelium. Cell. 1992;68(1):143–155. doi: 10.1016/0092-8674(92)90213-v. [DOI] [PubMed] [Google Scholar]
- Ross D, Ziff E. Defective synthesis of early region 4 mRNAs during abortive adenovirus infections in monkey cells. J. Virol. 1992;66(5):3110–3117. doi: 10.1128/jvi.66.5.3110-3117.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin BA, Rorke LB. Adenovirus vaccines. In: Plotkin S. A. a. M., Jr., et al., editors. Vaccines. W. B. Saunders Company; Philadelphia: 1994. pp. 475–502. [Google Scholar]
- Rutanen J, Markkanen J, et al. Gene therapy for restenosis: current status. Drugs. 2002;62(11):1575–1585. doi: 10.2165/00003495-200262110-00001. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning. Cold Spring Harbor Publishers; Cold Spring Harbor, NY: 1989. [Google Scholar]
- Sandig V, Youil R, et al. Optimization of the helper-dependent adenovirus system for production and potency in vivo. Proc. Natl. Acad. Sci. USA. 2000;97(3):1002–1007. doi: 10.1073/pnas.97.3.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandmair AM, Loimas S, et al. Thymidine kinase gene therapy for human malignant glioma, using replication-deficient retroviruses or adenoviruses. Hum. Gene Ther. 2000;11(16):2197–2205. doi: 10.1089/104303400750035726. [DOI] [PubMed] [Google Scholar]
- Sanlioglu S, Monick MM, et al. Rate limiting steps of AAV transduction and implications for human gene therapy. Curr. Gene Ther. 2001;1(2):137–147. doi: 10.2174/1566523013348788. [DOI] [PubMed] [Google Scholar]
- Saunders AM, Strittmatter WJ, et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology. 1993;43(8):1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- Schadeck EB, Partidos CD, et al. CTL epitopes identified with a defective recombinant adenovirus expressing measles virus nucleoprotein and evaluation of their protective capacity in mice. Virus Res. 1999;65(1):75–86. doi: 10.1016/s0168-1702(99)00103-3. [DOI] [PubMed] [Google Scholar]
- Schiedner G, Morral N, et al. Genomic DNA transfer with a high-capacity adenovirus vector results in improved in vivo gene expression and decreased toxicity. Nat. Genet. 1998;18(2):180–183. doi: 10.1038/ng0298-180. [DOI] [PubMed] [Google Scholar]
- Schwartz AR, Togo Y, et al. Clinical evaluation of live, oral types 1, 2, and 5 adenovirus vaccines. Annu. Rev. Respir. Dis. 1974;109(2):233–239. doi: 10.1164/arrd.1974.109.2.233. [DOI] [PubMed] [Google Scholar]
- Scinto LF, Daffner KR, et al. A potential noninvasive neurobiological test for Alzheimer’s disease. Science. 1994;266(5187):1051–1054. doi: 10.1126/science.7973660. [DOI] [PubMed] [Google Scholar]
- Segerman A, Mei YF, et al. Adenovirus types 11p and 35p show high binding efficiencies for committed hematopoietic cell lines and are infective to these cell lines. J. Virol. 2000;74(3):1457–1467. doi: 10.1128/jvi.74.3.1457-1467.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiger A, Nordberg A, et al. Intracranial infusion of purified nerve growth factor to an Alzheimer patient: The first attempt of a possible future treatment strategy. Behav. Brain Res. 1993;57(2):255–261. doi: 10.1016/0166-4328(93)90141-c. [DOI] [PubMed] [Google Scholar]
- Shayakhmetov DM, Papayannopoulou T, et al. Efficient gene transfer into human CD34(+) cells by a retargeted adenovirus vector. J. Virol. 2000;74(6):2567–2583. doi: 10.1128/jvi.74.6.2567-2583.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenk T. Adenoviridae: The viruses and their replication. In: Krupe D, Howley DM, editors. Virology. Raven Press; New York: 2001. [Google Scholar]
- Shering AF, Bain D, et al. Cell type-specific expression in brain cell cultures from a short human cytomegalovirus major immediate early promoter depends on whether it is inserted into herpesvirus or adenovirus vectors. J. Gen. Virol. 1997;78(pt. 2):445–459. doi: 10.1099/0022-1317-78-2-445. [DOI] [PubMed] [Google Scholar]
- Shewach DS, Zerbe LK, et al. Enhanced cytotoxicity of antiviral drugs mediated by adenovirus directed transfer of the herpes simplex virus thymidine kinase gene in rat glioma cells. Cancer Gene Ther. 1994;1(2):107–112. [PubMed] [Google Scholar]
- Shinoura N, Ohashi M, et al. Adenovirus-mediated overexpression of Fas induces apoptosis of gliomas. Cancer Gene Ther. 2000;7(2):224–232. doi: 10.1038/sj.cgt.7700110. [DOI] [PubMed] [Google Scholar]
- Shinoura N, Yoshida Y, et al. Adenovirus-mediated transfer of p53 and Fas ligand drastically enhances apoptosis in gliomas. Cancer Gene Ther. 2000;7(5):732–738. doi: 10.1038/sj.cgt.7700160. [DOI] [PubMed] [Google Scholar]
- Shono T, Tofilon PJ, et al. Apoptosis induced by adenovirus-mediated p53 gene transfer in human glioma correlates with site-specific phosphorylation. Cancer Res. 2002;62(4):1069–1076. [PubMed] [Google Scholar]
- Simon RH, Engelhardt JF, et al. Adenovirus-mediated transfer of the CFTR gene to lung of nonhuman primates: toxicity study. Hum. Gene Ther. 1993;4(6):771–780. doi: 10.1089/hum.1993.4.6-771. [DOI] [PubMed] [Google Scholar]
- Smith TA, Mehaffey MG, et al. Adenovirus mediated expression of therapeutic plasma levels of human factor IX in mice. Nat. Genet. 1993;5(4):397–402. doi: 10.1038/ng1293-397. [DOI] [PubMed] [Google Scholar]
- Smith-Arica JR, Morelli AE, et al. Cell-type-specific and regulatable transgenesis in the adult brain: Adenovirus-encoded combined transcriptional targeting and inducible transgene expression. Mol. Ther. 2000;2(6):579–587. doi: 10.1006/mthe.2000.0215. [DOI] [PubMed] [Google Scholar]
- Smith-Arica JR, Williams JC, et al. Switching on and off transgene expression within lactotrophic cells in the anterior pituitary gland in vivo. Endocrinology. 2001;142(6):2521–2532. doi: 10.1210/endo.142.6.8183. [DOI] [PubMed] [Google Scholar]
- Southgate TD, Bain D, Fairbanks LD, Morelli AE, Larrengina AT, Simmonds HA, Castro MG, Lowenstein PR. Adenoviruses encoding HPRT correct biochemical abnormalities of HPRT-deficient cells and allow their survival in negative selection medium. Metab. Brain Dis. 1999;14(4):205–21. doi: 10.1023/a:1020728924026. [DOI] [PubMed] [Google Scholar]
- Southgate TD, Stone D, et al. Long-term transgene expression within the anterior pituitary gland in situ: Impact on circulating hormone levels, cellular and antibody-mediated immune responses. Endocrinology. 2001;142(1):464–476. doi: 10.1210/endo.142.1.7898. [DOI] [PubMed] [Google Scholar]
- Spergel JM, Chen-Kiang S. Interleukin 6 enhances a cellular activity that functionally substitutes for E1A protein in transactivation. Proc. Natl. Acad. Sci. USA. 1991;88(15):6472–6476. doi: 10.1073/pnas.88.15.6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford-Perricaudet LD, Levrero M, et al. Evaluation of the transfer and expression in mice of an enzyme-encoding gene using a human adenovirus vector. Hum. Gene Ther. 1990;1(3):241–256. doi: 10.1089/hum.1990.1.3-241. [DOI] [PubMed] [Google Scholar]
- Stratford-Perricaudet LD, Makeh I, et al. Widespread long-term gene transfer to mouse skeletal muscles and heart. J. Clin. Invest. 1992;90(2):626–630. doi: 10.1172/JCI115902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhr ST, Gage FH. Gene therapy in the central nervous system: The use of recombinant retroviruses. Arch. Neurol. 1999;56(3):287–292. doi: 10.1001/archneur.56.3.287. [DOI] [PubMed] [Google Scholar]
- Takamiya Y, Short MP, et al. Gene therapy of malignant brain tumors: A rat glioma line bearing the herpes simplex virus type 1-thymidine kinase gene and wild type retrovirus kills other tumor cells. J. Neurosci. Res. 1992;33(3):493–503. doi: 10.1002/jnr.490330316. [DOI] [PubMed] [Google Scholar]
- Tamura K, Tamura M, et al. Eradication of murine brain tumors by direct inoculation of concentrated high titer-recombinant retrovirus harboring the herpes simplex virus thymidine kinase gene. Gene Ther. 2001;8(3):215–222. doi: 10.1038/sj.gt.3301371. [DOI] [PubMed] [Google Scholar]
- Tate MC, Shear DA, et al. Fibronection promotes survival and migration of primary neural stem cells transplanted into the traumatically injured mouse brain. Cell Transplant. 2002;11(3):283–295. [PubMed] [Google Scholar]
- Thomas CE, Abordo-Adesida E, Maleniak TC, Stone D, Gerdes G, Lowenstein PR. Gene transfer into rat brain using adenoviral vectors. In: Gerfen JN, McKay R, Rogawski MA, Sibley DR, Skolnick P, editors. Current Protocols in Neuroscience. John Wiley & Sons; New York: 2000a. pp. 4.23.1–4.23.40. [DOI] [PubMed] [Google Scholar]
- Thomas CE, Schiedner G, et al. Peripheral infection with adenovirus causes unexpected long-term brain inflammation in animals injected intracranially with first-generation, but not with high-capacity, adenovirus vectors: Toward realistic long-term neurological gene therapy for chronic diseases. Proc. Natl. Acad. Sci. USA. 2000b;97(13):7482–7487. doi: 10.1073/pnas.120474397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CE, Birkett D, et al. Acute direct adenoviral vector cytotoxicity and chronic, but not acute, inflammatory responses correlate with decreased vector-mediated transgene expression in the brain. Mol. Ther. 2001;3(1):36–46. doi: 10.1006/mthe.2000.0224. [DOI] [PubMed] [Google Scholar]
- Thomas CE, Schiedner G, et al. Preexisting antiadenoviral immunity is not a barrier to efficient and stable transduction of the brain, mediated by novel high-capacity adenovirus vectors. Hum. Gene Ther. 2001;12(7):839–846. doi: 10.1089/104303401750148829. [DOI] [PubMed] [Google Scholar]
- Thomas CE, Edwards P, et al. Adenovirus binding to the coxsackievirus and adenovirus receptor or integrins is not required to elicit brain inflammation but is necessary to transduce specific neural cell types. J. Virol. 2002;76(7):3452–3460. doi: 10.1128/JVI.76.7.3452-3460.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga-Yoshino K. Neurotoxic and neuroprotective effects of glutamate are enhanced by introduction of amyloid precursor protein cDNA. Brain Res. 2001;918(2):121–130. doi: 10.1016/s0006-8993(01)02983-3. [DOI] [PubMed] [Google Scholar]
- Tomko RP, Xu R, et al. HCAR and MCAR: The human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc. Natl. Acad. Sci. USA. 1997;94(7):3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Top FH, Jr., Grossman RA, et al. Immunization with live types 7 and 4 adenovirus vaccines. I. Safety, infectivity, antigenicity, and potency of adenovirus type 7 vaccine in humans. J. Infect. Dis. 1971a;124(2):148–154. doi: 10.1093/infdis/124.2.148. [DOI] [PubMed] [Google Scholar]
- Top FH, Jr., Buescher EL, et al. Immunization with live types 7 and 4 adenovirus vaccines. II. Antibody response and protective effect against acute respiratory disease due to adenovirus type 7. J. Infect. Dis. 1971b;124(2):155–160. doi: 10.1093/infdis/124.2.155. [DOI] [PubMed] [Google Scholar]
- Trask TW, Trask RP, et al. Phase I study of adenoviral delivery of the HSV-tk gene and ganciclovir administration in patients with current malignant brain tumors. Mol. Ther. 2000;1(2):195–203. doi: 10.1006/mthe.2000.0030. [DOI] [PubMed] [Google Scholar]
- Trotman LC, Mosberger N, et al. Import of adenovirus DNA involves the nuclear pore complex receptor CAN/Nup214 and histone H1. Nat. Cell Biol. 2001;3(12):1092–1100. doi: 10.1038/ncb1201-1092. [DOI] [PubMed] [Google Scholar]
- Ugolini G, Kuypers HG, et al. Transneuronal transfer of herpes virus from peripheral nerves to cortex and brainstem. Science. 1989;243(4887):89–91. doi: 10.1126/science.2536188. [DOI] [PubMed] [Google Scholar]
- Umana P, Gerdes CA, et al. Efficient FLPe recombinase enables scalable production of helper-dependent adenoviral vectors with negligible helper-virus contamination. Nat. Biotechnol. 2001;19(6):582–585. doi: 10.1038/89349. [DOI] [PubMed] [Google Scholar]
- Wade-Martins R, Smith ER, et al. An infectious transfer and expression system for genomic DNA loci in human and mouse cells. Nat. Biotechnol. 2001;19(11):1067–1070. doi: 10.1038/nbt1101-1067. [DOI] [PubMed] [Google Scholar]
- Wadsworth SC, Zhou H, et al. Adenovirus vector-infected cells can escape adenovirus antigen-specific cytotoxic T-lymphocyte killing in vivo. J. Virol. 1997;71(7):5189–5196. doi: 10.1128/jvi.71.7.5189-5196.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Fraefel C, et al. HSV-1 amplicon vectors. Methods Enzymol. 2002;346:593–603. doi: 10.1016/s0076-6879(02)46079-x. [DOI] [PubMed] [Google Scholar]
- Welsh MJ, Smith AE, et al. Cystic fibrosis gene therapy using an adenovirus vector: In vivo safety and efficacy in nasal epithelium. Hum. Gene Ther. 1994;5(2):209–219. doi: 10.1089/hum.1994.5.2-209. [DOI] [PubMed] [Google Scholar]
- Wickham TJ. Targeting adenovirus. Gene Ther. 2000;7(2):110–114. doi: 10.1038/sj.gt.3301115. [DOI] [PubMed] [Google Scholar]
- Wickham TJ, Mathias P, et al. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993;73(2):309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- Wickham TJ, Filardo EJ, et al. Integrin alpha v beta 5 selectively promotes adenovirus mediated cell membrane permeabilization. J. Cell Biol. 1994;127(1):257–264. doi: 10.1083/jcb.127.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson GWG. Gene therapy and viral vaccination. Rev. Med. Microbiol. 1994a;5:97–106. [Google Scholar]
- Wilkinson GWG. Viral vectors for gene therapy. In: Latchman D, editor. From Genetics to Gene Therapy. BioScientific Publishers; London: 1994b. pp. 161–192. [Google Scholar]
- Wilkinson GW, Borysiewicz LK. Gene therapy and viral vaccination: The interface. Br. Med. Bull. 1995;51(1):205–216. doi: 10.1093/oxfordjournals.bmb.a072947. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Engelhardt JF, et al. Gene therapy of cystic fibrosis lung disease using E1 deleted adenoviruses: A phase I trial. Hum. Gene Ther. 1994;5(4):501–519. doi: 10.1089/hum.1994.5.4-501. [DOI] [PubMed] [Google Scholar]
- Wold WS, Hermiston TW, et al. Adenovirus proteins that subvert host defenses. Trends Microbiol. 1994;2(11):437–443. doi: 10.1016/0966-842x(94)90801-x. [DOI] [PubMed] [Google Scholar]
- Wold WS, Doronin K, et al. Immune responses to adenoviruses: Viral evasion mechanisms and their implications for the clinic. Curr. Opin. Immunol. 1999;11(4):380–386. doi: 10.1016/S0952-7915(99)80064-8. [DOI] [PubMed] [Google Scholar]
- Wood MJ, Charlton HM, et al. Immune responses to adenovirus vectors in the nervous system. Trends Neurosci. 1996a;19(11):497–501. doi: 10.1016/S0166-2236(96)10060-6. [DOI] [PubMed] [Google Scholar]
- Wood MJA, Byrnes AP, McMenamin M, Kajiwara K, Vine A, Gordon I, Lang J, Wood KJ, Charlton HM. Immune responses to viruses: Practical implications for the use of viruses as vectors for experimental and clinical gene therapy. In: Lowenstein PR, et al., editors. Protocols for Gene Transfer in Neuroscience. John Wiley & Sons; New York: 1996b. [Google Scholar]
- Yang Y, Nunes FA, et al. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc. Natl. Acad. Sci. USA. 1994a;91(10):4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Nunes FA, et al. Inactivation of E2a in recombinant adenoviruses improves the prospect for gene therapy in cystic fibrosis. Nat. Genet. 1994b;7(3):362–369. doi: 10.1038/ng0794-362. [DOI] [PubMed] [Google Scholar]
- Yei S, Bachurski CJ, et al. Adenoviral-mediated gene transfer of human surfactant protein B to respiratory epithelial cells. Am. J. Respir. Cell Mol. Biol. 1994a;11(3):329–336. doi: 10.1165/ajrcmb.11.3.8086169. [DOI] [PubMed] [Google Scholar]
- Yei S, Mittereder N, et al. In vivo evaluation of the safety of adenovirus-mediated transfer of the human cystic fibrosis transmembrane conductance regulator cDNA to the lung. Hum. Gene Ther. 1994b;5(6):731–744. doi: 10.1089/hum.1994.5.6-731. [DOI] [PubMed] [Google Scholar]
- Zabner J, Couture LA, et al. Correction of cAMP-stimulated fluid secretion in cystic fibrosis airway epithelia: Efficiency of adenovirus-mediated gene transfer in vitro. Hum. Gene Ther. 1994;5(5):585–593. doi: 10.1089/hum.1994.5.5-585. [DOI] [PubMed] [Google Scholar]
- Zabner J, Chillon M, et al. A chimeric type 2 adenovirus vector with a type 17 fiber enhances gene transfer to human airway epithelia. J. Virol. 1999;73(10):8689–8695. doi: 10.1128/jvi.73.10.8689-8695.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemanick MC, Strick PL, et al. Direction of transneuronal transport of herpes simplex virus 1 in the primate motor system is strain-dependent. Proc. Natl. Acad. Sci. USA. 1991;88(18):8048–8051. doi: 10.1073/pnas.88.18.8048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zermansky AJ, Bolognani F, et al. Towards global and long-term neurological gene therapy: Unexpected transgene dependent, high-level, and widespread distribution of HSV-1 thymidine kinase throughout the CNS. Mol. Ther. 2001;4(5):490–498. doi: 10.1006/mthe.2001.0479. [DOI] [PubMed] [Google Scholar]
- Zhou H, Zhao T, et al. A Cre-expressing cell line and an E1/E2a double-deleted virus for preparation of helper-dependent adenovirus vector. Mol. Ther. 2001;3(4):613–622. doi: 10.1006/mthe.2001.0288. [DOI] [PubMed] [Google Scholar]
- Zhou H, Pastore L, et al. Helper-dependent adenoviral vectors. Methods Enzymol. 2002;346:177–198. doi: 10.1016/s0076-6879(02)46056-9. [DOI] [PubMed] [Google Scholar]
aReferences to Table II
- Abe T, Wakimoto H, et al. Intra-arterial delivery of p53-containing adenoviral vector into experimental brain tumors. Cancer Gene Ther. 2002;9(3):228–235. doi: 10.1038/sj.cgt.7700437. [DOI] [PubMed] [Google Scholar]
- Adachi Y, Tamiya T, et al. Experimental gene therapy for brain tumors using adenovirus-mediated transfer of cytosine deaminase gene and uracil phosphoribosyltransferase gene with 5-fluorocytosine. Hum. Gene Ther. 2000;11(1):77–89. doi: 10.1089/10430340050016175. [DOI] [PubMed] [Google Scholar]
- Adachi Y, Chandrasekar N, et al. Suppression of glioma invasion and growth by adenovirus-mediated delivery of a bicistronic construct containing antisense uPAR and sense p16 gene sequences. Oncogene. 2002;21(1):87–95. doi: 10.1038/sj.onc.1204999. [DOI] [PubMed] [Google Scholar]
- Akli S, Caillaud C, et al. Transfer of a foreign gene into the brain using adenovirus vectors. Nat. Genet. 1993;3(3):224–228. doi: 10.1038/ng0393-224. [DOI] [PubMed] [Google Scholar]
- Aoki M, Tamatani M, et al. Hypothermic treatment restores glucose regulated protein 78 (GRP78) expression in ischemic brain. Brain Res. Mol. Brain Res. 2001;95(1–2):117–128. doi: 10.1016/s0169-328x(01)00255-8. [DOI] [PubMed] [Google Scholar]
- Araki W, Yuasa K, et al. Pro-apoptotic effect of presenilin 2 (PS2) overexpression is associated with down-regulation of Bcl-2 in cultured neurons. J. Neurochem. 2001;79(6):1161–1168. doi: 10.1046/j.1471-4159.2001.00638.x. [DOI] [PubMed] [Google Scholar]
- Badie B, Hunt K, et al. Stereotactic delivery of a recombinant adenovirus into a C6 glioma cell line in a rat brain tumor model. Neurosurgery. 1994;35(5):910–915. doi: 10.1227/00006123-199411000-00016. discussion 915–916. [DOI] [PubMed] [Google Scholar]
- Bajocchi G, Feldman SH, et al. Direct in vivo gene transfer to ependymal cells in the central nervous system using recombinant adenovirus vectors. Nat. Genet. 1993;3(3):229–234. doi: 10.1038/ng0393-229. [DOI] [PubMed] [Google Scholar]
- Beer SJ, Matthews CB, et al. Poly (lactic-glycolic) acid copolymer encapsulation of recombinant adenovirus reduces immunogenicity in vivo. Gene Ther. 1998;5(6):740–746. doi: 10.1038/sj.gt.3300647. [DOI] [PubMed] [Google Scholar]
- Bemelmans AP, Horellou P, et al. Brain-derived neurotrophic factor-mediated protection of striatal neurons in an excitotoxic rat model of Huntington’s disease, as demonstrated by adenoviral gene transfer. Hum. Gene Ther. 1999;10(18):2987–2997. doi: 10.1089/10430349950016393. [DOI] [PubMed] [Google Scholar]
- Bennett J, Wilson J, et al. Adenovirus vector-mediated in vivo gene transfer into adult murine retina. Invest. Ophthalmol. Vis. Sci. 1994;35(5):2535–2542. [PubMed] [Google Scholar]
- Benraiss A, Chmielnicki E, et al. Adnoviral brain-derived neurotrophic factor induces both neostriatal and olfactory neuronal recruitment from endogenous progenitor cells in the adult forebrain. J. Neurosci. 2001;21(17):6718–6731. doi: 10.1523/JNEUROSCI.21-17-06718.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz AL, Yang GY, et al. Attenuation of stroke size in rats using an adenoviral vector to induce overexpression of interleukin-1 receptor antagonist in brain. J. Cereb. Blood Flow Metab. 1995;15(4):547–551. doi: 10.1038/jcbfm.1995.68. [DOI] [PubMed] [Google Scholar]
- Biroccio A, Bufalo DD, et al. Increase of BCNU sensitivity by wt-p53 gene therapy in glioblastoma lines depends on the administration schedule. Gene Ther. 1999;6(6):1064–1072. doi: 10.1038/sj.gt.3300935. [DOI] [PubMed] [Google Scholar]
- Boer GJ, van Esseveldt KE, et al. Long-term transgene expression in fetal rat suprachiasmatic nucleus neurografts following ex vivo adenoviral vector-mediated gene transfer. Exp. Neurol. 1997;145(2, pt. 1):536–545. doi: 10.1006/exnr.1997.6489. [DOI] [PubMed] [Google Scholar]
- Boviatsis EJ, Chase M, et al. Gene transfer into experimental brain tumors mediated by adenovirus, herpes simplex virus, and retrovirus vectors. Hum. Gene. Ther. 1994;5(2):183–191. doi: 10.1089/hum.1994.5.2-183. [DOI] [PubMed] [Google Scholar]
- Brust D, Feden J, et al. Radiosensitization of rat glioma with bromodeoxycytidine and adenovirus expressing herpes simplex virus-thymidine kinase delivered by slow, rate-controlled positive pressure infusion. Cancer Gene Ther. 2000;7(5):778–788. doi: 10.1038/sj.cgt.7700168. [DOI] [PubMed] [Google Scholar]
- Byrnes AP, Rusby JE, et al. Adenovirus gene transfer causes inflammation in the brain. Neuroscience. 1995;66(4):1015–1024. doi: 10.1016/0306-4522(95)00068-t. [DOI] [PubMed] [Google Scholar]
- Byrnes AP, MacLaren RE, et al. Immunological instability of persistent adenovirus vectors in the brain: Peripheral exposure to vector leads to renewed inflammation, reduced gene expression, and demyelination. J. Neurosci. 1996a;16(9):3045–3055. doi: 10.1523/JNEUROSCI.16-09-03045.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes AP, Wood MJ, et al. Role of T cells in inflammation caused by adenovirus vectors in the brain. Gene Ther. 1996b;3(7):644–651. [PubMed] [Google Scholar]
- Caillaud C, Akli S, et al. Adenoviral vector as a gene delivery system into cultured rat neuronal and glial cells. Eur. J. Neurosci. 1993;5(10):1287–1291. doi: 10.1111/j.1460-9568.1993.tb00914.x. [DOI] [PubMed] [Google Scholar]
- Cartmell T, Southgate T, et al. Interleukin-1 mediates a rapid inflammatory response after injection of adenoviral vectors into the brain. J. Neurosci. 1999;19(4):1517–1523. doi: 10.1523/JNEUROSCI.19-04-01517.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SH, Shine HD, et al. Gene therapy for brain tumors: Regression of experimental gliomas by adenovirus-mediated gene transfer in vivo. Proc. Natl. Acad. Sci. USA. 1994;91(8):3054–3057. doi: 10.1073/pnas.91.8.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi-Lundberg DL, Lin Q, et al. Dopaminergic neurons protected from degeneration by GDNF gene therapy. Science. 1997;275(5301):838–841. doi: 10.1126/science.275.5301.838. [DOI] [PubMed] [Google Scholar]
- Choi-Lundberg DL, Lin Q, et al. Behavioral and cellular protection of rat dopaminergic neurons by an adenoviral vector encoding glial cell line-derived neurotrophic factor. Exp. Neurol. 1998;154(2):261–275. doi: 10.1006/exnr.1998.6887. [DOI] [PubMed] [Google Scholar]
- Connor B, Kozlowski DA, et al. Differential effects of glial cell line-derived neurotrophic factor (GDNF) in the striatum and substantia nigra of the aged Parkinsonian rat. Gene Ther. 1999;6(12):1936–1951. doi: 10.1038/sj.gt.3301033. [DOI] [PubMed] [Google Scholar]
- Connor B, Kozlowski DA, et al. Glial cell line-derived neurotrophic factor (GDNF) gene delivery protects dopaminergic terminals from degeneration. Exp. Neurol. 2001;169(1):83–95. doi: 10.1006/exnr.2001.7638. [DOI] [PubMed] [Google Scholar]
- Corti O, Sabate O, et al. A single adenovirus vector mediates doxycycline-controlled expression of tyrosine hydroxylase in brain grafts of human neural progenitors. Nat. Biotechnol. 1999;17(4):349–354. doi: 10.1038/7901. [DOI] [PubMed] [Google Scholar]
- Cowsill C, Southgate TD, et al. Central nervous system toxicity of two adenoviral vectors encoding variants of the herpes simplex virus type 1 thymidine kinase: Reduced cytotoxicity of a truncated HSV1-TK. Gene Ther. 2000;7(8):679–685. doi: 10.1038/sj.gt.3301147. [DOI] [PubMed] [Google Scholar]
- Cregan SP, MacLaurin J, et al. Helper-dependent adenovirus vectors: Their use as a gene delivery system to neurons. Gene Ther. 2000;7(14):1200–1209. doi: 10.1038/sj.gt.3301208. [DOI] [PubMed] [Google Scholar]
- Cua DJ, Hutchins B, et al. Central nervous system expression of IL-10 inhibits autoimmune encephalomyelitis. J. Immunol. 2001;166(1):602–608. doi: 10.4049/jimmunol.166.1.602. [DOI] [PubMed] [Google Scholar]
- Davidson BL, Allen ED, et al. A model system for in vivo gene transfer into the central nervous system using an adenoviral vector. Nat. Genet. 1993;3(3):219–223. doi: 10.1038/ng0393-219. [DOI] [PubMed] [Google Scholar]
- Davidson BL, Doran SE, et al. Expression of Escherichia coli beta-galactosidase and rat HPRT in the CNS of Macaca mulatta following adenoviral mediated gene transfer. Exp. Neurol. 1994;125(2):258–267. doi: 10.1006/exnr.1994.1028. [DOI] [PubMed] [Google Scholar]
- Dewey RA, Morrissey G, et al. Chronic brain inflammation and persistent herpes simplex virus 1 thymidine kinase expression in survivors of syngeneic glioma treated by adenovirus-mediated gene therapy: Implications for clinical trials. Nat. Med. 1999;5(11):1256–1263. doi: 10.1038/15207. [DOI] [PubMed] [Google Scholar]
- Doran SE, Ren XD, et al. Gene expression from recombinant viral vectors in the central nervous system after blood-brain barrier disruption. Neurosurgery. 1995;36(5):965–970. doi: 10.1227/00006123-199505000-00012. [DOI] [PubMed] [Google Scholar]
- Dorsey SG, Bambrick LL, et al. Failure of brain-derived neurotrophic factor-dependent neuron survival in mouse trisomy 16. J. Neurosci. 2002;22(7):2571–2578. doi: 10.1523/JNEUROSCI.22-07-02571.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driesse MJ, Vincent AJ, et al. Intracerebral injection of adenovirus harboring the HSVtk gene combined with ganciclovir administration: Toxicity study in nonhuman primates. Gene Ther. 1998;5(8):1122–1129. doi: 10.1038/sj.gt.3300695. [DOI] [PubMed] [Google Scholar]
- Driesse MJ, Esandi MC, et al. Intra-CSF administered recombinant adenovirus causes an immune response-mediated toxicity. Gene Ther. 2000;7(16):1401–1409. doi: 10.1038/sj.gt.3301250. [DOI] [PubMed] [Google Scholar]
- Eck SL, Alavi JB, et al. Treatment of advanced CNS malignancies with the recombinant adenovirus H5.010RSVTK: A phase I trial. Hum. Gene Ther. 1996;7(12):1465–1482. doi: 10.1089/hum.1996.7.12-1465. [DOI] [PubMed] [Google Scholar]
- Eck SL, Alavi JB, et al. Treatment of recurrent or progressive malignant glioma with a recombinant adenovirus expressing human interferon-beta (H5.010CMVhIFN-beta): A phase I trial. Hum. Gene Ther. 2001;12(1):97–113. doi: 10.1089/104303401451013. [DOI] [PubMed] [Google Scholar]
- Fathallah-Shaykh HM, Zhao LJ, et al. Gene transfer of IFN-gamma into established brain tumors represses growth by antiangiogenesis. J. Immunol. 2000;164(1):217–222. doi: 10.4049/jimmunol.164.1.217. [DOI] [PubMed] [Google Scholar]
- Galanis E, Bateman A, et al. Use of viral fusogenic membrane glycoproteins as novel therapeutic transgenes in gliomas. Hum. Gene Ther. 2001;12(7):811–821. doi: 10.1089/104303401750148766. [DOI] [PubMed] [Google Scholar]
- Gerdes CA, Castro MG, et al. Strong promoters are the key to highly efficient, noninflammatory and noncytotoxic adenoviral-mediated transgene delivery into the brain in vivo. Mol. Ther. 2000;2(4):330–338. doi: 10.1006/mthe.2000.0140. [DOI] [PubMed] [Google Scholar]
- Ghodsi A, Stein C, et al. Extensive beta-glucuronidase activity in murine central nervous system after adenovirus-mediated gene transfer to brain. Hum. Gene Ther. 1998;9(16):2331–2340. doi: 10.1089/hum.1998.9.16-2331. [DOI] [PubMed] [Google Scholar]
- Glaser T, Castro MG, et al. Death receptor-independent cytochrome c release and caspase activation mediate thymidine kinase plus ganciclovir-mediated cytotoxicity in LN-18 and LN-229 human malignant glioma cells. Gene Ther. 2001;8(6):469–476. doi: 10.1038/sj.gt.3301415. [DOI] [PubMed] [Google Scholar]
- Gomez-Manzano C, Fueyo J, et al. Characterization of p53 and p21 functional interactions in glioma cells en route to apoptosis. J. Natl. Cancer Inst. 1997;89(14):1036–1044. doi: 10.1093/jnci/89.14.1036. [DOI] [PubMed] [Google Scholar]
- Goodman JC, Trask TW, et al. Adenoviral-mediated thymidine kinase gene transfer into the primate brain followed by systemic ganciclovir: Pathologic, radiologic, and molecular studies. Hum. Gene Ther. 1996;7(10):1241–1250. doi: 10.1089/hum.1996.7.10-1241. [DOI] [PubMed] [Google Scholar]
- Gupta A, Ho DY, et al. Neuroprotective effects of an adenoviral vector expressing the glucose transporter: A detailed description of the mediating cellular events. Brain Res. 2001;908(1):49–57. doi: 10.1016/s0006-8993(01)02572-0. [DOI] [PubMed] [Google Scholar]
- Hagan P, Barks JD, et al. Adenovirus-mediated over-expression of interleukin-1 receptor antagonist reduces susceptibility to excitotoxic brain injury in perinatal rats. Neuroscience. 1996;75(4):1033–1045. doi: 10.1016/0306-4522(96)00225-4. [DOI] [PubMed] [Google Scholar]
- Harada H, Nakagawa K, et al. Restoration of wild-type p16 down-regulates vascular endothelial growth factor expression and inhibits angiogenesis in human gliomas. Cancer Res. 1999;59(15):3783–3789. [PubMed] [Google Scholar]
- Horellou P, Vigne E, et al. Direct intracerebral gene transfer of an adenoviral vector expressing tyrosine hydroxylase in a rat model of Parkinson’s disease. Neuroreport. 1994;6(1):49–53. doi: 10.1097/00001756-199412300-00014. [DOI] [PubMed] [Google Scholar]
- Hughes SM, Moussavi-Harami F, et al. Viral-mediated gene transfer to mouse primary neural progenitor cells. Mol. Ther. 2002;5(1):16–24. doi: 10.1006/mthe.2001.0512. [DOI] [PubMed] [Google Scholar]
- Jomary C, Piper TA, et al. Adenovirus-mediated gene transfer to murine retinal cells in vitro and in vivo. FEBS Lett. 1994;347(2–3):117–122. doi: 10.1016/0014-5793(94)00512-5. [DOI] [PubMed] [Google Scholar]
- Kajiwara K, Byrnes AP, et al. Immune responses to adenoviral vectors during gene transfer in the brain. Hum. Gene Ther. 1997;8(3):253–265. doi: 10.1089/hum.1997.8.3-253. [DOI] [PubMed] [Google Scholar]
- Kajiwara K, Byrnes AP, et al. Humoral immune responses to adenovirus vectors in the brain. J. Neuroimmunol. 2000;103(1):8–15. doi: 10.1016/s0165-5728(99)00220-9. [DOI] [PubMed] [Google Scholar]
- Kim CD, Shin HK, et al. Gene transfer of Cu/Zn SOD to cerebral vessels prevents FPI-induced CBF autoregulatory dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2002;282(5):H1836–1842. doi: 10.1152/ajpheart.00590.2001. [DOI] [PubMed] [Google Scholar]
- Kitagawa H, Sasaki C, et al. Adenovirus-mediated gene transfer of glial cell line-derived neurotrophic factor prevents ischemic brain injury after transient middle cerebral artery occlusion in rats. J. Cereb. Blood Flow Metab. 1999;19(12):1336–1344. doi: 10.1097/00004647-199912000-00007. [DOI] [PubMed] [Google Scholar]
- Kosuga M, Sasaki K, et al. Engraftment of genetically engineered amniotic epithelial cells corrects lysosomal storage in multiple areas of the brain in mucopolysaccharidosis type VII mice. Mol. Ther. 2001b;3(2):139–148. doi: 10.1006/mthe.2000.0234. [DOI] [PubMed] [Google Scholar]
- Kosuga M, Takahashi S, et al. Widespread distribution of adenovirus-transduced monkey amniotic epithelial cells after local intracerebral injection: Implication for cell-mediated therapy for lysosome storage disorders. Cell Transplant. 2001a;10(4–5):435–439. [PubMed] [Google Scholar]
- Kozlowski DA, Connor B, et al. Delivery of a GDNF gene into the substantia nigra after a progressive 6-OHDA lesion maintains functional nigrostriatal connections. Exp. Neurol. 2000;166(1):1–15. doi: 10.1006/exnr.2000.7463. [DOI] [PubMed] [Google Scholar]
- Kozlowski DA, Bremer E, et al. Quantitative analysis of transgene protein, mRNA, and vector DNA following injection of an adenoviral vector harboring glial cell line-derived neurotrophic factor into the primate caudate nucleus. Mol. Ther. 2001;3(2):256–261. doi: 10.1006/mthe.2000.0256. [DOI] [PubMed] [Google Scholar]
- Kugler S, Meyn L, et al. Neuron-specific expression of therapeutic proteins: Evaluation of different cellular promoters in recombinant adenoviral vectors. Mol. Cell. Neurosci. 2001;17(1):78–96. doi: 10.1006/mcne.2000.0929. [DOI] [PubMed] [Google Scholar]
- Kuo H, Ingram DK, et al. Retrograde transfer of replication deficient recombinant adenovirus vector in the central nervous system for tracing studies. Brain Res. 1995;705(1–2):31–38. doi: 10.1016/0006-8993(95)01065-3. [DOI] [PubMed] [Google Scholar]
- Kurihara H, Zama A, et al. Glioma/glioblastoma-specific adenoviral gene expression using the nestin gene regulator. Gene Ther. 2000;7(8):686–693. doi: 10.1038/sj.gt.3301129. [DOI] [PubMed] [Google Scholar]
- Lammering G, Valerie K, et al. Radiosensitization of malignant glioma cells through overexpression of dominant-negative epidermal growth factor receptor. Clin. Cancer Res. 2001;7(3):682–690. [PubMed] [Google Scholar]
- Lawinger P, Venugopal R, et al. The neuronal repressor REST/NRSF is an essential regulator in medulloblastoma cells. Nat. Med. 2000;6(7):826–831. doi: 10.1038/77565. [DOI] [PubMed] [Google Scholar]
- Le Gal La Salle G, Robert JJ, et al. An adenovirus vector for gene transfer into neurons and glia in the brain. Science. 1993;259(5097):988–990. doi: 10.1126/science.8382374. [DOI] [PubMed] [Google Scholar]
- Li T, Adamian M, et al. In vivo transfer of a reporter gene to the retina mediated by an adenoviral vector. Invest. Ophthalmol. Vis. Sci. 1994;35(5):2543–2549. [PubMed] [Google Scholar]
- Lisovoski F, Cadusseau J, et al. In vivo transfer of a marker gene to study motoneuronal development. Neuroreport. 1994;5(9):1069–1072. doi: 10.1097/00001756-199405000-00013. [DOI] [PubMed] [Google Scholar]
- Mackler SA, Korutla L, et al. NAC-1 is a brain POZ/BTB protein that can prevent cocaine-induced sensitization in the rat. J. Neurosci. 2000;20(16):6210–6217. doi: 10.1523/JNEUROSCI.20-16-06210.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maleniak TC, Darling JL, et al. Adenovirus-mediated expression of HSV1-TK or Fas ligand induces cell death in primary human glioma-derived cell cultures that are resistant to the chemotherapeutic agent CCNU. Cancer Gene Ther. 2001;8(8):589–598. doi: 10.1038/sj.cgt.7700348. [DOI] [PubMed] [Google Scholar]
- Masada T, Hua Y, et al. Attenuation of intracerebral hemorrhage and thrombin-induced brain edema by overexpression of interleukin-1 receptor antagonist. J. Neurosurg. 2001;95(4):680–686. doi: 10.3171/jns.2001.95.4.0680. [DOI] [PubMed] [Google Scholar]
- Matsuoka N, Ishii K, et al. Overexpression of basic fibroblast growth factor and Bcl-xL with adenoviral vectors protects primarily cultured neurons against glutamate insult. Neurosurgery. 2002;50(4):857–862. doi: 10.1097/00006123-200204000-00032. discussion 862–863. [DOI] [PubMed] [Google Scholar]
- Millecamps S, Kiefer H, et al. Neuron-restrictive silencer elements mediate neuron specificity of adenoviral gene expression. Nat. Biotechnol. 1999;17(9):865–869. doi: 10.1038/12849. [DOI] [PubMed] [Google Scholar]
- Miller CR, Williams CR, et al. Intratumoral 5-fluorouracil produced by cytosine deaminase/5-fluorocytosine gene therapy is effective for experimental human glioblastomas. Cancer Res. 2002;62(3):773–780. [PubMed] [Google Scholar]
- Miyaguchi K, Maeda Y, et al. Neuron-targeted gene transfer by adenovirus carrying neural-restrictive silencer element. Neuroreport. 1999;10(11):2349–2353. doi: 10.1097/00001756-199908020-00024. [DOI] [PubMed] [Google Scholar]
- Morelli AE, Larregina AT, et al. Neuronal and glial cell type-specific promoters within adenovirus recombinants restrict the expression of the apoptosis-inducing molecule Fas ligand to predetermined brain cell types, and abolish peripheral liver toxicity. J. Gen. Virol. 1999;80(pt. 3):571–583. doi: 10.1099/0022-1317-80-3-571. [DOI] [PubMed] [Google Scholar]
- Muldoon LL, Nilaver G, et al. Comparison of intracerebral inoculation and osmotic blood-brain barrier disruption for delivery of adenovirus, herpesvirus, and iron oxide particles to normal rat brain. Am. J. Pathol. 1995;147(6):1840–1851. [PMC free article] [PubMed] [Google Scholar]
- Nanda D, Vogels R, et al. Treatment of malignant gliomas with a replicating adenoviral vector expressing herpes simplex virus-thymidine kinase. Cancer Res. 2001;61(24):8743–8750. [PubMed] [Google Scholar]
- Navarro V, Millecamps S, et al. Efficient gene transfer and long-term expression in neurons using a recombinant adenovirus with a neuron-specific promoter. Gene Ther. 1999;6(11):1884–1892. doi: 10.1038/sj.gt.3301008. [DOI] [PubMed] [Google Scholar]
- Nilaver G, Muldoon LL, et al. Delivery of herpesvirus and adenovirus to nude rat intracerebral tumors after osmotic blood-brain barrier disruption. Proc. Natl. Acad. Sci. USA. 1995;92(21):9829–9833. doi: 10.1073/pnas.92.21.9829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmoto Y, Wood MJ, et al. Variation in the immune response to adenoviral vectors in the brain: Influence of mouse strain, environmental conditions and priming. Gene Ther. 1999;6(4):471–481. doi: 10.1038/sj.gt.3300851. [DOI] [PubMed] [Google Scholar]
- Okada T, Shah M, et al. AV.TK-mediated killing of subcutaneous tumors in situ results in effective immunization against established secondary intracranial tumor deposits. Gene Ther. 2001;8(17):1315–1322. doi: 10.1038/sj.gt.3301526. [DOI] [PubMed] [Google Scholar]
- Ooboshi H, Ibayashi S, et al. Adenovirus-mediated gene transfer to ischemic brain: Ischemic flow threshold for transgene expression. Stroke. 2001;32(4):1043–1047. doi: 10.1161/01.str.32.4.1043. [DOI] [PubMed] [Google Scholar]
- Peltekian E, Garcia L, et al. Neurotropism and retrograde axonal transport of a canine adenoviral vector: A tool for targeting key structures undergoing neurodegenerative processes. Mol. Ther. 2002;5(1):25–32. doi: 10.1006/mthe.2001.0517. [DOI] [PubMed] [Google Scholar]
- Peltola M, Kyttala A, et al. Adenovirus-mediated gene transfer results in decreased lysosomal storage in brain and total correction in liver of aspartylglucosaminuria (AGU) mouse. Gene Ther. 1998;5(10):1314–1321. doi: 10.1038/sj.gt.3300740. [DOI] [PubMed] [Google Scholar]
- Perez-Cruet MJ, Trask TW, et al. Adenovirus-mediated gene therapy of experimental gliomas. J. Neurosci. Res. 1994;39(4):506–511. doi: 10.1002/jnr.490390417. [DOI] [PubMed] [Google Scholar]
- Plumb TJ, Bosch A, et al. Hypoxanthine-guanine phosphoribosyltransferase (HPRT) expression in the central nervous system of HPRT-deficient mice following adenoviral-mediated gene transfer. Neurosci. Lett. 1996;214(2–3):159–162. doi: 10.1016/0304-3940(96)12932-3. [DOI] [PubMed] [Google Scholar]
- Proescholdt MA, Heiss JD, et al. Vascular endothelial growth factor (VEGF) modulates vascular permeability and inflammation in rat brain. J. Neuropathol. Exp. Neurol. 1999;58(6):613–627. doi: 10.1097/00005072-199906000-00006. [DOI] [PubMed] [Google Scholar]
- Puumalainen AM, Vapalahti M, et al. Beta-galactosidase gene transfer to human malignant glioma in vivo using replication-deficient retroviruses and adenoviruses. Hum. Gene Ther. 1998;9(12):1769–1774. doi: 10.1089/hum.1998.9.12-1769. [DOI] [PubMed] [Google Scholar]
- Qiu J, Whalen MJ, et al. Upregulation of the Fas receptor death-inducing signaling complex after traumatic brain injury in mice and humans. J. Neurosci. 2002;22(9):3504–3511. doi: 10.1523/JNEUROSCI.22-09-03504.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph GS, Bienemann A, et al. Targeting of tetracycline-regulatable transgene expression specifically to neuronal and glial cell populations using adenoviral vectors. Neuroreport. 2000;11(9):2051–2055. doi: 10.1097/00001756-200006260-00048. [DOI] [PubMed] [Google Scholar]
- Ralph GS, Bienemann A, et al. Disruption of the GluR2-NSF interaction protects primary hippocampal neurons from ischemic stress. Mol. Cell. Neurosci. 2001;17(4):662–670. doi: 10.1006/mcne.2000.0959. [DOI] [PubMed] [Google Scholar]
- Ridoux V, Robert JJ, et al. Adenoviral vectors as functional retrograde neuronal tracers. Brain Res. 1994a;648(1):171–175. doi: 10.1016/0006-8993(94)91919-4. [DOI] [PubMed] [Google Scholar]
- Ridoux V, Robert JJ, et al. The use of adenovirus vectors for intracerebral grafting of transfected nervous cells. Neuroreport. 1994b;5(7):801–804. doi: 10.1097/00001756-199403000-00016. [DOI] [PubMed] [Google Scholar]
- Ross BD, Kim B, et al. Assessment of ganciclovir toxicity to experimental intracranial gliomas following recombinant adenoviral-mediated transfer of the herpes simplex virus thymidine kinase gene by magnetic resonance imaging and proton magnetic resonance spectroscopy. Clin. Cancer Res. 1995;1(6):651–657. [PubMed] [Google Scholar]
- Sandmair AM, Loimas S, et al. Thymidine kinase gene therapy for human malignant glioma, using replication-deficient retroviruses or adenoviruses. Hum. Gene Ther. 2000;11(16):2197–2205. doi: 10.1089/104303400750035726. [DOI] [PubMed] [Google Scholar]
- Sato N, Wang S, et al. A novel strategy for introducing exogenous bcl-2 into neuronal cells: The Cre/loxP system-mediated activation of bcl-2 for preventing programmed cell death using recombinant adenoviruses. Mol. Cell. Neurosci. 1998;12(1–2):65–78. doi: 10.1006/mcne.1998.0703. [DOI] [PubMed] [Google Scholar]
- Shen JS, Watabe K, et al. Intraventricular administration of recombinant adenovirus to neonatal twitcher mouse leads to clinicopathological improvements. Gene Ther. 2001;8(14):1081–1087. doi: 10.1038/sj.gt.3301495. [DOI] [PubMed] [Google Scholar]
- Shering AF, Bain D, et al. Cell type-specific expression in brain cell cultures from a short human cytomegalovirus major immediate early promoter depends on whether it is inserted into herpesvirus or adenovirus vectors. J. Gen. Virol. 1997;78(pt. 2):445–459. doi: 10.1099/0022-1317-78-2-445. [DOI] [PubMed] [Google Scholar]
- Shine HD, Wyde PR, et al. Neurotoxicity of intracerebral injection of a replication-defective adenoviral vector in a semipermissive species (cotton rat) Gene Ther. 1997;4(4):275–279. doi: 10.1038/sj.gt.3300397. [DOI] [PubMed] [Google Scholar]
- Shinoura N, Yoshida Y, et al. Highly augmented cytopathic effect of a fiber-mutant E1B-defective adenovirus for gene therapy of gliomas. Cancer Res. 1999;59(14):3411–3416. [PubMed] [Google Scholar]
- Shinoura N, Yamamoto N, et al. Adenovirus-mediated transfer of Fas ligand gene augments radiation-induced apoptosis in U-373MG glioma cells. Jpn. J. Cancer Res. 2000;91(10):1044–1050. doi: 10.1111/j.1349-7006.2000.tb00883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoura N, Sakurai S, et al. Co-transduction of Apaf-1 and caspase-9 highly enhances p53-mediated apoptosis in gliomas. Br. J. Cancer. 2002;86(4):587–595. doi: 10.1038/sj.bjc.6600061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JG, Raper SE, et al. Intracranial administration of adenovirus expressing HSV-TK in combination with ganciclovir produces a dose-dependent, self-limiting inflammatory response. Hum. Gene Ther. 1997;8(8):943–954. doi: 10.1089/hum.1997.8.8-943. [DOI] [PubMed] [Google Scholar]
- Smith RL, Traul DL, et al. Characterization of promoter function and cell-type-specific expression from viral vectors in the nervous system. J. Virol. 2000;74(23):11254–11261. doi: 10.1128/jvi.74.23.11254-11261.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Arica JR, Morelli AE, et al. Cell-type-specific and regulatable transgenesis in the adult brain: Adenovirus-encoded combined transcriptional targeting and inducible transgene expression. Mol. Ther. 2000;2(6):579–587. doi: 10.1006/mthe.2000.0215. [DOI] [PubMed] [Google Scholar]
- Soudais C, Laplace-Builhe C, et al. Preferential transduction of neurons by canine adenovirus vectors and their efficient retrograde transport in vivo. FASEB J. 2001;15(12):2283–2285. doi: 10.1096/fj.01-0321fje. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Manome Y, et al. Viral vector-mediated transduction of a modified platelet factor 4 cDNA inhibits angiogenesis and tumor growth. Nat. Med. 1997;3(4):437–442. doi: 10.1038/nm0497-437. [DOI] [PubMed] [Google Scholar]
- Thomas CE, Birkett D, et al. Acute direct adenoviral vector cytotoxicity and chronic, but not acute, inflammatory responses correlate with decreased vector-mediated transgene expression in the brain. Mol. Ther. 2001a;3(1):36–46. doi: 10.1006/mthe.2000.0224. [DOI] [PubMed] [Google Scholar]
- Thomas CE, Schiedner G, et al. Preexisting antiadenoviral immunity is not a barrier to efficient and stable transduction of the brain, mediated by novel high-capacity adenovirus vectors. Hum. Gene Ther. 2001b;12(7):839–846. doi: 10.1089/104303401750148829. [DOI] [PubMed] [Google Scholar]
- Thomas CE, Edwards P, et al. Adenovirus binding to the coxsackievirus and adenovirus receptor or integrins is not required to elicit brain inflammation but is necessary to transduce specific neural cell types. J. Virol. 2002;76(7):3452–3460. doi: 10.1128/JVI.76.7.3452-3460.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trask TW, Trask RP, et al. Phase I study of adenoviral delivery of the HSV-tk gene and ganciclovir administration in patients with current malignant brain tumors. Mol. Ther. 2000;1(2):195–203. doi: 10.1006/mthe.2000.0030. [DOI] [PubMed] [Google Scholar]
- Umana P, Gerdes CA, et al. Efficient FLPe recombinase enables scalable production of helper-dependent adenoviral vectors with negligible helper-virus contamination. Nat. Biotechnol. 2001;19(6):582–585. doi: 10.1038/89349. [DOI] [PubMed] [Google Scholar]
- Umegaki H, Ishiwata K, et al. In vivo assessment of adenoviral vector-mediated gene expression of dopamine D(2) receptors in the rat striatum by positron emission tomography. Synapse. 2002;43(3):195–200. doi: 10.1002/syn.10035. [DOI] [PubMed] [Google Scholar]
- Vincent AJ, Esandi MD, et al. Treatment of leptomeningeal metastases in a rat model using a recombinant adenovirus containing the HSV-tk gene. J. Neurosurg. 1996a;85(4):648–654. doi: 10.3171/jns.1996.85.4.0648. [DOI] [PubMed] [Google Scholar]
- Vincent AJ, Vogels R, et al. Herpes simplex virus thymidine kinase gene therapy for rat malignant brain tumors. Hum. Gene Ther. 1996b;7(2):197–205. doi: 10.1089/hum.1996.7.2-197. [DOI] [PubMed] [Google Scholar]
- Viola JJ, Ram Z, et al. Adenovirally mediated gene transfer into experimental solid brain tumors and leptomeningeal cancer cells. J. Neurosurg. 1995;82(1):70–76. doi: 10.3171/jns.1995.82.1.0070. [DOI] [PubMed] [Google Scholar]
- Wagenknecht B, Glaser T, et al. Expression and biological activity of X-linked inhibitor of apoptosis (XIAP) in human malignant glioma. Cell Death Differ. 1999;6(4):370–376. doi: 10.1038/sj.cdd.4400503. [DOI] [PubMed] [Google Scholar]
- Wang MS, Fang G, et al. The WldS protein protects against axonal degeneration: A model of gene therapy for peripheral neuropathy. Ann. Neurol. 2001;50(6):773–779. doi: 10.1002/ana.10039. [DOI] [PubMed] [Google Scholar]
- Xia H, Anderson B, et al. Recombinant human adenovirus: Targeting to the human transferrin receptor improves gene transfer to brain microcapillary endothelium. J. Virol. 2000;74(23):11359–11366. doi: 10.1128/jvi.74.23.11359-11366.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia XG, Harding T, et al. Gene transfer of the JNK interacting protein-1 protects dopaminergic neurons in the MPTP model of Parkinson’s disease. Proc. Natl. Acad. Sci. USA. 2001;98(18):10433–10438. doi: 10.1073/pnas.181182298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang GY, Zhao YJ, et al. Overexpression of interleukin-1 receptor antagonist in the mouse brain reduces ischemic brain injury. Brain Res. 1997;751(2):181–188. doi: 10.1016/s0006-8993(96)01277-2. [DOI] [PubMed] [Google Scholar]
- Yang GY, Mao Y, et al. Expression of intercellular adhesion molecule 1 (ICAM-1) is reduced in permanent focal cerebral ischemic mouse brain using an adenoviral vector to induce overexpression of interleukin-1 receptor antagonist. Brain Res. Mol. Brain Res. 1999;65(2):143–150. doi: 10.1016/s0169-328x(98)00335-0. [DOI] [PubMed] [Google Scholar]
- Yang GY, Pang L, et al. Attenuation of ischemia-induced mouse brain injury by SAG, a redox-inducible antioxidant protein. J. Cereb. Blood Flow Metab. 2001;21(6):722–733. doi: 10.1097/00004647-200106000-00010. [DOI] [PubMed] [Google Scholar]
- Yoon SO, Lois C, et al. Adenovirus-mediated gene delivery into neuronal precursors of the adult mouse brain. Proc. Natl. Acad. Sci. USA. 1996;93(21):11974–11979. doi: 10.1073/pnas.93.21.11974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H, Zhai P, et al. Recombinant adenovirus is an appropriate vector for endocytotic protein trafficking studies in cultured neurons. J. Neurosci. Methods. 1999;88(1):45–54. doi: 10.1016/s0165-0270(99)00011-4. [DOI] [PubMed] [Google Scholar]
- Zermansky AJ, Bolognani F, et al. Towards global and long-term neurological gene therapy: Unexpected transgene dependent, high-level, and widespread distribution of HSV-1 thymidine kinase throughout the CNS. Mol. Ther. 2001;4(5):490–498. doi: 10.1006/mthe.2001.0479. [DOI] [PubMed] [Google Scholar]
- Zheng C, Baum BJ, et al. Genomic integration and gene expression by a modified adenoviral vector. Nat. Biotechnol. 2000;18(2):176–180. doi: 10.1038/72628. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Yang GY, et al. Transforming growth factor-beta 1 increases bad phosphorylation and protects neurons against damage. J. Neurosci. 2002;22(10):3898–3909. doi: 10.1523/JNEUROSCI.22-10-03898.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L, Zhou H, et al. Prolonged transgene expression mediated by a helper-dependent adenoviral vector (hdAd) in the central nervous system. Mol. Ther. 2000;2(2):105–113. doi: 10.1006/mthe.2000.0104. [DOI] [PubMed] [Google Scholar]
- Zou L, Yuan X, et al. Helper-dependent adenoviral vector-mediated gene transfer in aged rat brain. Hum. Gene. Ther. 2001;12(2):181–191. doi: 10.1089/104303401750061249. [DOI] [PubMed] [Google Scholar]